Abstract
Abundant autologous proteins, like serum albumin, should be immunologically inert. However, individuals with no apparent predisposition to autoimmune disease can develop immune responses to autologous therapeutic proteins. Protein aggregation is a potential major trigger of these responses. Adsorption of proteins to particles provides macromolecular size and may generate structural changes in the protein, resembling aggregation. Using aldehyde/sulfate latex beads coated with murine serum albumin (MSA), we found that mice mounted MSA-specific IgG responses that were dependent on CD4+ T cells. IgG were specific for MSA adsorbed to solid surfaces and non-cross-reactive with human, bovine or pig albumins. T cells induced in response to MSA, augmented the primary and induced boosted secondary IgG and IgM responses specific for the T cell-independent antigen, capsular polysaccharide of Streptococcus pneumoniae type 14 (PPS14), when the latter was attached to the same bead. Similar to the anti-MSA IgG response, the boosted PPS14-specific IgG secondary response was CD4+ T cell-dependent, displayed a typical carrier effect, and was enhanced by, but did not require, Toll-like receptor stimulation. These results provide a potential mechanism for the induction of responses to autoantigens unable to induce specific T cell responses, and provide new insights into polysaccharide-specific immunity.
Keywords: Autoimmunity, Humoral responses, Immunity to bacteria, Autoreactive memory responses
INTRODUCTION
Autologous proteins are typically non-immunogenic. However, therapeutic autologous proteins often elicit antibody responses in non-autoimmune hosts. [1–3]. These responses reduce their efficacy [3], and in some instances, produce severe pathology[2; 4]. Degradation, modification or aggregation of the protein [5], or its contamination with TLR ligands [6; 7], are some of the possible factors responsible for inducing these antibody responses, although the underlying mechanisms are poorly understood.
Human serum albumin has a wide variety of clinical applications including intravascular volume stabilization [8] and as a stabilizer for protein therapeutics and vaccines [9]. Albumin constitutes nearly 60% of total plasma proteins [10]. It exhibits limited polymorphism, including no known phenotypic variation in inbred mouse strains [11]. In humans, although the gene for albumin is highly polymorphic, variations in the encoded protein sequence are rare [12]. Moreover, during its synthesis albumin is non-glycosylated, reducing its potential variability, although 6–15% may undergo nonenzymatic glycation in the blood [13] [14]. Glycation alters the conformation and function of albumin [15; 16]. Albumin also binds many ligands in serum [10; 17], and interacts specifically with a variety of host cells [18], and some bacterial pathogens [19–21]. Bacteria can also bind albumin passively or indirectly through receptors specific for other serum factors bound to albumin, like heme [22].
Extracellular bacteria are antigenically-complex particles in which capsular polysaccharide (CPS) and proteins are non-covalently associated. Antibody responses to CPS expressed by intact bacteria display features intermediate between purified CPS and CPS-protein conjugate vaccines [23; 24]. Thus, in contrast to the IgG response to isolated CPS of Streptococcus pneumoniae type 14 (PPS14), which is T cell-independent (TI), both the PPS14-specific IgG responses to intact S. pneumoniae and to PPS14-protein conjugate vaccines are dependent upon CD4+ T cell help. However, in contrast to conjugates, both purified and bacteria-linked PPS14 induce anti-PPS14 IgG responses with limited affinity maturation [25] [23]. Moreover, because of their particulate nature, and similarly to protein aggregates, bacteria concentrate within the marginal zone (MZ) of the spleen [26], and are more efficiently internalized by APCs [23; 27]. As a result, anti-PPS14 IgG responses induced by bacteria are largely elicited by MZB cells and dominated by the 44.1-idiotype [25]. In contrast, anti-PPS14 IgG responses to soluble conjugates arise from follicular B cells with only minor expression of this idiotype [25; 28]. We now show that murine serum albumin (MSA) attached to bacteria-size (1 µm) latex beads induce MSA-specific B and T cell responses, and that these responses can provide efficient help for antibody responses specific for CPS co-expressed non-covalently on the same bead. These results suggest a novel link between autoimmunity and anti-bacterial humoral immunity.
RESULTS
Autologous MSA attached to PPS14-coated beads induces CD4+ T cell help for boosted anti-PPS14 Ig response
Autologous therapeutic proteins often induce unwanted antibody responses potentially resulting from self-aggregation [1]. In light of albumin binding to bacterial surfaces, potentially mimicking this aggregation, we wished to determine whether MSA attached to bacteria-sized particles could induce an autoimmune response, and perhaps elicit CD4+ T cell help for a non-covalently associated TI antigen, such as bacterial CPS. Thus, PPS14 and MSA were both covalently attached to 0.96µm diameter latex beads, but not to each other (Supplemental figure 1; PPS14+[MSA]-beads). Additional beads, used as controls, were coated with similar amounts of MSA alone ([MSA]-beads) or PPS14 alone (PPS14+[Gly]-beads) or without any antigen ([Gly]-beads).
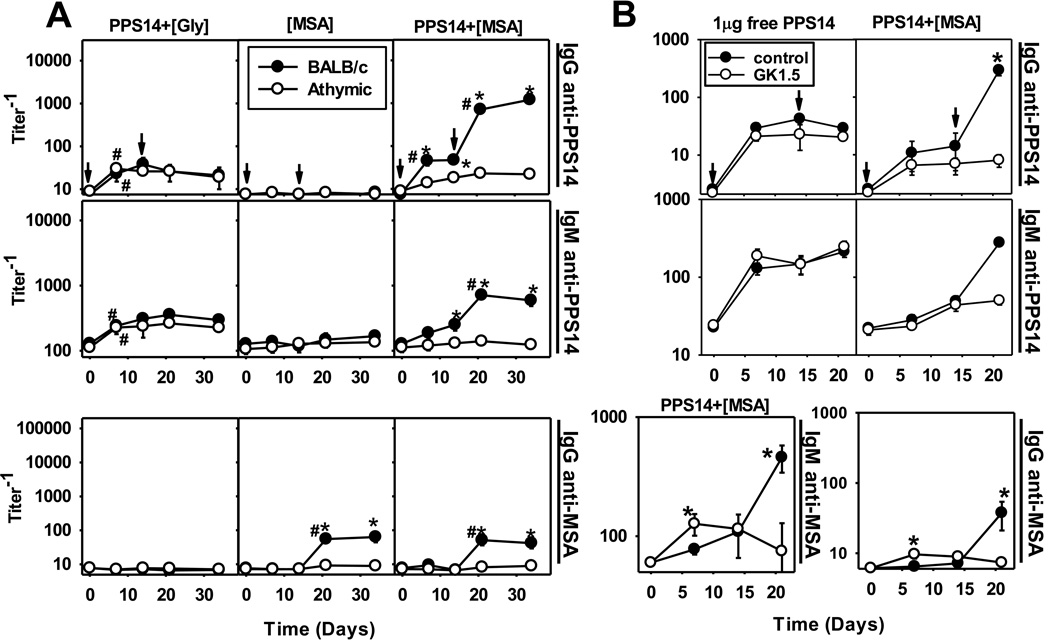
Both [MSA]- and PPS14+[MSA]-, but not PPS14+[Gly]-beads induced a modest but significant secondary anti-MSA IgG response in BALB/c, but not in athymic nude mice (Figure 1A). Further, PPS14+[MSA], but not [MSA] beads induced primary, and highly boosted secondary anti-PPS14 IgG responses in BALB/c, but not in athymic nude mice (Figure 1A), that included all IgG subclasses (Supplemental figure figure 2A). Primary and secondary PPS14-specific IgG responses to free PPS14 and PPS14+[Gly]-beads were mostly IgG1 and IgG3 (Supplemental figure 2). MSA-specific IgG were preferentially IgG2a and IgG3 (Supplemental figure 2B), although the MSA-specific IgG secondary responses kinetically mirrored the PPS14-specific IgG responses (Figure 1A). These results clearly indicate that the induction of boosted PPS14-specific IgG was T cell-dependent (TD). PPS14-specific IgM secondary responses were also boosted in a TD manner (Figure 1A). In contrast, PPS14+[Gly]-beads induced primary PPS14-specific IgG and IgM responses in BALB/c mice that were not significantly different in serum titer than the secondary response (p=0.11) or in nude mice (p=0.31; Figure 1A), indicating their strictly TI nature. These results demonstrate that MSA is directly involved in the induction of TD boosted responses to PPS14 when the two are co-expressed on the same bead.
Figure 1. PPS14 and autologous MSA co-attached to latex beads induce PPS14-specific antibody responses in a T cell-dependent manner.
(A) BALB/c and BALB/c athymic nude mice (n=7), were immunized on days 0 and 14 (arrows) with ≈ 2×108 latex beads, coated with PPS14 combined with glycine or MSA, in alum+CpG-ODN. Each dose of the corresponding beads was adjusted to contain approximately 230ng of serologically active PPS14. (B) Female BALB/c mice (n=5) were injected with 1mg of anti-CD4 mAb (GK1.5) or polyclonal rat IgG2b (control), 32h before injection with 1µg of free PPS14, or 2.2 × 108 PPS14+[MSA]-beads containing 80ng of PPS14, both admixed only with CpG-ODN. Mice received a secondary immunization 14 days later with the same antigen in the absence of anti-CD4 mAb or polyclonal rat IgG. Anti-MSA IgM responses are only shown for Panel A. Panel A and B shown different experiments using different bead preparations. Beads were always washed in PBS prior to immunization, to remove any antigen leaked free to the supernatant. Serum titers of IgG and IgM specific for PPS14 and MSA were determined by ELISA. Values are expressed as geometric mean ± SEM. *p < 0.05 (BALB/c versus athymic nude mice or controls relative to GK1.5 treated mice), #p< 0.05 (titer−1 relative to the previous bleeding of each mouse group); Student's t-test.
BALB/c mice were further acutely depleted of CD4+ T cells with anti-mouse CD4 mAb prior to primary immunization with PPS14+[MSA]-beads. These mice, in contrast to controls, failed to induce boosted PPS14-specific IgG and IgM responses upon secondary immunization (Figure 1B), although the primary PPS14-specific antibody responses were similar between the two groups (Figure 1B). Anti-CD4 mAb also inhibited the secondary IgG anti-MSA response to PPS14+[MSA]-beads (Figure 1B). Antibody responses to free PPS14, a TI antigen, were not boosted or affected by depletion of CD4+ T cells (Figure 1B). Thus, induction of boosted PPS14-specific Ig secondary responses to PPS14+[MSA]-beads required MSA-dependent priming of CD4+ T cells.
Secondary anti-PPS14 IgG responses to PPS14+[MSA]-beads are enriched in expression of 44.1-idiotype
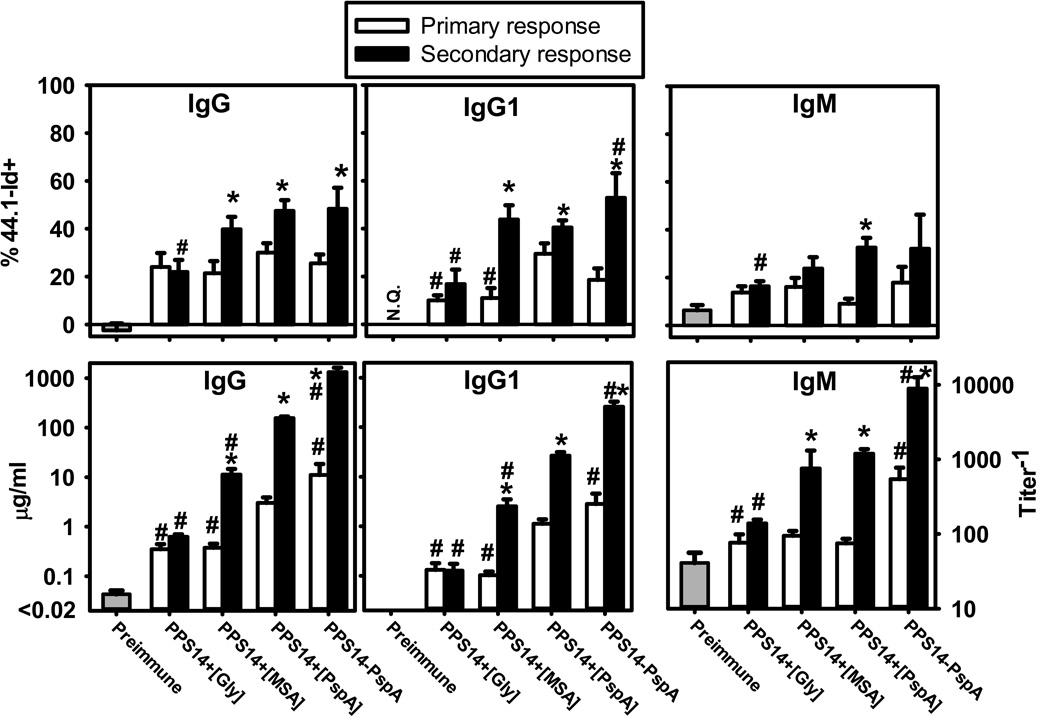
The idiotype 44.1-Id dominates the PPS14-specific IgG, but not IgM, responses of BALB/c mice to bacteria expressing PPS14 [25]. In distinct contrast, PPS14-specific IgG responses to soluble PPS14-PspA conjugate exhibited minimal usage of 44.1-Id, although significant 44.1-Id expression was elicited in response to bead-associated conjugate [25], or beads with non-covalent association of PPS14 and PspA ([PPS14+[PspA]) [29]. Thus, 44.1-Id dominates oligoclonal responses specific for PPS14 in particulate form. IgG1 and total IgG anti-PPS14 responses to both PPS14+[MSA] and PPS14+[PspA] beads exhibited similar 44.1-Id usage (p>0.152) (Figure 2). Relative to the primary, the contribution of 44.1-Id to the secondary IgG and IgG1 responses increased substantially for both (p<0.017), whereas no increase was noted in the secondary responses to PPS14+[Gly]-beads (Figure 2). Thus, bead-associated MSA, like the foreign protein PspA, recruit 44.1-Id+ B cell clones when associated with PPS14.
Figure 2. Secondary PPS14-specific IgG responses to PPS14+[MSA]-beads are highly enriched in the expression of the 44.1-Idiotype.
BALB/c mouse sera (n=21) collected 7–14 days after a primary (primary response) or 7 days after a secondary (secondary response) immunization at day 14 with latex beads coated with the antigen combinations indicated, were analyzed for the expression of the 44.1-Id in PPS14-specific IgG, IgG1 and IgM by inhibition ELISA. Sera were collected in three independent experiments. Mice received the primary immunization at day 0 and challenged at day 14. *p< 0.05 (primary relative to secondary response), #p< 0.05 (each group relative to the responses to PPS14+[PspA]-beads); Student's t-test.
PPS14-specific IgG responses to PPS14+[MSA] beads undergo minimal avidity maturation
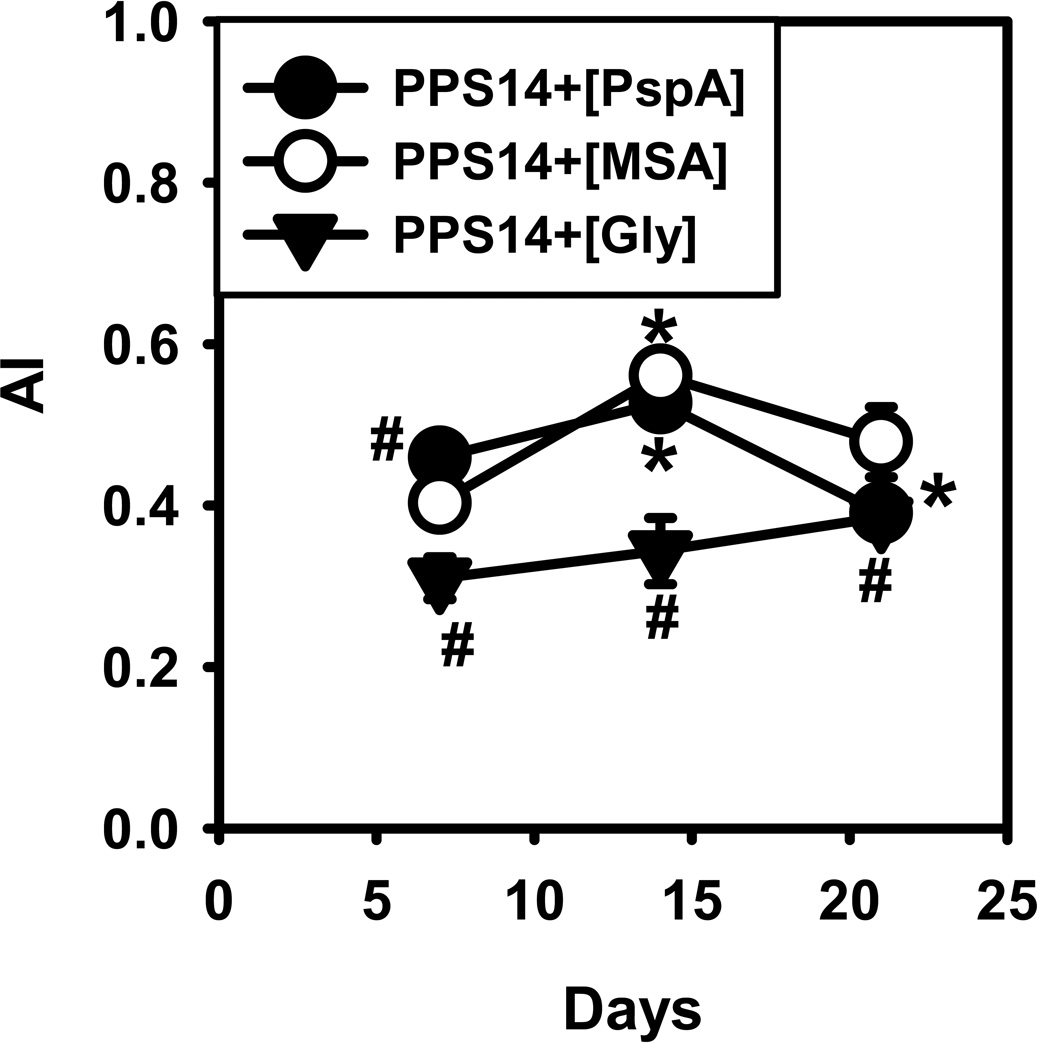
As shown in Figure 3, although the avidity of PPS14-specific IgG increases significantly during the primary response to PPS14+[MSA] beads (p=0.00006), this increase is rather modest and not sustained during the secondary response, similar to PPS14+[PspA]-beads. In contrast, the avidity in response to PPS14+[Gly]-beads was lower (p<0.023) and remained unchanged (p>0.36) (Figure 3). Thus, bead-associated MSA promotes a similar degree of affinity maturation as PspA for an IgG anti-PPS14 response.
Figure 3. Average avidity of PPS14-specific IgG responses to PPS14+[MSA]-beads.
The average avidity (AI) of PPS14-specific IgG in serum was estimated by elution with sodium thiocyanate (NaSCN). Mice were immunized at days 0 and 14 with ≈2×108 latex beads containing co-attached 230 ng of PPS14 and 202 ng PspA (PPS14+[PspA]) or with 42 ng MSA (PPS14+[MSA]) or in the absence of proteins, blocked with an excess of Gly (PPS14+[Gly]). All beads were admixed with CpG-ODN (without alum added). Sera tested (n=15–17) were from three independent experiments. Data show the avidity index (AI), as the molar concentration of NaSCN eluting 50% of the total content of PPS14-specific IgG in the serum sample. *p < 0.05 (titer−1 relative to serum samples from the previous time point of each mouse group), #p < 0.05 (each group relative to the responses to PPS14+[PspA]-beads); Student's t-test.
Bead-associated preimmune mouse sera (NMS) is also immunogenic
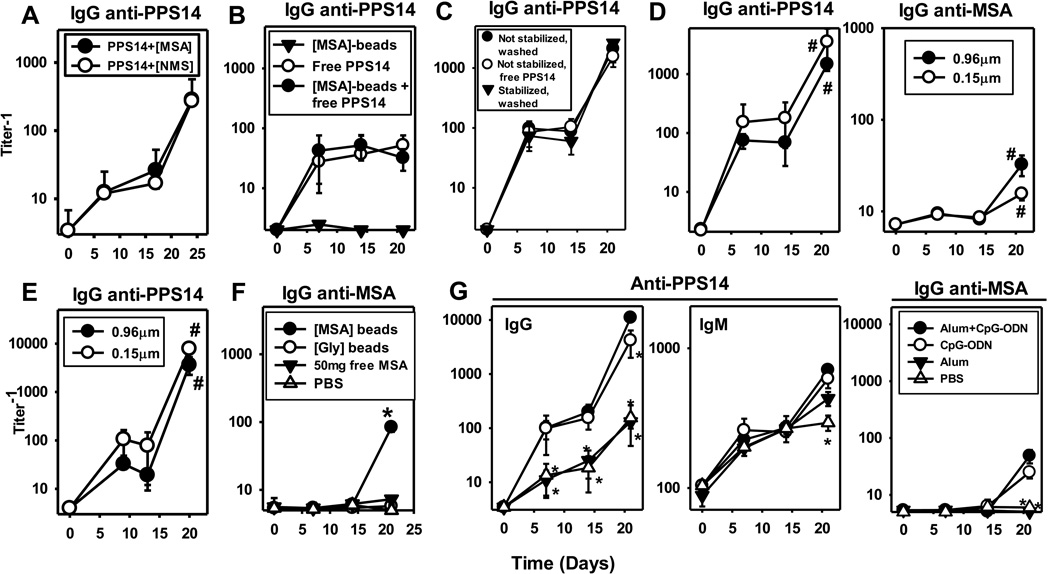
PPS14+[NMS]-beads were produced using sera from the same mice to be immunized, to rule out a potential role for contaminating exogenous or allotypic proteins in the MSA preparation. These beads contained only slightly less MSA (136±16ng/109 beads) than PPS14+[MSA]-beads (232±19 ng/109 beads; Supplemental Figure 1), with a considerably lower content of other major serum proteins, like IgG (16±2 ng/109 beads). PPS14+[NMS]-beads were equally efficient as PPS14+[MSA]-beads in inducing PPS14-specific IgG responses (Figure 4A), strongly ruling against a role for contaminants in the MSA preparation.
Figure 4. MSA-specific IgG and PPS14-specific IgG secondary responses to PPS14+[MSA]-beads are induced only by MSA and/or PPS14 attached to either micro- or nanobeads; enhancement of responses by CpG-ODN, but not alum.
Serum titers of PPS14-specific IgG in BALB/c mice (n=5) immunized at day 0 and 14 with (A) ≈2.2×108 latex beads containing 280ng of PPS14 attached to beads coated with MSA (PPS14+[MSA]) or normal mouse sera (PPS14+[NMS]), previously collected from the same mice receiving the beads. (B) 2×108 latex beads coated with MSA ([MSA]-beads), 30 ng of free PPS14, or a mixture of 2×108 [MSA]-beads and 30ng of free PPS14 ([MSA]-beads + free PPS14). (C) ≈2×108 PPS14+[MSA]-beads of a preparation in which the PPS14 attachment to the bead was not stabilized by reduction with cyanoborohydride and contained 209ng of PPS14 attached and 20ng of free PPS14, after >1year of storage. This preparation was used for immunization after washings with PBS that reduced the free PPS14 content to <0.8 ng (not stabilized, washed), or directly without washings (not stabilized, free PPS14), and compared to a preparation in which the Schiff’s bases were reduced with cyanoborohydride (stabilized, washed). (D) 2×108 0.96 µm in diameter, or 1.46 × 1011 0.15µm in diameter, PPS14+[MSA]-beads containing 200 ng of attached PPS14 (E) 3.5×108 0.96µm in diameter, or 4.5×109 0.15 µm in diameter, PPS14+[MSA]-beads containing 28 ng of attached PPS14. #p <0.05 (for C and D, primary [day 14] relative to secondary [day 21] responses); Student's t-test. (F) Anti-MSA IgG in sera of BALB/c mice (n=7) immunized at day 0 and 14 with 2×108 beads coated with 100 ng MSA ([MSA]-beads) or glycine ([Gly]-beads) combined with 25µg CpG-ODN, or 50µg of purified MSA (free MSA) admixed with 25µg CpG-ODN and 13µg of alum. A mixture of CpG-ODN and alum (PBS) was used as control. (G) Serum titers of MSA-specific IgG and PPS14-specific IgG and IgM from BALB/c mice (n=7) immunized at day 0 and 14, with 2×108 PPS14+[MSA]-beads containing 240ng of PPS14 attached to the beads. Beads were admixed for 1 hour at room temperature with 13µg of alum and 25µg CpG-ODN (alum+CpG-ODN), 25µg CpG-ODN alone (CpG-ODN), 13µg alum alone (alum), or in the same final volume of PBS (PBS).*p<0.05 (every group relative to mice immunized with PPS14+[MSA]-beads admixed with alum+CpG-ODN). Latex beads used in all the experiments, except for panel D and E, were 0.96µm in diameter. One of three (panel G), or two (panels A, D and F) independent experiments is shown.
PPS14 only attached to beads induced boosted anti-PPS14 IgG secondary response to PPS14+[MSA]-beads
Immunization with a mixture of 30ng free PPS14 and 2×108 [MSA]-beads, even when admixed with CpG-ODN+alum, induced no boosted, and statistically identical (p>0.51) PPS14-specific IgG (Figure 4B) and IgM responses (data not shown) to those induced by 30ng of free PPS14 in the absence of [MSA]-beads. Moreover, a preparation of PPS14+[MSA]- beads in which the PPS14-linkage was not stabilized with cyanoborohydride and contained >10% PPS14 released from the beads, after >1year in storage, induced identical PPS14-specific IgG responses as the same bead preparation following the removal of free PPS14 by centrifugation (Figure 4C) as well as PPS14+[MSA]-beads prepared at the same time, in which the PPS14-linkage was stabilized with cyanoborohydride (Figure 4C; <0.5% free PPS14). Thus, only the PPS14 directly attached to the bead, regardless of the linkage’s stability, participates in the induction of boosted PPS14-specific IgG responses, with no significant contribution or inhibitory effect induced by free PPS14.
Boosted anti-PPS14 IgG secondary responses to PPS14+[MSA]-beads are induced using micro- and nanobeads
Microparticles (0.96µm), in contrast to nanoparticles (0.15µm), cannot be phagocytized by conventional B cells, and have a limited ability to enter into the follicles [30–32]. However, PPS14− and MSA-specific IgG responses were not statistically different in mice immunized with the same amount (200ng) of PPS14 attached to 0.96µm and 0.15µm PPS14+[MSA]-beads (Figure 4D). Because the surface area is considerably lower on nanobeads, 730 times more nanobeads (1.46 × 1011) than microbeads (2×108) were needed to contain equal amounts of PPS14. Further, APCs need to internalize more nano- than microbeads to acquire similar amounts of antigen. To address this issue, microbeads were coated suboptimally with PPS14, whereas nanobeads were coated optimally. Nevertheless, mice immunized with the same amount of PPS14 (28ng), but this time contained in only 12 times more nano- (4.5×109) than microbeads (3.5×108) produced similar PPS14-specific IgG (Figure 4E) and IgM (data not shown) responses. These results suggest that professional APC are the major target and effector of the response to PPS14+[MSA]-beads, as a facilitated access of the beads to B cells had no major impact on the responses.
Anti-MSA IgG responses are induced only by MSA attached to the bead
Adsorption of MSA to the bead was required, as two immunizations with 50µg of free MSA in CpG-ODN+alum did not induce detectable MSA-specific antibodies (Figure 4F). In contrast, beads coated with only 100ng MSA induced anti-MSA IgG when combined with CpG (Figure 4F). Further, [NMS]-beads also induced anti-MSA IgG in the donor mice (data not shown). These results demonstrate that only bead-attached, but not soluble, MSA induces an immune response.
Anti-MSA Ig interacts specifically with solid phase adsorbed autologous, not xenogeneic, albumins
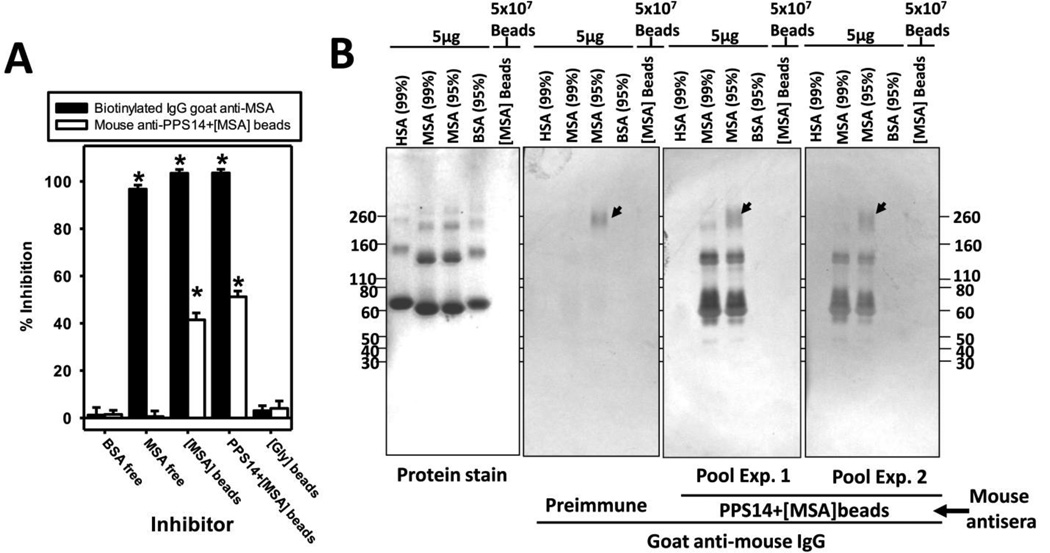
Detection of MSA-specific IgG was unexpected, not only because MSA is an autologous protein, but also because of the high levels of MSA in the bloodstream, that could block the binding of induced MSA-specific antibodies. However, this is consistent with the inability of soluble MSA (Figure 5A; 0.1–1000µg/ml) to inhibit the binding of MSA-specific IgG (Figure 5A) and IgM (data not shown) to MSA-coated plates. In contrast, >2500-times lower amounts of MSA attached to beads produced significant, although incomplete, inhibition (Figure 5A). Thus, these results strongly suggest that the interaction of MSA with mouse anti-MSA IgG is of low avidity, and detected only with MSA adsorbed to solid surfaces.
Figure 5. Sera from mice immunized with latex beads coated with MSA contain IgG that specifically recognized MSA adsorbed to solid surfaces.
(A) Inhibitory capacity of soluble and particulate forms of MSA. Mouse sera collected 7 days after a secondary immunization with 2×108 PPS14+[MSA]-beads were incubated overnight at 4°C with 100µg/well of soluble MSA, soluble BSA or 41ng/well MSA attached to beads, alone ([MSA]-beads) or in combination with PPS14 (PPS14+[MSA]-beads), all of them diluted in 2%OVA. This is the highest number of beads that can be reliably used in the assay. Sera incubated with the same amount of latex beads coated only with Gly ([Gly]-beads) were used as negative control. 1/20,000 dilutions of biotinylated polyclonal goat anti-MSA was used as positive control for inhibition. Unbound anti-MSA IgG was detected by ELISA in wells coated with 5µg/ml of MSA, and blocked with 2% OVA. *p <0.05 (relative to group immunized with [Gly]-beads); Student's t-test. Three independent experiments carried out in duplicate. (B) Purified serum albumins from human (HSA), bovine (BSA) and mouse (MSA) of two different degrees of purification (95% or 99% purity) were resolved in 4–20% SDS-PAGE Tris-Glycine gradient gels under non-reducing conditions. The supernatant of a boiled sample of [MSA]-beads is also included. The separated proteins were stained with Coomassie Blue (protein stain), or transferred to 0.45µm nitrocellulose membranes and detected by incubation with 1/100 dilution of a pool of five sera collected before immunization (preimmune), or 7 days after a secondary immunization with PPS14+[MSA]-beads. Immune sera from two independent experiments (Exp1 and Exp 2), using different PPS14+[MSA]-bead preparations are shown. Bound antibodies were detected with polyclonal goat anti-mouse IgG conjugated to horseradish-peroxidase followed by 4-chloronaphtol. The location of mouse globulins in the blot, which reacts with the secondary goat anti-mouse IgG(H+L), is indicated by arrows.
IgG induced in response to PPS14+[MSA] beads was specific for MSA, and not other autologous proteins, as demonstrated by the reactivity of immune, but not preimmmune, sera with all the protein bands corresponding to the electrophoretic pattern of MSA in Western blots (Figure 5B). Bands >67kDa (Figure 5B) are the result of the multimerization of albumin [15; 33]. Thus, MSA aggregation does not affect its recognition by autologous anti-MSA IgG. Mouse IgG was the only protein band detected that did not correspond to albumin (Figure 5B, arrows), but entirely due to reactivity with the secondary goat anti-mouse IgG. This was indicated by its presence also in preimmune sera and the decreased band intensity in MSA of 99% versus 95% purity. Antisera showed no reactivity with the supernatant of boiled [MSA]-beads (Figure 5B), suggesting that most of the MSA remains attached to beads, and that no free components in the [MSA]-bead produced this reactivity. More importantly, anti-MSA IgG was species-specific, and did not cross-react with human or bovine albumin (Figure 5B).
Anti-MSA and anti-PPS14 IgG responses to PPS14+[MSA]-beads are enhanced by distinct TLR agonists
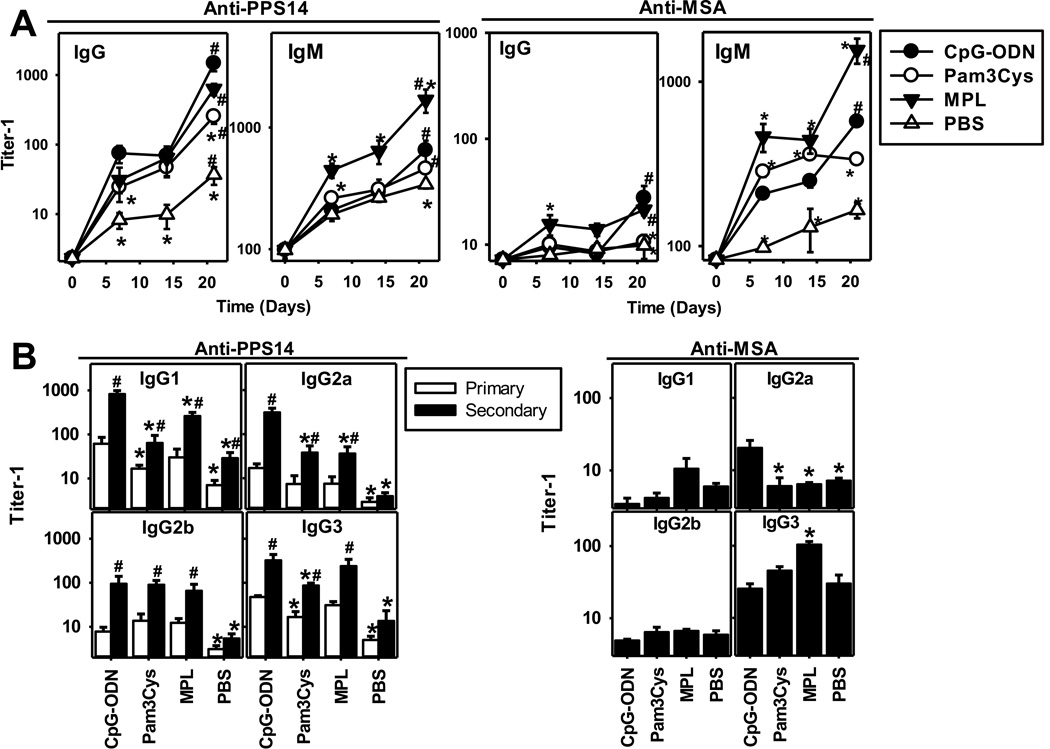
We next wished to determine the role of specific adjuvants in immunity to bead-associated antigens. Primary and boosted secondary IgG anti-PPS14, but not IgG anti-MSA, responses to PPS14+[MSA]-beads were induced in the absence of adjuvant (Figure 4G). However, addition of CpG-ODN, an agonist for the intracellular TLR9, markedly enhanced both primary (>8-fold) and secondary (>85-fold) IgG anti-PPS14 responses and induced a secondary IgG anti-MSA response. (Figure 4G). In contrast, alum alone had no significant effects on any of the IgG responses, nor did it further enhance CpG-ODN adjuvanticity (Figure 4G). These data suggest that latex beads, like alum, which are potent inflammasome activators[34] can replace the adjuvant properties of alum. Addition of synthetic agonists for the cell surface TLR4 (monophosphoryl lipid A; MPL [35]) or TLR2 (Pam3CSK4 [36]) also enhanced the primary and secondary PPS14-specific IgG responses to PPS14+[MSA]-beads (Figure 6; p<0.02). However, Pam3CSK4 was less efficient than either MPL or CpG-ODN (p=0.009; Figure 6A). Moreover, Pam3CSK4in contrast to CpG-ODN or MPL, failed to promote detectable secondary anti-MSA IgG or IgM responses to PPS14+[MSA] beads (Figure 6A). Consistent with the ability of MPL to enhance both polyclonal and antigen-specific IgM responses [37], PPS14− and MSA-specific IgM responses were significantly higher (p<0.011) and more strongly boosted than with CpG-ODN (Figure 6A), despite similar IgG responses (p>0.24). These three adjuvants also distinctively altered the IgG subclass response to PPS14+[MSA] beads relative to no adjuvant, where only IgG1 and IgG3 were detected (Figure 6B). Thus, TLR-mediated activation is required for the induction of anti-MSA and optimal anti-PPS14 antibodies responses to PPS14+[MSA], regardless of the specific TLR involved.
Figure 6. PPS14-specific IgG responses to PPS14+[MSA]-beads are enhanced by TLR ligand adjuvants, but not alum.
BALB/c mice (n=7) were immunized at day 0 and 14, with 2×108 PPS14+[MSA]-beads containing 240ng of PPS14 attached to the beads. Beads were admixed for 1 hour at room temperature before immunization with 25µg CpG-ODN, 50µg Pam3CSK4 (Pam3Cys) or 20µg synthetic monophosphoryl lipid A (MPL). No alum was added. (A) serum titers of MSA- and PPS14-specific IgG and IgM. (B) Serum titers of IgG isotypes during the primary (day 14) and secondary (day 21) responses. For serum titers of anti-MSA IgG, only secondary responses are shown because primary responses were mostly undetectable. One of two independent experiments is shown. *p < 0.05 (every group relative to mice immunized with PPS14+[MSA]-beads admixed with CpG-ODN). #p < 0.05 (primary [day 14] versus secondary [day 21] responses); Student's t-test.
Beads coated only with MSA prime for anti-PPS14 IgG responses to PPS14+[MSA]-beads
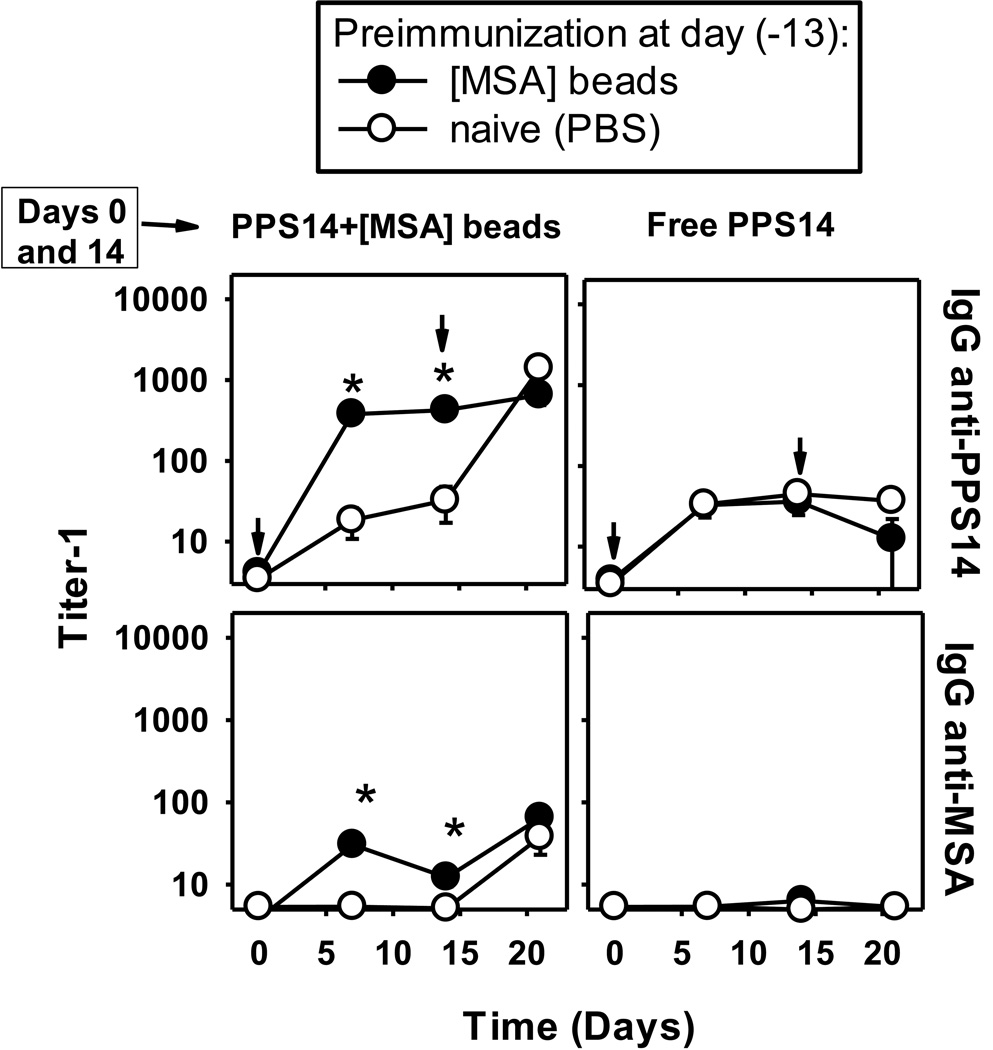
We next determined whether beads coated only with MSA could induce a typical carrier effect [38], enhancing PPS14-specific responses to subsequent immunization with PPS14+[MSA]-beads. Priming with [MSA] beads resulted in a higher and more rapid primary PPS14-specific IgG responses to PPS14+[MSA]-beads (Figure 7), but did not affect the PPS14-specific primary response to PPS14+[PspA]-beads (data not shown), or free PPS14 (Figure 7A, indicating that the effect was MSA-specific and unrelated to T or B cells with PPS14-specificity. Of note, PPS14− and MSA-specific IgG responses to a second immunization with PPS14+[MSA]-beads were not boosted in mice primed with [MSA]-beads (Figure 7).
Figure 7. Previous immunity to [MSA] beads, boosts the PPS14-specific IgG responses to PPS14+[MSA]-beads.
BALB/c mice (n=7) were pre-immunized with 2×108 beads coated with 37ng MSA ([MSA]-beads), 13 days before the start of the immunization schedule. Control mice received PBS (naïve). At day 0 and 14 (indicated by arrows), mice were immunized with 450ng of free PPS14 or ≈108 latex beads coated with, PPS14 and MSA (PPS14+[MSA]-beads), containing 160ng of PPS14 and 42ng MSA attached to the bead. Beads and free PPS14 were admixed for 1 hour with 25µg CpG-ODN, before immunization. Free PPS14 also included 13µg of alum. *p < 0.05 (groups preimmunized with [MSA]-beads, relative to naïve mice); Student's t-test One of two independent experiments is shown.
DISCUSSION
These results demonstrate that autologous serum albumin when expressed on a particulate surface induces antigen-specific B and T cell responses, in vivodespite it being an abundant and ubiquitous host protein. Remarkably, the CD4+ T cell response to albumin can provide help for a humoral immune response to a bacterial CPS attached to the same particle. In light of the ability of serum albumin to naturally adsorb onto a bacterial surface, this, breakdown in tolerance may serve to augment the immune response to bacterial antigens. Similar processes could be operative during the development of immune responses to cell-associated host glycan derivatives and glycan variants which are involved in many autoimmune and cancer-related pathologies [39; 40].
The induction of humoral immune responses specific for MSA is enigmatic. Anti-MSA antibody responses cannot be explained by simple allotypic variation. Albumin is a highly conserved protein[11; 12]. Moreover, anti-MSA antibodies were also induced in response to beads coated with truly autologous MSA from the preimmune sera of the same mice. This suggests a break in B cell tolerance induced by the albumin-coated beads. B cells specific for abundant soluble self proteins, like MSA[10], are likely anergic, as opposed to negatively selected, and thus excluded from the follicles [41]. In this regard, multivalent forms of a self antigen can reverse B cell anergy in vivo [42]. Therefore, the propensity of particles to be sequestered at extrafollicular locations, and the MSA multimerization provided by the bead, suggests that rescue from B cell anergy could be a major mechanism for the induction of MSA-specific antibodies by [MSA]-beads. Thus, our system may model the induction of autoimmune responses by aggregated/particulate or bacteria-associated forms of autologous antigens.
The detected anti-MSA antibodies induced by bead-associated MSA is unlikely to be specific for normally exposed epitopes in solution, since the overabundance of MSA in serum and its binding to antibody expressing such specificities would likely preclude their detection. Instead, our results suggest that the detected anti-MSA Ig are specific for epitopes better expressed when the MSA is bound to a solid surface. The simple interaction of MSA with the hydrophobic latex surface is potentially capable of producing enough structural rearrangements and conformational changes that expose new epitopes, similar to that following aggregation [16]. Under certain conditions, MSA has the propensity to aggregate in oligomeric or more complicated forms, like amyloid fibrils [16; 43]. The type of linkage to the bead is a less likely factor. The Schiff’s base formed between the aldehyde groups on the bead and ε-amino groups of Lys in the MSA, is the same bond linking glucose in glycated albumin [13]. However, the high content of glycated albumin (6–15%) in healthy individuals [14], suggests these epitopes will be similarly prone to anergy as the non-glycosilated forms. Because glucose blocks the ε-amino group of Lys, glycated albumin is less likely to be attached to our beads or directly involved in the responses.
MSA-coated beads induce MSA-specific CD4+ T cells, which enhance the secondary antibody responses specific for a non-covalently associated polysaccharide. These responses exhibited a classical carrier effect, indicating the involvement of MSA-specific memory T cells. Although T cell clones specific for autologous MSA, are likely to be negatively selected during development, the type of link and the interaction of MSA with the bead, as well as the distinctive compartmentalization in the APC of particles [44], could potentially alter the repertoire of peptides generated during processing. Thus, T cells specific for new or altered MSA epitopes could have escaped negative selection. Our results suggest that professional APC, rather than B cells, are directly involved in the recruitment of T cell help for PPS14-specific responses as suggested by the lack of a significant effect of the bead size. This is consistent with the limited avidity maturation of the PPS14-specific IgG secondary responses, which suggest a non-cognate interaction between the MSA-specific T cells and PPS14-specific B cells. Because many autologous antigens, such as MSA, interact with bacteria or form aggregates during inflammation, similar mechanisms could be involved in the development of autoimmune disorders.
Thus, it is noteworthy that PPS14-specific Ig responses to PPS14+[MSA]-beads are markedly enhanced in the presence of TLR ligands. The lower-level induction in the absence of exogenous adjuvants may be a consequence of the ability of latex beads to activate the inflammasome [34; 45], supporting a major role of professional APCs in these responses. Agonists for both, surface (TLR2 and TLR4) and endolysosome (TLR9) expressed TLRs [46] were effective in stimulating boosted PPS14-specific and MSA-specific IgG secondary responses to PPS14+[MSA]-beads. However, autologous ligands such as mammalian DNA, is a TLR9 activator, implicated in promoting autoimmunity [47], provided that it is directed to the endolysosomal compartment where TLR9 is located, and where particles localize. Thus, formation of protein aggregates or cell debris containing DNA during aseptic necrosis or cell lysis and inflammation could act as triggers of autoimmune responses.
MATERIALS AND METHODS
Mice
Female BALB/c mice (NCI, Frederick, MD) were used between 8–12 weeks. BALB/c athymic nude mice (CByJ.Cg-Foxn1nu/J) and background strain (BALB/cByJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). These experiments were approved by the USUHS Institutional Animal Use and Care Committees.
Reagents
Mouse IgG1κ anti-PPS14 mAb (clone 44.1) and IgG1κ mAb anti-44.1 Id (clone 2B6.2) have been characterized [25; 48]. Polyclonal rat IgG, mouse IgG2a anti-BSA mAb (clone BSA-33) and serum albumins from human (HSA; ≥99% purity), pig (≥99% purity), bovine (BSA; ≥95% purity) and mouse (MSA) of ≥99% purity (Cat#A3559) or ≥95% purity (Cat#A3139) were all purchased from Sigma (St. Louis, MO). Synthetic monophosphoryl lipid A (MPL) and Pam3CSK4 were purchased from InvivoGen (San Diego, CA).
Preparation of beads coated with PPS14 (PPS14+[Gly]-beads)
Surfactant-free aldehyde/sulfate latex beads (Invitrogen), 0.96µm diameter, were coated with PPS14 (ATCC, Manassas, VA) as described [29], and involved Schiff’s base formation with aldehyde groups on the bead without use of chemical activators. These bonds were stabilized by mild reduction with cyanoborohydride. Similar attachment of PPS14 to latex beads, 0.15µm diameter, used 41-times more beads during coupling to correct for the difference in surface area. Free binding sites on beads were blocked using 0.1% glycine.
Preparation of latex beads coated with PPS14 and autologous serum proteins
109 latex beads coated with PPS14 alone, but not blocked with Gly, were incubated for 24 hours with 50µg MSA (≈99% purity) (PPS14+[MSA]-beads) or 10µL preimmune mouse sera (NMS) [containing ~50µg MSA] from the same mice that will be immunized with beads (PPS14+[NMS]-beads). Pooled sera were passed through 10kDa ultrafiltration units to remove small molecular weight molecules. Beads were washed and resuspended in PBS containing 0.1% Gly. Content of free MSA and PPS14 was <0.04% and <0.13%, respectively. Control beads were coated only with MSA ([MSA]-beads) or NMS ([NMS]-beads). Mild attachment procedures prevented covalent linkage of proteins and PPS14.
Quantitation of PspA and PPS14 content
The content of bead-associated PspA and PPS14 was determined by ELISA as described [23]. PPS14 content was determined by quantitative sandwich ELISA in which mAb 44.1 specific for PPS14 was used for both capture and detection.
Quantitation of MSA content
MSA content was determined by ELISA using serial dilutions of beads in PBS, and MSA as standard. Wells were blocked with 2%BSA and MSA was detected using 0.3µg/ml of biotinylated goat IgG anti-MSA (Bethyl Laboratories Inc, Montgomery, TX), followed by detection with alkaline phosphatase-conjugated streptavidin. The content of MSA in PPS14+[MSA] beads was confirmed by quantitative sandwich ELISA using anti-PPS14 mAb 44.1 for capture and biotinylated goat IgG anti-MSA for detection.
Flow cytometric detection of PPS14 and MSA attached to latex beads
Beads were incubated overnight at 4°C with unlabeled 44.1 mouse IgG1 mAb and/or 1µg of biotinylated goat IgG anti-MSA, then washed and incubated for 30min with streptavidin-FITC and 0.5µg of phycoerthryin-goat anti-mouse IgG.
Mouse immunizations
Mice were immunized i.p. and boosted on day 14. Latex beads were washed in PBS pre-immunization to remove antigen released during storage. Beads were mixed with 25µg of a 30-mer CpG-containing oligodeoxynucleotide (CpG-ODN) as adjuvant, synthetized at USUHS [49].
In vivo depletion of CD4+ T cells
Mice received a single 1mg injection of depleting rat IgG anti-mouse CD4 mAb (clone GK1.5) given 32 hours prior to primary immunization, with polyclonal rat IgG used as control. Depletion was confirmed using two different anti-CD4 mAbs specific for the same (clone RM4-5) or a different CD4 epitope (clone RM4-4) than GK1.5
SDS-PAGE and Western blot
Albumins from mouse, bovine and human serum were boiled at 85°C for 2 minutes, fractionated in pre-casted 4–20% gradient SDS-PAGE (Invitrogen), and stained with Coomassie G-250 Blue (Invitrogen), Replicate gels were used for Western blot analysis, using 0.45µm nitrocellulose membranes. Membranes were blocked with 2% OVA in PBS for 1 hour and incubated overnight with 1/100 dilution of pooled mouse sera diluted in 2% OVA. Mouse antibodies were detected by incubation with polyclonal goat anti-mouse IgG (H+L)-HRP (Bio-Rad), followed by colorimetric development using 4-chloronaphtol as substrate.
Measurement of serum titers of antigen-specific Ig isotypes by ELISA
Serum PPS14− and PspA-specific isotype antibodies were determined by ELISA [29]. ELISA for MSA-specific antibodies, was essentially the same, except for use of 0.5µg/well of MSA in PBS for coating and 2% OVA for blocking and dilution.
Inhibition ELISA to quantify serum PPS14-specific Ig expressing the 44.1-Id
The inhibition ELISA for serum content of 44.1-Id+ PPS14-specific IgG1, IgG and IgM, has been previously described [25]
Determination of serum PPS14-specific IgG avidity
The average avidity of serum PPS14-specific IgG was determined by elution with sodium thiocyanate (NaSCN) [50], with modifications [25]. Avidities were expressed as avidity index (AI, the molar concentration of NaSCN eluting 50% of PPS14-specific IgG).
Inhibition ELISA for MSA specificity
Pre-titered serum dilutions in 2%OVA were incubated overnight at 4°C with decreasing amounts of soluble- or bead-attached MSA. Inhibition mixtures were then transferred into wells coated with 5µg/ml of MSA and blocked for 2 hours at 37°C with 2% OVA. After overnight incubation, MSA-bound IgG was detected with polyclonal goat anti-mouse IgG.
Statistics
Data were expressed as geometric mean ± SEM of the individual serum samples. Significance between groups was determined by the Student's t-test. p values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
This study was supported by NIH grant AI49192 and the USUHS Dean’s Research and Education Endowment Fund.
List of abbreviations
- AI
Avidity Index
- MSA
Murine serum albumin; [MSA]-beads, Beads coated with MSA
- PPS14
Capsular polysaccharide of Streptococcus pneumoniae type 14
- PPS14+[Gly]-beads
Beads coated with PPS14 and blocked with glycine
- PPS14+[MSA]-beads
Beads coated simultaneously with PPS14 and MSA
- PPS14-PspA beads
Beads coated with PPS14-PspA conjugate vaccine
- PspA
Pneumococcal surface protein A
- TD
T cell-dependent
- TI
T cell-independent
Footnotes
Conflict-of-interest disclosure: The authors declare no commercial or financial conflict of interest. The opinions expressed here are those of the authors and should not be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
References
- 1.De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N.Engl.J.Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 3.Scagnolari C, Casato M, Bellomi F, De PF, Turriziani O, Coviello R, et al. Serum interferon (IFN)-neutralizing antibodies and bioactivities of IFNs in patients with severe type II essential mixed cryoglobulinemia. Clin.Diagn.Lab Immunol. 2003;10:70–77. doi: 10.1128/CDLI.10.1.70-77.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 5.Moore WV, Leppert P. Role of aggregated human growth hormone (hGH) in development of antibodies to hGH. J.Clin.Endocrinol.Metab. 1980;51:691–697. doi: 10.1210/jcem-51-4-691. [DOI] [PubMed] [Google Scholar]
- 6.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 7.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J.Exp.Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastings GE, Wolf PG. The therapeutic use of albumin. Arch.Fam.Med. 1992;1:281–287. doi: 10.1001/archfami.1.2.281. [DOI] [PubMed] [Google Scholar]
- 9.Marth E, Kleinhappl B. Albumin is a necessary stabilizer of TBE-vaccine to avoid fever in children after vaccination. Vaccine. 2001;20:532–537. doi: 10.1016/s0264-410x(01)00329-2. [DOI] [PubMed] [Google Scholar]
- 10.Putnam FW. The plasma proteins: structure, function, and genetic control. Academic Press; 1984. [Google Scholar]
- 11.Petras ML. An inherited albumin variant in the house mouse, Mus musculus. Biochem.Genet. 1972;7:273–277. doi: 10.1007/BF00484827. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Takahashi Y, Blumberg BS, Putnam FW. Amino acid substitutions in genetic variants of human serum albumin and in sequences inferred from molecular cloning. Proc.Natl.Acad.Sci.U.S.A. 1987;84:4413–4417. doi: 10.1073/pnas.84.13.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iberg N, Fluckiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J.Biol.Chem. 1986;261:13542–13545. [PubMed] [Google Scholar]
- 14.Day JF, Thorpe SR, Baynes JW Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J.Biol.Chem. 1979;254:595–597. [PubMed] [Google Scholar]
- 15.Rondeau P, Navarra G, Cacciabaudo F, Leone M, Bourdon E, Militello V. Thermal aggregation of glycated bovine serum albumin. Biochim.Biophys.Acta. 2010;1804:789–798. doi: 10.1016/j.bbapap.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Rondeau P, Navarra G, Militello V, Bourdon E. On the aggregation of albumin: Influences of the protein glycation. In: Stein DA, editor. Protein Aggregation. Nova Science Publishers, Inc; 2011. pp. 139–159. [Google Scholar]
- 17.Lu J, Stewart AJ, Sadler PJ, Pinheiro TJ, Blindauer CA. Albumin as a zinc carrier: properties of its high-affinity zinc-binding site. Biochem.Soc.Trans. 2008;36:1317–1321. doi: 10.1042/BST0361317. [DOI] [PubMed] [Google Scholar]
- 18.Brunskill NJ. Albumin Receptors – Structure and Function. In: Duttaroy AK, Spener F, editors. Cellular Proteins and Their Fatty Acids in Health and Disease. Wiley; 2003. pp. 79–94. [Google Scholar]
- 19.Raeder R, Otten RA, Boyle MD. Comparison of albumin receptors expressed on bovine and human group G streptococci. Infect.Immun. 1991;59:609–616. doi: 10.1128/iai.59.2.609-616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wideback K, Kronvall G. Surface receptors for serum albumin in group C and G streptococci show three different types of albumin specificity. Infect.Immun. 1982;38:1154–1163. doi: 10.1128/iai.38.3.1154-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Retnoningrum DS, Cleary PP. M12 protein from Streptococcus pyogenes is a receptor for immunoglobulin G3 and human albumin. Infect.Immun. 1994;62:2387–2394. doi: 10.1128/iai.62.6.2387-2394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracken CS, Baer MT, Abdur-Rashid A, Helms W, Stojiljkovic I. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J.Bacteriol. 1999;181:6063–6072. doi: 10.1128/jb.181.19.6063-6072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colino J, Chattopadhyay G, Sen G, Chen Q, Lees A, Canaday DH, et al. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact gram-positive bacterium versus a soluble conjugate vaccine. J Immunol. 2009;183:1551–1559. doi: 10.4049/jimmunol.0900238. [DOI] [PubMed] [Google Scholar]
- 24.Snapper CM. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann.N.Y.Acad.Sci. 2012;1253:92–101. 92–101. doi: 10.1111/j.1749-6632.2011.06329.x. [DOI] [PubMed] [Google Scholar]
- 25.Colino J, Duke L, Arjunaraja S, Chen Q, Liu L, Lucas AH, et al. Differential idiotype utilization for the in vivo type 14 capsular polysaccharide-specific Ig responses to intact Streptococcus pneumoniae versus a pneumococcal conjugate vaccine. J.Immunol. 2012;189:575–586. doi: 10.4049/jimmunol.1200599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraal G, Ter HH, Meelhuizen C, Venneker G, Claassen E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur.J.Immunol. 1989;19:675–680. doi: 10.1002/eji.1830190416. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler HK, Orlin CA, Cluff CW. Differential requirements for the processing and presentation of soluble and particulate bacterial antigens by macrophages. Eur.J.Immunol. 1987;17:1287–1296. doi: 10.1002/eji.1830170911. [DOI] [PubMed] [Google Scholar]
- 28.Chattopadhyay G, Khan AQ, Sen G, Colino J, DuBois W, Rubtsov A, et al. Transgenic expression of Bcl-xL or Bcl-2 by murine B cells enhances the in vivo antipolysaccharide, but not antiprotein, response to intact Streptococcus pneumoniae. J.Immunol. 2007;179:7523–7534. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- 29.Colino J, Duke L, Snapper CM. Noncovalent Association of Protein and Capsular Polysaccharide on Bacteria-Sized Latex Beads as a Model for Polysaccharide-Specific Humoral Immunity to Intact Gram-Positive Extracellular Bacteria. J.Immunol. 2013;191:3254–3263. doi: 10.4049/jimmunol.1300722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int.Rev.Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catron DM, Pape KA, Fife BT, van RN, Jenkins MK. A protease-dependent mechanism for initiating T-dependent B cell responses to large particulate antigens. J.Immunol. 2010;184:3609–3617. doi: 10.4049/jimmunol.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahma A, Mandal C, Bhattacharyya D. Characterization of a dimeric unfolding intermediate of bovine serum albumin under mildly acidic condition. Biochim.Biophys.Acta. 2005;1751:159–169. doi: 10.1016/j.bbapap.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates NALP3 inflammasome. Proc.Natl.Acad.Sci.U.S.A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa T, Asai Y, Hashimoto M, Takeuchi O, Kurita T, Yoshikai Y, et al. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int.Immunol. 2002;14:1325–1332. doi: 10.1093/intimm/dxf097. [DOI] [PubMed] [Google Scholar]
- 36.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc.Natl.Acad.Sci.U.S.A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiernaux JR, Stashak PW, Cantrell JL, Rudbach JA, Baker PJ. Immunomodulatory activity of monophosphoryl lipid A in C3H/HeJ and C3H/HeSnJ mice. Infect.Immun. 1989;57:1483–1490. doi: 10.1128/iai.57.5.1483-1490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz DH, Paul WE, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J.Exp.Med. 1970;132:261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat.Rev.Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 40.Chui D, Sellakumar G, Green R, Sutton-Smith M, McQuistan T, Marek K, et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc.Natl.Acad.Sci.U.S.A. 2001;98:1142–1147. doi: 10.1073/pnas.98.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 42.Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J.Immunol. 2008;180:5816–5825. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouma B, Kroon-Batenburg LM, Wu YP, Brunjes B, Posthuma G, Kranenburg O, et al. Glycation induces formation of amyloid cross-beta structure in albumin. J.Biol.Chem. 2003;278:41810–41819. doi: 10.1074/jbc.M303925200. [DOI] [PubMed] [Google Scholar]
- 44.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu.Rev.Immunol. 1997;15:821–850. 821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 45.Vaine CA, Patel MK, Zhu J, Lee E, Finberg RW, Hayward RC, et al. Tuning innate immune activation by surface texturing of polymer microparticles: the role of shape in inflammasome activation. J.Immunol. 2013;190:3525–3532. doi: 10.4049/jimmunol.1200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Haas T, Metzger J, Schmitz F, Heit A, Muller T, Latz E, et al. The DNA sugar backbone 2' deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Colino J, Snapper CM. Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect.Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen G, Flora M, Chattopadhyay G, Klinman DM, Lees A, Mond JJ, et al. The critical DNA flanking sequences of a CpG oligodeoxynucleotide, but not the 6 base CpG motif, can be replaced with RNA without quantitative or qualitative changes in Toll-like receptor 9-mediated activity. Cell Immunol. 2004;232:64–74. doi: 10.1016/j.cellimm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J.Immunol.Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.