Abstract
Nonalcoholic steatohepatitis (NASH) is a progressive form of nonalcoholic fatty liver disease (NAFLD) and is a major cause of liver cirrhosis and hepatic failure. The methionine choline-deficient diet (MCD) is a frequently used hepatotoxicity animal model of NASH that induces hepatic transaminase (ALT, AST) elevations and hepatobiliary histological changes similar to those observed in human NASH. Liver-specific microRNA-122 (miR-122) has been shown as a key regulator of cholesterol and fatty acid metabolism in adult liver, and has recently been proposed as a sensitive and specific circulating biomarker of hepatic injury. The purpose of this study was to assess miR-122 serum levels in mice receiving an MCD diet for 0, 3, 7, 14, 28 and 56 days and compare the performance versus routine clinical chemistry when benchmarked against the histopathological liver findings. MiR-122 levels were quantified in serum using RT-qPCR. Both miR-122 and ALT/AST levels were significantly elevated in serum at all timepoints. MiR-122 levels increased on average by 40-fold after 3 days of initiating the MCD diet, while ALT and AST changes were 4.8- and 3.3-fold, respectively. In general, miR-122 levels remained elevated across all timepoints, whereas the ALT/AST increases were less robust but correlated with the progressive severity of NASH as assessed by histopathology. In conclusion, serum levels of miR-122 can potentially be used as a sensitive biomarker for early detection of hepatotoxicity and can aid in monitoring the extent of NAFLD-associated liver injury in mouse efficacy models.
Keywords: biomarker, liver toxicity, methionine and choline deficient diet, microRNA 122, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a progressive liver disease that ranges from simple steatosis to nonalcoholic steatohepatitis (NASH).Along with the obesity rates in the United States, the incidence of NAFLD and NASH have increased in recent years, and it is estimated that NAFLD affects between ~30% and 40% of the adult population (Ali and Cusi, 2009). NAFLD patients have an increased overall mortality rate and those who develop NASH have an increased rate of liver-related mortalities (Chalasani et al., 2012). The growing prevalence and increased mortality associated with these diseases have underscored the need for: 1) greater mechanistic understanding of disease progression and 2) development of more sensitive and reliable disease biomarkers.
Recently, there has been a significant amount of enthusiasm surrounding circulating microRNAs (miRNAs)as biomarkers for various diseases, including cancer, hepatitis, liver injury, and NAFLD(Osman, 2012;Cheung et al., 2008;Cermelli et al., 2011;Ding et al., 2012;Elfimova et al., 2012). miRNAs are non-coding RNAs approximately 22 nucleotides long involved in post-transcriptional gene regulation. Circulating miRNAs are remarkably stable in the ribonuclease rich environment of the blood because they can be incorporated into ribonucleoprotein complexes or vesicles (Arroyo et al., 2011). miR-122 has received considerable attention as a biomarker of liver disease because it comprises approximately 70% of miRNAs in the liver (Chang et al., 2004;Lagos-Quintana et al., 2002). High expression of miR-122 is observed specifically in the liver due to transcriptional regulation via hepatocyte-enriched transcription factors such as HNF1A, HNF3A, and HNF3B (Coulouarn et al., 2009). Within the liver, miR-122 is an important regulator of cholesterol and fatty-acid metabolism (Esau et al., 2006). Similarly miR-122 has been investigated clinically as a biomarker for hepatitis B and C infections, drug induced liver injury, and for NAFLD and has been benchmarked against traditional clinical chemistries (Bihrer et al., 2011;Cermelli et al., 2011;Cheung et al., 2008;Pogribny et al., 2010;Starkey Lewis et al., 2012;Tryndyak et al., 2012;Waidmann et al., 2012).
Multiple animal models of NALFD have been introduced to investigate the various aspects of NAFLD pathogenesis, each with specific strengths and weaknesses depending on the intent of the study (Hebbard and George, 2011). The methionine and choline deficient (MCD) diet is one dietary hepatotoxicity model of NASH that is increasingly used because it recapitulates many of the reported changes in human liver histopathology including steatosis, inflammation, and fibrosis (Hebbard and George, 2011;Anstee and Goldin, 2006;Hebbard and George, 2011). This model induces hepatotoxicity by impairing mitochondrial β-oxidation and is considered the best model for studying the inflammatory and fibrotic aspects of NAFLD (Anstee and Goldin, 2006). This and other types of hepatotoxicity can be induced by drugs such as Tamoxifen and acetaminophen and are a significant hurdle in drug development (Anstee and Goldin, 2006;James et al., 2003;Watkins, 2011). The purpose of this study was to assess the performance and timing of miR-122 as a biomarker of MCD-induced hepatotoxicity in the MCD murine model versus routine clinical chemistry when benchmarked against liver histopathological findings.
Materials and Methods
Animals
Six week old C57BL/6 male mice were purchased from Harlan Laboratories (Indianapolis, Indiana). All animals were housed in microisolator cages and maintained in 12 h light and 12 h dark cycles at 21–22 °C and 40–50% humidity in a University of Arizona animal facility, and were allowed water and standard chow ad libitum during the one week acclimation period. Housing and experimental procedures were performed according the University of Arizona Institute Animal Care and Use Committee approved guidelines. Mice were monitored daily for signs of pain or distress. Day zero mice (n=3) were euthanized on the same day the other mice began receiving the MCD diet (#518810) (Dyets Inc., Bethlehem, PA). This treatment diet replete with all nutrients found in standard rodent diets except it is completely devoid of L-methionine and choline. Mice (n=3 for each time point) were euthanized after 3, 7, 14, 28, and 56 days on a MCD diet. All mice were weighed immediately prior to euthanasia. Whole blood was collected by cardiac puncture, incubated at room temperature for one hour, transferred to 4°C over night, and centrifuged to collect serum. Livers were removed and weighed.
Clinical chemistry
ALT and AST levels in serum were analyzed on an Abbott Architect™ diagnostics instrument according to manufacturer instructions.
Histopathology
Hematoxylin and Eosin staining and Masson’s trichrome staining were performed on l paraffin-embedded slides according to established methods. Resulting slides were evaluated and scored independently by a board-certified pathologist.
RNA extraction
Total RNA, including small RNAs, were isolated from 100uL of serum using Qiazol extraction method followed by column purification with a miRNeasy Mini kit (Qiagen; Valencia, CA) in accordance with the manufacturer’s protocol. Briefly, 400µL of Qiazol and 80µL of chloroform were added to 100µL of serum, followed by centrifugation for 15min at 12,000×g at 4°C. 300µL of the RNA containing aqueous phase was transferred into a new tube, RNA was precipitated with 450uL of 100% ethanol and loaded on miRNeasy purification columns. Purified RNA was eluted from the column matrix with 20µL of RNase free water. Total RNA yield was quantified using a NanoDrop® photospectrometer (Thermo-Scientific; Wilmington, DE)
To control for extraction efficiency and variability, two synthetic Caenorhabditis elegans microRNAs, syn-miR-Cel-39 and syn-miR-Cel-54 (3×108 copies of each) were spiked into each sample during Qiazol step. To aid in improving RNA yield, 400ng of E. coli total RNA (Life Technologies; Carlsbad, CA) was added to each sample as a carrier.
MicroRNA detection and quantification
miR-122 in serum was quantified using SYBR Green based RT-qPCR assay from Qiagen (Valencia, CA). In brief, cDNA was synthesized using 5 µL of each total RNA sample(~80–100 ng of nucleic acid) by reverse transcription through utilization of a miRNA First Strand Kit from Qiagen (SA Biosciences, Valencia, CA) according to manufacturer’s instructions. Subsequent qPCR was performed in total reaction volume of 10µL using 3uL of diluted (1:10) cDNA on 7900HT real-time PCR instrument from Applied Biosystems (Foster City, CA). PCR conditions recommended by supplier included initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15s, annealing at 60°C for 40s and extension at 72°C for 30s. Dissociation curve analysis was performed at the end of each cycling program to control PCR specificity.
Data analysis
The ΔΔCt method was used to assess miR-122 fold change in animals receiving MCD diet over the mean of the corresponding non-diet (day 0) controls. For each sample, a mean Ct value of 2 spiked controls was used to normalize for extraction variability. Fold change over the control mean was assessed with ΔΔCt method. For each sample Ct values were normalized to spiked-in C. elegans controls. Linear regression analysis was performed using GraphPad Prism version 5.04.
Statistics
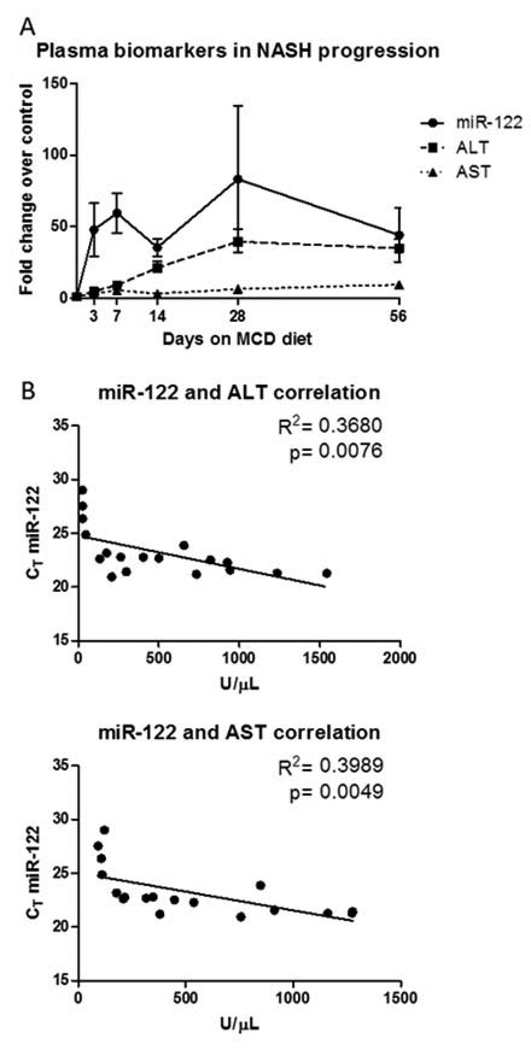
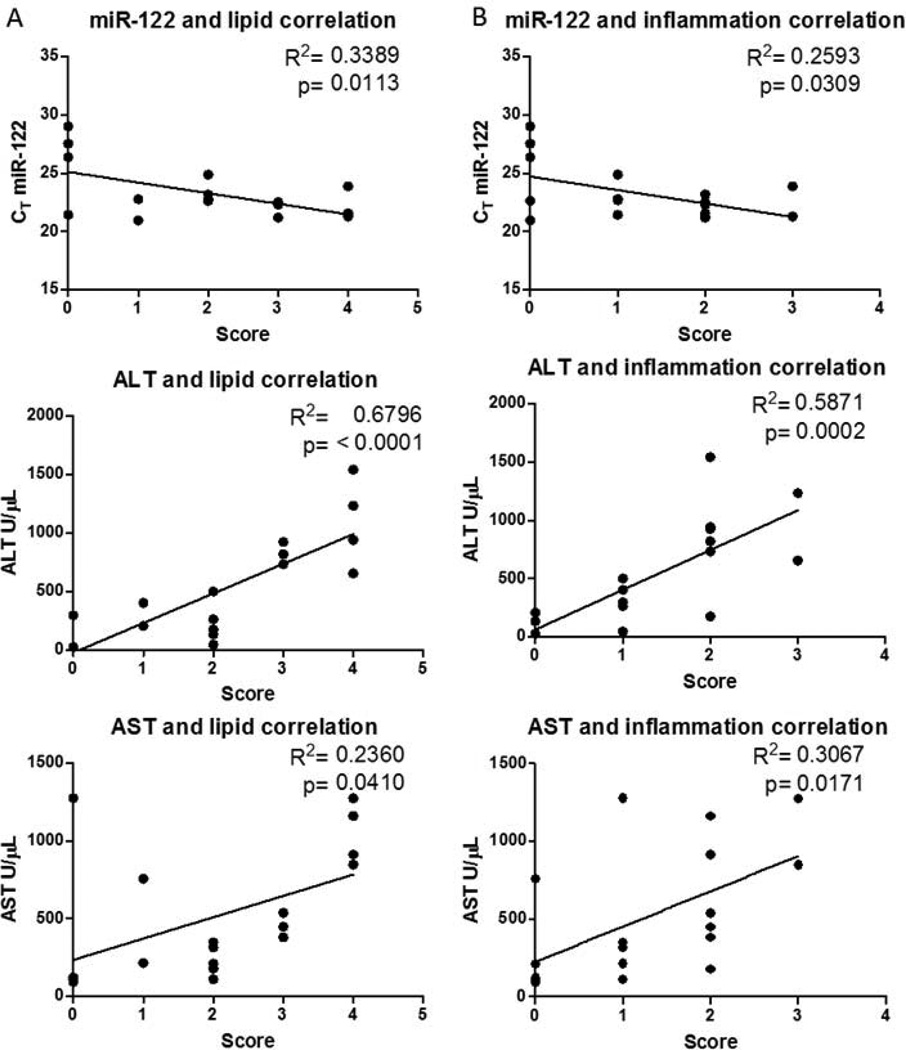
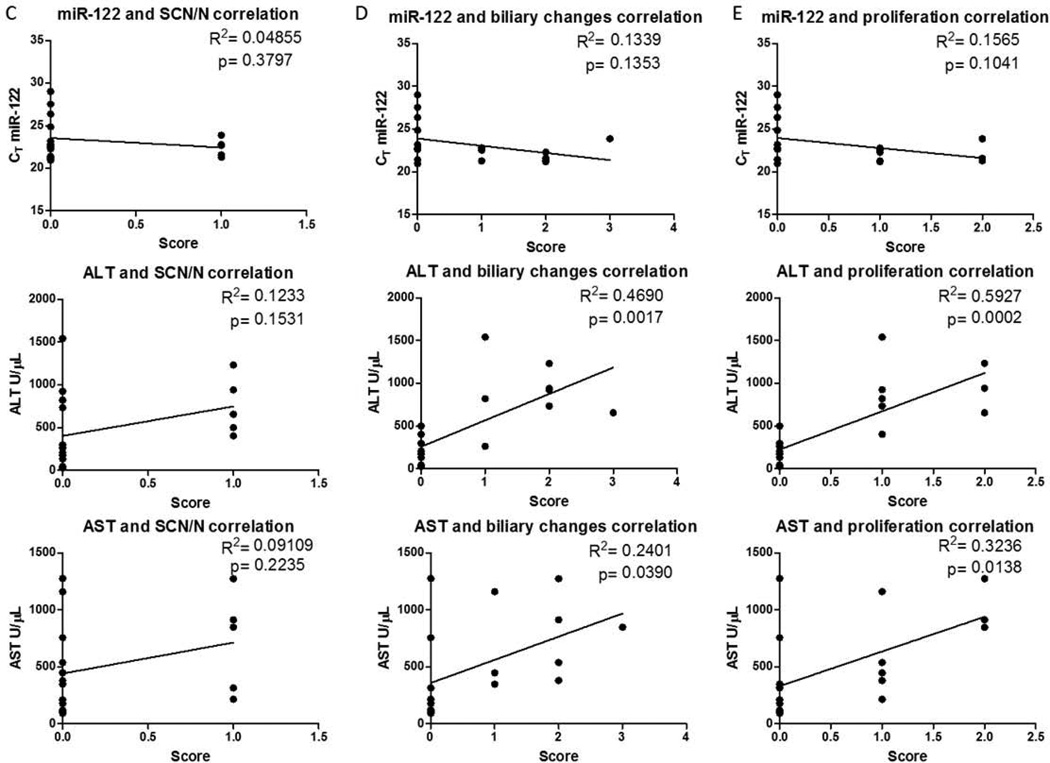
Linear regression analyses in figures 3 and 4 were performed in GraphPad prism. The R2 value for the line and the p-value from the test were used to determine whether the slope was significantly different from zero and are reported for each graph.
Figure 3.
Figure 4.
Results
Liver histopathology during 8 weeks of MCD diet
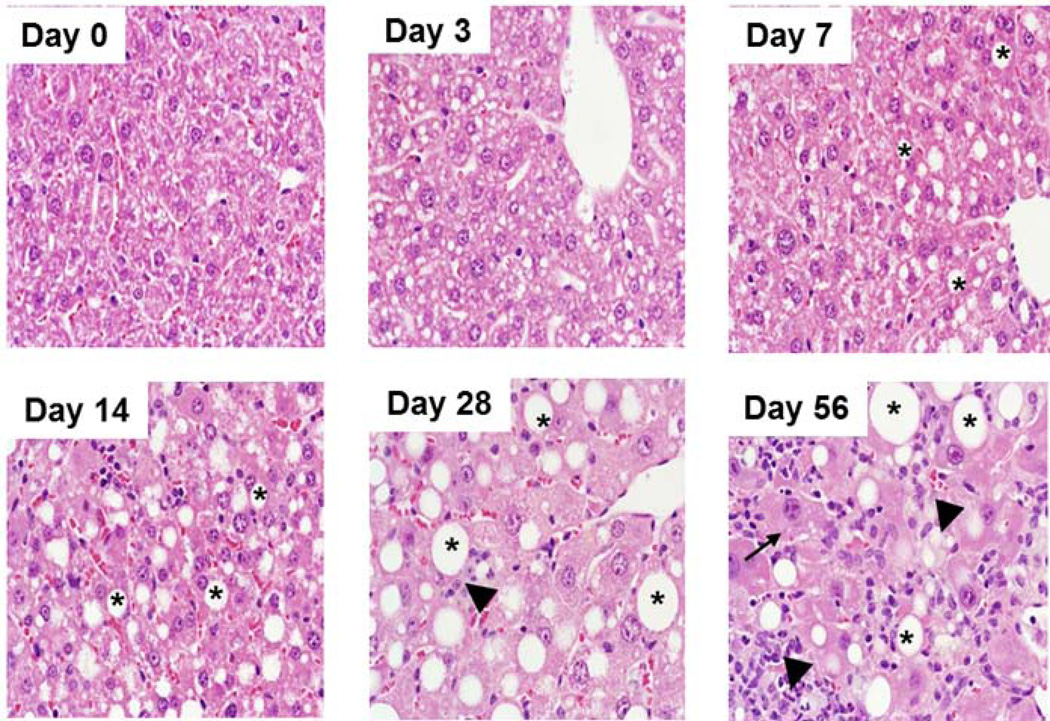
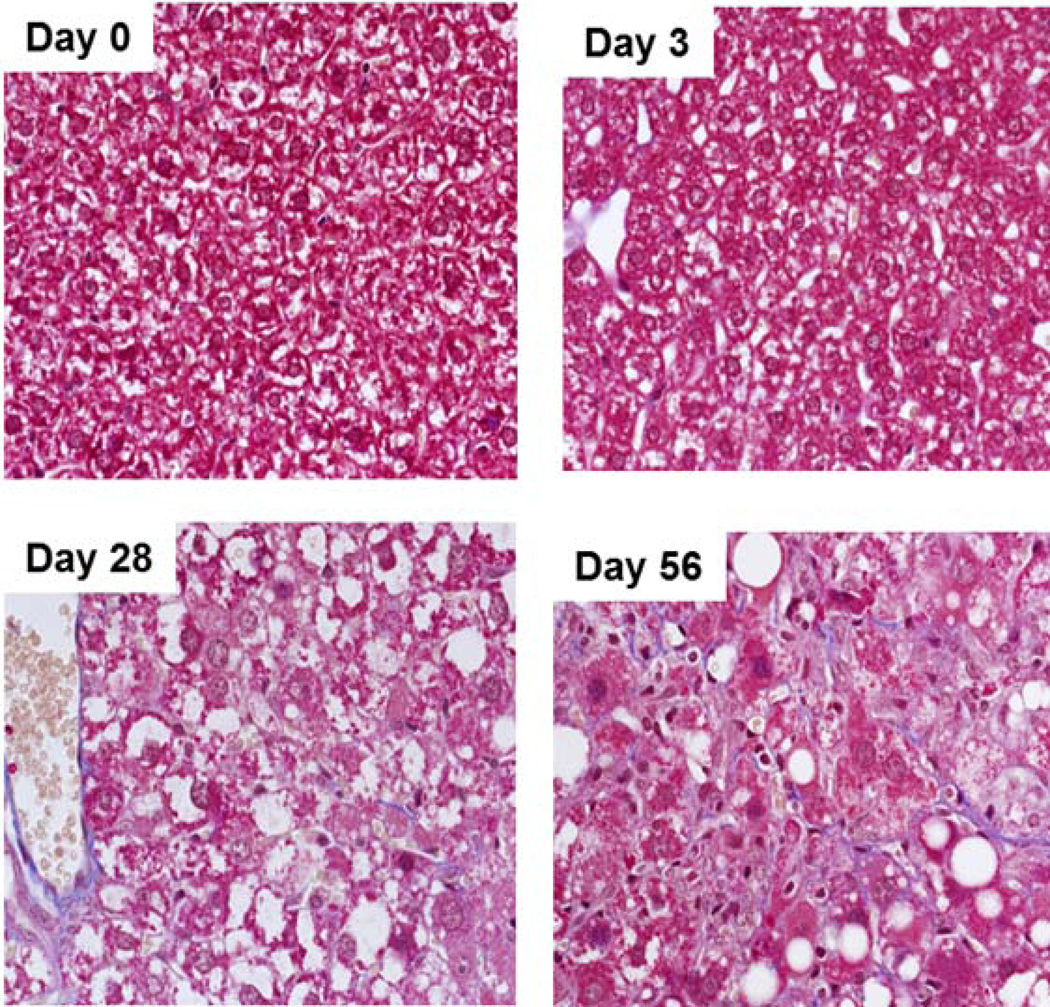
Livers from mice fed an MCD diet for 3, 7, 14, 28 and 56 days were scored for histopathological markers of NASH (Table 1). Steatosis was progressive across the study and reached maximal levels by day 28 (Fig. 1), while hepatocellular degeneration, characterized by cytomegaly, karyomegaly and binucleation, was most evident at day 56. Cellular infiltration composed of neutrophils, lymphocytes and activated macrophages was minimal at days 7 and 14 and progressed to moderate by day 56. Masson’s trichrome staining revealed increased collagen bundles along sinusoids on days 28 and 56 indicating minimal to mild fibrosis (Fig. 2). Consistent with previous reports for the MCD diet, the mice fed the MCD diet exhibited no clinical signs of distress or disease over the course of the study other than weight loss (Table 1) (Anstee and Goldin, 2006;Fan and Qiao, 2009;Schattenberg and Galle, 2010).
Table 1.
Comparison of miR-122 levels with benchmarked clinical chemistry and histopathology. Histopathology lexicon: Lipid-lipid accumulation, INF-inflammatory cell infiltrates, SCN/N-single cell necrosis/necrosis, Biliary-biliary hyperplasia and/or degeneration, Nuclear-increased mitotic figures (proliferation) and/or binucleate cells. Pathology severity scores: 0 - within normal limits, 1- minimal, 2 – mild, 3 – moderate, 4 – marked.
| Animal # |
Days on Diet |
Body weight (g) |
miR- 122 Fold Change |
Clinical Chemistry |
Histology Findings in Liver | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALT Fold Change |
AST Fold Change |
Lipid Accumulation |
Infiltration | Single Cell Necrosis/ Necrosis |
Biliary Injury |
Nuclear | ||||
| 1 | 0 | 19.8 | 1.13 | 1.04 | 1.00 | 0 | 0 | 0 | 0 | 0 |
| 2 | 21.6 | 0.59 | 1.00 | 0.86 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 22.1 | 1.54 | 0.96 | 1.13 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 3 | 21.0 | 81.20 | 4.96 | 1.95 | 2 | 0 | 0 | 0 | 0 |
| 5 | 23.8 | 45.58 | 7.70 | 7.03 | 1 | 0 | 0 | 0 | 0 | |
| 6 | 21.5 | 16.93 | 1.74 | 1.03 | 2 | 1 | 0 | 0 | 0 | |
| 7 | 7 | 18.0 | 31.99 | 6.52 | 1.66 | 2 | 2 | 0 | 0 | 0 |
| 8 | 17.8 | 74.33 | 11.04 | 11.85 | 0 | 1 | 0 | 0 | 0 | |
| 9 | 19.2 | 72.27 | 9.78 | 3.23 | 2 | 1 | 0 | 1 | 0 | |
| 10 | 14 | 18.8 | 43.24 | 30.37 | 4.16 | 3 | 2 | 0 | 1 | 1 |
| 11 | 18.7 | 23.25 | 14.96 | 2.01 | 1 | 1 | 1 | 0 | 1 | |
| 12 | 17.8 | 40.21 | 18.56 | 2.93 | 2 | 1 | 1 | 0 | 0 | |
| 13 | 28 | 17.4 | 185.51 | 57.11 | 10.77 | 4 | 2 | 0 | 1 | 1 |
| 14 | 16.5 | 32.38 | 34.30 | 5.00 | 3 | 2 | 0 | 2 | 1 | |
| 15 | 17.1 | 31.96 | 27.19 | 3.53 | 3 | 2 | 0 | 2 | 1 | |
| 16 | 56 | 16.8 | 41.88 | 34.89 | 8.48 | 4 | 2 | 1 | 2 | 2 |
| 17 | 14.8 | 78.42 | 45.70 | 11.83 | 4 | 3 | 1 | 2 | 2 | |
| 18 | 13.2 | 12.80 | 24.30 | 7.88 | 4 | 3 | 1 | 3 | 2 | |
Figure 1.
Figure 2.
miR-122 versus ALT/AST levels in NASH
MiR-122, ALT, and AST levels were measured in serum (Table 1) to compare the performance of miR-122 in assessing disease severity and progression. Serum miR-122 levels increased on average by 40-fold after 3 days of initiating the MCD diet, while ALT and AST changes were only 4.8- and 3.3-fold, respectively (Fig. 3A). miR-122 levels remained elevated at or above a 40 fold increase across all time points (Fig. 3A). Linear regression analysis between each clinical measure and each histopathological marker were determined in order to compare how serum miR-122 performed versus ALT/AST in assessing disease progression. When comparing changes in serum miR-122 to ALT and AST values, there was a significant linear relationship between these markers, indicating that all markers increase during disease progression. (Fig. 3B). There were linear relationships between each marker and both lipid accumulation (Fig. 4A) and the presence of inflammatory infiltrates (Fig. 4B), with the strongest relationships being with ALT. None of the markers correlated with biliary hyperplasia and/or degeneration (Fig. 4C). ALT and AST also correlated with biliary degeneration (Fig. 4D) and proliferation (Fig. 4E) but did not correlate with miR-122.
Discussion
Although NAFLD is becoming increasingly prevalent, the mechanisms that contribute to the development of NAFLD and progression to NASH are still only partially understood (Ali and Cusi, 2009). One theory for NASH development is the “two hit” model which includes an initial hit of fat accumulation in the liver and a second hit that leads to inflammation and potentially irreversible tissue injury. Drug-induced liver toxicities can contribute to either “hit” in NAFLD progression (Stravitz and Sanyal, 2003). Drug-induced hepatic steatosis, which is believed to be relatively benign and reversible, sets the stage for development of steatohepatitis if the liver is exposed to a second “hit”(Amacher, 2011). The ability to detect these toxicities early in liver injury will improve preclinical screening of drugs and identify them prior to proceeding into costly clinical trials. Also, due to the heterogeneity of human populations and the myriad of liver diseases that can alter biomarkers of disease, having a panel of easily obtained and measured biomarkers that can facilitate early diagnosis is valuable. Currently, serum ALT and AST levels are standard clinical chemistries used to assess liver injury but microRNAs are one group of serum biomarkers that have gained traction in assessing various liver diseases. miR-122 is highly expressed in the liver and has been investigated for various forms of liver injury. In this study, we show that miR-122 serum levels were robustly increased early on in a diet-induced (MCD) model of NAFLD and correlated with ALT and AST levels.
Several reports indicate that miR-122 may be a more sensitive and reliable biomarker of various forms of liver injury than the standard clinical chemistries. For example, it has been reported that miR-122 is more sensitive than ALT and AST for drug- (Wang et al., 2009), viral-, alcohol-, and chemical-induced liver injury, as it was detected earlier and at lower drug doses (Zhang et al., 2010). Furthermore, miR-122 is reported to be more specific for liver injury than ALT because, while ALT was elevated in muscle injury, miR-122 was not (Zhang et al., 2010). For NAFLD, liver enzyme levels are not reliable for diagnosis since they often remain at normal levels even in the presence of the disease (Greenfield et al., 2008). In our study, we observed a >40-fold elevation in miR-122 levels as early as three days after initiation of the MCD diet and miR-122 remained elevated throughout the entire study (Figure 3A). In contrast, ALT was increased only 5-fold after three days and did not reach its highest level until 28 days of MCD diet (Figure 3A). In fact, at early time points in some animals ALT/AST did not reach the three-fold increase threshold required by Hy’s law(Reuben, 2004), thereby making interpretation of these measures during the early stages of liver injury difficult. These data indicate that miR-122 is more sensitive for early detection of hepatic steatosis than ALT and AST, but was not sufficient for differentiating the pathological changes that define NAFLD and NASH.
It has been shown previously that hepatic injury and disease are reflected in decreased hepatic and increased circulating miR-122 levels. In mice fed a choline and folate deficient diet, serum miR-122 levels positively correlated with disease severity (Tryndyak et al., 2012). In agreement with these data, we also observed correlation of serum miR-122 levels with markers of NAFLD disease severity, mainly due to a dramatic increase in miR-122 levels early in disease progression. In NAFLD patients, miR-122 serum levels positively correlated with disease severity, liver enzyme levels, fibrosis stage, and inflammation activity (Cermelli et al., 2011). In NASH patients, a 63% reduction in hepatic miR-122 levels has been reported compared to control (Cheung et al., 2008). In a choline and folate deficient diet-induced model of NASH, lower liver and higher plasma levels of miR-122 compared to controls have been reported in C57Bl/6 mice (Pogribny et al., 2010;Tryndyak et al., 2012). These reports are in agreement with our current findings that serum miR-122 is dramatically increased at all timepoints in MCD-diet induced NAFLD progression. In drug-induced liver injury, 12 miRNAs, including miR-122, exhibited decreased liver and increased circulating plasma levels (Wang et al., 2009). Elevated serum miR-122 has also been reported in patients with hepatitis B virus (HBV) and hepatitis C virus (HCV) infections (Bihrer et al., 2011;Waidmann et al., 2012). These data clearly indicate that hepatic miR-122 expression and release of miR-122 into the blood is altered in various liver diseases, including NAFLD.
One approach to developing a reliable biomarker for injury/disease is to have redundant and mechanistically distinct biomarkers that can be used in conjunction to provide sensitive and specific diagnosis. Use of traditional clinical chemistry analyses such as ALT and AST in conjunction with miR-122 may increase the confidence when assessing liver injury and/or disease. Multiple reports show that traditional clinical chemistry analyses correlate with miR-122, indicating that both biomarkers are reliable for diagnosis of liver injury, HCV and HBV infections (Starkey Lewis et al., 2012;Bihrer et al., 2011;Waidmann et al., 2012). In congruence with these data, we observed correlations between miR-122 serum levels and liver enzyme levels, lipid accumulation, and inflammatory infiltrates in NAFLD progression (Figure 3B and 4A). Since ALT and AST are traditionally thought of as “leakage” markers associated with hepatocyte death, the correlation of ALT and AST with miR-122 indicate that miR-122 may also be a “leakage” marker. Importantly, these different biomarkers have distinct strengths and weaknesses that make their combination superior. From our data it is evident that ALT and AST correlate better with disease severity and progression, whereas the miR-122 changes occurred early and were robust across all time points acting as an “alarm” of injury. By utilizing the strengths of these distinct biomarkers there can be greater confidence in assessing the risk of hepatotoxicity during preclinical and clinical drug development.
These data indicate that serum levels of miR-122 can potentially be used as a sensitive biomarker for early detection hepatotoxicity and for monitoring NAFLD-associated liver injury in the MCD model. Whereas miR-122 offered no clear benefit in differentiating pathological events associated with NAFLD and NASH in the MCD mouse model, the utility of monitoring miR-122 could potentially add benefit to a traditional clinical chemistry panel due to improved: 1) early detection of steatotic changes 2) diagnostic accuracy due to better specificity, and 3) interpretation due to larger dynamic range of the assay. Since it has been shown previously that different strains of mice have variable hepatic miR-122 responses from hepatotoxicity (Tryndyak et al., 2012), it is important that future studies look at different strains of mice to determine if this same MCD diet-induced miR-122 profile is observed. In spite of this limitation, it is clear that miR-122 is a biomarker of liver injury that spans multiple models of hepatotoxicity, including MCD diet-induced toxicity. These data open the door for further investigation into the use of this biomarker for sensitive, early detection of drug toxicities and other liver pathologies.
Acknowledgments
The authors would like to thank Dr. Wayne Buck for his contributions to the histopathology and subsequent interpretation.
Funding and Declaration of Interest
This work was supported by The National Institute of Environmental Health Science Toxicology Training Grant [ES007091] and National Institutes of Health Grant [DK068039]. JMM, TS, and EAB are employees of AbbVie. AbbVie participated in the interpretation of data, review, and approval of the manuscript. Portions of the design, study conduct, and financial support for these studies was provided by AbbVie.
Abbreviations
- ALT
Alanine transaminase
- AST
aspartate aminotransferase
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- miRNA
microRNA
- MCD
methionine and choline deficient
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Reference List
- Ali R, Cusi K. New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD) Ann.Med. 2009;41:265–278. doi: 10.1080/07853890802552437. [DOI] [PubMed] [Google Scholar]
- Amacher DE. The mechanistic basis for the induction of hepatic steatosis by xenobiotics. Expert Opin.Drug Metab Toxicol. 2011;7:949–965. doi: 10.1517/17425255.2011.577740. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int.J Exp.Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc.Natl.Acad.Sci.U.S.A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihrer V, Friedrich-Rust M, Kronenberger B, Forestier N, Haupenthal J, Shi Y, Peveling-Oberhag J, Radeke HH, Sarrazin C, Herrmann E, Zeuzem S, Waidmann O, Piiper A. Serum miR-122 as a biomarker of necroinflammation in patients with chronic hepatitis C virus infection. Am.J Gastroenterol. 2011;106:1663–1669. doi: 10.1038/ajg.2011.161. [DOI] [PubMed] [Google Scholar]
- Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS.One. 2011;6:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the american association for the study of liver diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, Zaret KS, Taylor JM. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA.Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Ding J, Ning J, Yi F, Chen J, Zhao D, Zheng J, Liang Z, Hu Z, Du Q. Circulating microRNA-122 as a potential biomarker for liver injury. Mol.Med.Report. 2012;5:1428–1432. doi: 10.3892/mmr.2012.838. [DOI] [PubMed] [Google Scholar]
- Elfimova N, Schlattjan M, Sowa JP, Dienes HP, Canbay A, Odenthal M. Circulating microRNAs: promising candidates serving as novel biomarkers of acute hepatitis. Front Physiol. 2012;3:476. doi: 10.3389/fphys.2012.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Fan JG, Qiao L. Commonly used animal models of non-alcoholic steatohepatitis. Hepatobiliary.Pancreat.Dis.Int. 2009;8:233–240. [PubMed] [Google Scholar]
- Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr.Opin.Gastroenterol. 2008;24:320–327. doi: 10.1097/MOG.0b013e3282fbccf2. [DOI] [PubMed] [Google Scholar]
- Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat.Rev.Gastroenterol.Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr.Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Osman A. MicroRNAs in health and disease--basic science and clinical applications. Clin.Lab. 2012;58:393–402. [PubMed] [Google Scholar]
- Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, Beland FA. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab Invest. 2010;90:1437–1446. doi: 10.1038/labinvest.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A. Hy's law. Hepatology. 2004;39:574–578. doi: 10.1002/hep.20081. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig.Dis. 2010;28:247–254. doi: 10.1159/000282097. [DOI] [PubMed] [Google Scholar]
- Starkey Lewis PJ, Merz M, Couttet P, Grenet O, Dear J, Antoine DJ, Goldring C, Park BK, Moggs JG. Serum microRNA biomarkers for drug-induced liver injury. Clin.Pharmacol.Ther. 2012;92:291–293. doi: 10.1038/clpt.2012.101. [DOI] [PubMed] [Google Scholar]
- Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin.Liver Dis. 2003;7:435–451. doi: 10.1016/s1089-3261(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Tryndyak VP, Latendresse JR, Montgomery B, Ross SA, Beland FA, Rusyn I, Pogribny IP. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol.Appl.Pharmacol. 2012;262:52–59. doi: 10.1016/j.taap.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidmann O, Bihrer V, Pleli T, Farnik H, Berger A, Zeuzem S, Kronenberger B, Piiper A. Serum microRNA-122 levels in different groups of patients with chronic hepatitis B virus infection. J Viral Hepat. 2012;19:e58–e65. doi: 10.1111/j.1365-2893.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc.Natl.Acad.Sci.U.S.A. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PB. Drug safety sciences and the bottleneck in drug development. Clin.Pharmacol.Ther. 2011;89:788–790. doi: 10.1038/clpt.2011.63. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin.Chem. 2010;56:1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]