Abstract
Objectives
Women have been shown to have up to a four-fold higher risk of abdominal aortic aneurysm (AAA) rupture at any given aneurysm diameter compared to men, leading to recommendations to offer repair to women at lower diameter thresholds. Although this higher risk of rupture may simply reflect greater relative aortic dilatation in women who have smaller aortas to begin with, this has never been quantified. Our objective was therefore to quantify the relationship between rupture and aneurysm diameter relative to body size and to determine whether a differential association between aneurysm diameter, body size, and rupture risk exists for men and women.
Methods
We performed a retrospective review of all patients in the Vascular Study Group of New England (VSGNE) database who underwent endovascular or open AAA repair. Using each patient’s height and weight, body mass index (BMI) and body surface area (BSA) were calculated. Next, indices of each measure of body size (height, weight, BMI, BSA) relative to aneurysm diameter were calculated for each patient. To generate these indices, we divided aneurysm diameter (in cm) by the measure of body size [e.g. aortic size index (ASI) = aneurysm diameter (cm) / BSA (m2)]. Along with other relevant clinical variables, we used these indices to construct different age-adjusted and multivariable-adjusted logistic regression models to determine predictors of ruptured repair vs. elective repair. Models for men and women were developed separately and different models were compared using the area under the curve (AUC).
Results
We identified 4045 patients who underwent AAA repair (78% male, 53% EVAR). Women had significantly smaller diameter aneurysms, lower BSA, and higher BSA indices than men (Table 1). For men, the variable that increased the odds of rupture the most was aneurysm diameter (AUC = 0.82). Men exhibited an increased rupture risk with increasing aneurysm diameter (<5.5cm: OR 1.0; 5.5–6.4cm: OR 0.9, 95% CI 0.5–1.7, P=.771; 6.5–7.4cm: OR 3.9, 95% CI 1.9–1.0, P<.001; 7.5+ cm: OR 11.3, 95% CI 4.9–25.8, P<.001). In contrast, the variable most predictive of rupture in women was ASI (AUC = 0.81), with higher odds of rupture at higher ASI(ASI >3.5–3.9: OR 6.4, 95% CI 1.7–24.1, P=.006; ASI 4.0+: OR 9.5, 95% CI 2.3–39.4, P=.002). For women, aneurysm diameter was not a significant predictor of rupture after adjusting for ASI.
Conclusion
Aneurysm diameter indexed to body size is the most important determinant of rupture for women whereas aneurysm diameter alone is most predictive of rupture for men. Women with the largest diameter aneurysms and the smallest body sizes are at the greatest risk of rupture.
INTRODUCTION
Women have frequently been shown to have worse outcomes following abdominal aortic aneurysm (AAA) repair compared to men1–5. Though the reasons for this are likely multifactorial and include older age, higher operative risk due to undiagnosed cardiovascular comorbidity4, and smaller caliber vessels and challenging anatomy6, 7, one hypothesis has been that because women are generally smaller than men, an aneurysm of a certain size in a woman represents a greater relative dilatation of the aorta compared to the same sized aneurysm in a man. If this were true, women would effectively have more advanced disease at the time of treatment. Proponents of this theory cite the UK Small Aneurysm Trial8–10, which reported that rupture risk is 3–4 times higher in women and that women rupture at smaller aneurysm diameters than men. Largely in light of these findings, in their 2003 guidelines for AAA treatment,11 the Joint Council of the American Association of Vascular Surgery and the Society of Vascular Surgery (SVS) suggested that a lower threshold diameter for repair (4.5–5.5cm) could be considered for women. In the 2009 SVS Practice Guidelines, the suggestion was again made that women may benefit from early repair12.
However, opponents argue that the level of evidence to support differential treatment of women is lacking13. A recent Cochrane review of four randomized controlled trials that compared the long-term survival of patients with small aneurysms (4.0–5.5cm) undergoing either early repair or ultrasound surveillance concluded that there was no evidence to suggest a benefit to early repair14. However, out of the four trials, only the UK Small Aneurysm Trial (UKSAT)15 had a representative sample of women. Women were underrepresented in the Aneurysm Detection and Management (ADAM)16, 17, Comparison of Surveillance Versus Aortic Endografting for Small Aneurysm Repair (CEASAR)18, and Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL)19, 20 trial, in which the proportion of women was only 1% (n=10), 4% (n=15), and 15% (n=97), respectively.
We felt there would be value to using a large multicenter database with good female representation to quantify the relationship between rupture and aneurysm diameter relative to body size, and determine whether a differential association between aneurysm diameter, body size, and rupture risk exists for men and women.
METHODS
We performed a retrospective review of all patients in the Vascular Study Group of New England (VSGNE) database who underwent endovascular or open AAA repair. The VSGNE is a regional collaboration between 30 academic and community hospitals in the six New England states. Data on commonly performed vascular procedures from each participating institution are collected and maintained in a prospective registry. Details about this registry can be found at http://www.vsgne.org. At the time of this analysis, the registry included data on 1,887 open AAA repairs and 2,158 endovascular aortic aneurysm repair (EVAR) procedures. Presentation was categorized as ruptured if there was computed tomography (CT) or angiographic evidence of rupture or if rupture was found at exploration. Patients who underwent AAA repair as a planned or scheduled procedure, or those who had surgery within 24 hours of pain and/or tenderness but without radiographic evidence of rupture were categorized as non-ruptured.
The body mass index (BMI) and body surface area (BSA) of each patient were calculated using height and weight information. We used the standard formula for BMI:
The Dubois & Dubois formula21 was used to calculate BSA:
We next generated indices of each measure of body size (height, weight, BMI, and BSA) relative to the maximum antero-posterior aneurysm diameter, which was obtained from preoperative radiologic studies. If the antero-posterior diameter was not specified, the largest diameter was used. If more than one preoperative imaging study was obtained, the following hierarchy was used to obtain the diameter: CT, magnetic resonance imaging (MRI), echocardiogram, arteriogram. These indices were calculated by dividing aneurysm diameter (in cm) by each measure of body size [e.g. BSA index = aneurysm diameter (cm) / BSA (m2)]. The BSA index will hereafter be referred to as aortic size index (ASI) in order to establish consistency with previously published terminology22. Measures of body size and their respective aortic indices were divided into clinically relevant categories or based on quantiles (tertiles, quartiles, or quintiles). The BSA of men and women were compared to their gender-specific means (1.9m2 for men and 1.6m2 for women)23, 24.
Differences in categorical variables were compared using the Pearson χ2 and two-tailed Fisher’s exact test and differences in continuous variables were compared using student’s t-test. Using the indices of aneurysm size relative to body size, we constructed multivariable-adjusted logistic regression models using forward selection to determine predictors of ruptured vs. elective repair. We started with one model inclusive of gender as well as interaction terms between gender and ASI and gender and aortic diameter. Given that the interaction term between gender and ASI was borderline significant (p=.06), models for men and women were developed separately and the optimal model for each gender was chosen using the Area Under the Curve (AUC). Because covariates may potentially overlap in their ability to explain the variability in rupture rates we checked the beta coefficients and standard errors with and without suspected collinear covariates in the model. Covariates were considered confounders if they changed the coefficients by 20% and were included in the model. Covariates were classified as collinear if they did not substantially change the coefficient but increased the standard error by >20%. Additionally, cumulative distribution curves were generated to evaluate rupture as a function of either aneurysm diameter or ASI. The cumulative distribution curves between men and women were compared with the Kolmogorov-Smirnov test. Statistical analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC) and Stata version 12.0 (Stata Corp, College Station, Tex).
RESULTS
We identified 4,045 patients who underwent AAA repair, of which 2,158 (53%) underwent EVAR. In total, there were 440 ruptures (11%), comprising 18% of open repairs and 4% of EVARs. Women represented 22% of all patients. In general, women were older, less likely to have a smoking history and CAD, but more likely to have COPD than men. Women underwent EVAR for intact aneurysms less often than men (50% vs 60%, P<.001) but more underwent EVAR for rupture (26% vs 20%, P=.183) (Table I).
TABLE I.
Demographics and comorbidities of men and women undergoing ruptured and non-ruptured AAA repair.
| Total
|
P-value | Non-ruptured
|
P-value | Ruptured
|
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||||
| No, % | 3138 (76) | 907 (22) | 2,782 (69) | 823 (20) | 356 (8.8) | 84 (2) | |||
| EVAR, % | 55 | 47 | <.001 | 60 | 50 | <.001 | 20 | 26 | 0.183 |
| Age (y), mean ± SD | 72 ± 9 | 74.3 ± 8 | <.001 | 72 ± 9 | 74 ± 8 | <.001 | 72 ± 9 | 78 ± 7 | <.001 |
| Smoking History, % | |||||||||
| Never | 11 | 16 | 10 | 15 | 14 | 27 | |||
| Past | 53 | 47 | <.001 | 55 | 48 | <.001 | 45 | 35 | 0.025 |
| Current | 36 | 37 | 35 | 37 | 41 | 38 | |||
| HTN, % | 83 | 85 | 0.115 | 83 | 86 | 0.119 | 81 | 81 | 0.899 |
| CAD, % | 36 | 27 | <.001 | 36 | 26 | <.001 | 32 | 31 | 0.893 |
| Diabetes, % | 18 | 16 | 0.210 | 18 | 16 | 0.13 | 14 | 16 | 0.596 |
| COPD, % | 34 | 43 | <.001 | 34 | 43 | <.001 | 37 | 45 | 0.246 |
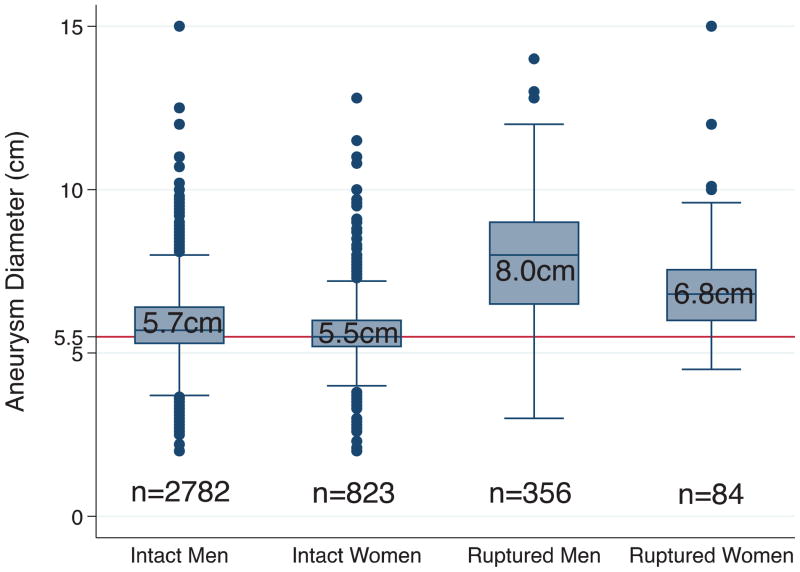
Compared to men, the mean aneurysm diameter in women was 2mm smaller for intact aneurysms and 7mm smaller for ruptured aneurysms(Table II). More women underwent repair of intact aneurysms at diameters<5.5cm (43% vs. 36%, P<.001) and <5.0cm (13% vs. 11%, P<.050) (Table II & Figure 1). A substantial proportion of aneurysms in both men and women ruptured at diameters <5.5cm. However, the difference between men and women did not reach statistical significance (10%vs. 17%, P=.093). A similar proportion of men and women ruptured at diameters <5.0cm (4% for both, P=1.000)and at diameters <4.5cm (3% vs. 0%, P=.361).
TABLE II.
Differences in measures of body size by gender and rupture status.
| Total
|
P-value | Non-ruptured
|
P-value | Ruptured
|
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||||
| AAA Diameter (cm), mean ± SD | 6.1 ± 1.4 | 5.8 ± 1.3 | <.001 | 5.9 ± 1.2 | 5.7 ± 1.1 | <.001 | 7.8 ± 1.9 | 7.1 ± 2.1 | 0.006 |
| <5.5, % | 33 | 41 | <.001 | 36 | 43 | <.001 | 10 | 17 | 0.093 |
| <5.0, % | 10 | 12 | 0.035 | 11 | 13 | 0.050 | 4 | 4 | 1.000 |
| <4.5, % | 5 | 5 | 0.671 | 6 | 5 | 0.796 | 3 | 0 | 0.361 |
| Height (m), mean ± SD | 1.8 ± 0.1 | 1.6 ± 0.1 | <.001 | 1.8 ± 0.1 | 1.6 ± 0.1 | <.001 | 1.8 ± 0.1 | 1.6 ± 0.1 | <.001 |
| Weight (kg), mean ± SD | 85 ± 18 | 71 ± 16 | <.001 | 85 ± 18 | 71 ± 17 | <.001 | 85 ± 21 | 70 ± 14 | <.001 |
| BSA (m2), mean ± SD | 2.0 ± 0.2 | 1.7 ± 0.2 | <.001 | 2.0 ± 0.2 | 1.7 ± 0.2 | <.001 | 2.0 ± 0.2 | 1.7 ± 0.2 | <.001 |
| BSA > gender-specific mean, % | 75 | 79 | 0.011 | 74 | 78 | 0.008 | 82 | 83 | 0.874 |
| BMI, mean ± SD | 28 ± 5 | 27 ± 6 | 0.004 | 28 ± 5 | 27 ± 6 | 0.007 | 28 ± 5 | 27 ± 6 | 0.297 |
| Underweight, % | 3 | 5 | 2 | 4 | 4 | 9 | |||
| Normal weight, % | 28 | 33 | 28 | 34 | 27 | 29 | |||
| Overweight, % | 40 | 33 | <.001 | 41 | 33 | <.001 | 35 | 41 | 0.174 |
| Obese, % | 22 | 20 | 21 | 20 | 27 | 14 | |||
| Morbidly obese, % | 7 | 9 | 7 | 9 | 8 | 7 | |||
Underweight, BMI <18.5; normal weight, BMI 18.5–24.9; overweight, 25.0–29.9; obese, 30–39.9; morbidly obese, ≥ 40
FIGURE 1. Aneurysm diameter of men and women undergoing repair for intact and ruptured AAA.
Box includes 25th to 75th percentiles (with median as number in box); whiskers include values within 1.5X the interquartile range; and remaining data are shown as individual data points.
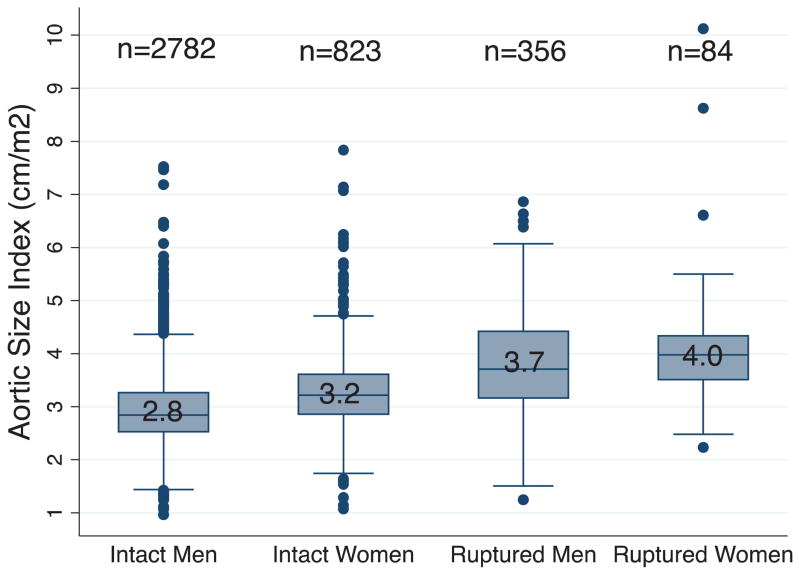
Men were generally taller and heavier and thus had significantly higher BSAs than women (Table II). However, women were more likely to have a BSA greater than their gender-specific mean. Based on BMI, women were more likely to be underweight and of normal weight compared to men, who were more likely to be obese. When aortic diameter was indexed to height, weight, BMI and BSA, women had larger aortas relative to height, weight, and BSA, but not BMI (Table III& Figure 2). These differences were non-significant between men and women with ruptured aneurysms, likely due to the small size of these subgroups.
TABLE III.
Measures of body size indexed to aortic diameter by gender and rupture status.
| Total
|
P-value | Non-ruptured
|
P-value | Ruptured
|
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Men (%) | Women (%) | Men (%) | Women (%) | Men (%) | Women (%) | ||||
| AD (cm) / height (cm), mean ± SD | 0.034 ± 0.008 | 0.036 ± 0.008 | <.001 | 0.034 ± 0.007 | 0.035 ± 0.007 | <.001 | 0.044 ± 0.011 | 0.044 ± 0.013 | 0.966 |
| < 0.030, % | 27 | 13 | <.001 | 28 | 14 | <.001 | 10 | 6 | 0.378 |
| 0.030–0.034, % | 38 | 40 | 41 | 41 | 11 | 15 | |||
| 0.035–0.044, % | 25 | 38 | 24 | 38 | 37 | 45 | |||
| ≥ 0.045, % | 10 | 9 | 7 | 7 | 42 | 34 | |||
| AD (cm) / weight (kg), mean ± SD | 0.075 ± 0.027 | 0.086 ± 0.030 | <.001 | 0.073 ± 0.025 | 0.085 ± 0.029 | <.001 | 0.099 ± 0.039 | 0.105 ± 0.041 | 0.275 |
| < 0.060, % | 28 | 13 | <.001 | 30 | 14 | <.001 | 8 | 4 | 0.389 |
| 0.060–0.079, % | 39 | 34 | 41 | 35 | 27 | 19 | |||
| 0.080–0.099, % | 19 | 29 | 18 | 29 | 27 | 30 | |||
| ≥ 0.100, % | 13 | 24 | 11 | 22 | 38 | 47 | |||
| AD (cm) / BMI (m/kg2), mean ± SD | 0.23 ± 0.07 | 0.22 ± 0.07 | 0.196 | 0.22 ± 0.06 | 0.22 ± 0.06 | 0.655 | 0.29 ± 0.09 | 0.27 ± 0.11 | 0.343 |
| < 0.20, % | 39 | 42 | 0.173 | 41 | 44 | 0.468 | 14 | 19 | 0.213 |
| 0.20–0.24, % | 32 | 31 | 33 | 31 | 23 | 31 | |||
| ≥ 0.25, % | 29 | 27 | 26 | 25 | 63 | 50 | |||
| AD (cm) / BSA (m2), mean ± SD | 3.0 ± 0.8 | 3.3 ± 0.8 | <.001 | 3.0 ± 0.7 | 3.3 ± 0.7 | <.001 | 3.8 ± 1.0 | 4.1 ± 1.3 | 0.109 |
| < 2.5, % | 21 | 9 | <.001 | 23 | 9 | <.001 | 6 | 4 | 0.135 |
| 2.5–2.9, % | 36 | 25 | 38 | 26 | 14 | 13 | |||
| 3.0–3.4, % | 22 | 33 | 22 | 35 | 19 | 6 | |||
| 3.5–3.9, % | 11 | 19 | 10 | 18 | 20 | 29 | |||
| ≥ 4.0, % | 10 | 14 | 7 | 12 | 41 | 48 | |||
AD, aortic diameter; BMI, body mass index; BSA, body surface area
FIGURE 2. Aortic size index (ASI) of men and women undergoing AAA repair by gender and rupture status.
Box includes 25th to 75th percentiles (with median as number in box); whiskers include values within 1.5X the interquartile range; and remaining data are shown as individual data points.
Multivariable-adjusted logistic regression models were constructed for men and women separately (Table IV). Men who had a past history of smoking had approximately half the odds of undergoing ruptured repair (OR 0.6, 95% CI 0.4–0.9, P=.014) but the same effect was not observed among men who reported to be current smokers. Similarly, there was no association between past or current smoking and intact vs. ruptured repair for women. For men, the strongest predictor of ruptured repair was aortic diameter. Compared to men with aneurysms <5.5cm, men with aneurysms 6.5–7.4cm had 4-fold higher odds of ruptured repair (95% CI 1.9–8.2, P<.001) and men with aneurysms ≥ 7.5cm had 12-fold higher odds of undergoing ruptured repair (95% CI 5.2–27.2, P<.001).
TABLE IV.
Multivariable-adjusted predictors of ruptured repair.
| Men*
|
Women**
|
|||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 1.0 (1.0, 1.1) | 0.140 | ||
| Smoking | ||||
| Never | - | - | ||
| Past | 0.6 (0.4, 0.9) | 0.014 | 0.6 (0.3, 1.2) | 0.142 |
| Current | 0.8 (0.5, 1.2) | 0.232 | 0.6 (0.3, 1.5) | 0.319 |
| Diabetes | 0.7 (0.5,1.1) | 0.116 | 0.9 (0.4, 2.1) | 0.789 |
| Aortic Diameter (cm) | ||||
| <5.5 | - | - | ||
| 5.5–6.4 | 0.9 (0.5, 1.7) | 0.774 | 1.1 (0.3, 3.7) | 0.852 |
| 6.5–7.4 | 4.0 (1.9, 8.2) | <.001 | 3.3 (0.8,12.7) | 0.089 |
| ≥ 7.5 | 12.0 (5.2, 27.2) | <.001 | 3.2 (0.7, 14.5) | 0.125 |
| ASI (cm/m2) | ||||
| < 2.5 | 1.3 (0.7, 2.8) | 0.413 | 3.9 (0.5, 28.2) | 0.175 |
| 2.5–2.9 | 1.6 (0.7, 3.7) | 0.284 | 3.3 (0.8, 14.5) | 0.111 |
| 3.0–3.4 | - | - | ||
| 3.5–3.9 | 1.3 (0.5, 3.3) | 0.597 | 6.4 (1.7, 24.1) | 0.006 |
| ≥ 40 | 2.2 (0.8, 5.7) | 0.118 | 9.5 (2.3, 39.4) | 0.002 |
AUC = 0.82
AUC = 0.81
In contrast, for women, the strongest predictor of ruptured repair was ASI (Table IV). Compared to women with a ASI between 3.0 and 3.4cm/m2, women with ASI 3.5–3.9cm/m2 had 6.4 times the odds (95% CI 1.7–24.1, P=.006) and women with ASI ≥ 4.0cm/m2had 9.5 times the odds (95% CI 2.3–39.4, P=.002) of undergoing AAA repair for ruptured aneurysms. In a multivariable model adjusting for ASI as well as aneurysm diameter and other covariates, aneurysm diameter alone was not a significant predictor of ruptured repair for women. Of note, the average aneurysm diameter of women with an ASI ≤ 3.5cm/m2 was 5.3cm. The corresponding average aneurysm diameter of men with an ASI ≤ 3.5cm/m2 was 6.0cm.
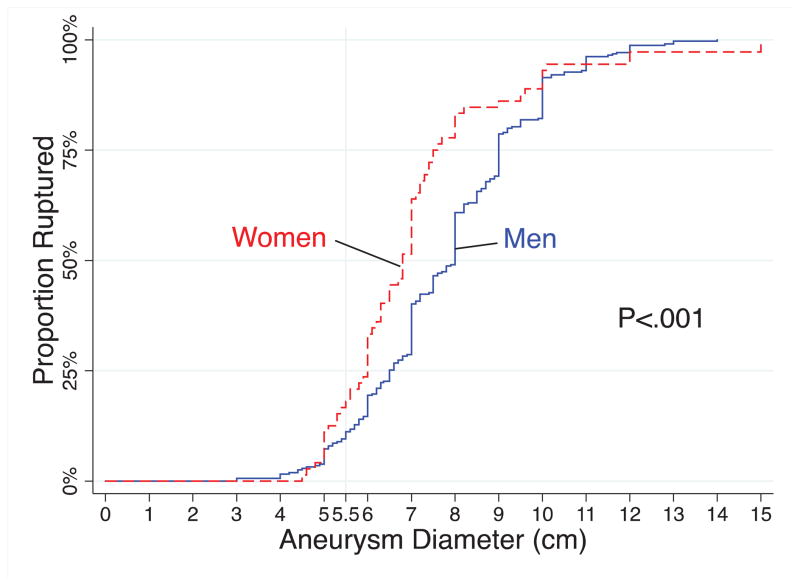
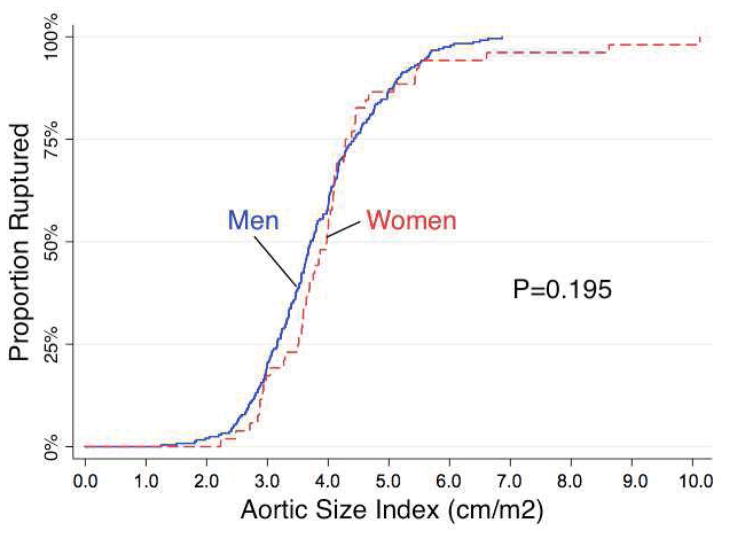
When the cumulative proportion of rupture repair was plotted against aneurysm diameter (Figure 3), we observed that in general, women had a 1.4 fold higher odds of ruptured repair at any given aneurysm diameter (95% CI 1.1–1.8, P=.014). When we adjusted aneurysm diameter for body size and plotted the cumulative proportion ruptured against ASI rather than aneurysm diameter (Figure 4), a difference between rates of ruptured repair between men and women was no longer observed (HR 0.9, 95% CI 0.6–1.2, P=0.315).
FIGURE 3.
Cumulative distribution of rupture repair as a function of aortic diameter.
FIGURE 4.
Cumulative distribution of rupture repair as a function of aortic size index.
There were 12 women who underwent repair of ruptured aneurysms that were smaller than 5.5cm (Table V). Of these 12, 10 (or 83%) may potentially have avoided rupture had an ASI threshold of ≥2.5cm/m2 been used to recommend elective repair (Table VI). Of the two that would have been missed, their aneurysm diameters were 4.8cm and 5.0cm. An additional 20 women underwent repair of symptomatic aneurysms <5.5cm. Of these, 14 had an ASI > 2.5cm/m2 (mean ASI 2.7 ± 0.4cm/m2).
Table V.
Size of aneurysms that ruptured under 5.5cm in women.
| Aneurysm Diameter (cm) | # |
|---|---|
| 4.5 | 1 |
| 4.6 | 1 |
| 4.8 | 1 |
| 5.0 | 5 |
| 5.1 | 1 |
| 5.3 | 2 |
| 5.4 | 1 |
|
| |
| Mean Diameter: 5.0 | Total: 12 |
Table VI.
ASI thresholds and the corresponding number (and percentage) of ruptured aneurysms <5.5cm that would have been selected for elective repair.
| ASI (cm/m2) | Ruptured Aneurysms <5.5cm # (%) |
|---|---|
| ≥ 4.0 | 3 (25) |
| ≥ 3.5 | 4 (33) |
| ≥ 3.0 | 5 (42) |
| ≥ 2.5 | 10 (83) |
| ≥ 2.0 | 12 (100) |
Conversely, of 322 women who underwent elective repair of intact, asymptomatic aneurysms that were smaller than 5.5cm, 61 had an ASI of <2.5cm/m2 This suggests that, if using size criteria alone as indication for elective repair, up to 19% of these women could potentially have safely continued to undergo surveillance. The mean aneurysm diameter of these women was 4.2cm.
DISCUSSION
The Joint Council of the American Association of Vascular Surgery and the Society of Vascular Surgery has suggested a lower diameter threshold for AAA repair in women11. This recommendation was made based on observations that women have a higher risk of aneurysm rupture at any given diameter and that women rupture at smaller diameters than men10, 25,26. Consistent with these previous reports, we found that a considerable proportion of patients who underwent rupture repair (10% of men and 17% of women in our series) have aneurysms smaller than 5.5cm and that the average diameter of ruptured aneurysms in women was 7mm smaller than in men. Using BSA, we were able to show that accounting for body size bridged the gender discrepancy in rupture rates. This gives credence to what many have previously suspected: that the reason women rupture at smaller aortic diameters is that their aneurysms are larger relative to their body size compared to men. It is important to note that BSA and ASI account for body size and are not simply measures of obesity. Notably, BMI, the standard measure of obesity, and its associated BMI index, were not predictive of rupture in the multivariable model. Thus, although obesity correlates with higher ASI, we cannot conclude that obesity is protective against rupture.
The results from our multivariable models indicated that ASI was a better predictor of rupture repair than aneurysm diameter alone for women. One might naturally wonder why there should be a differential importance of body size in predicting rupture repair for women versus men such that ASI is predictive for women but not men. We believe this is because the women in the VSGNE had a greater variation in body size than the men. Thus, indexing aneurysm diameter to body size provides little additional information than aneurysm size alone in men. In contrast, in women who have greater diversity in body size, ASI has more predictive power. If validated, using an ASI threshold of ≥2.5cm/m2 may help identify women with aneurysms <5.5cm who would benefit from early repair. For men, it appears aneurysm diameter alone remains the primary determinant of rupture risk.
ASI has also been shown to be a better predictor of rupture in thoracic aortic aneurysms. Davies et al. analyzed the association of ASI to the incidence of adverse events (rupture, dissection, or death)22. In all analyses, they found ASI to be a better predictor of adverse events than maximum aortic diameter alone. Using ASI, they stratified patients into three levels of risk. Those with ASI <2.75cm/m2were considered low risk (yearly risk approximately 4%), those with ASI between 2.75 and 4.25cm/m2were considered at moderate risk (yearly risk approximately 8%), and those with ASI above 4.25cm/m2 were categorized as high risk (yearly risk, approximating 20–25%).
Other groups have previously attempted indexing aneurysm diameter to other measures of body size. Forbes et al. calculated the relative dilatation of aneurysms (108 men, 21 women) undergoing elective EVAR by indexing maximum aneurysm diameter to suprarenal aortic diameter27. Using this index, they found relative dilatation to be greater in women and, through linear regression modeling, demonstrated that 5.5cm aneurysms in men translated to 5.2cm aneurysms in women. However, because their study included only patients undergoing elective repair, they could not comment on rupture risk prediction.
Ouriel et al. indexed aneurysm diameter to the transverse diameter of the third lumbar vertebral body28. They used this index to evaluate the CT scans of 100 patients undergoing elective AAA repair compared to 36 patients with ruptured aneurysms. When using a threshold value of 1.0, they found this measure to be a more accurate predictor of rupture than diameter alone. However, a gender-specific analysis was not performed.
Fillinger et al. have investigated the association of peak aortic wall stress to rupture risk. Using finite element analysis and three-dimensional (3D) CT reconstruction to measure peak mechanical wall tensile stress, they demonstrated first that wall stress was significantly different between intact and ruptured aneurysms29, and later that wall stress could reliably predict rupture risk in patients undergoing surveillance30. They are currently undertaking a large, prospective, multicenter study to validate this promising metric29 and one of their goals is to determine if there are any significant differences in aneurysm wall thickness, wall strength, or both between men and women since they initially observed that a higher proportion of aneurysms in women had high wall stress30. However, currently 3D CT reconstruction is not universally available and wall stress analysis is currently too complex for broad application. In contrast, ASI is quick and simple to calculate and therefore easily adoptable into clinical practice.
In addition to rupture risk, the decision to operate for AAAs should take into account the risk of the procedure and the patient’s predicted late survival. Opponents to adopting a lower aneurysm size threshold for women point out that women have frequently been shown to have significantly higher perioperative morbidity and mortality compared to men1–3, 31–33, effectively diminishing or even negating any benefit to earlier repair. However, in our previous analysis of gender differences in AAA presentation, management, and outcomes using the same database as this current study34, we observed gender disparities in perioperative and 1-year mortality only among patients undergoing ruptured repair. Within the VSGNE at the time of our analysis, 43% of women underwent elective AAA repair at aneurysm diameters <5.5cm and we found that both men and women who were repaired for such “small” aneurysms had significantly better perioperative and 1-year survival. On the surface these results may appear to contradict the conclusions made by the UKSAT and other randomized controlled trials16–18 that failed to show any benefit of early repair of small aneurysms. However, these women (and also the 35% of men undergoing AAA repair at aneurysm diameters <5.5cm) were clearly a group of carefully selected patients chosen for repair because of their more favorable risk profile. They were younger and had lower rates of congestive heart failure and chronic obstructive pulmonary disease and were more likely to undergo EVAR rather than open repair. Thus, when considering patients for “early repair,” appropriate patient selection is still the most important determinant of patient outcomes35. We therefore would not advocate repair of aneurysms <5.5cm in women who are not suitable surgical candidates.
Smoking has been shown to be a risk factor for AAA expansion, rupture, and poor long-term survival after repair36. Paradoxically, in our study, a positive smoking history was correlated with elective rather than ruptured repair in men. This may be perhaps because patients with a smoking history are more likely to be screened for AAA and subsequently referred for elective repair. It is also plausible that patients seen electively have smoking history more accurately recorded than patients who present emergently with rupture.
In addition to its retrospective design, our study is limited by the absence of information regarding patients treated non-operatively. It must be emphasized that since the VSGNE only captures patients who underwent repair, patients who were denied or who declined repair or died before reaching a hospital were excluded. The individuals included in the VSGNE are more likely to have smaller aneurysms and/or have a more favorable surgical risk profile, which has clearly led to some degree of selection bias. Furthermore, since patients in this database underwent repair when they reached appropriate size criteria or became symptomatic, they were eliminated from further analysis. This may in part explain why the relationship between repair for rupture and ASI is J-shaped rather than linear for women. Thus, a prospective study inclusive of patients with AAA managed with surveillance is necessary to validate the results of our study.
Additionally, the VSGNE lacks data on aneurysm expansion rate, tortuosity, diameter asymmetry, fusiform vs. saccular configuration, presence of thrombus, adequacy of blood pressure control, and other anatomic and clinical factors that have previously been shown to affect rupture risk26, 37, 38. Thus the prognostic utility of ASI is unclear in cases in which these factors are a major concern.
It can be argued that because the majority of men and women in the VSGNE had larger-than-normal body size indices (i.e. BMI, BSA) that our findings are limited to “larger” patients. However, most patients with AAA (and in fact most typical patients in America) tend to be overweight. Furthermore, recent studies have demonstrated associations between obesity and abdominal adiposity and increased incidence and expansion of AAA39, 40. We therefore do not think the overrepresentation of larger patients in our study jeopardizes the generalizability of our study’s results.
Finally, although the VSGNE captures more than 4,000 patients undergoing AAA repair, the number of men and women undergoing repair for small, ruptured aneurysms were comparatively small, potentially introducing instability in the multivariable models and increasing the risk of statistical error.
CONCLUSIONS
A significant proportion of patients with AAA rupture do so at aneurysm diameters less than 5.5cm. At the time of repair, women generally have larger aneurysms relative to their body size than men. For men, aneurysm size is still the strongest predictor of rupture. For women, aneurysm size indexed to body surface area is more predictive of rupture than aneurysm size alone. ASI may help identify women who would benefit from early repair of aneurysms <5.5cm.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery Grant HL00774.
Footnotes
Author Disclosures: RC Lo, none; B Lu, none; MTM Fokkema, none; M Conrad, none, VI Patel, none; M Fillinger, none; R Matyal, none; ML Schermerhorn, Endologix Consultant, Medtronic Consultant
Presented at the Society for Clinical Vascular Surgery’s 41st Annual Symposium on March 13, 2013 in Miami, FL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McPhee JT, Hill MH, Eslami JS. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. Journal of vascular surgery. 2007;45(5):891–899. doi: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Giles KA, Hamdan AD, Pomposelli FB, Wyers MC, Dahlberg ML, Schermerhorn SE. Population-based outcomes following endovascular and open repair of ruptured abdominal aortic aneurysms. J Endovasc Ther. 2009;16(5):554–564. doi: 10.1583/09-2743.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mureebe L, Egorova N, McKinsey JF, Kent KC. Gender trends in the repair of ruptured abdominal aortic aneurysms and outcomes. Journal of vascular surgery. 2010;51(4 Suppl):9S–13S. doi: 10.1016/j.jvs.2009.10.129. [DOI] [PubMed] [Google Scholar]
- 4.Egorova NN, Vouyouka AG, McKinsey JF, Faries PL, Kent KC, Moskowitz AJ, et al. Effect of gender on long-term survival after abdominal aortic aneurysm repair based on results from the Medicare national database. Journal of vascular surgery. 2011;54(1):1–12. e16. doi: 10.1016/j.jvs.2010.12.049. discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 5.Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PS, Kreienberg PB, et al. Women derive less benefit from elective endovascular aneurysm repair than men. Journal of vascular surgery. 2012;55(4):906–913. doi: 10.1016/j.jvs.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 6.Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Noel AA, Karla M, et al. Endovascular abdominal aortic aneurysm repair: does gender matter? Ann Vasc Surg. 2004;18(6):653–660. doi: 10.1007/s10016-004-0106-6. [DOI] [PubMed] [Google Scholar]
- 7.Sweet MP, Fillinger MF, Morrison D, Abel TM. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. Journal of vascular surgery. 2011;54(4):931–937. doi: 10.1016/j.jvs.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 8.Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–1452. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 9.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 10.Brown, Powell LCJT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999;230(3):289–296. doi: 10.1097/00000658-199909000-00002. discussion 296–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski JS, Matsumura WC. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37(5):1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 12.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. Journal of vascular surgery. 2009;50(4 Suppl):S2–49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Lederle FA. Should abdominal aortic aneurysm be managed differently in women? Scand J Surg. 2008;97(2):125–127. doi: 10.1177/145749690809700209. [DOI] [PubMed] [Google Scholar]
- 14.Filardo G, Powell JT, Martinez DJ, Ballard MA. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2012;3:CD001835. doi: 10.1002/14651858.CD001835.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown LC, Thompson SG, Greenhalgh JT, Powell RM. Fit patients with small abdominal aortic aneurysms (AAAs) do not benefit from early intervention. Journal of vascular surgery. 2008;48(6):1375–1381. doi: 10.1016/j.jvs.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. Journal of vascular surgery. 1997;26(4):595–601. doi: 10.1016/s0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- 17.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160(10):1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 18.Cao P, De Rango P, Verzini F, Parlani G, Romano E, Cieri L. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41(1):13–25. doi: 10.1016/j.ejvs.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Ouriel K. The PIVOTAL study: a randomized comparison of endovascular repair versus surveillance in patients with smaller abdominal aortic aneurysms. Journal of vascular surgery. 2009;49(1):266–269. doi: 10.1016/j.jvs.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Ouriel K, Clair DG, Kent CK, Zarins KC. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. Journal of vascular surgery. 2010;51(5):1081–1087. doi: 10.1016/j.jvs.2009.10.113. [DOI] [PubMed] [Google Scholar]
- 21.Du Bois, Du Bois DEF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. discussion 312–303. [PubMed] [Google Scholar]
- 22.Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81(1):169–177. doi: 10.1016/j.athoracsur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 24.Haycock GB, Schwartz DH, Wisotsky GJ. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls SC, Gardner JB, Meissner HK, Johansen MH. Rupture in small abdominal aortic aneurysms. Journal of vascular surgery. 1998;28(5):884–888. doi: 10.1016/s0741-5214(98)70065-5. [DOI] [PubMed] [Google Scholar]
- 26.Fillinger MF, Racusin J, Baker RK, Cronenwett JL, Teutelink A, Schermerhorn ML, et al. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: Implications for rupture risk. J Vasc Surg. 2004;39(6):1243–1252. doi: 10.1016/j.jvs.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Forbes TL, Lawlor DK, DeRose KA, Harris G. Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg. 2006;20(5):564–568. doi: 10.1007/s10016-006-9079-y. [DOI] [PubMed] [Google Scholar]
- 28.Ouriel K, Green RM, Donayre C, Shortell CK, Elliott JA, DeWeese J. An evaluation of new methods of expressing aortic aneurysm size: relationship to rupture. Journal of vascular surgery. 1992;15(1):12–18. doi: 10.1067/mva.1992.32982. discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 29.Fillinger MF, Raghavan ML, Marra SP, Cronenwett FE, Kennedy JL. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. Journal of vascular surgery. 2002;36(3):589–597. doi: 10.1067/mva.2002.125478. [DOI] [PubMed] [Google Scholar]
- 30.Fillinger MF, Marra SP, Raghavan FE, Kennedy ML. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. Journal of vascular surgery. 2003;37(4):724–732. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 31.Abedi NN, Davenport DL, Xenos E, Sorial E, Minion ED, Endean DJ. Gender and 30-day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. Journal of vascular surgery. 2009;50(3):486–491. 491 e481–484. doi: 10.1016/j.jvs.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Katz DJ, Stanley GB, Zelenock JC. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25(3):561–568. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 33.Dillavou ED, Muluk MS, Makaroun SC. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg. 2006;43(2):230–238. doi: 10.1016/j.jvs.2005.09.043. discussion 238. [DOI] [PubMed] [Google Scholar]
- 34.Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.11.039. TBD(TBD): p. TBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fillinger M. Who should we operate on and how do we decide: predicting rupture and survival in patients with aortic aneurysm. Semin Vasc Surg. 2007;20(2):121–127. doi: 10.1053/j.semvascsurg.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19(6):636–642. doi: 10.1053/ejvs.2000.1066. [DOI] [PubMed] [Google Scholar]
- 37.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. Journal of vascular surgery. 2009;50(4):880–896. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, Kalman BVPG. Saccular aortic aneurysms. Ann Vasc Surg. 1999;13(6):555–559. doi: 10.1007/s100169900297. [DOI] [PubMed] [Google Scholar]
- 39.Stackelberg O, Bjorck M, Sadr-Azodi O, Larsson SC, Orsini A, Wolk N. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100(3):360–366. doi: 10.1002/bjs.8983. [DOI] [PubMed] [Google Scholar]
- 40.Cronin O, Walker J, Golledge PJ. The association of obesity with abdominal aortic aneurysm presence and growth. Atherosclerosis. 2013;226(2):321–327. doi: 10.1016/j.atherosclerosis.2012.10.041. [DOI] [PubMed] [Google Scholar]