Abstract
Objectives
We postulated that ventilation-perfusion (V/Q) relationships within the lung might influence where lung cancer occurs. To address this hypothesis we evaluated the location of lung adenocarcinoma, by both tumor lobe and superior-inferior regional distribution, and associated variables such as emphysema.
Materials and Methods
One hundred fifty-nine cases of invasive adenocarcinoma and adenocarcinoma with lepidic features were visually evaluated to identify lobar or regional tumor location. Regions were determined by automated division of the lungs into three equal volumes: (upper region, middle region, or lower region). Automated densitometry was used to measure radiographic emphysema.
Results
The majority of invasive adenocarcinomas occurred in the upper lobes (69%), with 94% of upper lobe adenocarcinomas occurring in the upper region of the lung. The distribution of adenocarcinoma, when classified as upper or lower lobe, was not different between invasive adenocarcinoma and adenocarcinoma with lepidic features (formerly bronchioloalveolar cell carcinoma, P=0.08). Regional distribution of tumor was significantly different between invasive adenocarcinoma and adenocarcinoma with lepidic features (P = 0.001). Logistic regression analysis with the outcome of invasive adenocarcinoma histology was used to adjust for confounders. Tumor region continued to be a significant predictor (OR 8.5, P=0.008, compared to lower region), whereas lobar location of tumor was not (P=0.09). In stratified analysis, smoking was not associated with region of invasive adenocarcinoma occurrence (p=0.089). There was no difference in total emphysema scores between invasive adenocarcinoma cases occurring in each of the three regions (P=0.155). There was also no difference in the distribution of region of adenocarcinoma occurrence between quartiles of emphysema (P=0.217).
Conclusion
Invasive adenocarcinoma of the lung is highly associated with the upper lung regions. This association is not related to smoking, history of COPD, or total emphysema. The regional distribution of invasive adenocarcinoma may be due to V/Q relationships or other local factors.
Keywords: Non-small cell lung cancer, tumor location, emphysema
Introduction
Location of non-small cell lung cancer (NSCLC) has previously been evaluated based on lobe of the lung.(1),(2) Upper lobe nodules, for instance, are associated with an increased probability of lung cancer and a review of cases from the Surveillance, Epidemiology, and End Results (SEER) database reported an increased prevalence of adenocarcinoma in the upper lobes compared to the lower lobes.(3)(4)
Classification of tumor location based only on lobe of the lung may not accurately characterize the distribution when considering ventilation-perfusion (V/Q) relationships or other important regional factors. For instance the superior segment of the lower lobe on either side partially resides in the upper region of the chest resulting, in relatively lower perfusion compared to other regions of the lower lobe. This regional variation in V/Q also results in a physiologic gradient of alveolar oxygen tension of approximately 40mm Hg between the apex and the base of the lung.(5)
We hypothesized that ventilation-perfusion (V/Q) relationships or other local factors may predispose to the regional occurrence of lung cancer. Cases of invasive adenocarcinoma and the related histology of adenocarcinoma with lepidic features (formerly bronchioloalveolar cell carcinoma) were identified from a large lung cancer cohort and reviewed to identify lobar tumor location. Additionally, we used quantitative analysis of CT scans to divide the lung into equal volumetric thirds (upper region, middle region, and lower region) and classified tumor location based on region.
Materials and Methods
Cases
We conducted a cross-sectional analysis of lung cancer cases derived from a large prospectively enrolled lung cancer cohort: the Harvard-Massachusetts General Hospital Lung Cancer Susceptibility Study. Details of this cohort have been described previously.(2)(6) This study was approved by the Partners Human Research Committee (1999-P-004935/118).
Enrollment generally occurred at the initial visit in the multi-disciplinary Thoracic Oncology Clinic at Massachusetts General Hospital between the years 2002 and 2006. More than 85% of eligible patients were recruited in this cohort and 96% were Caucasians. Demographic information was collected by a trained research staff using a standardized questionnaire at the time of recruitment. In addition to demographic data, detailed smoking histories were collected for these patients based on the modified ATS questionnaire.(7)
Histology was determined by a trained pathologist and captured at the time of diagnosis. Bronchioloalveolar carcinoma (BAC) was defined by on the presence of lepidic growth and lack of invasion. Cases previously interpreted as BAC are referred to as adenocarcinoma with lepidic features since these cases were not interpreted under the contemporary definition.(8) The remaining cases are referred to as invasive adenocarcinoma.
We included cases that had a chest CT scan with intravenous contrast performed prior to initiation of surgery, radiation, or chemotherapy, and within three months of enrollment in the study. Additional exclusion criteria were lobar collapse or infiltrate, the presence of significant pulmonary fibrosis or lymphangitic spread, and inability to identify a primary lesion (e.g. isolated mediastinal disease or multiple lesions). Two hundred sixty cases of NSCLC were eligible for automated analysis. We limited our analysis to one hundred fifty-nine cases of adenocarcinoma and the related histology of adenocarcinoma with lepidic features to minimize the effects of differing distributions of other histologies.
Regional Division of the Lung and Quantitation of Emphysema
All CT scans were acquired with GE scanners (GE Healthcare, Waukesha,WI). Approximately half (50.3%) were obtained at a 5mm slice thickness with an additional 41% obtained with 2.5mm slices. The “Body” filter was used for 93.5% of the studies.
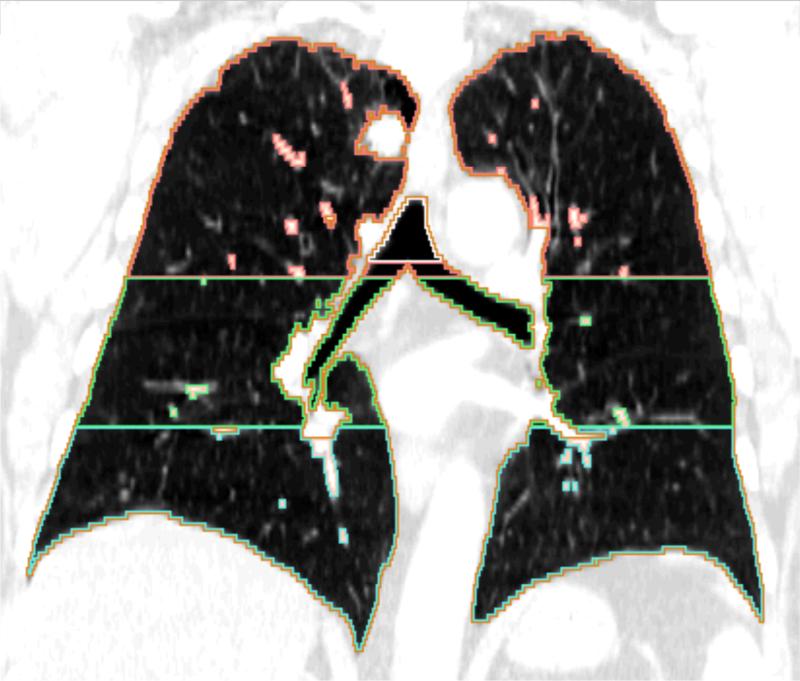
Automated densitometric analysis was performed using Airway Inspector Software (www.airwayinspector.org) as described previously.(9)(10) Total lung volume was divided into equal thirds in a superior-inferior fashion (Figure 1). Emphysema was calculated as the percentage of voxels with attenuation less than −910 Houndsfield units.
Figure 1.
Coronal CT image from a case with an upper region adenocarcinoma. The depicted regions were defined by automated segmentation of the lung into equal volumetric thirds.
Classification of Tumor Location
CTs were reviewed to identify the location of the tumor. “Upper lobe” classification included the right upper, right middle, and left upper lobes. “Lower lobe” classification included the right lower and left lower lobes. For tumors that spanned more than one lobe, location was designated as the lobe that contained the predominance of the tumor as measured in axial section. Tumor location by region (upper, middle, or lower), was identified in a similar fashion.
Statistical Analysis
All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., North Carolina) or JMP Pro version 10.0 (SAS Institute Inc., North Carolina). P values less than or equal to 0.05 were considered significant and all statistical tests were two-sided. Probabilities of tumor occurrence were calculated using the binomial distribution. Comparisons of continuous normally distributed data were analyzed using the Student's t-test and continuous non-normally distributed data were analyzed using the Wilcoxon rank sum test. Contingency tables were analyzed using the Fisher Exact test.
In order to assess the associations of lobar distribution and regional distribution with the histology of invasive adenocarcinoma, logistic regression was performed. The response variable was the dichotomous outcome of invasive adenocarcinoma compared to adenocarcinoma with lepidic features. Two logistic regression models were compiled using age, sex, stage, pack years, and either tumor region or tumor lobe.
Results
Tumor Distribution
Characteristics of the cohort have been described previously and are included in Supplemental Table I.(2),(6) The distribution of tumors by lobe (RUL 31.6%, RML 5.3%, RLL 19.1%, LUL 30.8%, LLL 13.4%) was nearly identical to that from aggregate data.(11)
Table I.
Characteristics of Cases of Invasive Adenocarcinoma and Adenocarcinoma with Lepidic Features
| Invasive AC | ACwLP | P value | |
|---|---|---|---|
| Age: y* | 65.2 (±11.0) | 69.2 (±9.0) | 0.046 |
| Sex: n (%) | |||
| Female | 69 (52.7) | 21 (75.0) | |
| Male | 62 (47.3) | 7 (25.0) | 0.074 |
| BMI:ψ | 26.0 (6.7) | 24.7 (6.5) | 0.156 |
| Smoking: n (%) | |||
| Never | 17 (13.3) | 6 (21.4) | |
| Former | 72 (56.2) | 14 (50.0) | |
| Current | 39 (30.5) | 8 (28.6) | 0.571 |
| Pack yearsψ | 38.0(40.0) | 18.7 (38.1) | 0.013 |
| PMH Chronic Bronchitis: n (%) | 17 (13.6) | 5 (20.8) | 0.355 |
| PMH Emphysema: n (%) | 21 (16.8) | 3 (12.5) | 0.766 |
| Emphysema: %ψ | 3.5 (11.9) | 2.9 (10.5) | 0.155 |
| Family History : n (%) | 28 (17.2) | 5 (15.2) | 0.605 |
| Stage: n (%) | |||
| Early (1-2) | 18 (13.7) | 24 (85.7) | |
| Late (3-4) | 113 (86.3) | 4 (14.3) | <0.001 |
| Lobar Tumor Location: n (%) | |||
| Upper lobe | 91 (69.5) | 14 (50.0) | |
| Lower lobe | 40 (30.5) | 14 (50.0) | 0.077 |
| Regional Tumor Location: n (%) | |||
| Upper region | 77 (58.8) | 11 (39.3) | |
| Middle region | 41 (31.3) | 6 (21.4) | |
| Lower region | 13 (9.9) | 11 (39.3) | 0.001 |
AC = adenocarcinoma, ACwLP = adenocarcinoma with lepidic features.
Percentages represent distribution within invasive AC or ACwLP groups.
denotes mean ± standard deviation presented.
denotes median and interquartile range presented.
PMH denotes history of disease as reported by the subject.
Given the known differing central-peripheral distributions of different NSCLC histologies (e.g. squamous cell carcinoma tends to occur more centrally), we next evaluated the distribution of cases of invasive adenocarcinoma. There was not a significant difference in the proportion of right-sided adenocarcinomas compared to left (54.1% vs. 45.9%, p=0.39). Invasive adenocarcinoma occurred more frequently in the upper lobes (69.5% vs 30.5%, P=5×10−6). The percentage of invasive adenocarcinomas occurring in the upper region was also significantly different (59%) from the expected distribution (30% based on volume, P= <1×10−6). Notably, 94% of upper lobe adenocarcinomas occurred in the upper third of the lung (upper region).
Tumor Distribution: Adenocarcinoma vs. Adenocarcinoma with Lepidic Features
To further characterize regional or lobar location of invasive adenocarcinoma, the related histology of adenocarcinoma with lepidic features was used as a comparator group. These two histologies are believed to exist on a pathologic spectrum.(8) Characteristics of cases of adenocarcinoma and adenocarcinoma with lepidic features are presented in Table 1. There were no significant differences in the distributions of sex, body mass index (BMI), smoking category, subject reported history of chronic bronchitis or emphysema, total emphysema score, or family history between cases of invasive adenocarcinoma and adenocarcinoma with lepidic features. Stage was significantly different between the two groups. This result was anticipated given that adenocarcinoma with lepidic features (formerly BAC) is predominantly early stage. Adenocarcinoma with lepidic features was more evenly distributed between the upper (39.3%), middle (21.1%), and lower (39.3%) regions.
There was not a significant difference in the lobar tumor distribution of adenocarcinoma compared to adenocarcinoma with lepidic features (P=0.077, Figure 2). In contrast, the distributions of adenocarcinoma and adenocarcinoma with lepidic features were significantly different between the three tumor regions (P=0.001, Figure 2).
Figure 2.
Comparison of distribution of adenocarcinoma by lobe or region, overlaid on sagittal section for illustration. The P values presented are in comparison to the distribution of adenocarcinoma with lepidic features (ACwLP, formerly referred to as BAC).
Logistic Regression Analysis to Predict Adenocarcinoma Histology
Logistic regression was performed to assess the association between regional tumor location and invasive adenocarcinoma (compared to adenocarcinoma with lepidic features) and that between lobar tumor location and invasive adenocarcinoma (compared to adenocarcinoma with lepidic features). Age, sex, stage, pack years, and either tumor lobe or tumor region were included in the final models. The unadjusted estimates for all covariates and adjusted estimates for the model including tumor lobe and the model including tumor region are presented in Table II.
Table II.
Logistic Regression Comparing Tumor Region or Tumor Lobe to Predict Invasive Adenocarcinoma vs. Adenocarcinoma with Lepidic Features.
| Unadjusted Estimates | Adjusted Model 1: Tumor Lobe | Adjusted Model 2: Tumor Region | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | OR | CI | P | |
| Age | 0.99 | [0.96, 1.01] | 0.272 | 0.97 | [0.91, 1.02] | 0.323 | 0.97 | [0.90, 1.03] | 0.302 |
| Sex | |||||||||
| Female | 0.56 | [0.20, 1.54 | 0.261 | 0.55 | [0.16, 1.81] | 0.327 | 0.28 | [0.06, 1.14] | 0.089 |
| Male | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Pack Years | 1.02 | [1.00, 1.03] | 0.026 | 1.02 | [0.99, 1.04] | 0.154 | 1.01 | [0.98, 1.04] | 0.219 |
| Stage | |||||||||
| Early (I-II) | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Late (III-IV) | 24.04 | [9.15, 67.98] | <.0001 | 34.76 | [11.33, 135.92] | <.0001 | 49.43 | [13.42, 271.31] | <.0001 |
| Tumor Lobe | |||||||||
| Upper | 2.21 | [0.99, 5.01] | 0.052 | 2.59 | [0.86, 8.33] | 0.092 | |||
| Lower | Ref | Ref | Ref | Ref | Ref | Ref | |||
| Tumor Region | |||||||||
| Upper | 6.16 | [2.22, 17.45 | <.0001 | 8.48 | [1.69, 51.71] | 0.008 | |||
| Middle | 5.92 | [1.89, 20.31] | 0.002 | 9.25 | [1.55, 68.16] | 0.014 | |||
| Lower | Ref | Ref | Ref | Ref | Ref | Ref | |||
OR= odds ratio, CI= confidence interval, P= p value
Unadjusted analysis did not reveal a statistically significant relationship between tumor lobe and the outcome of invasive adenocarcinoma compared to adenocarcinoma with lepidic features (P=0.052). Tumor region, in contrast, was statistically significant for comparisons of both the upper region to the lower, and the middle region to the lower (P=0.0005 and P=0.002, respectively).
Tumor lobe was also not a significant predictor in adjusted analysis (P=0.09). The adjusted model that included tumor region revealed a statistically significant association between regional tumor location and the histology of invasive adenocarcinoma (e.g. p=0.008 for upper region compared to lower region tumor location). As compared to adenocarcinoma with lepidic features, there was an odds ratio of 8.5 for invasive adenocarcinoma occurring in the upper lung region compared to the lower, while controlling for age, sex, stage, and pack years of smoking. Additionally, the ability of the logistic model to discriminate (by area under the curve) between invasive adenocarcinoma and adenocarcinoma with lepidic features was superior when tumor region was included as compared to tumor lobe (C=0.95 and C=0.88, respectively). In a model that included both tumor region and tumor lobe (in addition to age, sex, pack years, and stage), tumor region remained a statistically significant predictor (P=0.029) despite the modest correlation between the tumor region and tumor lobe variables (Cramer's V=0.68).
Stratified Analysis Comparing Regions of Invasive Adenocarcinoma Occurrence
Cases of invasive adenocarcinoma were compared by region of tumor occurrence to attempt to identify possible associated factors. The distribution of covariates between cases of invasive adenocarcinoma that occurred in the upper, middle, and lower regions is shown in Table III. There were no significant differences between any of the included covariates.
Table III.
Characteristics of Invasive Adenocarcinoma Cases by Region of Occurrence
| Upper | Middle | Lower | P Value | |
|---|---|---|---|---|
| Age: y* | 64.0 (±10.4) | 66.6 (±11.9) | 67.5 (±10.3)) | 0.321 |
| Sex: n (%) | ||||
| Female | 38 (53.5) | 27 (38.0) | 6 (8.5) | |
| Male | 42 (65.6) | 15 (23.4) | 7 (10.9) | 0.187 |
| BMI:ψ | 26.2 (6.3) | 25.4 (10.4) | 25.9 (3.9) | 0.962 |
| Smoking: n (%) | ||||
| Never | 11 (64.7) | 3 (17.7) | 3 (17.7) | |
| Former | 38 (52.1) | 26 (35.6) | 9 (12.3) | |
| Current | 29 (69.1) | 12 (28.1) | 1 (2.4) | 0.124 |
| Pack yearsψ | 39.1 (45.6) | 35.1 (35.9) | 34.4 (53.3) | 0.641 |
| PMH Chronic Bronchitis: n (%) | 7 (41.2) | 6 (35.3) | 4 (23.4) | 0.102 |
| PMH Emphysema: n (%) | 12 (57.1) | 6 (28.6) | 3 (14.3) | 0.829 |
| Emphysema: %ψ | 4.6 (10.6) | 2.8 (7.4) | 12.3 (14.7) | 0.155 |
| Family History : n (%) | 16 (57.1) | 7 (25.0) | 5 (17.9) | 0.235 |
| Stage: n (%) | ||||
| Early (1-2) | 11 (61.1) | 5 (27.8) | 2 (11.1) | |
| Late (3-4) | 69 (59.0) | 37 (27.4) | 11 (9.4) | 0.932 |
Mean ± standard deviation
Median and interquartile range
PMH denotes history of disease as reported by the subject
The distribution of stage of invasive adenocarcinoma was not different between cases with tumors occurring in the three different lung regions (P= 0.752). Similarly, when limited to invasive adenocarcinoma, the association of smoking category to tumor location was not significant (p=0.12). Even in never smokers, the upper region remained the predominant location of invasive adenocarcinoma (64.7%).
Total emphysema score was not different between cases with invasive adenocarcinoma in the upper, middle, or lower regions. We also divided total emphysema score into quartiles and compared the regional distribution of invasive adenocarcinoma cases. Table 4 depicts the percent of invasive adenocarcinoma cases by tumor region versus quartile of total emphysema score. There was no difference in regional invasive adenocarcinoma distribution amongst quartiles of emphysema (P=0.217).
Table IV.
Invasive Adenocarcinoma Location (%) vs. Quartile of Total Emphysema Score
| Region of Adenocarcinoma Location |
|||
|---|---|---|---|
| Lower | Middle | Upper | |
| Quartile 1 | 3.0 | 39.4 | 57.6 |
| Quartile 2 | 12.8 | 35.9 | 51.3 |
| Quartile 3 | 3.5 | 24.1 | 72.4 |
| Quartile 4 | 17.7 | 19.0 | 58.9 |
P=0.217
Discussion
This lung cancer cohort is one of the largest analyzed by automated methods and the first to evaluate tumor location by region. The overall lobar distribution of NSCLC tumors in this cohort is nearly identical to that noted from over 200,000 cases evaluated in the SEER database.(11) Prior work from that database has revealed a statistically significant association of adenocarcinoma for the upper lobes.(3) The primary finding of this analysis was a predilection of invasive adenocarcinoma for the upper lung regions. When compared to the more even distribution of adenocarcinoma with lepidic features, invasive adenocarcinoma was significantly associated with upper region location (in unadjusted and adjusted analyses) but not upper lobe location. This finding is not contradictory to that from the larger SEER database as adenocarcinoma region and adenocarcinoma lobe are correlated variables. However, based on our data, invasive adenocarcinoma is more highly associated with an upper region distribution than with an upper lobe distribution.
Multiple reports from smaller cohorts of cases have described an association of emphysema and lung cancer based on visual analysis.(12)(13) We performed automated densitometric analysis of emphysema to avoid bias associated with visualizing the location of the tumor. Although CT scans in this study were clinically acquired, the variation in slice thickness here is less than that from CT scans used for automated densitometric analysis from the well established National Emphysema Treatment Trial cohort.(14) We found no significant difference in total emphysema score between cases of invasive adenocarcinoma and adenocarcinoma with lepidic features. There was also no difference in emphysema score between cases of invasive adenocarcinoma that occurred in the upper, middle, or lower regions or in the regional distribution of invasive adenocarcinoma amongst quartiles of total emphysema score. We cannot rule out an association of emphysema and adenocarcinoma region based solely on densitometric analysis(15-17) but there is no evidence of a statistical relationship in this analysis.
Airflow obstruction is a well-established risk factor for lung cancer.(18)(19) Patient reported history of COPD has been shown to correlate with spirometry-diagnosed COPD. (20) Although spirometry is not available for the majority of the cohort, patient reported histories of emphysema and chronic bronchitis were not associated with region of invasive adenocarcinoma occurrence.
It is possible that patterns of smoke inhalation may result in the upper region predominance of invasive adenocarcinoma. The volume of the lower lobes is slightly greater than the upper (including the right middle lobe) with some equalization in the setting of upper lobe emphysema.(21)(22) Ventilation is also greater in the lower lung regions.(5) However, the relatively poor perfusion of the upper lung regions does result in slower lymphatic drainage along the peribronchial system. Gurney postulated that this process might result in higher particle concentrations and subsequently predispose to lung disease in those regions (e.g. pneumoconiosis, emphysema).(23) This hypothesis could explain the predilection of invasive adenocarcinoma for the upper regions. However, we also observed that invasive adenocarcinoma was associated with an upper region distribution even after controlling for smoking. In never-smokers, the distribution of invasive adenocarcinoma remained upper region predominant and was not significantly different from the distribution in smokers. We cannot account for unknown particulates or other exposures that may lead to lung cancer in never-smokers and could possibly be susceptible to this mechanism. Alternatively, physiologic variables such as differences in perfusion could lead to variation in immune surveillance, angiogenesis, or other mechanisms that affect early tumor development and invasion.
Our study has several limitations. We only analyzed data on invasive adenocarcinoma and the related histology of adenocarcinoma with lepidic features (formerly BAC) to minimize the effect of other histologies, which are known to have different distributions (e.g. central vs. peripheral). The predilection for the upper lung regions may not be limited to adenocarcinoma. Larger sample sizes would allow for further analysis of the distribution of other invasive histologies.
Several secular trends since enrollment of this cohort also deserve comment. Bronchioloalveolar cell carcinoma has undergone pathologic reclassification.(8) It is possible that some cases referred to as adenocarcinoma with lepidic features would be classified as invasive adenocarcinoma under the contemporary system. The favorable (and significantly different) stage distribution of adenocarcinoma with lepidic features argues that this would likely be a small number. More importantly, misclassification would bias toward the null hypothesis that there is no difference between the regional distributions of the two histologies.
In 2009 the 7th Edition of the TNM staging system was published.(24)(25) We found no association of stage with invasive adenocarcinoma distribution, thus changes to the staging system would be unlikely to affect the findings. Additionally, cases with multiple tumors (one prominent change in the TNM system) were excluded.
This analysis revealed a predilection of the histology of invasive adenocarcinoma for the upper regions of the lung. Tumor region was a significant predictor of the histology of invasive adenocarcinoma whereas tumor lobe was not. The distribution of invasive adenocarcinoma was not associated with smoking, severity of emphysema, or history of COPD. Further studies will clarify if these findings can be attributed to differences in physiologic or other regional factors.
Supplementary Material
Acknowledgements
Conflict of Interest Statement: The above authors receive no personal or financial support and have no involvement with any organization(s) with financial interest in the subject matter, and report no conflicts of interest in the subject matter.
Research Support: Supported by Grants No. CA092824(D.C.C.), CA074386(D.C.C.), and CA090578(D.C.C.)
Abbreviations List
- ACwLF
adenocarcinoma with lepidic features
- BAC
bronchioloalveolar carcinoma
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- NSCLC
non-small cell lung cancer
- SEER
Surveillance, Epidemiology, and End Results
- V/Q
ventilation-perfusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors receive no personal or financial support and have no involvement with any organization(s) with financial interest in the subject matter, and report no conflicts of interest in the subject matter.
Contributor Information
C. Matthew Kinsey, Pulmonary and Critical Care Unit, Massachusetts General Hospital 55 Fruit Street, Boston MA 02114 Department of Environmental Health, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115
Raul San Jose Estepar, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston MA 02115
Yang Zhao, Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu, China
Xiaojin Yu, Department of Epidemiology and Biostatistics, School of Public Health, Southeast University, Nanjing, Jiangsu, China
Nancy Diao, Department of Environmental Health , Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115.
Rebecca Suk Heist, Division of Hematology and Oncology, Massachusetts General Hospital, 55 Fruit Street, Boston MA 02114
John C. Wain, Division of Thoracic Surgery, Massachusetts General Hospital, 55 Fruit Street, Boston MA 02114
Eugene J. Mark, Department of Pathology, Massachusetts General Hospital, 55 Fruit Street, Boston MA 02114
George Washko, Pulmonary and Critical Care Medicine, Brigham and Women's Hospital, 75 Francis Street, Boston MA 02115
David C. Christiani, Pulmonary and Critical Care Unit, Massachusetts General Hospital, 55 Fruit Street, Boston MA 02114 Departments of Environmental Health and Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115
References
- 1.Whitson BA, Groth SS, Andrade RS, Habermann EB, Maddaus MA, D'Cunha J. T1/T2 non–small-cell lung cancer treated by lobectomy: Does tumor anatomic location matter? J Surg Res. 2012;177:185–90. doi: 10.1016/j.jss.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Lee BW, Wain JC, Kelsey KT, Wiencke JK, Christiani DC. Association of cigarette smoking and asbestos exposure with location and histology of lung cancer. Am J Resp Crit Care Med. 1998;157:748–55. doi: 10.1164/ajrccm.157.3.9707025. [DOI] [PubMed] [Google Scholar]
- 3.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiographically indeterminate nodules. Arch Intern Med. 1997;157:849–55. [PubMed] [Google Scholar]
- 4.Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: an epidemiologic inquiry. JNCI. 1984;72:1271–75. [PubMed] [Google Scholar]
- 5.West JB. Regional differences in gas exchange in the lung of erect man. J Appl Physiol. 1962;17:893–98. doi: 10.1152/jappl.1962.17.6.893. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Heist RS, Liu G, et al. Polymorphisms of vitamin D receptor and survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2239–45. doi: 10.1158/1055-9965.EPI-06-0023. [DOI] [PubMed] [Google Scholar]
- 7.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz AA, Come CE, Ross JC, et al. Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT scan in subjects with COPD. Chest. 2012;141:736–44. doi: 10.1378/chest.11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ. Cancer survival among adults: US SEER Program, 1988-2001: patient and tumor characteristics. National Cancer Institute; Bethesda, MD: 2007. SEER Program NIH Pub. No. 07-6215. 2007. [Google Scholar]
- 12.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–38. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–44. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMeo DL, Hersh CP, Hoffman EA, et al. Genetic Determinants of Emphysema Distribution in the National Emphysema Treatment Trial. Am J Resp Crit Care Med. 2007;176:42–48. doi: 10.1164/rccm.200612-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138:1295–302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 16.Gierada DS, Guniganti P, Newman BJ, et al. Quantitative CT assessment of emphysema and airways in relation to lung cancer risk. Radiology. 2011;261:950–59. doi: 10.1148/radiol.11110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: A systematic review and meta-analysis. Lung cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986;105:503–07. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 19.Wasswa-Kintu S, Gan WQ, Man SFP, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570–75. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr RG, Herbstman J, Speizer FE, Camargo CA. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidem. 2002;155:965–71. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 21.Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ. A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med. 1998;158:1644–55. doi: 10.1164/ajrccm.158.5.9802003. [DOI] [PubMed] [Google Scholar]
- 22.Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, et al. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Resp J. 2008;32:1443–50. doi: 10.1183/09031936.00056008. [DOI] [PubMed] [Google Scholar]
- 23.Gurney JW, Schroeder BA. Upper lobe lung disease: physiologic correlates. Review. Radiology. 1988;167:359–66. doi: 10.1148/radiology.167.2.3282257. [DOI] [PubMed] [Google Scholar]
- 24.UICC . TNM classification of malignant tumors. 7th edition. Wiley Blackwell; New York: 2009. [Google Scholar]
- 25.Raptis CA, Bhalla S. The 7th Edition of the TNM staging system for lung cancer: what the radiologist needs to know. Radiol Clin North Am. 2012;50:915–33. doi: 10.1016/j.rcl.2012.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.