Abstract
Objectives
A new radiotracer, 124I-epidepride, has been developed for the imaging of dopamine D2/3 receptors (D2/3Rs). 124I-epidepride (half-life of 124I = 4.2days) allows imaging over extended periods compared to 18F-fallypride (half-life of 18F = 0.076days) and may maximize visualization of D2/3Rs in the brain and pancreas (allowing clearance from adjacent organs). D2/3Rs are also present in pancreatic islets where they co-localize with insulin to produce granules and may serve as a surrogate marker for imaging diabetes.
Methods
124I-Epidepride was synthesized using N-[[(2S)-1-ethylpyrrolidin-2-yl]methyl]-5-tributyltin-2,3-dimethoxybenzamide and 124I-iodide under no carrier added condition. Rats were used for in vitro and in vivo imaging. Brain slices were incubated with 124I-epidepride (0.75μCi/cc) and nonspecific binding measured with 10 μM haloperidol. Autoradiograms were analyzed by OptiQuant. 124I-Epidepride (0.2 to 0.3 mCi, iv) was administered to rats and brain uptake at 3 hours, 24 hours, and 48 hours post injection was evaluated.
Results
124I-Epidepride was synthesized with 50% radiochemical yield and high radiochemical purity (>95%). 124I-Epidepride localized in the striatum with a striatum to cerebellum ratio of 10. Binding was displaced by dopamine and haloperidol. Brain slices demonstrated localization of 124I-epidepride up until 48 hr in the striatum. However, the extent of binding was reduced significantly.
Conclusions
124I-Epidepride is a new radiotracer suitable for extended imaging of dopamine D2/3 receptors and may have applications in imaging of receptors in the brain and monitoring pancreatic islet cell grafting.
Keywords: Epidepride, Fallypride, Dopamine D2/3 receptor, Iodine-124
1. Introduction
Besides the brain, dopamine D2-like receptors are also found in peripheral organs, such as the pancreas, where they colocalize with insulin to produce beta cells and hence may serve as a surrogate marker for imaging islets (Rubi et al 2005). Loss of insulin producing cells in the pancreatic islets leads to an inability to manage blood sugar levels, which results in diabetes mellitus, Type 1 (T1DM) or Type 2 (T2DM). In the field of diabetes research, search for a proper biomarker to image transplanted islets non-invasively is being pursued by various research groups using diverse imaging technologies, in order to predict the development of clinical T1DM or other islet cell disorders. We have recently reported our efforts for imaging dopamine D2/3 receptors in pancreatic islets using 18F-fallypride (Garcia et al., 2011).
Our findings with 18F-fallypride suggested a higher degree of nonspecific binding in the pancreas than in the brain (Garcia et al., 2011; Mukherjee et al., 2002). In an attempt to reduce the nonspecific binding and enhance clearance from organs adjacent to the pancreas, imaging of the pancreas may benefit by using a longer half-life PET radioisotope. Although F-18 has a higher resolution (Yu et al., 2009), I-124 with half-life of 4.2 days compared to F-18 and its half-life of 0.076 days may be a better choice when taking extended imaging into account. Epidepride (de Paulis 2003), radiolabeled with I-123 in clinical research setting for SPECT imaging of dopamine D2/3 receptors (Barth et al., 2013; Fagerlund et al., 2012; Van Laere et al., 2010) can be radiolabeled with I-124 for extended PET imaging.
Improvement of the power and reliability of imaging methods to predict diabetes would raise the possibility for pharmacological intervention during the preclinical phase and the honeymoon period to either slow down or arrest the ongoing destruction of the remaining islet cells in the pancreas (Sweet et al., 2004; Wu and Kandeel, 2010). Currently, the only accepted end-point for drug trials is the clinical diagnosis of T1DM. Development and use of a new and novel PET imaging agent such as 124I-epidepride which has a longer half-life than 18F-fallypride may have improved capabilities for noninvasive imaging of diabetes.
In this paper we report synthesis and radiosynthesis of (S)-N-[(1-ethyl-2-pyrrolidinylmethyl]-5-124I-iodo-2,3-dimethoxybenzamide, or 124I-epidepride. This PET radiotracer is synthesized for longitudinal imaging of dopamine and dopamine-like receptors found in the central and peripheral nervous system. The half-life of 124I-epidepride (100.2 hrs) greatly outlast those of 76Br-isoremoxipride (16.2 hrs), 18F-fallypride (1.83 hrs), and 123I-epidepride (13.3 hrs). In vitro binding studies were carried out in the rat brain slices to illustrate receptor-mediated binding. In vitro binding was also carried out in isolated rat islet cells to show selecting of binding. MicroPET imaging evaluation of 124I-epidepride binding in the rat was carried out in order to evaluate time-course of extended binding.
2. Materials and Methods
2.1 General Methods
Chemicals were purchased from Sigma-Aldrich. All other reagents and solvents were of analytical grade and used as such. Sodium 124I- iodide was purchased from IBA Molecular, Richmond, VA. HPLC was carried out on a Gilson System consisting of isocratic solvent {60/40:CH3CN/H2O(0.01%Et3N)} and two detectors, a UV detector fixed at 287 nm and a radiation flow detector with a NaI(Tl) crystal. Semi-prep C18 column (250 × 10 mm; 10μm) from Alltech Assoc Inc. were used for reverse phase HPLC. All animal studies were approved by the Institutional Animal Care and Use Committee of University of California-Irvine.
2.2 Radiochemistry
Epidepride ((S)-N-[((l-ethyl-2-pyrrolidinynmethyl-5-iodo-2,3-dimethoxybenzamide) and (S)-N-[(l-ethyl-2-pyrrolidinynmethyll-5-tri-n-butyltin-2,3-dimethoxybenzamide were prepared by using previously reported methods (Clanton et al., 1991; Kessler et al., 1991). Radiolabeling of the tributyltin precursor to produce (S)-N-[(l-ethyl-2-pyrrolidinyl)methyl]-5-124I-iodo-2,3-dimethoxybenzamide was carried out using the following procedures: To the vial of no-carrier-added Na124I (commercially supplied in 0.02 M NaOH, IBA Molecular) (3.0 mCi, approx 30μL), 0.5N aq. HCl (10μL), tributyltin precursor (0.2 mg, 50μL EtOH), glacial AcOH /(30%aq.)H2O2 : (3/1) (50μL) were added in sequence and the reaction mixture was incubated at room temp for about 20 min. The reaction was quenched by adding NaHSO3 (100μL of 1.0mg/mL) and saturated NaHCO3 soln. (200μL) was added. The crude was loaded on HPLC column (Econosil C18; 10μm, 250 × 10 mm) and purified by using CH3CN/H2O(1%Et3N): 60/40 at a flow rate of 2.5mL/min. The desired radioactive peak/ product was collected (retention time =37 min), rotavaped to dryness, and formulated in 10%EtOH in saline for biological (in vitro/ in vivo) experiments. The radiochemical yield was 50%, radiochemical purity 98% and specific activity was 100 Ci/mmol.
In another separate radiolabeling experiment chloramine–T (50μL, 1mM solution) was used in place of glacial acetic acid /30% aqueous H2O2. The radiochemical yield was 50%, radiochemical purity 98% and specific activity was 30 Ci/mmole.
2.3 In Vitro 124I-Epidepride Autoradiographic Studies
Male Sprague–Dawley rats (200 – 250 g) were decapitated and the brain was rapidly removed and frozen in isopentane at −20° C. Horizontal sections (40 μm thick) containing the cortex, striatum, thalamus, hippocampus and cerebellum were prepared using LEICA CM 1850 cryotome. The temperature for harvesting the brain sections was −20° C; sections were stored at −20° C until use. For competitive binding studies, slides were thawed for approx. 15 mins at ambient temperature and were subsequently pre-incubated for 10 mins at ambient temperature in buffer (50mmol/L Tris HCl containing 120mmol/L NaCl, 5mmol/L KCl, 2mmol/L CaCl2,1 mmol/L MgCl2, 1mmol/L EDTA, 0.1mmol/L Sodium acsorbate, pH 7.4). The preincubation buffer was then discarded. Subsequently, the slices were treated with incubation buffer containing 124I-epidepride (0.2μCi/mL) at 37°C for 60 min. Nonspecific binding was measured in the presence of 100 μmol of haloperidol. Competitive binding assay with different concentrations of dopamine (endogenous ligand for dopamine receptor) were also carried out. For each drug concentration, several adjacent brain sections (n=5 or more) were used. After incubation, slides were washed twice (2 mins each) with ice-cold incubation buffer, followed by a quick rinse in cold (0–5 °C) deionized water. The slides were then air-dried and apposed to phosphor screens (Perkin Elmer medium type, MS, films) for at least 16 hours and read by the Cyclone Phosphor Imaging System (Packard Instruments Co). The amount of bound 123I-niodene in the autoradiograms was evaluated in various brain regions (as digital lights units (DLU]/mm2) using the OptiQuant acquisition and analysis program (Packard Instruments Co.). Data from sets of brain sections were pooled to provide average values and standard deviation of 124I-epidepride binding.
2.4 In Vivo and Ex Vivo 124I-Epidepride Studies
MicroPET 124I-Epidepride Studies
Male Sprague-Dawley rats (650g) were fasted 24 hours prior to time of the scan. On the day of the study, rats were anesthetized and induced with 4.0% isoflurane. The preparation of dose injection was as follows: 50–250μCi of 124I-epidepride was collected into a 1mL syringe with a 25 gauge needle. Tracer compound was then diluted with saline to a final volume of 0.3ml. The dose was injected via IV into the tail vein of the rat. Isoflurane was reduced and maintained at 2.5% following injection. Scans were carried out for 90 minutes and acquired by the Inveon MicroPET in full list mode. Rats were also imaged at 24 and 48 hrs post injection for 90 min. List mode data was collected dynamically, 3 dimensional sinogram, which were rebinned using a Fourier Rebinning algorithm. The images were reconstructed using 2 dimensional Filter Back Projection using a Hanning Filter with a Nyquist cut off at 0.5, and corrected for attenuation using the Co-57 attenuation scan data.
Ex vivo MicroPET
After completion of whole body in vivo MicroPET scans, male Sprague-Dawley rats (650g) were sacrificed and the brain was pulled out for ex vivo scanning of an additional 60 minutes. Images were collected by the Inveon MicroPET scanner in full list mode. List mode was collected in a single frame while reconstruction of images was identical to the in vivo studies. Images were analyzed using Acquisition Sinogram Image Processing IDL's virtual machine (ASIPRO VM) and PMOD 3.0 softwares.
Ex vivo Autoradiograph studies
After the ex vivo microPET scan, the brain was removed and placed in isopentane for preparation of sectioning. Horizontal sections were acquired using the Leica CM1850 (40μm thick) and exposed to phosphor films overnight. Films were read using the Cyclone Phosphor Imaging System. ROI's of same size were drawn and analyzed on brain regions rich in dopamine D2/3 receptors using OptiQuant software and measured in digital light units/mm2.
In Vitro Autoradiograph studies on Pancreatic Islets
Islets were isolated from Sprague–Dawley rats using collagenase digestion of the pancreas and density-gradient purification of the islets. Isolated islets were stained with dithizone to quantify the yield and to estimate purity. Another aliquot of isolated islets was stained with SYTO Green (Invitrogen)/Ethidium Bromide (Sigma-Aldrich) to quantify viability. Purified rat islets (50 IEQs) were incubated with 0.2 μCi/mL of 124I-epidepride in the absence or presence of 100 μM haloperidol for one hour in a 37°C water bath. The islets were then collected from incubation test tubes onto Whatman filter paper that had been presoaked in 0.1% polyethylenamine using a 24-sample Brandel Cell Harvester and were washed 3 times with cold incubation buffer. Filters were first exposed to phosphor screens for 30 min, and 124I-epidepride activity was determined by autoradiography. Films were read using the Cyclone Phosphor Imaging System. Region of interest of the same size were drawn and analyzed to evaluate 124I-epidepride binding to dopamine D2/3 receptors using OptiQuant software and measured in digital light units/mm2.
3. Results
Radiosynthesis
Two methods of 124I-sodium iodide radiolabeling of the tributyltin precursor for 124I-epidepride were tried using different oxidants. When using hydrogen peroxide in glacial acetic acid, a 50% radiochemical yield was obtained with high radiochemical purity. The specific activity of 124I-epidepride was found to be 100 Ci/mmol. The reaction with chloramine-T as an oxidant provided similar yields. However, the chemical purity was lower and resulted in lower specific activity (30 Ci/mmol) when using chloramine-T as the oxidant. The identity of 124I-epidepride was confirmed by analytical HPLC using pure epidepride.
In Vitro Binding
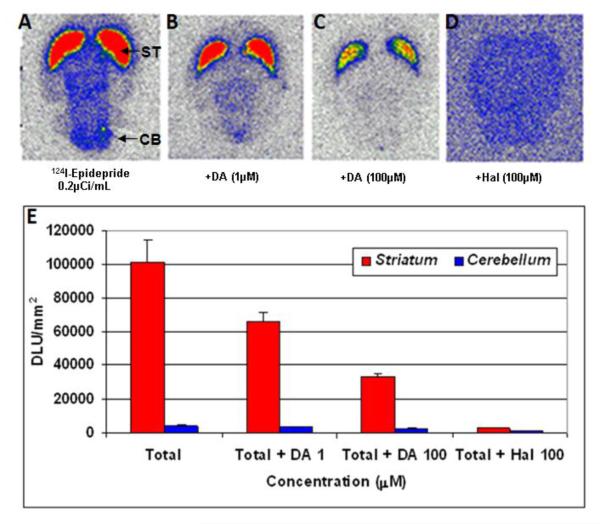
In vitro binding of 124I-epidepride to rat brain slices is shown in Fig-2. Localization of 124I-epidepride was evident in the striatum while other extrastriatal regions of the brain had lower binding as seen in Fig-2A. Ratio of striatum to cerebellum (a region known to have few dopamine receptors, Mukherjee et al., 1999) was 10. In the presence of dopamine, binding in the striatum was reduced by 36% (with 1 μM dopamine) and by 66% (with 100 μM dopamine). Haloperidol (100 μM) displaced most of the binding from the striatum (Fig-2D and 2E).
Figure-2.
In vitro autoradiography results. Specificity of 124I-epidepride for D2/3 receptors in the striatum of Sprague Dawley rat brain with thickness of 40 μm. A: total binding; B: in the presence of 1 μM dopamine; C: in the presence of 100 μM dopamine; D. Nonspecific binding in the presence of 100 μM haloperidol. E. The graph shows degree of displacement of 124I-epidepride under different drug conditions.
In Vivo Binding
Whole body imaging of rats resulted in distribution of 124I-epidepride in the brain as well as other parts of the body. Localization of 124I-epidepride was evident in the striatum in vivo while clearing out from other parts of the brain. Ex vivo brain uptake of 124I-epidepride indicated localization in the striatum (Fig-3A–C) while cerebellum exhibited lower retention of the radiotracer as expected. In comparison to 124I-epidepride, 18F-fallypride exhibited higher uptake in the striatum.
Figure-3.
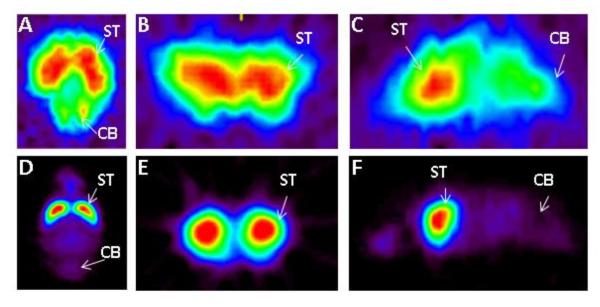
Comparison of 124I-epidepride with 18F-fallypride. Ex vivo MicroPET results of excised Sprague Dawley rat brain 3 hours post injection in separate studies of 124I-epidepride (IV injection of 0.15 mCi at specific activity of 80 Ci/mmol) and 18F-fallypride (IV injection of 0.5 mCi at specific activity of 2000 Ci/mmol). Orthogonal slices of 124I-epidepride (A–C) were compared those of 18F-fallypride (D–F) showing striatal binding of the two radiotracers.
Extended Time Imaging
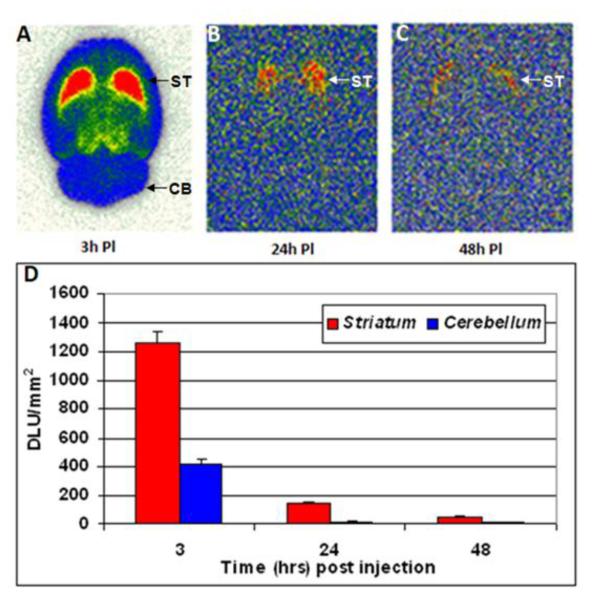
Rat brains excised at different time points were sectioned and shown in Figure-4 are brain slices at 3hr, 24hr and 48 hrs. Selective retention of 124I-epidepride was observed in the striatum up until 48 hrs. Ratio of striatum to cerebellum was 3 initially (3 hrs post injection) and increased over time to 10 in 24hrs. Subsequently, the ratio went down to 5 after 48 hrs. However there was significant clearance from the striatum and cerebellum overtime. There was >80% decrease in 124I-epidepride binding in 24 hrs which further reduced by 96% in 48 hrs compared to the initial binding of 124I-epidepride at 3 hrs.
Figure-4.
Ex vivo brain slice autoradiography results for epidepride uptake after A) 3 hours, B) 24 hours and C) 48 hours postinjection of 124I-epidepride (after IV injection of 0.1–0.15 mCi at specific activity of 100 Ci/mmol in a normal rat under 3% isoflurane) showing binding in the striatum (ST) and nonspecific binding in the cerebellum (CB). The plot D) shows the comparison of 124I-epidepride uptake in rat striatum and cerebellum at the different time points.
Whole body distribution of 124I-epidepride is shown in Figure-5. Initial distribution (2 hrs postinjection) showed excessive activity in the regions corresponding to the stomach and the urinary bladder on CT (Fig-5A, B). Due to high amount of activity in the stomach it was difficult to delineate the pancreas. Over time, in 48 hrs postinjection, most of the activity from the stomach and urinary bladder had cleared (Fig-5C). The remaining activity was found predominantly in the intestines and very little activity was discernable in the vicinity of the pancreas.
Figure-5.
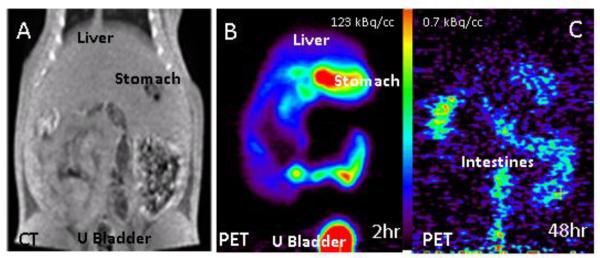
A. CT scan of a normal rat showingliver, stomach and urinary bladder. B. PET scan of 124I-epidepride after IV injection of 0.22 mCi at specific activity of 100 Ci/mmol in a normal rat under 3% isoflurane. C. PET scan of the same animal as in B, 48 hrs postinjection.
Islet cell In Vitro Binding
Significant in vitro binding of 124I-epidepride in isolated islet cells was observed which was displaceable by haloperidol. Figure-6 shows a >80% reduction in the binding of 124I-epidepride to the islet cells shown in Figure-6A.
Figure-6.
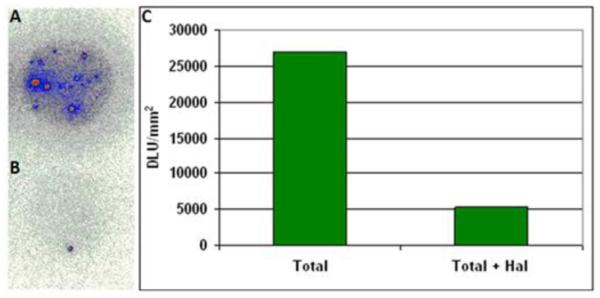
In vitro results for specificity of 124I-epidepride for D2/3 receptors in rat pancreatic islets for A) total binding and B) total + haloperidol (100 μm). C) The plot shows an 80% decrease in 124I-epidepride binding in the presence of haloperidol.
4. Discussion
Radiolabeling of 124I-epidepride proceeded smoothly using the tributyltin precursor and sodium 124I-iodide using two separate procedures. In the two oxidative methods used, radiochemical yields were similar but the specific activity was higher (100 Ci/mmol) using hydrogen peroxide and glacial acetic acid while with chloramine-T the specific activity was 30 Ci/mmol.
Similar to the binding characterisitics of 123I-epidepride and 18F-fallypride, 124I-epidepride exhibited selectivity to the striatum and other extrastriatal regions. Dopamine was able to displace >66% the binding of 124I-epidepride which further suggested binding of the radiotracer to dopamine receptors. It also indicated the reversibility of binding of 124I-epidepride similar to observations made with 18F-fallypride (Mukherjee et al., 1999).
PET imaging studies of the rat brain using 124I-epidepride resulted in regional striatal localization. However, in comparison to 18F-fallypride PET, the lower resolution of 124I- was evident as can be seen in Fig-3. This issue of lower resolution with 124I-radiotracers has been reported previously (Yu et al., 2009). Kinetics of binding of 124I-epidepride in brain slices suggested clearance from both striatum and cerebellum over the period of 48 hrs, suggesting that 124I-epidepride may not be superior to 123I-epidepride or 18F-fallypride for brain imaging. However, the ratio of binding between striatum and cerebellum increased over time, suggesting that there may be utility of the longer half-life 124I-epidepride in certain applications. In particular, this may apply to more clearly visualize islet cells in the pancreas, since 124I-epidepride, like 18F-fallypride (Garcia et al., 2011), labels D2/D3 receptors in isolated islet cells in vitro consistent with the presence of dopamine receptors (Shankar et al., 2006) (Fig-4).
Preliminary evaluation of rat whole body distribution of 124I-epidepride reflected significant amount of activity in the abdominal cavity. This activity cleared out over the 48 hr period with very little binding in the pancreatic region. This may be due to the dissociation and clearance of receptor-bound 124I-epidepride from the pancreas, as was seen in the brain. Thus, delineation of 124I-epidepride in the pancreas will require further analyses. Additionally, compared to 18F-fluoride (approx.. 97% positron emission branching fraction), 124I-iodide has only approx.. 23% branching fraction which further affects the quality of the PET data. This adds to the challenges of characterization of selective binding of 124I-epidepride in the pancreas. The presence of D2-like receptors in the intestines has been reported to affect motility in mice (Liz et al., 2006). It remains to be confirmed if the 124I-epidepride in the intestines at late times (48hr, Fig-5C) is D2-like receptor bound. Evaluation of whole body PET data at extended times using 124I-epidepride in pancreas and intestines is currently in progress.
5. Conclusion
124I-Epidepride, prepared for longitudinal imaging of dopamine D2/3 receptor, was found to be selective, high affinity antagonist for the dopamine D2/3 receptor. The use of 124I-epidepride potentially provides a non-invasive method of monitoring the levels of dopamine receptors in the body, especially the brain and pancreas over extended periods. However, the relatively faster clearance of 124I-epidepride from the receptor sites diminishes the value of the long physical half life of 124I-epidepride.
Figure-1.
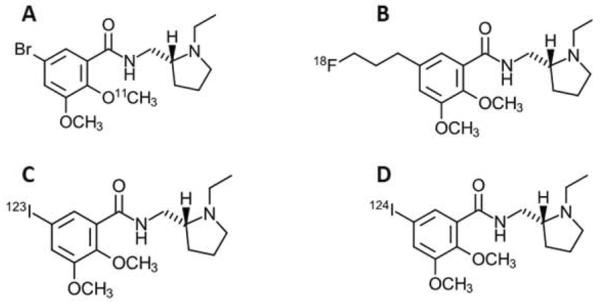
A comparison of the structure and half-life (in minutes) of four different compounds, including A. 11C-FLB 457 (76Br-Isoremoxipride), B. 18F-Fallypride, C. 123I-Epidepride, D. 124I-Epidepride. .
Acknowledgements
This research was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) with award numbers RC1DK087352 and R21DK092917. We would like to thank Dr. Tomas de Paulis for a gift sample of epidepride and Dr. Jonathan Lakey for helpful discussions and for providing the rat pancreas islet cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Rubí B, Ljubicic S, Pournourmohammadi S, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- Clanton JA, de Paulis T, Schmidt DE, Ansari MS, Manning RG, Baldwin RM, Kessler RM. Preparation of 123I-and 125I-epidepride: A dopamine D-2 receptor antagonist radioligand. J. Label. Compd. Radiopharm. 1991;29:745–751. [Google Scholar]
- de Paulis T. The discovery of epidepride and its analogs as high-affinity radioligands for imaging extrastriatal dopamine D2 receptors in Human Brain. Current Pharmaceutical Design. 2003;9:673–696. doi: 10.2174/1381612033391135. [DOI] [PubMed] [Google Scholar]
- Garcia A, Mirbolooki MR, Constantinescu C, Pan ML, Sevrioukov E, Milne N, Wang PH, Lakey J, Chandy KG, Mukherjee J. 18F-Fallypride PET of pancreatic islets: in vitro and in vivo rodent studies. J Nucl Med. 2011;52:1125–1132. doi: 10.2967/jnumed.111.088583. [DOI] [PubMed] [Google Scholar]
- Liz ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006;26(10):2798–2807. doi: 10.1523/JNEUROSCI.4720-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-Fallypride in normal volunteers: blood analysis, distribution, test-retest studies and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Pinborg LH, Mortensen EL, Friberg L, Baaré WF, Gade A, Svarer C, Glenthøj BY. Relationship of frontal D2/3 binding potentials to cognition: a study of antipsychotic-naive schizophrenia patients. Int. J. Neuropsychopharmacol. 2012;16:1–14. doi: 10.1017/S146114571200003X. [DOI] [PubMed] [Google Scholar]
- Barth V, Need AB, Tzavara ET, Giros B, Overshiner C, Gleason SD, Wade M, Johansson AM, Perry K, Nomikos GG, Witkin JM. In vivo occupancy of dopamine D3 receptors by antagonists produces neurochemical and behavioral effects of potential relevance to attention-deficit-hyperactivity disorder. J Pharmacol Exp Ther. 2013;344(2):501–510. doi: 10.1124/jpet.112.198895. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Ansari MS, Schmidt DE, de Paulis T, Clanton JA, Innis R, al-Tikriti M, Manning RG, Gillespie D. High affinity dopamine D2 receptor radioligands. 2. [125I]epidepride, a potent and specific radioligand for the characterization of striatal and extrastriatal dopamine D2 receptors. Life Sci. 1991;49(8):617–628. doi: 10.1016/0024-3205(91)90261-9. [DOI] [PubMed] [Google Scholar]
- Yu AR, Kim JS, Kim KM, Lee YS, Woo SK, Lee WH, Kim JG, Park JA, Kim HJ, Cheon GJ. Optimal PET acquisition setting of 1–124 with Siemens Inveon PET: comparative simulation study with F-18 and microPET R4. IEEE NSS MIC Conf. Rec..2009. [Google Scholar]
- Wu Z, Kandeel F. Radionuclide probes for molecular imaging of pancreatic beta-cells. Adv Drug Deliv Rev. 2010;62:1125–1138. doi: 10.1016/j.addr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Cook DL, Lernmark A, et al. Systemic screening of potential b-cell imaging agents. Biochem. Biophys. Res. Commun. 2004;314:976–983. doi: 10.1016/j.bbrc.2003.12.182. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Varrone A, Booij J, et al. EANM procedure guidelines for brain transmission SPECT/PET using dopamine D2 receptor ligands, version 2. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:434–442. doi: 10.1007/s00259-009-1265-z. [DOI] [PubMed] [Google Scholar]
- Shankar E, Santosh KT, Paulose CS. Dopaminergic regulation of glucose-induced insulin secretion through dopamine D2 receptors in the pancreatic islets in vitro. IUBMB Life. 2006;58:157–163. doi: 10.1080/15216540600687993. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Brown T, et al. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and non-human primate brains using the high affinity radioligand, [F-18]fallypride. Nucl. Med. Biol. 1999;26:519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]