Abstract
Individuals carrying DRB1*0401 who smoke cigarettes are at an increased risk of developing severe seropositive RA. To determine how cigarette smoke (CS) interacts with host genetic factors in the induction of RA-associated autoimmunity, we used transgenic mice carrying the RA-susceptible HLA genes DR4 and DQ8, but lacking all endogenous murine class II molecules. Cigarette smoke exposure augmented peptidylarginine deiminase (PAD) enzyme expression, and enhanced immune responses to citrullinated collagen and vimentin. Here we show for the first time that DQ molecules can present citrullinated peptides much more efficiently than native peptides. Interestingly, CS exposure suppressed collagen-induced arthritis (CIA) in DRB1*0401 mice although innate immune response was enhanced. On the other hand, CS exposure exacerbated CIA in DQ8 mice, which was accompanied by an increased expression of Th17 gene transcripts in lungs. These observations suggest that cigarette smoke promotes antigen-specific autoimmunity that is profoundly influenced by host genetic factors.
Keywords: Smoking, Rheumatoid arthritis, HLA transgenic mice, Citrullination, Innate immune response
1. Introduction
Predisposition to rheumatoid arthritis (RA) is associated with both genetic and environmental factors [1,2]. Genetic studies have estimated a 50–60% risk of developing RA attributable to host genetic factors with the remaining contributed by environmental factors [1,2]. Genes in the major histocompatibility complex (MHC) region are the most important determinant of risk to RA. An association with DRB1*0401 and other DR alleles sharing the 3rd hypervariable region – so called ‘shared epitope’ (SE) alleles – provides an important genetic determinant of RA risk [1]. Among environmental factors, cigarette smoke (CS) exposure is the major risk factor for RA development, and interacts with host genetic factors to induce up to 21-fold increase in the relative risk of developing seropositive RA [2].
RA is characterized by the presence of circulating autoantibodies like rheumatoid factor (RF) and antibodies to citrullinated peptides (ACPA) of synovial antigens. While ACPA are more specific to RA than RF [3], the specific target autoantigen/s that promote ACPA generation in RA are not well defined. Antibodies to citrullinated vimentin occur in 75% of RA patients and are associated with DR-SE genes [4]. Citrullination occurs in both synovial and extra-articular tissues in RA. For example, in approximately half of cases with interstitial lung involvement in RA (which occurs in about 20% of RA patients throughout the course of disease) there is in situ evidence of enhanced citrullination on lung biopsies [5]. Smoking may be a key factor associated with increased citrullination in the lungs, potentially by inducing the expression of peptidylarginine deiminase (PAD) enzymes [6].
Smoking increases the risk of extra-articular RA occurring in the lung resulting in complications that carry high morbidity and mortality in RA [7]. Smoking is likely to play a critical role in the onset of RA-associated autoimmunity, as autoantibodies precede the onset of clinical disease by decades in some cigarette smokers carrying DR4 [4,8]. In humans, DR and DQ occur in linkage and it is difficult to assess their interaction separately with environmental factors. Using transgenic mice that carry RA-associated susceptible DR and DQ alleles provides an opportunity to determine how specific host genes interact with CS in the induction of autoimmunity.
Smoking has been reported to modulate immunity in complex ways, but the effect of CS on adaptive immunity in the context of specific HLA genes relevant to human RA is not known. To define the interaction between CS and RA susceptibility HLA genes on adaptive immunity, HLA transgenic mice carrying human DR and DQ alleles were generated. Our observations show that CS suppresses arthritis and immunity in arthritis-susceptible HLA-DRB*0401 (DR4) mice while increasing the severity of arthritis in HLA-DQ8 mice, suggesting that epistatic interactions between gene-environmental factors may differ for DR and DQ molecules. The current model provides novel insights in the interactions between smoking and arthritis-associated HLA-DR/DQ haplotype.
2. Materials and methods
2.1. Transgenic mice
The generation of HLA-DRB1*0401 (DR4) transgenic mice has been described previously [9]. AβoDRB1*0401 mice were mated with MHCIIΔ/Δ (AEo) mice [10] to generate DR4. AEo mice that lack all endogenous class II chains. Similarly, we generated AEo DQA1*0301/DQB1*0302 (AEoDQ8) mice [11]. Mice of both sexes (8–12 weeks of age) used in this study were bred and maintained in the institutional pathogen-free Immunogenetics Mouse Colony in accordance with guidelines established by the local Institutional Animal Use and Care Committee (IACUC). Transgene negative littermates were used as controls. Transgene negative controls did not develop disease or generate antigen-specific T cell response. For convenience, AE*0401 mice will be referred to as DR4 and AEoDQ8 mice as DQ8.
2.2. Flow cytometry
The expression of DRβ, H2E, and DQ chains on peripheral blood leukocytes of transgenic mice were analyzed by flow cytometry using mAbs: L227 (anti-DR), IVD12 (anti-DQ), 14-4-4 s (anti-Eα), and Conjugated antibodies for CD3, CD4, CD8, B220, CD11b and CD11c (BD Biosciences, CA) were also used. All cell surface markers were done with cells pooled from 2 mice/strain and experiments were repeated 2–3 times.
2.3. Induction and evaluation of collagen-induced arthritis (CIA)
CIA was induced in transgenic animals by immunization with chick type II collagen (Chondrex Inc) (100µg of CII emulsified in complete Freund's adjuvant) according to the standard protocol as described [12]. The arthritic severity of mice was evaluated with a grading system for each paw of 0–3. The mean arthritic score was determined using arthritic animals only. Mice were sacrificed after 10–12 weeks of immunization and the paws were decalcified and fixed. Sections were stained with H& E and examined for infiltration and erosions. Lungs were harvested, frozen and sectioned.
2.4. CS exposure
Mice were exposed to CS 2 weeks before immunization with CII and continued for up to 10 weeks after immunization for in vivo studies. Chronic CS exposure was performed in a smoking chamber (Teague enterprises, CA) that enables exposure of mice to high levels of CS inhalation generated by 3R4F Kentucky research cigarettes on a daily basis, as previously described [13]. In this system, mice are exposed to a mixture of mainstream and side-stream cigarette smoke and enables sufficient exposure that results in generation of murine blood nicotine levels analogous to levels attained in heavy human smokers (range of 45–181; mean 112 ng/ml; measurements using liquid chromatography-tandem mass spectrometry) [13,14].
2.5. T cell proliferation assay
Mice were immunized with 200 µg of chick CII emulsified 1:1 in CFA (Difco) intradermally at the base of the tail and one hind footpad. Ten days post immunization, draining lymph node cells (LNCs) and spleen were removed and cultured in vitro in the presence or absence of CII (50 µg/ml). In some experiments recombinant Vimentin (rVim) as well as citrullinated rVim (Cit-rVim) were used. During the last 18 h, cells were pulsed with 3H-thymidine (1µCi/well) and cell proliferation determined by thymidine incorporation. In other experiments, Lipopolysaccharide (LPS) (5 µg/ml) and Cytosine-phosphate-guanine (CPG)-ODN (1 µg/ml) were used. Lungs were digested with collagenase for isolating DCs and B cells as previously described [13]. CD4+ cells were sorted by FACS from spleens harvested from primed mice. CD4+ cells were cultured in vitro in the presence or absence of antigen-presenting cells (APCs). Up to 5 × 105 CD4+ sorted cells were used for culturing alone with CII (50 µg/ml). Stimulation index of 2 or more was taken as positive response. Experiments were repeated 3–5 times with cells pooled from 2 mice/experiment. A total of 4–8 mice in each group were tested.
2.6. Auto antibodies
2.6.1. Anti-chick CII antibodies
Levels of anti-CII IgG antibodies were measured in sera obtained 35 days following CII immunization by a standard ELISA using ELISA grade CII as per instructions (Chondrex Inc). In addition antibodies to citrullinated CII (cit-CII) and Cit rVim were measured. IgM and IgG RF were measured by ELISA using sera from 35-day post-primed mice as previously described [9].
2.7. Citrullination of proteins
Type II collagen and human recombinant vimentin (Research Diagnostics Inc.) were citrullinated in vitro by incubating with rabbit muscle PAD (Sigma; 50 U PAD per mg of vimentin/CII) for 3 h at 55 °C in a buffer (0.1 M Tris–HCl, 10 mM Cacl2 and 5 mM dithioerythritol). EGTA was added to stop the reaction.
2.8. Identification of citrullinated proteins by MASS spectrophotometry
The protein solution was digested with chymotrypsin in 50 mM Tris/0.025% Protease Max (Promega, Madison WI) at room temp for 12 h and analyzed by nano-flow liquid chromatography electrospray tandem mass spectrometry (nanoLC-ESI-MS/MS) using an Orbitrap Elite (Thermo Scientific, Bremen Germany) coupled to a Dionex NCS 3500RS Nano-Cap System. Chromatography was performed using 0.2% formic acid in both the A solvent (98%water/2%acetonitrile) and B solvent (80% acetonitrile/10% isopropanol/10% water), and a 5%B to 50%B gradient over 30 min at 350 nl/min through a hand packed PicoFrit (New Objective, Woburn, MA) 100µm × 120 mm column (Agilent Poroshell120 EC C18). The Elite mass spectrometer experiment was set to perform a FT full scan from 340 to 1500 m/z with resolution set at 120,000 (at 400 m/z), followed by linear ion trap MS/MS scans on the top 15 ions. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.2.04), and X! Tandem (www.thegpm.org; version 2006.09.15.3). X! Tandem was set up to search the Swissprot database (699052 entries) assuming the digestion enzyme chymotrypsin. Mascot was set up to search the Swissprot database (699052 entries) assuming the digestion enzyme chymotrypsin. Mascot and X! Tandem were searched with a fragment ion mass tolerance of 0.60 Da and a parent ion tolerance of 10.0 PPM. Oxidation of methionine, deamidation of asparagine, glutamine, and arginine are specified in Mascot, and X! Tandem as variable modifications. Scaffold (version Scaffold_4_2_1, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide identifications and modification assignments were manually validated.
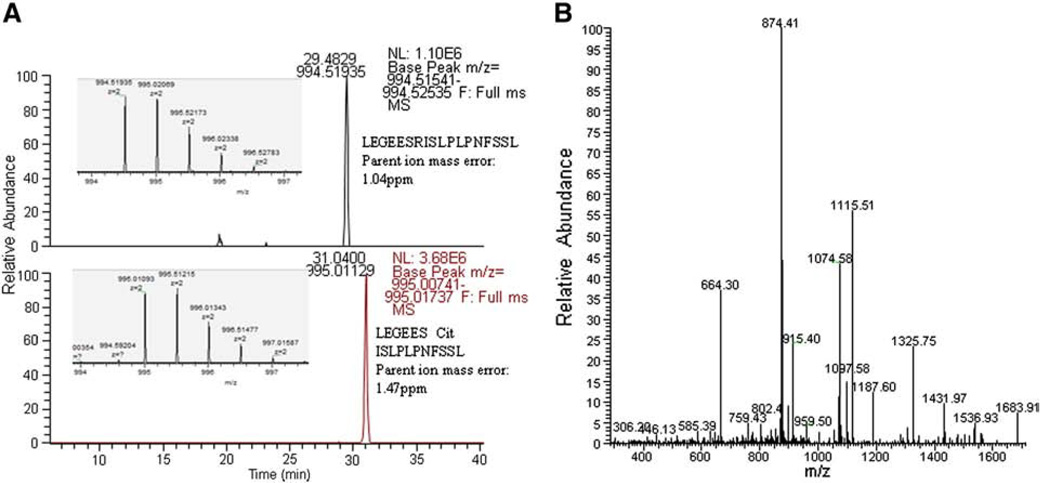
High resolution mass spectrometry was used to demonstrate the presence of citrullination in PAD treated CII and vimentin. An example of the retention time shift of one of the citrulline peptides when compared to the arginine peptide is shown (Fig. 1A). The extracted ion chromatograms of the [M + 2H]2+ ions for the sequence LEGEES(R/cit)SLPLPNFSSL, showed a retention time shift of 1.6 min with the citrulline peptide eluting later, due to the loss of the positive charge. The expected monoisotopic masses representing the arginine or citrulline modification are observed to less than 2 ppm. The MS/MS spectrum for the citrulline peptide is shown in Fig. 1B. The observed fragment ions clearly support the assignment of the deimination at the arginine site (Fig. 1B).
Figure 1.
High resolution mass spectrometry was used to demonstrate the citrullination of protein. A) Orbitrap Elite extracted chromatogram base peaks of the [M + 2H]2+ ions for arginine and citrulline versions of the sequence: LEGEES (R/cit) ISLPLPNFSSL showing a retention time shift of 1.6 min for the modification. The inserts illustrate the [M + 2H]2+ parent ion corresponding to each peptide. B) MS/MS spectra of the citrullinated Vimentin peptide with the matched b-ions and matched y-ions.
2.9. Measurement of cytokines
Cytokines were measured using the Bio-Plex protein array system with the mouse cytokine 23-plex panel as per manufacturer's instructions and analyzed with Bio-Plex manager 2.0 software (Bio-Rad laboratories, Hercules, CA). Some cytokines were also tested by Capture ELISA using commercial kits (BD biosciences).
2.10. Real time PCR
Expression of IL-10, IL-13, IL-17, TGF-β, thymic stromal lymphopoietin (TSLP), Peptidyl arginine deiminase2 (PAD2) and Peptidyl arginine deiminase4 (PAD4), Collagen and fibrinogen mRNA in lungs was analyzed by real time PCR. RNA was extracted from cells using RNAeasy columns (Qiagen) and cDNA was prepared using RNase H-reverse transcriptase (Invitrogen). cDNA was analyzed by real-time quantitative PCR in triplicates by using SYBR® GreenER™ qPCR reagent system (Invitrogen). The expression level of each gene was quantified using the threshold cycle (Ct) method normalized for the house keeping gene GAPDH.
2.11. Statistical analysis
The difference in the incidence of arthritis between groups was analyzed using the Chi square test. A p-value of less than 0.05 was considered significant. Cytokine levels, T cell proliferation comparisons, expression of transgenes and onset of arthritis for arthritic mice between male and female and among transgenes were compared using 2 tailed Student's t test. All values depicted are mean ± SEM.
3. Results
3.1. Genotype-specific interactions between cigarette smoke and HLA molecules determine arthritis outcome
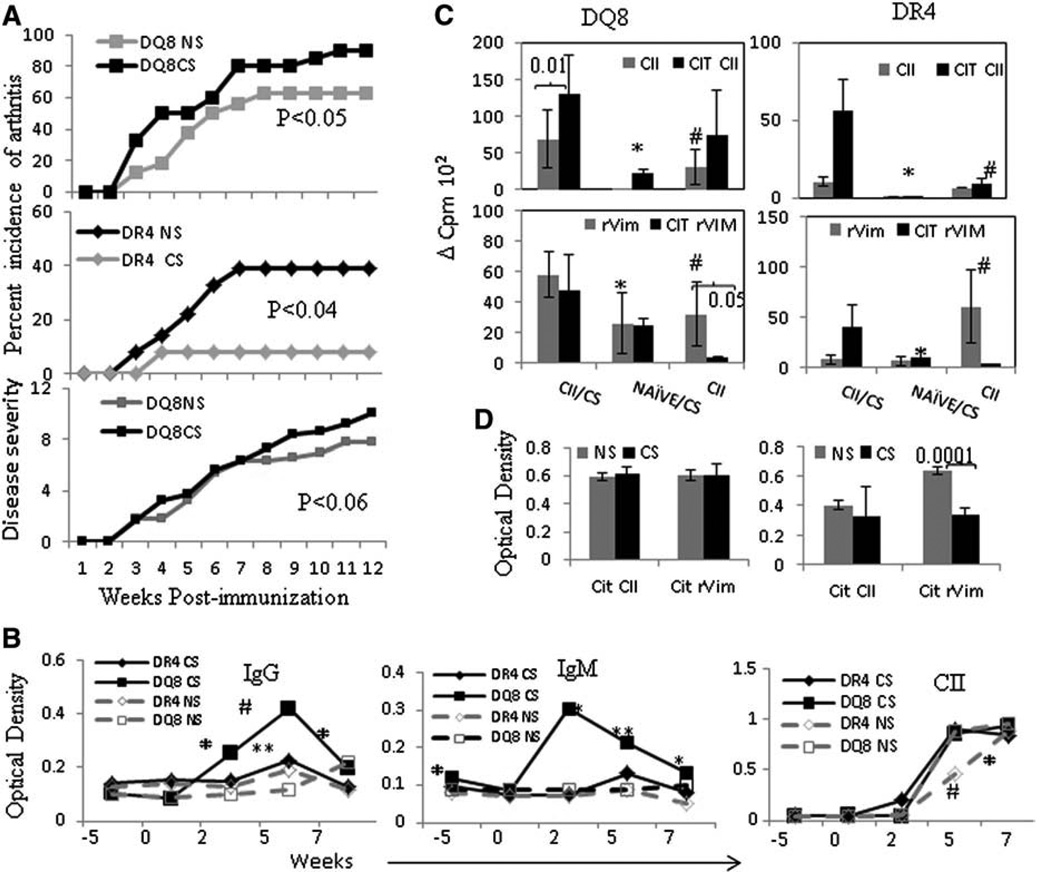
As suggested for human arthritis, we hypothesized that CS exposure promotes severe arthritis with earlier onset in DR4 mice. Taking into account that predominantly female DR4 mice develop arthritis [9], we exposed male DR4 mice to CS and then challenged with CII to induce arthritis. Contrary to our original hypothesis, male DR4 mice did not show any increase in incidence or severity of arthritis compared to controls. Female DR4 mice exposed to CS also showed lower incidence compared to controls (CS-exposed DR4 mice vs. control mice exposed to air or “non-smokers” (NS), X2 = 4.1, P < 0.04) (Fig. 2A). Since HLA-DQ8 occurs in linkage with DR4 in humans, we also tested the effect of CS exposure on CIA in arthritis-susceptible DQ8 mice [12]. Chronic CS exposure in CII-immunized DQ8 mice induced CIA with increased incidence (Fig. 2A, X2 = 3.89, P < 0.05) and severity compared to controls (10 ± 2.7 and 7.8 ± 2.09 respectively, P < 0.05). DQ8 mice do not show sex-bias. Next we evaluated the effect of CS on humoral immunity by measuring antibodies. Control and CS exposed mice immunized with CII produced anti-CII antibodies and RF (Fig. 2B). In keeping with the observation that CS augmented disease severity in DQ8 mice, RF production was also enhanced in DQ8 mice (Fig. 2B). Interestingly, although CS did not enhance disease severity in DR4 mice, anti-CII Abs were significantly increased in primed DR4 mice.
Figure 2.
Cigarette smoke suppresses collagen-induced arthritis in DR4 (N = 36, NS = 23, CS = 13) mice but exacerbates in DQ8 (N = 36, NS = 16 and CS = 20) mice. A) DQ8 and DR4 mice were induced for CIA and onset and progression of arthritis was monitored. Cigarette smoke (CS) and control “non-smoker” (NS) exposed mice. The upper panel shows female DQ8 mice, middle panel shows female DR4 mice and the lower panel shows disease severity in DQ8 mice. B) Kinetics of autoantibody production in CS and NS mice; rheumatoid factor (RF) (IgG and IgM) and anti-CII antibodies were tested from sera obtained at time points indicated in the graph (N = 7–12 mice/group). Mice were exposed to CS starting at week 0, immunized with CII at week 2 and continually exposed to CS, CS vs. NS * p < 0.05, **p < 0.005, #p < 0.001. C) Lymph node cell responses to native and citrullinated CII (CIT CII) and recombinant Vimentin (rVim) in CS and NS and CS exposed naïve mice (N = 4–7 mice/group). DQ8 mice; *CII/CS vs. Naïve/CS: CIT CII—p < 0.03, CII—p < 0.01, rVim—p < 0.02, CIT rVIm—p < 0.002. # CII/CS vs. CII: CIT CII p < 0.01, rVim—p < 0.04, CIT rVim—p < 0.008 DR4 mice; *CII/CS vs. Naïve/CS: Cit rVim—p < 0.03. # CII/CS vs. CII: CIT CII p < 0.01, rVim p < 0.04. D) Anti-Cit CII and Cit rVim antibodies in CS and NS mice (N = 6–10 mice/group). Antibodies were normalized with transgene negative littermates.
Since CIA is dependent on antigen-specific cellular and humoral immune responses, we measured T cell responses to CII and cit-CII in CS exposed mice and controls at the termination of the in vivo experiments. Lymph node cells from CS exposed DR4 and DQ8 mice generated significantly higher proliferation to cit-CII when compared with control mice; however the difference in proliferation between CS exposed and control T cells was more marked in the DR4 mice (Fig. 2C). Lymph node cells isolated from naïve DR4 mice exposed to CS did not show any response to either native or cit-CII; however a mild proliferative response to cit-CII was observed in DQ8 mice, suggesting that CS alone may have promoted priming of immunity in these mice (Fig. 2C upper panel, p < 0.05). However, exposure to CS alone did not cause development of arthritis in transgenic mice.
Smoking has been correlated with citrullination of, and antibodies to Vimentin in RA patients [2,15–17]. To determine whether CS modifies immunity to Vimentin in our murine model, we measured the immune response to recombinant Vimentin (rVim) and citrullinated rVimentin (cit-rVim). Interestingly, LNCs from naïve mice exposed to CS generated T cell responses when challenged in vitro with rVim and cit-rVim, particularly in the DQ8 mice (Fig. 2C, lower panel). CS suppressed anti Cit-rVim antibodies significantly as compared to NS DR4 mice but not in the DQ8 mice (Fig. 2D). Lymph node cells from CII-primed DQ8 and DR4 mice generated a response to rVim, but not cit-rVim, suggesting that native Vimentin may have a significant role in the pathogenesis of arthritis in this model. On the other hand, LNCs from CII-primed mice exposed to CS mounted a robust response to cit-rVim in both strains, implying a role for CS in priming adaptive immunity to cit-rVim, analogous to what has been observed in RA patients.
3.2. Cigarette smoke augments Th2/Th17 gene expression in lungs of DQ8 mice but suppresses expression in DR4 mice
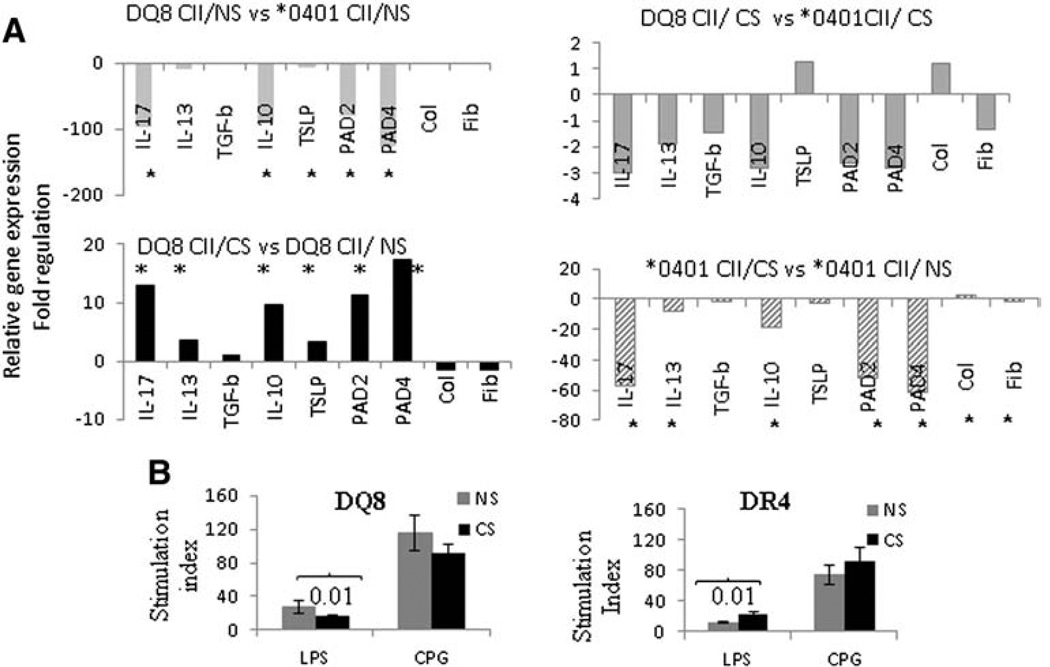
Since CS exposure affected the arthritis phenotype differently in DQ8 (worse in “smokers”) and DR4 mice (less arthritis in female “smokers”), we compared mRNA transcripts for Th2 (IL13) and Th17 (IL17) cytokines involved in chronic inflammation, and, IL10, TSLP and TGFβ, cytokines associated with immunomodulation (Fig. 3A). The gene expression levels in lungs of CII-immunized DQ8 mice were significantly lower compared to CII-immunized DR4 mice (P < 0.05 for all) (Fig. 3A, upper left panel). CS enhanced expression of IL10, IL13, IL17 and TSLP significantly in CII-immunized DQ8 mice compared with controls (Fig. 3A, lower left panel, P < 0.05 for all). In contrast, CS exposure of CII-immunized DR4 mice was associated with significant reduction in the expression of IL10, IL-13 and IL17 (Fig. 3, lower right panel). Comparison of cytokine mRNA transcripts between the DQ8 and DR4 CS exposed mice showed no statistically significant differences (Fig. 3A, upper right panel). We also determined the gene expression of enzymes that control citrullination, PAD2 and PAD4. CS significantly induced PAD2 and PAD4 gene expression in DQ8 mice compared to controls but these differences were significant as compared to DR4 mice (Fig. 3A).
Figure 3.
Smoking augments immune responses in DQ8 but not DR4 mice. A) Fold regulation in mRNA transcripts in lung tissues isolated from cigarette smoke exposed (CS) and not smoker (NS) mice induced for CIA (N = 4 each group).* P ≤ 0.05 comparison between CS and NS mice B). Lymph node cells from naïve mice exposed to CS or not (NS) (N = 4 mice/group) were tested for proliferation after culturing with LPS and CPG for 24 h.
3.3. Cigarette smoke augments antigen-specific responses but suppresses innate responses in DQ8 mice
CS is known to modulate immunity, but the effect of CS on immune responses in the context of specific genetic backgrounds is not well defined [18]. To determine the interactions between CS and host HLA genes on innate immune functions, LNCs of naïve or CS exposed mice were cultured in the presence of LPS and CPG. The proliferative response to LPS was significantly suppressed in CS exposed DQ8 mice when compared with non-smoker(NS) controls while LNCs from CS exposed DR4 mice showed significantly increased proliferation (Fig. 3B, p = 0.01 for both). Conversely, the LNCs response to CPG did not differ between CS and NS mice, although a trend similar to LPS was observed for both strains (Fig. 3B).
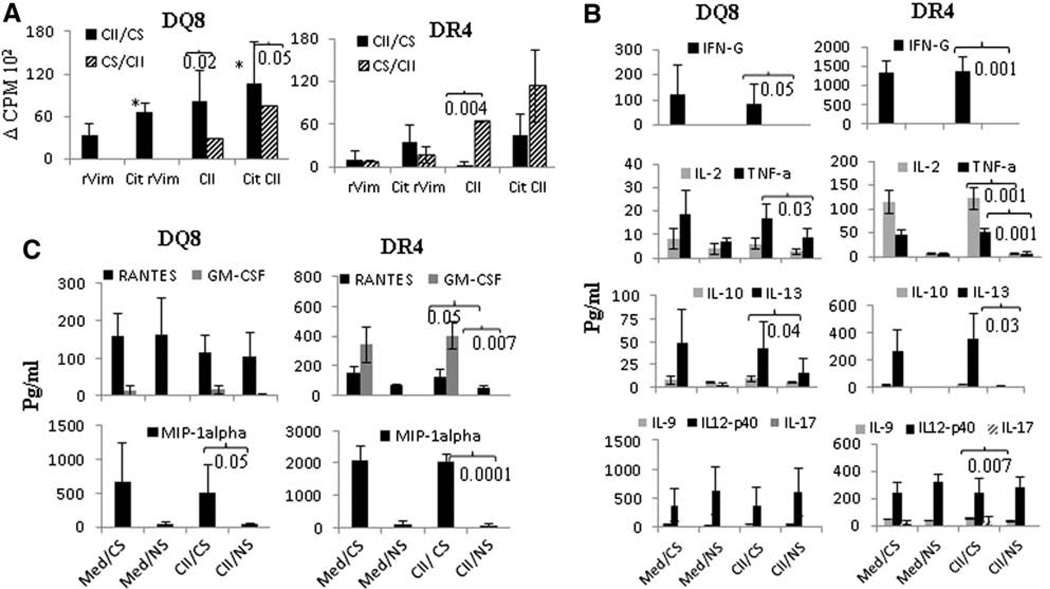
We next sought to determine whether CS-induced changes in antigen specific adaptive immunity may also be dependent on HLA polymorphism. Mice were exposed to CS for 2 weeks, immunized with CII and continued to be exposed to CS for 2 weeks (CS/CII) (Fig. 4A). In other experiments, mice were immunized with CII and then exposed to CS for 2 weeks (CII/CS). At the end of the experiment, in vitro T cell responses to native and citrullinated CII and rVim were tested. CS exposure prior to antigen priming (CS/CII) led to significantly higher responses to native CII (P = 0.004) with no significant differences in responses to rVim and cit-rVim between the CS/CII and CII/CS groups in DR4 mice suggesting that CS exposure prior to priming with antigen promotes subsequent antigen specific adaptive immunity (Fig. 4A). On the other hand, CS exposure after priming with antigen generated higher proliferative responses to native and citrullinated proteins in DQ8 mice (Fig. 4A). This data suggested that immune responses to CII may be important in initiation of arthritis while Vimentin may be a secondary antigen in this model.
Figure 4.
Smoking enhances production of pro-inflammatory cytokines systemically. Timing of exposure to CS determines the antigenic response. A) Lymph node cells from mice exposed to CS prior to immunization with CII (CS/CII) or post-immunization (CII/CS) were tested in vitro for response to native and CIT CII and rVim. *DQ8, P < 0.03 CII/CS vs CS/CII DQ8 B) Cytokine and C) chemokine production in culture supernatants of LNCs challenged with CII from CII-primed mice with or without CS (N = 4 mice/group). Med/CS—the media control for mice exposed to CS, Med/NS—media control for non-smoker mice.
3.4. Cigarette smoke increases antigen-specific Th1 and immuno-modulatory cytokines
To determine the influence of CS on systemic immune responses, ex vivo cytokine production in the supernatants from LNCs cultured in the presence or absence of CII were analyzed. In DQ8 mice, CS exposure enhanced production of IFNγ (P = 0.05), TNFα (P = 0.03) and IL-10 (P = 0.04) as compared with NS (Fig. 4B). The augmented release of these cytokines was observed in the absence of CII, suggesting that autoreactive Th1/Th2 cells are expanded by CS exposure in DQ8 mice. On the other hand, the Th17 cytokines, IL-23 (p40) and IL-17, did not differ between CS and control DQ8 mice (Fig. 4B). A similar cytokine profile was observed for Th1 and Th2 cytokines in DR4 mice, although DR4 mice produced very high amounts of IFNγ compared to DQ8 mice. Unlike DQ8 mice, LNCs from CS exposed DR4 mice produced higher levels of the Th17 cytokine, IL-9, compared to NS mice (P = 0.007). Overall, CS augmented Th1 and Th2 cytokine production in both strains with less significant changes in Th17 cytokines.
Among chemokines, RANTES (Regulated on activation normal T cell expressed and secreted, renamed as CCL5) and granulocyte macrophage stimulating factor (GMCSF) were significantly increased in CS exposed DR4 mice (P = 0.05 and P = 0.007 respectively as compared to NS mice) but not DQ8 mice (Fig. 4C). Another relevant chemokine, macrophage inflammatory protein (MIP-1A), was significantly elevated in both strains after CS exposure suggesting a potential role for macrophages in the influx and accumulation of activated T and B cells ensuing in an enhanced inflammatory response.
3.5. Cigarette smoke suppresses function of lung antigen presenting cells
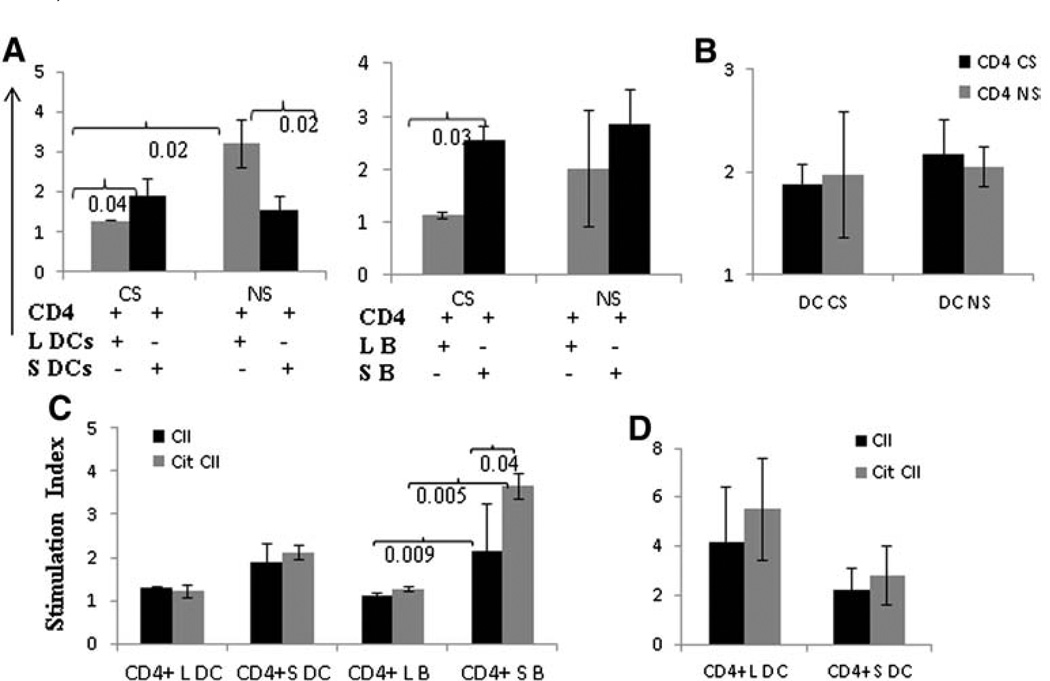
Since CS differentially modulated innate and adaptive immune responses, we tested whether these differences were due to modulation of APCs (DCs and B cells) function (Fig. 5). Although CS augmented the severity of arthritis in DQ8 mice, splenic CD4 T cells cultured with lung DCs (L DCs) isolated from CS exposed DQ8 mice showed reduced proliferation when compared with lung DCs isolated from control DQ8 mice (Fig. 5, P = 0.02). A similar phenomenon was observed with lung and splenic B cells (Fig. 5A, P = 0.03). Conversely, T cell proliferative responses induced by lung DCs of control (NS) DQ8 mice were robust when compared with splenic DC-induced T cell proliferation. B cells from the lung and spleen showed similar responses in control (NS) mice (Fig. 5A). To determine if the observed suppression of T cell proliferation in Fig. 5 A was a result of DCs or suppressive CD4 T cells, splenic DCs isolated from CS and control (NS) DQ8 mice were cultured with CD4 T cells isolated from spleen (Fig. 5B). No difference in T cell proliferation was observed between CS and NS mice.
Figure 5.
Cigarette smoke (CS) suppresses lung APC-induced splenic T cell proliferation but enhances systemic APC function in DQ8 mice. A) Splenic CD4+ T cells from CII-primed mice exposed to CS or control “non-smokers” (NS) were cultured with dendritic cells (DCs) and B cells isolated from lungs (L) and spleen (S) and challenged with CII (20 µg/ml). T cell response was suppressed when DCs and B cells from lungs were used as APCs in CS mice. In NS mice, DCs from lungs (L DC) led to a significantly higher T cell response than splenic DCs (S DC), while B cells did not show any significant difference. B) Splenic DCs from CS mice were cultured with CD4 cells from CS or NS mice. Similarly, DCs from NS mice were cultured with CD4 T cells from CS and NS mice. C) DCs and B cells isolated from lungs and spleen of CS mice were tested as APCs for native and Citrullinated type II collagen (CIT-CII) in DQ8 mice. D) DCs from lungs and spleen were cultured with splenic CD4 T cells in DR4 mice exposed to CS. Enough B cells from lungs of DR4 mice could not be obtained. All experiments were done 2–3 times with cells pooled from 2 to 3 mice/each experiment.
Since CS promotes immunity to citrullinated proteins, we next determined if CS promoted Cit-CII related immunity in the CII-immunized DQ8 mice exposed to CS. Citrullinated proteins are known to bind DR4 with higher efficiency compared to native proteins [19]; however whether citrullinated proteins bind with higher efficiency to DQ8 is unknown. DCs isolated from lungs and spleen induced similar proliferative responses to native and cit-CII, though responses were comparatively higher with splenic DCs (Fig. 5). On the other hand, B cells isolated from the spleens of CS DQ8 mice generated significantly higher T cell responses to native and Cit-CII compared to lung B cells (Fig. 5C). This implies that CS promotes humoral and cellular responses to citrullinated proteins in the DQ8 mice. Contrary to the observations with DQ8 mice, DR4 mice showed an increased response to Cit-CII when presented by DCs from lungs or spleen (Fig. 5D). However these differences were not significant due to high variability in response observed in various mice.
3.6. Cigarette smoke increases co-stimulatory receptors on lung DCs
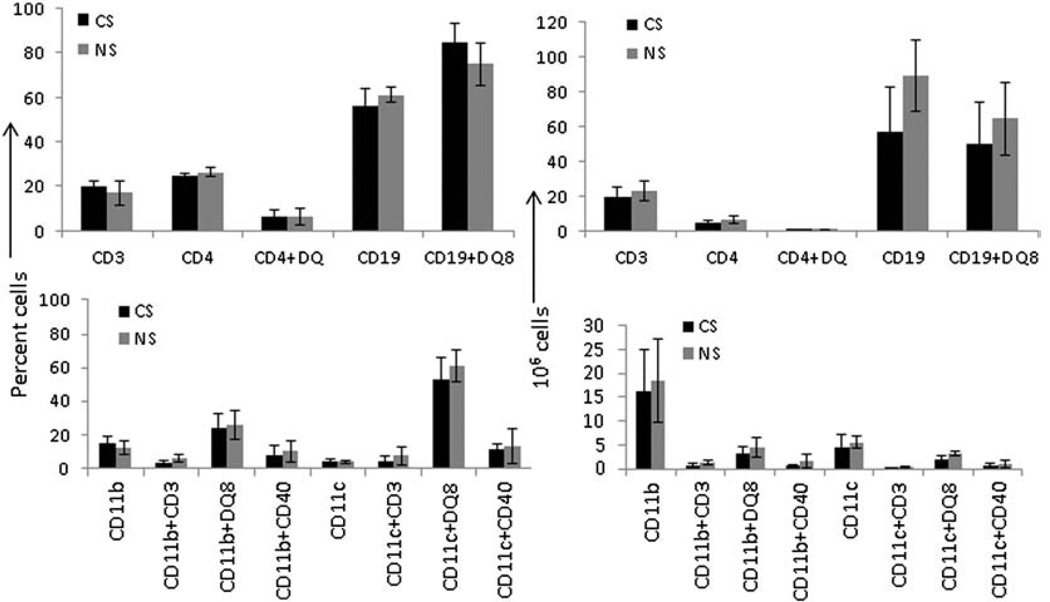
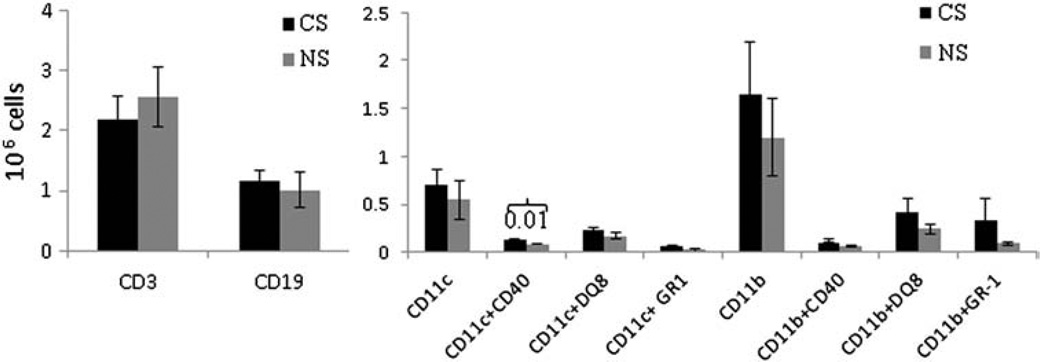
To determine whether CS modified antigen specific responses by altering APC numbers or surface receptor expression, we enumerated absolute numbers and relative proportions of immune cells in the lung and spleen of DQ8 mice. There were no significant differences in the percentage and absolute numbers of splenic T and B cell populations in CS and NS mice (Fig. 6). Similarly, there was no difference in various subsets of DCs and macrophages between CS and control NS mice. CS exposure led to a significant increase in the numbers of DCs in the lungs that was reflected in the expression of CD40 on DCs, suggesting a role for these APCs in augmenting the local lung inflammatory response after CS exposure (Fig. 7, P < 0.01).
Figure 6.
A comparison of percent (left panel) and absolute numbers (right panel) of various immune cells in spleens of cigarette smoke (CS) and non-smoker (NS) DQ8 mice immunized with type II collagen. The cells were stained with relevant conjugated antibodies and analyzed by FACS (N = 3–4 mice/group).
Figure 7.
Absolute numbers of CD3+ and CD19 cells in the lungs of CS and NS CII-immunized DQ8 mice (left panel). The right panel shows the absolute numbers of CD11c and CD11b expressing lung cells and their subsets. Subsets were enumerated after gating on CD11c and CD11b (N = 4–6 mice each group).
4. Discussion
The mechanism(s) by which CS promotes autoimmunity are largely unknown, but likely involve modulation of systemic immune response as smokers who carry HLA-DR4 are at a higher risk of developing seropositive RA [2]. Our previous data has shown that DR4 and DQ8 mice develop collagen induced arthritis that mimics human RA in multiple aspects, including development of autoimmunity with autoantibodies analogous to human disease [9,12,20]. Here we demonstrate that chronic exposure to CS suppresses arthritis in DR4 mice while enhancing it in DQ8 mice. Precise mechanisms by which CS promotes autoimmune RA are not well defined. Our observations using HLA transgenic mice expressing RA-associated DR4 and DQ8 alleles show that HLA genes determine the influence of CS in arthritis development.
The current model provides important insight into potential mechanisms by which CS and DR4/DQ8 interact to promote RA-related autoimmunity. CS exacerbated CIA in DQ8 mice by augmenting antigen-specific adaptive T cell responses to native and citrullinated proteins. In addition, CS augmented co-stimulatory molecule expression on lung DCs, and enhanced IL-10 and IL-17 gene expression in the lungs of DQ8 mice. On the other hand, CS suppressed CIA severity in DR4 mice. DR4 has been shown to bind and present citrullinated proteins with higher affinity than native protein [19]. In keeping with that observation, CS augmented immune responses to cit-CII in DR4 mice although immune response to Cit-rVim was suppressed. In the current study we show, for the first time, that DQ8 mice immunized with CII generate a higher response to citrullinated CII than native protein which was further augmented by CS exposure. Our observations imply that DQ8, similar to DR4, can present citrullinated peptides and that CS augments immunity to cit-CII. Interestingly, naïve mice exposed to CS also generate a mild response to citrullinated but not native CII confirming human studies that CS enhances host responses to citrullinated protein. Further, our data suggests that seropositive RA could be associated with the DQ8 genetic background.
Smoking is associated with an increase in cellular and humoral responses to cit-rVim in 75% of RA patients and a recent study identified cit-rVim epitopes in DR4 positive RA patients [21,22]. Our study shows that mice immunized with CII generate T cell responses to native as well as cit-rVim, while CS enhances T cell responses to cit-Vim only in DR4 mice. Interestingly, T cells extracted from naïve DR4 and DQ8 mice with prolonged CS exposure also respond to rVim and cit-rVim, even though the mice do not have arthritis, suggesting that CS alone is sufficient in the induction of adaptive immunity to native and cit-rVim. The current study shows that CS significantly alters murine responses to autoantigens. Our data suggests that the influence of CS on adaptive immunity to specific peptides is influenced also by the timing of CS exposure relative to the generation of specific adaptive immune responses. Our study does not define whether autoreactivity to vimentin or CII are epiphenomena or essential steps in the generation of autoimmunity, but does demonstrate the critical effects of CS on T cell dependent immunity generated to native and cit-rVim and CII.
CS has been shown to modulate immunity by altering innate and adaptive immune responses [18]. Previous studies have shown suppression of Th17 immunity by CS [23,24]. Rheumatoid arthritis and CIA appear to be at least partially Th17-dependent diseases [20,25]. It is possible that the reduced severity of arthritis observed in CS exposed DR4 mice may be associated with CS-mediated suppression of Th17 lung immunity. However, DQ8 mice showed the opposite response with higher expression of IL-17 as well as other inflammatory cytokines like TSLP and IL-13. The relative roles of Th1, 2 and 17 factors in the mediation of CS effects on autoimmunity will need to be addressed in future studies.
Our study suggests that DR4 may be involved in autoimmunity by enhancing immune response to bacterial antigens like LPS and CPG while DQ8 contributes to antigen-specific autoreactive responses. CS did not induce production of IL-17 but led to increased production of TNF-α and IL-13 in both strains. IL-13 is a prototypic Th2 cytokine which can contribute to the inflammatory process in the model by facilitating antigen presentation (potentially by enhancing B cell function) and stimulating auto-antibody production by B cells [26,27]. Indeed, RA patients show higher levels of IL-13 than healthy individuals and elevated levels of IL-13 in sera correlate with CCP positivity [28]. Similarly TNFα is important in RA pathogenesis as evidenced by the clinical success of TNFα inhibitors in RA patients.
CS is known to alter APC function [23]. Our data is consistent with prior studies that showed CS-induced modulation of DC function [13]. In the current study, we show suppression of antigen presentation by murine lung DC and B cells in CS exposed DQ8 mice. This would seem to contradict the observation of enhanced arthritis in primed DQ8 mice after CS exposure. In the current model, CS exposure modulated costimulatory functions as DCs from mice exposed to CS had higher expression of CD40 compared to controls. Additionally, CS augmented PAD enzyme expression as well as TNFα and IL-13 gene expression levels in the lings, potentially, these may be key mechanisms by which CS enhanced response to cit-CII and promoted arthritis in DQ8 mice. As previously shown, CS has complex effects on local lung and systemic immunity, which are best described as immunomodulatory rather than suppressive or stimulatory [13,23,29]. Mice immunized with CII generate higher responses to cit-CII suggesting that B cells are involved in generation of cellular and humoral immune response to citrullinated proteins and is consistent with previous studies [30,31]. Our study shows for the first time that B cells from DQ8 mice can present citrullinated proteins with higher efficiency than native proteins. Binding studies to prove that citrullinated proteins can bind DQ8 molecules with increased affinity are an area of important future investigation.
These studies imply that prolonged CS enhances innate immune responses especially in DR4 mice. Augmented innate immune responses may promote abnormal antigen-specific adaptive immune responses which in genetically predisposed individuals, may lead to a break in tolerance and autoimmunity. In humans, DR4 is implicated in severity of disease in association with CS. Our studies provide an insight into the role of DQ8 involvement in exacerbation of murine CIA and presentation of citrullinated proteins. The current study did not identify the specific components of CS involved in the modulation of immunity in the murine model of arthritis used. Nicotine, one of the chemicals found in CS with important immunomodulatory functions, has been shown to aggravate arthritis when administered prior to arthritis induction while suppress arthritis when delivered after induction of disease [32]. The importance of nicotine in modulation of arthritis in the murine model described in our study is not known, but it would appear highly likely that other CS constituents, including reactive oxidant species, play an important role in modulating APC function and other aspects of innate and adaptive immunity in the arthritis mouse model. Overall these studies provide important insight into how CS and host genetic factors interact in the development of autoimmunity.
Acknowledgment
We thank Julie Hanson and her staff in the Mayo Immunogenetics mouse colony for breeding and care of the mice and Michele Smart for tissue typing of transgenic mice. The study was supported by the NIH grants AR60077, AI 075262 and AR30752.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 2.Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat. Clin. Pract. 2006;2:425–433. doi: 10.1038/ncprheum0249. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal R, Liao K, Nair R, Ringold S, Costenbader KH. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009;61:1472–1483. doi: 10.1002/art.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 5.Bongartz T, Cantaert T, Atkins SR, Harle P, Myers JL, Turesson C, et al. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2007;46:70–75. doi: 10.1093/rheumatology/kel202. [DOI] [PubMed] [Google Scholar]
- 6.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 2008;67:1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 7.Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat. Clin. Pract. Rheumatol. 2007;3:707–715. doi: 10.1038/ncprheum0655. [DOI] [PubMed] [Google Scholar]
- 8.Szodoray P, Szabo Z, Kapitany A, Gyetvai A, Lakos G, Szanto S, et al. Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun. Rev. 2010;9:140–143. doi: 10.1016/j.autrev.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum. 2007;56:69–78. doi: 10.1002/art.22213. [DOI] [PubMed] [Google Scholar]
- 10.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, et al. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng S, Smart M, Hanson J, David CS. Characterization of HLA DR2 and DQ8 transgenic mouse with a new engineered mouse class II deletion, which lacks all endogenous class II genes. J. Autoimmun. 2003;21:195–199. doi: 10.1016/s0896-8411(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 12.Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra H, et al. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J. Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- 13.Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R. Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J. Immunol. 2008;181:1536–1547. doi: 10.4049/jimmunol.181.2.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol. Biochem. Behav. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg K, Bengtsson C, Kharlamova N, Reed E, Jiang X, Kallberg H, et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann. Rheum. Dis. 2013;72:652–658. doi: 10.1136/annrheumdis-2012-201484. [DOI] [PubMed] [Google Scholar]
- 16.Klareskog L, Padyukov L, Alfredsson L. Smoking as a trigger for inflammatory rheumatic diseases. Curr. Opin. Rheumatol. 2007;19:49–54. doi: 10.1097/BOR.0b013e32801127c8. [DOI] [PubMed] [Google Scholar]
- 17.Lee HS, Irigoyen P, Kern M, Lee A, Batliwalla F, Khalili H, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56:1745–1753. doi: 10.1002/art.22703. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J. Dent. Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 20.Taneja V, Behrens M, Basal E, Sparks J, Griffiths MM, Luthra H, et al. Delineating the role of the HLA-DR4 “shared epitope” in susceptibility versus resistance to develop arthritis. J. Immunol. 2008;181:2869–2877. doi: 10.4049/jimmunol.181.4.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW, et al. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:117–125. doi: 10.1002/art.25059. [DOI] [PubMed] [Google Scholar]
- 22.Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. Ther. 2004;6:R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J. Immunol. 2005;175:2684–2691. doi: 10.4049/jimmunol.175.4.2684. [DOI] [PubMed] [Google Scholar]
- 24.Vassallo R, Walters PR, Lamont J, Kottom TJ, Yi ES, Limper AH. Cigarette smoke promotes dendritic cell accumulation in COPD; a lung tissue research consortium study. Respir. Res. 2010;11:45. doi: 10.1186/1465-9921-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 2013;72(Suppl. 2):ii116–ii123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 26.Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, et al. Interleukin 13 is a B cell stimulating factor. J. Exp. Med. 1994;179:135–143. doi: 10.1084/jem.179.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 28.Hitchon CA, Alex P, Erdile LB, Frank MB, Dozmorov I, Tang Y, et al. A distinct multicytokine profile is associated with anti-cyclical citrullinated peptide antibodies in patients with early untreated inflammatory arthritis. J. Rheumatol. 2004;31:2336–2346. [PubMed] [Google Scholar]
- 29.Vassallo R, Kroening PR, Parambil J, Kita H. Nicotine and oxidative cigarette smoke constituents induce immunemodulatory and pro-inflammatory dendritic cell responses. Mol. Immunol. 2008;45:3321–3329. doi: 10.1016/j.molimm.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behrens M, Trejo T, Luthra H, Griffiths M, David CS, Taneja V. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. J. Autoimmun. 2010;35:1–9. doi: 10.1016/j.jaut.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taneja V, Krco CJ, Behrens MD, Luthra HS, Griffiths MM, David CS. B cells are important as antigen presenting cells for induction of MHC-restricted arthritis in transgenic mice. Mol. Immunol. 2007;44:2988–2996. doi: 10.1016/j.molimm.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H, Yang YH, Rajaiah R, Moudgil KD. Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum. 2011;63:981–991. doi: 10.1002/art.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]