Abstract
Development of anxiety disorders is associated with neurobiological changes in areas that are a critical part of the fear neurocircuitry. Fear conditioning paradigms can offer insight into the mechanisms underlying the neurobiological ontogeny of anxiety. A small number of studies have focused on the effects of age and anxiety separately in school age children. The present study aimed to investigate these effects in 8-13 year old children with higher and lower trait anxiety. We examined differential fear conditioning and extinction using skin conductance responses and fear-potentiated startle in 60 children recruited from a low-income urban population. The results indicated that children under 10 years of age show poor discrimination of conditioned stimuli, and that anxiety increases fear responses during fear acquisition. After controlling for age and trauma exposure, fear-potentiated startle to the safety cue predicted child anxiety levels suggesting that impaired safety signal learning may be a risk factor for anxiety disorders in adulthood. Identifying risk phenotypes in children may provide opportunities for early intervention and prevention of illness.
Keywords: Fear Conditioning, Extinction, Children, Development, Anxiety
Introduction
The prevalence of anxiety disorders is known to increase during late childhood and early adolescence, suggesting that this period may be developmentally critical in identifying individuals at risk for adult anxiety (Cohen, Cohen, & Brook, 1993). A recent review of development of brain structures in children at risk for anxiety reported dysfunction in amygdala and prefrontal regions associated with anxiety (Blackford & Pine, 2012). In addition, anxiety disorders are associated with larger amygdala volume in children and adolescents (De Bellis et al., 2000). Given that these neurobiological changes are observed in areas that are a critical part of the fear neurocircuitry (Whalen, 1998), fear conditioning paradigms can offer insight into the mechanisms underlying the neurobiological ontogeny of anxiety (Britton, Lissek, Grillon, Norcross, & Pine, 2011). In human fear conditioning experiments, the two most commonly used peripheral measures of fear are an increase in skin conductance response (SCR) and fear-potentiated startle. Skin conductance, which reflects changes in sweat gland activity that alters the electrical conductivity of the skin, is a direct index of sympathetic nervous system activation, and an excellent peripheral metric for arousal. Importantly, the magnitude of the SCR reliably increases during presentations of a reinforced conditioned stimulus (CS+) that was previously paired with an aversive unconditioned stimulus (US), making it a good index of conditioned fear (Pattwell et al., 2012; Waters, Henry, & Neumann, 2009). In fear-potentiated startle, the magnitude of the startle reflex increases during aversive CS presentations (Glenn et al., 2011; Grillon & Davis, 1997; Jovanovic et al., 2010), a phenomenon that has been extensively modelled in animals (Davis, 1992; Falls & Davis, 1994). In fear extinction paradigms, the stimulus that was previously paired with the US (that is, the CS+) is then repeatedly presented without the US, so that it no longer elicits a fear response (Quirk, 2006). Whereas fear acquisition refers to learning that something is dangerous, extinction is a mechanism by which an individual learns that something that was previously dangerous has become safe. Neuroimaging studies in humans have implicated the amygdala activation during fear conditioning (Phan, Wager, Taylor, & Liberzon, 2002) and amygdala, hippocampus, and ventromedial prefrontal cortex (vmPFC) activation in fear extinction (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Milad et al., 2007).
A small number of studies have investigated developmental trends in activity in the above neural structures. One study used functional magnetic resonance imaging (fMRI) during fear conditioning in adolescents and adults, and found that compared to adults, the CS+ evoked greater responses in the amygdala and hippocampus relative to the CS− in adolescents (Lau et al., 2011). Although no other studies specifically examined fear conditioning using fMRI in children and adolescents, several studies have used fear-relevant cues, such as fearful faces, to activate these structures. For example, a recent study found a developmental shift in functional connectivity between the amygdala and the medial prefrontal cortex (mPFC) during the viewing of fearful faces (Gee et al., 2013). The cross-sectional study included children from 4 years of age to adults and found that these areas were positively connected prior to age 10 years, and negatively connected after age 10 years (Gee et al., 2013).
Similar age effects have been found in a fear conditioning studies using fear potentiation of the acoustic startle response with faces as CSs and the sound of a woman’s scream as the aversive US that was paired to one face (CS+), but not a second face (CS−). The study found that fear-potentiated startle to the CS+ was greater in the 10-13 year old group compared to the 8-9 year olds (Glenn et al., 2011). Furthermore, the study suggested that generalized fear responses to safety cues occurs around age 10, since children in the 8-9 age group showed higher responses to the CS− and poor generalization between the CS+ face and a generalization stimulus face. A developmental study of extinction examined SCR in children, adolescents, and adults and found normal (adult-like) levels of extinction to the CS+ in children (Pattwell et al., 2012). Interestingly, adolescent showed suppressed extinction compared to both children and adults (Pattwell et al., 2012). The results of this study indicated that there may be a reduction in extinction during this developmental stage due to a lack of synaptic plasticity in the vmPFC. It is also possible that hormonal changes during puberty impact extinction, as data from animal and human studies suggest that estrogen levels play a role in extinction (Glover et al., 2012; Zeidan et al., 2011).
A very small number of studies have examined the effect of anxiety on fear conditioned responses in children. Waters and colleagues included anxious and non-anxious children between 8 and 12 years of age in their study of fear conditioning, using a loud tone as the US. Anxious children showed greater fear responses to all CSs during conditioning compared to controls, and did not discriminate between danger (CS+) and safety (CS−) signals on SCR. A similar fear conditioning study using the scream US found that pediatric anxiety was associated with higher ratings of fear to all CSs in the experiment (Lau et al., 2008). Finally, a study of fear extinction in anxious and non-anxious children found that anxious children had higher fear responses measured with fear-potentiated startle, SCR and fear ratings (Liberman, Lipp, Spence, & March, 2006). Increased fear responses may be a biomarker of risk for psychopathology: a study of adolescent offspring of adults with anxiety disorders found that these adolescents had higher fear-potentiated startle responses compared to low-risk adolescents (Grillon, Dierker, & Merikangas, 1998). More specifically, increased fear-potentiated startle in the presence of safety signals may be an early indicator of a propensity for anxiety disorders, as it has been associated with higher anxiety in adolescents who were categorized as behaviorally inhibited as toddlers (Reeb-Sutherland et al., 2009). To date, no studies have specifically examined the association between anxiety and fear responses in children under 10 years old as compared to those 10 years of age and older. Such information can point to developmental windows of opportunity for early intervention.
The objective of the current study was to investigate fear acquisition and extinction using SCR and fear-potentiated startle in in school-age children, focusing on the effects of age and child anxiety. Our laboratory has developed a fear conditioning paradigm which uses neutral stimuli (geometric shapes appearing on a computer monitor) as CSs and an aversive airblast to the larynx as the US (Jovanovic et al., 2005). Given our previous findings in adults with PTSD and the prior studies of children in the literature (Glenn et al., 2011), we hypothesized that children 10 years and older would show better discrimination between danger cues (CS+) and safety cues (CS−), and that higher anxiety would be associated with greater fear responses in the presence of the CS−. On the other hand, we hypothesized that older children may show reduced fear extinction as observed in studies of adolescents (Pattwell et al., 2012).
Material and Methods
Participants
The study included 60 participants between 8 and 13 years of age (mean age=10.3, SD=1.58), of which 31 were female. The participants were recruited from the waiting rooms of the Primary Care or Obstetrics Gynecology clinics at the Grady Health System in Atlanta, GA. Eligible participants were between 8 and 13 years of age willing to participate; exclusion criteria were autism spectrum disorders, bipolar or psychotic disorders, or cognitive disability. The mothers and children were recruited from a low-income urban population with high trauma exposure (Jovanovic et al., 2011; Kamkwalala et al., 2012). We used the Violence Exposure Scale for Children-Revised (VEX-R) to assess the participants’ exposure to traumatic events. The scale comes in a male and female version. Internal consistency ranges from 0.80-0.86 (Fox & Leavitt, 1995). Prior to their participation, all mothers signed informed consent as well as parental permission for their children, and the children provided study assent approved by the Emory University Institutional Review Board and the Grady Research Oversight Committee.
Psychological Assessments
Anxiety in the participants was assessed using the Behavioral Assessment System for Children – Second Edition (BASC-2; Reynolds, 2004). This instrument has a parent report and child report section. This 161-item scale for children ages 6-11 encompasses 8 clinical subscales which combine to yield composite scores on 2 child psychopathology dimensions: Externalizing (Aggression, Attention Problems, Conduct Disorder, and Hyperactivity scales) and Internalizing (Anxiety, Atypicality, Depression, Somatization, and Withdrawal scales). The results are normalized by age. For the present study we focused on child-reported Anxiety ratings. Test-retest reliability, internal consistency, and convergent validity of the scales are very high (Reynolds & Kamphaus, 2004). IQ was assessed using the Reynolds Intellectual Assessment Scale (RIAS; Reynolds & Kamphaus, 2003), and participants with cognitive disabilities were excluded from further analyses. Psychological data were available for 54 participants.
Psychophysiological Assessment
The psychophysiological data was collected using Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA). Electromyographic (EMG) and skin conductance (SC) data were sampled at 1000 Hz and amplified using the respective modules of the Biopac system. The acquired data were filtered, rectified, and smoothed in MindWare software (MindWare Technologies, Inc) and exported for statistical analyses. EMG activity was recorded from two 5 mm Ag/AgCl electrodes placed over the orbicularis oculi muscle, approximately 1 cm under the pupil and 1 cm below the lateral canthus. The impedances for all participants were less than 6 kilo-ohms. The EMG signal was filtered with low- and high- frequency cutoffs at 28 and 500 Hz, respectively. Startle magnitude was assessed as the peak amplitude of the EMG contraction 20 to 200 ms following the acoustic stimulus. SC was measured using two electrodes on the hypothenar surface of the non-dominant hand. The SCR was defined as the average increase (from a 1 s pre-CS onset baseline) from 3 to 6 s after the CS onset.
Experimental Design
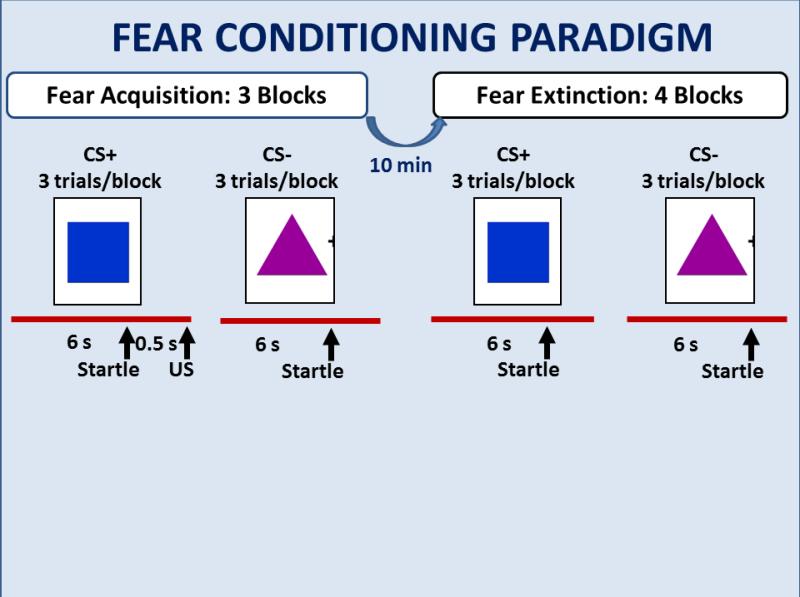
The experimental paradigm assessed differential fear conditioning and extinction, based on a paradigm used successfully in adult trauma populations (Norrholm et al., 2011, see Figure 1). Participants were seated in a sound attenuated booth and asked to remain still and look at a computer monitor approximately 1 m in front of them. The startle probe (noise burst) was a 106-dB [A] SPL, 40-ms burst of broadband noise delivered binaurally through headphones. In order to adapt the paradigm to children, we reduced the intensity of the airblast directed to the larynx to 80 p.s.i. The experimental protocol consisted of two phases: fear acquisition and extinction. The sessions were separated by 10 minutes. The acquisition phase consisted of 3 blocks, each with 3 CS+ trials, 3 CS− trials, and 3 noise alone (NA, no CS presented during startle probe) trials, for a total of 27 startle trials. Both CSs were colored shapes presented on a computer monitor using Superlab presentation software (Cedrus, Inc.) for 6000 ms prior to the delivery of the startle probe, and co-terminated with the US 500 ms after the presentation of the startle stimulus. The CS+ was reinforced with the airblast 100% of the time. The extinction phase consisted of 4 blocks with 3 trials of each type. The CSs were same as above, except that the CS+ was no longer paired with the airblast. In all phases of the experiment, inter-trial intervals will be randomized between 9 and 22 seconds. A response keypad (Cedrus, Inc.) was incorporated in the experiment: at the end of each block of acquisition and at the end of extinction a question appeared on the screen asking if a particular shape was followed by an airblast. The child was asked to respond to the question be pressing “Yes”, “No”, of “I don’t know”. US-expectancy was scored as 1 if the child answered “Yes”, and −1 if the child answered “No”, “I don’t know” was scored as 0.
Figure 1.
Schematic diagram of the fear conditioning and extinction protocol.
Data Analyses
As noted above, SCR was calculated as the average response during the 3 to 6 seconds following CS onset minus the pre-CS baseline. In some cases this subtraction resulted in a negative value as SCR habituated from baseline to CS onset. The SCR data for each individual were square root transformed in order to normalize the data. In cases with negative raw data, the square root transformation was performed on the absolute value and the resulting value was returned to a negative state indicating a level below that observed at baseline. The startle magnitude was compared between the three trial types; the degree of change from baseline startle (NA), i.e., fear-potentiated startle (FPS) was indexed by calculating percent potentiation for each CS type, in order to account for individual differences in startle magnitude as well as startle habituation. This value was derived as follows: Percent Startle Potentiation = 100 × (startle magnitude during CS trials – NA startle magnitude during same session) / (NA startle magnitude during same session). In cases where startle to a CS was lower than startle to the NA the resulting value was negative.
We used a repeated measures analysis of variance (RM-ANOVA) with the within-subject factors of Trial Type (2 levels for SCR: CS+, CS−; 3 levels for startle: NA, CS+, CS−) and Block (3 levels for acquisition and 4 levels for extinction). In order to assess contingency awareness, we compared average response pad responses during acquisition to CS+ and CS−, and to the CS+ at the end of extinction.
In order to assess the effects of age, we categorized children into 2 groups (under 10 years of age, and 10 and over, based on prior work (Gee et al., 2013; Glenn et al., 2011)). The younger group included 21 participants referred to as “children”, while the older group had 39 participants referred to as “youth”. We repeated the above analyses with Age as the between-groups variable. The participants were also divided into Lower and Higher Anxiety groups based on a median split of the self-report anxiety ratings (which was equal to a T score of 50). In subsequent RM-ANOVAs, Age and Anxiety were used a between-groups variables. The participants were distributed across the four groups as follows: 8 children with low anxiety, 11 children with high anxiety, 19 youth with low anxiety, and 16 youth with high anxiety. To assess the association between fear responses and anxiety, after controlling for age and trauma exposure, we conducted stepwise regression analyses with fear conditioned responses to the CS+ and CS− as predictor variables and anxiety as the dependent variable. We repeated the same analysis with extinction to the CS+ as the predictor variable. The number of individuals for different analyses varied due to data loss from either noisy EMG or SCR data, missing responses on the response keypad, or drop-out between acquisition and extinction. Ten participants (5 children and 5 youth, 3 in the Higher Anxiety group) discontinued the experiment after acquisition. All analyses were performed using SPSS 20.0 for Windows, with alpha set at .05.
Results
Participants
As noted above the participants were recruited from an inner-city population with high rates of trauma exposure. The average trauma exposure score on the VEX-R for all participants was 24.09 (range 1 through 54.95, of a maximum possible score of 66). The average anxiety ratings T score was 50.12 (range 36 to 81, median of 50, SD of 10). The median split resulted in a Higher Anxiety group, with a mean T score of 57.50 and Lower Anxiety group, with a mean T score of 41.91. Anxiety was positively correlated with trauma exposure, r=.31, p<.05).
Fear Conditioning and Extinction in the Entire Sample
The contingency awareness data collected from the response keypad indicated that the participants had higher US-expectancy on the CS+ (M=.51, SE=.08) relative to the CS− (M=−.41, SE=.09) trials, F(1, 50)=44.56, p<.001. In addition, US-expectancy on the CS+ trials decreased significantly from late acquisition (M=.50, SE=.13) to the end of extinction (M=−.61, SE=.13), F(1, 32)=36.00, p<.001. There were no significant main or interaction effects of Age or Anxiety on US-expectancy, indicating that all participants showed successful learning and extinction on a cognitive level. Figure 2 shows the SCR for all participants across Block and Trial Type during a) acquisition and b) extinction. During acquisition there was a significant Block by Trial Type interaction, F(2, 118)=8.04, p=.001, with SCR to the CS+ increasing across blocks and SCR to the CS− decreasing across blocks. During extinction, there was a significant main effect of Block, F(3, 135)=4.24, p<.01, but no interaction with Trial Type.
Figure 2.
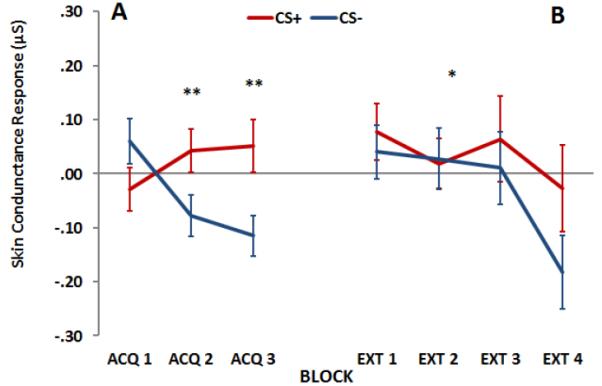
Skin conductance responses (SCR) during fear acquisition (A) and extinction (B) to the CS+ and CS−. There was a significant Block by Trial Type interaction during acquisition, F(2, 118)=8.04, p=.001, and a significant effect of Block during extinction, F(3, 135)=4.24, p<.01. Abbreviations: CS+=reinforced conditioned stimulus; CS−=non-reinforced conditioned stimulus.
**=p<.001 effect of Trial Type, CS+ vs CS− in Acq 2 and 3;
*=p<.01 main linear effect of Extinction Block (Ext1, Ext2, Ext3, and Ext4) across Trial Type
Figure 3 shows the same analysis using startle magnitude as the dependent variable during acquisition and extinction. The analysis of the acquisition phase revealed a significant main effect of Trial Type, F(2, 92)=5.89, p<.005, and a significant interaction of Block by Trial Type, F (4, 184)=2.99, p<.05. Contrasts comparing NA to CS+ and CS+ to CS− indicated that both comparisons changed across blocks (both p’s <.05). The RM-ANOVA of the extinction phase showed a significant effect of Block, F(3, 120)=3.15, p<.05, and no interaction with Trial Type. Given that discrimination between the CS+ and CS− developed across blocks, in further analyses we focused on late acquisition (average blocks 2 and 3, as in our previous work with adults (Norrholm et al., 2013)), when fear conditioning was maximal (see Figure 2a and 3a).
Figure 3.
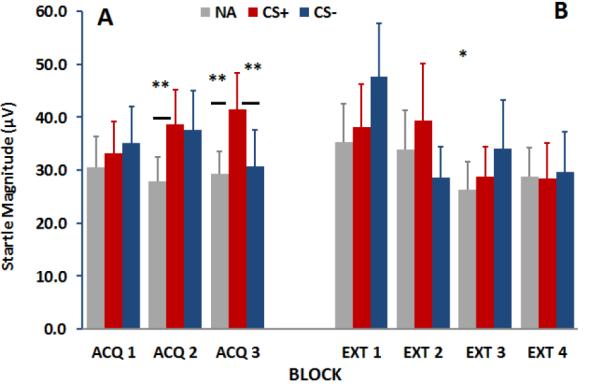
Startle magnitude responses during fear acquisition (A) and extinction (B) to the NA, CS+, and CS−. There was a significant Block by Trial Type interaction during acquisition, F (4, 184)=2.99, p<.05. Contrasts comparing NA to CS+ and CS+ to CS− indicated that both comparisons changed across blocks (both p’s <.05). The RM-ANOVA of the extinction phase showed a significant effect of Block, F(3, 120)=3.15, p<.05. Abbreviations: NA=noise alone trials; CS+=reinforced conditioned stimulus; CS−=non-reinforced conditioned stimulus
**=p<.001 effect of Trial Type, NA vs CS+; in Acq 2 and 3; CS+ vs CS− in Acq3
*=p<.01 main linear effect of Extinction Block (Ext1, Ext2, Ext3, and Ext4) across Trial Type
Main Effects of Age on Fear Conditioning and Extinction
We compared Trial Type during late acquisition across Age categories (children vs. youth). With regard to SCR during acquisition, we found a significant effect of Trial Type, F(1, 58)=10.02, p<.005, and a trend for children to have higher SCR compared to youth, F(1, 58)=3.68, p=.06, but no interaction effects. For extinction we examined the effect of Block on CS+ trials as the within-subject variable and Age category as the between-groups variable. We found a significant effect of Block, F(3, 132)=2.91, p<.05, and a trend for Age, F(1, 44)=3.60, p=.06 but no interaction of the two. In order to account for individual differences in baseline startle, we calculated percent fear-potentiated startle (FPS) to each CS using the above formula. There were no Age-related differences in baseline startle, with the younger group mean and SE equal to 24.03±20.17 μV, and the older group mean and SE equal to 33.15±34.44 μV, p>.1. An RM-ANOVA of late acquisition FPS across Age categories showed significantly higher FPS to the CS+ compared to the CS−, F(1, 46)=7.15, p=.01, but no main or interaction effects with Age. Examining extinction to the CS+ we found a significant effect of Block, F(3, 117)=2.98, p<.05, but again no main or interaction effects with Age.
Interaction Effects of Age and Anxiety on Fear Conditioning and Extinction
In order to examine the association between child Anxiety and fear conditioning measures we used a median split of the child-reported BASC T-score to divide children into Low and High Anxiety groups (the median value was a T-score of 50). The Anxiety groups did not differ in age. We then examined CS+ vs. CS− during late acquisition across Age and Anxiety groups. SCR results showed a significant effect of Trial Type, F(1, 50)=5.85, p<.05, and a trend for an interaction of Anxiety and Trial Type, F(1, 50)=3.71, p=.06. There were no other main or interaction effects. With regard to extinction we no longer found an effect of Block, nor any other effects of Age or Anxiety, see Figure 4.
Figure 4.
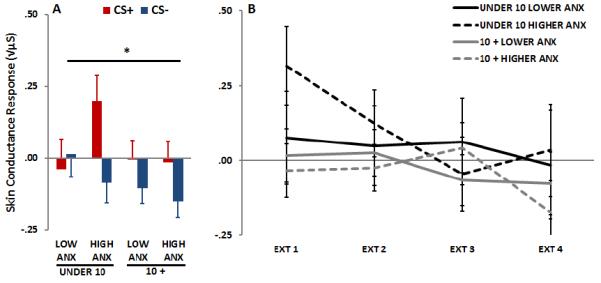
SCR during late acquisition of CS+ and CS− (A) and extinction to the CS+ (B) across Age and Anxiety groups. During late acquisition (blocks 2 and 3), there was a significant effect of Trial Type, F(1, 50)=5.85, p<.05, and a trend for an interaction of Anxiety and Trial Type, F(1, 50)=3.71, p=.06. There were no significant effects during extinction. Abbreviations: CS+=reinforced conditioned stimulus; CS−=non-reinforced conditioned stimulus
*=p<.05 interaction effect of Age and Anxiety
Analyses of the effects of Age and Anxiety on FPS between the CS+ and CS− during late acquisition revealed a significant effect of Trial Type, F(1, 40)=8.89, p=.005, as well as an interaction of Age and Anxiety, F(1, 40)=6.44, p<.05. Analyses of each Age category separately showed that only youth discriminated between the CS+ and CS−, F(1, 25)=10.48, p<.005, while the children did not, F(1, 15)=1.50, p>.1. Although Anxiety did not interact with Trial Type within each Age category, there was a main effect of Anxiety in the youth, F(1, 25)=5.06, p<.05. As seen in Figure 5a, higher levels of anxiety increased FPS to both Trial Types in in older children. A follow-up analysis of each CS separately showed that the interaction effect was significant only for the CS−, F(1, 40)=5.54, p<.05, and only at trend level for the CS+(1, 40)=3.70, p=.06. Breaking down the interaction effect for FPS to the CS−, we found that there were significant effects of Age in the Lower Anxiety group, F(1, 21)=8.59, p<.01, with youth showing reduced FPS to the CS− compared to children (Figure 5a). There were no effects of Age in the Higher Anxiety group.
Figure 5.
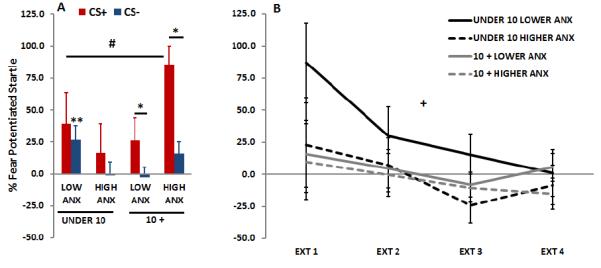
Fear-potentiated startle (FPS) during late acquisition of the CS+ and CS− (A) and extinction to the CS+ (B) across Age and Anxiety groups. During late acquisition (blocks 2 and 3), there was a significant effect of Trial Type, F(1, 40)=8.89, p=.005, as well as an interaction of Age and Anxiety, F(1, 40)=6.44, p<.05. Analyses of each Age category separately showed that only children over 10 years old discriminated between the CS+ and CS−, F(1, 25)=10.48, p<.005, while the younger children did not, F(1, 15)=1.50, p>.1. There was a main effect of Anxiety in the older children, F(1, 25)=5.06, p<.05. During extinction, there was a significant effect of extinction Block, F(3, 99)=4.28, p<.01 and a significant main effect of Anxiety, F(1, 33)=3.97, p=.05. FPS was derived as follows: Percent Startle Potentiation = 100 x (startle magnitude during CS trials – NA startle magnitude during same session) / (NA startle magnitude during same session). Abbreviations: CS+=reinforced conditioned stimulus; CS−=non-reinforced conditioned stimulus.
*=p<.01 effect of Trial Type (CS+ vs CS−); **=p<.01 effect of age on the CS−; #=p<.05 interaction of Age and Anxiety; +=p<.01 main effect of Extinction Block
An analysis of Age and Anxiety on FPS to the CS+ during extinction showed a significant effect of extinction Block, F(3, 99)=4.28, p<.01 and a significant main effect of Anxiety, F(1, 33)=3.97, p=.05, but no main or interaction effects with Age. As shown in Figure 5b, the Lower Anxiety group had higher FPS levels; this effect appears to be more pronounced in younger participants, who also showed the steepest extinction curve.
Regression Analysis with Trauma Exposure
As mentioned above, the participants were recruited from a highly traumatized population. Trauma exposure was positively correlated with Age, r=.49, p<.001, and Anxiety, r=.31, p<.05. In order to examine whether fear conditioning measures were associated with anxiety after controlling for age and childhood exposure to traumatic events, we conducted stepwise regression analyses with late acquisition and extinction variables. The first regression used the fear conditioning variables during acquisition as predictors of anxiety. We entered Age and Trauma level in the first step, SCR to the CS+ in the second step, SCR to the CS− in the third step, FPS to the CS+ in the fourth step, and FPS to the CS− in the final step. Anxiety was included as the dependent variable. The overall model was significant, F(6, 35)=2.61, p<.05, and accounted for 19% of the variance in Anxiety. FPS to the CS− during acquisition predicted 9.5% of the variance after controlling for the other variables. Table 1 shows the regression statistics for the acquisition phase. A second stepwise regression focused on extinction and included Age and Trauma in the first step, with SCR entered in the second step, and FPS entered in the last step, with Anxiety as the dependent variable. This analysis did not show any significant predictive effects of extinction on Anxiety after controlling for Age and Trauma, F(6, 35)=1.07, p>.1.
Table 1.
Regression analysis of fear conditioning measures during acquisition predicting child anxiety
| Outcome: Child Anxiety Level |
|||||
|---|---|---|---|---|---|
| Predictors: | R2 | R2
Change |
F Change | p | |
|
Age and
Trauma Exposure |
0.15 | 0.15 | 3.39 | 0.04* | |
| SCR to CS+ | 0.15 | 0.00 | 0.00 | 0.97 | |
| SCR to CS− | 0.17 | 0.02 | 0.80 | 0.38 | |
| FPS to CS+ | 0.21 | 0.05 | 2.18 | 0.15 | |
| FPS to CS− | 0.31 | 0.10 | 4.84 | 0.03* | |
Discussion
The current study investigated the effects of age and anxiety on fear acquisition and extinction using SCR and fear-potentiated startle in school-age children and youth. We hypothesized that children 10 years and older would show better discrimination between danger cues (CS+) and safety cues (CS−), and that higher anxiety would be associated with impaired safety signal learning to the CS−. On the other hand, we hypothesized that older children may show reduced fear extinction. The primary findings of the study were 1) children under 10 years of age show poor discrimination, replicating earlier work by Glenn and colleagues (Glenn et al., 2011); 2) anxiety in children under 10 years of age increases SCR responses to danger cues during conditioning and extinction, consistent with findings by Waters and colleagues (Waters et al., 2009); 3) anxiety in youth (10-13 years of age) increases fear-potentiated startle to danger and safety cues; 4) after controlling for age and trauma exposure, fear-potentiated startle to the safety cue predicted child anxiety levels; 5) anxiety reduced fear-potentiated startle during extinction in the children under 10.
In addition to supporting earlier findings on the effects of age and anxiety on conditioned fear responses, the current study indicates that dysregulated safety signal processing may be associated with anxiety in children and youth, and serve as a risk factor for anxiety disorders such as posttraumatic stress disorder (PTSD) in adulthood. Our previous studies have found that impaired safety signal learning may be a specific biomarker of PTSD (Christianson et al., 2012; Jovanovic & Norrholm, 2011). However these studies were conducted in patients who already suffered from PTSD, and were not able to distinguish whether impaired safety signal learning was a predisposing risk factor for PTSD or a consequence of the illness itself. Recently described genetic influences on responses to a safety signal (ie., CS−) in PTSD patients implicate the former (Norrholm et al., 2013). Similarly, studies of behaviorally inhibited adolescents with high anxiety also implicate increased fear-potentiated startle to safety signals as a phenotype of risk for anxiety disorders (Reeb-Sutherland et al., 2009). Longitudinal studies of fear conditioning and genotype in children at high risk for PTSD may provide insight into safety signal processing as a risk factor for the disorder.
Interestingly, our data support a developmental shift in safety signal processing around age 10, in that only participants in the 10 and older group showed significant discrimination between the CS+ (danger signal) and the CS− (safety signal). Other studies of healthy children have found improved discrimination between danger and safety signals at this age (Glenn et al., 2011), which may correspond to a shift in connectivity between the amygdala and prefrontal cortex which occurs around age 10 (Gee et al., 2013). It is important to note that this age-related shift was dependent on anxiety levels, inasmuch as older participants with lower anxiety showed reduced FPS to the safety signal compared to younger participants with lower anxiety. There were no age-related differences in FPS to the safety signal in the children with higher anxiety. Data from our study showed that reduced fear-potentiated startle in the presence of safety signals was associated with activation in the mPFC (Jovanovic et al., 2013). Higher Anxiety had different effects on SCR and FPS, depending on Age category. The children with higher anxiety had higher SCR levels to the CS+, which may have reflected higher arousal and higher sympathetic nervous system activation in that group. On the other hand, anxiety increased FPS to both CS+ and CS− in the youth; in fact, increased FPS to the CS− was predictive of increased anxiety after controlling for age and trauma exposure. It is interesting that anxiety appeared to decrease FPS in the younger participants; as this group shows the highest levels of fear on the SCR measure it is possible that the neural substrates of these metrics are different. Previous studies with adults have indicated discrepant findings between FPS and SCR, especially with regard to psychopathology (Glover et al., 2011). Fear-potentiated startle may be a more direct index of amygdala activity which may have a different developmental trajectory and be influenced by anxiety differently compared to the sympathetic system. It is also possible that the younger group with Higher Anxiety was not engaging the CSs during fear conditioning; however, the SCR data suggests that these children were attending to the CS+.
In this study we found that extinction learning was not robustly associated with age, in either SCR or FPS measures. Anxiety appeared to decrease FPS during extinction; however, this effect was stronger in the younger group. The anxious youth appeared to have blunted levels of fear during early extinction on both measures of fear. The low rates of extinction in the Higher Anxiety older group may have been due to increases in startle to NA trials at the start of extinction (see Figure 3). Because percent potentiation was calculated relative to a different baseline in the acquisition and extinction sessions, the FPS during early extinction was reduced. Although most of the participants in the older group (ages 10-13 years) in our study were younger than the adolescents (ages 12-17 years) who showed suppressed extinction in the study by Pattwell and colleagues (Pattwell et al., 2012), it is possible that the older participants in our study were showing effects of early puberty often observed in high stress environments (Braithwaite et al., 2009). Future longitudinal studies should track hormonal changes during puberty to investigate putative effects of estrogen on extinction (Glover et al., 2012; Milad et al., 2010).
In the present study we saw an increase in SCR and FPS to the CS− from late acquisition to the beginning of the extinction phase. This observation is consistent with our previous work in adults (Norrholm et al., 2006; Norrholm et al., 2008). Based on participant responses on the exit interview, this change in response to the CS− often reflects an expected contingency shift during the early extinction phase (in the absence of the reinforcement of the original CS+). In other words, participants initially expected the extinction session to be an additional acquisition session in which the CS−US contingency was reversed.
One of the limitations of the present study was its cross-sectional design which did not allow for true analyses of developmental effects of age. Furthermore, the absence of hormonal data in the participants and information about puberty status limits any conclusions on the effects of early adolescence. Finally, the study recruited participants from a high trauma population and may not generalize to normally developing children growing up in non-trauma environments. Age was positively correlated with trauma exposure potentially confounding the effects of age. However, the regression analysis found that safety signal processing was predictive of child and youth anxiety above and beyond the effects of age and trauma. A primary strength of the study is the concurrent assessment of fear acquisition and extinction using SCR (as a measure of sympathetic activation), fear-potentiated startle (as an index of amygdala-driven fear circuitry), and on-line contingency awareness. Moreover, the study of a high trauma population offers the potential for identifying at-risk children and youth, and providing early intervention or prevention approaches for these individuals.
Acknowledgements
We thank Allen Graham, Angelo Brown, and the Grady Trauma Project staff for their assistance with participant recruitment and data collection. This research was supported by National Institute of Mental Health Grants MH070129 and MH100122 (PI, T.J.), the Emory Medical Care Foundation, the Atlanta Clinical Translational Science Institute, the NIH National Centers for Research Resources (M01 RR00039). This work was funded in part by the Brain and Behavior Foundation (formerly NARSAD; S.D.N. and T.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Drs. Jovanovic, Smith, Davis, Norrholm, and Bradley report no financial disclosures. Ms. Nylocks and Gamwell have no conflicts of interest.
References
- Blackford Jennifer Urbano, Pine Daniel S. Neural Substrates of Childhood Anxiety Disorders: A Review of Neuroimaging Findings. Child and Adolescent Psychiatric Clinics of North America. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite Dejana, Moore Dan, Lustig Robert, Epel Elissa, Ong Ken, Rehkopf David, Hiatt Robert. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes and Control. 2009;20(5):713–720. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28(1):5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson John P., Fernando Anushka B. P., Kazama Andy M., Jovanovic Tanja, Ostroff Linnaea E., Sangha Susan. Inhibition of Fear by Learned Safety Signals: A Mini-Symposium Review. The Journal of Neuroscience. 2012;32(41):14118–14124. doi: 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Brook J. An epidemiological study of disorders in late childhood and adolescence--II. Persistence of disorders. J Child Psychol Psychiatry. 1993;34(6):869–877. doi: 10.1111/j.1469-7610.1993.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992;13(1):35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- De Bellis Michael D., Casey BJ, Dahl Ronald E., Birmaher Boris, Williamson Douglas E., Thomas Kathleen M., Ryan Neal D. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2000;48(1):51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Falls WA, Davis M. Fear-potentiated startle using three conditioned stimulus modalities. Animal Learning and Behavior. 1994;22:379–383. [Google Scholar]
- Fox NA, Leavitt LA. The Violence Exposure Scale for Children - VEX (preschool version) Department of Human Development, University of Maryland; College Park, MD: 1995. [Google Scholar]
- Gee Dylan G., Humphreys Kathryn L., Flannery Jessica, Goff Bonnie, Telzer Eva H., Shapiro Mor, Tottenham Nim. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala–Prefrontal Circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn Catherine R., Klein Daniel N., Lissek Shmuel, Britton Jennifer C., Pine Daniel S., Hajcak Greg. The development of fear learning and generalization in 8–13 year-olds. Developmental Psychobiology. 2011;54(7):675–84. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen Levels Are Associated with Extinction Deficits in Women with Posttraumatic Stress Disorder. Biological Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety. 2011;28(12):1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Effects of explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44(10):990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Ressler KJ. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49(7):1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan E. Fear Potentiation and Fear Inhibition in a Human Fear-Potentiated Startle Paradigm. Biological Psychiatry. 2005;57(12):1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience. 2011;5 doi: 10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M, Ressler K. Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology. 2010;35:846–857. doi: 10.1016/j.psyneuen.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Smith A, Kamkwalala A, Poole JM, Samples T, Norrholm SD, Bradley B. Physiological markers of anxiety are increased in children of abused mothers. Journal of Child Psychology and Psychiatry. 2011;52(8):844–852. doi: 10.1111/j.1469-7610.2011.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkwalala A, Norrholm SD, Poole JM, Brown A, Donely S, Duncan E, Jovanovic T. Dark-Enhanced Startle Responses and Heart-Rate Variability in a Traumatized Civilian Sample: Putative Sex-Specific Correlates of PTSD. Psychosom Med. 2012;74(2):153–159. doi: 10.1097/PSY.0b013e318240803a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human Amygdala Activation during Conditioned Fear Acquisition and Extinction: a Mixed-Trial fMRI Study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek Shmuel, Nelson Eric E., Lee Yoon, Roberson-Nay Roxann, Poeth Kaitlin, Pine Daniel S. Fear Conditioning in Adolescents With Anxiety Disorders: Results From a Novel Experimental Paradigm. Journal of Amer Academy of Child & Adolescent Psychiatry. 2008;47(1):94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman Lisa C., Lipp Ottmar V., Spence Susan H., March Sonja. Evidence for retarded extinction of aversive learning in anxious children. Behaviour Research and Therapy. 2006;44(10):1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Smith A, Binder EB, Klengel T, Conneely K, Ressler KJ. Differential Genetic and Epigenetic Regulation of Catechol-O-Methyl-Transferase (COMT) is Associated with Impaired Fear Inhibition in Posttraumatic Stress Disorder. Frontiers in Behavioral Neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behavioral Neuroscience. 2008;122(5):1016–30. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell Siobhan S., Duhoux Stéphanie, Hartley Catherine A., Johnson David C., Jing Deqiang, Elliott Mark D., Lee Francis S. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K. Luan, Wager Tor, Taylor Stephan F., Liberzon Israel. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Quirk Gregory J. Extinction: new excitement for an old phenomenon. Biological Psychiatry. 2006;60(4):317–318. doi: 10.1016/j.biopsych.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Perez-Edgar K, Henderson HA, Lissek S, Fox NA. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(6):610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Reynolds Intellectual Assessment Scales: Professional manual. PAR; Lutz, FL: 2003. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment Systemfor Children-Second Edition (BASC-2) Pearson; Bloomington, MN: 2004. [Google Scholar]
- Waters Allison M., Henry Julie, Neumann David L. Aversive Pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. Journal of Abnormal Psychology. 2009;118(2):311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Zeidan Mohamed A., Igoe Sarah A., Linnman Clas, Vitalo Antonia, Levine John B., Klibanski Anne, Milad Mohammed R. Estradiol Modulates Medial Prefrontal Cortex and Amygdala Activity During Fear Extinction in Women and Female Rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]