The actomyosin network of trabecular meshwork (TM) cells influences intraocular pressure (IOP) and aqueous humor drainage resistance1 and represents an important therapeutic target for glaucoma. The biology of actin in the TM and effect of agents that alter actin have been studied primarily in cultured TM cells. We are developing a tissue-based model of the human TM in which live cells and cellular interactions can be directly observed in situ.2,3 Here we report our initial novel observations of actin in live cells within the human TM following in situ baculovirus transduction with actin-RFP.
Human donor corneoscleral tissue was received in Optisol GS (Bausch & Lomb, Rochester, NY). For institutional reasons, age, death and other patient information were not available to us. Typical age at death ranged from 40-70 years and typical post-mortem age was 7-days (oral communication, Dr. Martin Heur), with experiments begun within a day of receipt.2-5
TM was cut into segments (Fig. 1) and representative segments were randomly selected for viability analysis, as previously described,2 prior to incubations for F-actin labeling. Briefly, tissue was co-incubated with Calcein AM and propidium iodide at 37°C and 8% CO2 prior to live cell imaging. Tissues with at least 50% Calcein-positive cells were considered viable.2 Viable tissue was incubated with Cellular Lights™ Actin-RFP (Life Technologies; n=5) following manufacturer's instructions. Cellular Lights uses a baculovirus delivery vector (BacMam technology) that transduces mammalian cells and directs fluorescence expression by TagRFP fusion to the N-terminus of beta-actin. Some specimens were co-incubated with Hoechst 33342 to label cell nuclei. For comparison, different tissue segments were fixed (4% parformaldehyde), permeabilized in 5% Triton X-100 (2h, 4°C), and incubated with Alexa Fluor 568®-conjugated phalloidin (n=40).4
Figure 1.
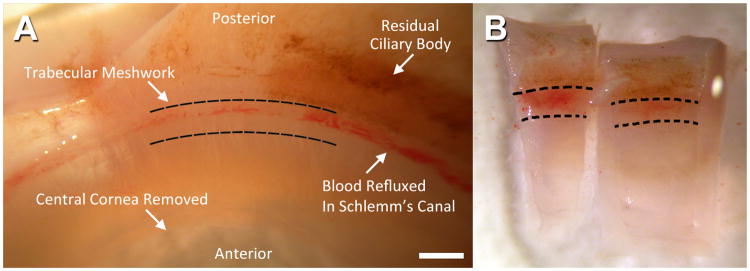
A: Location of trabecular meshwork (TM) in human corneoscleral tissue. Bar=1mm. B: Examples of wedges cut from corneoscleral donor tissue. Hashed lines indicate the anterior and posterior borders of the TM. Blood is present in Schlemm's canal, immediately deep to the TM.
The tissue was imaged on a PerkinElmer™ Ultraviewer spinning disk confocal microscopy system with 63× water immersion objective. Excitation/emission: 488/525nm (autofluorescence); 555/584nm (Actin-RFP; phalloidin) and 350/460nm (Hoechst)
Following baculovirus transduction, cell clusters expressing actin-RFP (red fluorescence) were seen associated with autofluorescent TM uveal beams (Fig. 2A), corneoscleral pores (Fig. 2B,C) and juxtacanalicular fibers (Fig. 2D). Actin-RFP had a primarily cortical distribution and outlined cell borders, comparable with phalloidin labeling (compare figs. 2E-H). Actin distribution in the cytosol was perinuclear (Figs. 2D, 2H; closed arrowheads), punctate (Figs. 2A, 2C, 2D-H; open arrowheads) and filamentous (Figs. 2B-D; open arrows). In some sections, actin filaments were aligned along uveal beams (Figs. 2A, 2E) and corneoscleral pores (Figs. 2B, 2F). Some cell borders had an appearance resembling membrane ruffles typically seen in cultured cells (Fig. 2B, 2C; closed arrows). These ruffle-like structures were not observed in phalloidin-labeled cells. Nuclei were closely associated with fluorescence-labeled actin (Figs. 2A, 2D-H; asterisks). No nuclear fragmentation was seen.
Figure 2.
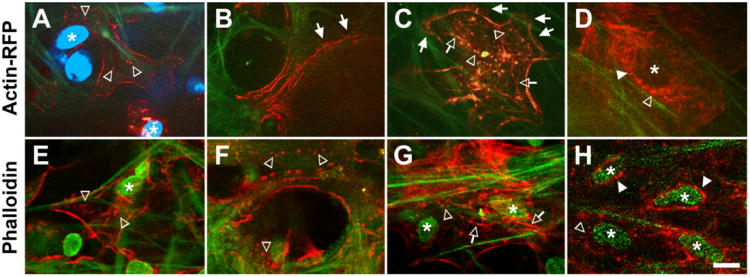
Clusters of live TM cells expressing Actin-RPF (red; A-D) or fixed, phalloidin-labeled (red) TM cells in the uveal (A, E), corneoscleral (B, C, F, G) and juxtacanalicular (D, H) regions. Membrane ruffle-like structures (closed arrows) were apparent in Actin-RFP labeled, but not phalloidin-labeled, cells. Green fibers: TM autofluorescence. Blue or green ovals: Hoechst-labeled nuclei. Asterisks: nuclei associated with fluorescent actin. Open arrows: filamentous cytosolic actin. Open arrowheads: punctate cytosolic actin. Closed arrowheads: perinuclear actin. Bar=10μm.
We have observed the actin cytoskeleton of live cells in the human TM following baculovirus transduction with actin-RFP. Optical sections captured various aspects of the actin cytoskeleton at different TM depths. Actin distribution was perinuclear, punctate, filamentous, and prominent in cell cortices and borders. Notably, prominent stress fibers were not seen. This may be due to the tissue micro-environment that differs from that of rigid-surfaced 2D culture; lack of serum or endogenous factors that enhance actin polymerization; or optical sectioning of cells in 3D tissue that masks stress fibers. Alternatively, the lack of uveal and posterior tissue attachments in donor tissue rims could result in decreased tensions across the TM, and explain the lack of stress fibers.
Actin-RFP labeling showed similarities with phalloidin-labeled actin with one caveat. Actin-RFP revealed the presence of membrane protrusions reminiscent of ruffles that were not evident in fixed and permeabilized phalloidin-labeled cells. It could be that Actin-RFP (or GFP) labeling has particular benefits for visualizing less stable actin structures (lamellipodia, filopodia) in live cells, a possibility we plan to explore in future studies using 2-photon microscopy.
We used spinning disk laser confocal microscopy that limits phototoxicity during live cell imaging. We are now optimizing our transduction protocols and using 2-photon microscopy that is less phototoxic and penetrates deeper than 1-photon microscopy. We have reported the utility of our human TM tissue-based system for live cell analysis and analysis of protein induction and expression, extracellular matrix and glaucoma markers.2-6 Our present report expands the findings of our prior studies with evidence for the model's potential use in tissue-based real-time studies of actin dynamics and testing of actin-targeting glaucoma therapies.
Acknowledgments
Special thanks to doctors of the Doheny Eye Institute Corneal Service for providing human corneoscleral donor rim tissue; Drs. Jim Burford and Janos Peti-Peterdi (USC Multiphoton Core) and Ernesto Barron and Dr. David Hinton (Doheny Vision Research Imaging Core) for sharing imaging expertise.
Funding sources: National Institutes of Health, Bethesda, MD EY020863 (JCHT); EY03040 (Doheny Vision Research Institute Imaging Core); 1S10RR024754 (USC Multiphoton Core); Kirchgessner Foundation Research Grant (JCHT); American Glaucoma Society Mentoring for Physician Scientists Award and Young Clinician Scientist Award; Career Development Award from Research to Prevent Blindness (JCHT); and an unrestricted grant (USC Department of Ophthalmology) from the Research to Prevent Blindness, Inc. New York, New York; Karl Kirchgessner Foundation (JCT); American Glaucoma Society (Mentoring for Physician Scientists and Young Clinician Scientist Awards [JCT]).
Footnotes
Competing/conflicts of interest: No stated conflict of interest
References
- 1.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in regulation of Aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41(3):619–623. [PubMed] [Google Scholar]
- 2.Gonzalez JM, Jr, Hamm-Alvarez S, Tan JC. Analyzing live cellularity in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2013;54(2):1039–47. doi: 10.1167/iovs.12-10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez JM, Jr, Heur M, Tan JC. Two-photon immunofluorescence characterization of the trabecular meshwork in situ. Invest Ophthalmol Vis Sci. 2012;53(7):3395–404. doi: 10.1167/iovs.11-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez JM, Jr, Tan JC. In situ 3D analysis of actin and its disruption in human trabecular meshwork. ARVO Meeting Abstracts. 2012 Mar 26;53:2746. [Google Scholar]
- 5.Tan JC, Gonzalez JM., Jr In situ autofluorescence visualization of human trabecular meshwork structure. Invest Ophthalmol Vis Sci. 2012;53(4):2080–8. doi: 10.1167/iovs.11-8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang AS, Gonzalez JM, Jr, Le PV, Heur M, Tan JC. Sources of structural autofluorescence in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2013;54(7):4813–20. doi: 10.1167/iovs.12-11235. [DOI] [PMC free article] [PubMed] [Google Scholar]


