Abstract
Sodium butyrate (NaBt) is the byproduct of anaerobic microbial fermentation inside the gastro-intestinal tract that could reach upto 20 mM, and has been shown to inhibit the growth of various cancers. Herein, we evaluated its effect on mitochondrial fusion and associated induction of apoptosis in colorectal cancer cells (CRC). NaBt treatment at physiological (1-5 mM) concentrations for 12 and 24 h decreased the cell viability and induced G2-M phase cell cycle arrest in HCT116 (12h) and SW480 human CRC cells. This cell cycle arrest was associated with mitochondria-mediated apoptosis accompanied by a decrease in survivin and Bcl-2 expression, and generation of reactive oxygen species (ROS). Furthermore, NaBt treatment resulted in a significant decrease in the mitochondrial mass which is an indicator of mitochondrial fusion. Level of dynamin-related protein 1 (DRP1), a key regulator of mitochondrial fission and fusion where its up-regulation correlates with fission, was found to be decreased in CRC cells. Further, at early treatment time, DRP1 down-regulation was noticed in mitochondria which later became drastically reduced in both mitochondria as well as cytosol. DRP1 is activated by cyclin B1-CDK1 complex by its ser616 phosphorylation in which both cyclin B1-CDK1 complex and phospho-DRP1 (ser616) were strongly reduced by NaBt treatment. DRP1 was observed to be regulated by apoptosis as pan-caspase inhibitor showing rescue from NaBt-induced apoptosis also caused the reversal of DRP1 to the normal level as in control proliferating cells. Together, these findings suggest that NaBt can modulate mitochondrial fission and fusion by regulating the level of DRP1 and induce cell cycle arrest and apoptosis in human CRC cells.
Keywords: Colon cancer, sodium butyrate, apoptosis, DRP1, mitochondrial fusion
1. Introduction
Thirteen million individuals lost their life around the world in 2008 due to cancer (Jemal et al., 2011). In developed country like United States of America (USA) every year 22% of total deaths occur due to the different types of cancers (Siegel et al., 2013). Cancer etiology is complex and attributable to a variety of factors including exposure to chemicals and viruses present in food and environment, hormonal and physiological conditions, and radiation as well as genetic factors. Among all, colorectal cancer (CRC) is the second most commonly diagnosed cancer in females and the third in males, which caused 1.2 million new cancer cases and 608,700 deaths in 2008 (Jemal et al., 2011). Five year survival rate for colon cancer was approximately 65% in USA during 2002 to 2008 (Siegel et al., 2013) and less than 60% in Europe (Cunningham et al., 2010). Several gene mutations are common in CRC including the most prevalent mutations in adenomatous polyposis coli (APC) gene (Markowitz and Bertagnolli, 2009).
Mitochondrion is a crucial organelle, which plays an important role in a range of events starting from embryonic development to the control of cell death. It also plays a role in cancer growth and progression, as cancer cells express metabolic instability and show resistance to mitochondrial apoptosis. There are two approaches to regulate it, first to triumph on glycolysis to revert the Warburg’s effect and another by inducing apoptosis by targeting mitochondrial proteins and/or membranes (Kroemer, 2006). This organelle dynamically and constantly undergoes fission and fusion events. Many human disorders including neurodegenerative diseases are related with deficiencies in the proteins that regulate mitochondrial dynamics (Westermann, 2010). Dynamin-related protein 1(DRP1) or dynamin-1-like protein (DNM1L) functions as mitochondrial and peroxisomal division machinery. It facilitates the membrane fission via oligomerization into ring-like structures which wrap around the scission site to constrict and sever the mitochondrial membrane through a GTP-dependent mechanism (Shin et al., 1997). DRP1 plays an important role in normal brain development by facilitating the regulated apoptosis, which takes place during neural tube development (Chen et al., 2000; Parone et al., 2006). It is also required for cytochrome c release and consequently activation of caspases during apoptosis. During mitosis, it is required for mitochondrial fission and in addition, it can also be involved in programmed necrosis execution and vesicle transport. Inhibition of DRP1 is reported to cause ATM-dependent G2/M arrest and aneuploidy (Qian et al., 2012).
Human body has at least 10 times more bacteria than the number of human cells present in the body, and most of them are in the human gastrointestinal tract (Savage, 1977). They produce certain molecules which play crucial role in various metabolic events including digestion and homeostasis maintenance. Short-chain fatty acids produced by microbial flora, by anaerobic fermentation of dietary fibers, have active role in homeostasis regulation. Short-chain fatty acids including butyric acid, propionic acid and acetic acid are present in mM concentrations in gastrointestinal tract. One of which, the butyric acid, is previously shown to withdraw cells from cell cycle or to promote cell differentiation, and finally to induce programmed cell death (Pajak et al., 2007). The focus of present study was to investigate the effect of physiological concentrations of butyric acid on human CRC cell death and mitochondrial dynamics.
2. Methods
2.1 Cell lines and chemicals
SW480 and HCT116 colorectal cell lines were purchased from the American Type Culture Collection (Manassas, VA) and grown in RPMI 1640 (HiMedia, Mumbai, India) supplemented with 10% fetal bovine serum (FBS, Invitrogen-Life Technologies, Grand Island, NY), 100 units/mL penicillin, and 100 μg/mL streptomycin under standard culture conditions. Sodium butyrate (NaBt) was purchased from Sigma-Aldrich (St. Louis, MO). Anti-survivin, CDK1, DRP1, phospho-DRP1(ser616), Bcl-2, total PARP [Poly (ADP-ribose) polymerase], cleaved-PARP, total caspase-3 and cleaved-caspase-3 antibodies were purchased from Cell Signaling Technology (Danvers, MA) and cyclin B1 and CDC25C were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Dallas, Texas). Anti-beta-actin antibody was from Sigma-Aldrich. Mouse and rabbit secondary horseradish peroxidase (HRP)-conjugated antibodies were from Cell Signaling Technology.
2.2 Cell viability assay
Cells were plated at 105 cells/60-mm cell culture dishes, and after 24 h, treated with fresh medium containing different concentrations of NaBt (1, 2.5 and 5 mM) in complete medium. After 6, 12 and 24 h of these treatments, total cells were collected by brief trypsinization, and washed with phosphate-buffered saline (PBS) twice. Live cell number was determined by trypan blue dye exclusion assay (Agarwal et al., 2003; Nambiar et al., 2012). Each treatment and time point had three independent plates. The concentrations of NaBt used in this study were within the range observed in normal human GI tract (Pellizzaro et al., 2002) that is below 20 mM.
2.3 Flow cytometric analysis for cell cycle phase distribution
The effect of NaBt treatment on cell cycle distribution was determined by flow cytometry after staining the cells with propidium iodide (PI) as described earlier (Nambiar et al., 2012; Stan et al., 2008)). Briefly, 1 × 105 cells were seeded, allowed to attach by overnight incubation, and exposed to desired concentrations of NaBt for specified time periods. Both floating and adherent cells were collected, washed with PBS twice, and fixed in 70% ethanol overnight at 4°C. The cells were then treated with 80 μg/mL RNase A and 50 μg/mL propidium iodide in saponin-EDTA solution for 30 min at 37°C and analyzed using a FACS Aria III (BD Biosciences, San Jose, CA). Percentage of cells in different phases of the cell cycle was computed for control and NaBt-treated samples.
2.4 Immunoblotting
Cells were seeded in 100-mm culture dishes and treated with NaBt at ~60% confluency for specified time periods. Both floating and attached cells were collected and lysed as described (Hahm and Singh, 2012; Lee et al., 2010). In brief, cells were lysed on ice with a lysis solution (50 mM Tris, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 150 mM NaCl) containing protease and phosphatase inhibitors. The cell lysate was cleared by centrifugation at 14,000 rpm for 30 min. The lysate proteins were resolved by 10 or 12.5% sodium-dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto the membrane. The membrane was blocked with tris-buffered saline containing 0.05% Tween-20, and 5% (weight/volume) non-fat dry milk for 1 h. The membrane was then incubated with the desired primary antibody for overnight at 4°C. Following incubation with an appropriate secondary antibody, the immunoreactive bands were visualized using enhanced chemiluminescence method. The blots were stripped and re-probed with anti-beta-actin antibody to normalize for differences in protein loading.
2.5 Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from control and NaBt treated cells was isolated using Trizol reagent (Life Technologies, USA) and was reverse-transcribed using a blue print 1st strand cDNA synthesis kit. PCR amplification was done as follows: initial denaturation at 95°C for 5 min, followed by 25 cycles of 94°C for 30 sec denaturation, annealing (respective temperature for each primer) for 30 sec and extension at 72°C for 1 min, followed by final extension at 72°C for 5 min. Primer sequences used are for survivin forward primer: GCCCAGTGTTTCTTCTGCTT, and reverse primer: GACAGAAAGGAAAGCGCAAC, for Bcl-2 forward primer: AAGCGGTCCCGTGGATAG, and reverse primer: TCCGGTATTCGCAGAAGTCC and for GAPDH forward primer: GCCTTCCGTCTCCCCACTGC, and reverse primer: CAATGCCAGCCCCAGCGTCA. The PCR products were analyzed on 1.2% agarose gel containing ethidium bromide (Stan and Singh, 2009).
2.6 Annexin V apoptosis assay
Cells were seeded and treated with NaBt as done for cell viability assay. At the end of the treatments, both floating and attached cells were collected and processed for annexin V and PI staining using FITC Annexin V apoptosis detection kit (BD Pharmingen, San Jose, CA) following the manufacturer’s protocol. The flow analysis for annexin V and PI stained cells was carried out by FACS using FACS Aria III (BD Biosciences). For caspase inhibitor assay, cells (1 × 105) were seeded in 60-mm dishes, allowed to attach overnight incubation and then treated with or without 25 μM z-VAD-FMK (pan-caspase inhibitor, BD Pharmingen), 2.5 mM NaBt or their combination for 24 h. At the end of treatments, both floating and adherent cells were collected and washed with PBS. Cells were analyzed for apoptosis by annexin V and PI staining as mentioned above.
2.7 Determination of mitochondrial mass
Cells (1 × 105) were seeded in 60-mm dishes, allowed to attach by overnight incubation, and then treated with desired concentrations of NaBt for 12 and 24 h. After completion of the treatment time periods, cells were exposed to 200 nM MitoTracker Red for 60 min. Thereafter, cells were collected by brief trypsinization and washed with PBS. Pelleted cells were resuspended in 0.5 mL PBS and mitochondrial degradation was determined by FACS Aria III (BD Biosciences) (Tal et al., 2009).
2.8 Mitochondrial reactive oxygen species (ROS) determination
ROS production was measured by flow cytometry following staining of cells with MitoSOX Red. Cells were treated with desired concentration of NaBt (2.5 mM) for 24 h and then incubated with 5 μM MitoSOX Red for 30 min. Cells were collected, washed with PBS and fluorescence was detected using FACS Aria III (BD Biosciences) (Hahm et al., 2011; Mukhopadhyay et al., 2007).
2.9 Sub-cellular fractionation
Mitochondrial and cytosolic fractionation was carried out using protocol described earlier (Jan et al., 2004). Briefly, SW480 cells were collected after treatment with NaBt for 24 h using cell lifter. Cells were washed with PBS and resuspended in 1 mL isotonic mitochondrial buffer (250 mM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM HEPES [pH 7.5]). Suspension was homogenized using Dounce homogenizer with 50 strokes. Nuclei and unbroken cells were removed by centrifugation at 500 × g for 5 min, this was followed by centrifugation at 10,000 ×g for 30 min at 4°C to collect mitochondrial pellet. The pellet was resuspended in 50 μL isotonic mitochondrial buffer and supernatant was collected as cytosolic fraction. These fractions were resolved on SDS-PAGE followed by immunoblotting.
2.10 Immunoprecipitation
The interaction between cyclin B1 and CDK1 was determined by co-immunoprecipitation by using protocol described earlier (Hahm and Singh, 2007). In brief, cells were collected and washed with ice-cold PBS, and lysed with lysis buffer containing 50 mmol/L Tris (pH, 8.0), 150 mmol/L NaCl, and 1% NP40. Lysate containing 300 μg of protein was incubated overnight at 4°C with 10 μg of anti-cyclin B1 antibody. Protein A/G plus agarose (50 μL, Santa Cruz Biotechnology) was subsequently added to each sample, and the incubation was continued for an additional 3 h at 4°C with gentle shaking. The immunoprecipitates were subjected to SDS-PAGE followed by immunoblotting using anti-CDK1 or anti-cyclin B1 antibody (Hahm and Singh, 2007; Singh et al., 2003).
2.11 Statistical analysis
Statistical analysis was done with GrapPad Prism 5 software version 5. Quantitative data are presented as mean ± SD. All the experiments were repeated two-three times, and representative data are shown. Statistical significance of difference between control and treated group was determined by Student t test and one-way ANOVA followed by Dunnett’s test or Bonferroni’s multiple comparison test and P<0.05 was considered significant.
3. Results
3.1 NaBt decreased survival of human colorectal cancer cells
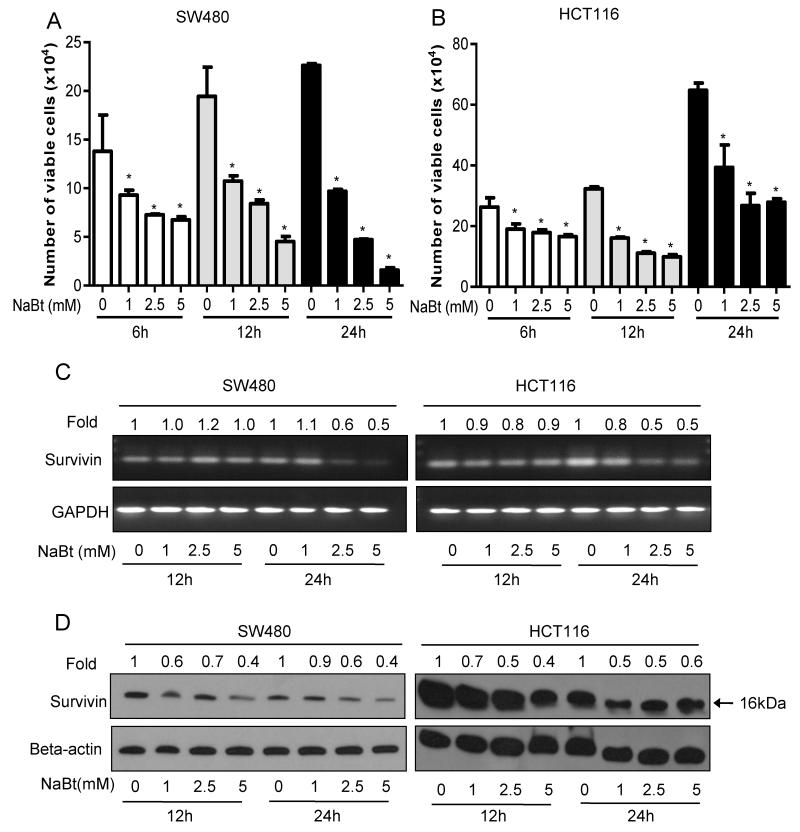
The effect of NaBt treatment on cell viability of CRC cell lines SW480 and HCT116, which respectively represent second and third stages of the cancer, was determined by trypan blue dye exclusion assay (Fig. 1A and B). The NaBt concentrations used in this study are within the physiological range in normal human GI tract. NaBt treatment at 1-5 mM concentrations for 12 and 24 h decreased viability of both CRC cell lines in a concentration- as well as time-dependent manner. This accounted for 47-93 % (P<0.005-0.001) and 40-61% (P<0.005-0.001) decrease in viability in SW480 and HCT116 cells, respectively (Fig. 1A and B). We assessed the level of survivin, an endogenous apoptosis inhibitor, following NaBt treatment. It decreased the expression of survivin at both mRNA level (Fig. 1C) as well as protein level (Fig. 1D). These results suggest that NaBt decreases the survival of CRC cells which may involve the down-regulation of survivin.
Figure 1.
Effects of NaBt on survival of human CRC SW480 and HCT116 cells. For the studies assessing the effect of NaBt on survival of exponentially growing SW480 and HCT116 cells, 105 cells were plated in 60-mm dishes and next day, treated with vehicle (distilled water) alone or 1-5 mM of NaBt in fresh medium. After 6, 12 and 24 h of these treatments, viable cells were counted using trypan blue staining and hemocytometer. The cell number data shown was mean ± SD of three independent plates; each sample was counted in duplicate. Data was analyzed using one-way ANOVA Dunnett’s test. *, P<0.05, significantly different compared with corresponding control. (A) Represents the viable cell number for SW480 cells; (B) Represents the viable cell number for HCT116 cells. After treatment with NaBt for 12 and 24 h, RNA and protein were extracted as detailed in Material and Methods, and (C) semiquantitative reverse transcription (RT)-PCR and (D) western blot analysis was done for survivin in both of the cell lines along with the loading controls, GAPDH and beta-actin, respectively. Band intensity was analyzed by densitometry in each case and was represented as fold change to that of their respective control, and shown at the top of each band (C-D).
3.2 NaBt treatment caused G2-M phase cell cycle arrest in human CRC cells
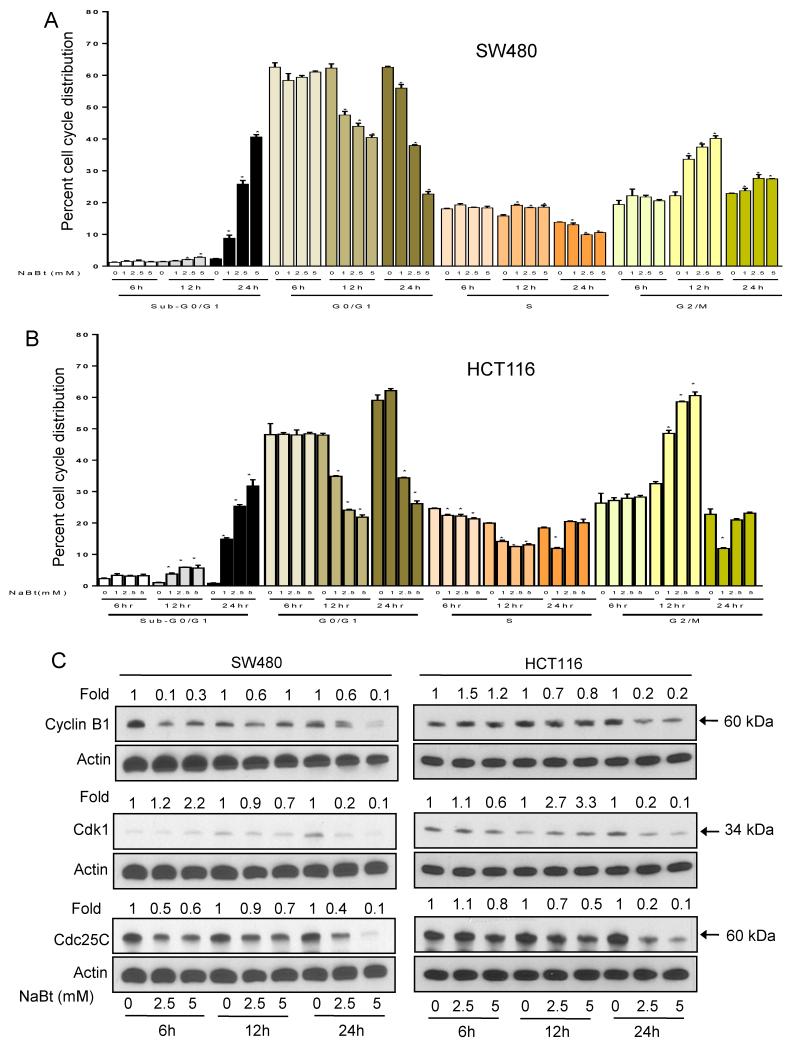
Graphical representation of the cell cycle distribution without or with treatment with 1-5 mM NaBt shows that it causes G2-M arrest at 12 h in both SW480 and HCT116 cells which is accompanied by a decrease in both G0/G1 and S phases (Fig. 2A and B). This effect is followed by significant (32-41%, P<0.001) increase in sub-G0/G1 population at the 24 h time point reflective of apoptosis. We did not observe any significant effect at 6 h of treatment. In SW480 cells, the percentage of cells arrested in G2-M phase showed decline with an increase in sub-G1 cell population (Fig. 2A). In HCT116 cells, the G2-M phase cell cycle arrest mostly disappeared at 24 h time point and was taken over by sub-G0/G1 phase, an indicator of cell death (Fig. 2B).
Figure 2.
Effect of NaBt on cell cycle distribution and cell cycle regulators in human CRC cells. (A) SW480 and (B) HCT116 cells were treated with vehicle (distilled water) or 1, 2.5 and 5 mM concentrations of NaBt for 6, 12 and 24 h. At the end of treatments, cells were collected and analyzed for cell cycle phase distribution as detailed in Materials and Methods. Data are mean ± SD of triplicate samples for each treatment. *, P<0.05, significantly different compared with control by one-way ANOVA followed by Dunnett’s test. (C) Immunoblotting for cyclin B1, CDK1 and CDC25C using lysates from SW480 and HCT116 cells treated with NaBt as detailed in Materials and Methods. Membranes were stripped and re-probed with anti-beta actin antibody to ensure equal protein loading. Numbers on top of the bands represent changes in protein levels as determined by densitometric analysis of the immunoreactive bands and corrected for beta-actin loading control.
3.3 NaBt altered the levels of proteins involved in regulation of G2-M transition
Eukaryotic cell cycle progression involves sequential activation of cyclin-dependent kinases (CDKs) whose association with corresponding regulatory cyclins is necessary for their activity (Molinari, 2000). The G2-M is regulated by a complex between cyclin B1 and CDK1. NaBt treatment (1-5 mM for 12 and 24 h) caused a marked decrease (especially at 24 h) in protein levels of cyclin B1 and CDK1 in both the CRC cells. These results indicated that NaBt mediated cell cycle arrest in G2-M phase in SW480 and HCT116 cells could be mediated via a decrease in protein levels of cyclin B1 and CDK1. The CDK1 is dephosphorylated by the phosphatase, CDC25C that results in leads the entry of cells in M phase. NaBt treatment decreased the level of CDC25C as early as 6 h after treatment (Fig. 2C).
3.4 NaBt treatment induced apoptosis in human CRC cells
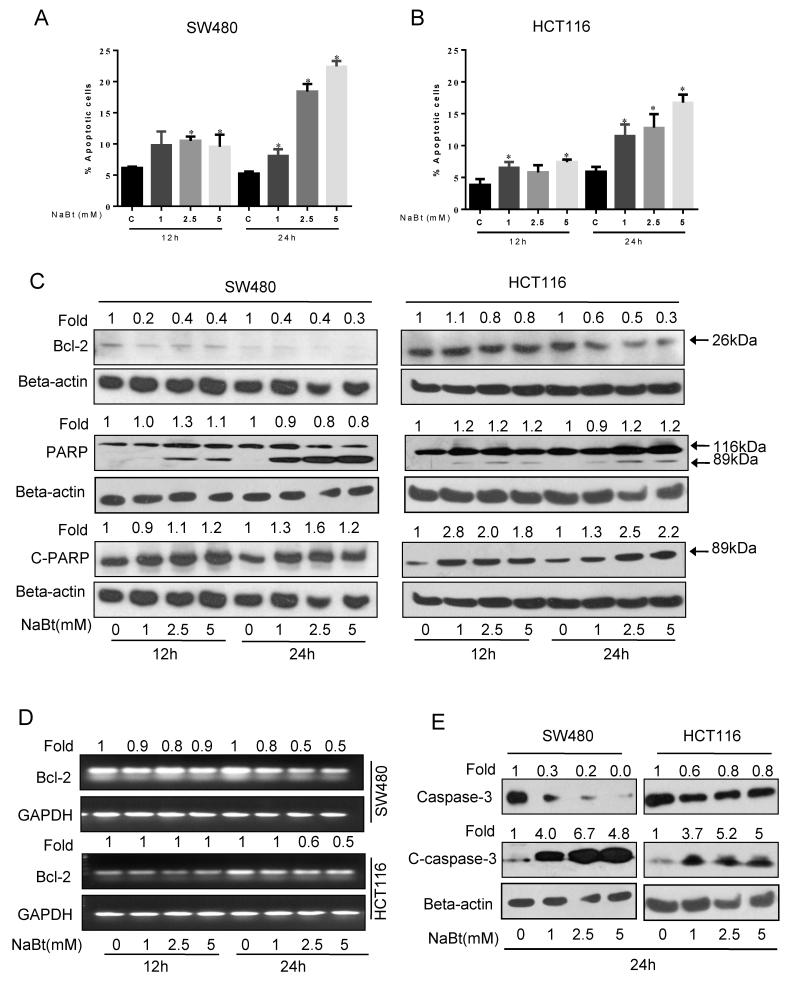
Because the flow cytometric analysis of cell cycle distribution suggested apoptosis induction due to accumulation of sub-G0-G1 cells, we proceeded to characterize proapoptotic response to NaBt. NaBt treatment for 12 and 24 h showed a significant increase (10-17%, P<0.05-0.001) in apoptotic cells in both the CRC cell lines (Fig. 3A and B). SW480 cells showed 1.5 fold increase in apoptotic cells at 12 h time point which was increased by 3-4 fold after 24 h treatment (Fig. 3A). Similarly, the HCT116 cells showed 1.5 and 3 fold enrichment of apoptotic cells at 12 h and 24 h after NaBt treatments, respectively (Fig. 3B). To further confirm the proapoptotic effect of NaBt, western blot analysis and RT-PCR were performed for the levels of total PARP, cleaved-PARP, total caspase-3, cleaved-caspase-3 and Bcl-2 protein and mRNA. NaBt increased the level of cleaved-PARP and cleaved-caspase-3 and decreased the level of Bcl-2 (Fig. 3C-3E), suggesting the possible involvement of mitochondrial apoptosis.
Figure 3.
The apoptotic effect of NaBt on human CRC cells. SW480 and HCT116 cells were treated with 1, 2.5 and 5 mM NaBt for 12 and 24 h. (A-B) At the end of treatments, total cells were collected and stained with annexin V/PI and analyzed for apoptotic cell population as mentioned in ‘Materials and Methods’. Data are mean ± SD of triplicate samples in each case. *, P<0.05, significantly different from control by one-way ANOVA followed by Dunnett’s test. (C) In similar treatments as detailed above, total cell lysates were prepared as described in ‘Materials and Methods’. SDS-PAGE and western blot analysis were performed for Bcl-2 and total as well as cleaved PARP. Membranes were stripped and re-probed with anti-beta-actin antibody to ensure equal protein loading. (D) In similar treatment, semiquantitative RT-PCR was done for mRNA levels of Bcl-2 and GAPDH in both cell lines as detailed in ‘Materials and Methods’. (E) Effect of NaBt treatment on cleavage of caspase-3 and its total level were analyzed in SW480 and HCT116 at 24 h using specific antibody by SDS-PAGE and western blot analysis and membrane was stripped and re-probed with anti-beta-actin. Numbers on top of the bands represent fold changes in band intensity as compared to control as determined by densitometric analysis of the bands and corrected for beta-actin or GAPDH loading control for western blot or PCR band, respectively (C-E).
3.5 NaBt caused caspase-mediated apoptosis in CRC cells
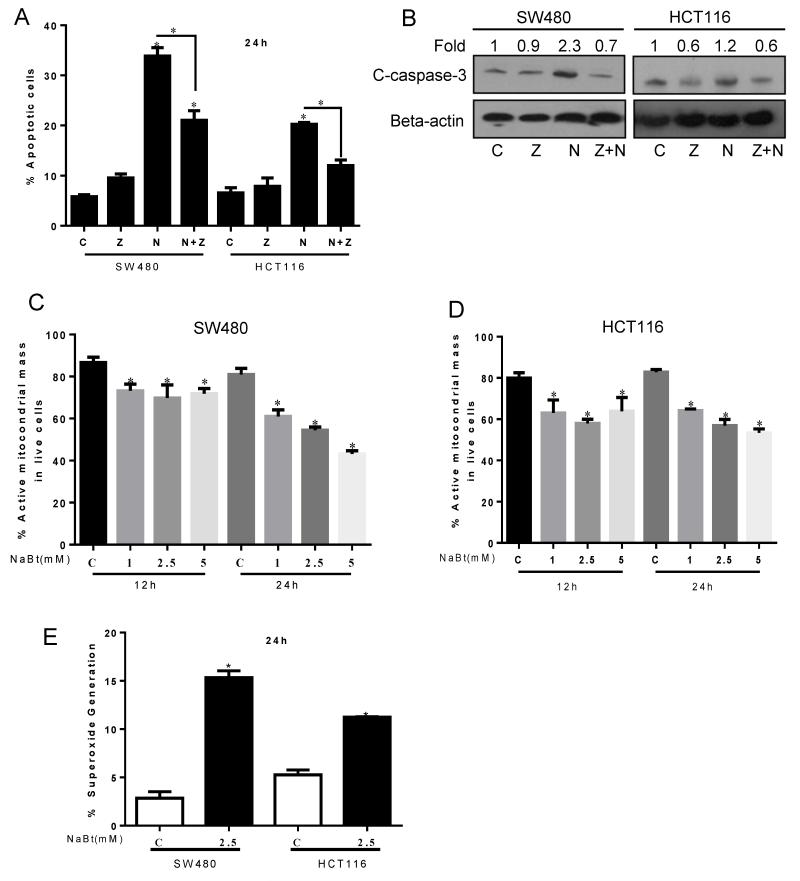
The role of caspase-mediated apoptosis was addressed by using z-VAD-FMK, a pan-caspase inhibitor, which causes irreversible inhibition to caspases and inhibits mitochondrial apoptosis. The quantitative apoptotic cell death assay using annexin V/PI staining of both of the cell lines showed that in caspase inhibiting condition, NaBt induced apoptosis (28% increase over that of control, P<0.05) was strongly reduced (11.6% increase over that of z-VAD-FMK treatment, P<0.05) in SW480 cells (Fig. 4A). Similar effects were observed in HCT116 cells in which NaBt caused increased in apoptotic cells i.e. 15.7% (P<0.05) was reduced to 4.1% (P<0.05) increase in caspase inhibiting condition (Fig. 4A). The protein level of cleaved caspase-3 was analyzed. NaBt showed an increase in cleaved caspase-3 level in both the cell lines which was drastically reduced in the presence of caspase inhibitor (Fig. 4B). These results indicated that NaBt mediated apoptosis is mainly mediated by caspases potentially involving mitochondria-mediated apoptotic pathway.
Figure 4.
Effect of NaBt on apoptosis, mitochondrial mass and ROS generation in human CRC cells. SW480 and HCT116 cells were first either pre-treated for 1 h with 25 μM z-VAD-FMK or left untreated and were then either left untreated again or were treated with 2.5 mM NaBt for 24 h. (A) Then cells were stained with annexin V and PI and analyzed for number of apoptotic cells by flow cytometric analysis. Data are shown as mean ± SD of triplicate samples. *, P<0.05, significantly different as compared with control by one-way ANOVA followed by Bonferroni’s multiple comparison test. (B) In similar treatments as detailed above, SDS-PAGE and western blot analysis were performed for cleaved-caspase-3, and membranes were stripped and reprobed with anti-beta-actin to ensure equal protein loading. Numbers on top of the bands represent fold changes in band intensity as compared to control as determined by densitometric analysis of the bands and corrected for beta-actin. (C-D) Cells were treated with 1, 2.5 and 5 mM NaBt for 12 h and 24 h. At the end of treatments, total cells were collected and stained with MitoTracker Red CMXRos and analyzed by flow cytometry as mentioned in ‘Materials and Methods’. Data are shown as mean ± SD of triplicate samples. *, P<0.05, significantly different compared with respective controls by one-way ANOVA followed by Dunnett’s test. (E) Cells were treated with 2.5 mM NaBt for 24 h and analyzed by flow cytometry for MitoSOX Red fluorescence in both SW480 and HCT116 cells as detailed in ‘Materials and Methods’. Data are shown as mean ± SD of triplicate samples. *, P<0.05, significantly different compared with respective controls by Student t-test. C, control; Z, 25 μM z-VAD-FMK; N, 2.5 mM NaBt and Z+N, 25 μM z-VAD-FMK + 2.5 mM NaBt.
3.6 NaBt decreased active mitochondrial mass and enhanced ROS production in CRC cells
The effect of NaBt on mitochondrial mass was investigated using MitoTracker. MitoTracker is a fluorescent dye which gets accumulated in active mitochondria in live cells and it is dependent upon mitochondrial membrane potential (Chazotte, 2011). NaBt (1-5 mM) caused 15-27% reduction (P<0.001) in mitochondrial mass after 12 h of treatment in CRC cells (Fig. 4C and D). A further decrease (25-47%, P<0.001, in SW480; 22-35%, P<0.001, in HCT-116) in mitochondrial mass was observed after 24 h of NaBt treatment (Fig. 4C and D). These results indicated that NaBt can cause the lysis or fusion of mitochondria accompanied with the reduction of total surface area.
Mitochondria-mediated ROS generation was determined by MitoSOX which specifically detects mitochondrial superoxides. Superoxide is also an indirect marker of hypoxia (Gao and Wolin, 2008; Vanden Hoek et al., 1998). NaBt (2.5 mM) treatment for 24 h showed 5 fold (P<0.001) increase in mitochondrial superoxide/ROS production in SW480 cells, whereas this increase was 2 fold (P<0.001) in HCT-116 cells (Fig. 4E). These observations indicated for potential mitochondrial fusion by NaBt treatment.
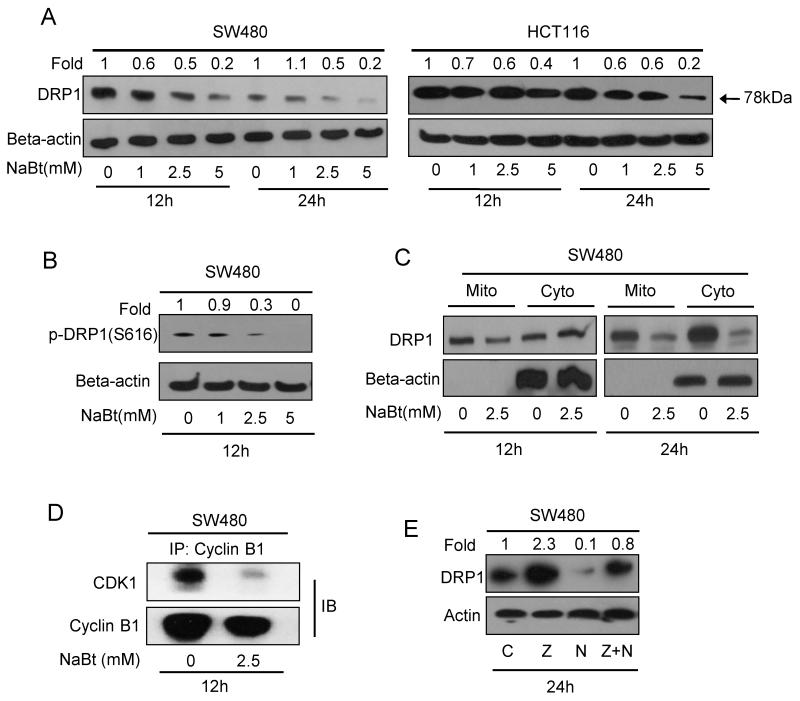
3.7 NaBt caused DRP1-mediated mitochondrial fusion in human CRC cells
DRP1 is the key player in regulation of mitochondrial fission and fusion during cell cycle progression (Yamano and Youle, 2011). To examine the effect of NaBt on mitochondrial fusion, first we assessed the protein level of DRP1 in both the CRC cells. NaBt (1-5 mM) treatment for 12 and 24 h showed both time- and dose-dependent decrease in DRP1 in CRC cells (Fig. 5A). Next, using SW480 cell lysates, we observed that NaBt strongly decreased the phosphorylation of DRP1 at serine 616 position (Fig. 5B), which is required for its translocation into the mitochondrial membrane. Further, the localization of DRP1 was assessed in mitochondrial and cytosolic fractions of SW480 cells following 2.5 mM NaBt treatment for 12 and 24 h. At early time point, the decrease in DRP1 localization was noticed only in mitochondrial fraction which later became predominant in both mitochondrial as well as cytosolic fractions (Fig. 5C).
Figure 5.
Effect of NaBt on DRP1 expression and its localization in human CRC cells. (A) SW480 and HCT116 cells were treated with 1, 2.5 and 5 mM NaBt for 12 and 24 h, and cell lysates were analyzed by SDS-PAGE and western blot analysis for DRP1 levels. Membranes were stripped and reprobed with anti-beta-actin. (B) Cell lysate from SW480 cells treated with 1, 2.5 and 5 mM NaBt for 12 h was analyzed for phospho-DRP1(serine 616) using specific antibody through western blotting. (C) Mitochondrial and cytosolic fractions for control and 2.5 mM NaBt treated SW480 cells for 12 and 24 h were prepared as described in ‘Materials and Methods’. SDS-PAGE and western blot analysis were performed for DRP1 and membrane was stripped and reprobed with anti-beta-actin. (D) Effect of NaBt treatment on cyclin B1-CDK1 complex was analyzed in SW480 cells by co-immunoprecipitation with anti-cyclin- B1 followed by immunoblotting for CDK1 and cyclin B1 as described in ‘Materials and Methods’. (E) Effect of caspase inhibitor and NaBt caused decrease in DRP1 in SW480 cells was analyzed by immunoblotting. Cells were treated as described in Fig. 4A and membrane was probed with anti-DRP1 and anti-beta-actin. Numbers on top of the bands represent fold changes in band intensity as compared to control as determined by densitometric analysis of the bands and corrected for beta-actin (A-C, E).
Cyclin B1-CDK1 complex is associated with the activating phosphorylation of DRP1 (pDRP1-ser616) during mitotic division (Yamano and Youle, 2011), which was examined by co-immunoprecipitation and looking at the complex formation of cyclin B1 with CDK1, known for its kinase activity. NaBt reduced the level of cyclin B1-CDK1 complex formation (Fig. 5D), and thereby it can inhibit DRP1 activation and its mitochondrial localization. In another experiment, we examined the level of DRP1 in caspase inhibiting condition. Pan-caspase inhibitor treatment showed an increase in DRP1 level and it also reversed NaBt-caused decrease in DRP1 level (Fig. 5E). This observation suggests for a novel mechanism of DRP1 regulation in caspase-dependent manner.
4. Discussion
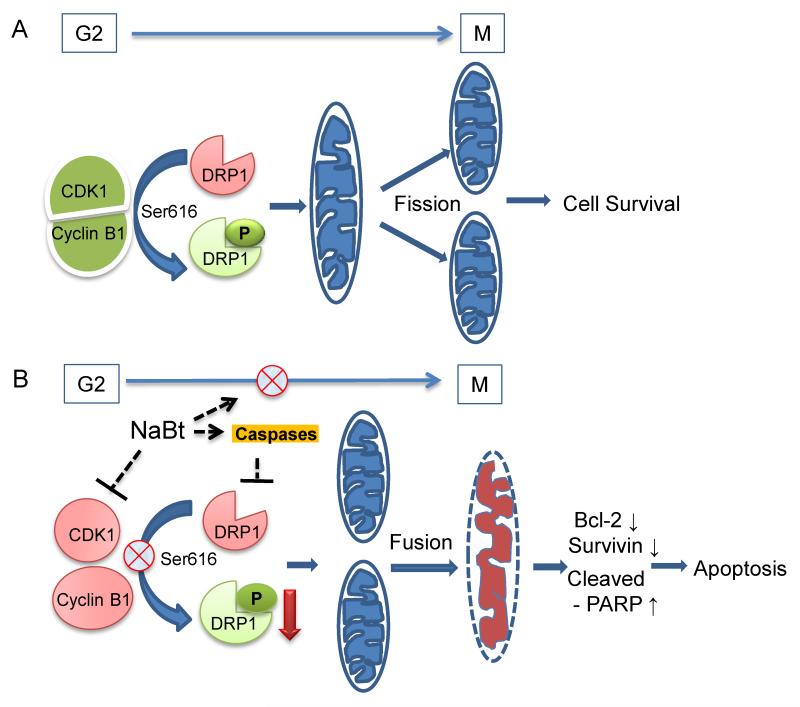
Mitochondrial dynamics, which is regulated by fission and fusion, is a key event during mitotic division for segregation of equal number of mitochondria in each daughter cell. This is regulated by fusion to fission ratio and strongly associated with normal embryonic development to neurodegenerative disorders (Detmer and Chan, 2007). Mitochondrial fission depends upon cyclin B1-CDK1 mediated phosphorylation of DRP1 at S616 and its localization on outer mitochondrial membrane (Taguchi et al., 2007).
Butyric acid is mostly produced by anaerobic fermentation of dietary fibers by microbial flora in GI tract. It plays an important role in normal homeostasis maintenance of epithelial lining of colon and intestine (Vanhoutvin et al., 2009). Butyrate is the primary source of energy for the colonocytes, however, its absence causes apoptosis, but when these colonocytes get transformed into cancer cells, the presence of butyrate can cause cell death in these cancer cells (Cuff et al., 2005; Pajak et al., 2007). Currently, it is poorly understood, especially with respect to mitochondria, how butyrate induces apoptosis in cancer cells of GI tract. In present study, we explored the relationship of mitochondrial dynamics and apoptosis after butyric acid exposure to human CRC cells.
SW480 and HCT116 human CRC cell lines, representing early and advanced stages of the cancer, respectively, were used in the study. Treatment with NaBt upto 5 mM concentration showed reduction in cell viability starting as early as from 6 h in a time- and dose-dependent manner and showed ~90% decrease in cell survival by 24 h. This observation was consistent with earlier reported effects of NaBt on cancer cells (Wang et al., 2009). Survivin is a small molecular weight antiapoptotic protein which is overexpressed in cancer cells, and known to provide surviving capability by inhibiting the activation of apoptotic machinery (Altieri et al., 2008). Our studies showed that NaBt decreases both mRNA as well protein levels of survivin supporting the assumption that decrease in CRC cell survival by NaBt could be mediated via apoptosis in our culture conditions.
Usually, induction of apoptosis is linked with cell cycle arrest which is also a means to provide cells enough time to adjust with the external environmental as well as intracellular changes in the cells. Thus, cell cycle check-points play the important role in cell survival and proliferation. Interestingly, NaBt treatment to CRC cells first showed an increase in G2-M phase cell population which later substituted by an increase in sub-G0/G1 phase cell population. The increase in sub-G0/G1 is an indication of cells going under cell death involving apoptosis. The cyclin B1 and CDK1 are key players of G2-M transition in which CDK1 gets activated a phosphatase CDC25C (Sanchez et al., 1997). In our study, NaBt caused G2-M phase arrest was accompanied by the decrease in protein levels of cyclin B1, CDK1 as well as CDC25C providing an evidence of molecular changes associated with the biological effect.
We further explored the extent of apoptotic cells and associated molecular changes following NaBt treatment. As expected, dose- and time-dependent increases in number of apoptotic cells were observed. The increase in apoptosis was accompanied by an increase in PARP and caspase-3 cleavage and down-regulation of Bcl-2 expression. The Bcl-2 is anti-apoptotic mitochondrial protein and known for its survival response whereas the cleavage of PARP at Asp214 that helps in cellular disassembly is an indicator of apoptosis (Oliver et al., 1998). The role of caspase pathway in apoptosis induced by NaBt treatment was investigated by using pan caspase inhibitor, z-VAD-FMK that irreversibly binds to the catalytic site of caspase proteases and inhibits apoptosis. The results of this study suggested that decrease in CRC cells survival is mostly mediated via caspase-mediated apoptosis which was validated by measuring the level of cleaved caspase 3. Together, the cell cycle arrest at G2-M phase and caspase mediated apoptosis by NaBt indicated for the role of mitochondria in cell death.
G2-M phase cell cycle transition is also associated with mitochondrial division which can be assessed by the active mitochondrial mass in live cells (Margineantu et al., 2002). The result of MitoTracker experiment showed the decrease in overall population/mass of active mitochondria in CRC cells and that could also be an indicator of mitochondrial fusion. Decrease in active mitochondrial mass is associated with fusion of mitochondria because of overall surface area of mitochondria gets decreased. In neuronal disorders when mitochondrial division gets blocked, it leads to elongation of organelles due to imbalanced and excess fusion (Kageyama et al., 2012). Accumulation of these elongated tubules leads to oxidative damage, which causes swelling of mitochondria accompanied with instability in the electron transport chain leading to a decrease in respiratory competence. Eventually, this decline in respiration causes neuronal cell death (Kageyama et al., 2012). Consistent with this, our MitoSOX data showed an increase in mitochondrial superoxide production confirming the mitochondria-mediated ROS generation which could be leading to oxidative damage and cell death.
The NaBt-induced mitochondrial fusion was further investigated by DRP1 level which is key regulator of mitochondrial fission and fusion process. Its higher level and specifically in mitochondrial membrane favors mitochondrial fission or otherwise its lower level promotes mitochondrial fusion (Youle and van der Bliek, 2012). NaBt down-regulated the level of DRP1 in both the CRC cell lines. Further, we also observed that it strongly reduces the localization of DRP1to the mitochondrial membrane. Further, it also decreased the complex formation of cyclin B1 with CDK1, the active complex of which is required for the DRP1 Ser616 phosphorylation which is needed for its translocation to mitochondrial membrane (Taguchi et al., 2007). Together these findings suggest that DRP1 could be a potential molecular target to induce mitochondrial fusion mediated by NaBt treatment in CRC cells. Importantly, we observed that DRP1 protein level is also regulated in a caspase-dependent manner.
In summary, our findings suggest that NaBt-caused decrease in survival of CRC cells is associated with down-regulation of survivin and Bcl-2, and G2-M phase cell cycle arrest and apoptosis. Further, it is mediated by an increase in mitochondrial fusion owing to down-regulation of DRP1 as well as its mitochondrial translocation likely via a decrease in cyclin B1-CDK1 complex and an activation of caspase pathway. This study is a further advancement in the understanding of mechanisms, specifically the identification of a novel molecular target of NaBt, the DRP1, by which it can inhibit the survival of cancer cells.
Figure 6.
Proposed mechanisms for the role of DRP1 in mitochondrial fusion in response to NaBt treatment of CRC cells. (A) High expression of DRP1 during G2-M transition and its activation through cyclin B1-CDK1 complex ensures mitochondrial fission during cell division. (B) NaBt inhibits G2-M phase progression and formation of active cyclin B1-CDK1 complex and thereby inhibits the activation/phosphorylation of DRP1 and its translocation to mitochondrial membrane, on the other hand NaBt also decreased DRP1 level via inhibition of caspase activation. The combined effects for lowering the DRP1 level by NaBt would lead to mitochondrial fusion and subsequently respiratory distress and apoptosis in CRC cells.
Acknowledgments
The work was supported by the funds from UGC-Central University of Gujarat. Authors acknowledge University Grant Commotion (UGC), India, Central University of Gujarat, India and National Cancer Institute (NIH), USA Grant No. CA101753 for fellowship to DT.
Abbreviations
- NaBt
sodium butyrate
- CDK
cyclin-dependent kinase
- DRP1
dynamin-related protein 1
- z-VAD-FMK
pan-caspase inhibitor
- ROS
reactive oxygen species
Footnotes
No conflict to disclose.
References
- Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- Chazotte B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb Protoc. 2011;2011:990–992. doi: 10.1101/pdb.prot5648. [DOI] [PubMed] [Google Scholar]
- Chen CH, Howng SL, Hwang SL, Chou CK, Liao CH, Hong YR. Differential expression of four human dynamin-like protein variants in brain tumors. DNA Cell Biol. 2000;19:189–194. doi: 10.1089/104454900314573. [DOI] [PubMed] [Google Scholar]
- Cuff M, Dyer J, Jones M, Shirazi-Beechey S. The human colonic monocarboxylate transporter Isoform 1: its potential importance to colonic tissue homeostasis. Gastroenterology. 2005;128:676–686. doi: 10.1053/j.gastro.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Gao Q, Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2008;295:H978–H989. doi: 10.1152/ajpheart.00316.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ER, Singh SV. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol Cancer Ther. 2007;6:2686–2695. doi: 10.1158/1535-7163.MCT-07-0217. [DOI] [PubMed] [Google Scholar]
- Hahm ER, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N, Kensler TW, Reddy PH, Iijima M, Sesaki H. Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol. 2012;197:535–551. doi: 10.1083/jcb.201110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G. Mitochondria in cancer. Oncogene. 2006;25:4630–4632. doi: 10.1038/sj.onc.1209589. [DOI] [PubMed] [Google Scholar]
- Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–1998. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margineantu DH, Gregory Cox W, Sundell L, Sherwood SW, Beechem JM, Capaldi RA. Cell cycle dependent morphology changes and associated mitochondrial DNA redistribution in mitochondria of human cell lines. Mitochondrion. 2002;1:425–435. doi: 10.1016/s1567-7249(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar D, Prajapati V, Agarwal R, Singh RP. In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- Pajak B, Orzechowski A, Gajkowska B. Molecular basis of sodium butyrate-dependent proapoptotic activity in cancer cells. Adv Med Sci. 2007;52:83–88. [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzaro C, Coradini D, Daidone MG. Modulation of angiogenesis-related proteins synthesis by sodium butyrate in colon cancer cell line HT29. Carcinogenesis. 2002;23:735–740. doi: 10.1093/carcin/23.5.735. [DOI] [PubMed] [Google Scholar]
- Qian W, Choi S, Gibson GA, Watkins SC, Bakkenist CJ, Van Houten B. Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J Cell Sci. 2012;125:5745–5757. doi: 10.1242/jcs.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Shin HW, Shinotsuka C, Torii S, Murakami K, Nakayama K. Identification and subcellular localization of a novel mammalian dynamin-related protein homologous to yeast Vps1p and Dnm1p. J Biochem. 1997;122:525–530. doi: 10.1093/oxfordjournals.jbchem.a021784. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal C, Agarwal R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis. 2003;24:555–563. doi: 10.1093/carcin/24.3.555. [DOI] [PubMed] [Google Scholar]
- Stan SD, Singh SV. Transcriptional repression and inhibition of nuclear translocation of androgen receptor by diallyl trisulfide in human prostate cancer cells. Clin Cancer Res. 2009;15:4895–4903. doi: 10.1158/1078-0432.CCR-09-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60(Suppl 1):51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One. 2009;4:e6759. doi: 10.1371/journal.pone.0006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luo HS, Xia H. Sodium butyrate induces human colon carcinoma HT-29 cell apoptosis through a mitochondrial pathway. J Int Med Res. 2009;37:803–811. doi: 10.1177/147323000903700323. [DOI] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- Yamano K, Youle RJ. Coupling mitochondrial and cell division. Nat Cell Biol. 2011;13:1026–1027. doi: 10.1038/ncb2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]