Abstract
Tumor cells shed gangliosides and populate their microenvironment with these biologically active membrane glycosphingolipids. In vitro, ganglioside enrichment amplifies receptor tyrosine kinase signaling and activation of vascular endothelial cells. However, a long-standing question is whether in the actual microenvironment of a neoplasm, in vivo, tumor cell ganglioside shedding stimulates angiogenesis. Here we tested the hypothesis that tumor gangliosides have a critical proangiogenic role in vivo using novel murine tumor cells (DKO) genetically completely incapable of ganglioside synthesis and impaired in tumor growth vs. wild-type (WT) ganglioside-rich cells. We studied angiogenesis during tumor formation by these ganglioside-depleted cells, quantifying vessel formation, angiogenic factor production/release, and consequences of reconstitution with purified WT gangliosides. DKO cells formed virtually avascular tumors, much smaller than ganglioside-rich WT tumors and displaying a striking paucity of blood vessels, despite levels of VEGF and other angiogenic factors that were similar to those of WT cells. Transient enrichment of the ganglioside milieu of the DKO cell inoculum by adding purified WT gangliosides partially restored angiogenesis and tumor growth. We conclude that tumor gangliosides trigger robust angiogenesis important for tumor growth. Our findings suggest strategies to eliminate their synthesis and shedding by tumor cells should be pursued.
Keywords: gangliosides, shedding, tumor angiogenesis, vascular endothelial cells
INTRODUCTION
Tumor angiogenesis is recognized as a process that is critical to tumor growth[1–3]. Yet, fully effective approaches to control angiogenesis and thereby ultimate tumor progression are still elusive. For example, counteracting VEGF and its signaling as a treatment to interfere with tumor growth by inhibiting angiogenesis has met only with partial success[4–6]. With better understanding of the dynamics of tumor angiogenesis, it is increasingly recognized that complex interactions between the tumor cell and the host play an important role[7–10]. To an important degree, soluble factors especially those released by the tumor cell into the tumor microenvironment (TME), can shape these interactions[7, 11, 12].
Tumor cell gangliosides, amphiphatic cell surface glycosphingolipids released into the extracellular milieu in strikingly high quantities by shedding specifically from tumor cell membranes, are one such factor. Shedding rates of up to 50% of total tumor cell gangliosides, or 12 nmol/108 cells/24 hours, have been measured in vitro[13]. Directly as a result of this shedding process, gangliosides can interact with and bind to normal stromal cells present in the TME[14]. Consequent modification of normal cell function, including enhancement of growth factor binding to and activation of stromal fibroblasts and vascular endothelial cells, has been extensively shown in vitro[15–19]. In vivo, manipulation of the ganglioside content of the TME has shown that most gangliosides [except possibly GM3[20–22]] positively influence tumor growth. As examples, tumor growth was enhanced by addition of purified tumor gangliosides to a tumor cell inoculum[23], and conversely, tumor growth was suppressed by transient inhibition of ganglioside synthesis by pharmacological blockade of glucosylceramide synthase[24], or by the administration of ganglioside-specific IgG antibodies that can bind and neutralize shed gangliosides in the TME[25]. Together with findings of augmentation of stromal cell function by addition of complex exogenous gangliosides in assays linked to angiogenesis, such as VEGF-induced human vascular endothelial cell (HUVEC) activation, proliferation, migration, and tube formation[18, 20, 26–28], the question arises as to whether tumor gangliosides, i.e., those gangliosides actually synthesized by the tumor cells in vivo and shed into the TME, impact tumor angiogenesis, and whether they do so in such a way as to enhance tumor growth.
It is now possible to answer this question directly. To do this, we used a recently developed novel, genetically determined, tumor model[29] in which specific and complete ganglioside deficiency resulted from knockout of activities of two key ganglioside synthetic genes, Siat9 (encoding GM3 synthase) and Galgt1 (encoding GM2 synthase). Embryonic fibroblasts (MEF) from these double knockout mice and littermate wild type mice were stably transformed with c-myc and H-Ras. This for the first time generated tumor cells in which gangliosides were constitutively completely depleted and therefore unable to condition the TME. Tumor growth of the resulting ganglioside-deficient knockout (DKO) tumor cells was reduced compared to that of the ganglioside-rich wild type (WT) tumor cells[29], despite their identical cell proliferation kinetics in vitro. This model provided the basis for directly testing the hypothesis that ganglioside synthesis and shedding impacts tumor angiogenesis.
Our results show that angiogenesis, robust in WT tumors, was markedly impeded in the ganglioside-poor DKO tumors, and that this was not attributable to a difference between the WT and DKO tumor cells in VEGF (or other angiogenic factor) production. Together with substantial restoration of angiogenesis and an increase in tumor growth caused by addition of purified WT tumor cell gangliosides to the DKO cell inoculum, the findings directly implicate shed tumor gangliosides in modifying normal cell responses involved in tumor angiogenesis. Inhibition of human tumor ganglioside synthesis could be a novel therapeutic target for human cancer.
MATERIALS AND METHODS
Materials and cell culture
6 week old C57B6/L mice were obtained from Jackson Laboratory (Bar Harbor, Maine). CD34 rat IgG2a was from Biolegend (San Diego, CA), CD31 rat IgG2a and the DAB substrate kit were from BD Pharmingen (San Jose, CA). The murine VEGF ELISA kit was from R&D Systems, Minneapolis, MN. c-Myc/h-Ras oncogene-transformed GM3S/GM2S double knockout (DKO) and oncogene transformed control (WT) murine embryonic fibroblasts [29] were cultured in DMEM with 4.5g/L glucose (Lonza, Walkersville, MD) containing 10% FCS, 2mM L-glutamine, and 1% non-essential amino acids (NEAA).
Tumors
105–106 oncogene-transformed WT or DKO cells were injected s.c. to groups of 4–6 normal syngeneic c57Bl/6 female mice. Tumor growth was monitored 3x/week and tumor volumes calculated according to the formula: (HxWxL)/2. Mice were cared for according to approved IACUC protocol 61–96–12. Results shown are representative of 3–5 separate experiments.
Immunohistochemistry
10µm cryosections of OGT-embedded tumor tissues were fixed, H2O2 treated, blocked with donkey serum for 30 min and incubated at 4°C overnight with primary anti-CD31 and anti-CD34 antibodies at 1:50 dilution followed by HRP conjugated second antibody IgG at 1:100 dilution[30]. In parallel, 5µm sections of the same tumors, formalin-fixed, were stained by H&E.
Vessel quantification
Vessels were quantified in the most vascular areas, i.e., “hot spots” [31], as identified by scanning tumor sections at low power (x40) with AxioVision 4 software. The number of fully formed large tumor vessels (≥100 µm length) seen on H&E stained slides or identified by CD31 staining in 5–8 hot spots/tumor, three tumors/group, at 200× magnification (0.22mm2 area fields), were counted and the vessel density determined.
Ganglioside purification
Total tumor cell gangliosides were purified as previously described [32]. Briefly, gangliosides in the chloroform:methanol total lipid extracts of WT and DKO cells were partitioned in aqueous DIPE/butanol, further purified by Sephadex G-50 gel filtration, characterized by HPTLC, compared to standard gangliosides, and quantified by scanning densitometry.
VEGF and other angiogenic factor detection
5×104 DKO or WT cells were cultured in 3ml complete medium in 6-well culture plates. After 24 hours the medium was replaced with fresh medium, and 16 hours later the cells and culture supernatants were collected, and the cells were counted. VEGF content and release by DKO and WT cells and tumors were quantified by ELISA. Results were expressed as pg VEGF/106 cells, or the supernatant of 106 cells[33]. Angiogenesis factors of DKO and WT cells and culture supernatants were also screened by Western blotting using the TranSignalTM Mouse Angiogenesis Antibody Array (Panomics, Redwood, CA).
Statistical Analysis
The statistical significance of differences between groups was assessed by Student’s t-test, two-tailed. A p value of less than 0.05 was considered statistically significant. All bars shown in the figures and data in the tables are presented as the mean ± SD.
RESULTS
Tumor ganglioside depletion and tumor formation
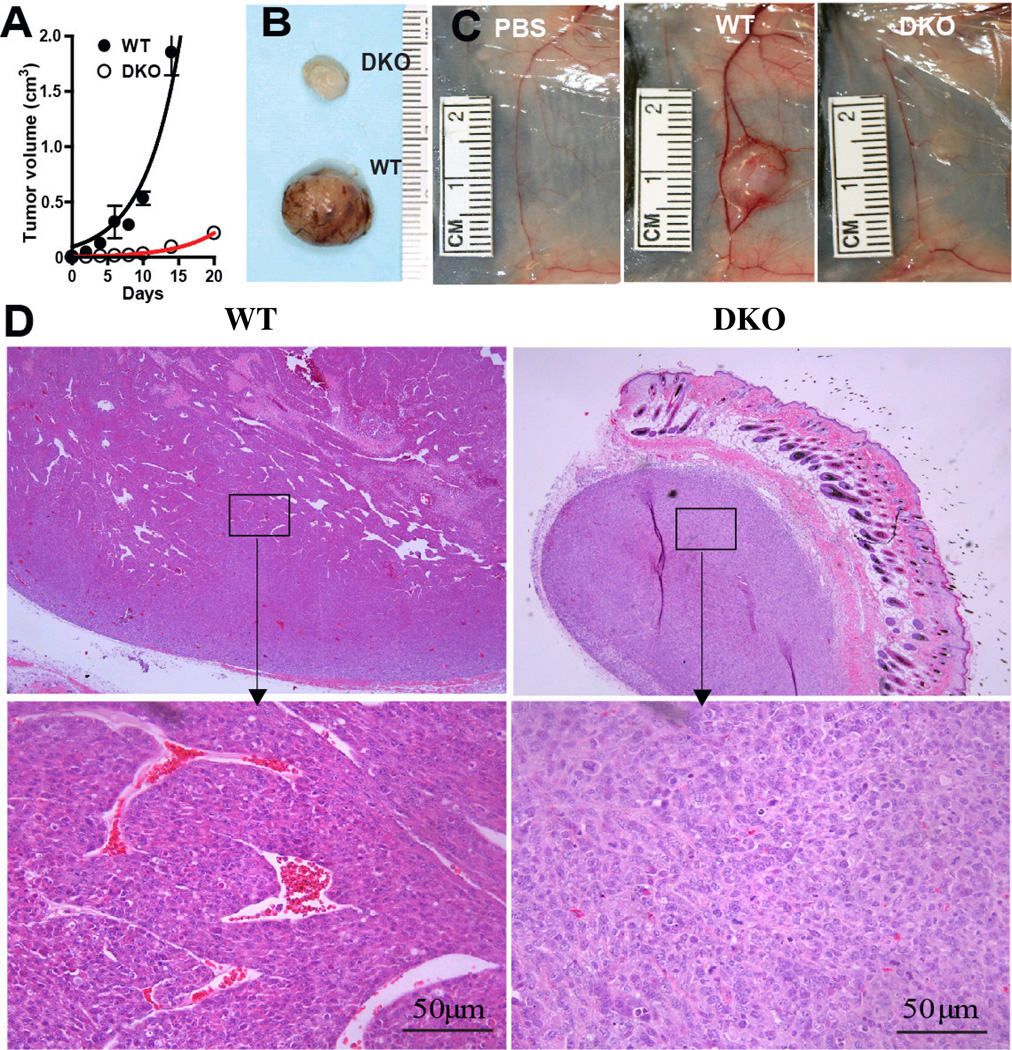
Initial experiments calibrated the influence of tumor gangliosides on tumor formation, by assessing that of ganglioside-poor DKO cells and ganglioside-rich (control) WT cells, to confirm our original findings in the tumor model[29]. In the representative experiment shown (Fig. 1A), tumors resulting from s.c. injection of 106 WT cells in syngeneic normal C57Bl/6 mice grew rapidly, reaching a mean volume of >1000 mm3 in less than 14 days. The ganglioside-poor DKO tumors, in contrast, had a tumor volume of only 93±15 mm3 by 14 days (p=0.0001) (Fig. 1A).
Figure 1. Ganglioside depletion impedes tumor vascularization.
Panel A: Tumor growth (5 mice/group), representative of three separate experiments. Panel B: Visual appearance 10 days after s.c. injection of 10 6 WT or DKO cells. Panel C: Vasculature of the tumor site day 14 after injection (from left to right) of PBS (vehicle control), 105 WT, or 105 DKO cells. Panel D: Tumor histopathology (H&E stain), 10 days after injection of 106 cells. Upper: 50× magnification; lower: 400× magnification; left: WT tumor; right: DKO tumor.
By explanting and culturing WT and DKO cells after in vivo passage we also established that during in vivo growth, the original ganglioside profile of the WT cells was maintained and importantly, explanted DKO tumor cells remained completely ganglioside-depleted (not shown). Together, these results confirm that in the absence of active ganglioside synthesis and shedding by the tumor cells, tumor growth is significantly impeded.
Tumor ganglioside depletion and angiogenesis
Examination of the DKO tumors revealed a striking visual appearance (compared to WT tumors) that accompanied their impeded growth: The surface of the DKO tumors was almost avascular, appearing pearly white in comparison to well-vascularized, red, WT tumors (Fig. 1B). Dissection of the tumor site on day 14 after injection of 105 cells uncovered a poorly supplied DKO tumor bed in contrast to the well supplied WT tumor bed (Fig. 1C). The major blood vessel serving the DKO tumor (Fig. 1C right) was less prominent than the one supplying the WT tumor (Fig. 1C, middle) and similar in size to the same vessel in the tumor-free control mouse (Fig. 1C, left), clearly demonstrating robust angiogenesis in the ganglioside-rich WT but not in the ganglioside-poor DKO tumor sites.
Assessment of the intra-tumor vascularity confirmed and expanded this novel finding. Low power views of WT tumors showed many large vessels and at higher power, well-formed vessels with patent lumens (containing clearly visible erythrocytes) were seen in abundance in the WT tumors (Fig. 1D). DKO tumors, in contrast, exhibited few if any well-formed vessels. Only punctuate collections of a few cells were seen at higher power (Fig. 1D).
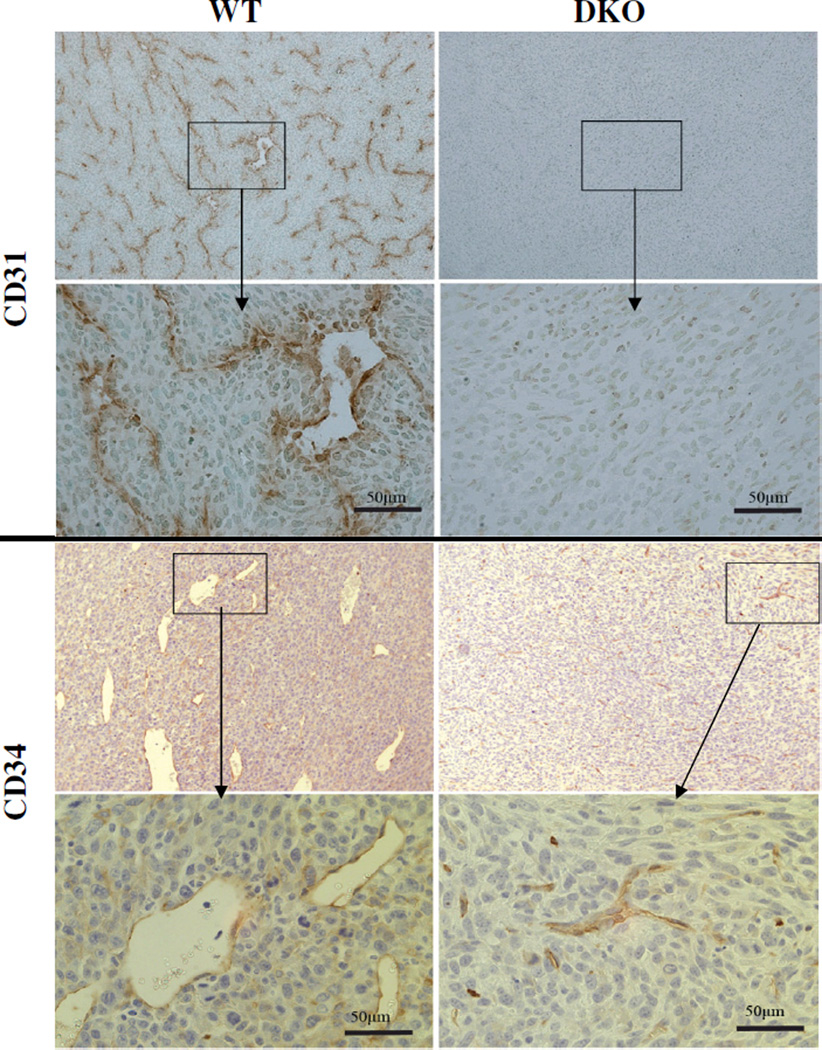
Imunohistochemical staining for the angiogenesis markers CD31 and CD34 underscored the differences in vasculature. WT tumors stained for CD31 exhibited well-formed vessels while DKO tumors had almost none (Fig. 2, upper panels). Likewise, CD34 staining[30, 34] showed that the WT tumors had many well-formed new vessels whereas DKO tumors had many fewer, mostly undeveloped, vessels as well as punctate staining possibly representing nascent vessels (Fig. 2, lower panels).
Figure 2. Immunohistochemical detection of blood vessels of WT and DKO tumors.
Frozen sections of tumors harvested 10 days after s.c. injection of 105 WT or DKO cells were stained for CD31 or CD34. Left panels: WT tumors; right panels: DKO tumors. Magnification: 50× and 400×.
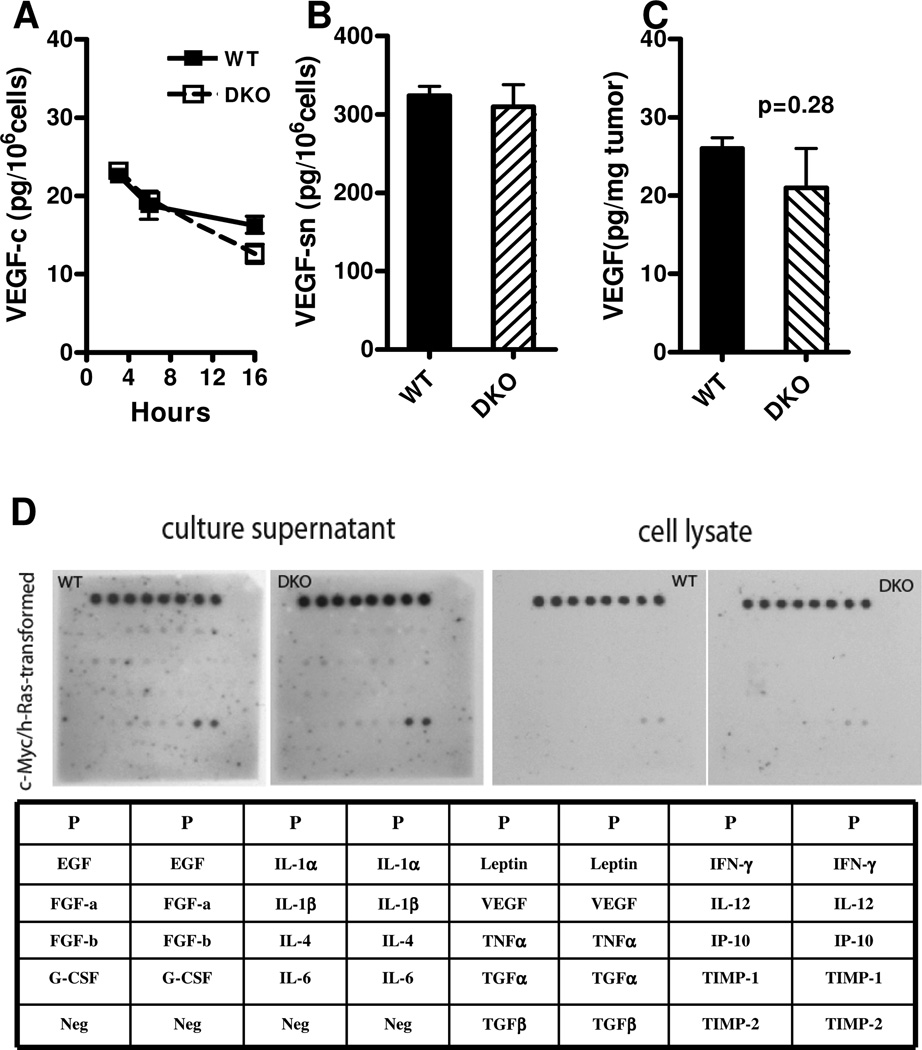
Finally, quantitative evaluation of vascularity two weeks after tumor cell injection corroborated these observations. The many large vessels visible upon H&E staining in WT tumors were in contrast to the very few in DKO tumors (10±3.6 vs. 0.4±0.6 vessels/0.22mm2 field, p<0.0001). Similarly, as detected by immunostaining, DKO tumors displayed a striking paucity of CD31+ tumor blood vessels (0.1±0.2 vs. 8.9±3.2 vessels/0.22mm2, p<0.0001). Importantly, these differences in angiogenesis occurred in the absence of any significant difference in either VEGF production (Fig. 3A) or release (Fig. 3B) between the two cell types, or in VEGF content of the tumors (Fig. 3C), or in the cellular expression of a series of other angiogeneic factors (Fig. 3D).
Figure 3. VEGF and other proangiogenic factors of WT and DKO cells.
Neither the VEGF content in cell lysates (panel A; WT vs. DKO at 16 hours: 16.2±1.9 vs. 12.6±2.2 pg/106 cells, p=0.1) nor in the corresponding 16-hour cell culture supernatants (panel B; WT vs. DKO, 324±23 vs. 310±51 pg released/106 cells, p=0.7) were significantly different. Panel C: Tumor VEGF content. Small WT (day 9, mean 87mm3, n=3) and DKO (day 14, 84mm3, n=3) tumors following injection of 105 cells were studied. VEGF was assayed by ELISA. The tumor VEGF contents were not significantly different (26±1.4 vs. 21±5 pg/mg tumor, p=0.3). Panel D: Angiogenesis factor screen of DKO and WT cells and culture supernatants by Western blotting. The table is the key for the western blot of angiogenesis factors, among which there were no differences in concentrations between DKO and WT cells or supernatants.
Exogenous addition of WT gangliosides enhances growth and angiogenesis of DKO tumors
This clear link between ganglioside deficiency, impeded angiogenesis, and tumor growth prompted us to evaluate the effect of reconstitution of the ganglioside-poor DKO tumor cell inoculum with the “missing” (WT) gangliosides. Total WT tumor cell gangliosides consist of 9% GM3, 29% GM2, 16% GM1, and 46% GD1a (not shown). While recognizing that adding gangliosides to the DKO tumor cell inoculum would only transiently enrich the ganglioside content in the tumor microenvironment, we asked whether at least temporarily providing tumor gangliosides to the TME during the early stages of ganglioside-deficient DKO tumor development would result in some restoration of angiogenesis and possibly enhancement of tumor growth.
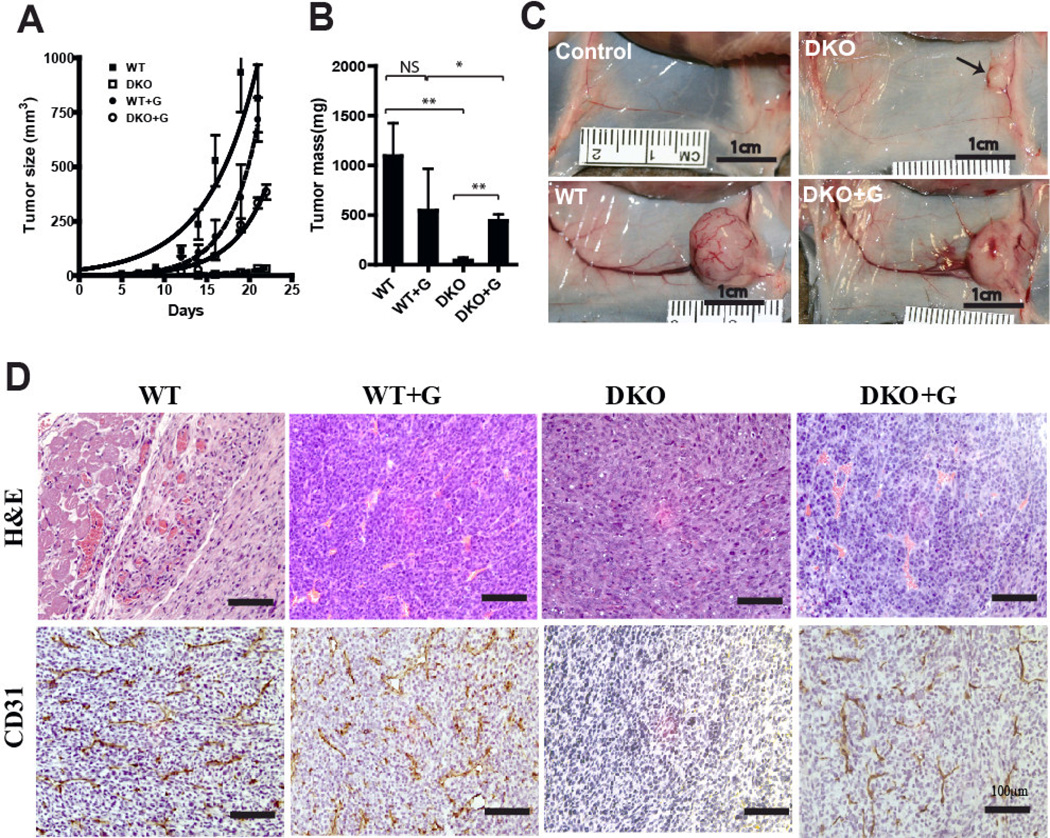
We injected 100 pmol of purified WT cell gangliosides together with an inoculum of 105 WT or DKO tumor cells. This transient enrichment of the ganglioside milieu by the WT gangliosides significantly increased DKO tumor volume (Fig. 4A) and tumor mass (Fig. 4B): Ganglioside enrichment of the DKO cell inoculum increased the volume of DKO tumors on day 19 (Fig. 4A) from 10±9 to 278±69 mm3 (p<0.0001). In contrast, identical ganglioside enrichment of the (already ganglioside replete) WT tumor cell inoculum did not enhance growth of WT tumors (Fig 4A).
Figure 4. Effect of purified exogenous WT tumor gangliosides on tumor angiogenesis.
105 WT or DKO cells were co-injected s.c. with or without 100 pmol WT tumor gangliosides (G) into groups of five mice. Tumor growth curves (Panel A) and tumor weights at the day 21/22 endpoint (Panel B) are shown (key: NS: p>0.05; *: p=0.07; **: P≤0.0001). Panel C, tumor site vasculature (day 14): (control) PBS alone, (DKO) DKO tumor identified by the arrow, (WT) WT tumor, (DKO+G) DKO tumor cells co-injected with 100 pmol WT tumor gangliosides. Panel D: histopathology of equal size (small) WT and DKO tumors. Upper row, H&E staining; lower row, CD31 staining. Tumors were harvested on days 7–9 (WT), (WT+G), and (DKO+G), and day 14 (DKO). Magnification: 200×; bars represent 100µm.
Examination of the tumor bed (Fig. 4C) clearly captures the angiogenic effect of the added gangliosides on the tumor feeding circulation. The major vessels in the site of the ganglioside-enriched DKO (i.e., DKO+G) tumors were almost as robust as those in the WT tumor site, and much larger than those in the tumor bed of unenriched DKO cells, in turn not very different from those the tumor free (control) site.
Fig. 4D shows both by H&E staining and by CD31 immunostaining that vascularity in the WT tumors was not affected by enrichment with WT tumor gangliosides while WT tumor ganglioside addition to the DKO tumor cell inoculum clearly enhanced vessel formation. This was confirmed by quantifying tumor blood vessels of WT and DKO tumors that were of the same size (Table 1). Strikingly, the vessel number in DKO tumors was increased from 0.1±0.3 to 8.0±5.2 CD31+ vessels/0.22mm2 field, p<0.0001. Thus, while ganglioside enrichment of ganglioside-rich WT tumor cells did not alter the already robust angiogenesis of WT tumors, ganglioside enrichment of the DKO tumor microenvironment restored vessel density in the DKO tumors, implicating gangliosides specifically as causing the enhanced angiogenesis.
Table.
Effect on tumor angiogenesis of cell enrichment with WT tumor gangliosides
| Cells | WT | P | DKO | P | ||
|---|---|---|---|---|---|---|
| WT Gangliosides | − | + | − | + | ||
| Tumor vessels | ||||||
| H&E | 3.8±1.7 | 4.2 ±3.9 | N.S. | 0.4 ±0.6 | 3.6 ±2.8 | <0.0001 |
| CD31 | 12.9±2.7 | 12.8 ±3.1 | N.S. | 0.1 ±0.3 | 8.0 ±5.2 | <0.0001 |
105 WT or DKO cells were injected s.c. with or without 100 pmol WT tumor gangliosides, as in Fig. 3. Tumors were harvested and analyzed on days 7–9, except for DKO alone tumors (day 14). Vessels ≥100 µm long/0.22mm2 field were quantified (mean ± SD, 3 mice/group, 5–8 fields/tumor). N.S. = not significant. Other comparisons: WT vs. DKO (H&E or CD31), P<0.0001; WT vs. DKO + G (H&E), N.S; WT vs. DKO + G (CD31), p<0.01.
DISCUSSION
The critical roles of angiogenesis in supporting/facilitating tumor growth and progression[35] and of the TME in shaping this angiogenic response[7] stress the importance of identifying the factors, especially soluble factors derived from the tumor cell, that impact this microenvironment. Here we have discovered that tumor gangliosides that are released into the TME have potent activity affecting this critical component of tumor progression such that complete elimination of ganglioside synthesis and shedding by the tumor cell caused a dramatic reduction in angiogenesis in vivo. Unique characteristics of the model tumor system we used are that only ganglioside metabolism, and only ganglioside metabolism of the tumor cell (and not of the host) is blocked, accompanied by only minor (not statistically significant) increases of the precursor molecules glucosylceramide, lactosylceramide, and ceramide [29]. Thus gangliosides and specifically those emanating from the tumor itself are implicated in accelerating tumor angiogenesis in vivo. Supporting this conclusion is the finding that even brief ganglioside enrichment of the TME of ganglioside-poor DKO tumors increased angiogenesis and tumor growth. The striking effects in vivo were observed despite identical WT and DKO cell growth kinetics in vitro[29] and no difference in production or release of VEGF or expression of other angiogenic factors.
Because this tumor model is constitutive and causes blockade of ganglioside synthesis only by the tumor cell, it provides a novel and accurate view of a dynamic interaction between tumor cell gangliosides and the host that influences angiogenesis. What we found was that even at relatively early stages of tumor development, the ganglioside-replete small WT tumors showed robust vasculature, whereas the small ganglioside poor DKO tumors did not. Thus, the low density of tumor blood vessels in the DKO tumors is not explicable simply by small tumor size. Also, minor amounts of gangliosides or other molecules that could be adsorbed from the TME [36] by stromal or tumor cells, were, if present, clearly not sufficient to overcome the effect on angiogenesis caused by of lack of ganglioside synthesis and shedding by the tumor cells. Furthermore, the detection in DKO tumors of many small punctuate collections of CD31+ cells that may represent undeveloped vessels is of particular interest and suggests a pathophysiological mechanism of action of the tumor cell gangliosides. That is, the finding of such collections of cells may mean that the absence of tumor gangliosides in the TME in vivo holds back the further development of nascent blood vessels (in turn impeding growth of the tumor that is supported by them), and conversely that tumor cell gangliosides accelerate angiogenesis by stimulating the activation of these cells. This hypothesis that gangliosides may promote angiogenesis by an effect on vessel maturation, originally proposed by Guillino[26, 37], is further supported by our findings of the amplifying effect of ganglioside enrichment on VEGF-induced signaling of vascular endothelial cells[18], enabling them to respond to even trace, subthreshold concentrations of growth factors such as VEGF. In the clinical setting, by increasing vascular endothelial cell sensitivity to VEGF[18], the process of ganglioside shedding could also impair the therapeutic efficacy of specific pharmacological inhibitors targeting VEGF or its receptor.
The overall findings illuminate a novel explanation, at the cellular level, for how ganglioside synthesis and shedding by tumor cells acts to affect tumor angiogenesis: Tumor cell shedding will result in substantial concentrations of gangliosides into the TME. In the TME, these molecules can bind to the membranes of normal vascular endothelial cells critical for vessel formation. This ganglioside enrichment amplifies growth factor-induced cell signaling[15, 17, 18] even causing subthreshold (non-activating) concentrations of VEGF to become potent in promoting vascular endothelial cell signaling, activation, proliferation, and migration. [In vivo, this lowering of the threshold for stimulation by the presence of tumor gangliosides in the TME could even compensate for low concentrations of VEGF in the TME that would themselves be insufficient to stimulate angiogenesis.] Accelerated angiogenesis in comparison to that in the absence of tumor cell gangliosides is the end result of this process.
How does ganglioside structure influences angiogenic activity? This may be of interest because as a result of structural variations in both the carbohydrate and lipid (ceramide) portions of the molecule, gangliosides are a highly diverse family of many molecular species. And, in actuality, tumor cells do not synthesize and shed only a single ganglioside. Rather, they frequently express a multiplicity of ganglioside species. Structure-activity relationships, as established in various in vitro assays, indicate that both carbohydrate and ceramide structural details (e.g., number of sialic acids in the carbohydrate portion[38] and fatty acyl group chain length in the ceramide[39], respectively) may affect activity. Studies of the relative activity of individual gangliosides, exogenously added to in vitro and other assay systems related to angiogenesis, have shown that with the possible exception of GM3[21, 22], all gangliosides studied, including the GM1, GD1a, and GD3 found in WT tumor cells, enhance cellular binding of growth factors to normal stromal cells and the consequent cell responses (activation and function)[15, 17, 18, 20–22]. These results are embodied in the proposal of a model[26–28] in which the relative concentrations of the simple ganglioside GM3 vs. that of other, complex, gangliosides such as GD1a determine the effect on angiogenesis, with higher concentrations GM3 relative to that of the other gangliosides being associated with less angiogenesis.
The central clinically relevant issue, however, is probably not whether or how much one or another particular ganglioside, or a change in the ratio of individual gangliosides in the tumor cell to one another, modifies the degree of angiogenesis, but how would complete elimination of ganglioside synthesis and shedding by the tumor cell affect tumor angiogenesis. Our studies have been able to answer this question for the first time. The result, marked inhibition of angiogenesis, identifies a highly pro-angiogeneic effect of the composite (total) WT tumor gangliosides (GM3, GM1, GD1a, and GD3) in vivo. Therefore, in a potential clinical application of these findings, it is likely that the inhibition of tumor cell ganglioside synthesis or action as a cancer therapeutic intervention would be most effective in the adjuvant setting therapy early on, or in the circumstance of minimal residual disease.
In summary, we have directly demonstrated, for the first time in a well characterized and fully in vivo system (in which only the tumor cell has altered ganglioside synthesis) that the gangliosides that tumor cells synthesize and release have critical proangiogenic activity in vivo, and that this is associated with enhanced tumor growth. Arguably not the only factor influencing tumor growth and progression, the action of gangliosides nevertheless clearly accelerates the process of angiogenesis and of tumor formation. Thus, selectively abrogating the synthesis and shedding of tumor cell gangliosides into the TME in the clinical setting of human cancer (as was accomplished genetically in the DKO tumor model) could be an important achievement. This could be achieved by targeted delivery of an agent, such as a small molecule inhibitor, that selectively blocks the enzymatic activity GM3 synthase, since in human cells, as has been observed already in human fibroblasts[40], complete ablation of ganglioside synthesis and shedding can be accomplished by the inactivation of this single enzyme, GM3 synthase[40, 41]. The argument for developing such an approach is especially compelling because numerous types of human tumors shed gangliosides[13, 42–48].
ACKNOWLEDGEMENTS
The authors thank Yiwen Chen and Yi Zhang for assistance with these studies.
This work was supported by the National Cancer Institute at the National Institutes of Health (grant R01 CA61010).
REFERENCES
- 1.Folkman J. What is the evidence that tumors are angiogenesis dependent? Journal of the National Cancer Institute. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Roodink I, Leenders WP. Targeted therapies of cancer: Angiogenesis inhibition seems not enough. Cancer letters. 2010;299:1–10. doi: 10.1016/j.canlet.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shojaei F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer letters. 2012;320:130–137. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix MJ, Seftor EA, Kirschmann DA, Quaranta V, Seftor RE. Remodeling of the microenvironment by aggressive melanoma tumor cells. Annals of the New York Academy of Sciences. 2003;995:151–161. doi: 10.1111/j.1749-6632.2003.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Stolarska MA, Othmer HG. The role of the microenvironment in tumor growth and invasion. Prog Biophys Mol Biol. 2011;106:353–379. doi: 10.1016/j.pbiomolbio.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Liu J. Tumor stroma as targets for cancer therapy. Pharmacology & therapeutics. 2013;137:200–215. doi: 10.1016/j.pharmthera.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein LR, Liotta LA. Molecular mediators of interactions with extracellular matrix components in metastasis and angiogenesis. Current opinion in oncology. 1994;6:106–113. doi: 10.1097/00001622-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Ladisch S, Gillard B, Wong C, Ulsh L. Shedding and immunoregulatory activity of yac-1 lymphoma cell gangliosides. Cancer Res. 1983;43:3808–3813. [PubMed] [Google Scholar]
- 14.Olshefski R, Ladisch S. Intercellular transfer of shed tumor cell gangliosides. FEBS Lett. 1996;386:11–14. doi: 10.1016/0014-5793(96)00392-4. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Liu Y, Ladisch S. Enhancement of epidermal growth factor signaling and activation of src kinase by gangliosides. J Biol Chem. 2001;276:42782–42792. doi: 10.1074/jbc.M101481200. [DOI] [PubMed] [Google Scholar]
- 16.Hakomori Si SI. The glycosynapse. Proc Natl Acad Sci U S A. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Li R, Ladisch S. Exogenous ganglioside gd1a enhances epidermal growth factor receptor binding and dimerization. J Biol Chem. 2004;279:36481–36489. doi: 10.1074/jbc.M402880200. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, McCarthy J, Ladisch S. Membrane ganglioside enrichment lowers the threshold for vascular endothelial cell angiogenic signaling. Cancer Res. 2006;66:10408–10414. doi: 10.1158/0008-5472.CAN-06-1572. [DOI] [PubMed] [Google Scholar]
- 19.Regina Todeschini A, Hakomori SI. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta. 2008;1780:421–433. doi: 10.1016/j.bbagen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung TW, Kim SJ, Choi HJ, Kim KJ, Kim MJ, Kim SH, Lee HJ, Ko JH, Lee YC, Suzuki A, Kim CH. Ganglioside gm3 inhibits vegf/vegfr-2-mediated angiogenesis: Direct interaction of gm3 with vegfr-2. Glycobiology. 2009;19:229–239. doi: 10.1093/glycob/cwn114. [DOI] [PubMed] [Google Scholar]
- 21.Seyfried TN, Mukherjee P. Ganglioside gm3 is antiangiogenic in malignant brain cancer. J Oncol. 2010;2010:961243. doi: 10.1155/2010/961243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Isaji T, Satoh M, Li D, Arai Y, Gu J. Antitumor effects of exogenous ganglioside gm3 on bladder cancer in an orthotopic cancer model. Urology. 2013;81:210 e211–210 e215. doi: 10.1016/j.urology.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Ladisch S, Kitada S, Hays EF. Gangliosides shed by tumor cells enhance tumor formation in mice. J Clin Invest. 1987;79:1879–1882. doi: 10.1172/JCI113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng W, Li R, Ladisch S. Influence of cellular ganglioside depletion on tumor formation. Journal of the National Cancer Institute. 2000;92:912–917. doi: 10.1093/jnci/92.11.912. [DOI] [PubMed] [Google Scholar]
- 25.Katano M, Irie RF. Human monoclonal antibody to tumor-associated ganglioside gd2: Suppressed growth of human melanoma in nude mice. Immunology letters. 1984;8:169–174. doi: 10.1016/0165-2478(84)90072-5. [DOI] [PubMed] [Google Scholar]
- 26.Gullino PM. Prostaglandins and gangliosides of tumor microenvironment: Their role in angiogenesis. Acta Oncol. 1995;34:439–441. doi: 10.3109/02841869509094005. [DOI] [PubMed] [Google Scholar]
- 27.Alessandri G, Cornaglia-Ferraris P, Gullino PM. Angiogenic and angiostatic microenvironment in tumors--role of gangliosides. Acta Oncol. 1997;36:383–387. doi: 10.3109/02841869709001284. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee P, Faber AC, Shelton LM, Baek RC, Chiles TC, Seyfried TN. Thematic review series: Sphingolipids. Ganglioside gm3 suppresses the proangiogenic effects of vascular endothelial growth factor and ganglioside gd1a. J Lipid Res. 2008;49:929–938. doi: 10.1194/jlr.R800006-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Yan S, Wondimu A, Bob D, Weiss M, Sliwinski K, Villar J, Notario V, Sutherland M, Colberg-Poley AM, Ladisch S. Ganglioside synthase knockout in oncogenetransformed fibroblasts depletes gangliosides and impairs tumor growth. Oncogene. 2010;29:3297–3306. doi: 10.1038/onc.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garlanda C, Berthier R, Garin J, Stoppacciaro A, Ruco L, Vittet D, Gulino D, Matteucci C, Mantovani A, Vecchi A, Dejana E. Characterization of mec 14.7, a new monoclonal antibody recognizing mouse cd34: A useful reage for identifying and characterizing blood vessels and hematopoietic precursors. European journal of cell biology. 1997;73:368–377. [PubMed] [Google Scholar]
- 31.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 32.Ladisch S, Gillard B. A solvent partition method for microscale ganglioside purification. Analytical biochemistry. 1985;146:220–231. doi: 10.1016/0003-2697(85)90419-1. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho JF, Blank M, Shoenfeld Y. Vascular endothelial growth factor (vegf) in autoimmune diseases. J Clin Immunol. 2007;27:246–256. doi: 10.1007/s10875-007-9083-1. [DOI] [PubMed] [Google Scholar]
- 34.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circulation research. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Tumor angiogensis: Role in regulation of tumor growth. Symp Soc Dev Biol. 1974;30:43–52. [PubMed] [Google Scholar]
- 36.Ecsedy JA, Holthaus KA, Yohe HC, Seyfried TN. Expression of mouse sialic acid on gangliosides of a human glioma grown as a xenograft in scid mice. Journal of neurochemistry. 1999;73:254–259. doi: 10.1046/j.1471-4159.1999.0730254.x. [DOI] [PubMed] [Google Scholar]
- 37.Ziche M, Alessandri G, Gullino PM. Gangliosides promote the angiogenic response. Laboratory investigation; a journal of technical methods and pathology. 1989;61:629–634. [PubMed] [Google Scholar]
- 38.Ladisch S, Becker H, Ulsh L. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of t cell responses. Biochim Biophys Acta. 1992;1125:180–188. doi: 10.1016/0005-2760(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 39.Ladisch S, Li R, Olson E. Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proc Natl Acad Sci U S A. 1994;91:1974–1978. doi: 10.1073/pnas.91.5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Su Y, Wiznitzer M, Epifano O, Ladisch S. Ganglioside depletion and egf responses of human gm3 synthase-deficient fibroblasts. Glycobiology. 2008;18:593–601. doi: 10.1093/glycob/cwn039. [DOI] [PubMed] [Google Scholar]
- 41.Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, Reinkensmeier G, Wang H, Wiznitzer M, Gurtz K, Verganelaki A, Pryde A, Patton MA, Dwek RA, Butters TD, Platt FM, Crosby AH. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of gm3 synthase. Nature genetics. 2004;36:1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- 42.Ladisch S, Wu ZL. Detection of a tumour-associated ganglioside in plasma of patients with neuroblastoma. Lancet. 1985;1:136–138. doi: 10.1016/s0140-6736(85)91906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernhard H. Meyer zum Buschenfelde, K.H. Dippold, W.G. Ganglioside gd3 shedding by human malignant melanoma cells, International journal of cancer. Journal international du cancer. 1989;44:155–160. doi: 10.1002/ijc.2910440127. [DOI] [PubMed] [Google Scholar]
- 44.Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990;75:1564–1567. [PubMed] [Google Scholar]
- 45.Portoukalian J, David MJ, Gain P, Richard M. Shedding of gd2 ganglioside in patients with retinoblastoma. International journal of cancer. Journal international du cancer. 1993;53:948–951. doi: 10.1002/ijc.2910530614. [DOI] [PubMed] [Google Scholar]
- 46.Merritt WD, Der-Minassian V, Reaman GH. Increased gd3 ganglioside in plasma of children with t-cell acute lymphoblastic leukemia. Leukemia. 1994;8:816–822. [PubMed] [Google Scholar]
- 47.Chang F, Li R, Ladisch S. Shedding of gangliosides by human medulloblastoma cells. Experimental cell research. 1997;234:341–346. doi: 10.1006/excr.1997.3619. [DOI] [PubMed] [Google Scholar]
- 48.Uzzo RG, Clark PE, Rayman P, Bloom T, Rybicki L, Novick AC, Bukowski RM, Finke JH. Alterations in nfkappab activation in t lymphocytes of patients with renal cell carcinoma. Journal of the National Cancer Institute. 1999;91:718–721. doi: 10.1093/jnci/91.8.718. [DOI] [PubMed] [Google Scholar]