INTRODUCTION
Investigating the effects of antimicrobial products on microorganisms is a common procedure carried out in many microbiology laboratory courses, often using antibiotics or disinfectants against common bacterial species. As the issue of antimicrobial resistance continues to grow, there is a renewed interest in deriving antimicrobial products from natural compounds, particularly extracts from plant material (8). This article describes a procedure for retrieving the antimicrobial compounds (essential oils) from the plant thyme (Thymus vulgaris) and testing it against a variety of microorganisms, both Gram-negative and Gram-positive, for antimicrobial effect (4). The aim of this activity is to investigate the antimicrobial effects of plant material after extracting compounds using the relatively simple Soxhlet method (6). This article presents information ensuring the existing extraction method is achievable in the teaching laboratory, while allowing students hands-on investigative experience. As well as supporting scientific thinking, laboratory skill and competency, this activity allows students to partake in an investigation with cross-discipline approaches to the very relevant and current healthcare issue of antimicrobials.
PROCEDURE
Performing the Soxhlet method of ethanol extraction
Allowing the students to carry out this section of the investigation provides an added layer to what would be a standard microbiology assay. It takes the students from ‘start’ to ‘finish’ in terms of extracting and testing their own antimicrobial compounds. Students should set up and perform the extraction to gain first-hand experience of extraction methods.
Plant material can be fresh (for example, a plant leaf) or dried. It needs to be crushed, using a pestle and mortar, to provide a greater surface area. The plant material should be sufficient to fill the porous cellulose thimble (in our experiments we use an average of 14 g of thyme in a 25- x 80-mm thimble).
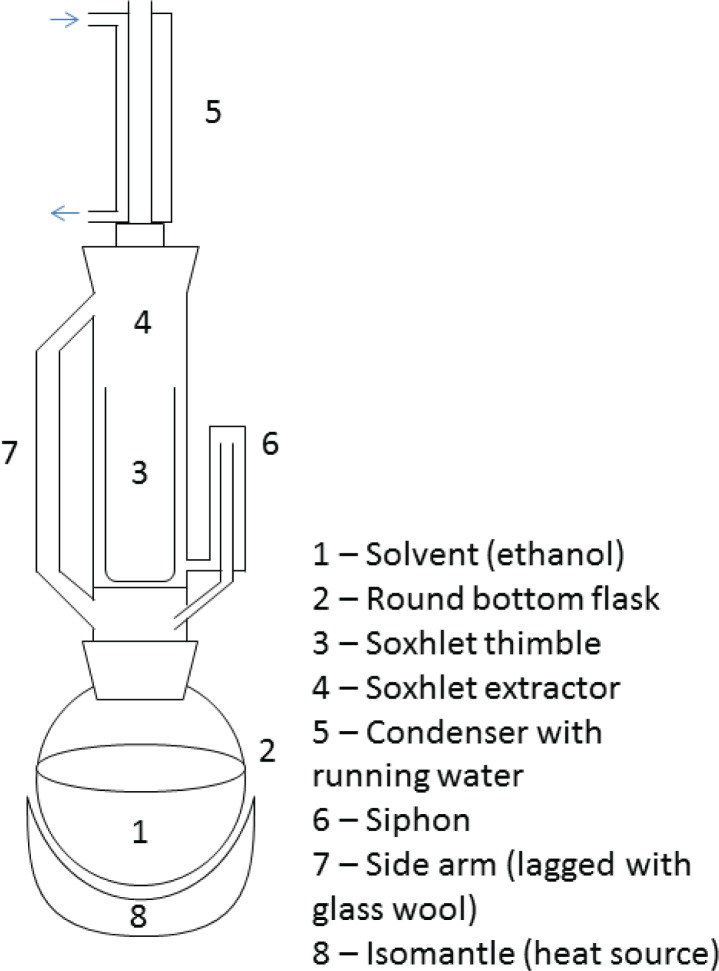
All equipment should be provided for students to assemble. Allowing students to build the extraction apparatus may give them a greater appreciation for the process of extraction, as opposed to testing an antimicrobial compound out of a purchased bottle. The students should begin by building a rig using stands and clamps to support the extraction apparatus. Following this, the solvent (250 ml of ethanol) is added to a round bottom flask, which is attached to a Soxhlet extractor and condenser (Fig. 1) on an isomantle. The crushed plant material is loaded into the thimble, which is placed inside the Soxhlet extractor. The side arm is lagged with glass wool. The solvent is heated using the isomantle and will begin to evaporate, moving through the apparatus to the condenser. The condensate then drips into the reservoir containing the thimble. Once the level of solvent reaches the siphon it pours back into the flask and the cycle begins again. The process should run for a total of 16 hours. Once the student has set up the extraction it can be left to run without direct supervision. It is not advised to leave the equipment completely alone due to the mix of running water and an electrical appliance, so a technician or other lab user should be made aware. The equipment can be turned on and off when overnight running is not permitted, and the time split over a number of days. For good practice, a control should be added. This could be plant material that has no known antimicrobial effect (for example, a carrier oil such as sunflower oil) at the testing stage.
FIGURE 1.
Diagram of Soxhlet extraction equipment.
Once the process has finished, the ethanol should be evaporated using a rotary evaporator, leaving a small yield of extracted plant material (about 2 to 3 ml) in the glass bottom flask.
Testing the extract for antimicrobial effect
Antimicrobial properties of the plant extract should be performed using the agar disc diffusion technique using antibiotic assay discs (1). It is suggested students test against a Gram-positive microorganism (e.g. Staphylococcus aureus) and a Gram-negative microorganism (e.g. Escherichia coli). Plates should be incubated at 37°C for 24 to 48 hours. The activity can be modified to meet your curriculum requirements. Examples of a modification can be found in Appendix 1.
Safety issues
The Soxhlet extraction process heats the solvent (ethanol) to boiling temperature (>78°C). The evaporated ethanol is contained within the apparatus by the condenser unit; however the apparatus should be placed under a fume hood in case of escape. Due to the continuous running of water and a heat source, it is not advisable to leave the apparatus unattended overnight. Personal protective equipment, including gloves, should be used when handling the plant extract, as it may be an irritant to the skin (7).
CONCLUSION
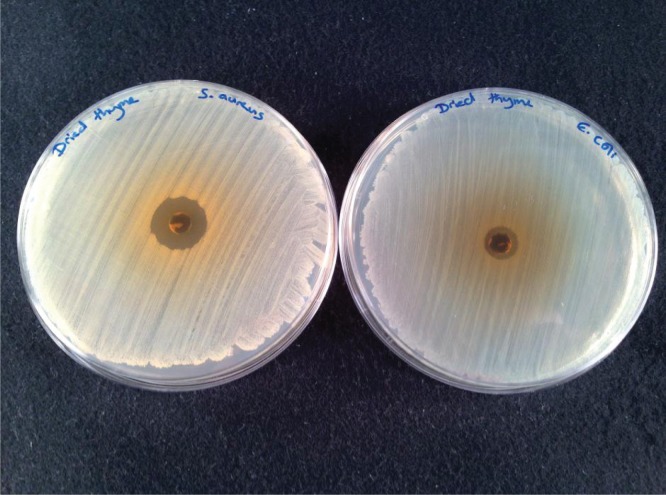
If an antimicrobial compound has been successfully extracted, a clear zone (no growth) around the test substance is observed. This is known as the ‘zone of inhibition’ (ZoI). Comparisons can be made between different test organisms (3), plant extracts, and other variables (described below). In our lab, average ZoI for the essential oil extracted from dried thyme is 9 mm diameter when tested against E. coli and 16 mm diameter when tested against S. aureus (Fig. 2).
FIGURE 2.
An example of zones of inhibition produced from extracted thyme plant material after 16 hours ethanol Soxhlet extraction on test organisms S. aureus (left) and E. coli (right).
SUPPLEMENTAL MATERIALS
Appendix 1: Potential modifications to the activity
Acknowledgments
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Burdass D, Grainger J, Hurst J, editors. Basic practical microbiology. Society for General Microbiology, Reading; United Kingdom: 2006. [Google Scholar]
- 2.Cavanagh HMA, Wilkinson JM. Biological activities of lavender essential oil. Phytotherapy Res. 2002;16(4):301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 3.Haitham Q. Antibacterial activity in vitro of Thymus capitatus from Jordan. Pak. J. Pharm. Sci. 2009;22(3):247–251. [PubMed] [Google Scholar]
- 4.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86(6):985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 5.Hawthorne SB, Krieger MS, Miller DJ. Analysis of flavor and fragrance compounds using supercritical fluid extraction coupled with gas chromatography. Analytical Chem. 1988;60(5):472–477. doi: 10.1021/ac00156a020. [DOI] [PubMed] [Google Scholar]
- 6.Luque de Castro MD, García-Ayuso LE. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Analytica Chimica Acta. 1998;369(1–2):1–10. doi: 10.1016/S0003-2670(98)00233-5. [DOI] [Google Scholar]
- 7.Metcalfe J. Culpper guides: herbs and aromatherapy. Bloomsbury Books; London, United Kingdom: 1993. [Google Scholar]
- 8.Thormar H. Front matter. In: Thormar H, editor. Lipids and essential oils as antimicrobial agents. John Wiley & Sons, Ltd; Chichester, United Kingdom: 2011. pp. i–xvii. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Potential modifications to the activity