Abstract
Hypoxia and anoxia are important microenvironmental stresses that contribute to pathological events such as solid-tumor development. We have been investigating the effects of hypoxia and anoxia on expression of the proto-oncogene c-jun and the regulation of c-Jun/AP-1 transcription factors. In earlier work using genetically manipulated mouse embryo fibroblasts (mEFs), we found a functional relationship among c-jun expression, c-Jun N-terminal phosphorylation, and the presence of hypoxia-inducible factor 1α (HIF-1α), the oxygen-regulated subunit of the HIF-1 transcription factor. Both the induction of c-jun mRNA expression and c-Jun N-terminal phosphorylation in cells exposed to hypoxia or anoxia were found to be dependent on the presence of HIF-1α, but this was not the case in cells exposed to less-severe hypoxia. Here we describe new findings concerning HIF-1-dependent c-Jun N-terminal phosphorylation in cells exposed to hypoxia or anoxia. Specifically, we report that hypoxia-inducible c-Jun N-terminal kinase (JNK) activity, which involves JNKs or stress-activated protein kinases (SAPKs), is dependent on enhanced glucose utilization mediated by HIF-1. These results suggest a model in which hypoxia-inducible JNK activity is connected to oxygen sensing through increased glucose absorption and/or glycolytic activity regulated by the HIF-1 system. We also found that basal threonine and tyrosine phosphorylation (within the TEY motif) of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and the corresponding ERK1/2 activity were defective in hypoxic HIF-1α-null mEFs but not in wild-type mEFs, independently of glucose uptake. Therefore, the activities of both JNKs/SAPKs and ERK1/2 are sensitive to HIF-1-dependent processes in cells exposed to hypoxia or anoxia.
The c-Jun protein is a subunit of AP-1 transcription factors, pleiotropic regulators that influence the proliferation, survival, and differentiation of both normal and transformed cells (reviewed in references 34, 52, and 63). We have been investigating the response of c-Jun/AP-1 to low-oxygen conditions (hypoxia and anoxia), particularly those present within the microenvironments of solid tumors (3, 27-29). The transcriptional and posttranscriptional activation of c-Jun/AP-1 by hypoxic or anoxic stress has been reported for various normal and transformed cells (2, 3, 5, 36, 45, 60, 64, 65), indicating that it is generally sensitive to changes in ambient oxygen concentration. Recently we demonstrated that phosphorylation of c-Jun within its N-terminal region in cells exposed to hypoxia or anoxia is dependent on the presence of hypoxia-inducible factor 1 (HIF-1) (27), the principal transcriptional regulator of hypoxia-responsive gene expression in mammalian cells (recently reviewed in references 19, 51, and 61). In general, we found that the pattern of hypoxia-inducible c-Jun N-terminal phosphorylation is biphasic, consisting of early HIF-1-independent and late HIF-1-dependent components (27). The functional relationship demonstrated between c-Jun/AP-1 and HIF-1 in hypoxic cells (2, 27) suggests a high level of organization—a network ensuring that hypoxic or anoxic signals are interpreted appropriately in a particular cell or tissue. Little is known, however, of the pathways responsible for the activation of c-Jun/AP-1 by hypoxic signals.
Protein kinases that directly or indirectly modulate c-Jun/AP-1 activity in vivo include members of the mitogen-activated protein kinase (MAPK) family (c-Jun N-terminal kinases [JNKs]/stress-activated protein kinases [SAPKs] and extracellular signal-regulated kinases 1 and 2 [ERK1/2]), glycogen synthase kinase 3 (GSK3), casein kinase II (CKII), and c-Abl (6, 26, 30, 31, 41). It has been established that phosphorylation of the c-Jun N-terminal region (e.g., by JNKs/SAPKs) is important for its transactivation function in an AP-1 complex, whereas phosphorylation of the C-terminal region (e.g., by GSK3 or CKII) inhibits the specific DNA binding function of c-Jun (26, 41). Both GSK3 and CKII have been reported to be sensitive to hypoxia (11, 23), although we have not been able to detect changes in their activities in cells exposed to the low oxygen conditions normally used in our studies (pO2, ≤0.1%). To further investigate the mechanism of HIF-1-dependent N-terminal phosphorylation of c-Jun in cells exposed to hypoxia or anoxia, we compared the phosphorylation of the c-Jun N-terminal region in normoxic and hypoxic cultures of wild-type (wt) and HIF-1α-null mouse embryo fibroblasts (mEFs). In addition, we investigated whether hypoxia-inducible c-Jun phosphorylation is dependent on the presence of factors within the extracellular medium of hypoxic cultures. Here we describe the major finding that glucose utilization mediated by HIF-1 activity is a critical factor determining the N-terminal phosphorylation response of c-Jun to hypoxia or anoxia.
MATERIALS AND METHODS
Materials.
A glutathione S-transferase (GST) fusion protein containing the c-Jun N-terminal region [GST-c-Jun(1-141)] was expressed in Escherichia coli from a pGEX-2T plasmid [GST-c-Jun(1-141)-pGEX-2T] and purified from lysates by capture on glutathione-Sepharose 4B beads (Amersham Biosciences), as described in detail in reference 29. Mutated versions of the GST-c-Jun(1-141) protein described below were purified by the same protocol. Where needed, free GST-c-Jun(1-141) protein was prepared by elution from the beads with a glutathione (GSH) solution (10 mM GSH, 25 mM Tris-HCl [pH 7.5], 1 mM EDTA, 1 mM dithiothreitol [DTT], 5% glycerol) followed by dialysis at 4°C in a buffer containing 10 mM MgCl2, 50 mM Tris-HCl (pH 7.5), 5 mM NaF, and 1 mM Na3VO4. Site-specific mutations were made in GST-c-Jun(1-141)-pGEX-2T by using a QuikChange site-directed mutagenesis kit (Invitrogen) according to the supplier's instructions. The following primers were used to produce mutations in c-Jun N-terminal phosphorylation sites from this template (mutated bases are underlined): for S63A, 5′-CTCGGACCTCCTCACCGCGCCCGACGTGGGGCT-3′ (forward) and 5′-AGCCCCACGTCGGGCGCGGTGAGGAGGTCCGAG-3′ (reverse); for T9193A, 5′-CACATCACCACCGCGCCAGCCCCCACCCAGTTC-3′ (forward) and 5′-GAACTGGGTGGGGGCTGGCGCGGTGGTGATGTG-3′ (reverse). The S63 S73A mutation was prepared similarly by using the S63A mutant as a template and the following primers for constructing an S73A mutation: 5′-TGCTCAAGCTGGCGGCGCCCGAGCTGGAG-3′ (forward) and 5′-CTCCAGCTCGGGCGCCGCCAGCTTGAGCA-3′ (reverse). All changes to GST-c-Jun(1-141)-pGEX-2T were confirmed by conventional DNA sequencing using commercially available primers for pGEX-2T plasmids (Amersham Biosciences).
The following antibodies were obtained from commercial sources: a rabbit polyclonal anti-JNK1/SAPKγ antibody (JNK1 [FL]; immunizing antigen, full-length recombinant human JNK1; cross-reactive with mouse JNK1/SAPKγ, JNK2/SAPKα, and JNK3/SAPKβ; Santa Cruz Biotechnology), a monoclonal anti-JNK2 antibody (JNK2 [D-2]; immunizing antigen, full-length recombinant human JNK2; cross-reactive with mouse JNK1 to -3; Santa Cruz Biotechnology), a rabbit polyclonal anti-ERK1/2 antibody (ERK1/2-CT; immunizing antigen, C-terminal amino acids of rat ERK2; cross-reactive with mouse ERK1/2; Upstate Cell Signaling Solutions), a rabbit polyclonal anti-p38 MAPK antibody (C-20; immunizing antigen, C-terminal amino acids of mouse p38 MAPK; Santa Cruz Biotechnology), a monoclonal anti-phospho-ERK1/2 antibody (immunizing antigen, a synthetic peptide containing amino acids of human ERK1/2 phosphorylated on Thr202 and Tyr204; cross-reactive with phosphorylated mouse ERK1/2; catalog no. 9106S; New England Biolabs), a monoclonal anti-phospho-p38 MAPK antibody (immunizing antigen, a synthetic peptide containing amino acids of human p38 MAPK phosphorylated on Thr180 and Tyr182; cross-reactive with phosphorylated mouse p38 MAPK; catalog no. 9211S; New England Biolabs), a monoclonal anti-phospho-c-Jun antibody (KM-1; immunizing antigen, amino acids 56 to 69 of human c-Jun; recognizes c-Jun phosphorylated on Ser63; Santa Cruz Biotechnology), a rabbit polyclonal anti-c-Jun antibody (H79; immunizing antigen, amino acids 1 to 79 of human c-Jun; Santa Cruz Biotechnology), and a rabbit polyclonal anti-GLUT1 antibody (AB1340; immunizing antigen, C-terminal amino acids of mouse/rat GLUT1; Chemicon International).
The following reagents were obtained from commercial sources: JNK inhibitor I (L)-Form (Calbiochem), recombinant human epidermal growth factor (EGF) (Gibco Invitrogen), 2-deoxy-d-[2,6-3H]glucose (53.0 Ci/mmol, 1.0 mCi/ml; Amersham Biosciences), PD 98059, a selective pharmacological inhibitor of the ERK1/2 pathway (1) (Calbiochem), and myelin basic protein (Upstate Cell Signaling Solutions). The photolabile cross-linking agent for glucose receptors, Bio-LC-ATB-BGPA {4,4′-O-[2-[2-[2-[2-[2-[6-(biotinylamino)hexanolyl]-amino]-ethoxy]-ethoxy]-ethoxy]-4-(1-azi-2,2,2-trifluoroethylbenzoyl]amino-1,3-propanediyl]-bis-d-glucose}, was a kind gift from Geoffrey Holman (University of Bath, Bath, United Kingdom).
Cell culture and hypoxia-anoxia protocols.
The origin and culture of immortalized, wt, and HIF-1α-null mEFs have been described in detail elsewhere (27). Briefly, cells were usually plated in 60- or 100-mm-diameter plastic culture dishes (400 to 500 cells/mm2) in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; Sigma) and 25 mM HEPES buffer (pH 7.4) (DMEMH-10% FBS). Thus, both bicarbonate and HEPES buffers regulated extracellular pH. Hypoxia or anoxia experiments (called hypoxia here) were performed according to a standard protocol in which cells were incubated under an air-5% CO2 atmosphere at 37°C overnight and then placed in aluminum gas-exchange chambers maintained at 37°C (27). The chambers containing the cells were then placed in a 37°C circulating water bath, and the original atmosphere was repeatedly exchanged with 5% CO2-95% N2 by using a manifold equipped with a vacuum pump and a gas cylinder. Atmospheric pO2 values of approximately 1 to ≤0.01% (relative to air at a pO2 of ≈21%) can be achieved inside the chambers by using this system. For example, atmospheric pO2 values of approximately 1 and 0.01% can be achieved at 0.5 and 2 h, respectively, following the initiation of hypoxia. We have determined that after 2 h of hypoxia, atmospheric pO2 is less than or equal to 0.01% (the studies described here were all performed at a pO2 of ≤0.01%). Alternatively, cells were incubated on a warm surface maintained at 37°C inside an anaerobic glove box (Bactron X; Sheldon Manufacturing Inc., Cornelius, Oreg.) maintained at 5% CO2-95% N2. Atmospheric oxygen levels in both the chambers and the glove box have been measured and calibrated by using a polarographic oxygen electrode (Oxygen Sensors, Inc., Norristown, Pa.).
Following various hypoxic exposures, the chambers were opened in the anaerobic glove box to prepare cell lysates without significant reoxygenation. For studies involving glucose stress, cells were cultured in normal or glucose-free DMEMH-10% FBS and incubated in air-5%CO2 or exposed to hypoxia for 8 h (glucose-free medium was added immediately before the hypoxic exposure). Then a degassed glucose solution (0.2 mg/ml in sterile water) was added to dishes of normoxic and hypoxic cells to a standard concentration (4.5 g of glucose/liter, or 25 mM), and the cells were incubated at 37°C for 1 h before being harvested for immunoblot analysis. All manipulations of hypoxic cells were performed in the anaerobic glove box.
JNK assays.
Pulldown or affinity kinase assays of JNK activity in cell lysates were performed by using GST-c-Jun(1-141) as a substrate (29). Briefly, dishes of normoxic and hypoxic cells were placed on ice in air or on Super Ice cold packs in the anaerobic glove box, and the medium was removed. Each dish was washed with ice-cold phosphate-buffered saline (PBS), and then 250 μl of ice-cold lysis buffer (LB; 50 mM Tris-HCl [pH 7.4], 250 mM NaCl, 15 mM Na4P2O7, 50 mM NaF, 0.5% NP-40, 1 mM Na3VO4, 25 mM β-glycerophosphate, 100 nM okadaic acid, 1 mM DTT, 1× Protease Inhibitor Cocktail Set III [PIC III; Calbiochem]) was added (solutions were degassed before protein was harvested from hypoxic cells). Cells in each dish were disrupted by scraping with a plastic spatula, and cell lysates were transferred to microcentrifuge tubes. Lysates were spun at 9,000 × g for 5 min at 4°C, and the protein concentrations of the supernatants were determined by a bicinchoninic acid (BCA) assay (Pierce Biotechnology). Protein concentrations were normalized with LB.
Pulldown kinase assays were performed by first adding 20-μl suspensions of glutathione-Sepharose 4B beads with adsorbed normal or mutated GST-c-Jun(1-141) protein in PBST (20 mM sodium phosphate [pH 7], 150 mM NaCl, 1% Triton X-100, 100 μM Na3VO4, 50 mM NaF, 1 mM benzamidine hydrochloride, 1× PIC III) to tubes of supernatants each containing 100 to 200 μg of total cell protein. Then 20 μl of freshly prepared kinase buffer (KB; 25 mM HEPES [pH 7.4], 25 mM MgCl2, 1 mM Na3VO4, 25 mM β-glycerophosphate, 100 nM okadaic acid, 1× PIC III, 2 mM DTT) containing normal and radioactively labeled ATP (20 μM ATP; 3 to 5 μCi of [γ-32P]ATP [5,000 Ci/mmol]; Amersham Biosciences) was added to each tube. After incubation for 30 min at 30°C, the beads in each sample were washed three times with PBST; then 40 μl of 2× sodium dodecyl sulfate (SDS) sample buffer (125 mM Tris-HCl [pH 6.8], 4.6% SDS, 10% mercaptoethanol, 20% glycerol) was added, and the samples were boiled for 5 min. Boiled samples were resolved in SDS-10% polyacrylamide gels, and the gels were stained with colloidal Coomassie brilliant blue R-250 to visualize protein loading. Gels were dried and exposed to Kodak X-Omat AR film to prepare autoradiographs for digital imaging. This type of assay detects any kinase activity capable of phosphorylating the c-Jun N-terminal region in a cell lysate.
In an alternative pulldown kinase assay, 20 μg of purified GST-c-Jun(1-141) protein was added to samples of whole-cell lysates in microcentrifuge tubes, and the tubes were gently rotated at 4°C overnight. Each tube then received a 20-μl suspension of glutathione-Sepharose 4B beads, and the tubes were rotated at 4°C for 2 h. The beads in each tube were washed twice with ice-cold LB and three times with ice-cold KB, and then JNK inhibitor I was added to a final concentration of 1.7 μM in KB (8) (controls received KB only). The tubes were incubated at 30°C for 10 min, and then kinase reactions and analyses were performed as described above. This type of assay detects any tightly bound kinase activity capable of phosphorylating the c-Jun N-terminal region and is diagnostic for activated JNKs/SAPKs (see, e.g., reference 48).
Immunocomplex kinase assays of JNK activity were performed using cell lysates prepared by essentially the same protocol as that described above for pulldown kinase assays. Each sample of lysate supernatant (containing 100 to 200 μg of protein) was divided into two equal fractions in microcentrifuge tubes. One fraction received an antibody (1 μg of an anti-JNK/SAPK or anti-ERK1/2 antibody), and the other fraction was used as a control for nonspecific protein binding to Protein A/G Plus agarose beads (Santa Cruz Biotechnology). Each tube then received 20 μl of glutathione-Sepharose 4B beads, and the tubes were gently tumbled for at least 1 h at 4°C. Tubes were spun at 600 × g and 4°C for 5 min, and beads were washed twice with ice-cold LB and three times with ice-cold KB. The KB from each sample of beads was removed and replaced with the same buffer containing ATP (20 μM ATP; 3 to 5 μCi of [γ-32P]ATP [5,000 Ci/mmol]) and 5 μg of GST-c-Jun(1-141) protein. Samples were incubated for 30 min at 30°C, and reactions were stopped by addition of 40 μl of 2× SDS sample buffer, followed by boiling for 5 min. Samples were resolved in SDS-10% polyacrylamide gels, and autoradiographs were prepared as described above.
Immunoblot analysis.
Immunoblotting protocols have been described in detail elsewhere (27). Briefly, cells were placed on ice in air or on Super Ice cold packs in the anaerobic glove box, and the medium was removed. Cells were lysed by addition of 200 μl of ice-cold LB (degassed before protein was harvested from hypoxic cells). After spinning at 9,000 × g for 5 min at 4°C, the protein concentrations of the supernatants were determined as described above. Equal protein samples (typically 10 to 15 μg) were resolved in freshly prepared discontinuous SDS-10% polyacrylamide gels and electroblotted onto Immobilon P membranes (Millipore). We find that these gels provide better resolution of c-Jun phospho-isoforms than commercially available preformed protein gels. Blots were blocked in 5% nonfat dry milk in PBS containing 0.1% Tween 20 at 4°C overnight. For protein detection, blots were incubated at room temperature with a primary antibody, typically diluted 1:1,000 in PBS-0.1% Tween 20 containing 5% nonfat driy milk, and a secondary anti-mouse or anti-rabbit immunoglobulin G (IgG) antibody conjugated with horseradish peroxidase (IgG-HRP; Santa Cruz Biotechnology), diluted 1:10,000 or 1:5,000, respectively, in PBS-0.1% Tween 20. Primary antibody binding was detected and visualized by using the ECL Plus Western Blotting Detection system (Amersham Pharmacia Biotech, Inc.) according to the supplier's instructions.
ATP assay.
Equal numbers of cells were plated in 35-mm-diameter plastic culture dishes (5 × 105 cells/dish) in DMEMH-FBS and then incubated in 5% CO2-air or exposed to hypoxia (pO2, ≤0.01%) for as long as 8 h (triplicate dishes were harvested at each time point). Cells in each well were washed twice with PBS and lysed on ice by addition of 0.5 ml of 5% trichloroacetic acid (TCA) and scraping with a plastic spatula. Lysates were spun at 9,000 × g for 5 min at 4°C, and the pellets were kept. Pellets were dissolved in 0.5 ml of 0.1 N NaOH, incubated at 65°C for 10 min, and vortexed vigorously to shear genomic DNA. Total cellular ATP was measured by using a luciferase-luciferin assay (ATP Bioluminescent Assay kit; Sigma) according to the supplier's instructions.
Glucose uptake assay.
Cellular uptake or absorption of the glucose analog 2-deoxyglucose (2-DG) was measured essentially according to the protocol described in reference 32. Briefly, wt and HIF-1α-null cells were plated into the wells of two 24-well plates (105 cells/well; 0.5 ml of DMEMH-FBS) and incubated at 37°C overnight. One plate was placed inside the anaerobic glove box in a humidified acrylic box in contact with the anaerobic atmosphere and incubated on a 37°C warm plate for 8 h. The other plate was incubated at 37°C in air-5% CO2 for 8 h. Hypoxic cells in the anaerobic glove box were washed with degassed PBS, and then 1.0 ml of the following solution in 1× Dulbecco's PBS (D-PBS; Sigma) was added to each well: 0.1 mM 2-DG, 4 μCi of 2-deoxy-d-[2,6-3H]glucose/ml, and 1% bovine serum albumin, with or without 20 nM cytochalasin B. Cells were incubated at 37°C for 5 min, and the plate was placed on an ice-cold surface. Then 0.5 ml of ice-cold D-PBS containing 110 μg of phloretin/ml was added to each well to stop 2-DG uptake, and the plate was removed from the anaerobic glove box and placed on ice. Cells were washed three times with D-PBS containing 55 μg of phloretin/ml and lysed by adding 300 μl of 0.1% SDS to each well and gently agitating the plate for 5 min at room temperature. Normoxic cells were handled identically. The supernatant was removed from each well without scraping, and the protein concentration of each supernatant was determined by a BCA assay. The radioactivity of each sample was determined by counting 200 μg of total protein in 5 ml of CytoScint (ICN). Values of 2-DG uptake that could be inhibited by cytochalasin B were calculated and expressed as picomoles per 5 min per milligram of total cellular protein.
Detection of cell surface glucose receptors by photoaffinity labeling.
Bio-LC-ATB-BGPA is a member of a series of membrane-impermeant photoaffinity labels for cell surface glucose receptors (GLUT receptors) (7, 9). Briefly, cells were plated in DMEMH-10% FBS at 3 × 106/100-mm-diameter plastic culture dish (two dishes for each treatment), and experiments were performed the next day. A fresh solution of the Bio-LC-ATB-BGPA cross-linker (0.2 mg/ml in deionized water) was prepared under low light conditions and stored in the dark until needed. Normoxic and hypoxic cells were washed twice with ice-cold D-PBS (hypoxic cells were washed in the anaerobic glove box) and immediately placed on an ice-cold surface to inhibit GLUT receptor endocytosis. One plate for each treatment received 1 ml of the cross-linker solution; the other plate received 1 ml of D-PBS. Cells for each treatment were directly exposed on ice to 350-nm light for 30 s (approximately 2 mJ/s) for a total of 2 min of exposures, and then the cells were washed three times with ice-cold D-PBS. Cells were lysed in PBS containing 1% Triton X-100 and PIC III, the lysates were spun at 9,000 × g for 5 min at 4°C, and the supernatants were recovered. Samples of the lysates were adjusted to an equal protein concentration with the lysis solution, and then each sample received 75 μl of streptavidin-agarose beads (ImmunoPure immobilized streptavidin gel; Pierce Chemical Co.). Samples were tumbled overnight at 4°C and then washed three times with ice-cold PBS-0.1% Triton X-100 and twice with ice-cold PBS. Each sample received 30 μl of SDS sample buffer, and samples were heated at 95°C for 10 min. Samples were resolved in protein gels (NuPAGE 10% Bis-Tris gels; Invitrogen) and immunoblotted, as described above.
Coculturing assay.
In order to investigate a potential autocrine mechanism of hypoxia-inducible c-Jun phosphorylation and c-jun mRNA expression, wt and HIF-1α-null cells were plated in either the wells or the inserts of multiwell cell culture plates (Falcon 24-multiwell cell culture insert systems; pore size, 3 μm; Becton Dickinson). All combinations of wt and HIF-1α-null cells were separately plated in wells and companion inserts in triplicate (2 × 105 cells per well or per insert) in 2 ml of DMEMH-FBS and were incubated at 37°C overnight. Plates were either exposed to hypoxia (pO2, ≤0.01%) in the anaerobic glove box on a 37°C warm plate for 8 h or incubated in 5% CO2-air at 37°C for 8 h. Whole-cell lysates were prepared from the wells as described above for immunoblot analysis. Total RNA was harvested from the wells of identical plates, purified by the RNeasy method (Qiagen), and used for quantitative real-time reverse transcriptase PCR (RT-PCR) analysis of c-jun expression.
Quantitative real-time RT-PCR analysis.
Quantitative real-time RT-PCR analysis was performed by using a LightCycler System instrument (Roche Diagnostics) according to the manufacturer's protocols for the SYBER Green method. The following primers were used: the c-jun sense primer (5′-CATGGAGTCTCAGGAGCGGATCA-3′) and the c-jun antisense primer (5′-TGAGTACGATTGCGTCGTCAACG-3′); the GLUT1 sense primer (5′-AGTATGTGGAGCAACTGTGCGG-3′) and the GLUT1 antisense primer (5′-GGTGTCTTGTCACTTTGGCTGG-3′); the GLUT4 sense primer (5′-TCGTCATTGGCATTCTGGTTG-3′) and the GLUT4 antisense primer (5′ CAGGGGCTTTAGACTCTTTCGG 3′). Mouse heart total RNA (BD Biosciences Clontech) was used to provide a positive control for amplification of mouse GLUT4 mRNA.
RESULTS
HIF-1-dependent phosphorylation of the c-Jun N-terminal region in response to hypoxia or anoxia involves JNK/SAPK activity.
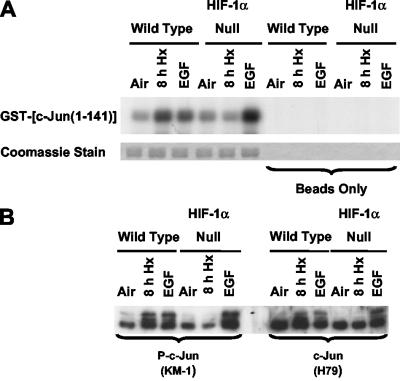
Previously we used anti-c-Jun antibodies specific for N-terminal phosphorylation sites and c-jun knock-in mEFs (S63A S73A and T91A T93A) to show that most, if not all, of the N-terminal sites can be phosphorylated in response to hypoxia or anoxia (27). Using wt and HIF-1α-null cells, we also showed that under these low-oxygen conditions, N-terminal phosphorylation was dependent on HIF-1 (27). Transient N-terminal phosphorylation was detectable between 0.5 and 2 h of hypoxia, but this response was not dependent on HIF-1 (23). To further investigate HIF-1-dependent c-Jun N-terminal phosphorylation, we compared the abilities of whole-cell lysates of wt and HIF-1α-null cells to phosphorylate a wt fusion protein substrate [GST-c-Jun(1-141)]. Figure 1 confirms previous findings for HIF-1-dependent c-Jun N-terminal phosphorylation. Lysates of normoxic and hypoxic wt and HIF-1α-null cells were used in pulldown kinase assays to show a direct correlation between JNK activity (Fig. 1A) and levels of endogenous c-Jun phosphorylation determined by immunoblotting (Fig. 1B). EGF treatment was used as a control for both c-Jun N-terminal phosphorylation and endogenous c-Jun phosphorylation (27). GST alone was not phosphorylated in any of the kinase assays described here (data not shown).
FIG. 1.
Inducible JNK activity in cells exposed to hypoxia or anoxia is impaired in the absence of HIF-1α. (A) Autoradiograph showing phosphorylation of a GST-c-Jun(1-141) fusion protein substrate in pulldown kinase assays using whole-cell lysates of wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). EGF exposure (50 ng/ml; 5 min) in air-5% CO2 was used as a positive control. The beads-only control refers to identical lysate samples that received only clean agarose beads before the addition of radiolabeled ATP (for details, see Materials and Methods). Coomassie staining of the substrate bands is shown here and elsewhere to confirm equal loading of the original SDS-polyacrylamide gels used to obtain autoradiographs. This autoradiograph is representative of multiple independent experiments. (B) Replicate immunoblots of total protein from wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h) or EGF (50 ng/ml; 5 min). Blots were probed with an anti-phospho-c-Jun antibody (left panel; KM-1) or with an antibody that recognizes nonphosphorylated c-Jun (right panel; H79). In this experiment and in all others, hypoxic cells to be used for protein kinase or phosphorylation assays were harvested under anaerobic conditions.
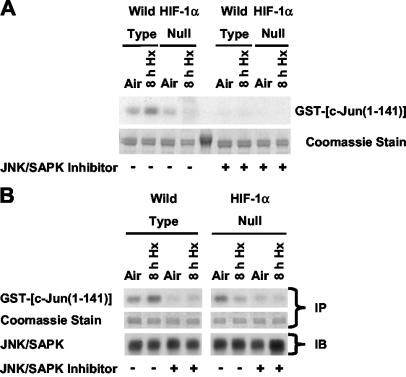
Phosphorylation of a substrate such as GST-c-Jun(1-141) in a pulldown kinase assay is considered diagnostic of total JNK/SAPK activation (17, 48). To confirm that JNKs/SAPKs contributed to the activity exemplified in Fig. 1A, identical lysates of normoxic and hypoxic wt and HIF-1α-null cells were incubated with free GST-c-Jun(1-141), extracted with GSH-agarose beads, and then exposed to a peptide inhibitor of JNKs/SAPKs (JNK inhibitor I) before kinase assays were performed (8). Figure 2A shows that the JNK/SAPK inhibitor suppressed phosphorylation of GST-c-Jun(1-141) complexes extracted from lysates of both cell types, indicating that the fusion protein captured JNKs/SAPKs. We also performed immunoprecipitation or immunocomplex kinase assays using similar lysates with an anti-JNK antibody that has broad specificity for mammalian JNK/SAPK family members (JNK1 to -3) (26) and with the GST-c-Jun(1-141) substrate. Figure 2B shows that the inhibitor suppressed basal and/or inducible JNK/SAPK activity immunoprecipitated from normoxic and hypoxic wt and HIF-1α-null cells. In general, JNKs/SAPKs immunoprecipitated from lysates of aerobic and hypoxic wt and HIF-1α-null cells had the same pattern of activity toward GST-c-Jun(1-141) as activities detected in corresponding pulldown kinase assays: JNK/SAPK activity was induced in hypoxic wt but not HIF-1α-null cells. Together the findings presented in Fig. 2 demonstrate that JNK/SAPK activity contributes both to HIF-1-dependent c-Jun N-terminal phosphorylation in hypoxia-exposed cells and to basal c-Jun phosphorylation in normoxic wt and HIF-1α-null cells. Because of the potential for reduced specificity or potency in cell-based assays (4), we did not use the JNK/SAPK inhibitor to investigate hypoxia-inducible c-Jun phosphorylation in intact wt and HIF-1α-null cells.
FIG. 2.
HIF-1-dependent JNK activity in cells exposed to hypoxia or anoxia involves JNKs/SAPKs. (A) Autoradiograph of pulldown kinase assays and the corresponding stained gel showing phosphorylation of the GST-c-Jun(1-141) fusion protein substrate extracted from whole-cell lysates of wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). Beads with adsorbed fusion protein were thoroughly washed, and then kinase assays were performed in the absence (−) or presence (+) of a peptide JNK/SAPK inhibitor (1.7 μM). For details, see Materials and Methods. (B) (Top) Representative autoradiographs showing phosphorylation of the GST-c-Jun(1-141) fusion protein substrate by JNKs/SAPKs immunoprecipitated from whole-cell lysates of wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). Kinase assays were performed in the absence (−) or presence (+) of the peptide JNK/SAPK inhibitor (1.7 μM). (Center) Corresponding stained gels for the immunoprecipitation assays (immunocomplex kinase assays) shown above. (Bottom) Immunoblots of total JNKs/SAPKs immunoprecipitated from the same whole-cell lysates of wt and HIF-1α-null mEFs used for the immunocomplex kinase assays shown above. Blots were probed with a primary anti-JNK2 monoclonal antibody (cross-reactive with mouse JNK1 and JNK3) and a secondary anti-mouse IgG antibody conjugated with horseradish peroxidase. IP, immunoprecipitation assay; IB, immunoblot assay.
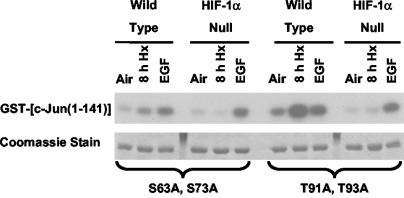
The contribution of JNK/SAPK activity to both basal and hypoxia-inducible phosphorylation of endogenous c-Jun was demonstrated previously by immunoblotting studies using specific anti-phospho-c-Jun antibodies (27). However, it was not obvious from these studies whether c-Jun N-terminal phosphorylation in hypoxic cells involves a preference for any of the known phospho-acceptor sites (Ser63, Ser73, Thr91, and Thr93) (6, 44). To address this issue, we generated GST-c-Jun(1-141) constructs containing site-specific mutations (replacement of Ser or Thr with Ala) at these positions and used these molecules as substrates for pulldown assays of JNK/SAPK activity in lysates of normoxic, hypoxic, or EGF-treated wt and HIF-1α-null cells. Figure 3 shows representative results from kinase assays involving the substrate mutants GST-c-Jun(1-141) S63A S73A and GST-c-Jun(1-141) T91A T93A. Although there were variations in the relative levels of phosphorylation between each set of results for the wt and mutated GST-c-Jun(1-141) substrates, essentially identical patterns of kinase activity were obtained for all substrates; hypoxia-inducible JNK activity was detected in lysates of wt but not HIF-1α-null cells, and EGF stimulated this activity in both cell types. As a potential caveat, it is possible that the wt and mutated GST-c-Jun(1-141) substrates are inherently less specific than the N-terminal region of endogenous c-Jun toward specific protein kinases. However, the trend revealed by the pulldown kinase assays shown in Fig. 1 to 3 agree with our previous findings with c-jun knock-in mEFs (S63A S73A and T91A T93A), which indicated that all sites in the c-Jun N-terminal region can be phosphorylated in cells exposed to hypoxia or anoxia (27). In addition, a recent report has demonstrated that all of these N-terminal sites in endogenous c-Jun can be phosphorylated by MAPKs (preferentially by JNKs/SAPKs), based on immunoblot analyses involving a comprehensive panel of phosphospecific anti-c-Jun antibodies (38). Taken together, these published findings and our present results suggest that all sites in the c-Jun N-terminal region are susceptible to hypoxia-inducible phosphorylation.
FIG. 3.
Autoradiograph showing phosphorylation of the GST-c-Jun(1-141) S63A S73A or GST-c-Jun(1-141) T91A T93A mutant fusion protein substrate in pulldown kinase assays using whole-cell lysates of wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h) or EGF (50 ng/ml; 5 min). These results are representative of at least three independent experiments.
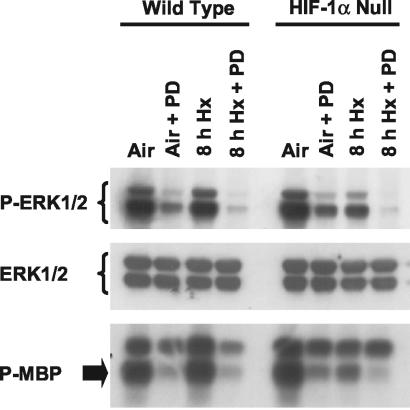
Finally, to determine whether the dependence of hypoxia-inducible JNK/SAPK activity on HIF-1α is unique among MAPK family members, we used specific anti-phospho-MAPK antibodies to evaluate the levels of ERK1/2 and p38 MAPKs phosphorylated on the TXY activation motif (TEY and TGY for ERK1/2 and p38 MAPK, respectively) (26) in lysates of normoxic and hypoxic wt and HIF-1α-null cells. Figure 4 shows that there was no induction of ERK1/2 phosphorylation above the basal level in hypoxic compared with normoxic wt cells. However, the basal level of phosphorylated ERK1/2 was strongly decreased in hypoxic compared with normoxic HIF-1α-null cells. Consistent with this suppression of basal ERK1/2 phosphorylation in hypoxic HIF-1α-null cells, basal ERK1/2 immunocomplex kinase activity was also significantly decreased in hypoxic but not in normoxic HIF-1α-null cells. The levels of phosphorylated p38 MAPKs were increased in response to the same hypoxic exposure, but this increase was essentially the same on immunoblots of lysates of both normoxic and hypoxic wt and HIF-1α-null cells (data not shown). The findings shown in Fig. 4 demonstrate that basal ERK1/2 phosphorylation and associated kinase activities are HIF-1 dependent, but unlike JNKs/SAPKs, these activities are not inducible in response to hypoxia or anoxia.
FIG. 4.
Basal ERK1/2 phosphorylation and activity are defective in HIF-1α-null mEFs exposed to hypoxia or anoxia. ERK1/2 is not inducible in wt mEFs. (Top and center) Immunoblots of total protein from wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). Replicate blots were probed with an anti-phospho-ERK1/2 antibody (specific for phospho-Thr202 and phospho-Tyr204 in the TEY activation motif) (top) or with an antibody that recognizes total ERK1/2 (center). (Bottom) Autoradiograph showing phosphorylation of myelin basic protein (MBP) by ERK1/2 immunoprecipitated from identical whole-cell lysates of wt and HIF-1α-null mEFs harvested under normoxic conditions (5% air-CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). PD 98059 (PD) was used as a control for ERK1/2 activity in these experiments. PD (final concentration, 50 μM) was added just before initiation of hypoxia.
HIF-1-dependent phosphorylation of the c-Jun N-terminal region in response to hypoxia or anoxia is dependent on glucose utilization.
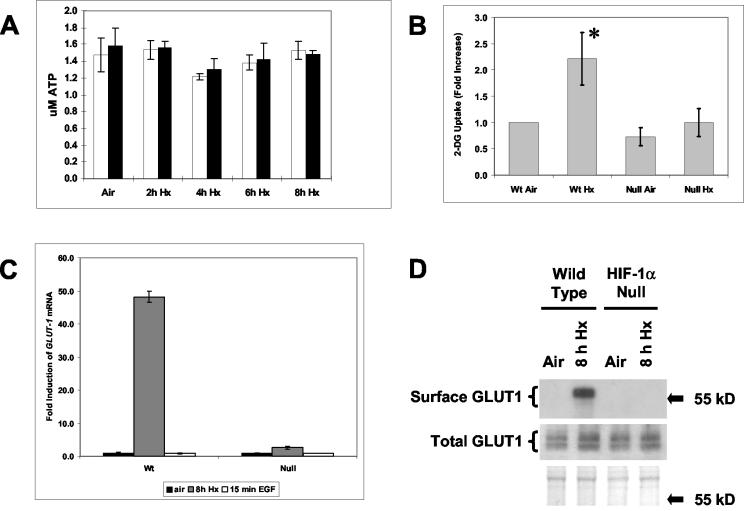
HIF-1 activity is critical for physiological adaptations to low oxygen and glucose conditions (18, 51). Therefore, we reasoned that the deficient responses of MAPK signaling pathways involving JNKs/SAPKs and ERK1/2 found in hypoxic HIF-1α-null cells (Fig. 2 and 4) could be evidence of global defects in HIF-1-regulated processes rather than specific defects in gene expression. To investigate this possibility, we determined whether the absence of HIF-1α expression influenced total cellular ATP production and glucose uptake in HIF-1α-null versus wt cells under the same hypoxic conditions that induced c-Jun N-terminal phosphorylation in wt cells (Fig. 1).
Figure 5A shows that total cellular ATP levels were not significantly different in wt versus HIF-1α-null cells during hypoxic exposure times of 2 to 8 h. This finding agrees with the results of a previous study in which exposure to hypoxia for at least 24 h was required to produce statistically significant decreases in ATP production in immortalized mEFs (50). Thus, total cellular ATP production, which is expected to be dependent on anaerobic glycolysis under these hypoxic conditions (18), did not differ between the wt and HIF-1α-null cells at the maximum exposure time (8 h) used in the present study. There were also no significant differences between wt and HIF-1α-null cells in the ratios of reduced to oxidized pyridine nucleotide reducing equivalents (NADPH/NADP+, NADH/NAD+) following 8 h of hypoxia (data not shown), indicating indirectly that the cellular redox states were similar under these stress conditions (14, 42). Therefore, hypoxia-inducible JNK activity was not stimulated in response to depletion of total ATP in wt cells exposed to hypoxia or anoxia. However, Fig. 5B shows that hypoxic wt cells absorbed significantly more extracellular glucose than HIF-1α-null cells, which were unable to change their glucose uptake between the normoxic and hypoxic states investigated here. This finding is reasonable considering that HIF-1 is an essential regulator of both glucose absorption and flux through the glycolytic pathway, biochemical processes underlying the positive Pasteur effect (18). Figure 5C shows that hypoxia strongly induced expression of GLUT1 mRNA in wt but not HIF-1α-null cells, suggesting that hypoxia-inducible glucose absorption by wt cells was mediated at least in part by upregulated GLUT1 receptors (reviewed in reference 22). Figure 5D indicates that HIF-1-dependent GLUT1 expression ultimately promoted enhanced surface expression of this receptor, consistent with the significant increase in the rate of extracellular glucose uptake detected under these hypoxic conditions (Fig. 5B). HIF-1 has been reported to be the primary transcriptional regulator of the mouse GLUT1 gene in response to hypoxia (15, 40). We have not been able to detect expression of GLUT4, another prominent hypoxia-inducible glucose receptor in some cell types (e.g., cardiac myocytes [56), by using quantitative RT-PCR analysis of total RNA from wt or HIF-1α-null cells (data not shown). Taken together with the information, found at the Entrez-GEO database (http://www.ncbi.nlm.nih.gov/, data set GDS405), that GLUT1 is the major GLUT isoform expressed in cultured normal mEFs, our present findings indicate that inducible GLUT1 expression is mainly responsible for the increased rate of glucose absorption stimulated in wt cells by hypoxia.
FIG. 5.
(A) Total cellular ATP levels are not significantly different between wt and HIF-1α-null mEFs exposed to hypoxia or anoxia. Histogram shows total cellular ATP levels in lysates of wt (open bars) and HIF-1α-null (closed bars) mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia for the indicated times. Error bars, sample standard deviations from triplicate measurements. (B) wt but not HIF-1α-null mEFs exhibit an increased demand for extracellular glucose in response to hypoxia or anoxia. wt and HIF-1α null mEFs were assayed for uptake of 2-deoxy-d-[2,6-3H]glucose (2-DG; 4 Ci/ml) after a 10-min exposure to the label under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). Error bars, sample standard deviations from three independent experiments; *, P ≤ 0.04. For details, see Materials and Methods. (C) Hypoxia or anoxia strongly induces expression of the GLUT1 gene in wt mEFs. Histogram shows relative amounts (fold induction relative to normoxic levels) of GLUT1 mRNA detected by quantitative RT-PCR amplification of total RNA harvested from wt and HIF-1α-null mEFs after exposure to normoxic conditions (air-5% CO2) or hypoxia (pO2, ≤0.01%; 8 h). Error bars, sample standard deviations from triplicate measurements. (D) Hypoxia or anoxia induces surface expression of the GLUT1 receptor on wt mEFs. (Top) Immunoblot of cross-linked GLUT1 protein captured from lysates of wt and HIF-1α-null mEFs exposed to the cell-impermeant cross-linking agent for glucose receptors, Bio-LC-ATB-BGPA. Cells were harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h). Darker exposures show very light bands in the three lanes that appear empty. (Center) Immunoblot of total protein from the wt and HIF-1α-null mEFs used for the cross-linking experiment. The blot was probed with an anti-GLUTI antibody. (Bottom) Ponceau S staining of total protein on the immunoblot shown above. Arrows indicate the position of a marker with an electrophoretic mobility within the range corresponding to GLUT1 (55 kDa).
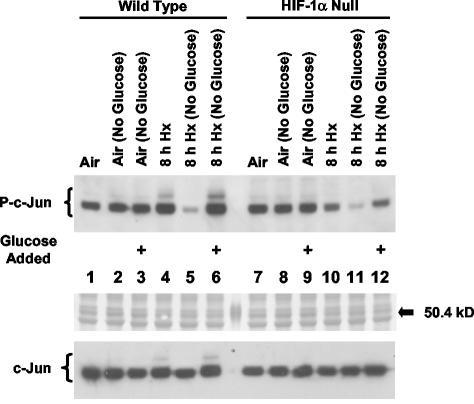
Previously we determined that HIF-1-dependent N-terminal phosphorylation of c-Jun in cells exposed to hypoxia or anoxia is serum independent (27), which suggested that this phenomenon is independent of extracellular factors. However, the glucose concentration in the cell culture medium was a constant in the earlier study. Taken together with the present finding that the ability of hypoxic HIF-1α-null cells to increase glucose uptake from the medium was impaired, this fact suggested that hypoxia-inducible c-Jun phosphorylation and glucose uptake in wt cells are functionally related. To test this possibility, we performed immunoblotting studies with an anti-phospho-c-Jun antibody specific for phospho-Ser63 and/or -Ser73 in order to evaluate the effect on c-Jun N-terminal phosphorylation of the addition of glucose to normoxic and hypoxic wt and HIF-1α-null cells cultured in glucose-free medium for approximately 8 h. Figure 6 shows two important findings of this study. First, the normal induction of c-Jun N-terminal phosphorylation in hypoxic wt but not in hypoxic HIF-α-null cells (Fig. 6; compare lane 4 with lane 10) was eliminated in glucose-free medium (lanes 5 and 11), showing a dependence of this induction on the presence of extracellular glucose. However, basal c-Jun phosphorylation was also greatly inhibited in both cell types in the absence of extracellular glucose (Fig. 6, lanes 5 and 11). Thus, while this particular study suggests that HIF-1-dependent induction of GLUT1 expression is necessary for hypoxia-inducible c-Jun N-terminal phosphorylation, it is unlikely that expression of the receptor alone is sufficient to induce the phosphorylation response. This finding demonstrates that extracellular glucose is essential for both basal and inducible c-Jun N-terminal phosphorylation in hypoxic wt cells. Second, the addition of degassed glucose to hypoxic wt and HIF-1α-null cells cultured in glucose-free medium restored basal and hypoxia-inducible c-Jun N-terminal phosphorylation in wt cells but only basal phosphorylation in HIF-1α-null cells (Fig. 6; compare lane 6 with lane 12). This finding confirms that extracellular glucose is required for basal c-Jun N-terminal phosphorylation in both cell types upon exposure to hypoxia or anoxia, and it demonstrates that the contribution of extracellular glucose to hypoxia-inducible c-Jun N-terminal phosphorylation is HIF-1 dependent. Importantly, because the addition of the same amount of extracellular glucose to HIF-1α-null cells cultured in glucose-free medium had no effect on c-Jun phosphorylation, a contribution of increased osmolality to the induction of c-Jun N-terminal phosphorylation in hypoxic wt cells can be eliminated. Together with the finding that wt cells exhibited an increased uptake of extracellular glucose in response to an identical hypoxic exposure (Fig. 5B), this immunoblotting study suggests that the initial signal responsible for HIF-1-dependent c-Jun N-terminal phosphorylation is the enhanced glucose utilization associated with the transition from normoxic to anaerobic metabolism.
FIG. 6.
HIF-1-dependent c-Jun N-terminal phosphorylation requires the presence of extracellular glucose. (Top) Immunoblot of total protein from wt and HIF-1α-null mEFs harvested under normoxic conditions (air-5% CO2) or following exposure to hypoxia (pO2, ≤0.01%; 8 h) in normal or glucose-free medium (No Glucose; glucose starvation was for 8 h only). The blot was probed with the anti-phospho-c-Jun antibody KM-1 (P-c-Jun). Plus signs indicate glucose-starved cultures to which degassed glucose was added to a final concentration of 4.5 g/liter 1 h before protein was harvested. This immunoblot is representative of two independent experiments. (Center) Ponceau S staining of total protein on the immunoblot shown above. Arrow indicates the position of a marker (between lanes 6 and 7) with an electrophoretic mobility within the range corresponding to c-Jun phospho-isoforms (50.4 kDa). (Bottom) A replicate immunoblot probed with an antibody that recognizes nonphosphorylated c-Jun (H79).
HIF-1-dependent induction of c-Jun N-terminal phosphorylation and c-jun expression in response to hypoxia or anoxia is not an autocrine phenomenon.
Previously we demonstrated that induction of both c-Jun N-terminal phosphorylation and c-jun expression in cells exposed to hypoxia or anoxia is independent of serum (27), indicating that these responses do not require extracellular signals such as polypeptide growth factors. However, this earlier study did not exclude the possibility that a HIF-1-dependent autocrine mechanism could regulate the responses of the c-jun system to hypoxia or anoxia. HIF-1 has been reported to influence gene expression through both autocrine and paracrine signaling pathways (24, 46, 55, 66). To detect a putative HIF-1-dependent autocrine factor(s), we used immunoblotting and quantitative RT-PCR to investigate the hypoxic response of c-Jun N-terminal phosphorylation and c-jun mRNA expression, respectively, in cocultured wt and HIF-1α-null cells. Stimulation of c-Jun N-terminal phosphorylation is generally associated with increased expression of the c-jun gene (26, 59).
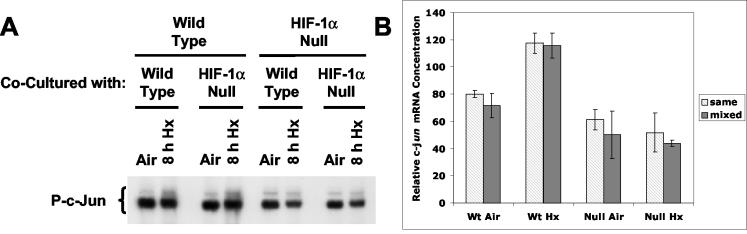
wt and HIF-1α-null cells were cocultured in the same medium in the inserts or wells of modified Boyden chambers, and normoxic and hypoxic cells in the wells were harvested for total cellular protein or RNA. By coculturing wt and HIF-1α-null cells in this arrangement, we expected that a putative autocrine factor expressed from wt cells during hypoxia or anoxia would be detected by its ability to complement the defect in hypoxia-inducible c-Jun N-terminal phosphorylation and/or c-jun mRNA expression in HIF-1α-null cells (27). Figure 7A shows that cocultured wt cells had no effect on c-Jun N-terminal phosphorylation in HIF-1α-null cells during 8 h of hypoxia; only wt cells exhibited the normal hypoxic induction of endogenous c-Jun phosphorylation. Figure 7B shows that cocultured wt cells also had no effect on c-jun mRNA expression in HIF-1α-null cells during 8 h of hypoxia. In agreement with the findings of our previous study (27), only wt cells exhibited hypoxic induction of c-jun mRNA. These findings indicate that neither the induction of c-Jun N-terminal phosphorylation nor c-jun mRNA expression in cells exposed to hypoxia or anoxia involves an autocrine mechanism. Rather, the findings shown in Fig. 5B and 6 support a glucose-dependent mechanism of hypoxia-inducible JNK activity in wt cells.
FIG. 7.
HIF-1-dependent induction of c-Jun N-terminal phosphorylation and c-jun expression in cocultured wt and HIF-1α-null cells exposed to hypoxia or anoxia. (A) Immunoblot of total protein harvested from mEFs in the wells of modified Boyden chambers after exposure to normoxic conditions (air-5% CO2) or hypoxia (pO2, ≤0.01%; 8 h). wt and HIF-1α-null mEFs were plated on either cell-impermeant inserts or companion wells of the chambers and were cocultured in the same medium before and during exposure to hypoxia. The blot was probed with the anti-phospho-c-Jun antibody KM-1 (P-c-Jun). (B) Histogram of relative amounts of c-jun mRNA detected by quantitative RT-PCR amplification of total RNA harvested from mEFs in the wells of modified Boyden chambers after exposure to normoxic conditions (air-5% CO2) or hypoxia (pO2, ≤0.01%; 8 h). Open bars (same), identical genotypes in the chamber or insert; shaded bars (mixed), different genotypes in the chamber or insert. Error bars, sample standard deviations for triplicate measurements. For details, see Materials and Methods.
DISCUSSION
The major conclusion of the present study is that HIF-1-dependent phosphorylation of the c-Jun N-terminal region in cells exposed to hypoxia or anoxia is functionally coupled to glucose utilization (Fig. 1, 5, and 6). This conclusion is biochemically reasonable considering that energy metabolism in hypoxic or anoxic cells is sustained by HIF-1 through positive transcriptional control of both glucose receptor (e.g., GLUT1) and glycolytic enzyme expression (50, 51). Thus, increased demand for glucose during the normal physiological transition from normoxic to glycolytic metabolism (the positive Pasteur effect) generates a signal leading to increased N-terminal phosphorylation of c-Jun. An attractive feature of this model is that it provides a physiological explanation for how the induction of HIF-1 activity in hypoxic or anoxic cells could influence a network of signaling pathways regulating multiple downstream targets, including c-Jun/AP-1.
We determined that JNKs/SAPKs contribute to hypoxia-inducible c-Jun N-terminal phosphorylation in wt cells (Fig. 2). This finding is also biochemically reasonable considering the established importance of the JNK/SAPK pathway for regulating the c-jun gene and c-Jun/AP-1 activity in general (26). ERK1/2 can also phosphorylate the c-Jun N-terminal region (see, e.g., reference 30), but JNKs/SAPKs have been reported to preferentially phosphorylate c-Jun under conditions where both the JNK/SAPK and ERK1/2 pathways are activated (38). We did not detect an induction of ERK1/2 activity in hypoxic wt cells in the present study (Fig. 4). Interestingly, the addition of glucose to hypoxic HIF-1α-null cells did not restore basal ERK1/2 phosphorylation (data not shown). There have been several reports of the activation of MAPKs in various cell types by hypoxia, including JNKs/SAPKs (13, 21, 25, 29, 32, 35, 36, 49, 58), ERK1/2 (10, 13, 21, 33, 39, 43, 53, 58), and p38 MAPK (10, 12, 13, 21, 23, 29, 43). Our present findings indicate at least one pathway by which hypoxia can stimulate JNK/SAPK activity but do not explain the induction of p38 MAPKs by this stress. Potential molecular mechanisms that couple JNK/SAPK activity to changes in intracellular glucose concentrations are under investigation by many groups, although this research is mainly focused on normoxic or oxidative metabolism (37, 54, 62). In terms of hypoxic signaling mechanisms, overexpression of GLUT1 in vascular smooth muscle cells, with artificially increased glucose uptake as a consequence, has been reported to decrease rather than increase hypoxia-inducible JNK/SAPK activation and to inhibit apoptosis, possibly through the effect of enhanced glucose levels on MEKK1 activity (32). We have been unable to detect significant cell death in either wt or HIF-1α-null cells exposed to the same low oxygen conditions that induce c-Jun N-terminal phosphorylation, suggesting that the hypoxic response of JNKs/SAPKs is not simple but may depend on the degree or duration of stress in a particular cell type. AMP-activated protein kinase (AMPK) has been reported to directly stimulate glycolysis in hypoxic or anoxic cells, and sustained AMPK activity can induce JNK/SAPK activity in normoxic liver cells (20). However, the pathways that transmit signals directly associated with glucose uptake to a stress-responsive network containing AMPK are not clear (reviewed in reference 47). Determining exactly how a signal(s) arising from stimulated glucose utilization is able to induce c-Jun N-terminal phosphorylation in cells exposed to hypoxia or anoxia would significantly advance our understanding of the adaptation of both tumor and normal cells to this common physiological and pathophysiological stress.
In summary, using genetically manipulated cells that are wt or null for the hypoxia-responsive transcription factor HIF-1α, we have demonstrated that c-Jun phosphorylation in wt cells exposed to hypoxia or anoxia is directly correlated with enhanced glucose utilization. There was no hypoxia-inducible c-Jun phosphorylation in the absence of extracellular glucose, but the addition of glucose to hypoxic wt cells cultured in glucose-free medium restored this induction. Increased glucose uptake in response to hypoxia was dependent on the expression of HIF-1α and therefore on HIF-1 activity, indicating a pathway in which intracellular glucose levels connect hypoxia-inducible JNK activity with oxygen sensing. It remains to be determined whether an increased rate of glucose uptake or the combination of this uptake and enhanced glycolytic activity is critical for the activation of kinases such as JNKs/SAPKs. wt and HIF-1α-null cells exhibit significant differences in vitro in terms of metabolic phenotypes such as the Pasteur effect (50) and in vivo as experimental fibrosarcomas (16, 57). In the context of the tumor microenvironment, identifying c-Jun/AP-1-dependent genes responsive to HIF-1-dependent glucose flux and elucidating their contribution to tumor development are important areas for further research that will increase our understanding of the role of hypoxia in malignant progression.
Acknowledgments
This work was supported by grants CA73807 and CA82515 from the NIH.
REFERENCES
- 1.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 2.Alfranca, A., M. Gutierrez, A. Vara, J. Aragones, F. Vidal, and M. Landazuri. 2002. c-Jun and hypoxia-inducible factor 1 functionally cooperate in hypoxia-induced gene transcription. Mol. Cell. Biol. 22:12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausserer, W. A., B. Bourrat-Floeck, C. J. Green, K. R. Laderoute, and R. M. Sutherland. 1994. Regulation of c-jun expression during hypoxic and low-glucose stress. Mol. Cell. Biol. 14:5032-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain, J., H. McLauchlan, M. Elliott, and P. Cohen. 2003. The specificities of protein kinase inhibitors: an update. Biochem. J. 371:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandyopadhyay, R. S., M. Phelan, and D. V. Faller. 1995. Hypoxia induces AP-1-regulated genes and AP-1 transcription factor binding in human endothelial and other cell types. Biochim. Biophys. Acta 1264:72-78. [DOI] [PubMed] [Google Scholar]
- 6.Barila, D., R. Mangano, S. Gonfloni, J. Kretzschmar, M. Moro, D. Bohmann, and G. Superti-Furga. 2000. A nuclear tyrosine phosphorylation circuit: c-Jun as an activator and substrate of c-Abl and JNK. EMBO J. 19:273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, K., J. C. Ingram, O. H. Porras, L. F. Barros, E. R. Hudson, L. G. Fryer, F. Foufelle, D. Carling, D. G. Hardie, and S. A. Baldwin. 2002. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J. Cell Sci. 115:2433-2442. [DOI] [PubMed] [Google Scholar]
- 8.Barr, R. K., T. S. Kendrick, and M. A. Bogoyevitch. 2002. Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem. 277:10987-10997. [DOI] [PubMed] [Google Scholar]
- 9.Bentley, J., D. Itchayanan, K. Barnes, E. McIntosh, X. Tang, C. P. Downes, G. D. Holman, A. D. Whetton, P. J. Owen-Lynch, and S. A. Baldwin. 2003. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J. Biol. Chem. 278:39337-39348. [DOI] [PubMed] [Google Scholar]
- 10.Blaschke, F., P. Stawowy, S. Goetze, O. Hintz, M. Grafe, U. Kintscher, E. Fleck, and K. Graf. 2002. Hypoxia activates β1-integrin via ERK 1/2 and p38 MAP kinase in human vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 296:890-896. [DOI] [PubMed] [Google Scholar]
- 11.Chen, E. Y., N. M. Mazure, J. A. Cooper, and A. J. Giaccia. 2001. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 61:2429-2433. [PubMed] [Google Scholar]
- 12.Conrad, P. W., R. T. Rust, J. Han, D. E. Millhorn, and D. Beitner-Johnson. 1999. Selective activation of p38α and p38γ by hypoxia. Role in regulation of cyclin D1 by hypoxia in PC12 cells. J. Biol. Chem. 274:23570-23576. [DOI] [PubMed] [Google Scholar]
- 13.Das, M., D. M. Bouchey, M. J. Moore, D. C. Hopkins, R. A. Nemenoff, and K. R. Stenmark. 2001. Hypoxia-induced proliferative response of vascular adventitial fibroblasts is dependent on G protein-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 276:15631-15640. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson, D. A., and H. J. Forman. 2002. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 64:1019-1026. [DOI] [PubMed] [Google Scholar]
- 15.Ebert, B. L., J. D. Firth, and P. J. Ratcliffe. 1995. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J. Biol. Chem. 270:29083-29089. [DOI] [PubMed] [Google Scholar]
- 16.Grunstein, J., W. G. Roberts, O. Mathieu-Costello, D. Hanahan, and R. S. Johnson. 1999. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 59:1592-1598. [PubMed] [Google Scholar]
- 17.Hall, J. P., and R. J. Davis. 2002. Analysis of c-Jun N-terminal kinase regulation and function. Methods Enzymol. 345:413-425. [DOI] [PubMed] [Google Scholar]
- 18.Hochachka, P. W., and G. N. Somero. 2002. Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, London, United Kingdom.
- 19.Huang, L. E., and H. F. Bunn. 2003. Hypoxia-inducible factor and its biomedical relevance. J. Biol. Chem. 278:19575-19578. [DOI] [PubMed] [Google Scholar]
- 20.Hue, L., C. Beauloye, L. Bertrand, S. Horman, U. Krause, A. S. Marsin, D. Meisse, D. Vertommen, and M. H. Rider. 2003. New targets of AMP-activated protein kinase. Biochem. Soc. Trans. 31:213-215. [DOI] [PubMed] [Google Scholar]
- 21.Jin, N., N. Hatton, D. R. Swartz, X. Xia, M. A. Harrington, S. H. Larsen, and R. A. Rhoades. 2000. Hypoxia activates Jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am. J. Respir. Cell Mol. Biol. 23:593-601. [DOI] [PubMed] [Google Scholar]
- 22.Joost, H. G., and B. Thorens. 2001. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol. Membr. Biol. 18:247-256. [DOI] [PubMed] [Google Scholar]
- 23.Kayyali, U. S., C. Donaldson, H. Huang, R. Abdelnour, and P. M. Hassoun. 2001. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J. Biol. Chem. 276:14359-14365. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamachary, B., S. Berg-Dixon, B. Kelly, F. Agani, D. Feldser, G. Ferreira, N. Iyer, J. LaRusch, B. Pak, P. Taghavi, and G. L. Semenza. 2003. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 63:1138-1143. [PubMed] [Google Scholar]
- 25.Kunz, M., S. Ibrahim, D. Koczan, H. J. Thiesen, H. J. Kohler, T. Acker, K. H. Plate, S. Ludwig, U. R. Rapp, E. B. Brocker, G. N. van Muijen, E. Flory, and G. Gross. 2001. Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth Differ. 12:137-145. [PubMed] [Google Scholar]
- 26.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 27.Laderoute, K. R., J. M. Calaoagan, C. Gustafson-Brown, A. M. Knapp, G. C. Li, H. L. Mendonca, H. E. Ryan, Z. Wang, and R. S. Johnson. 2002. The response of c-Jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1α dependent. Mol. Cell. Biol. 22:2515-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laderoute, K. R., J. M. Calaoagan, H. L. Mendonca, W. A. Ausserer, E. Y. Chen, A. J. Giaccia, and R. M. Sutherland. 1996. Early responses of SiHa human squamous carcinoma cells to hypoxic signals: evidence of parallel activation of NF-κB and AP-1 transcriptional complexes. Int. J. Oncol. 8:875-882. [DOI] [PubMed] [Google Scholar]
- 29.Laderoute, K. R., H. L. Mendonca, J. M. Calaoagan, A. M. Knapp, A. J. Giaccia, and P. J. Stork. 1999. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J. Biol. Chem. 274:12890-12897. [DOI] [PubMed] [Google Scholar]
- 30.Leppa, S., R. Saffrich, W. Ansorge, and D. Bohmann. 1998. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 17:4404-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, A., J. Frost, T. Deng, T. Smeal, N. al-Alawi, U. Kikkawa, T. Hunter, D. Brenner, and M. Karin. 1992. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell 71:777-789. [DOI] [PubMed] [Google Scholar]
- 32.Lin, Z., J. M. Weinberg, R. Malhotra, S. E. Merritt, L. B. Holzman, and F. C. Brosius III. 2000. GLUT-1 reduces hypoxia-induced apoptosis and JNK pathway activation. Am. J. Physiol. Endocrinol. Metab. 278:E958-E966. [DOI] [PubMed] [Google Scholar]
- 33.Lo, L. W., J. J. Cheng, J. J. Chiu, B. S. Wung, Y. C. Liu, and D. L. Wang. 2001. Endothelial exposure to hypoxia induces Egr-1 expression involving PKCα-mediated Ras/Raf-1/ERK1/2 pathway. J. Cell. Physiol. 188:304-312. [DOI] [PubMed] [Google Scholar]
- 34.Mechta-Grigoriou, F., D. Gerald, and M. Yaniv. 2001. The mammalian Jun proteins: redundancy and specificity. Oncogene 20:2378-2389. [DOI] [PubMed] [Google Scholar]
- 35.Minet, E., T. Arnould, G. Michel, I. Roland, D. Mottet, M. Raes, J. Remacle, and C. Michiels. 2000. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 468:53-58. [DOI] [PubMed] [Google Scholar]
- 36.Minet, E., G. Michel, D. Mottet, J. P. Piret, A. Barbieux, M. Raes, and C. Michiels. 2001. c-JUN gene induction and AP-1 activity is regulated by a JNK-dependent pathway in hypoxic HepG2 cells. Exp. Cell Res. 265:114-124. [DOI] [PubMed] [Google Scholar]
- 37.Moley, K. H., and M. M. Mueckler. 2000. Glucose transport and apoptosis. Apoptosis 5:99-105. [DOI] [PubMed] [Google Scholar]
- 38.Morton, S., R. J. Davis, A. McLaren, and P. Cohen. 2003. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 22:3876-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mottet, D., G. Michel, P. Renard, N. Ninane, M. Raes, and C. Michiels. 2003. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J. Cell. Physiol. 194:30-44. [DOI] [PubMed] [Google Scholar]
- 40.Murakami, T., T. Nishiyama, T. Shirotani, Y. Shinohara, M. Kan, K. Ishii, F. Kanai, S. Nakazuru, and Y. Ebina. 1992. Identification of two enhancer elements in the gene encoding the type 1 glucose transporter from the mouse which are responsive to serum, growth factor, and oncogenes. J. Biol. Chem. 267:9300-9306. [PubMed] [Google Scholar]
- 41.Plyte, S. E., K. Hughes, E. Nikolakaki, B. J. Pulverer, and J. R. Woodgett. 1992. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim. Biophys. Acta 1114:147-162. [DOI] [PubMed] [Google Scholar]
- 42.Powis, G., J. R. Gasdaska, and A. Baker. 1997. Redox signaling and the control of cell growth and death. Adv. Pharmacol. 38:329-359. [DOI] [PubMed] [Google Scholar]
- 43.Premkumar, D. R., G. Adhikary, J. L. Overholt, M. S. Simonson, N. S. Cherniack, and N. R. Prabhakar. 2000. Intracellular pathways linking hypoxia to activation of c-fos and AP-1. Adv. Exp. Med. Biol. 475:101-109. [DOI] [PubMed] [Google Scholar]
- 44.Rahmsdorf, H. J. 1996. Jun: transcription factor and oncoprotein. J. Mol. Med. 74:725-747. [DOI] [PubMed] [Google Scholar]
- 45.Rupec, R. A., and P. A. Baeuerle. 1995. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-κB. Eur. J. Biochem. 234:632-640. [DOI] [PubMed] [Google Scholar]
- 46.Ruscher, K., D. Freyer, M. Karsch, N. Isaev, D. Megow, B. Sawitzki, J. Priller, U. Dirnagl, and A. Meisel. 2002. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J. Neurosci. 22:10291-10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutter, G. A., G. Da Silva Xavier, and I. Leclerc. 2003. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 375:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez, I., R. T. Hughes, B. J. Mayer, K. Yee, J. R. Woodgett, J. Avruch, J. M. Kyriakis, and L. I. Zon. 1994. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372:794-798. [DOI] [PubMed] [Google Scholar]
- 49.Scott, P. H., A. Paul, C. M. Belham, A. J. Peacock, R. M. Wadsworth, G. W. Gould, D. Welsh, and R. Plevin. 1998. Hypoxic stimulation of the stress-activated protein kinases in pulmonary artery fibroblasts. Am. J. Respir. Crit. Care Med. 158:958-962. [DOI] [PubMed] [Google Scholar]
- 50.Seagroves, T. N., H. E. Ryan, H. Lu, B. G. Wouters, M. Knapp, P. Thibault, K. Laderoute, and R. S. Johnson. 2001. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells. Mol. Cell. Biol. 21:3436-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semenza, G. L. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3:721-732. [DOI] [PubMed] [Google Scholar]
- 52.Shaulian, E., and M. Karin. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131-E136. [DOI] [PubMed] [Google Scholar]
- 53.Song, M. S., Y. K. Park, J. H. Lee, and K. Park. 2001. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase C-ɛ/ERK/AP-1 signaling cascade. Cancer Res. 61:8322-8330. [PubMed] [Google Scholar]
- 54.Spitz, D. R., J. E. Sim, L. A. Ridnour, S. S. Galoforo, and Y. J. Lee. 2000. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann. N. Y. Acad. Sci. 899:349-362. [DOI] [PubMed] [Google Scholar]
- 55.Stoeltzing, O., W. Liu, N. Reinmuth, F. Fan, A. A. Parikh, C. D. Bucana, D. B. Evans, G. L. Semenza, and L. M. Ellis. 2003. Regulation of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am. J. Pathol. 163:1001-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun, D., N. Nguyen, T. R. DeGrado, M. Schwaiger, and F. C. Brosius III. 1994. Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation 89:793-798. [DOI] [PubMed] [Google Scholar]
- 57.Unruh, A., A. Ressel, H. G. Mohamed, R. S. Johnson, R. Nadrowitz, E. Richter, D. M. Katschinski, and R. H. Wenger. 2003. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene 22:3213-3220. [DOI] [PubMed] [Google Scholar]
- 58.Vasilevskaya, I. A., and P. J. O'Dwyer. 1999. Effects of geldanamycin on signaling through activator-protein 1 in hypoxic HT29 human colon adenocarcinoma cells. Cancer Res. 59:3935-3940. [PubMed] [Google Scholar]
- 59.Vogt, P. K. 2001. Jun, the oncoprotein. Oncogene 20:2365-2377. [DOI] [PubMed] [Google Scholar]
- 60.Webster, K. A., D. J. Discher, and N. H. Bishopric. 1993. Induction and nuclear accumulation of Fos and Jun proto-oncogenes in hypoxic cardiac myocytes. J. Biol. Chem. 268:16852-16858. [PubMed] [Google Scholar]
- 61.Wenger, R. H. 2002. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16:1151-1162. [DOI] [PubMed] [Google Scholar]
- 62.Widegren, U., J. W. Ryder, and J. R. Zierath. 2001. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol. Scand. 172:227-238. [DOI] [PubMed] [Google Scholar]
- 63.Wisdom, R. 1999. AP-1: one switch for many signals. Exp. Cell Res. 253:180-185. [DOI] [PubMed] [Google Scholar]
- 64.Xu, L., K. Xie, N. Mukaida, K. Matsushima, and I. J. Fidler. 1999. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res. 59:5822-5829. [PubMed] [Google Scholar]
- 65.Yao, K. S., S. Xanthoudakis, T. Curran, and P. J. O'Dwyer. 1994. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol. Cell. Biol. 14:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zudaire, E., A. Martinez, and F. Cuttitta. 2003. Adrenomedullin and cancer. Regul. Pept. 112:175-183. [DOI] [PubMed] [Google Scholar]