Abstract
Neuregulin 1 (NRG1) is induced in granulosa cells by LH and acts on granulosa and cumulus cells during ovulation. In this study, we sought to determine the role of NRG1 in oocyte maturation by generating a granulosa cell–specific Nrg1 knockout mouse (Nrg1flox/flox;Cyp19a1Cre mice [gcNrg1KO]). In the gcNrg1KO mice, meiosis was induced 2 hours earlier than in control mice. More than 60% of the oocytes in the mutant mice spontaneously re-resumed meiosis beyond the MII stage. The percentage of successful fertilization was comparable in oocytes of both genotypes collected at 14 or 16 hours after human chorionic gonadotropin injection but was significantly lower in oocytes of the gcNrg1KO mice at 18 or 20 hours. The number of pups per litter was significantly decreased in gcNrg1KO mice. To determine the molecular events associated with the abnormal progression of meiosis in the gcNrg1KO mouse oocytes, the defects of cumulus/granulosa cell functions were analyzed. The expression of genes involved in luteinization and cumulus expansion was significantly higher at 2 hours after human chorionic gonadotropin injection in the gcNrg1KO mice; this was related to abnormal activation of protein kinase C (PKC) and phosphorylation of connexin-43 in cumulus cells. Changes in connexin-43 by PKC might lead to early meiotic resumption of oocytes in gcNrg1KO mice. We conclude that NRG1 is induced by LH in mural granulosa cells and exerts an important regulatory role in oocyte meiotic maturation and competence by reducing PKC activation in cumulus cells and preventing premature progression to the MII stage that leads to abnormal fertilization and fertility.
The surge of LH stimulates ovulation, a process that permits the release of the cumulus cell–oocyte complex (COC) from the ovarian surface. The LH surge also leads to the reinitiation of meiosis and the development of oocytes competent for fertilization. Recent studies have revealed that the effects of LH on ovulation and oocyte maturation are mediated, in part, by the induced expression of the epidermal growth factor (EGF)–like factors, amphiregulin (AREG), epiregulin (EREG), and β-cellulin (BTC) in mural granulosa cells (1). These ligands then bind ERBB1, the EGF receptor (EGFR) present in granulosa and cumulus cells, leading to activation of a RAS-MEK1-ERK1/2 pathway (2–5). In our previous study (4), we showed that targeted disruption of ERK1/2 in granulosa cells and cumulus cells did not affect follicular development to the preovulatory stage but did suppress events initiated by the LH surge: granulosa cell luteinization, cumulus cell differentiation, and oocyte meiotic maturation. These results indicated that activation of ERK1/2 is required for the induction of the ovulation process. Targeted deletion of the ligands (Areg and Ereg) or the EGFR (Erbb1 and Egfr) suppressed the activation of ERK1/2 in cumulus cells and led to a subfertile phenotype (2, 6). These reports suggested that an EGF-like ligand acts on ERBB expressed on granulosa cells and cumulus cells to regulate ovulation or oocyte quality.
Neuregulin 1 (NRG1) is a member of the EGF-like factor family that binds ERBB3, leading to the formation of a heterodimeric complex between ERBB3 and ERBB2 (7, 8) but does not bind the EGFR (ERBB1). Nrg1 is expressed in the brains of embryonic and adult mice (9, 10) but is also induced in mural granulosa cells of periovulatory follicles (11). LH activation of ERBB2 and ERBB3 occurs as early as 2 hours after human chorionic gonadotropin (LH) injections in vivo and thus concomitantly with the induction of Nrg1 type III expression in mural granulosa cells but not in cumulus cells (11, 12), indicating that NRG1 might affect early events in ovulation and/or oocyte maturation. This is supported by the observation that NRG1 can suppress the spontaneous resumption of meiosis that occurs when COCs are isolated from preovulatory follicles in the absence of any hormone treatments and can improve the developmental competence of oocytes in in vitro fertilization (IVF) (11). However, the mechanisms by which NRG1 regulates oocyte functions remain to be determined. Therefore, the following studies were designed to determine the mechanisms by which NRG1 acts on somatic cells to regulate proper progression of oocyte maturation. For this we have used an Nrg1 mutant mouse model (13).
Materials and Methods
Materials
Pregnant mare serum gonadotropin/equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG) were purchased from Asuka Seiyaku. AREG and NRG1 were obtained from R&D Systems, Inc. DMEM/F12 medium and penicillin-streptomycin were from Invitrogen, fetal bovine serum was from Life Technologies, Inc, oligonucleotide poly(dT) was from Invitrogen, avian myeloblastosis virus reverse transcriptase was from Promega, and routine chemicals and reagents were obtained from Sigma-Aldrich or Nakarai Chemical Co. Anti-neuregulin 1 antibody (catalog no. ab53104) was purchased from Abcam. Anti-StAR antibody (catalog no. K1209) was purchased from Santa Cruz Biotechnology. Anti-phosphorylated cAMP response element-binding protein (CREB) antibody (catalog no. 9198), anti-connexin-43 (total Cx43, catalog no. 3512) antibody, anti-phosphorylated (Ser368) connexin-43 (pCx-43 S368, catalog no. 3511), anti-phosphorylated ERK1/2 antibody (phospho-p44/42 MAPK [Thr202/Tyr204], catalog no. 4376), and anti-total ERK1/2 antibody (p44/42 MAPK mAb, catalog no. 4696) were purchased from Cell Signaling Technology, Inc. Anti-acetylated tubulin antibody (catalog no. 081M4760) and anti-β-actin antibody (catalog no. 128K4805) were from Sigma-Aldrich.
Animals
Wild-type (WT) C57BL/6j female mice were obtained from Charles River Laboratories Japan, Inc. Mice lacking NRG1 in granulosa cells (gcNrg1KO) were generated by crossing Cyp19-Cre (3) mice with previously reported Nrg1flox/flox (13) mice (a kind gift from Dr. Carmen Birchmeier, Max Delbrueck Center for Molecular Medicine, Berlin, Germany). Animals were housed under a 14-hour light, 10-hour dark schedule, were provided food and water ad libitum and were treated in the Experiment Animal Center at Hiroshima University or the Center for Comparative Medicine at Baylor College of Medicine and provided with food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the animal care and use committee at Baylor College of Medicine or at Hiroshima University.
To study ovarian responses to exogenous gonadotropins, 21-day-old immature females were analyzed to avoid the complexity of ovarian functions associated with estrous cycles and endogenous surges of gonadotropins. Immature mice were injected ip with 4 IU of eCG (Asuka Seiyaku) to stimulate preovulatory follicle development followed 48 hours later with 5 IU of hCG (Asuka Seiyaku) to stimulate ovulation and luteinization.
To determine the formation of functional corpora lutea, more than 8-week-old female mice were mated with adult males. The ovaries were collected at day 5 after mating (observation of plug formation), and the number of corpora lutea was counted under a stereomicroscope. The ovaries were used for immunohistochemistry or RT-PCR study.
COC isolation to examine the kinetic changes of meiotic resumption and progression in vivo
Immature WT or gcNrg1KO mice were injected ip with 4 IU of eCG followed 48 hours later with 5 IU of hCG. The ovaries or oviducts were collected before or at 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, or 24 hours after hCG injection. COCs were isolated from antral follicles or oviducts by needle puncture and collected by pipette. The COCs were treated with 10 IU/mL hyaluronidase and stripped of cumulus cells by repeated pipetting. The denuded oocytes were fixed in 4% (w/v) paraformaldehyde to determine their meiotic status. The procedure was done within 10 minutes after killing of each animal.
Mating test and in vivo fertility test
The mating experiment was conducted using 5 females of each genotype (Nrg1flox/flox, WT, and Nrg1flox/flox;Cyp19a1Cre, gcNrg1KO mice) that were 8 weeks old at the outset. Adult males were placed in the cages for 6 months, and the number of pups in each litter and the number of litters were recorded.
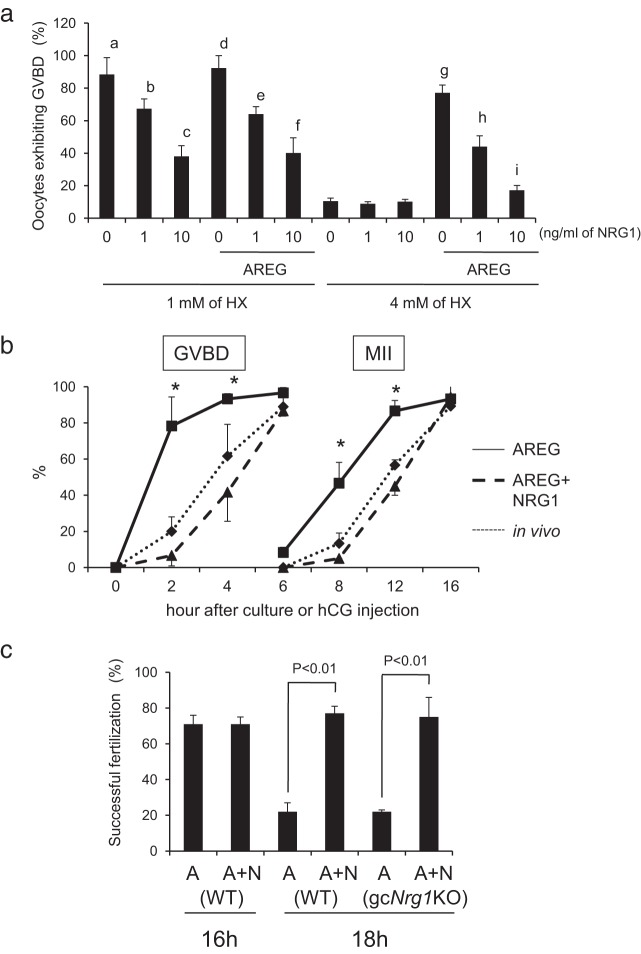
To examine the fertilization ability of oocytes in vivo, immature females were injected ip with 4 IU of eCG followed 48 hours later with 5 IU of hCG. An adult proven fertile male was placed in each cage (mated with an hCG-injected female mice) at 0, 5, 10, or 15 hours after hCG injection. The mice were checked for the plug formation every hour to determine the time of mating. At 30 hours after hCG injection, the eggs were recovered from oviducts and stained with immunofluorescent tubulin to determine the numbers of pronuclei and sperm tails in each egg. The relationship between the percentage of oocytes that were successfully fertilized (1 female pronuclei and 1 male pronuclei with sperm tail) is shown on the y-axis, and the time of plug formation after the hCG injection shown on the x-axis was analyzed in both genotypes (Figure 3D).
Figure 3.
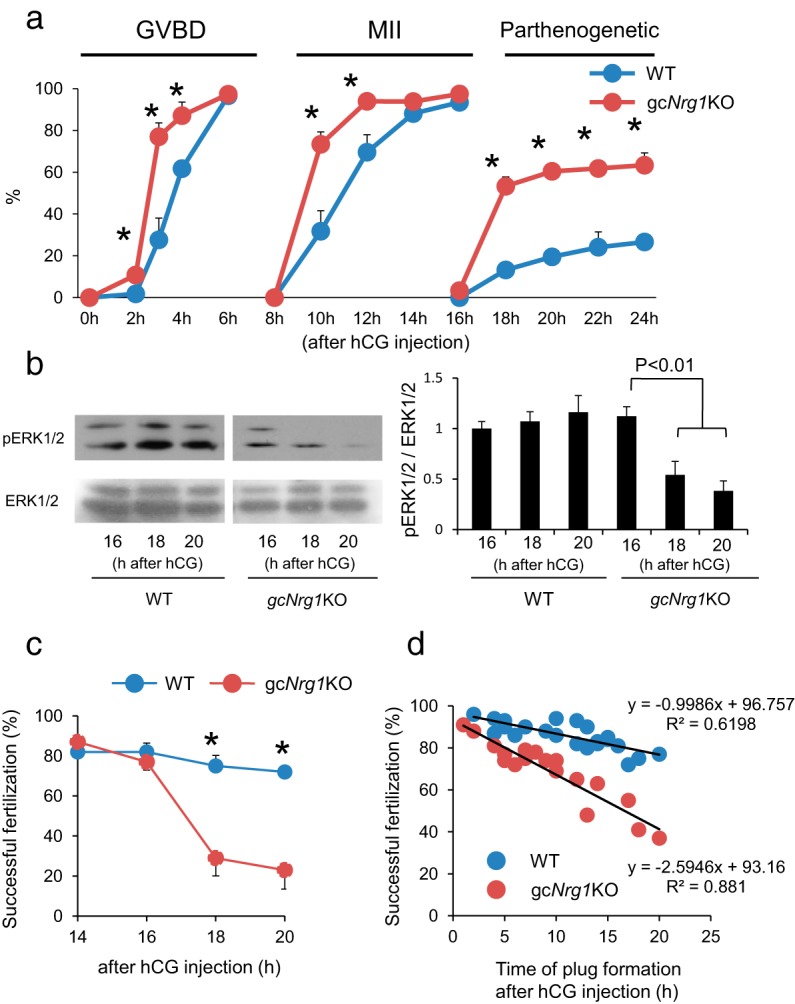
The kinetic changes of meiotic progression and fertilization of oocytes in gcNrg1KO mice. A, Timing of GVBD, arrest at the MII stage, or remeiotic resumption beyond the MII stage in oocytes of gcNrg1KO and WT mice after hCG was analyzed. Oocytes were collected from preovulatory follicles, periovulatory follicles, or oviducts of hormone (eCG-hCG)-primed female mice and stained by DAPI and anti-acetylated tubulin. Oocytes at each time point were collected from at least 5 female mice of each genotype (at the 2, 3, or 4 hour point; 10 female mice were used for each time point). *, P < .05, significant differences were observed between the genotypes at each time point. Values are means ± SEM of data from more than 5 female mice. % GVBD, the percentage of oocytes exhibiting germinal vesicle break down; MII, the percentage of oocytes arrested at meiotic metaphase II stage; parthenogenetic, the percentage of oocytes that reresumed meiosis from the MII stage to anaphase II, telophase II, or pronuclear stage. B, Phosphorylation level of ERK1/2 (p44/p42MAPK, MAPK3/1) in ovulated oocytes recovered from WT or gcNrg1KO mice at 16, 18, or 20 hours after hCG injection. The intensity of the bands was analyzed using a Gel-Pro Analyzer. Values are means ± SEM of 3 replicates. The phosphorylation level of ERK1/2 in the16 hours WT oocytes was set as 1. Significant differences were observed in oocytes of gcNrg1KO mice by the additional period (18 or 20 hours point) after hCG injection as compared with that at the 16 hour point. C, IVF of oocytes was reduced in a time-dependent manner in gcNrg1KO but not in WT mice. The ovulated oocytes were recovered from the oviducts of at least 3 female mice of each genotype at 14, 16, 20, or 24 hours after hCG and then analyzed by IVF protocols. The y-axis shows the percentage of successful fertilization (2 pronuclei and sperm tail). *, P < .05, significant differences were observed between the genotypes. Values are means ±SEM of 3 replicates. D, Relationship between the time of mating (plug formation) and successful in vivo fertilization of oocytes (1 female pronuclei and 1 male pronuclei with sperm tail as described in image A in Supplemental Figure 3) was determined in gcNrg1KO and WT mice mated with WT male mice. WT female mice (n = 22) or KO female mice (n = 18) were injected with eCG followed 48 hours later by hCG. An adult proven fertile male was placed in each cage at 0, 5, 10, or 15 hours after hCG injection. The mice were checked for the plug formation every 1 hour to determine the time of mating. The rate of successful fertilization (1 female pronuclei and 1 male pronuclei with sperm tail) was shown in y-axis and the time of plug formation after hCG injection was shown in x-axis.
IVF
IVF was analyzed as described previously (14). COCs were collected from oviducts at 14, 16, 18, or 20 hours after hCG injection in vivo or were matured in vitro as described below for 16 or 18 hours. The COCs were placed in 50 μL of human tubal fluid medium. Spermatozoa were collected from the cauda epididymis of 4-month-old ICR mice into 500 μL of human tubal fluid medium. After a 60-minute incubation to induce sperm capacitation, the spermatozoa were introduced into the fertilization medium at a final concentration of 1000 spermatozoa/μL. Twelve hours after insemination, some oocytes were examined for the numbers of pronuclei and sperm tails as described below. Other gametes were further cultured for an additional day in the developing medium (KSOM+AA; Millipore) to check for development to the blastocyst stage.
COC isolation for culture
Ovaries of immature mice primed with eCG for 48 hours contain multiple preovulatory follicles. COCs were isolated from these follicles by needle puncture and collected by pipette. Nonexpanded COCs were selected, and groups of 50 COCs were cultured in a 50-μL drop of defined medium containing 1% fetal bovine serum with or without 100 ng/mL AREG and/or 1 or 10 ng/mL NRG1 in a 35-mm dish in the presence of 1 or 4 mM hypoxanthine (HX) (Sigma-Aldrich) (11). The meiotic status (disappearance of germinal vesicle [GV] or emission of the first polar body) was observed every 2 hours for up to 16 hours. COCs cultured for 1 or 2 hours were used to prepare protein lysates or to extract RNA (see below). COCs cultured for 16 hours were used for IVF analyses.
RNA extraction and quantitative PCR analyses
Total RNA was obtained from mural granulosa cells or cumulus cells of COCs using the RNAeasy Mini Kit (Qiagen Sciences) according to the manufacturer's instructions. Total RNA was reverse transcribed using 500 ng of poly(dT) and 0.25 U of avian myeloblastosis virus–RT at 42°C for 75 minutes and 95°C for 5 minutes. Quantitative real-time PCR analyses were performed as described previously (15). In brief, cDNA and primers shown in Supplemental Table 1 were added to 15 μL of the total reaction volume of the Power SYBR Green PCR Master Mix (Applied Biosystems). PCR reactions were then performed using a StepOne real-time PCR system (Applied Biosystems). Conditions were set to the following parameters: 10 minutes at 95°C followed by 45 cycles each of 15 seconds at 95°C and 1 minute at 64°C. L19 was used as a control for reaction efficiency and variations in concentrations of mRNA in the original RT reaction.
Western blot analyses
Whole ovaries or COCs were lysed with radioimmunoprecipitation assay buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% [v/v] Nonidet P-40, 0.5% [w/v] sodium deoxycholate, 1 mM EDTA, and 0.1% [w/v] SDS) containing complete protease inhibitors (Roche Diagnostics GmbH). Twenty oocytes were lysed with 10 μL of SDS sample buffer. Western blot analyses were performed according to our previous study (11). In brief, extracts (10 μg of protein) or oocyte lysates were resolved by SDS-polyacrylamide gel (12.5%) electrophoresis and transferred to polyvinylidene difluoride membranes (GE Bioscience). Membranes were blocked in Tris-buffered saline and Tween 20 (TBST; 10 mM Tris [pH 7.5], 150 mM NaCl, and 0.05% [v/v] Tween 20) containing 5% (w/v) nonfat Carnation instant milk (Nestle Co). Blots were incubated with primary antibodies. The primary antibodies were used at 1:1000 dilutions except for anti-EGF domain of NRG1 antibody (1:5000) or anti-tublin antibody (1:10,000) as shown in Supplemental Table 2 overnight at 4°C. After washing in TBST, enhanced chemiluminescence detection was performed using an enhanced chemiluminescence system according the manufacture's specifications (GE Bioscience) and appropriate exposure of the blots to Fuji x-ray film (Fujifilm). The intensity of the bands was analyzed using a Gel-Pro analyzer (Media Cybernetics).
Generation of EGF domain of NRG1 antibodies
Rabbit polyclonal antibodies were raised against the peptide (amino acid sequence, PNEFTGDR) that is unique to the EGF domain of NRG1 compared with other EGF-like factor family members and is encoded by exon 3 of the Nrg1 gene. Because in Nrg1flox/flox mice, a floxed allele that contained loxP sites inserted into intron 2 and intron 5 of type III Nrg1 (accession no. NM_178591.2) was generated (13), the antibody selectively detects the WT of NRG1 but not the mutant type of NRG1. Polyclonal antibodies were generated by Scrum. In brief, peptides were coupled to keyhole limpet hemocyanin for subcutaneous inoculation of rabbits. After 4 and 6 weeks, the serum was harvested, and the titer of the serum was measured against the immunogen peptide (data not shown).
Immunohistochemistry
Ovaries were collected and fixed in 4% (w/v) paraformaldehyde (Nakarai) overnight, dehydrated in 70% (v/v) ethanol, and embedded in paraffin. The fixed sections (7 μm) embedded in paraffin were deparaffinized in xylene washes and quenched with 3% hydrogen peroxide in methanol. The sections were incubated with 20% (v/v) nonimmune goat serum/PBS to block nonspecific sites followed by incubation with the primary anti-StAR antibody (dilution rate of 1:500 as shown in Supplemental Table 2) overnight at 4°C. The positive signals were visualized using a VECTASTAIN Life ABC Rabbit IgG Kit (Vector Laboratories) according to the manufacturer's recommendations.
Immunofluorescence
Ovaries were collected and fixed in 4% paraformaldehyde, embedded in paraffin, and processed by routine procedures as described above. Sections were probed with the EGF domain of NRG1 antiserum (dilution rate of 1:5000) and visualized with Cy3-conjugated goat anti-rabbit IgG (1:200, Sigma-Aldrich) and 4′,6-diamidino-2-phenylindole (DAPI) (VECTASHIELD Mounting Medium with DAPI; Vector Laboratories). Digital images were captured using a BZ-9000 microscope (Keyence) with a ×20 objective. For all experiments, the exposure times were kept the same for hCG-injected ovaries in WT or gcNrg1KO mice for 0- and 2-hour samples.
For detection of the nuclear status of oocytes, denuded oocytes were fixed in 4% (w/v) paraformaldehyde and then were treated with 0.1% (v/v) Triton X-100 for permeabilized. The oocytes were incubated in 4% (w/v) BSA containing PBS for 1 hour and then probed with anti-acetyltublin antibody (dilution rate 1:200) overnight at 4°C. After washing by 0.01% (v/v) Tween 20–containing PBS, the oocytes were visualized with Cy3-conjugated goat anti-rabbit IgG (1:200) and DAPI. Digital images were captured using a Keyence BZ-9000 microscope to analyze the meiotic status, germinal vesicle (GV) stage, MI stage, MII stage, and anaphase or telophase II stage and the numbers of pronuclei and sperm tails in fertilized eggs.
Transient transfection and luciferase reporter assay
Granulosa cells from eCG-primed WT or gcNrg1KO mice were cultured as described previously (15, 16). After 3 hours, cells were transfected with 0.5 μg of Ptgs2 promoter-reporter construct (4), Areg promoter-reporter construct (17), Ereg promoter-reporter construct (18), or Snap25 promoter-reporter construct (15) and 10 ng of pRL Renilla luciferase control vector (Promega) using FuGENE 6 (Roche Molecular Biochemicals), according to the manufacturer's instructions. After overnight culture, cells were washed in serum-free medium and then were placed in the same medium containing 10 μM forskolin, 20 nM phorbol 12-myristate 13-acetate (PMA), or both. After 4 hours of agonist treatment, the luciferase activity was measured using the Dual-Glo Luciferase Assay System (Promega). Firefly luciferase activities were normalized by Renilla luciferase activities. Each experiment was performed in triplicate at least 3 times.
Protein kinase A (PKA) and protein kinase C (PKC) activity measurement in granulosa cells of preovulatory and periovulatory follicles
Before or at 2 hours after hCG injection, granulosa cells were collected from preovulatory or periovulatory follicles and stored at −80°C. The COCs cultured with AREG and/or NRG1 for 2 hours were also collected. All samples were dissolved in whole-cell lysis buffer with both protein inhibitor cocktail and phosphatase inhibitor cocktail (Roche Diagnostics GmbH). Protein samples were sonicated, and the protein concentration was measured using a DC Protein Assay Kit (Bio-Lad laboratories). A quantity of 10 μg of each extract was used for the PKA or PKC kinase assay with a PKA kinase activity kit (Enzo Life Sciences) or a PKC kinase activity kit (Enzo Life Sciences), according to the instruction manual. In brief, the protein samples were incubated with ATP in each well of a 96-microtiter plate on which PKA substrate or PKC substrate was precoated. After incubation, the phospho-specific substrate antibodies were added to each well. The conjugation of phosphorylated substrates and phospho-specific substrate antibodies were reacted with secondary antibody conjugated with peroxidase. The assay is developed with tetramethylbenzidine substrate, and the intensity of the color is measured as PKA or PKC phosphotransferase activity in a microplate reader at 450 nm (Bio-Rad Laboratories).
Detection of intracellular Ca2+ ([Ca2+]i) in cumulus cells of COCs
Intracellular Ca2+ ([Ca2+]i) in cumulus cells of COCs was detected as described previously (19). In brief, 50 COCs were incubated in a 50 -μL drop with Fura-2/AM (final concentration 5 μM; Calbiochem) and 0.02% Pluronic F-127 (Invitrogen). After 30 minutes in culture under dark conditions, the COCs were washed 2 times to remove excess Fura-2/AM in the medium. The COCs were moved to 100 μL of DMEM/F12 medium in 96-well plates and then AREG and/or NRG1 was added to each well. The intensity of fluorescence was measured as the level of the 334 nm/365 nm Fura-2 fluorescence ratio every 15 seconds by a fluorescence microplate reader.
Calpain activity assay
Calpain activity was measured using a SensoLyte 520 fluorimetric calpain activity assay kit (AnaSpec) according to our previous article (19). In brief, granulosa cells were lysed with buffer included in the assay kit. Then 10 μg of protein sample was incubated with an internally quenched 5-FAM/OXLTM 520 FRET substrate. Activated calpain cleaves the FRET substrate, yielding the release of fluorescent 5-FAM, which was monitored at 520 nm emission and 490 nm excitation by a 96-well fluorescence microplate reader (BD Biosciences).
Progesterone assay
Progesterone in the cultured medium was measured by a specific AIA-1800 system (Tosoh) as described previously (20).
Statistics
Statistical analyses of all data from 3 or 4 replicates for comparison were performed by one-way ANOVA followed by a Duncan multiple-range test (Statview; Abacus Concepts, Inc).
Results
Fertilization is impaired in the gcNrg1KO mice
Genomic depletion of Nrg1 leads to lethality at embryonic day 10 (21), precluding any studies of NRG1 functions in adult mice. To bypass this severe limitation, we generated mural granulosa and cumulus cell–specific Nrg1 knockout mice (gcNrg1KO mice) by mating Nrg1flox/flox mice to Cyp19a1Cre mice expressing Cre recombinase driven by Cyp19a1 (granulosa cell and cumulus cell–specific) promoter (3, 13). In the Nrg1flox/flox mice, loxP sites were inserted into intron 2 and intron 5 of type III Nrg1 (accession no. NM_178591.2) (13). By using Cyp19a1Cre female mice (3), the functional EGF domain that is encoded by exons 3 to 5 of Nrg1 gene was deleted in both cell types.
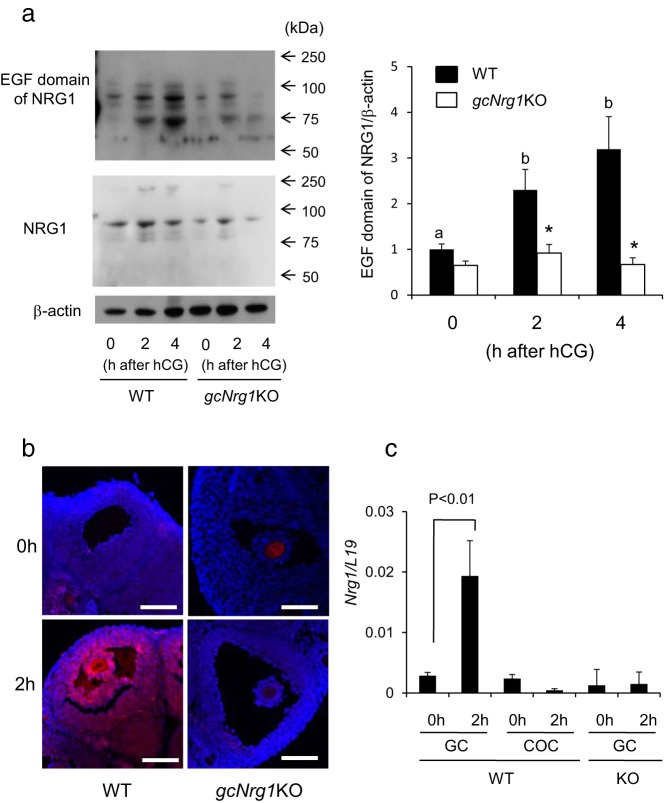
To determine the induction of NRG1 in response to hCG, ovarian extracts were prepared from WT and gcNrg1KO mice at 2 and 4 hours after hCG injection and analyzed by Western blot. Two different antibodies were used: (1) a commercial antibody that was used previously (11) and recognizes both the IgG repeat region and EGF domain of NRG1 and (2) an antiserum we generated against a unique peptide (PNEFTGDR) within the EGF domain of NRG1 (see Materials and Methods). As shown in Figure 1A, both antibodies recognized 1 band at about 90 kDa, predicted to be the membrane-bound form. However, the EGF domain–specific antiserum recognized a second band at 75 kDa predicted to be the secreted form of NRG1 including the EGF domain. These immunoreactive bands increased and were more intense in the ovarian extracts of WT mice than in those of the gcNrg1KO mice (Figure 1A). The level of the EGF domain of NRG1 in extracts of the gcNrg1KO mice at 4 hours after hCG injection was about 25% of that observed in extracts of WT mice (Figure 1A). This decrease in the EGF domain of NRG1 driven byCyp19a1-Cre is similar to that observed for the EGFR in granulosa cells of Egfrflox/flox;Cyp19a1Cre mice described by Hsieh et al (6). Immunofluorescent analyses using the anti-EGF NRG1 domain antisera showed minimal staining in ovaries of WT mice before hCG but intense labeling of both the mural granulosa cells and cumulus cells of periovulatory follicles at 2 hours after hCG injection (Figure 1B). Because Nrg1 mRNA is increased in granulosa cells but not induced in cumulus cells by hCG in vivo (Figure 1C), we conclude that the immune-positive signal on the cumulus cells is derived from NRG1 being secreted from the granulosa cells. Importantly, positive signals were not detected in granulosa or cumulus cells of periovulatory follicles of gcNrg1KO mouse ovaries (Figure 1B). The expression level of Nrg1 mRNA in mural granulosa cells of gcNrg1KO mice was dramatically lower than that in WT mice (Figure 1C).
Figure 1.
Expression and localization of NRG1 in gcNrg1KO mice. A, Western blotting analysis of NRG1 protein levels in whole ovaries recovered from mice primed with eCG followed by hCG injection. NRG1, the blotted membrane was incubated with the commercial anti-NRG1 polyclonal antibody that recognized the IgG repeat and EGF domain of NRG1 (Abcam); EGF domain of NRG1, the blotted membrane was reacted with the anti-EGF domain produced by NRG1 antiserum. The intensity of the bands was analyzed using a Gel-Pro Analyzer. Values are means ± SEM of 3 replicates. The EGF domain of NRG1/β-actin at 0 hours ratio in WT ovary was set as 1. B, Localization of the EGF domain of NRG1 was detected by immunofluorescence using anti-EGF domain of NRG1 antiserum. Cross sections of mouse ovary recovered from before or at 2 hours after hCG injection were stained with antiserum to visualize either the EGF domain of NRG1 (red) or nuclei (blue). Scale bars correspond to 100 μm. C, Expression of Nrg1 in mural granulosa cells (GC) and cumulus cells (COC) recovered from WT and gcNrg1KO (KO) mice primed with eCG followed by hCG injection. The primer sets recognized exon 3 to exon 5 of the Nrg1 gene that encoded the EGF domain of NRG1. The expression level of the gene was normalized according to that of L19. Values are means ± SEM of 3 replicates.
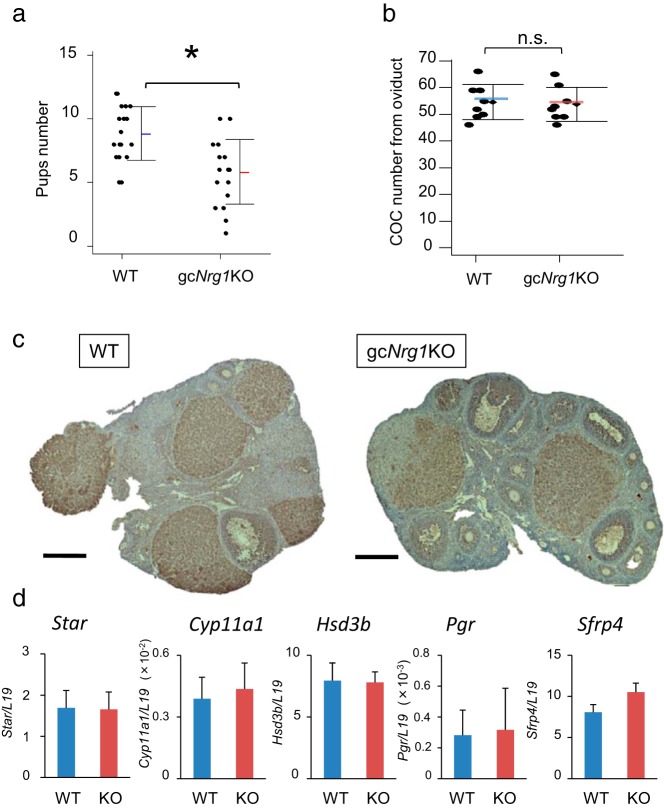
The fertility in the mutant female mice was tested by mating gcNrg1KO female mice with WT fertile males. The average number of pups born per litter was significantly decreased in gcNrg1KO mice compared with that in Nrg1flox/flox (WT) mice (Figure 2A). Litter size was also highly variable in gcNrg1KO mice (1–10 pups/litter) compared with that for the WT mice. However, the number of oocytes ovulated with a superovulation regimen of hormone treatments was similar in both genotypes (Figure 2B). These results suggested that events controlling the ovulation process itself were intact but that oocyte maturation, the developmental competence of oocytes, and/or corpora lutea formation were defective in gcNrg1KO mice.
Figure 2.
Fertility is impaired in A, Numbers of pups born per litter in the Nrg1flox/flox;Cyp19a1Cre (gcNrg1KO) and Nrg1flox/flox (WT) female mice was determined by mating with WT male mice for 6 months. Five pairs were prepared in each genotype. *, P < .05, significant differences were observed between the genotypes. B, Numbers of oocytes ovulated in the gcNrg1KO and WT mice primed with eCG + hCG superovulation regimen was determined. Immature female mice were injected ip with 4 IU of eCG followed 48 hours later with 5 IU of hCG. After 16 hours, the ovulated COCs were collected from their oviducts and the number of ovulated oocytes counted. C, Immunohistochemical staining for StAR shows its expression in corpora lutea in gcNrg1KO and WT mice at day 5 after mating of natural cycle. Scale bars correspond 200 μm. D, Expression levels of luteal cell marker genes, Cyp11a1, Hsd3b, Star, Pgr, and Sfrp4, were determined in ovarian RNA prepared from gcNrg1KO (KO) and WT mice at day 5 after mating. The expression levels of genes were normalized according to that of L19. There were no significant differences between genotypes in the expression of each gene. Values are means ± SEM of 3 replicates.
To determine whether corpora lutea were present and functional in the Nrg1 mutant mice, histological and immunohistochemical analyses were done using ovaries recovered from both genotypes 5 days after mating. The number of corpora lutea in gcNrg1KO mice (9.3 ± 1.2) was similar to that in WT mice (10.0 ± 1.0). As indicated in Figure 2C, the morphology and expression of StAR in corpora lutea of the gcNrg1KO mice was similar to that of WT mice. Furthermore, the expression levels of luteal cell–specific genes were similar in the ovaries of the gcNrg1KO and WT mice (Figure 2D). The serum levels of progesterone were also similar in the gcNrg1KO and WT mice (15.58 ± 3.20 vs 15.33 ± 2.09 ng/mL). Because the formation and function of luteal cells appeared normal, we next analyzed oocyte functions.
Progression of meiosis and fertilization of oocytes is altered in gcNrg1KO mice
Visual inspection of oocytes in COCs recovered from preovulatory follicles before exposure to hCG determined that the oocytes in gcNrg1KO mice and those in the control Nrg1flox/flox (WT) female mice were all in the germinal vesicle (GV stage. However, after exposure to hCG, the resumption of meiosis and meiotic progression to the MII stage were accelerated in gcNrg1KO mice compared with those in WT mice (Figure 3A). Specifically, we noted that in control WT female mice, most oocytes exhibited germinal vesicle breakdown (GVBD) at 4 hours and meiotic progression to the MII stage by 12 hours (Figure 3A). In contrast, in the gcNrg1KO mice, meiosis was induced as early as 2 hours, and meiotic progression was not arrested at the MII stage (Figure 3A). More than half of the mutant oocytes progressed beyond the MII stage to the anaphase or telophase II stages (Figure 3A) and then about 20% of oocytes formed pronuclei (were parthenogenic) at 24 hours after hCG injection (Supplemental Figure 1, A and B). When oocytes were recovered from oviducts of gcNrg1KO mice at 20 hours after hCG injection and cultured for 4 days, 15% of the oocytes developed to the blastocyst stage, whereas about half of them (51%) became arrested at the 2- to 16-cell stage and 28% of them degenerated (Table 1). On the other hand, most oocytes (89%) in WT mice remained arrested at MII (Table 1) and did not show signs of parthenogenic activation.
Table 1.
Developmental Competence of Parthenogenic Activated Oocytes
| Genotype | No. of Oocytes | Oocytes, % |
Morula, % | Blastocyst, % | Fragmentation, % | Degenerate, % | ||
|---|---|---|---|---|---|---|---|---|
| 1 cell | 2–4 cells | 8–16- cells | ||||||
| WT | 95 | 85 (89) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 5 (5) |
| KO | 100 | 16 (16) | 26 (26) | 6 (6) | 4 (4) | 15 (15) | 4 (4) | 28 (28) |
Oocytes were recovered from oviducts of WT or gcNrg1KO (KO) mice at 20 hours after hCG injection. The oocytes were cultured for 4 days in the developing medium (KSOM+AA) to check for development to the blastocyst stage.
It is known that the meiotic arrest at the MII stage is controlled by ERK1/2 in the oocyte (22, 23). Suppression of ERK1/2 by inhibitor or knockdown studies induced spontaneous activation of oocytes to resume meiosis from the MII stage to the pronuclear stage (24). Thus, we analyzed the phosphorylated status of ERK1/2 in oocytes of both genotypes. When oocytes were recovered from oviducts of WT and gcNrg1KO mice at 16 hours after hCG injection, the phosphorylated forms of ERK1/2 were detected in both genotypes (Figure 3B). The level was not significantly different between genotypes at 16 hours and did not change from 16 to 20 hours after hCG injection in WT mice (Figure 3B). However, levels of phospho-ERK1/2 decreased dramatically at 18 and 20 hours in oocytes of the gcNrg1KO mice (Figure 3B). These results indicate that the loss of Nrg1 in somatic cells led to abnormal meiotic progression and defective oocytes.
It has been reported that each ovulated oocyte is fertilized sequentially in vivo and that the last oocyte is fertilized at approximately 18 hours after hCG injection (25). To assess the fertilization competence of oocytes from mutant and WT mice, COCs were collected from oviducts at 14, 16, 18, or 20 hours after hCG, and the oocytes were isolated for IVF analyses. The fertilization ability of oocytes recovered from gcNrg1KO mice at 14 and 16 hours was similar to that in WT female mice. However, the percentage of oocytes that were successfully fertilized (2 pronuclei and sperm tail) was significantly lower at 18 hours in gcNrg1KO mice than that in WT mice (Figure 3C). Half of the sperm-penetrated oocytes in mutant mice exhibited no sperm tail and/or had 3 pronuclei (Supplemental Figure 2 and Supplemental Table 3). The developmental competence of fertilized eggs to the blastocyst stage in gcNrg1KO female mice was reduced when the oocytes were recovered from the oviducts at 18 hours after hCG and then used for IVF (Supplemental Figure 3).
To determine the relationship between oocyte functions and fertilization ability in the gcNrg1KO mice in vivo, a fertile male mouse was placed with an individual mutant or WT female mouse at different time intervals (0, 10, and 15 hours) after hCG injection. The mice were checked for the plug formation every hour to determine the time of mating. At 30 hours after hCG injection, the eggs were recovered from the oviduct and inspected for the pronuclear formation. The percentage of oocytes that were successfully fertilized (1 female pronuclei and 1 male pronuclei with sperm tail) is shown on the y-axis and the time of plug formation after hCG injection is on the x-axis. The percentage of successfully fertilized oocytes decreased linearly with time in gcNrg1KO mice (Figure 3D and Supplemental Table 4.1). However, no reduction in fertilization was observed in oocytes of WT female mice at the same time intervals (Figure 3D and Supplemental Table 4.2), documenting a significant loss in oocyte quality in the gcNrg1KO mice compared with that in the WT mice. Thus, one potential reason for the reduced litter size in gcNrg1KO mice appears to be the limited time during which the mutant oocytes remain capable of proper fertilization and before they have progressed beyond the MII stage.
Expression of genes involved in granulosa cell luteinization and COC expansion are altered in the gcNrg1KO mice
In our previous study, the highest levels of NRG1 protein in mural granulosa cells and activation of the receptor heterodimer of ERBB2/3 in both mural granulosa cells and cumulus cells were detected at 2 hours after hCG (11), suggesting that an NRG1-induced signaling pathway, and the expression of genes in granulosa cells and cumulus cells might affect the timing of oocyte meiotic resumption at that time. Based on this consideration, we analyzed the expression of well-known genes (Has2, Tnfaip6, and Ptx3) that are markers of COC expansion or granulosa cell luteinization (Star and Pgr) by quantitative RT-PCR. The expression levels of the COC expansion-related genes and the luteinization-related genes were increased at 2 hours after hCG injection in WT mice (Figure 4). The expression levels of these same genes were increased more in samples of the gcNrg1KO mice at 2 hours after hCG injection (Figure 4). Expression of the EGF-like factors (Areg, Ereg, and Btc) and a member of the exocytosis system (Snap25) were also up-regulated in Nrg1-depleted cells (Figure 4).
Figure 4.

The expression of genes involved in granulosa cell luteinization and COC expansion in periovulatory follicles of WT and gcNrg1KO mice. Real-time PCR analyses validate the expression of genes involved in COC expansion and luteinization at 2 hours after hCG injection. The granulosa cells and cumulus cells were recovered from ovaries before or at 2 hours after hCG injection. The expression levels were detected by quantitative RT-PCR. The expression level of genes was normalized according to that of L19. Values represent means ± SEM of 3 replicates. *, P < .05, significant differences were observed.
A PKC pathway is up-regulated in granulosa cells of gcNrg1KO mice
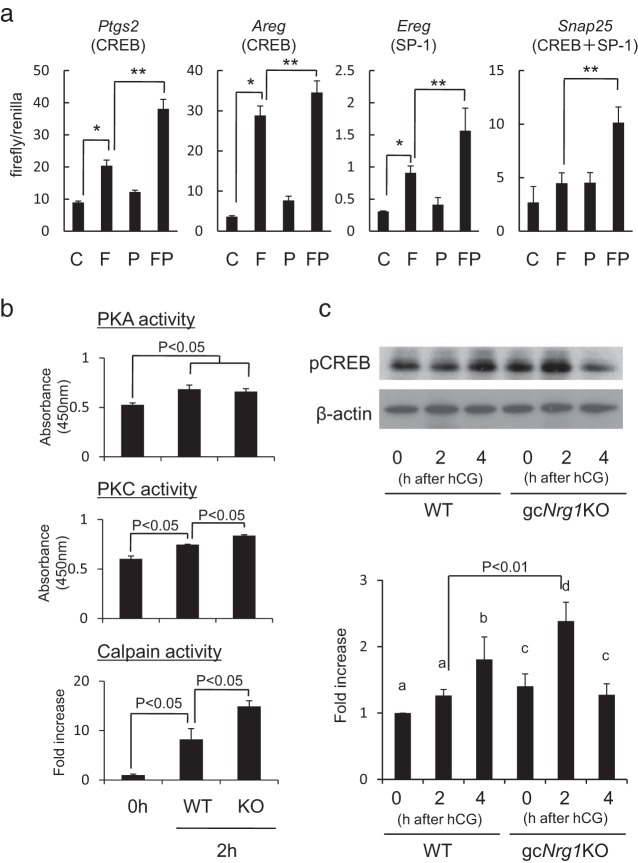
In our previous studies, we showed that the Pgr and Snap25 promoters contain DNA elements that (1) bind SP1, (2) are activated in rat granulosa cells by forskolin, an inducer of adenylyl cyclase activity and cAMP production, and (3) are further activated by the phorbol ester, PMA, that activates PKC (15, 26). Moreover, our microarray data showed that in granulosa/cumulus cells of the gcNrg1KO female mice, 205 genes induced by hCG were further up-regulated (more than 2-fold in gcNrg1KO mice) at 2 hours after hCG injection compared with those at 2 hours after hCG injection in WT mice (unpublished data T. Umehara and M. Shimada). Most (88%) of the genes that increased in the Nrg1-depleted cells have putative CREB response elements (CRE sites) and/or SP1 binding sites. Because both CREB and SP1 can be phosphorylated by PKA and PKC (27–31) and because the deletion of the SP1 sites within the Pgr and Snap25 promoters decreased their activities (15, 26), we hypothesized that the enhanced induction of genes in Nrg1-depleted granulosa cells might be mediated by enhanced activation of PKA and/or PKC. To test this, Ptgs2, Areg, Ereg, and Snap25 promoter-luciferase reporter constructs were transfected individually into mouse granulosa cells, and the cells were stimulated with forskolin, PMA, or both. The Ptgs2 and Areg constructs were used as models for CREB binding and activation, Ereg for SP1 activation, and Snap25 for CREB and SP1 activation. Strikingly, forskolin increased the activity of all promoter-luciferase constructs, whereas PMA alone had no affect (Figure 5A). However, PMA enhanced the forskolin-induced promoter activities (Figure 5A). With use of the granulosa cells recovered from gcNrg1KO mice, the promoter activities of Ptgs2, Areg, or Ereg were significantly increased by forskolin and further induced by PMA (Supplemental Figure 4), indicating that PKC activity can affect the activation of these genes and that PKC might be abnormally elevated in the Nrg1-depleted cells of mutant mice after hCG.
Figure 5.
A PKC pathway is up-regulated in granulosa cells of gcNrg1KO mice. A, Promoter activity of genes that are expressed at elevated levels in granulosa cells of gcNrg1KO mice was analyzed by transfection of promoter-luciferase reporter constructs of each gene. The Ptgs2-, Areg-, Ereg-, and Snap25-luciferase constructs were transfected into cultured mouse granulosa cells and then treated with forskolin (F), PMA (P), or both. Firefly luciferase activities were normalized by Renilla luciferase activities. Values represent means ± SEM of 3 replicates. *, P < .05, treatment with forskolin significantly increased the promoter activities; **, P < .05, Additional PMA added to forskolin-containing medium significantly enhanced the promoter activities compared with those by forskolin alone., Enzyme activity of PKA, PKC, or calpain was determined in granulosa cells of WT or gcNrg1KO (KO) mice before (0 hours) or at 2 hours after hCG injection. PKA or PKC activity was measured by a PKA kinase activity kit or PKC kinase activity kit. In both assay systems, the intensity of color derived from the immunoreaction is measured as PKA or PKC phosphotransferase activity in a microplate reader at 450 nm. The enzyme activity of PKA or PKC was revealed as absorbance at 450 nm. Calpain enzyme activity was assessed by fluorescent determination of the cleavage of a specific calpain substrate (5-FAM/OXLTM). For reference, the value of calpain enzyme activity of the 0-hour sample was set as 1, and the data are presented as fold increase. Values are means ± SEM of 3 replicates. All enzyme activities were significantly increased by hCG injection, and the levels were significantly higher in gcNrg1KO mice than in those in WT mice at 2 hours after hCG injection (P < .05). C, Phosphorylation status of CREB in granulosa cells of WT or gcNrg1KO mice before (0 hours) or after (2 or 4 hours) hCG injection. The intensity of the bands was analyzed using a Gel-Pro Analyzer. For reference, the value of calpain enzyme activity of the 0-hour WT sample was set as 1, and the data are presented as fold increase. Values represent means ± SEM of 3 replicates. Different superscripts within each genotype denote significant differences (P < .05).
The increase in PKA activity is known to be dependent on cAMP and is observed in mural granulosa cells and cumulus cells after hCG stimulation (32–34). Although PKC activation induces gene expression and PKC inhibitors suppress meiotic resumption and COC expansion (35), there is relatively little information about PKC activity in mural granulosa cells during the ovulation process. Therefore, we collected mural granulosa cells from female mice before or at 2 hours after hCG in vivo and analyzed them for either PKA or PKC activity using specific kinase assays. As shown in Figure 4B, PKA activity was significantly increased by hCG (Figure 5B) as expected from many previous studies (32–34). There was no significant difference in PKA activity in mural granulosa cells of the gcNrg1KO mice compared with those of WT mice (Figure 5B). In contrast, PKC activity was not only significantly increased by hCG but also was higher in mural granulosa cells of the gcNrg1KO mice than in those of WT mice (Figure 5B). Calpain enzyme activity is directly regulated by intracellular calcium (36) and is increased in granulosa cells during ovulation (19). Therefore, calpain activity was analyzed as a positive maker of intracellular calcium levels of granulosa cells in both genotypes. The enzyme activity was significantly increased in granulosa cells of WT mice 2 hours after hCG injection; calpain activity was even higher in granulosa cells of the gcNrg1KO mice (Figure 5B). Moreover, the phosphorylation of CREB was enhanced in granulosa cells of the gcNrg1KO mice at 2 hours after hCG injection (Figure 5C), suggesting that the Ca2+-PKC pathway might up-regulate CREB phosphorylation in the hCG-stimulated Nrg-1 depleted granulosa cells and that NRG1 might suppress PKC activity in the hCG-treated WT mice.
High level of PKC activity in gcNrg1KO mice accelerates meiotic progression and decreases fertilization ability by premature closure of gap junctions
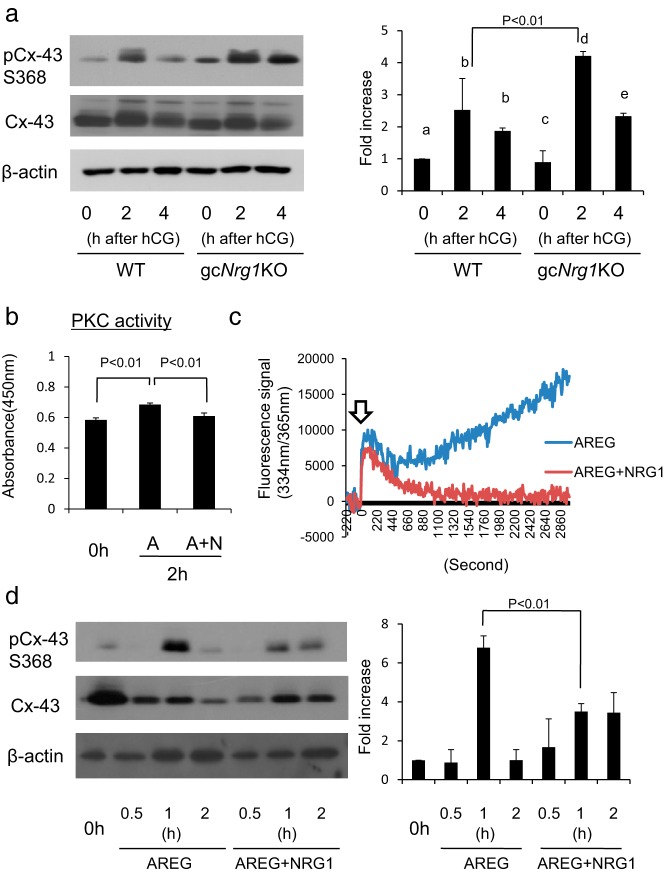
The resumption of meiosis in oocytes is associated with the rapid closure of gap junctions between the cumulus cells and the oocyte (37, 38). Gap junction closure (1) is mediated by the phosphorylation of connexin-43 (39), (2) blocks the transfer of cGMP from cumulus cells to oocytes (40–42), which leads to activation of phosphodiesterase 3A (43), and (3) thereby reduces intra-oocyte levels of cAMP (40–43). Reduction of cAMP leads to the initiation of meiosis (40–45). Because oocyte maturation was abnormally advanced in the Nrg1 mutant mice, we next sought to determine whether the increased PKC activity in cumulus cells might enhance meiotic resumption in gcNrg1KO mice by regulating gap junctions. Although the phosphorylation of connexin-43 in cumulus cells is mediated, in part, by ERK1/2, connexin-43 can also be phosphorylated at serine 368 by PKC although at a lower level in LH-stimulated follicles (46). We hypothesized that the rapid and elevated phosphorylation of connexin-43 by PKC in cumulus cells of gcNrg1KO mice might enhance gap junction closure and thereby accelerate the resumption of meiosis. To test this, we analyzed PKC-mediated phosphorylation of connexin-43 using a PKC (Ser368)–specific antibody. Total connexin-43 was present at similar levels in cumulus cells of COCs recovered from the WT and gcNrg1KO mice before and after hCG (Figure 6A). However, the phosphorylation of connexin-43 (Ser368) was significantly higher at 2 and 4 hours after hCG in the cells of the gcNrg1KO mice than in those of WT mice (Figure 6A). Furthermore, when COCs were cultured with AREG to induce meiosis and expansion, PKC activity and intracellular calcium in COCs increased, whereas the addition of NRG1 blocked these AREG-mediated effects (Figure 6, B and C). Likewise, AREG dramatically increased PKC-induced phosphorylation of connexin-43, whereas the addition of NRG1 suppressed this effect (Figure 6D).
Figure 6.
The high level of Ca2+ and PKC activity in granulosa/cumulus cells of gcNrg1KO mice enhances phosphorylation of connexin-43. A, Phosphorylation status of connexin-43 (Cx-43) in granulosa/cumulus cells of gcNrg1KO mice was analyzed before and after hCG. The intensity of the bands was analyzed using a Gel-Pro Analyzer. Values represent means ± SEM of 3 replicates. Different superscripts within each genotype denote significant differences (P < .05). B, Activity of PKC was measured in cumulus cells of COCs cultured for 2 hours with AREG (A) and/or NRG1 (N). The enzyme activity of PKC was revealed as absorbance at 450 nm. Values represent means ± SEM of 3 replicates. C, Level of Ca2+ in COCs cultured with AREG and/or NRG1. COCs that were preincubated with Fura-2/AM and then cultured with AREG and/or NRG1 for up to 60 minutes. The Ca2+ level in COCs was measured as the level of 334 nm/365 nm Fura-2 fluorescence ratio every 15 second. Arrow indicates the time of the treatment with AREG and/or NRG1. Results are representative of 3 separate experiments. D, Phosphorylation of Cx-43 in cumulus cells is regulated by NRG1. COCs were cultured with AREG and/or NRG1 for 30 or 60 minutes. The intensity of the bands was analyzed using a Gel-Pro Analyzer. For reference, the value of calpain enzyme activity of the 0-hour sample was set as 1, and the data are presented as fold increase. Values represent means ± SEM of 3 replicates. 0h, samples were collected before culture.
When COCs were cultured in the presence of a low concentration of HX (1 mM) with or without AREG, meiotic resumption was induced within 2 hours. The addition of NRG1 alone to the culture medium (1 mM HX condition) significantly decreased the percentage of oocytes exhibiting meiotic resumption in a dose-dependent manner (Figure 7A). When COCs were cultured with AREG, the rate of oocytes exhibiting GVBD was also significantly decreased by the addition of NRG1 in a dose-dependent manner (Figure 7A). The resumption of meiosis in oocytes of COCs cultured with AREG and 10 ng/mL of NRG1 occurred in a temporal pattern similar to that observed in vivo (Figure 7B). Furthermore, the oocytes within the COCs that were cultured with AREG and 10 ng/mL of NRG1 under a 1 mM HX condition exhibited successful fertilization after 16 and 18 hours of culture, whereas oocytes cultured with AREG alone were highly fertilized at 16 hours but not at 18 hours (Figure 7C). In addition, when COCs were recovered from eCG-primed gcNrg1KO mice and cultured with AREG and 10 ng/mL of NRG1 under the 1 mM HX condition, more oocytes were successfully fertilized after 18 hours than when Nrg1 mutant COCs were cultured with AREG alone (Figure 7C).
Figure 7.
The addition of NRG1 delays meiotic progression to the MII stage and prolongs duration of oocyte fertilization competence. A, Effects of NRG1 on oocyte meiotic resumption (exhibiting GVBD). Some COCs were cultured with or without AREG and/or NRG1 (1 or 10 ng/mL) for 3 hours in the presence of 1 mM HX. Other COCs were cultured with or without AREG or NRG1 (1 or 10 ng/mL) in the presence of 4 mM HX for 6 hours. No common letters are significant (P < .05). Values represent means ± SEM of 3 replicates. B, Kinetic changes in meiotic progression occur in vivo and in vitro with AREG and/or NRG1. The COCs were collected from preovulatory follicles of eCG-injected WT mice. The COCs were cultured with AREG and/or NRG1 in the presence of 1 mM HX, and oocytes were stained by DAPI and anti-acetylated tubulin at each time point. The experiments were repeated 3 times at each time point. Values represent means ± SEM of data. *, P < .05, significant differences were observed in oocytes of COCs cultured with AREG compared with those with AREG and NRG1 at each time point. % GVBD, the rate of oocytes exhibiting germinal vesicle break down; % MII, the rate of oocytes arrested at meiotic metaphase II stage. C, Successful fertilization of oocytes matured in vitro changes with AREG (A) and/or NRG1 (N). COCs were recovered from eCG-injected WT or gcNrg1KO mice and then cultured with AREG and/or NRG1 in the presence of 1 mM of HX 16 or 18 hours. The COCs were cocultured with sperm (IVF) for 10 hours, and then the oocytes were stained by DAPI and anti-acetylated tubulin antibody to visualize the number of pronuclei and sperm tail in each putative egg. The y-axis shows the percentage of successful fertilization (2 pronuclei and sperm tail). Values represent means ± SEM of 3 replicates.
When COCs were cultured in the presence of a high concentration of HX (4 mM), the resumption of meiosis occurred in less than half of oocytes at 6 hours (Figure 7A). However, the addition of AREG increased meiotic resumption in the presence of a high concentration of HX. In contrast, the combination of AREG and NRG1 decreased the rate of meiotic resumption in an NRG1 dose-dependent manner (Figure 7A). After 16 hours of culture with AREG and 10 ng/mL NRG1, most oocytes (85.7 ± 7.1%) arrested at the MII stage, similar to oocytes cultured with AREG alone (89.7 ± 3.5%).
Discussion
Our analyses of the gcNrg1KO mouse model have revealed that disruption of the Nrg1 gene in mural granulosa cells and cumulus cells in vivo accelerates the oocyte meiotic process and thereby shortens the period of time during which sperm can successfully penetrate the oocyte and induce normal fertilization and embryo development. Specifically, although follicular development, ovulation (shown in Supplemental Figure 5) and corpora lutea formation are normal in the gcNrg1KO mice, the resumption of meiosis occurs significantly earlier after the LH/hCG surge, and the ability of oocytes to arrest at the MII stage is severely impaired; most ovulated oocytes in the gcNrg1KO mice are prematurely activated and hence are resistant to sperm penetration or contain multiple pronuclei. These results indicate that rapid induction and activation of NRG1 in mural granulosa cells at 2 hours after hCG (11) control a critical event(s) during the initial response of mural granulosa cells and cumulus cells to LH/hCG. We show herein that a PKC signaling pathway is abnormally activated in mural granulosa cells and cumulus cells of gcNrg1KO mice at 2 hours after hCG. This activation of PKC is probably associated with elevated intracellular calcium because NRG1 reduces intracellular calcium and PKC activity in COCs cultured with AREG. Moreover, the direct effects of NRG1 on oocyte maturation were observed in cultured COCs. Specifically, NRG1 (1) blocks PKC-induced phosphorylation of connexin-43 (Ser368), (2) delays the rapid and premature resumption of meiosis, and (3) restores successful fertilization of oocytes cultured for 18 hours. Thus, NRG1 appears to reduce or modulate events that are mostly activated by the AREG and EGFR pathways in response to LH/hCG. We propose that NRG1 appears to play an important role in oocyte maturation via cumulus cell- and mural granulosa cell–mediated mechanisms that involve the regulation of PKC activity and intracellular calcium.
The developmental competence of matured oocytes is partly determined by the timing of meiotic resumption. When oocytes were recovered from eCG-primed mice and then cultured in vitro, meiotic resumption is rapidly induced within 2 hours, and then the oocytes progress to the MII stage (47, 48). However, the matured oocytes have less developmental competence than oocytes that are matured in vivo. In contrast, when intact COCs were cultured with FSH, the onset of meiotic resumption was delayed (49), and the oocytes exhibited improved developmental competence in IVF (50–52). Thus, it has been thought that at the GV stage during the in vitro culture and ovulation process, oocytes acquire the full maturation potential, including polyadenylation of maternal mRNA and transportation of intercellular organelles such as mitochondria and endoplasmic reticulum (53–56). However, the in vivo mechanism of how to delay the timing of meiotic resumption has remained unclear.
Specifically, in mice, several articles showed that meiotic resumption was observed from 2 to 4 hours after hCG injection (43, 57, 58). In the present study, the rate of meiotic resumption at 3 and 4 hours after hCG injection was slightly slower than that reported by other groups (43, 58). However, the meiotic resumption occurred around 4 hours after hCG injection, and the time of polar body emission was almost the same in the present study as that reported by others (59–61). On the other hand, in the gcNrg1KO mice, the onset of meiotic resumption and the progression to the MII stage were accelerated compared with those in WT mice. Moreover, the matured oocytes exhibited impaired fertilization and developmental competence. Thus, there appears to be a critical window of time between exposure to hCG (ovulation stimuli) and initiation of meiotic resumption during which specific events occur that determine normal meiotic progression and developmental competence. Our studies indicate that NRG1 is a key modulator of these events that determine the onset of meiotic resumption during ovulation in vivo.
Recently, Wang et al (62) reported that the rapid elevation of intracellular calcium in cumulus cells induced meiotic resumption of mouse oocytes when COCs were cultured with natriuretic precursor peptide C and EGF. In addition, the increase in intracellular calcium activates calpain activity in cumulus cells (19), which is required for hCG-induced COC expansion in vivo or AREG-induced expansion in vitro. Thus, EGFR-mediated release of Ca2+ in cumulus cells leads to the differentiation of cumulus cells and oocyte meiotic resumption. The present study has also revealed that the rapid induction of NRG1 at 2 hours after hCG provides a critical negative regulatory mechanism to reduce the AREG-EGFR–induced PKC activity in cumulus cells and granulosa cells at early stages in the ovulation process and to fine-tune the temporal progression of meiosis. Thus, moderate but not elevated and sustained levels of Ca2+ and PKC activity in cumulus cells appear to be required for successful meiotic progression. Intracellular calcium release also appears to be important in granulosa cells for successful ovulation because depletion of the G proteins (Gαq/α11) (Gqflox/flox;G11−/−;Cyp19a1Cre mice) that activate the phospholipase C family in granulosa cells blocks ovulation and oocytes remain entrapped within corpora lutea. Failure of ovulation in this mouse model is associated with reduced expression of progesterone receptor in mural granulosa cells (63). Thus, precise spatial, temporal, and ligand-specific increases in intracellular calcium occur in cumulus cells and mural granulosa cells and are essential for coordinating oocyte meiosis and development with ovulation.
The ovulated oocytes in gcNrg1KO mice reach the MII stage, but the cytostatic activity needed to arrest meiosis at the MII stage is impaired and leads to spontaneous oocyte activation in these mutant mice. The loss of cytostatic activity has been described in knockout mouse models and by the injection of antisense RNA or small interfering RNA into oocytes in vitro. Thus, all of these studies were dependent on the disruption of oocyte-specific genes (64–66). For example, the knockdown and knockout of the kinase Mos in oocytes allowed the oocytes to progress to the MII stage but, in the absence of sufficient ERK1/2 activation, they became spontaneously activated without arresting at the MII stage (67, 68). In gcNrg1KO mice, the disruption of the Nrg1 gene in somatic cells led to low cytostatic activity that was also associated with rapid reduction of phosphorylated (active) ERK1/2 in oocytes at 18 or 20 hours after hCG injection. Oocytes that were recovered from WT mice at 18 hours after hCG injection were successfully fertilized and developed to the blastocyst stage. However, oocytes recovered from the gcNrg1KO mice exhibited severely reduced fertilization and developmental competence (Supplemental Figure 3 and Supplemental Table 2.2). Therefore, this is the first report to document that the induction of NRG1 in follicular somatic cells is essential to control the progression of events in the oocyte, including the onset of meiosis, the acquisition of cytostatic activity, and thereby successful sperm penetration. Thus, the normal meiotic progression of oocytes is essential for successful fertilization and embryo development.
These results are relevant to current fertility, IVF, and oocyte maturation practices that are being used in human fertility clinics. The collection of matured oocytes used for IVF is a routine procedure for human infertility care. In most cases, the matured oocytes are retrieved from periovulatory follicles just before ovulation at 35 hours after hCG or GnRH agonist injection (68, 69). At that time, most of the oocytes have not yet reached the MII stage and additional culture of the oocytes is required for IVF. However, in some patients, successful fertilization (2 pronuclei) is low even when the sperm is directly injected into oocyte. Often only 1 pronuclei or >3 are observed (70, 71). In addition, in some oocytes, the spindle at the metaphase plate is not fully formed immediately after emission of the first polar body and the rate of fertilization is low when these premature oocytes are used for intracytoplasmic sperm injection (72, 73). Our data from the gcNrg1KO mice provide evidence that the time required to reach the MII stage (or progression beyond this stage) may be different in each oocyte recovered not only from different patients but also from different follicles of same patient. Our results indicate that early induction of Nrg1 and its target PKC signaling pathway after hCG stimulation are important and if impaired may explain why abnormal fertilization occurs repeatedly in some patients.
In vitro maturation of oocytes (IVM) is another procedure used to obtain matured oocytes, especially from low responder patients or patients with polycystic ovary syndrome (74). The maturation of oocytes is normally similar to that in the in vivo matured oocytes; however, the developmental competence of these in vitro mature oocytes remains limited (75). Our previous study showed that cumulus cells express the EGF-like factors, Areg, Ereg, and Btc, to act on EGFR in cumulus cells (5). On the other hand, Nrg1 mRNA and protein are not induced in cumulus cells either in vivo or in vitro after hCG or AREG stimulation. These results indicate that the IVM conditions may be similar to that of the COCs of the gcNrg1KO mice; ie, they are NRG1 depleted. Moreover, as shown in this study, the meiotic progression is accelerated in oocytes in the IVM conditions unless NRG1 and HX were added. Because meiotic progression is also accelerated in human oocytes in IVM conditions (76) and because HX is not commonly used for human IVM (20, 76–79), it is possible that the addition of NRG1 will improve human IVM procedures and enhance oocyte developmental competence.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Dr Carmen Birchmeier, Max Delbrueck Center for Molecular Medicine (Berlin, Germany) for providing us with Nrg1flox/flox mice. Dr Y. Yamahsita, Hiroshima Prefecture University (Hiroshima, Japan) and Dr Z. Liu, Baylor College of Medicine (Houston, TX) provided outstanding assistance and good suggestions. We are grateful to Dr T. Mihara, M. Enjoji, T. Kawai, R. Hiramatsu, Kie Fukuma, and Y. Inzen at Hiroshima University for technical assistance.
This work was supported in part by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research 21688019, 24688028, and 25132708 [to M.S.] and Young Research Fellowship 09J04118 [to I.K.] and the National Institutes of Health (Grants HD-16229 and HD-07495, Project II, Specialized Cooperative Program in Reproductive Research [to J.S.R.]).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AREG
- amphiregulin
- BTC
- β-cellulin
- COC
- cumulus cell–oocyte complex
- CREB
- cAMP response element-binding protein
- DAPI
- 4′,6-diamidino-2-phenylindole
- eCG
- equine chorionic gonadotropin
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- EREG
- epiregulin
- gcNrg1KO
- granulosa cell–specific Nrg1 knockout mouse
- GV
- germinal vesicle
- GVBD
- germinal vesicle breakdown
- hCG
- human chorionic gonadotropin
- HX
- hypoxanthine
- IVF
- in vitro fertilization
- IVM
- In vitro maturation of oocytes
- NRG1
- neuregulin 1
- PKA
- protein kinase A
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- WT
- wild-type.
References
- 1. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 2. Hsieh M, Lee D, Panigone S, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan HY, Shimada M, Liu Z, et al. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan HY, Liu Z, Shimada M, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. [DOI] [PubMed] [Google Scholar]
- 6. Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One. 2011;6:e21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315:611–618. [DOI] [PubMed] [Google Scholar]
- 10. Meyer D, Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc Natl Acad Sci USA. 1994;91:1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noma N, Kawashima I, Fan HY, et al. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2011;25:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimada M, Yamashita Y. The key signaling cascades in granulosa cells during follicular development and ovulation process. J Mamm Ova Res. 2011;28:25–31. [Google Scholar]
- 13. Yang X, Arber S, William C, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. [DOI] [PubMed] [Google Scholar]
- 14. Shimada M, Yanai Y, Okazaki T, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. [DOI] [PubMed] [Google Scholar]
- 15. Doyle KM, Russell DL, Sriraman V, Richards JS. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol. 2004;18:2463–2478. [DOI] [PubMed] [Google Scholar]
- 16. Shimada M, Yanai Y, Okazaki T, et al. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–2502. [DOI] [PubMed] [Google Scholar]
- 17. Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. β-Catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010;24:1529–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sekiguchi T, Mizutani T, Yamada K, et al. Transcriptional regulation of the epiregulin gene in the rat ovary. Endocrinology. 2002;143:4718–4729. [DOI] [PubMed] [Google Scholar]
- 19. Kawashima I, Liu Z, Mullany LK, Mihara T, Richards JS, Shimada M. EGF-like factors induce expansion of the cumulus cell-oocyte complexes by activating calpain-mediated cell movement. Endocrinology. 2012;153:3949–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sato C, Shimada M, Mori T, et al. Assessment of human oocyte developmental competence by cumulus cell morphology and circulating hormone profile. Reprod Biomed Online. 2007;14:49–56. [DOI] [PubMed] [Google Scholar]
- 21. Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. [DOI] [PubMed] [Google Scholar]
- 22. Verlhac MH, de Pennart H, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993;158:330–340. [DOI] [PubMed] [Google Scholar]
- 23. Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. [DOI] [PubMed] [Google Scholar]
- 24. Tong C, Fan HY, Chen DY, Song XF, Schatten H, Sun QY. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: microtubule organization, asymmetric division and metaphase II arrest. Cell Res. 2003;13:375–383. [DOI] [PubMed] [Google Scholar]
- 25. Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59:105–107. [DOI] [PubMed] [Google Scholar]
- 26. Sriraman V, Sharma SC, Richards JS. Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility. Mol Endocrinol. 2003;17:436–449. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez GA, Yamamoto KK, Fischer WH, et al. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989;337:749–752. [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. [DOI] [PubMed] [Google Scholar]
- 29. Xie H, Rothstein TL. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. J Immunol. 1995;154:1717–1723. [PubMed] [Google Scholar]
- 30. Pal S, Claffey KP, Cohen HT, Mukhopadhyay D. Activation of Sp1-mediated vascular permeability factor/vascular endothelial growth factor transcription requires specific interaction with protein kinase C zeta. J Biol Chem. 1998;273:26277–26280. [DOI] [PubMed] [Google Scholar]
- 31. Rohlff C, Ahmad S, Borellini F, Lei J, Glazer RI. Modulation of transcription factor Sp1 by cAMP-dependent protein kinase. J Biol Chem. 1997;72:21137–21141. [DOI] [PubMed] [Google Scholar]
- 32. Hunzicker-Dunn M, Jungmann RA. Rabbit ovarian protein kinases. I. Effect of an ovulatory dose of human chorionic gonadotropin or luteinizing hormone on the subcellular distribution of follicular and luteal protein kinases. Endocrinology. 1978;103:420–430. [DOI] [PubMed] [Google Scholar]
- 33. Hunzicker-Dunn M, Jungmann RA. Rabbit ovarian protein kinases. II. Effect of an ovulatory dose of human chorionic gonadotropin or luteinizing hormone on the multiplicity of follicular and luteal protein kinases. Endocrinology. 1978;103:431–440. [DOI] [PubMed] [Google Scholar]
- 34. Hunzicker-Dunn M, Jungmann RA. Rabbit ovarian protein kinases. III. Gonadotrophin-induced activation of soluble adenosine 3′,5′-monophosphate-dependent protein kinases. Endocrinology. 1978;103:441–451. [DOI] [PubMed] [Google Scholar]
- 35. Downs SM, Cottom J, Hunzicker-Dunn M. Protein kinase C and meiotic regulation in isolated mouse oocytes. Mol Reprod Dev. 2001;58:101–115. [DOI] [PubMed] [Google Scholar]
- 36. Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. [DOI] [PubMed] [Google Scholar]
- 37. Larsen WJ, Wert SE, Brunner GD. A dramatic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol. 1986;113:517–521. [DOI] [PubMed] [Google Scholar]
- 38. Larsen WJ, Wert SE, Brunner GD. Differential modulation of rat follicle cell gap junction populations at ovulation. Dev Biol. 1987;122:61–71. [DOI] [PubMed] [Google Scholar]
- 39. Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. [DOI] [PubMed] [Google Scholar]
- 40. Norris RP, Ratzan WJ, Freudzon M, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han SJ, Vaccari S, Nedachi T, et al. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178:393–402. [DOI] [PubMed] [Google Scholar]
- 45. Downs SM, Eppig JJ. Cyclic adenosine monophosphate and ovarian follicular fluid act synergistically to inhibit mouse oocyte maturation. Endocrinology. 1984;114:418–427. [DOI] [PubMed] [Google Scholar]
- 46. Norris RP, Freudzon M, Mehlmann LM, et al. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cross PC, Brinster RL. In vitro development of mouse oocytes. Biol Reprod. 1970;3:298–307. [DOI] [PubMed] [Google Scholar]
- 48. Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. [DOI] [PubMed] [Google Scholar]
- 49. Schultz RM, Montgomery RR, Ward-Bailey PF, Eppig JJ. Regulation of oocyte maturation in the mouse: possible roles of intercellular communication, cAMP, and testosterone. Dev Biol. 1983;95:294–304. [DOI] [PubMed] [Google Scholar]
- 50. Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. [DOI] [PubMed] [Google Scholar]
- 51. Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–810. [DOI] [PubMed] [Google Scholar]
- 52. Sirard MA, Parrish JJ, Ware CB, Leibfried-Rutledge ML, First NL. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod. 1988;39:546–552. [DOI] [PubMed] [Google Scholar]
- 53. Gebauer F, Xu W, Cooper GM, Richter JD. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994;13:5712–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72:1218–1223. [DOI] [PubMed] [Google Scholar]
- 55. Ajduk A, Małagocki A, Maleszewski M. Cytoplasmic maturation of mammalian oocytes: development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod Biol. 2008;8:3–22. [DOI] [PubMed] [Google Scholar]
- 56. Chen J, Torcia S, Xie F, et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol. 2013;15:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Edwards RG, Gates AH. Timing of the stages of the maturation divisions, ovulation, fertilization and the first cleavage of eggs of adult mice treated with gonadotrophins. J Endocrinol. 1959;18:292–304. [DOI] [PubMed] [Google Scholar]
- 58. Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2-derived prostaglandin E2 directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem. 2006;281:37117–37129. [DOI] [PubMed] [Google Scholar]
- 59. Polański Z. In-vivo and in-vitro maturation rate of oocytes from two strains of mice. J Reprod Fertil. 1986;78:103–109. [DOI] [PubMed] [Google Scholar]
- 60. Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci USA. 2005;102:9206–9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Su YQ, Wu X, O'Brien MJ, et al. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276:64–73. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Kon N, Li N, et al. Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology. 2013;154:3401–3409. [DOI] [PubMed] [Google Scholar]
- 63. Breen SM, Andric N, Ping T, et al. Ovulation involves the luteinizing hormone-dependent activation of Gq/11 in granulosa cells. Mol Endocrinol. 2013;27:1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hashimoto N, Watanabe N, Furuta Y, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71. [DOI] [PubMed] [Google Scholar]
- 65. Colledge WH, Carlton MB, Udy GB, Evans MJ. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370:65–68. [DOI] [PubMed] [Google Scholar]
- 66. Verlhac MH, Kubiak JZ, Weber M, et al. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development. 1996;122:815–822. [DOI] [PubMed] [Google Scholar]
- 67. Svoboda P, Stein P, Hayashi H, Schultz RM. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development. 2000;127:4147–4156. [DOI] [PubMed] [Google Scholar]
- 68. Perry AC, Verlhac MH. Second meiotic arrest and exit in frogs and mice. EMBO Rep. 2008;9:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lopata A. Successes and failures in human in vitro fertilization. Nature. 1980;288:642–643. [DOI] [PubMed] [Google Scholar]
- 70. Porter RN, Smith W, Craft IL, Abdulwahid NA, Jacobs HS. Induction of ovulation for in-vitro fertilisation using buserelin and gonadotropins. Lancet. 1984;2:1284–1285. [DOI] [PubMed] [Google Scholar]
- 71. Munné S, Cohen J. Chromosome abnormalities in human embryos. Hum Reprod Update. 1998;4:842–855. [DOI] [PubMed] [Google Scholar]
- 72. Winston NJ, Braude PR, Johnson MH. Are failed-fertilized human oocytes useful? Hum Reprod. 1993;8:503–507. [DOI] [PubMed] [Google Scholar]
- 73. De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14:1859–1863. [DOI] [PubMed] [Google Scholar]
- 74. Hyun CS, Cha JH, Son WY, et al. Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum Reprod. 2007;22:1991–1995. [DOI] [PubMed] [Google Scholar]
- 75. Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353–362. [DOI] [PubMed] [Google Scholar]
- 76. Piquette GN. The in vitro maturation (IVM) of human oocytes for in vitro fertilization (IVF): is it time yet to switch to IVM-IVF? Fertil Steril. 2006;85:833–835. [DOI] [PubMed] [Google Scholar]
- 77. Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. [DOI] [PubMed] [Google Scholar]
- 78. Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16:675–689. [DOI] [PubMed] [Google Scholar]
- 79. Coticchio G, Dal-Canto M, Guglielmo MC, Mignini-Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol. 2012;56:909–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.