Abstract
T4 (3,5,3′,5′-tetraiodo-l-thyronine) is classically viewed as a prohormone that must be converted to the T3 (3,5,3′-triiodo-l-thyronine) form for biological activity. We first determined that the ability of reporter genes to respond to T4 and to T3 differed for the different thyroid hormone receptor (TR) isoforms, with TRα1 generally more responsive to T4 than was TRβ1. The response to T4 vs T3 also differed dramatically in different cell types in a manner that could not be attributed to differences in deiodinase activity or in hormone affinity, leading us to examine the role of TR coregulators in this phenomenon. Unexpectedly, several coactivators, such as steroid receptor coactivator-1 (SRC1) and thyroid hormone receptor-associated protein 220 (TRAP220), were recruited to TRα1 nearly equally by T4 as by T3 in vitro, indicating that TRα1 possesses an innate potential to respond efficiently to T4 as an agonist. In contrast, release of corepressors, such as the nuclear receptor coreceptor NCoRω, from TRα1 by T4 was relatively inefficient, requiring considerably higher concentrations of this ligand than did coactivator recruitment. Our results suggest that cells, by altering the repertoire and abundance of corepressors and coactivators expressed, may regulate their ability to respond to T4, raising the possibility that T4 may function directly as a hormone in specific cellular or physiological contexts.
Thyroid hormones (THs) are regulatory molecules with crucial roles in vertebrate development and physiology (1, 2). THs exert these roles by binding to their receptors (thyroid hormone receptors [TRs]), which function primarily as hormone-regulated transcription factors. TRs bind to specific target DNA sequences (thyroid hormone response elements [TREs]) and regulate transcription of adjacent target genes through the recruitment and release of accessory proteins denoted corepressors and coactivators (3–6). Corepressors and coactivators, in turn, alter the chromatin template or interact with the general transcription machinery to produce the appropriate transcriptional output (7). Best understood is the ability of TRs to bind corepressors and thus repress target gene transcription in the absence of TH and to release corepressors, bind coactivators, and activate gene expression in the presence of TH (3, 6–9). Nonetheless, TRs are known to possess the capability of mediating a wide diversity of regulatory outcomes that depend on the nature of the TRE, the presence of additional transcription factors arrayed on the same target gene, the effect of posttranslational modifications, and the nature of the “hormone” ligand. Further, the TRs are themselves diversified by being encoded by 2 genetic loci and, through alternative mRNA splicing, to generate multiple distinct isoforms. The 2 most abundant TR isoforms, TRα1 and TRβ1, are expressed at different times in development, predominate in different adult tissues, display distinct regulatory properties, and play partially overlapping, yet distinguishable biological roles (10–15).
Two major derivatives of TH are found in the circulation: T3 (3,5,3′-triiodo-l-thyronine) and T4 (3,5,3′,5′-tetraiodo-l-thyronine) (1, 2). T3 binds in vitro to TRs with a significantly greater affinity than T4 (16). As a result, T3 has generally been viewed to be the functional form of TH (16), with T4 serving as an inactive prohormone that is converted to active T3 in the target cell by tissue-specific deiodinases (1, 17–20). Nonetheless, it is notable that most of the TH produced in the thyroid is secreted in the form of T4 and normal serum concentrations of T4 are many-fold greater than those of T3 (the exact fold excess depends on comparing free vs bound levels) (1, 2, 21). In addition, once bound to its receptors, T4 can invoke conformational changes similar to those of T3 (21–24). Finally, mice genetically engineered to lack the deiodinases that convert T4 to T3 in the periphery are surprisingly normal in their development and physiology (25). Therefore, the precise role of T4 as a prohormone and the possibility that T4 might function directly as an active hormone in at least certain cellular contexts, remain incompletely answered questions.
In this study, we explored in more detail the ability of T4 to directly function as a TR agonist. Interestingly, we found that the different TR isoforms differed in their ability to respond to T4, with TRα1 generally exhibiting a much stronger response to T4 than did TRβ1. The ability of TRα1 and TRβ1 to respond to T4 vs T3 differed widely in different cell types in a manner that could not be attributed simply to cellular deiodinase activity or the innate affinity of the different TR isoforms for either hormone derivative. Instead, we observed that coactivators, such as steroid receptor coactivator-1 (SRC1) and thyroid hormone receptor-associated protein 220 (TRAP220) (26, 27), were recruited to TRα1 in vitro nearly equally by either T4 or T3, whereas other coactivators and corepressors, such as nuclear receptor corepressor (NCoR) ω (28), were affected by T4 much less efficiently than by T3. We propose that different cell types may modulate their relative ability to respond to T4 vs T3 by altering the relative abundance of different coactivators and corepressors and raise the possibility that T4 may be able to function as a direct-acting hormone agonist in certain contexts.
Materials and Methods
Hormones
T4 (catalog number T2376; lot number 1439478) and T3 (catalog number T2877; lot number 016K1628V) were purchased from Sigma-Aldrich. Each preparation was confirmed by gas chromatography to be greater than 99% pure overall. The T4 stock contained less than 0.27% T3, whereas the T3 stock contained less than 0.57% T4. The concentrations of all stock solutions were confirmed by both gravimetric and spectroscopic analysis.
DNA constructs
The pSG5 expression vectors for human TRα1 and TRβ1 and the tk-luciferase reporter vectors were described previously; response elements inserted within the latter include a direct repeat-4 composed of a consensus TAAGGTCA half-site (DR4-CON), a direct repeat-4 derived from murine leukemia virus (DR4-MLV), a direct repeat-4 derived from the myosin heavy chain promoter (DR4-MHC), or a divergent-6 repeat derived from the chicken lysozyme-F2 promoter (DIV6-F2) (29, 30). The pGEX-SG vectors for SRC1 codons 568 to 891, glutamate receptor-interacting protein 1 (GRIP) codons 544 to 767, cAMP response element–binding protein–binding protein (CBP) codons 1 to 451, TRAP220 codons 486 to 723, or NCoRω codons 1817 to 2453 represent the known receptor interaction domains of these coregulators (containing LXXLL or CoRNR motifs for the coactivators and corepressors, respectively) and were also described previously (26, 31–34).
Oligonucleotides and DNA probes
Oligonucleotides were obtained as complementary, single-stranded DNAs (Integrated DNA Technologies) and were annealed to create double-stranded DNAs with 4-bp overhangs. The overhangs were filled in with [32P-alpha]-d-GTP (3000 Ci/mmol; PerkinElmer) plus the 3 remaining unlabeled dNTPs using Klenow DNA polymerase (35). The oligonucleotides used in the EMSAs included the following: DR4 top strand, 5′-TGCAATAAGGTCAAATAAGGTCAGAG-3′; and DIV6-F2 top strand, 5′-TCGACTTATTGACCCCAGCTGAGGTCAAGTTAG-3′ (half-sites underscored).
EMSAs
Human TRα and TRβ1 were expressed as full-length proteins via recombinant baculovirus infection of SF9 cells and isolated from nuclear extracts as described previously (35, 36). Glutathione S-transferase (GST)-coactivator and GST-corepressor fusions were expressed in Escherichia coli strain BL21 (DE3)-RP transformed by the pGEX vectors described above. In brief, the bacterial transformants were grown in LB broth at 37°C to an A600 value of 0.8 to 1.0, induced with 1 mM isopropyl β-d-1-thiogalactopyranoside, shifted to 16°C, and harvested after 16 hours (37, 38). The GST fusion proteins were subsequently purified by binding to and elution from a glutathione-agarose matrix (Sigma-Aldrich). EMSAs were performed by mixing the nuclear receptor proteins of interest, 1 pmol of 32P-radiolabeled oligonucleotide probe, and 2 μL of coregulator protein in 20 μL of binding buffer containing the hormone concentration desired (T3, T4, or ethanol vehicle) (39). After a 25°C incubation for 15 minutes, the protein-DNA complexes were resolved from free probe by nondenaturing gel electrophoresis at 200 V for 75 minutes in a 4% polyacrylamide gel polymerized over an 8% polyacrylamide gel cushion in 0.5× TBE (45 mM Tris-borate and 1 mM EDTA) buffer. Gels were dried and visualized via STORM phosphorimager analysis (GE Healthcare Bio-Sciences Corp.) and quantified using ImageQuant Software (Molecular Dynamics).
GST pulldown assays
Radiolabeled TRα and TRβ1 were prepared by in vitro transcription/translation of the corresponding pSG5 clones in the presence of [35S]methionine using a TNT T7 quick-coupled transcription/translation system (Promega) and the manufacturer's protocol. GST-coactivator and GST-corepressor fusion proteins were expressed in and purified from the corresponding pGEX transformants of E. coli strain BL21 (DE3)-RP. GST pulldown assays were performed using a 96-well plate protocol (38). Typically, 1 μL of full-length 35S-labeled TRα1 or TRβ1 TNT expressed protein was incubated with 400 to 600 ng of a given GST-coactivator or GST-corepressor in 100 μL of binding buffer A in the presence of a range of T3 or T4 concentrations. After a 1.5-hour incubation at 4°C, the wells were washed 3 × with 200 μL of binding buffer A at 4°C (T3 or T4 was maintained in the wash buffers in the coactivator binding assays to retain the TRs in a liganded state). Radiolabeled TR proteins remaining bound to the GST-coregulators after the washes were eluted by incubation of each sample with 50 μL of 20 mM glutathione and 100 mM Tris-HCl (pH 8.0), at 4°C for 45 minutes, were resolved by SDS-PAGE and were visualized and quantified via STORM phosphorimager analysis and Prism 5 statistical/plotting software (GraphPad Software, Inc.).
Protease protection assays
35S-labeled TRα1 or TRβ1 proteins were synthesized in vitro using the coupled Promega TNT system. One microliter of each TNT reaction was diluted to 18 μL in ice-cold 50 mM Tris-HCl (pH 7.5) containing either carrier alone or the desired T3 or T4 hormone concentration (40–42). After addition of 0.025 U (for TRα1) or 0.05 U (for TRβ1) of elastase (pancreatic type IV, E0258; Sigma-Aldrich), the tubes were transferred to 25°C and were incubated for 10 minutes. The reactions were rapidly terminated by adding 7 μL of SDS-PAGE sample buffer (125 mM Tris-HCl [pH 6.8], 20% glycerol, 4% SDS, 10% β-mercaptoethanol, and 0.5 mg/mL bromphenol blue) and flash freezing the samples to −70°C. The accumulated samples were subsequently quickly thawed and denatured at 95°C for 10 minutes. Samples were then resolved by SDS-12% PAGE and were visualized/quantified via STORM phosphorimager/ImageQuant analysis as above.
Transient transfections
Human embryonic kidney (HEK) 293T, CV1, HeLa, Hep G2, and Chinese hamster ovary (CHO) cells were maintained in Dulbecco's modified Eagle's medium formulated with 25 mM glucose and 4 mM l-glutamine (11965–092; Invitrogen) and supplemented with either 5% (HEK293T) or 9% (CV1, HeLa, Hep G2, and CHO cells) fetal bovine serum. 3T3-L1 cells were maintained in Dulbecco's modified Eagle's medium containing 25 mM glucose, 4 mM l-glutamine, and 1 mM sodium pyruvate (11995–065; Invitrogen) and supplemented with 5% calf bovine serum. All cells were incubated at 37°C in a 5% CO2 atmosphere. For each transfection, 3 × 104 cells (HEK293T, HeLa, and CV1) or 2 × 104 cells (CHO and 3T3L1) were plated per well in a 24-well tissue culture plate. After 24 hours, the medium was removed, the wells were washed with 0.5 mL of × PBS each, and medium containing 5% hormone-stripped fetal bovine serum was added to each well. Transfections were initiated using Effectene (QIAGEN) following the manufacturer's protocol. Each transfection cocktail contained 4 ng of pSG5-TRα1 or pSG5-TRβ1, 50 ng of luciferase reporter, 5 ng of a pCMV-LacZ reporter (used as an internal transfection control), and sufficient pUC18 carrier to bring the total DNA concentration to 250 ng/well. Twenty-four hours after transfection, the medium was replaced with fresh hormone-stripped medium containing the desired hormone concentration (T3, T4, or ethanol vehicle). After an additional 24 hours, the cells were washed with 0.5 mL of 1× PBS per well and lysed with 100 μL of Triton lysis buffer, rocking the plates for 15 minutes at 25°C. Luciferase activity was then measured by adding 20 μL of Luciferase Assay Reagent from the Luciferase Assay System (Promega) to 5 μL of cell lysate and by use of a Plate Chameleon V luminometer (Hidex). β-Galactosidase activity was measured by addition of 200 μL of chlorophenol red–β-d-galactopyranoside (Roche) reagent to 20 μL of cell lysate and use of a Molecular Devices SpectraMax 250 microplate reader (29).
Two-hybrid mammalian assays
The mammalian 2-hybrid assay was performed as described previously (43, 44).
Results
TRα1 exhibits a higher relative affinity for T4 than for T3 compared with that for TRβ1
T4 is known to bind to TRs with a lower affinity than does T3 using a radiolabeled hormone-binding assay (1, 16). We first sought to confirm that we could recapitulate this difference using our specific conditions and recombinant proteins. We therefore used a protease-protection methodology that detects the known ability of hormone agonists to induce a compact, degradation-resistant conformation on their cognate nuclear receptors (40–42). This method allows the affinity for hormone to be determined for each receptor by measuring the hormone concentration required to confer half-maximal protease protection, whereas the broad conformation changes invoked by the hormone can be visualized by comparing the pattern of proteolytic fragments released at a given hormone concentration (40–42). This method is highly reproducible and yields measures of affinity that agree well with those derived from radiolabeled or immunological hormone binding assays but avoids the high background of these more traditional approaches (40–42). We believe that the T3 and T4 binding to TR would be expected to equilibrate rapidly under our conditions (16).
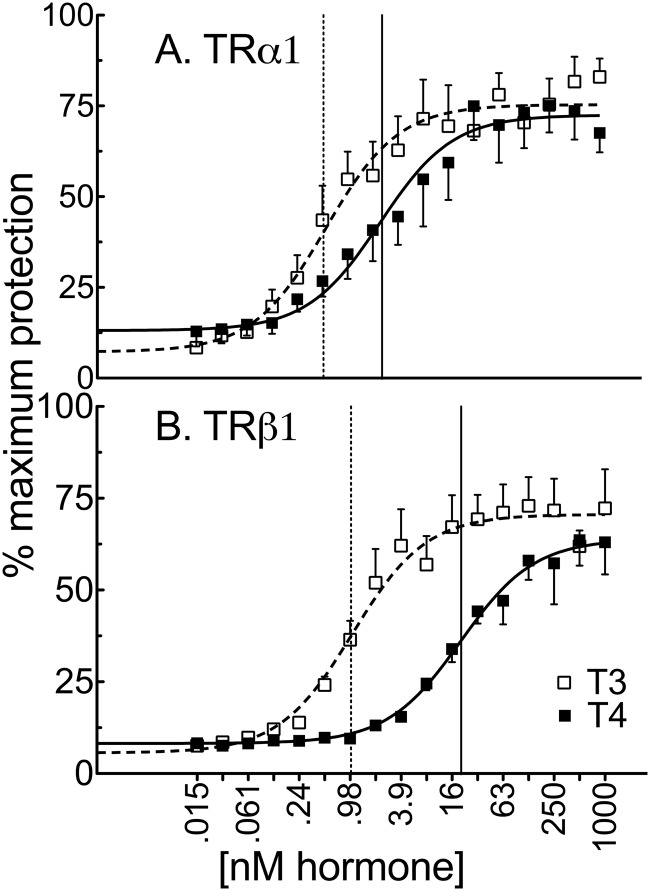
In agreement with previous reports (16, 45), it required significantly more T4 than T3 to bind to and thus confer protease resistance on both TRα1 and TRβ1. However, the specific hormone concentrations required differed for the different TR isoforms: TRα1 displayed a 4.4-fold difference between T4 vs T3 (2.4 nM vs 0.54 nM) for half-maximal protection, whereas TRβ1 displayed a near 20-fold difference (20 nM vs 1.0 nM for half-maximal protection) (Figure 1 and Supplemental Figure 1). Notably, our protease resistance numbers agree with but do not match specifically the published values obtained using radiolabeled hormone binding (16, 45). No differences in the size or relative abundance of the elastase-resistant fragments were observed for a given receptor isoform in response to T4 vs T3 (not shown), indicating that both hormone derivatives invoke similar global, if not necessarily identical, conformations on binding (45).
Figure 1.
TRα1 (A) exhibits a greater affinity for T4 vs T3 than does TRβ1 (B). Radiolabeled in vitro transcribed and translated TRs were incubated with 0.025 U (TRα1) or 0.05 U (TRβ1) of elastase and the indicated concentrations of vehicle, T3, or T4 for 10 minutes. The samples were analyzed by SDS-PAGE, and the percent recovery of the receptor protease-resistant core (maximum = 100%) is indicated for each T3 or T4 concentration. Error bars indicate SEMs of at least 3 biological replicate experiments. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
Ability of TRs to respond to T4 vs T3 differs in different cells and can greatly exceed the differences in affinity observed in vitro
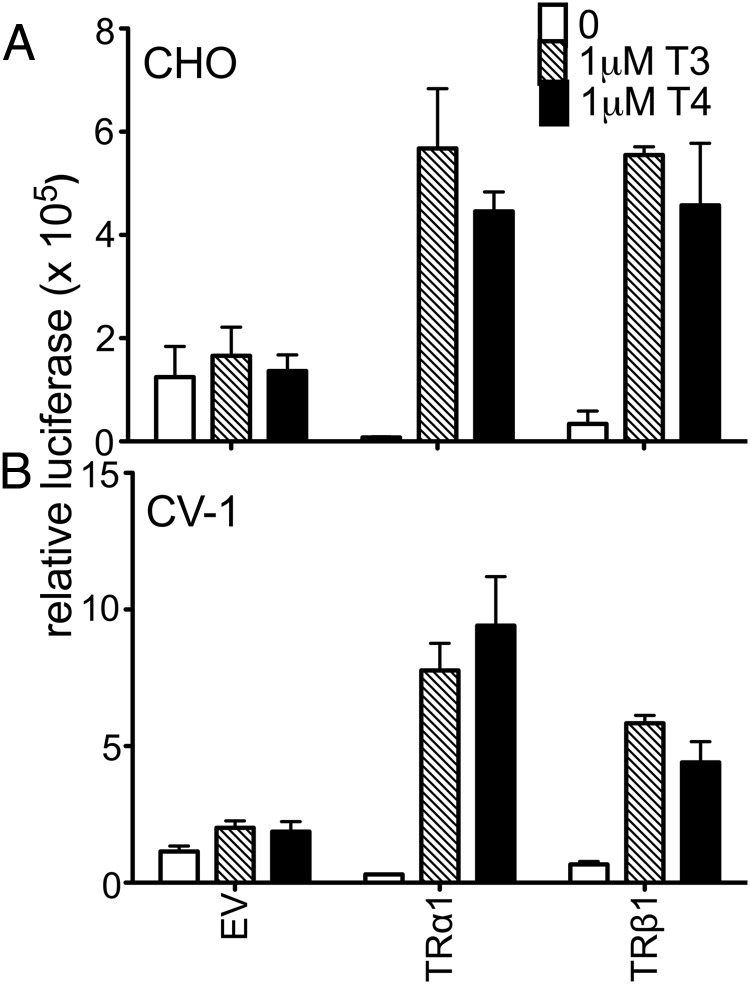
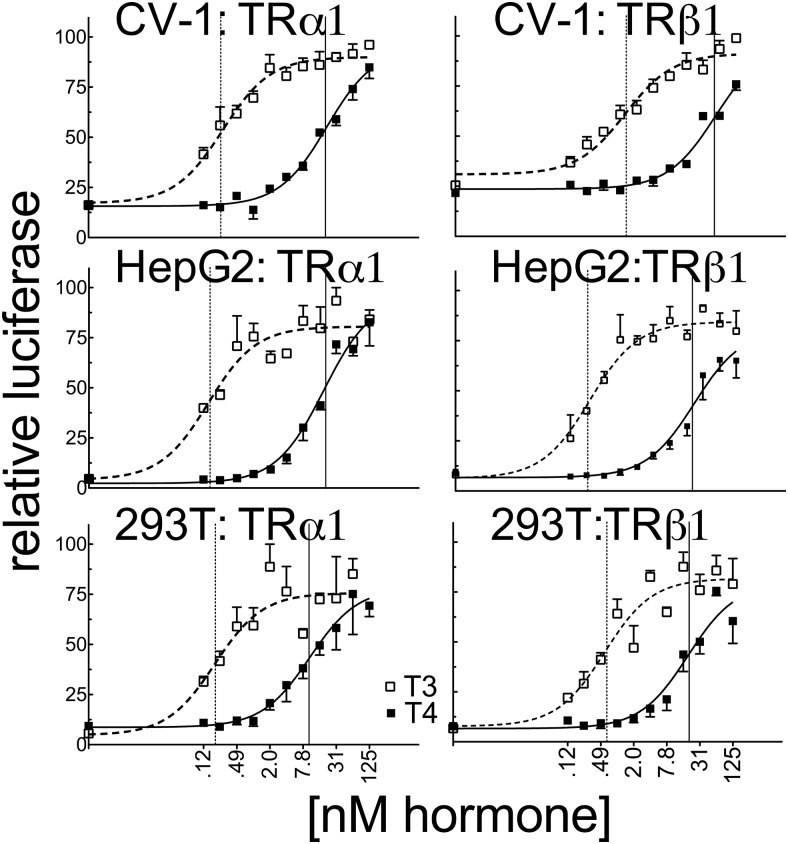
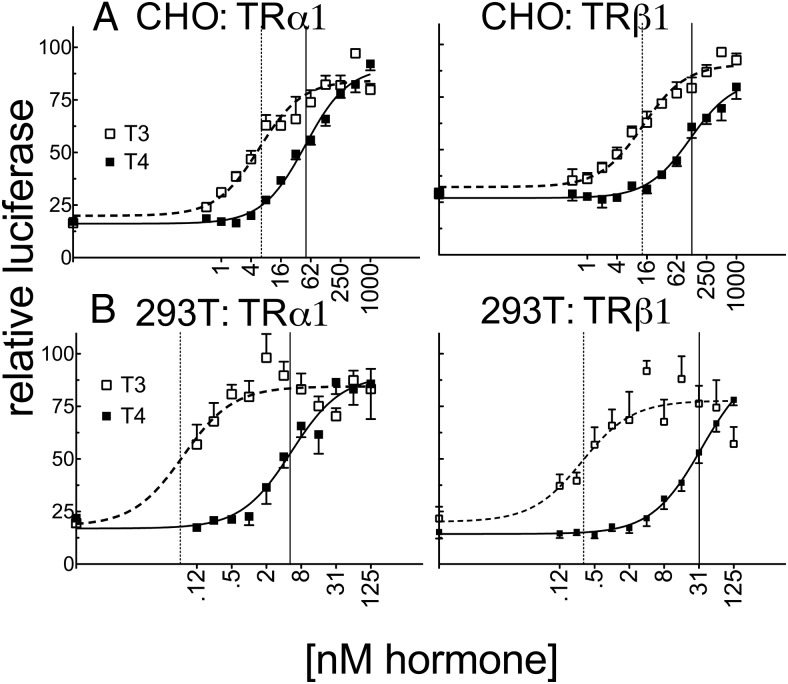
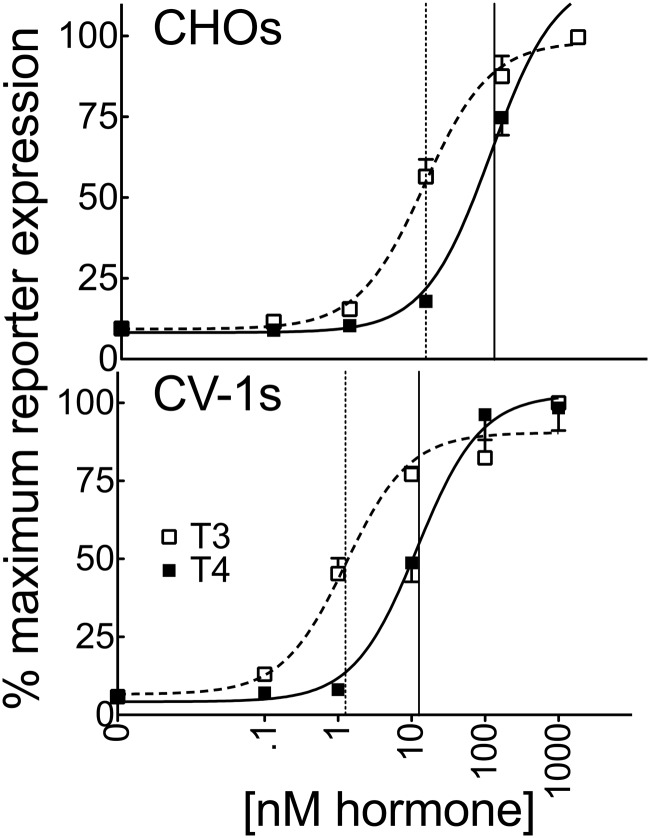
Consistent with the low endogenous expression of TRs in CHO and CV-1 cells, introduction of a consensus DR4-CON-tk-luciferase reporter into these cells in the absence of an ectopic TR construct (and an empty expression vector [EV]) produced only basal levels of luciferase expression, which were relatively unaffected by T4 or T3 (EV; Figure 2). Addition of ectopic TRα1 or TRβ1 to these transfections produced a robust activation of luciferase reporter expression in response to T3 and T4 (Figure 2). Unexpectedly, the ability of either isoform to respond to T4 in CV-1 cells was significantly more impaired than would be predicted from the ligand affinity criteria alone, with TRα1 requiring 78-fold more T4 and TRβ1 requiring 41-fold more T4 to yield a transcriptional response equal to that observed for T3 (Figure 3 and Supplemental Table 1). An analogous result was obtained in HEK293T and HepG2 cells with TRα1 requiring 53- to 125-fold more T4 and TRβ1 requiring 33- to 79-fold more T4 to obtain a transcriptional response equal to that of T3 (Figure 3 and Supplemental Table 1). This attenuated reporter gene response to T4 compared with that to T3 is unlikely to simply be the consequence of cellular deiodinase activity for several reasons. HEK293T cells have very low or undetectable levels of deiodinase activity (46). Further, deiodinase (DIO) 1 and DIO2 are T4-activating enzymes and would therefore narrow, rather than broaden, the differences in EC50 observed between T4 and T3, whereas DIO3, the primary TH-inactivating enzyme, recognizes both T4 and T3 equally. We conclude that there are mechanisms operating in CV-1, HepG2, and HEK293T cells that permit a robust TR response to T3 while conferring an attenuated response to T4 relative to the TR affinities for these ligands in vitro.
Figure 2.
Very low endogenous expression of TRs in CV-1 and CHO cells induced very low basal activity on a DR4-CON-tk reporter. CHO (A) or CV-1 cells (B) were transiently transfected via lipofection with an empty expression vector (EV) or expression vectors containing TRα1 or TRβ1, a consensus DR4-CON-tk-luciferase reporter, and β-galactosidase control in hormone-depleted medium. After 24 hours in hormone-depleted medium, the cells were exposed to the indicated T3 or T4 concentrations. Cells were incubated an additional 24 hours before harvest, and lysates were analyzed for luciferase activity and β-galactosidase levels. Luciferase values were normalized against the β-galactosidase control for each sample. Error bars indicate SEMs for at least 3 biological replicate experiments.
Figure 3.
The ability of TRα1 to respond to T4 vs T3 differs in CV-1, HepG2, and HEK293T cells and substantially exceeds observed affinity differences determined in vitro. CV-1, HepG2, and HEK293T cells were transiently transfected as described for Figure 2 with the consensus DR4-CON-tk-luciferase reporter. Luciferase values were normalized against the β-galactosidase control for each sample and plotted as percent T3 maximum. Error bars indicate SEMs of at least 3 biological replicate experiments. Unfortunately, we could not measure the TR protein levels expressed in these transfected cells due to limitations arising from the relatively low levels of expression and technical limitations as to the commercially available anti-TR antibodies. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
Although traditionally our EC50 values would be independent of the level of expression of the TRs in the different cells, these EC50 values, as well as the maximum activity, might conceivably depend on the ability of our TRs to interact with specific limiting cofactors in these cells; we therefore note that identical results were obtained over a wide range of TR expression levels, at least in the CV-1 cells (data not shown) and that the relative amounts of T4 to T3 required to activate the reporter were obtained using identical TR expression for each cell line. The estimated Hill coefficients under these conditions were approximately 1 for all our assays (although we note that our data were not analyzed so as to allow an extremely precise calculation of the Hill coefficient).
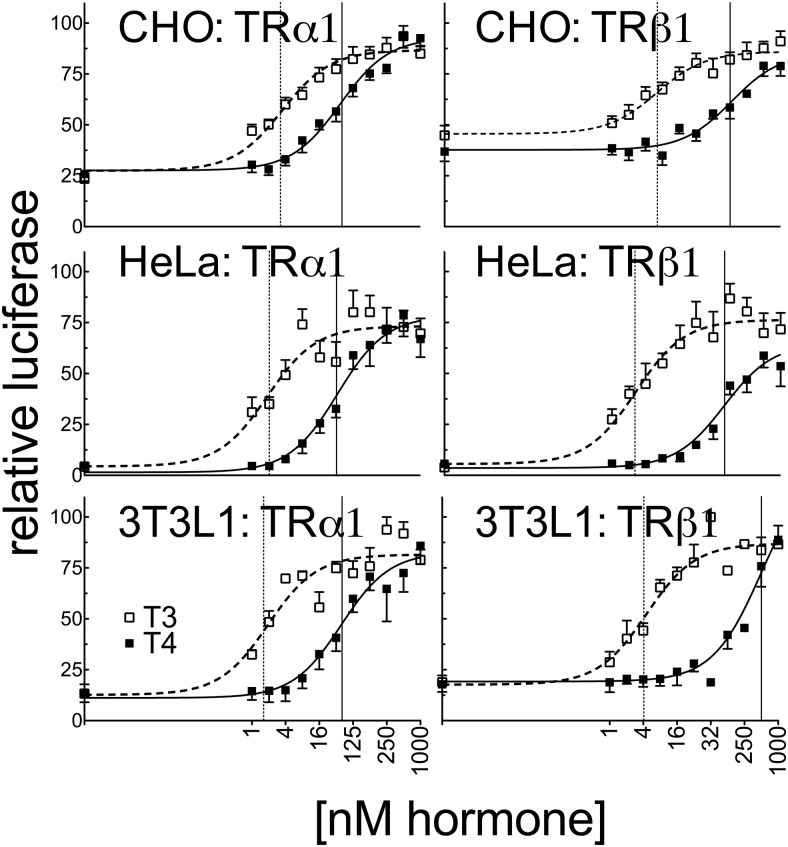
Expanding our reporter gene assays to CHO, HeLa, and 3T3L1 cells, we found that the ability of TRα1 and TRβ1 to respond to T3 differed notably among the different cell lines, with EC50 values ranging from 0.14 to 3.4 nM T3 (for TRα1) and from 0.28 to 6.2 nM (for TRβ1) (Figure 4). These differences in T3 response have been observed before (47) and are likely to reflect differences in T3 transport, sequestration, or turnover. Notably, the T4 response in the different cell lines varied independent of the T3 response. For example, both TRα1 and TRβ1 displayed a much stronger relative ability to respond to T4 than T3 when expressed in CHO cells than when expressed in the previously discussed cell lines; TRα1 exhibited only a 10- to 11-fold differential in its EC50 for T4 vs T3 (37 nM T4 vs 3.4 nM T3) and TRβ1 exhibited a 22-fold differential between the 2 ligands (135 nM T4 vs 6.2 nM T3) in CHO cells (Figure 4). Notably, we observed an intermediate response to T4 with TRα1 in both the HeLa and 3T3-L1 cell lines. HeLa cells were found to have an 18-fold difference between T4 and T3 for transcriptional activation of the DR4-CON-tk-luciferase reporter with TRα1 (33 nM T4 vs 1.9 nM T3) compared with a 38-fold difference with TRβ1 (99 nM T4 vs 2.6 nM T3) (Figure 4 and Supplemental Table 1). Similarly, 3T3-L1 cells require 22-fold more T4 than T3 to induce comparable expression of the DR4-CON-tk-luciferase reporter with TRα1 (39 nM T4 vs 1.8 nM T3), whereas TRβ1 required 112-fold more T4 than T3 (472 nM T4 vs 4.2 nM T3) under the same circumstances (Figure 4 and Supplemental Table 1). CHO cells, like HEK293T cells, have low or undetectable deiodinase activities (45, 48), making it unlikely that the relative T4 sensitivity of the former vs the insensitivity of the latter simply reflects differences in T4 conversion into T3. Further, the relative ability to respond to T4 vs T3 differed independently for the 2 different TR isoforms: for example, TRβ1 had a narrower difference in its T4 vs T3 response than did TRα1 when these receptors were expressed in CV-1 cells, whereas the reverse was true in 3T3-L1 cells. This result further indicates that the cell type–specific nature of the T4 response does not simply reflect selective conversion, inactivation, import, or export of one hormone derivative over the other, any of which would have equal effects on both receptor isoforms (see also our mammalian 2-hybrid results below).
Figure 4.
The ability of TRα1 to respond to T4 vs T3 differs much less dramatically in CHO, HeLa, and 3T3L1 cells. CHO, HeLa, and 3T3–L1 cells were transiently transfected in the same manner as in Figure 3 with the DR4-CON-tk-luciferase reporter. Error bars indicate SEMs of at least 3 biological replicate experiments. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
We expanded our transfections to include reporters containing a DR4 element derived from the murine leukemia virus retrovirus (DR4-M-tk-luciferase), a DR4 element derived from the αMHC gene (DR4-αMHC-tk-luciferase), or a DIV6 element derived from the chicken lysozyme F2 promoter (DIV6-F2-tk-luciferase) (49–51). The relatively strong response to T4 vs T3 observed for the CHO cells with use of the DR4-CON-tk-luciferase reporter was also observed when these additional reporter constructs were used (Figure 5A and data not shown). Conversely, the relatively weak response of TRα1 and TRβ1 to T4 vs T3 observed in HEK293T cells with use of the DR4-CON-tk-luciferase reporter was also recapitulated when the nonconsensus DR4-MLV-tk-luciferase reporter was used (Figure 5B). These observations further indicate that the cell type–specific nature of the T4 vs the T3 response is not simply due to T4 availability or the nature of the TRE.
Figure 5.
A known nonconsensus TRE exhibits an ability similar to that of the consensus DR4 TRE to respond to T4 vs T3 in CHO and HEK293T cells. A, CHO cells were transiently transfected as described for Figure 3 except with a DR4-M-tk-luciferase reporter derived from the murine leukemia virus. Luciferase values were normalized against the β-galactosidase control for each sample and plotted as percent T3 maximum. Error bars indicate SEMs of at least 3 replicate experiments. B, Same experiment as for panel A was repeated using HEK293T cells. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
Both T4 and T3 induce TRα1 to bind certain coactivators with near equal potency in vitro, whereas substantially more T4 than T3 is required for other coactivators
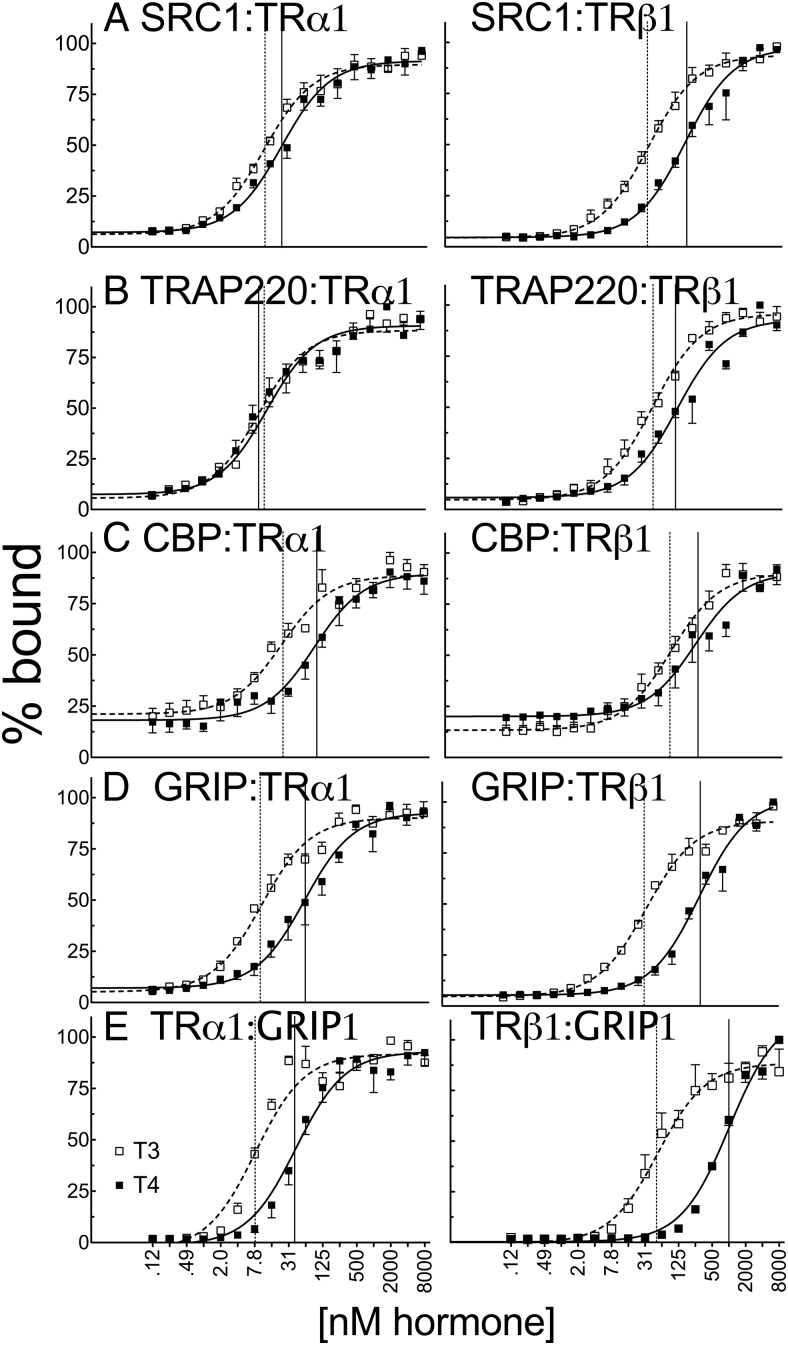
The ability of TRs to regulate transcription is conferred through the ability of these receptors to interact with a diverse panel of coactivator proteins that vary in abundance in different cell types. We next asked whether the varied TR response to T4 vs T3 in different cell lines might reflect, in part, differences in the ability of the 2 ligands to induce TRs to bind these different coactivators. Using a GST pulldown protocol, we found that the relative ability of T4 and T3 to induce coactivator binding depended both on the TR isoform and on the coactivator. For most of the coactivators tested, TRα1 displayed a stronger relative ability to respond to T4 than did TRβ1. For example, only 2-fold more T4 than T3 (25 nM vs 12 nM) was required to induce SRC1 (52) binding by TRα1, whereas 5-fold more T4 than T3 (188 nM vs 42 nM) was required to induce SRC1 binding by TRβ1 under the same conditions (Figure 6A and Supplemental Table 1). Similarly, only 6-fold more T4 than T3 was required to induce GRIP1 (52) binding by TRα1 (61 nM vs 11 nΜ) (Figure 6D). The generally strong relative response of TRα1 to T4 vs T3 was also observed when the reciprocal assay was performed by using GST-TR fusions and full-length GRIP1 (Figure 6E).
Figure 6.
T4 recruits certain coactivators to TRα1 with potency that was nearly equal to that of T3 in vitro. Full-length in vitro–transcribed and translated, 35S-labeled human TRα1 or TRβ1 were incubated with glutathione-agarose bead immobilized GST fusions of coactivator proteins (LXXLL receptor interaction motifs) indicated within each panel: SRC1 (amino acids 568–891) (A), TRAP220 (486–723) (B), CBP (1–451) (C), and GRIP (544–767) (D). Incubations were conducted in the presence of the indicated T3 or T4 concentration. The immobilized GST constructs were washed, and the nuclear receptors remaining bound to each construct were eluted. The resulting coactivator-TR complexes were characterized by SDS-PAGE and phosphorimager analysis and plotted as the percent maximum binding for each coactivator. Error bars indicate SEs of at least 3 replicate experiments. E, Reverse experiment was conducted with full-length in vitro–transcribed and translated 35S-labeled GRIP1 with immobilized full-length GST-TRα1 or GST-TRβ1, with vehicle alone or the indicated T3 or T4 hormone concentration. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
Surprisingly, the ability of TRα1 to bind to one coactivator, TRAP220 (53), in response to T4 was so potent as to be indistinguishable from, or even slightly stronger than, its response to T3 (Figure 6B). TRAP220 was bound by TRα1 with an EC50 of 9.9 nM for T4 vs 14 nM for T3, whereas TRAP220 binding by TRβ1 required 2.7-fold more T4 (136 nM) than T3 (50 nM) (Figure 6B and Supplemental Table 1). Taken as a whole, our results indicate that TRα1 binds to all the coactivators tested at lower absolute T4 and T3 concentrations than does TRβ1, that the difference in the T4 vs T3 response of TRα1 is also generally narrower than that of TRβ1, and that this latter effect is sufficient for TRα1 and for certain coactivators, such as TRAP220, to allow binding of TRAP220 by T4 equal to that of T3. The one exception observed in our TRα1 > TRβ1 sensitivity to T4 vs T3 for coactivator binding was for CBP (6), which required 3.8-fold more T4 than T3 (91 nM vs 24 nM) for binding by TRα1 compared with 2.8-fold more T4 than T3 for binding by TRβ1 (285 nM vs 103 nM) (Figure 6C). We note that the levels of TRα1 and TRβ1 were identical in all these assays.
Notably, the similar abilities of T4 and T3 to induce TRαl binding to SRC1 was also observed in mammalian 2-hybrid assays in both CV-1 and CHO cells (Figure 7). It was observed that much lower T4 concentrations (12 nM) were required to bind SRC1 to TRα1 in CV-1 cells by the 2-hybrid assay than to activate reporter gene transcription in the same cells (21.2 nM) (Figure 7), indicating the ready availability of both forms of TH in the CV-1 cells despite the large discrepancy in the T4 vs T3 driven reporter gene response in these cells.
Figure 7.
SRC1 is recruited efficiently by both T3 and T4 to TRα1 in CHO and CV-1 cells by a mammalian 2-hybrid reaction. An expression vector encoding a Gal4 DNA binding domain (GBD)–SRC1 (LXXLL domains) fusion and an expression vector encoding a Gal4 activation domain–TRα1 fusion was transfected into CHO or CV-1 cells together with a GAL-17-mer luciferase reporter as described previously. The graphs represent means of 4 replicates. Error bars indicate SEMs. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
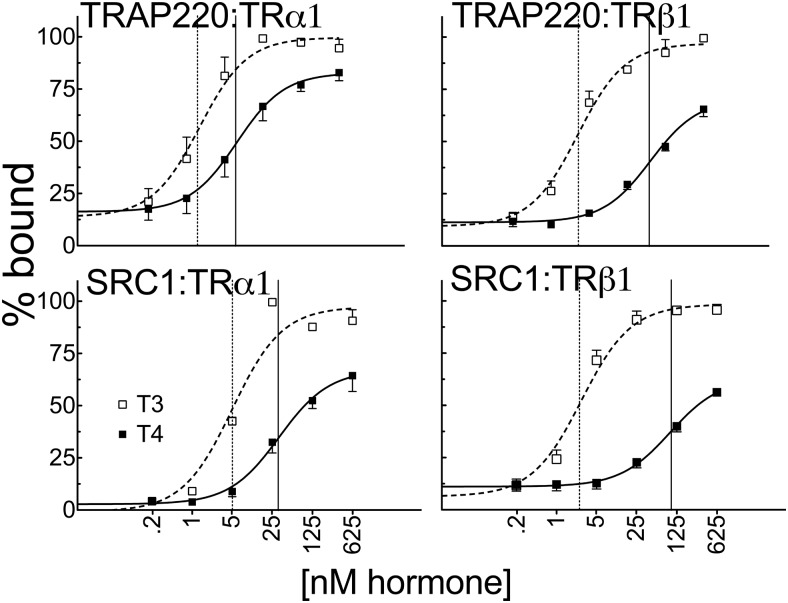
We next used EMSAs to determine whether this pattern of coactivator recruitment by T4 or T3 was altered when the TRs were bound to cognate DNA recognition sites. As expected, TRα1 and TRβ1 formed receptor homodimers on both a DR4-CON and DIV6-F2 DNA probe. Addition of SRC1 or TRAP220 in the presence of T4 or T3 shifted the mobility of the receptor homodimers to a slower migrating species characteristic of formation of a DNA/TR/coactivator complex, whereas these DNA/TR/coactivator complexes did not form in the absence of either ligand. Using the EMSA protocol, we found much the same overall pattern as was observed in the GST pulldowns: TRα1 recruited both SRC1 and TRAP220 at lower absolute T4 concentrations and relatively more strongly in response to T4 vs T3 than did TRβ1, with the ability of TRα1 to respond to T4 being more readily observed for TRAP220 than for SRC1 (Figure 8 and Supplemental Figure S2). Consistent with our reporter gene experiments, changing the nature of the TRE did not substantially affect the relative response of either TR isoform to T4 vs T3 nor did performing the assay with retinoid X receptor-α/TRα1 heterodimers (data not shown). We conclude that the TRα1 isoform, in particular, possesses an inherent, strong ability to recruit certain coactivators in response to T4 and that, in several circumstances, the ability of TRα1 to recruit coactivators in response to T4 approaches or overlaps its response to T3. These results raise the possibility that TRα1 may be able to serve as a dual sensor for both T4 and T3 in certain contexts.
Figure 8.
T4 and T3 recruit certain coactivators to TR-DNA complexes with almost the same potency as do uncomplexed TRs. A DR4 consensus TRE shows that TRα1 homodimers bound to DNA require less T4 than does TRβ1 to supershift TRAP220 or SRC1. EMSAs were conducted by incubating human TRα1 or TRβ1 derived from a recombinant baculovirus/SF9 cell system with 1 pmol of 32P-labeled TA-DR4 oligonucleotide probe and 400 ng of glutathione-purified GST-coactivator proteins (TRAP220 [486–723] or SRC1 [568–891]) in the presence of the indicated hormone concentrations or vehicle alone. The resulting TR-DNA complexes were resolved by native acrylamide gel electrophoresis and were visualized using phosphorimager analysis. The TR-DNA complexes were not supershifted by GST alone and were quantified relative to the amount of unshifted TR homodimer. The graphs represent a mean of n > 3 replicates. Error bars indicate SEMs. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
Failure to release corepressors, as well as differences in binding coactivators, helps explain the impaired T4 response in certain cell types
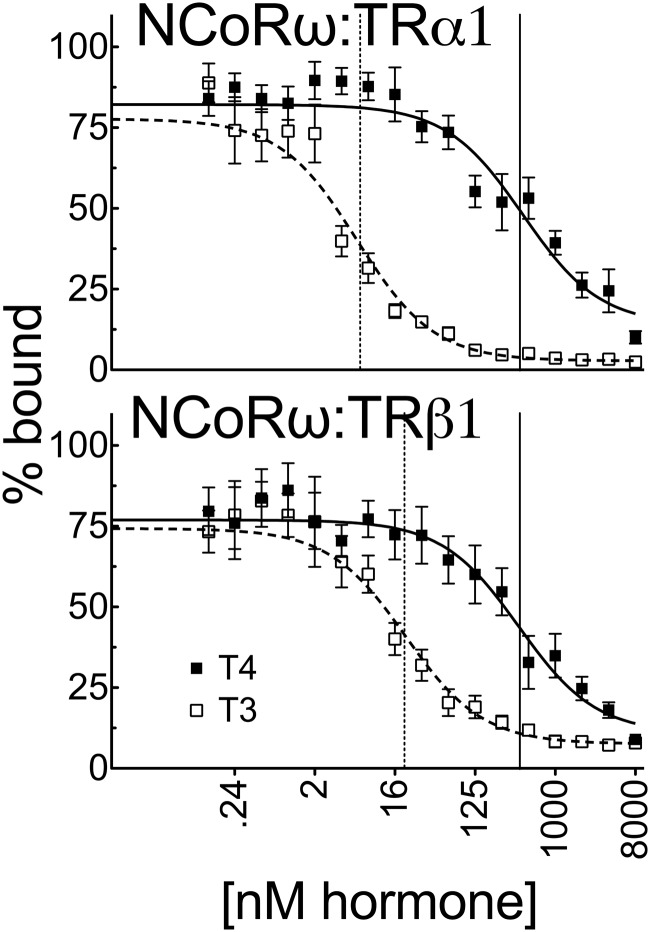
Our experiments indicated that the severely impaired T4 vs T3 response observed in the CV-1 or HEK293T cells could not be readily explained on the basis of coactivator recruitment: although the different coactivators tested displayed different abilities to be recruited by TRα1 and TRβ1 in response to T4 vs T3, the relative response to T4 vs T3 in all cases approximated or was somewhat narrower than the corresponding receptor affinities for these ligands in vitro. We therefore examined the ability of T4 and T3 to operate conversely by inducing corepressor release rather than coactivator binding. We used GST pulldown assays with NCoRω, which has been identified as a physiologically important corepressor for TRs (28). Interestingly, although T3 induced NCoRω release from both TRα1 and TRβ1 at concentrations only slightly higher than those required to confer coactivator binding, the ability of T4 to release NCoRω from either TR isoform was much more inefficient, with TRα1 requiring 74-fold more T4 than T3 (432 nM vs 5.8 nM) and TRβ1 requiring 19-fold more T4 than T3 (389 nM vs 21 nM) to release this corepressor (Figure 9). The requirement for much higher T4 concentrations than T3 for release of NCoRω from TRα1 was also seen in EMSAs and mammalian 2-hybrid assays (data not shown).
Figure 9.
A much greater concentration of T4 than T3 is required to release NCoRω from TRs in vitro. Full-length 35S-labeled human TRα1 or TRβ1 was incubated with immobilized GST fusions of NCoRω (amino acids 1817–2453) in the presence of increasing T3 or T4 as indicated for coactivators in Figure 6. The resulting corepressor-TR complexes were washed, and the corepressor remaining bound at each hormone concentration was released by incubation with soluble glutathione and was characterized by SDS-PAGE and phosphorimager analysis. The graphs represent means of n > 3 replicates. Error bars indicate SEMs. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.
Certain endogenous genes respond to low concentrations of T4 as well as to T3
Most of our prior studies were performed in vitro or using ectopic reporter genes. We therefore examined whether certain endogenous TR target genes might be equally responsive to T4 as to T3, assuming this would depend on the cell type and the particular cofactors used at that particular target gene. We therefore used quantitative RT-PCR to study the expression of a variety of endogenous genes in GH3 cells and TRα1-transfected HepG2 cells (data not shown). We found several of these highly T4-responsive genes by these criteria (eg, KLF9; Supplemental Figure 3). Of course, other, more traditional T3-driven genes were also discovered (eg, SHH) that required far less T3 than T4 for activation; these genes may be regulated by the cofactors that we have found to be relatively nonresponsive to binding of T4 by TRs. These data further support our theory that certain genes in certain cells may be directly regulated by T4 without its conversion to T3.
Discussion
Different TR isoforms differ in their ability to bind to T4
Thyroid hormones play multiple important roles in vertebrate physiology by regulating homeostasis, development, and metabolism (2, 3, 6, 9, 12, 13, 15, 54). Based primarily on affinity and reporter gene assays, T3 has generally been considered to be the only biologically active form of thyroid hormone, with T4 viewed as a prohormone that only activated its receptors through conversion into T3 by target tissue–level deiodinases (1, 17, 18, 20, 55). Consistent with prior studies, we found that TRs in general bound T4 in our in vitro assays with a lower affinity than T3. However, we also report here that the ability to bind T4 was TR isoform dependent and that TRα1 bound T4 with only a 4.4-fold lower affinity than T3. Given that TRα1 also exhibited a 2-fold higher affinity for T3 than did TRβ1, these results suggest that the ability of TRα1 to respond to T4 is substantially greater than that of TRβ1. Further, once bound, T4 induced global conformational changes in TRα1 (and in TRβ1) that were indistinguishable from those mediated by T3 (21–24). Notably, free T4 is present in serum at approximately 3-fold the concentration of free T3 and when considering total T4 in serum, bound plus free, T4 is 100-fold the concentration of total T3 (56). Although it is difficult to extrapolate the effective T4 and T3 concentrations in target tissues from these serological measurements, our results raise the possibility that T4 may be able to function directly as an active hormone under certain circumstances and that tissues that express primarily the TRα1 isoform (such as heart or brain) may have a greater potential to respond directly to T4 than do tissues that express primarily the TRβ1 isoform (such as liver). We discuss this possibility in more detail below.
Relative ability of T4 vs T3 to regulate reporter gene expression differs for different cell types and is determined by factors other than simple affinity
In general, the higher affinity of TRα1 vs TRβ1 for T4 in vitro was paralleled by a stronger T4 response by TRα1 vs TRβ1 in reporter gene assays. However, the ability of either TR isoform to respond to T4 was strongly dependent on the cell line, was typically impaired compared with that expected from the corresponding affinities in vitro, and was divergent than that seen for T3. CHO cells displayed the strongest relative T4 vs T3 response: introducing TRα1 into CHO cells resulted in an 11-fold difference in reporter gene response to T4 vs T3. In contrast, when tested on the same reporter gene, TRα1 required 125-fold more T4 than T3 when expressed in HepG2 cells and 78-fold more T4 than T3 when expressed in CV-1 cells. These effects were also observed using a variety of reporter genes and cannot be readily attributed to the actions of deiodinases such as DIO2, which would be expected to sensitize, rather than suppress, the response to T4 by causing conversion to T3. Although we cannot completely rule out the possibility that the cell type–specific differences in the reporter gene response to T4 vs T3 reflect differences in the uptake, degradation, or sequestration of the T4 and T3 forms of TH, it is important to note that the effects of cell type differed for the two different TR isoforms, and our 2-hybrid results indicate that T4 is readily biologically available for coactivator acquisition even when not able to induce reporter transcription. Our results also indicate that the cell-specific nature of the T4 response must include mechanisms beyond those that affect T4 vs T3 intracellular levels per se.
T4 efficiently recruits many coactivators to TRα1, with certain coactivators exhibiting a T4 response equal or near equal to that of T3
To determine the molecular basis behind the cell specificity of the T4 vs T3 response, we examined the ability of these ligands to recruit a panel of coactivators to TRα1 and TRβ1 (5, 23, 26, 52, 57, 58). Consistent with our affinity and cell transfection data, TRα1 generally displayed a stronger ability to recruit coactivators in response to T4 than did TRβ1. Interestingly, many coactivators were recruited surprisingly efficiently in response to T4. This phenomenon was most pronounced for TRAP220, which by GST pulldown was recruited by TRα1 better or equally well in response to T4 as to T3. Similarly, SRC-1 was recruited by TRα1 in response to concentrations of T4 only modestly higher than those required for T3. This efficient recruitment of these coactivators by TRα1 and T4 was also observed in EMSAs using 2 distinct DNA binding sites and in mammalian 2-hybrid assays.
TRAP220 is the major subunit by which the Mediator complex is recruited by the TRs, and SRC1 is well established as playing a physiologically important role in TR function (26). Both TRAP200 and SRC1 are recruited primarily through the interaction of LXXLL motifs in the coactivator with a hydrophobic groove in the TR that is exposed by an agonist-induced reorientation of receptor helix 12; presumably, T4 operates this conformational switch in much the same manner as does T3 (24). Significantly, none of the coactivators tested displayed the 78-fold difference in the concentrations of these T4 vs T3 required for reporter gene activation in CV-1 cells. We conclude that TRα1, in particular, has an innate ability to recognize T4 as an agonist and can do so at concentrations approximating those required for the T3 agonist response. Conversely, we conclude that the weak reporter gene response in cells such as CV-1 cells is not likely to be due to an impaired T4-mediated recruitment of the coactivators tested here.
The ability of TRAP220 to be recruited by TRα1 at virtually equal concentrations of T4 and T3 might appear at first to conflict with the 4.4-fold difference in the measured in vitro affinity of TRα1 for these 2 hormone derivatives. It should be noted, however, that just as hormone binding can alter the affinity of a receptor for its coregulators, the reciprocal also applies, such that association of coactivators and corepressors can alter the receptor's affinity for hormone. By this model, TRAP220 may induce a TRα1 conformation that possesses a higher affinity for T4 than observed for TRα1 alone and NCoR may decrease the affinity for T4. Alternatively, it is known that T4 makes subtly different contacts with TRα1 than does T3 (24) and thus conceivably may be able to induce a TRα1 conformation possessing an affinity for TRAP220 that was higher than that induced by T3; if so, fewer TRα1 molecules bound to T4 may exhibit an equal overall TRAP220 recruitment as do more TRα1 molecules bound to T3.
Impaired response to T4 vs T3 in certain cell types parallels an impaired ability of T4 vs T3 to release corepressors
Given that T4 can efficiently bind to and induce recruitment of certain coactivators by TRα1, why is T4 such a weak agonist compared with T3 in cell lines such as CV-1? It is notable that in contrast to coactivators, release of corepressors such as NCoRω required much higher concentrations of T4 than did T3. This inefficient release of NCoRω by T4 vs T3 was observed by multiple approaches, including GST pulldown, EMSA, and the mammalian 2-hybrid assay. We propose that this inefficient release of corepressors by T4 may constrain the ability of the TRs to activate gene expression in cells compared with the relatively more efficient corepressor release by T3. We have knocked-down NCoR expression by siRNA methodology and noted an apparent decrease in the ratio of T4 to T3 necessary to activate a reporter in our transfected HEK293T cell assay, as predicted from our hypothesis; however, the contributions of all coregulators, including coactivators, to the relative T4/T3 response still needs to be examined (data not shown). Alternatively, for example, certain yet-to-be-examined coactivators may require much more T4 than T3 for their recruitment. We suggest that the status of T4 as an inactive prohormone is not an absolute or autonomous property of the TRs themselves, but rather that differences in the precise nature and abundance of the different corepressors and coactivators expressed in a cell may serve to modulate that cell's ability to respond to T4 vs T3.
Why does corepressor release by TRα1 or TRβ1 require 180-fold more T4 than expected from hormone binding affinity measurements, even when these assays are performed in vitro using identical TR preparations? As noted in our coactivator discussion, the affinity of a receptor/coregulator complex can differ from that of the receptor alone; thus, the presence of the corepressor might itself inhibit T4 binding and thereby shift the EC50 for corepressor release to higher T4 concentrations (59). We have seen this in preliminary experiments (data not shown). Notably the EC50 for corepressor release by T3 and the in vitro binding constant for T3 are also nonidentical (although less dramatically so than for T4). Alternatively, it is possible that T4 may be able to bind in more than one location or orientation within the hormone-binding pocket of the TRs, with different binding modes favored at different T4 concentrations. Multiple binding modes for a given ligand have been observed for other members of the nuclear receptor family (60), and these distinct binding modes, if they exist for T4, could invoke distinct conformations that could account for coactivator acquisition at low T4 concentrations vs corepressor release at high T4 concentrations.
Although TRs required significantly more T4 to release corepressor than to bind coactivator, T3 displayed the converse behavior: releasing corepressor at lower T3 concentrations than required to bind several coactivators. T3 may therefore function in a bimodal fashion, with corepressors released and target genes derepressed at low T3 concentrations and with coactivator bound and overt (ie, above basal) target gene activation observed only at the higher T3 concentrations required for coactivator binding. This phenomenon may contribute to the diversity of the T3 response seen on different genes and in different cell types.
Are there physiological contexts in which T4 is a direct-acting TRα1 agonist?
Several recent studies have indicated that T4 can play nongenomic roles that do not require conversion to T3 (61) but have generally not challenged the view that T3, not T4, is the biologically relevant agonist for nuclear TR function. Our own experiments in vitro, however, indicate that TRα1 has the potential to act as a dual sensor of both T4 and T3, although TRβ1 appears to be primarily a T3 sensor. Are there in vivo contexts in which the T4 response of TRα1 is sufficient to produce physiologically relevant gene regulation in the absence of conversion to T3? Our most T4-responsive cell line, CHO cells, required approximately 37 nM T4 for half-maximal reporter gene activation, whereas total T4 in total normal human serum ranges from 64 to 154 nM (56). Although the effective concentration of T4 in a given target tissue is difficult to determine, it is plausible from these values that T4 levels in vivo are sufficient to induce, at least in certain tissues, activation of TRα1-regulated genes in the absence of T3. Therefore, the normal mix of T4 and T3 in circulation may actually confer a mixed T4/T3 response mediated primarily by TRα1, together with a more pure T3 response mediated primarily by TRβ1. Notably, mice in which both DIO1 and DIO2 have been genetically ablated, which thus lack the target tissue conversion of T4 to T3, display only very mild defects in their physiology (25). If, as indicated by these DIO1/2 knockouts, T4 is not absolutely required in its traditional role as a prohormone, the dominance of T4 to T3 in the circulation may instead reflect a novel role of T4 as a direct-acting hormone, and this direct role may be helping to ameliorate the effects of the DIO1/2 knockout.
In contrast to CHO, CV-1 and HepG2 cells, our least T4-responsive cell lines required nearly 80- to 125-fold more T4 than T3 for TRα1-mediated reporter gene activation. Cell-specific differences in coactivator and corepressor levels or function are likely to contribute to these cell type–specific differences in hormone response and may suggest a means by which the T4 sensitivity of a given cell type can be regulated in response to biologically important internal or external signals. The NCoR corepressor, for example, is targeted for proteolytic degradation by mSiah2, which is itself expressed in a cell type– and differentiation-specific manner (60); thus, regulation of corepressor turnover provides one potential mechanism by which cells may customize or modify their T4 response. MAPK signaling cascades cause dissociation of corepressors from their nuclear receptor partners and/or nuclear export (43, 44), whereas CK2 increases the interaction between corepressors and the TRs (61); these kinase-based signals therefore represent additional mechanisms by which cells may potentially adjust their response to T4 vs T3. Finally, both NCoR and SMRT are expressed through alternative mRNA splicing as a series of distinct corepressor variants that differ in molecular architecture and in biological function (28, 31). Preliminary results indicate that 2 distinct splice forms of NCoR, denoted ω and δ, are released from TRα1 at comparable T3 concentrations, but that much more T4 is required to release NCoRω from TRα1 than NCoRδ (data not shown); thus, the specific corepressor splice forms expressed in a cell are likely to also influence the T4 response of that cell.
We have, as predicted from these studies, also found endogenous genes in TRα1-expressing HepG2 and GH3 cells that appear to demonstrate an equal or near equal response to T4 as to T3 (Supplemental Figure 3 and data not shown). It is likely, therefore, that the ability to respond to T4 varies with the target gene, depending on the coregulators used for gene regulation at that locus, as well as the cell type. We are next seeking to use RNA sequencing to broadly determine the T4-responsive genes in these cells and chromatin immunoprecipitation to analyze the coregulators used by each TR target gene.
It is also interesting to consider the implications of these findings to sick euthyroid syndrome, the early stages of which are characterized by a fall in T3 levels, whereas T4 levels remain normal or are further elevated (62, 63). If some genes and tissues are directly responsive to T4, then this disease might reflect a shift in target gene expression, rather that an overall decrease in all TR regulation. The validity of this proposal will need to be studied further.
In conclusion, TH endocrinology is tightly regulated at multiple tiers. Negative feedback loops in the hypothalamus and pituitary control T3 and T4 output by the thyroid gland itself. Further, multiple phenomena function together to modulate the response to circulating TH at the target cell level. These include the rate of uptake of T3 and T4 into the cell, conversion of intracellular T4 into T3 by DIO1 and DIO2, inactivation/turnover of T3 and T4 by DIO3, and, as noted here, the ability of different TR isoforms and different coregulators to respond differently to T4 vs T3. Operating jointly, these mechanisms serve to maintain proper endocrine homeostasis while permitting the system to respond to developmental and physiological needs.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We sincerely thank Liming Liu for superb technical help and Drs David Furlow and Brenda Mengeling for isolating and helping to provide appropriate primers for the analysis of the stably transfected GH3-TRE luciferase cells.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R0153518). A.C.S. was supported in part by the National Science Foundation Graduate Research Fellowship Program (Grant GRFP 1148897). R.J. was supported in part by the National Institutes of Health (Training Grant T32 GM007377).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CBP
- cAMP response element–binding protein–binding protein
- CHO
- Chinese hamster ovary
- DIO
- deiodinase
- GRIP
- glutamate receptor-interacting protein 1
- GST
- glutathione S-transferase
- HEK
- human embryonic kidney
- NCoR
- nuclear receptor corepressor
- SRC1
- steroid receptor coactivator-1
- TR
- thyroid hormone receptor
- TRAP220
- thyroid hormone receptor-associated protein 220
- TRE
- thyroid hormone response element.
References
- 1. Larsen PR, Davies TF, Schlumberger MJ, Hay ID. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. 10th ed Philadelphia: Saunders; 2003:331–373. [Google Scholar]
- 2. Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. [DOI] [PubMed] [Google Scholar]
- 3. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flamant F, Baxter JD, Forrest D, et al. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev. 2006;58:705–711. [DOI] [PubMed] [Google Scholar]
- 5. Wu Y, Koenig RJ. Gene regulation by thyroid hormone. Trends Endocrinol Metab. 2000;11:207–211. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. [DOI] [PubMed] [Google Scholar]
- 7. Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol. 2004;66:315–360. [DOI] [PubMed] [Google Scholar]
- 8. Rosen MD, Privalsky ML. Thyroid hormone receptor mutations in cancer and resistance to thyroid hormone: perspective and prognosis. J Thyroid Res. 2011;2011:361304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wondisford FE. Thyroid hormone action: insight from transgenic mouse models. J Investig Med. 2003;51:215–220. [DOI] [PubMed] [Google Scholar]
- 10. Chan IH, Privalsky ML. Isoform-specific transcriptional activity of overlapping target genes that respond to thyroid hormone receptors α1 and β1. Mol Endocrinol. 2009;23:1758–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chatonnet F, Guyot R, Benoît G, Flamant F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc Natl Acad Sci USA. 2013;110:E766–E775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flamant F, Gauthier K. Thyroid hormone receptors: the challenge of elucidating isotype-specific functions and cell-specific response. Biochim Biophys Acta. 2013;1830:3900–3907. [DOI] [PubMed] [Google Scholar]
- 13. Forrest D, Vennstrom B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10:41–52. [DOI] [PubMed] [Google Scholar]
- 14. Gil-Ibañez P, Morte B, Bernal J. Role of thyroid hormone receptor subtypes alpha and beta on gene expression in the cerebral cortex and striatum of postnatal mice. Endocrinology. 2013;154:1940–1947. [DOI] [PubMed] [Google Scholar]
- 15. Murata Y. Multiple isoforms of thyroid hormone receptor: an analysis of their relative contribution in mediating thyroid hormone action. Nagoya J Med Sci. 1998;61:103–115. [PubMed] [Google Scholar]
- 16. Samuels HH, Tsai JS, Casanova J, Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974;54:853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Köhrle J. The selenoenzyme family of deiodinase isozymes controls local thyroid hormone availability. Rev Endocr Metab Disord. 2000;1:49–58. [DOI] [PubMed] [Google Scholar]
- 18. Maia AL, Goemann IM, Meyer EL, Wajner SM. Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodinase in human physiology and disease. J Endocrinol. 2011;209:283–297. [DOI] [PubMed] [Google Scholar]
- 19. St Germain DL, Galton VA. The deiodinase family of selenoproteins. Thyroid. 1997;7:655–668. [DOI] [PubMed] [Google Scholar]
- 20. Williams GR, Bassett JH. Deiodinases: the balance of thyroid hormone: local control of thyroid hormone action: role of type 2 deiodinase. J Endocrinol. 2011;209:261–272. [DOI] [PubMed] [Google Scholar]
- 21. Galton VA. The role of 3,5,3′-triiodothyronine in the physiological action of thyroxine in the premetamorphic tadpole. Endocrinology. 1989;124:2427–2433. [DOI] [PubMed] [Google Scholar]
- 22. Apriletti JW, Ribeiro RC, Wagner RL, et al. Molecular and structural biology of thyroid hormone receptors. Clin Exp Pharmacol Physiol Suppl. 1998;25:S2–S11. [DOI] [PubMed] [Google Scholar]
- 23. Ribeiro RC, Apriletti JW, Wagner RL, et al. Mechanisms of thyroid hormone action: insights from X-ray crystallographic and functional studies. Recent Progr Horm Res. 1998;53:351–392; discussion 392–354. [PubMed] [Google Scholar]
- 24. Ribeiro RC, Apriletti JW, Wagner RL, et al. X-ray crystallographic and functional studies of thyroid hormone receptor. J Steroid Biochem Mol Biol. 1998;65:133–141. [DOI] [PubMed] [Google Scholar]
- 25. Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150:2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ito M, Roeder RG. The TRAP/SMCC/mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab. 2001;12:127–134. [DOI] [PubMed] [Google Scholar]
- 27. Spencer TE, Jenster G, Burcin MM, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 1997;389:194–198. [DOI] [PubMed] [Google Scholar]
- 28. Goodson M, Jonas BA, Privalsky MA. Corepressors: custom tailoring and alterations while you wait. Nucl Recept Signal. 2005;3:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahm JB, Privalsky ML. Research resource: identification of novel coregulators specific for thyroid hormone receptor-beta2. Mol Endocrinol. 2013;27:840–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosen MD, Privalsky ML. Thyroid hormone receptor mutations found in renal clear cell carcinomas alter corepressor release and reveal helix 12 as key determinant of corepressor specificity. Mol Endocrinol. 2009;23:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodson ML, Jonas BA, Privalsky ML. Alternative mRNA splicing of SMRT creates functional diversity by generating corepressor isoforms with different affinities for different nuclear receptors. J Biol Chem. 2005;280:7493–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties. Mol Endocrinol. 2005;19:863–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee S, Young BM, Wan W, Chan IH, Privalsky ML. A mechanism for pituitary-resistance to thyroid hormone (PRTH) syndrome: a loss in cooperative coactivator contacts by thyroid hormone receptor (TR)β2. Mol Endocrinol. 2011;25:1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang Z, Privalsky ML. Isoform-specific transcriptional regulation by thyroid hormone receptors: hormone-independent activation operates through a steroid receptor mode of co-activator interaction. Mol Endocrinol. 2001;15:1170–1185. [DOI] [PubMed] [Google Scholar]
- 35. Mengeling BJ, Pan F, Privalsky ML. Novel mode of deoxyribonucleic acid recognition by thyroid hormone receptors: thyroid hormone receptor beta-isoforms can bind as trimers to natural response elements comprised of reiterated half-sites. Mol Endocrinol. 2005;19:35–51. [DOI] [PubMed] [Google Scholar]
- 36. Phan TQ, Jow MM, Privalsky ML. DNA recognition by thyroid hormone and retinoic acid receptors: 3,4,5 rule modified. Mol Cell Endocrinol. 2010;319:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodson ML, Farboud B, Privalsky ML. An improved high throughput protein-protein interaction assay for nuclear hormone receptors. Nucl Recept Signal. 2007;5:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodson ML, Farboud B, Privalsky ML. High throughput analysis of nuclear receptor-cofactor interactions. Methods Mol Biol. 2009;505:157–169. [DOI] [PubMed] [Google Scholar]
- 39. Mengeling BJ, Phan TQ, Goodson ML, Privalsky ML. Aberrant corepressor interactions implicated in PML-RAR(alpha) and PLZF-RAR(alpha) leukemogenesis reflect an altered recruitment and release of specific NCoR and SMRT splice variants. J Biol Chem. 2011;286:4236–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan IH, Privalsky ML. Thyroid hormone receptors mutated in liver cancer function as distorted antimorphs. Oncogene. 2006;25:3576–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farboud B, Hauksdottir H, Wu Y, Privalsky ML. Isotype-restricted corepressor recruitment: a constitutively closed helix 12 conformation in retinoic acid receptors beta and gamma interferes with corepressor recruitment and prevents transcriptional repression. Mol Cell Biol. 2003;23:2844–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoh SM, Privalsky ML. Molecular analysis of human resistance to thyroid hormone syndrome. Methods Mol Biol. 2002;202:129–152. [DOI] [PubMed] [Google Scholar]
- 43. Jonas BA, Privalsky ML. SMRT and N-CoR corepressors are regulated by distinct kinase signaling pathways. J Biol Chem. 2004;279:54676–54686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jonas BA, Varlakhanova N, Hayakawa F, Goodson M, Privalsky ML. Response of SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) and N-CoR (nuclear receptor corepressor) corepressors to mitogen-activated protein kinase kinase kinase cascades is determined by alternative mRNA splicing. Mol Endocrinol. 2007;21:1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sandler B, Webb P, Apriletti JW, et al. Thyroxine-thyroid hormone receptor interactions. J Biol Chem. 2004;279:55801–55808. [DOI] [PubMed] [Google Scholar]
- 46. Steinsapir J, Bianco AC, Buettner C, Harney J, Larsen PR. Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme's active center. Endocrinology. 2000;141:1127–1135. [DOI] [PubMed] [Google Scholar]
- 47. Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Warner GJ, Berry MJ, Moustafa ME, Carlson BA, Hatfield DL, Faust JR. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J Biol Chem. 2000;275:28110–28119. [DOI] [PubMed] [Google Scholar]
- 49. Ikeda M, Rhee M, Chin WW. Thyroid hormone receptor monomer, homodimer, and heterodimer (with retinoid-X receptor) contact different nucleotide sequences in thyroid hormone response elements. Endocrinology. 1994;135:1628–1638. [DOI] [PubMed] [Google Scholar]
- 50. Izumo S, Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988;334:539–542. [DOI] [PubMed] [Google Scholar]
- 51. Baniahmad A, Steiner C, Köhne AC, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–514. [DOI] [PubMed] [Google Scholar]
- 52. Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. [DOI] [PubMed] [Google Scholar]
- 53. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135. [DOI] [PubMed] [Google Scholar]
- 54. O'Shea PJ, Williams GR. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J Endocrinol. 2002;175:553–570. [DOI] [PubMed] [Google Scholar]
- 55. St Germain DL, Galton VA, Hernandez A. Minireview: Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams RH, Larsen PR, eds. Williams Textbook of Endocrinology. 10th ed Philadelphia: Saunders; 2003. [Google Scholar]
- 57. Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5:307–324. [DOI] [PubMed] [Google Scholar]
- 58. Jenster G. Coactivators and corepressors as mediators of nuclear receptor function: an update. Mol Cell Endocrinol. 1998;143:1–7. [DOI] [PubMed] [Google Scholar]
- 59. Wang Q, Blackford JA, Jr, Song LN, Huang Y, Cho S, Simons SS., Jr Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Mol Endocrinol. 2004;18:1376–1395. [DOI] [PubMed] [Google Scholar]
- 60. Zhang J, Guenther MG, Carthew RW, Lazar MA. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 1998;12:1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou Y, Gross W, Hong SH, Privalsky ML. The SMRT corepressor is a target of phosphorylation by protein kinase CK2 (casein kinase II). Mol Cell Biochem. 2001;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McIver B, Gorman CA. Euthyroid sick syndrome: an overview. Thyroid. 1997;7:125–132. [DOI] [PubMed] [Google Scholar]
- 63. Stathatos N, Wartofsky L. The euthyroid sick syndrome: is there a physiologic rationale for thyroid hormone treatment? J Endocrinol Invest. 2003;26:1174–1179. [DOI] [PubMed] [Google Scholar]
- 64. Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro. 2011;25:257–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.