Abstract
Although 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] is considered the most biologically active vitamin D3 metabolite, the vitamin D3 prohormone, 25-hydroxyvitamin D3 [25(OH)D3], is metabolized into other forms, including 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3]. Herein we show that 24R,25(OH)2D3 is fundamental for osteoblastic differentiation of human mesenchymal stem cells (hMSCs). Our approach involved analyses of cell proliferation, alkaline phosphatase activity, and pro-osteogenic genes (collagen 1A1, osteocalcin, vitamin D receptor [VDR], vitamin D3-hydroxylating enzymes [cytochrome P450 hydroxylases: CYP2R1, CYP27A1, CYP27B1 and CYP24A1]) and assessment of Ca2+ mineralization of extracellular matrix. 24R,25(OH)2D3 inhibited hMSC proliferation, decreased 1α-hydroxylase (CYP27B) expression, thereby reducing the ability of hMSCs to convert 25(OH)D3 to 1α,25(OH)2D3, and promoted osteoblastic differentiation through increased alkaline phosphatase activity and Ca2+ mineralization. 24R,25(OH)2D3 decreased expression of the 1α,25(OH)2D3 receptor, VDR. 24R,25(OH)2D3 but not 1α,25(OH)2D3 induced Ca2+ mineralization dependent on the absence of the glucocorticoid analog, dexamethasone. To elucidate the mechanism(s) for dexamethasone-independent 1α,25(OH)2D3 inhibition/24R,25(OH)2D3 induction of Ca2+ mineralization, we demonstrated that 1α,25(OH)2D3 increased whereas 24R,25(OH)2D3 decreased reactive oxygen species (ROS) production. 25(OH)D3 also decreased ROS production, potentially by conversion to 24R,25(OH)2D3. Upon inhibition of the vitamin D3-metabolizing enzymes (cytochrome P450s), 25(OH)D3 increased ROS production, potentially due to its known (low) affinity for VDR. We hypothesize that vitamin D3 actions on osteoblastic differentiation involve a regulatory relationship between 24R,25(OH)2D3 and 1α,25(OH)2D3. These results implicate 24R,25(OH)2D3 as a key player during hMSC maturation and bone development and support the concept that 24R,25(OH)2D3 has a bioactive role in the vitamin D3 endocrine system.
The vitamin D3 endocrine system is fundamental to health maintenance and disease prevention. Bone homeostasis and repair are regulated by a network of vitamin D3 metabolites, of which 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] is considered the most biologically active and relevant in bone health. Vitamin D3 is metabolized into other forms, including 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3], a metabolite that circulates in the blood at concentrations about 1000 times higher than those of 1α,25(OH)2D3 (1), similar to 25-hydroxyvitamin D3 [25(OH)D3] serum levels in humans (2). Moreover, 24R,25(OH)2D3 and 25(OH)D3 bind the vitamin D–binding protein with a greater affinity (Kd = 0.02 μM) than that of 1α,25(OH)2D3 (Kd = 0.1 μM) in humans (described in Ref. 3 as reported in Ref. 4; for a review, see Ref. 5). These properties of 24R,25(OH)2D3 are similar to those of 25(OH)D3 in human serum, which is considered to be the best indicator of vitamin D status (for an extensive review, see Refs. 6–8). Furthermore, early studies in anephric humans and pigs showed impaired renal metabolism of 25(OH)D3 and 24R,25(OH)2D3, further supporting the view that 24R,25(OH)2D3 is an important circulating vitamin D3 metabolite.
A recent study on multiple sclerosis demonstrated that the ratio of serum 25(OH)D3 to 24R,25(OH)2D3 and not the ratio of 25(OH)D3 to 1α,25(OH)2D3 correlated with higher disability and increased disease progression and brain atrophy (9, 10). These studies further support the notion that systemic 24R,25(OH)2D3 levels are important for proper human vitamin D endocrine system function, suggesting that 24R,25(OH)2D3 may have a direct effect on bone formation, unique from that of 1α,25(OH)2D3.
Circulating serum levels of 24R,25(OH)2D3 have been assessed during bone injury and have been found to increase during bone injury and promote fracture repair in chicks, due to increased renal 24-hydroxylase (24-OHase) activity (11). Contrary to this finding in chicks, serum 24R,25(OH)2D3 levels and vitamin D–binding protein-bound 24R,25(OH)2D3 did not change after long bone fractures in humans (3). These studies take into account systemic levels of 24R,25(OH)2D3 yet are limited because of the lack of data on tissue-specific levels of 24R,25(OH)2D3 and the cytochrome P450 hydroxylases in the recovering bone.
In humans with chronic renal insufficiency, combined 1α,25(OH)2D3/24R,25(OH)2D3 treatments increased osteoblast recruitment while decreasing the risk of hypercalcemia, whereas 1α,25(OH)2D3 treatment alone led to an increased risk of hypercalcemia (12). These studies suggest a sequential or coupling of 1α,25(OH)2D3/24R,25(OH)2D3 effects in bone repair (for a review, see Ref. 1).
Mesenchymal stem cells (MSCs) reside in a specialized bone niche and are precursors for the generation of different mesenchymal cell lineages, including osteoblasts, adipocytes, myoblasts, chondrocytes, and fibroblasts (13). MSCs show great potential to repair and maintain bone homeostasis. First reported in humans in 1981 (14) and confirmed in more recent studies (15, 16), human bone cells and human mesenchymal stem cells (hMSCs) metabolize 25(OH)D3 into both 1α,25(OH)2D3 and 24R,25(OH)2D3, demonstrating the ability of bone cells to convert 25(OH)D3 in vitro. These studies spanning more than 30 years indicate the importance of systemic and tissue-specific 24R,25(OH)2D3 actions and suggest a role in osteoblastic differentiation and prompted our study in which we assess the concept that the hydroxylation of the prohormone 25(OH)D3 leads to 2 bioactive forms of vitamin D3, 24R,25(OH)2D3 and 1α,25(OH)2D3, with unique functions.
Our main hypothesis is that 24R,25(OH)2D3 is a biologically active vitamin D3 metabolite and promotes the osteoblastic differentiation of hMSCs. Here we assessed the role of the vitamin D3 metabolites 24R,25(OH)2D3 and 1α,25(OH)2D3 and the prohormone 25(OH)D3 during the process of osteoblastic differentiation. Osteoblastic differentiation is defined as the developmental process by which naive cells undergo a morphogenic, phenogenic, and genotypic change toward committed bone-forming cells.
To provide an unbiased assessment of the function(s) of 24R,25(OH)2D3 in our in vitro model system of human MSC osteoblastic differentiation, we strove to take a more naive approach in these studies. Specifically, there is conclusive data from the literature demonstrating that the glucocorticoid analog, dexamethasone, which historically has been used to promote in vitro osteoblastic differentiation, increases expression of the 1α,25(OH)2D3 receptor (vitamin D receptor [VDR]) and alters vitamin D3 metabolism through decreasing the expression of the 24-hydroxylase (CYP24A1) (17–19). These findings suggest that inclusion of dexamethasone during in vitro osteoblastic differentiation would favor a direct 1α,25(OH)2D3 response (through increased VDR) as well as increased 1α,25(OH)2D3 bioavailability (due to decreased 24-hydroxylation, which inactivates 1α,25(OH)2D3). In addition, even though dexamethasone promotes osteoblastic differentiation in vitro, glucocorticoid-like drugs such as dexamethasone (Decadron, Hexadrol), prednisone (Deltasone, Orasone), prednisolone (Prelone), and cortisone (Cortone) have all been linked to glucocorticoid-induced osteoporosis and are known to inhibit bone formation in vivo (20, 21). For these reasons, we assessed the effects of the prohormone 25(OH)D3 and the metabolites 24R,25(OH)2D3 and 1α,25(OH)2D3 without the addition of dexamethasone, unless otherwise stated.
The main aim of this study was to assess the function(s) of 24R,25(OH)2D3 compared with that of 1α,25(OH)2D3 during osteoblastic differentiation of primary hMSCs through analysis of (1) cell proliferation, (2) alkaline phosphatase activity, (3) pro-osteogenic genes, such as collagen 1A1, osteocalcin, and VDR, and (4) vitamin D3-hydroxylating enzymes (cytochrome P450 hydroxylases CYP2R1, CYP27A1, CYP27B1, and CYP24A1) and (5) through the assessment of Ca2+ mineralization of extracellular matrix, which is considered a hallmark characteristic of bone-forming osteoblastic cells. Our secondary and tertiary aims were to assess whether dexamethasone does indeed regulate pro-osteogenic genes independent of vitamin D3, and based on our initial studies, to determine the possible mechanisms for our observed differences seen between 24R,25(OH)2D3 and 1α,25(OH)2D3 during osteoblastic differentiation. The studies described herein provide to the best of our knowledge an unbiased assessment of the function of the vitamin D3 metabolites during osteoblastic differentiation of early-passage hMSCs in vitro and because of the exclusion of dexamethasone, which inhibits bone in vivo, provide a more clinically translational assessment of in vitro bone formation.
Materials and Methods
Bone marrow–derived MSC isolation and cell culture
hMSCs were isolated and cultured as described previously (22) from postmortem thoracolumbar (T1–L5) vertebral bodies of donors of various ages (7–30 years old) immediately after death from traumatic injury. hMSCs from a 7-year-old male subject and a 22-year-old male subject were used in these studies. There were no significant differences seen between young and old cells in regard to osteoblastic differentiation and treatment with the various vitamin D3 metabolites. Guidelines were followed as outlined by the Committee on the Use of Human Subjects in Research at the University of Miami. Expansion medium consisted of DMEM-low glucose medium, containing 5% fetal bovine serum (FBS) (lot no. 300039; HyClone), 20 mM ascorbic acid (49752; Fluka/Sigma), an essential fatty acid solution (described in Reference 23), and antibiotics (100 U/mL penicillin and 0.1 mg/mL streptomycin) (15140; Gibco) on 10 ng/mL fibronectin (F2518; Sigma-Aldrich)–coated flasks (Nunclon) in 21% O2, 5% CO2, and 92% N2. The medium was changed every 3 days, and cells were subcultured on reaching 80% confluence. For expansion, cell growth, and 5-bromo-2′-deoxyuridine (BrdU) experiments, hMSCs were seeded at 500 to 1000 cells/cm2.
Osteoblastic differentiation
hMSCs were initially seeded at 10 000 cells/cm2 overnight in expansion medium (22). Osteogenic medium (α-MEM, 10% FBS, 100 U/mL penicillin and 0.1 mg/mL streptomycin, 200 μM ascorbate, and 10 mM β-glycerophosphate) was used to promote osteoblastic differentiation. All flasks and wells were coated with 10 ng/mL fibronectin. Fibronectin was used consistently during expansion and osteoblastic differentiation of hMSCs for 2 main reasons: (1) it helps to maintain cell adhesion to the plates and (2) it promotes long-term mineralization of hMSCs in vitro (24). Osteogenic medium was added on day 0 and changed every 3 days (Figure 1). The inclusion of dexamethasone (1 or 10 nM) is noted per experimental figure or result.
Figure 1.

Schematic representation depicting vitamin D3 metabolite treatment(s) and subsequent time points for analysis during in vitro osteoblastic differentiation.
Vitamin D3 metabolite treatment
25(OH)D3 (H4014; Sigma-Aldrich), 24R,25(OH)2D3 (17943; Sigma-Aldrich), and 1α,25(OH)2D3 (679101; Calbiochem) were reconstituted in ethanol (100 μM) and stored in dark Eppendorf tubes at −80°C until addition to the culture medium. 24R,25(OH)2D3 and 1α,25(OH)2D3 were used at 10 nM final concentrations, and 25(OH)D3 was used at a 20 nM final concentration, unless otherwise indicated. All vitamin D3 metabolite treatments started on day 0 with medium changes every 3 days (Figure 1) for all experiments. We used 10 nM 1α,25(OH)2D3 for our in vitro osteoblastic differentiation of hMSC studies because (1) 10 nM is close to the Kd (1 nM) for 1α,25(OH)2D3 binding to VDR (for an extensive review, see Ref. 5) and (2) 10 nM is the physiologically relevant concentration of 1α,25(OH)2D3 needed to modulate cellular activity in osteoblasts (15, 25, 26).
RNA sample preparation and cDNA synthesis
RNA was isolated using the RNAqueous-4PCR kit (AM1914; Ambion) according to the manufacturer's directions. RNA was quantified on an ND-1000 spectrophotometer (NanoDrop), followed by reverse transcription of 2 μg of RNA to cDNA using a High-Capacity cDNA Reverse Transcription Kit (4368814; Applied Biosystems).
Real-time quantitative PCR (RT-qPCR)
For RT-qPCR analysis of mRNA expression studies, vitamin D3 metabolite treatments were done every 3 days starting with day 0. Therefore, for the day 0 time point, there was no vitamin D3 treatment, the day 1 time point is 1 day after treatment, the day 3 time point is 3 days after treatment, the day 7 time point is 1 day after the last treatment on day 6, and the day 14 time point is 2 days after the last treatment on day 12 (Figure 1). RT-qPCR was done using 10 μL of 1:20 diluted cDNA on an Mx3005P Multiplex Quantitative PCR System (401513; Stratagene) using RT-qPCR SYBR Green reagents (Brilliant II SYBR Green QPCR Master Mix; Agilent Technologies) with ROX reference dye. A 2 μM stock solution containing both forward and reverse primer pairs was used for RT-qPCR analyses. RT-qPCR cycling conditions were 95°C for 10 minutes followed by 40 cycles at 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 15 seconds. RT-qPCR data were analyzed using the ΔΔCP method (27) and normalized against 1 negative control and 2 reference genes, eukaryotic translational elongation factor 1α (EF1α, NM_001402) and ribosomal protein L13a (RPL13a, NM_001242) (23, 28). Results are displayed as fold change relative to the results for untreated samples set to the value of 1 (dashed line in the respective figures).
Primer pairs used for RT-qPCR analysis
Primer pairs were from Eurofins MWG Operon. Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome) was used to construct primer pair sequences. Primer pair sequences were as follows: EF1α (forward, [F] 5′-AGGTGATTATCCTGAACCATCC-3′; reverse [R], 5′-AAAGGTGGATAGTCTGAGAAGC-3′); RPL13a (F, 5′-CATAGGAAGCTGGGAGCAAG-3′; R, 5′-GCCCTCCAATCAGTCTTCTG-3′); CYP24A1 (F, 5′-GCAGCCTAGTGCAGATTT-3′; R, 5′-ATTCACCCAGAACTGTTG-3′); CYP27A1 (F, 5′-GGAAAGTACCCAGTA CGG-3′; R, 5′-AGCAAATAGCTTCCAAGG-3′); CYP27B1 (F, 5′-GCGGACTGCTCACTGCGGAA-3′; R, 5′-GCCGCACAAGGTCGCAGACT-3′); CYP2R1 (F, 5′-TTTGCAGACAGACCATGCCT-3′; R, 5′-ATCTGGAATTGAGTAAGCCTCCC-3′); VDR (F, 5′-TTGACCGGAACGTGCCCCGGATC-3′; R, 5′-CCTCATCTGTCAGAATGAACTCC-3′); osteocalcin (F, 5′-CACTCCTCGCCCTATTGGC-3′; R, 5′-CCCTCCTGCTTGGACACAAAG-3′); collagen 1A1 (F, 5′-GTCGAGGGCCAAGACGAAG-3′; R, 5′-CAGATCACGTCATCGCACAAC-3′); osterix (F, 5′-CCCAGGCAACACTCCTACTC-3′; R, 5′-GGCTGGATTAAGGGGAGCAAA-3′); alkaline phosphatase (F, 5′-CACCCACGTCGATTGCATCT-3′; R, 5′-TAGCCACGTTGGTGTTGAGC-3′); and Runx2 (F, 5′-TCCTATGACCAGTCTTACCCCT-3′; R, 5′-GGCTCTTCTTACTGAGAGTGGAA-3′).
Alkaline phosphatase activity and staining
For alkaline phosphatase enzyme activity studies, vitamin D3 metabolite treatments were done every 3 days starting with day 0. Therefore, for the day 0 time point, there was no vitamin D3 treatment, the day 1 time point is 1 day after treatment, the day 3 time point is 3 days after treatment, the day 5 time point is 2 days after treatment, the day 7 time point is 1 day after the last treatment on day 6, and the day 10 time point is 1 day after the last treatment on day 9 (Figure 1). Cells were assessed for alkaline phosphatase enzyme activity as described previously (22), using conversion of p-nitrophenyl phosphate to p-nitrophenol formed as determined at 405 nm. Total p-nitrophenol formed was normalized against time (minutes) and against total cell number, allowing for the determination of alkaline phosphatase activity, or total p-nitrophenol formed per minute per cell, allowing for comparison between experiments regardless of cell number variations. Cells were also stained for alkaline phosphatase activity. In brief, cells were fixed in 2% paraformaldehyde-0.2% glutaraldehyde for 1 hour and then were incubated with substrate solution for 30 minutes at 37°C. The substrate solution contained 8 mg of naphthol AS-TR (Sigma-Aldrich) in 0.3 mL of N,N′-dimethylformamide (Sigma-Aldrich) mixed with 24 mg of Fast Blue BB (Sigma-Aldrich) in 30 mL of 100 mM Tris-HCl (pH 9.6). Subsequently, 10 mg of MgCl2 was added, the pH was adjusted to 9.0, and then the entire solution was filtered (0.2 μM pore size).
Ca2+ mineralization assay
For Ca2+ mineralization studies, vitamin D3 metabolite treatments were done every 3 days starting with day 0. Therefore, treatments were performed on days 0, 3, 6, 9, and 12, and the day 14 time point is 2 days after the last treatment on day 12 (Figure 1). Cells were fixed with 2% paraformaldehyde-0.2% glutaraldehyde for 1 hour and then were stained with 40 mM Alizarin Red S as described previously (22). The staining was quantified (550 nm) after solubilizing in 10% cetylpyridinium chloride (10 mM sodium phosphate, pH 7.0) (29). For 24-well plates, 1 mL of 10% cetylpyridinium chloride was used per well. Because of the wide range of staining, undiluted samples and diluted samples (1:10 or 1:100) were used to determine OD550 readings. OD550 readings were normalized against total cell numbers and graphed as OD at 550 nm/mg of protein, which takes into account changes in cell number and allows for comparison between experiments.
Protein preparation, Western blot analysis, and quantification
For protein analysis studies, vitamin D3 metabolite treatments were done every 3 days starting with day 0. Therefore, treatments were performed on days 0, 3, and 6 and the day 7 time point is 1 day after the last treatment on day 6 (Figure 1). Cell pellets were resuspended in NP40 lysis buffer (50 mM Tris, 1.0% NP40, 150 mM NaCl, 2 mM EGTA, 2 mM EDTA, 50 mM NaF, and 0.1 mM NaVO4, pH 8.0). Protease inhibitor cocktail (P8340; Sigma-Aldrich) was added (2 μL/mL lysis buffer) to NP40 lysis buffer before protein extracts were collected. The protein concentration was determined with the BCA protein assay (Pierce). Blocking solution consisted of either 2% to 5% BSA or dried milk in Tris-buffered saline solution with 1% to 2.5% Tween 20. Antibodies used were α-tubulin (Santa Cruz Biotechnology) (dilution 1:2000) and VDR (NeoMarkers).
BrdU assay
For BrdU studies, vitamin D3 metabolite treatments were done every 3 days starting with day 0. Therefore, for the day 0 time point, there was no vitamin D3 treatment, the day 3 time point is 3 days after treatment, the day 7 time point is 1 day after the last treatment on day 6, and the day 10 time point is 1 day after the last treatment on day 9 (Figure 1). Cells were seeded in 96-well plates at 500 to 1000 cells/cm2 in expansion medium. Medium with treatment was changed every 3 days. The BrdU assay (Cell Signaling Technology) was performed according to the manufacturer's protocol. Absorbance (450 nm) was quantified on a microplate reader. The control (day 3) was set to the value of 1 for comparison.
In vitro biosynthesis of 1α,25(OH)2D3 from 25-(OH)D3
hMSCs were seeded at 10 000 cells/cm2 in expansion medium and then were pretreated with 24R,25(OH)2D3 (10 nM) for 14 days before assessment of 1α,25(OH)2D3 biosynthesis from 25(OH)D3. Treatment with 24R,25(OH)2D3 started on day 0, and medium was changed every 3 days (Figure 1). Before addition of 25(OH)D3, cells were rinsed 2 times with PBS and then were switched to serum-free α-MEM (Gibco) supplemented with the antioxidant 1,2-dianilinoethane (N,N′-diphenylethylenediamine) (10 μM; D27004; Sigma-Aldrich) (16, 30). The medium was collected from each well at 12, 24, and 48 hours after 25(OH)D3 treatment. 1α,25(OH)2D3 levels in the medium were quantitatively determined using a 1α,25(OH)2D3 ELISA kit (USA Immunodiagnostic Systems Ltd). Biosynthesis of 1α,25(OH)2D3 from 25(OH)D3 requires 1α-hydroxylase (1α-OHase) activity and is represented as biosynthesized 1α,25(OH)2D3 (femtomoles) per milligram of protein per hour after addition of 25(OH)D3. Cellular protein lysates were collected and quantified using NP40 lysis buffer as described above. Total cellular protein was used as a normalization factor to account for changes in total cell number at the different time points. To calculate a more stringent representation of 1α,25(OH)2D3 biosynthesis, the average rate of production (femtomoles of 1α,25(OH)2D3) at 12, 24, and 48 hours was calculated and normalized against the same day total cellular protein (per milligram of protein) to account for changes in cell number.
Measurement of reactive oxygen species (ROS)
For ROS studies, vitamin D3 metabolite treatments were done every 3 days starting with day 0. Therefore, treatments were performed on days 0, 3, 6, 9, and 12; the day 14 time point is 2 days after the last treatment on day 12 (Figure 1). ROS are defined as chemically reactive ions formed by the incomplete 1-electron reduction of oxygen, encompassing a variety of species including superoxide anions, hydroxyl radicals, and hydrogen peroxide (for a review, see Ref. 31). ROS production was measured using the fluorescent probe dihydrorhodamine 123 (DHR) (32). In brief, after 14 days of osteogenic conditions, cells were rinsed 1 time with PBS, and then the medium was replaced with osteogenic medium without serum. The medium was subsequently collected after 24 hours. Then 20 μL of culture medium was added to 1 ml of PBS, mixed with 20 μM DHR, and incubated at 37°C for 15 minutes. Fluorescence was measured using an excitation wavelength of 485 mm and an emission wavelength of 535 nm. The change in ROS is expressed as a percentage over control and normalized per milligram of protein, which takes into account changes in cell number and allows for comparison between experiments.
Statistical analysis
All data sets contained n = ≥3 experiments (triplicate samples per condition per experiment). A one-way ANOVA followed by a Tukey post hoc analysis was used to calculate the statistical significance between treatment conditions compared with untreated controls (GraphPad Software). A Student t test was used for direct comparisons of significance (*, P ≤ .05). Error bars represent SDs.
Results
24R,25(OH)2D3 regulates the proliferation and increases the Ca2+ mineralization of hMSCs in vitro
Initial studies to determine the effects of 1α,25(OH)2D3 and 24R,25(OH)2D3 on hMSC proliferation showed that both 1α,25(OH)2D3 and 24R,25(OH)2D3 significantly (P < .05) decreased hMSC proliferation compared with that of controls as assessed by cell counts (day 10) and BrdU incorporation (day 5) (Figure 2, A and B). 1α,25(OH)2D3 significantly (P < .05) decreased proliferation (day 10) and BrdU incorporation (day 5) to a greater extent than 24R,25-(OH)2D3 (Figure 2, A and B). Based on these results, we used RT-qPCR analysis to assess the gene expression of the cell cycle inhibitor p21/p27 after 1, 2, 3, and 7 days of treatment; no changes were observed (data not shown).
Figure 2.

24R,25(OH)2D3 regulates the osteoblastic differentiation of hMSCs. 24R,25(OH)2D3 (10 nM) and/or 1α,25(OH)2D3 (10 nM) were added at day 0 and every 3 days when medium was changed (Figure 1). A and B, hMSCs were cultured under expansion conditions (seeded at 500–1000 cells/cm2) and collected for cell counting (A) or BrdU incorporation (B). B, BrdU incorporation was assayed at days 3 and 5. Relative fold change compared with day 3 control samples is shown (set to equal 1). Abs, absorbance. C, hMSCs were cultured in osteogenic medium (OG) with no dexamethasone, unless otherwise stated (seeded at 10 000 cells/cm2) and assayed for alkaline phosphatase activity. pNP, p-nitrophenol. D, hMSCs were seeded at 10 000 cells/cm2 in osteogenic medium with or without dexamethasone (Dex; 10 nM). Alizarin Red S stain was used to determine Ca2+ mineralization. ★, P ≤ .05 compared with the respective “linked samples” (A and B) or relative to the respective day controls (B).
To assess the effects of vitamin D3 metabolites on osteoblastic differentiation, we assayed alkaline phosphatase staining and activity and Ca2+ mineralization after 3 to 14 days of vitamin D3 treatment. Because of the regulatory relationship between dexamethasone and VDR (18, 19), dexamethasone was excluded unless indicated otherwise, so as not to skew the results toward a pro-1α,25(OH)2D3 response. Under osteogenic conditions without dexamethasone, 24R,25(OH)2D3 significantly (P < .05) increased alkaline phosphatase activity (Figure 2C) and staining (data not shown) compared with those for no treatment. 1α,25(OH)2D3 alone or in combination with 24R,25(OH)2D3 treatment inhibited hMSC alkaline phosphatase activity and Ca2+ mineralization (Figure 2, C and D). In addition, the inclusion of 1 to 10 nM dexamethasone negated the differences seen between vitamin D3 treatments (ie, no differences were seen; data not shown).
24R,25(OH)2D3 and 25(OH)D3 promote and 1α,25(OH)2D3 inhibits Ca2+ mineralization
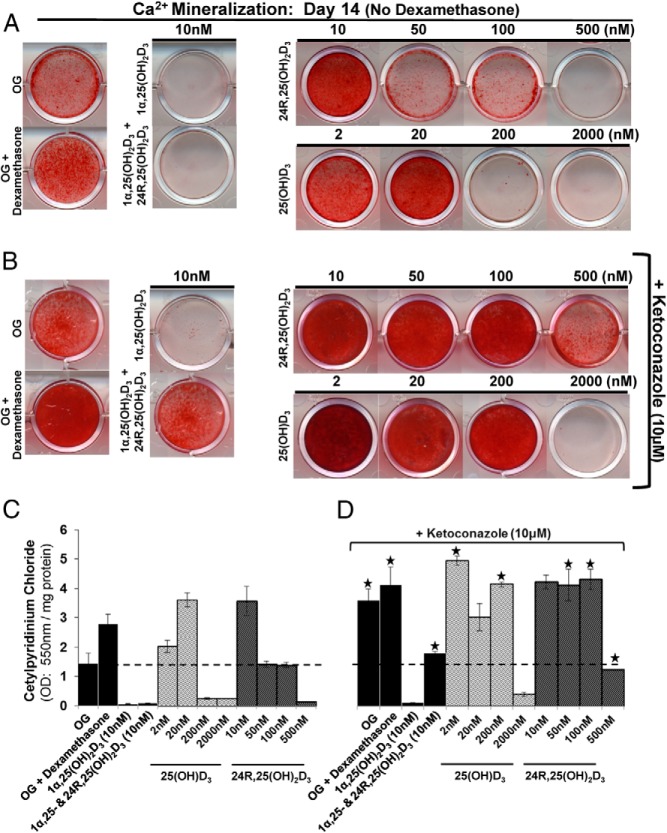
After demonstrating that 24R,25(OH)2D3 decreased hMSC proliferation and increased alkaline phosphatase activity and Ca2+ mineralization, we performed a dose-response experiment using 24R,25(OH)2D3 and the precursor 25(OH)D3 to further assess Ca2+ mineralization. Low doses of 24R,25(OH)2D3 (10 nM) and 25(OH)D3 (2–20 nM) significantly (P < .05) induced Ca2+ mineralization of hMSCs compared with that for no treatment or inclusion of dexamethasone (Figure 3, A and C). Higher doses of 24R,25(OH)2D3 (50, 100, and 500 nM) and 25(OH)D3 (200 and 2000 nM) inhibited Ca2+ mineralization similar to the inhibitory effect seen with 1α,25(OH)2D3 (10 nM) (Figure 3, A and C). At lower 24R,25(OH)2D3 (1 pM–1 nM) concentrations, we did not observe a change in Ca2+ mineralization compared with that for the untreated osteogenic controls (day 14) (data not shown).
Figure 3.
24R,25(OH)2D3 and 25(OH)D3 but not 1α,25(OH)2D3 induces Ca2+ mineralization in the absence of dexamethasone. A and B, hMSCs were seeded at 10 000 cells/cm2 in osteogenic medium (OG) with no dexamethasone, unless otherwise stated, and 5% FBS. Treatments were started at day 0, and medium was changed every 3 days (Figure 1). Alizarin Red S stain was used to assess Ca2+ mineralization at day 14. B, Cytochrome P450 inhibitor ketoconazole (10 μM) was added 1 hour before treatment or medium change at day 0 and at every medium change. C, Quantification of Ca2+ mineralization (from A): cetylpyridinium chloride was used to solubilize Alizarin Red S staining, and the OD550 readings were normalized against total protein for each well (per milligram of protein) to account for differences in cell number. D, Quantification of Ca2+ mineralization (from B) with addition of ketoconazole. n = ≥3 independent experiments. ★, P ≤ .05 compared with osteogenic conditions without ketoconazole. 1α,25-, 1α,25(OH)2D3.
Inhibition of vitamin D3–metabolizing cytochrome P450 enzymes increases 24R,25(OH)2D3- and 25(OH)D3-induced Ca2+ mineralization
To determine whether the dose-dependent effect of 24R,25(OH)2D3 and 25(OH)D3 on Ca2+ mineralization was due to direct effects or was the result of their metabolism into other vitamin D3 metabolites, ketoconazole, which has classically been used to inhibit the family of vitamin D hydroxylases known as cytochrome P450s in hMSCs (16), was used. Ketoconazole (10 μM) was added 1 hour before each treatment with vitamin D3 metabolites, throughout the duration of osteoblastic differentiation studies. There was a significant (P < .05) increase in 25(OH)D3- and 24R,25(OH)2D3-induced Ca2+ mineralization (Figure 3, B and D), compared with that for no 25(OH)D3 and 24R,25(OH)2D3 treatments (Figure 3A) without ketoconazole. The enhanced Ca2+ mineralization observed concomitant with blocked vitamin D metabolism using ketoconazole suggests unique roles for 25(OH)D3 and 24R,25(OH)2D3 in independently enhancing/increasing Ca2+ mineralization. Note that 1α,25(OH)2D3 inhibited Ca2+ mineralization in these studies (Figure 3). There was also a significant (P < .05) increase in vitamin D3 untreated (osteogenic/osteogenic + dexamethasone) control Ca2+ mineralization, which might be accounted for by 24R,25(OH)2D3 or 25(OH)D3 present in the FBS. Also of note, the addition of ketoconazole together with 24R,25(OH)2D3 significantly (P < .05) rescued Ca2+ mineralization from the inhibitory effects of 1α,25(OH)2D3 (Figure 3, B and D), further supporting a role for 24R,25(OH)2D3 as a bioactive metabolite with a direct role in promoting increased matrix mineralization.
Dexamethasone increases pro-osteogenic genes and decreases CYP24A1 expression
Dexamethasone has previously been shown to regulate and increase the expression of pro-osteogenic genes and the 1α,25(OH)2D3 receptor, VDR. To verify and test the effects of dexamethasone, we treated hMSCs with dexamethasone (1–10 nM) for 3 days. Treatments were done on days 0 and 2, with collection of hMSCs for analysis on day 3. Dexamethasone (10 nM) significantly (P < .05) increased the expression of VDR (fold increase of 2.10 ± 0.1), alkaline phosphatase (fold increase of 6.6 ± 0.37) and osteocalcin (fold increase of 1.95 ± 0.2) mRNA. Dexamethasone (1 nM) significantly (P < .05) increased the expression of alkaline phosphatase (fold increase of 4.32 ± 0.2) but did not significantly increase VDR nor osteocalcin mRNA at this lower concentration. Dexamethasone (1 and 10 nM) significantly (P < .05) decreased CYP24A1 expression (fold decreases of 0.25 ± 0.06 and 0.33 ± 0.05, respectively) and increased CYP27A1 expression (fold increases of 1.50 ± 0.21 and 2.2 ± 0.31, respectively).
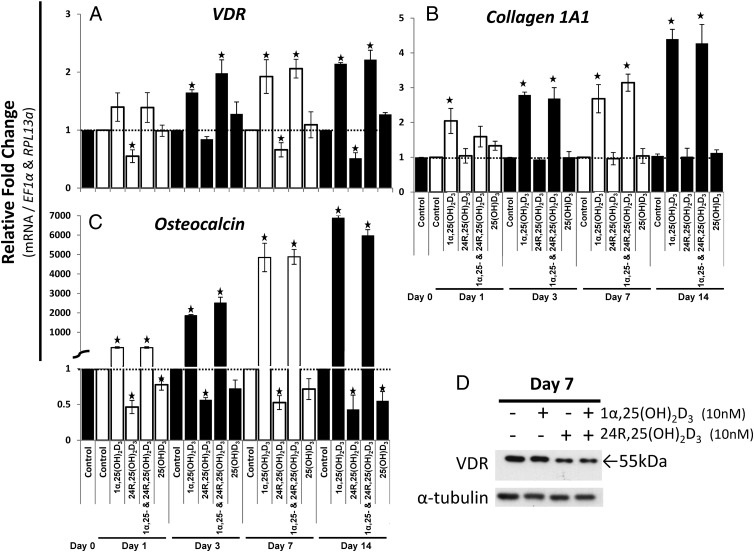
24R,25(OH)2D3 decreased 1α,25(OH)2D3 target genes: VDR and osteocalcin
To further characterize the effects of 24R,25(OH)2D3, we assessed its effect on genes associated with osteoblastic differentiation, over a time course ending with mineralization (day 14). 24R,25(OH)2D3 significantly decreased the mRNA expression and protein of VDR, suggesting that 24R,25(OH)2D3 decreased 1α,25(OH)2D3 bioactivity (Figure 4, A and D). At lower concentrations (1 pM, 10 pM, 0.1 nM, and 1 nM), 24R,25(OH)2D3 did not decrease VDR mRNA expression (data not shown). 1α,25(OH)2D3 increased VDR mRNA alone or in combination with 24R,25(OH)2D3 (Figure 4A), yet there was no increase observed in VDR protein (Figure 4D) with 1α,25(OH)2D3 treatment alone. 1α,25(OH)2D3 treatment in combination with 24R,25(OH)2D3 decreased VDR protein (Figure 4D) similar to 24R,25(OH)2D3 treatment alone. There was no significant change in VDR mRNA expression in the controls (data not shown).
Figure 4.
24R,25(OH)2D3 decreased 1α,25(OH)2D3-stimulated genes: osteocalcin and VDR. A–C, Using RT-qPCR, the mRNA expression of osteogenic genes was assessed after vitamin D3 treatment up to the time of mineralization (day 14). hMSCs were seeded at 10 000 cells/cm2. A 10 nM concentration was used for all vitamin D3 metabolites. Treatments started at day 0, and medium was change every 3 days (Figure 1). B, Western blot analysis of the 1α,25(OH)2D3 receptor (VDR) levels. α-Tubulin was used as a loading control. n = 3 independent experiments in triplicate. ★, P ≤ .05 compared to the respective day control. 1α,25-, 1α,25(OH)2D3.
24R,25(OH)2D3 treatment decreased the expression of osteocalcin, a known target of 1α,25(OH)2D3 (Figure 4C). At lower concentrations (1 pM, 10 pM, 0.1 nM, and 1 nM), 24R,25(OH)2D3 did not decrease osteocalcin mRNA expression (data not shown). Even though for RT-qPCR analysis, we set the controls to equal the value 1, the basal expression of osteocalcin significantly (P < .05) increased during osteoblastic differentiation: day 1, fold increase of 2.91 ± 0.36; day 3, fold increase of 4.16 ± 0.47; day 7, fold increase of 4.61 ± 0.21;, and day 14, fold increase of 7.97 ± 0.12. Collagen 1A1, another target of 1α,25(OH)2D3 and required for bone matrix formation, was increased by 1α,25(OH)2D3 but not by 24R,25(OH)2D3 (Figure 4B). Again, we observed a significant (P < .05) increase in basal collagen 1A1 mRNA expression at day 14 only (fold increase of 2.65 ± 0.31). No change was observed for Runx2 or alkaline phosphatase gene expression either with treatment or in the controls (data not shown). Also of note, the prohormone 25(OH)D3 significantly (P < .05) decreased osteocalcin expression (days 1 and 14) similar to the effects of and possibly due to conversion to 24R,25(OH)2D3 (Figure 4C). We also observed a nonsignificant decrease in osteocalcin mRNA at days 3 and 7, which fit with the overall trend of an 25(OH)D3-induced decrease in osteocalcin mRNA at days 1 and 14 (Figure 4C).
24R,25(OH)2D3 decreases CYP27B1 and the biosynthesis of 1α,25(OH)2D3 from 25(OH)D3 by hMSCs
Vitamin D3 is metabolized in the liver and kidney in addition to localized tissue-specific biosynthesis. Bone cells are known to metabolize 25(OH)D3 into both 1α,25(OH)2D3 and 24R,25(OH)2D3 via 1α-OHase and 24-OHase, respectively (Figures 1 and 6A). To further characterize the bioactivity of 24R,25(OH)2D3, its involvement in osteoblastic differentiation, and the decrease it exerts on the expression of VDR, we assessed its effect on the expression of vitamin D hydroxylases (cytochrome P450 enzymes) (Figure 5).
Figure 6.

24R,25(OH)2D3 decreases the biosynthesis of 1α,25(OH)2D3 from 25(OH)D3 in hMSCs. A, Diagram of the cytochrome P450 hydroxylases (OHases) involved in vitamin D3 metabolism. B, Biosynthesis of 1α,25(OH)2D3 from 25(OH)D3 was assessed after 14 days of 24R,25(OH)2D3 pretreatment, with treatments on day 0 and every 3 days (Figure 1). hMSCs were seeded at 10 000 cells/cm2. Medium was collected 12, 24, and 48 hours after 25(OH)D3 (1 μM) stimulation and assayed for 1α,25(OH)2D3. The rate of 1α,25(OH)2D3 biosynthesis is represented as femtomoles of 1α,25(OH)2D3, normalized against total cellular protein per hour, and is the average from the 12-, 24-, and 48-hour time points. Normalization against total cellular protein (per milligram of protein) was used to account for changes in cell number over time. N.D., none detected. C, RT-qPCR analysis was used to assess the levels of cytochrome P450s (CYP24A1, 24-OHase; CYP27A1 and CYP2R1, 25-OHases; and CYP27B1, 1α-OHase), after pretreatment with 24R,25(OH)2D3 (10 nM) for 14 days under confluent conditions with treatments on day 0 and every 3 days. D, RT-qPCR analysis was used to assess the level of 24-OHase (CYP24A1) mRNA expression after treatment with 24R,25(OH)2D3 (1 pM, 10 pM, 0.1 nM, 1 nM, and 10 nM) for 7 days. Treatments started at day 0 and continued every 3 days. ★, P ≤ .05 compared with the untreated control.
Figure 5.
24R,25(OH)2D3 decreases 25(OH)D3-1α-hydroxylase (CYP27B1), whereas 1α,25(OH)2D3 increases 25-hydroxylase (CYP2R1) expression. With use of RT-qPCR, mRNA expression of the cytochrome P450 genes (CYP27A1 and CYP2R1, 25-OHases [A and C]; CYP27B1, 1α-OHase [B]; and CYP24A1, 24-OHase [D]) involved in vitamin D3 metabolism was assessed after vitamin D3 treatment up to the time of mineralization (day 14). hMSCs were seeded at 10 000 cells/cm2. A 10 nM concentration was used for all vitamin D3 metabolites. Treatments started with day 0, and medium was changed every 3 days (Figure 1). ★, P ≤ .05 compared with the respective day control. 1α,25-, 1α,25(OH)2D3.
We observed that over a time course ending with concurrent mineralization (day 14), 1α,25(OH)2D3 increased expression of 24-OHase (CYP24A1) (Figure 5D). Surprisingly, 1α,25(OH)2D3 increased 25-hydroxylase (25-OHase) (CYP2R1) expression to a significant extent in the later stages of osteoblastic differentiation concurrent with mineralization (days 7 and 14) (Figure 5A), suggesting a possible mechanism for tissue-specific vitamin D3 25-hydroxylation in mature matrix-producing osteoblasts. We also observed a novel 24R,25(OH)2D3-mediated decrease in the expression of 1α-OHase (CYP27B1) (Figure 5B) and to a lesser extent of 24-OHase (CYP24A1) (Figure 5D) after treatment with 10 nM 24R,25(OH)2D3. 24R,25(OH)2D3 did not change the expression of either of the 25-OHases (CYP2R1 or CYP27A1) (Figure 5, A and C) at 10 nM nor at lower concentrations (1 pM–1 nM). Also of note, the basal expression of 24-OHase (CYP24A1) and 25-OHase (CYP2R1 and CYP27A1) did not change, yet there was a significant increase (P < .05) in 1α-OHase (CYP27B1) mRNA expression during osteoblastic differentiation in the controls: day 1, fold increase of 2.53 ± 0.31; day 3, fold increase of 4.34 ± 0.59; day 7, fold increase of 3.61 ± 0.41; and day 14, fold increase of 4.41 ± 0.42.
The recognized ability of bone cells to metabolize 25(OH)D3 into 1α,25(OH)2D3 prompted us to question whether the 24R,25(OH)2D3-mediated decrease in 1α-OHase expression subsequently decreased the ability of hMSCs to biosynthesize 1α,25(OH)2D3 from 25(OH)D3 (Figures 1 and 6A). After expansion of hMSCs for 14 days with continuous 24R,25(OH)2D3 treatment, we observed a significant decrease in the rate of 1α,25(OH)2D3 biosynthesis from 25(OH)D3 compared with that for untreated hMSCs (Figure 6B), which corresponded to a significant decrease in 1α-OHase (CYP27B1) (Figure 6C). In addition, to follow up on and confirm our previous findings, which demonstrated that 24R,25(OH)2D3 (10 nM) regulated cytochrome P450 expression through decreased 1α-OHase (CYP27B1) and 24-OHase (CYP24A1) mRNA expression, we treated hMSCs with lower concentrations (1 pM–1 nM) of 24R,25(OH)2D3 for 7 days with treatments every 3 days starting with day 0 (Figure 1). There was no observed change in 1α-OHase (CYP27B1) expression when the cells were treated with lower concentrations of 24R,25(OH)2D3 (1 pM, 10 pM, 0.1 nM, and 1 nM) (data not shown). We did observe a significant decrease (P < .05) in 24-OHase (CYP24A1) mRNA expression when cells were treated with 1 and 10 nM 24R,25(OH)2D3 by days 3 and 7, but observed no decrease with lower concentrations of 24R,25(OH)2D3 (1 pM–0.1 nM) (Figure 6D).
24R,25(OH)2D3 decreases and 1α,25(OH)2D3 increases ROS
We have shown that without dexamethasone, 1α,25(OH)2D3 inhibited hMSC Ca2+ mineralization, whereas 25(OH)D3 and 24R,25(OH)2D3 appear to independently enhance/increase Ca2+ mineralization (Figures 2D and 3A). ROS have been reported by others to directly inhibit Ca2+ mineralization of mouse osteoblast MC3T3-E1 cells, whereas 1α,25(OH)2D3-induced osteocalcin gene expression was maintained (33), similar to our findings. Hence, we reasoned that production of ROS may account for the observed inhibition of Ca2+ mineralization. We report here that 1α,25(OH)2D3 significantly (P < .05) increased ROS in the culture medium, whereas 24R,25(OH)2D3 and 25(OH)D3 significantly (P < .05) decreased ROS (Figure 7A). Furthermore, 24R,25(OH)2D3 blunted the 1α,25(OH)2D3 increase in ROS. There was a significant (P < .05) increase in ROS (in the culture medium) when ketoconazole was added to hMSCs in the presence of 25(OH)D3. This suggests that the 25(OH)D3 reduction in ROS could be due to 25(OH)D3 conversion to 24R,25(OH)2D3 (reduced ROS) and not 1α,25(OH)2D3 (increased ROS) in this context (Figure 7A). The addition of ketoconazole had no effect on the 1α,25(OH)2D3 increase nor the 24R,25(OH)2D3 decrease of ROS production in hMSCs, suggesting direct 1α,25(OH)2D3/24R,25(OH)2D3 effects (Figure 7B).
Figure 7.
24R,25(OH)2D3 decreases whereas 1α,25(OH)2D3 increases ROS production. ROS production in the culture media was measured after 14 days of osteoblastic differentiation conditions (osteogenic medium [OG]). hMSCs were seeded at 10 000 cells/cm2. Treatments were started at day 0 and continued every 3 days (A and B) (Figure 1). B, Cytochrome P450 inhibitor ketoconazole (10 μM) was added 1 hour before the treatment/medium change. The medium was changed to serum-free conditions 24 hours before the collection for ROS measurement. The medium was passed through a 10-kDa exclusion filter. The flowthrough was used for analysis of ROS. ★, P ≤ .05 compared with the no ketoconazole treatment; #, P ≤ .05 comparing 1α,25(OH)2D3 vs the combination of 1α,25(OH)2D3 + 24R,25(OH)2D3 treatment groups; $, P ≤ .05 comparing treatments vs the untreated osteogenic control.
The blunting of 1α,25(OH)2D3-induced ROS production by 24R,25(OH)2D3, decreased ROS production by 25(OH)D3 and 24R,25(OH)2D3, and a marked increase in 25(OH)D3 ROS production with ketoconazole all suggest that 1α,25(OH)2D3 directly increased whereas 24R,25(OH)2D3 [or 25(OH)D3 conversion to 24R,25-(OH)2D3] decreased ROS production by hMSCs.
Discussion
The current understanding of the vitamin D3 endocrine system has maintained a 1α,25(OH)2D3-centric dogma in which systemic or tissue-specific 1α-hydroxylation of prohormone 25(OH)D3 to 1α,25(OH)2D3 leads to the only bioactive form of vitamin D3, whereas 24-hydroxylation of prohormone 25(OH)D3 to 24R,25(OH)2D3 is considered the first step in catabolism and inactivation of vitamin D3.
In an early attempt to understand the complex story of vitamin D3 in bone health, studies in rachitic chicks demonstrated that 24R,25(OH)2D3, and not 1α,25(OH)2D3, was required for bone healing (34). In addition, during chicken egg formation and calcium/phosphorus homeostasis and in chick models of fracture healing, a dependence on 24R,25(OH)2D3 without 1α,25(OH)2D3 was demonstrated (35–37; for a review, see Ref. 38), challenging the 1α,25(OH)2D3-centric dogma of vitamin D3 activity in osteogenesis.
Moreover, classic studies in ovariectomized rats showed that 1α,25(OH)2D3 increased bone mineral density, yet 24R,25(OH)2D3 was required for proper bone physical tolerance and function (39). 1α,25(OH)2D3/VDR signaling is also required for 24-hydroxylation of 25(OH)D3 and 1α,25(OH)2D3 as seen in VDR knockout mice (40).
In further support of direct 24R,25(OH)2D3 effects are studies in rat long bones (41, 42), fracture-healing chick callus (43, 44), and rat resting zone cartilage cells (45), demonstrating a possible plasma membrane–bound 24R,25(OH)2D3 receptor (as reviewed by Norman et al [37]). Recent findings by St-Arnaud (46) in 1α,25(OH)2D3/25-hydroxyvitamin D3 24-hydroxylase (CYP24A1: 24-OHase) knockout mice demonstrated the importance of 24R,25(OH)2D3 in fracture healing. These findings also support direct 24R,25(OH)2D3/receptor effects in bone formation and maintenance. The positive actions of 1α,25(OH)2D3 and 24R,25(OH)2D3 on bone formation have also been established in various in vivo animal models (chickens [36, 38] and trout [47]) and shown to require 1α,25-(OH)2D3/VDR as seen in VDR knockout mice (40). Additional in vitro studies conducted in human osteoblast cell lines (SV-HFO) and human osteosarcoma cell lines (MG63 and SaOS-2) also demonstrated positive actions of 1α,25(OH)2D3 and the 24-hydroxylated metabolites, 24R,25(OH)3D3 and 1α,24R,25(OH)2D3, on mineralization, alkaline phosphatase activity, and osteocalcin production (48).
Certainly 1α,25(OH)2D3 is an active vitamin D3 metabolite, yet it is abundantly clear from the literature over the past 30 to 40 years that we do not completely understand the role of other vitamin D3 metabolites in bone health. Using an unbiased approach, we renewed studies on the role of vitamin D3 metabolites and their involvement during in vitro hMSC osteoblastic differentiation to further our understanding of the vitamin D endocrine system and bone health.
A literature review of the mainstream methods for in vitro bone formation studies revealed that dexamethasone (glucocorticoid analog) has long been used as an osteogenic supplement to enhance in vitro bone formation, both decreasing the time of osteoblastic differentiation and increasing the extent of Ca2+ mineralization (49, 50). Dexamethasone enhanced 1α,25(OH)2D3 effects through numerous mechanisms including up-regulation of VDR expression (18, 19) and increased 1α,25(OH)2-D3/VDR binding to target gene promoters through enhanced VDR/glucocorticoid receptor (GR) interaction (17), and further enhanced 1α,25(OH)2D3/VDR actions through up-regulation of GRs (19). In addition, dexamethasone enhanced the 1α,25(OH)2D3/VDR/GR–induced up-regulation of 24-OHase, leading to increased 24-hydroxylation and altered vitamin D metabolism (17, 19). Dexamethasone, independent of vitamin D stimulation, also induced alkaline phosphatase expression/activity in hMSCs in vitro (51). In agreement with these studies, we demonstrated that dexamethasone (1–10 nM) treatment of hMSCs (3 days) significantly increased VDR, osteocalcin, alkaline phosphatase, and 25-OHase (CYP27A1), whereas, in contrast, dexamethasone decreased 24-OHase (CYP24A1) mRNA expression.
Dexamethasone did enhance but was not required for Ca2+ mineralization or osteoblastic differentiation of hMSCs without vitamin D3 treatment. Inclusion of dexamethasone blunted the effects seen with 25(OH)D3, 1α,25(OH)2D3, and 24R,25(OH)2D3 on Ca2+ mineralization and alkaline phosphatase activity. In contrast to reports in the literature that 1α,25(OH)2D3 promoted Ca2+ mineralization in the presence of dexamethasone (48), 1α,25(OH)2D3 completely inhibited Ca2+ mineralization in the absence of dexamethasone, whereas 24R,25(OH)2D3 and 25(OH)D3 showed increased Ca2+ mineralization compared with that in osteogenic medium with or without dexamethasone. These results imply that continuous treatment with 1α,25(OH)2D3 can both induce and/or inhibit Ca2+ mineralization based on glucocorticoid status, whereas 24R,25(OH)2D3 and 25(OH)D3 enhance Ca2+ mineralization and alkaline phosphatase activity. These data also suggest that previously published studies over the past 30 years using glucocorticoid analogs, such as dexamethasone, skew the results toward a 1α,25(OH)2D3 response and/or mask the effects of 24R,25(OH)2D3 and 25(OH)D3. This could be due to independent direct dexamethasone effects such as stimulating an increase in the 1α,25(OH)2D3 receptor VDR (18, 19), which could lead to a skewed 1α,25(OH)2D3 response. Dexamethasone has also been shown to induce extracellular matrix Ca2+ mineralization and increase alkaline phosphatase activity (49) independent of vitamin D3.
In a dose-response experiment, with and without the cytochrome P450 inhibitor ketoconazole (inhibits general vitamin D3 metabolism in hMSC [15, 16]), we observed increased Ca2+ mineralization with 24R,25(OH)2D3 (10 nM) and 25(OH)D3 (2–20 nM). At higher 24R,25(OH)2D3 (50, 100, and 500 nM) and 25(OH)D3 (200 and 2000 nM) concentrations we observed inhibition of Ca2+ mineralization. Further reductions in the concentration of added 24R,25(OH)2D3 (1 pM–1 nM) did not produce any additional increase in Ca2+ mineralization over that for the untreated osteogenic control, leading to the conclusion that 10 nM is within the range of 24R,25(OH)2D3 bioactivity. Inhibition of Ca2+ mineralization at higher concentrations, as seen with 1α,25(OH)2D3, may be due to further conversion of 24R,25(OH)2D3 and 25(OH)D3 to other metabolites such as 1α,24R,25(OH)3D3. This is unclear, however, because a previous study in a human osteoblast cell line (SV-HFO) treated with dexamethasone and 1α,24R,25(OH)3D3, 24R,25(OH)2D3, or 1α,25(OH)2D3 demonstrated that the 24-hydroxylated metabolites increased both osteocalcin production and Ca2+ mineralization to the same or a greater extent than 1α,25(OH)2D3 (48), challenging the idea that 1α,24R,25(OH)3D3 may be inhibiting mineralization in our studies.
Inhibition of vitamin D3 metabolism by ketoconazole leads to a dose-dependent increase or plateau effect on Ca2+ mineralization by 24R,25(OH)2D3 and 25(OH)D3, suggesting direct actions on promotion of Ca2+ mineralization and not the effects of metabolism of 24R,25(OH)2D3 and 25(OH)D3. In addition, in the presence of ketoconazole, 24R,25(OH)2D3 ameliorated the 1α,25(OH)2D3 inhibition of Ca2+ mineralization. These observations further confirm that 24R,25(OH)2D3 and the prohormone 25(OH)D3 both directly play a positive role in hMSC mineralization, whereas 1α,25(OH)2D3 can both inhibit (no dexamethasone) and promote (with dexamethasone) mineralization. It is unclear though, whether the observed inhibitory effects of 1α,25(OH)2D3 are dexamethasone-specific effects or can be extrapolated to general glucocorticoid status and other glucocorticoid-like drugs. In addition, 24R,25(OH)2D3 reduced whereas 1α,25(OH)2D3 inhibited cell proliferation (in agreement with other studies [15]), further suggesting unique functions of 24R,25(OH)2D3.
Localized metabolism of vitamin D3 in bone cells has previously been reported to be regulated by cytochrome P450 enzymes (14–16, 52). 24-OHase and 1α-OHase are both known downstream targets of 1α,25(OH)2D3/VDR activity. In agreement with previous findings, we observed that 1α,25(OH)2D3 increased 24-OHase (CYP24A1) expression (5, 16, 52, 53). Further, we report here that 1α,25(OH)2D3 had no effect on 1α-OHase (CYP27B1) expression, a result similar to that of another study with primary human bone-derived cells (52), yet in contrast to other reports that demonstrated a 1α,25(OH)2D3-stimulated decrease in 1α-OHase expression (16, 54).
We observed that 1α,25(OH)2D3 alone or in combination with 24R,25(OH)2D3 increased VDR mRNA, yet there was no change seen in VDR protein, whereas in combination with 24R,25(OH)2D3, there was a decrease in VDR protein, as well as in the expression of 1α-OHase (CYP27B1). This finding suggests that although 24R,25(OH)2D3 can decrease VDR mRNA expression, 1α,25(OH)2D3 was the dominant positive regulator of VDR mRNA transcription, whereas 24R,25(OH)2D3 appears to be the dominant negative regulator of VDR protein levels, possibly through translational and/or degradation mechanisms.
Long-term treatment of hMSCs with 24R,25(OH)2D3 demonstrated that the 24R,25(OH)2D3-induced down-regulation of 1α-OHase led to a reduction in the rate of 1α,25(OH)2D3 biosynthesis from prohormone 25(OH)D3 in hMSCs. This finding suggests that 24R,25(OH)2D3 directly decreased 1α,25(OH)2D3 bioactivity through both decreased 1α,25(OH)2D3 receptor VDR expression and decreased 1α,25(OH)2D3 biosynthesis via down-regulation of 1α-OHase in hMSCs. In addition, 1α,25(OH)2D3 stimulated the increased expression of 25-OHase (CYP2R1) during the later stages of differentiation concurrent with mineralization, but not in naive hMSCs, suggesting that 1α,25(OH)2D3 may increase 25-OHase and subsequent 25(OH)D3 biosynthesis only in mature osteoblasts. This is in contrast to findings in dermal fibroblasts and prostate cancer LNCaP cells in which it was reported that 1α,25(OH)2D3 suppressed CYP2R1 (55). Hence, 1α,25(OH)2D3 may in fact promote the tissue-specific stage-dependent 25-hydroxylation of vitamin D3, prompting a positive feedback loop for increased vitamin D3 activity only in mature matrix-producing osteoblasts. Unfortunately, the in vivo relevance of this suggestion is unclear, because most systemic vitamin D3 is 25-hydroxylated in the liver, and it is not known whether significant quantities of vitamin D3 would interact directly with hMSCs residing in bone.
Assessment of genes involved in osteoblastic differentiation revealed that 24R,25(OH)2D3 treatment decreased the expression of osteocalcin, a known target of and increased by 1α,25(OH)2D3 (48, 52, 56). In contrast to our findings, treatment of cells from a human osteoblast cell line (SV-HFO) or human osteosarcoma cell lines (MG-63 and SaOS-2) with 24R,25(OH)2D3 or 1α,24R,25(OH)3D3, in the presence of dexamethasone, led to an increase, not a decrease, in osteocalcin mRNA and osteocalcin accumulation in the matrix (48). It is not clear whether this difference in osteocalcin mRNA regulation by the 24-hydroxylated metabolites of vitamin D3 is due to the constant inclusion of dexamethasone or to innate differences in cell types. Collagen 1A1, another target of 1α,25(OH)2D3 and required for bone matrix formation (57), was increased by 1α,25(OH)2D3 but not by 24R,25(OH)2D3. No change was observed for Runx2 or alkaline phosphatase gene expression by either 1α,25(OH)2D3 or 24R,25(OH)2D3. These findings are in complete contrast to the observed promoting and inhibitory effects, respectively, of 24R,25(OH)2D3 and 1α,25(OH)2D3 on Ca2+ mineralization without dexamethasone. Specifically, in the absence of dexamethasone, 1α,25(OH)2D3 up-regulated collagen 1A1 (matrix) and osteocalcin, yet completely inhibited Ca2+ mineralization, whereas 24R,25(OH)2D3 decreased collagen 1A1 and osteocalcin, yet enhanced Ca2+ mineralization. Osteocalcin and collagen 1A1 are both important acidic bone matrix proteins with known roles in matrix synthesis and are characteristic of mature matrix-forming osteoblasts. Collagen 1A1 is the most abundant matrix protein, and osteocalcin is thought to be responsible for the control of matrix synthesis (58). From these results, we speculate that (1) 1α,25(OH)2D3 may prime hMSCs by up-regulating matrix proteins required for mineralization (ie, collagen 1A1 and osteocalcin), whereas (2) 24R,25(OH)2D3 is required for the actual initiation of mineralization and osteoblastic differentiation of 1α,25(OH)2D3-primed hMSCs, followed by (3) a 24R,25(OH)2D3 feedback loop decreasing 1α,25(OH)2D3 activity (decreased VDR and 1α,25(OH)2D3 biosynthesis) as differentiation progresses. This scenario could account for the complete inhibitory effects of 1α,25(OH)2D3 alone, whereas the addition of 24R,25(OH)2D3 is required to trigger the hMSCs to mineralize. The studies and results presented here do not directly show a sequential stimulation or priming by 1α,25(OH)2D3 followed by 24R,25(OH)2D3 but raise the idea that sequential vitamin D3 metabolite stimulation, metabolism, and/or feedback inhibition could play a role in osteoblastic differentiation and warrant future investigation.
To further investigate possible mechanisms that would explain the observed inhibition of mineralization by 1α,25(OH)2D3 and subsequent increase in mineralization by 24R,25(OH)2D3, we assessed the levels of ROS in the medium. The presence and regulation of ROS, ie, a variety of chemical compounds encompassing superoxide anions, hydroxyl radicals, and hydrogen peroxide (31), were evaluated with a general assay involving the oxidation of DHR to determine the level of and change in ROS (32). There are 2 main schools of thought for how ROS affect a cell: (1) the accumulation of ROS leads to random and cumulative oxidation, or (2) ROS act as specific signaling molecules and can modulate the activation and/or inhibition of signaling cascades through direct oxidation of target proteins. With this in mind, we asked whether 1α,25(OH)2D3, 24R,25(OH)2D3, and/or the prohormone 25(OH)D3 alter the levels of ROS.
ROS have been shown to completely inhibit mineralization of mouse osteoblastic MC3T3-E1 cells, yet ROS directly induced osteocalcin gene expression at the same time (33), similar to what we report here with 1α,25(OH)2D3 inhibition of Ca2+ mineralization concurrent with up-regulation of osteocalcin. In addition, 24R,25(OH)2D3 has been shown to increase ROS production through the inhibition of catalase, which leads to inhibition of 1α,25(OH)2D3 effects (59), again suggesting a possible role for ROS in hMSC osteoblastic differentiation. Here we observed increased ROS in the medium of hMSCs treated with 1α,25(OH)2D3, whereas 25(OH)D3- and 24R,25(OH)2D3-treated cells showed a decrease in ROS. Ketoconazole reversed the effect of 25(OH)D3, leading to a dose-dependent increase in ROS, suggesting that the observed 25(OH)D3-induced decrease could have been due to 25(OH)D3 conversion to 24R,25(OH)2D3. It is also well known that 25(OH)D3 at higher concentrations (Kd = ∼100–1000 nM) (for a detailed review, see Ref. 5) can bind and activate VDR compared with the higher-affinity binding of 1α,25(OH)2D3 to VDR (Kd = ∼0.1–1 nM) (5, 60, 61). This could explain the high-dose 25(OH)D3 increase in ROS in the presence of ketoconazole and further supports the concept that 1α,25(OH)2D3/VDR activity leads to increased ROS production. Hence, it is conceivable that prohormone 25(OH)D3 conversion to 24R,25(OH)2D3 by hMSCs leads to a decrease in ROS production and is the default action of 25(OH)D3, not its conversion to 1α,25(OH)2D3.
The combination of 24R,25(OH)2D3 with 1α,25(OH)2D3 treatment also blocked the 1α,25(OH)2D3 increase in ROS, with no observed changes with further inclusion of ketoconazole. One possibility to account for these observations is that 1α,25(OH)2D3 increases ROS, leading to inhibition of Ca2+ mineralization [mechanism(s) unknown], yet up-regulates osteocalcin expression, similar to a previous report in MC3T3-E1 cells (33). Addition of 24R,25(OH)2D3 to hMSCs reduced ROS in the medium, potentially allowing subsequent Ca2+ mineralization to occur. Unfortunately, this does not account for the on-off complete inhibition of Ca2+ mineralization by 1α,25(OH)2D3 at such a low concentration, whereas 24R,25(OH)2D3 shows increased Ca2+ mineralization at 10 nM with a dose-dependent decrease at higher concentrations.
These observations demonstrate a correlation between 1α,25(OH)2D3 inhibition/24R,25(OH)2D3 stimulation of mineralization with respective increased/decreased ROS, setting the stage for future studies. Specifically, ROS have been shown to directly activate the ERK and phosphatidylinositol 3-kinase/Akt pathways via mimicking growth factor receptor–ligand interactions. ROS have also been shown to directly activate the p38 MAPK signal cascades, the nuclear factor-κB signaling system, and the p53 family of transcription factors (for a review, see Ref. 31). Of interest, but outside the scope of the current work, future studies could be focused on using general antioxidants, superoxide scavengers, nitric oxide synthase inhibitors, and hydrogen peroxide scavengers, ie, vitamin E, N-acetyl-l-cysteine, N-nitro-l-arginine-methyl ester, manganese superoxide dismutase, or N,N′-dimethylthiourea, to attenuate the 1α,25(OH)2D3-stimulated increase in ROS and assess the link between 1α,25(OH)2D3-ROS inhibition of mineralization and subsequent activation of signaling pathways. Conversely, oxidants, such as hydrogen peroxide, could be added during osteoblastic differentiation to determine whether there is an observed inhibition of mineralization, similar to what we see with 1α,25(OH)2D3. These studies would provide a better understanding of how vitamin D3 metabolites and ROS mediate Ca2+ mineralization and potentially bone formation.
The studies presented here demonstrate that 24R,25(OH)2D3 is an active form of vitamin D3 with a heretofore unappreciated essential role in osteoblast maturation, Ca2+ mineralization, gene expression, and the regulation of cytochrome P450 expression, resulting in decreased 1α,25(OH)2D3 biosynthesis. In addition, it appears that the prohormone 25(OH)D3 has 2 independent modes of action: (1) a direct positive role in mineralization (not dependent on conversion to 24R,25(OH)2D3); and (2) an indirect inhibitory effect on ROS production and gene expression of 24-OHase and osteocalcin, potentially due to metabolism into 24R,25(OH)2D3. We hypothesize that the actions of vitamin D3 and the prohormone 25(OH)D3 on osteoblastic differentiation and bone repair are due to a regulatory relationship between unique 24R,25(OH)2D3 and 1α,25(OH)2D3 effects, possibly through ROS-mediated mechanisms. These results support a generalized paradigm implicating 24R,25(OH)2D3 as a key player during hMSC maturation and bone development and support the concept that the hydroxylation of the prohormone 25(OH)D3 by hMSCs leads to the formation of not 1 but 2 active metabolites: 24R,25(OH)2D3 and 1α,25(OH)2D3.
Acknowledgments
We thank David Vazquez and B. Nubia Rodriguez for excellent technical assistance. We also thank Dr A. R. Jayakumar for assessing ROS production.
This work was funded through the Department of Veterans Affairs (Merit Review Grants to B.A.R. and G.A.H.) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Award 7F32AR062990 to K.M.C.). G.A.H. is the recipient of a Senior Research Career Scientist award from the Department of Veterans Affairs.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-bromo-2′-deoxyuridine
- DHR
- dihydrorhodamine 123
- F
- forward
- FBS
- fetal bovine serum
- GR
- glucocorticoid receptor
- hMSC
- human mesenchymal stem cell
- MSC
- mesenchymal stem cell
- 1α-OHase
- 1α-hydroxylase
- 24-OHase
- 24-hydroxylase
- 25-OHase
- 25-hydroxylase
- 25(OH)D3
- 25-hydroxyvitamin D3
- 24R,25(OH)2D3
- 24R,25-dihydroxyvitamin D3
- 1α,25(OH)2D3
- 1α,25-dihydroxyvitamin D3
- R
- reverse
- ROS
- reactive oxygen species
- RT-qPCR
- real-time quantitative PCR.
References
- 1. van Leeuwen JP, van den Bemd GJ, van Driel M, Buurman CJ, Pols HA. 24,25-Dihydroxyvitamin D3 and bone metabolism. Steroids. 2001;66:375–380 [DOI] [PubMed] [Google Scholar]
- 2. Lambert PW, Stern PH, Avioli RC, Brackett NC, Turner RT, Greene A, et al. Evidence for extrarenal production of 1α ,25-dihydroxyvitamin D in man. J Clin Invest. 1982;69:722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briggs AD, Kuan V, Greiller CL, Maclaughlin BD, Ramachandran M, Harris T, et al. Longitudinal study of vitamin D metabolites after long bone fracture. J Bone Miner Res. 2013;28:1301–1307 [DOI] [PubMed] [Google Scholar]
- 4. Kawakami M, Imawari M, Goodman DS. Quantitative studies of the interaction of cholecalciferol (vitamin D3) and its metabolites with different genetic variants of the serum binding protein for these sterols. Biochem J. 1979;179:413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones G. Vitamin D analogs. Endocrinol Metab Clin North Am. 2010;39:447–472, table of contents [DOI] [PubMed] [Google Scholar]
- 6. Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am. 2010;39:243–253, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LoPiccolo MC, Lim HW. Vitamin D in health and disease. Photodermatol Photoimmunol Photomed. 2010;26:224–229 [DOI] [PubMed] [Google Scholar]
- 8. Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235:1034–1045 [DOI] [PubMed] [Google Scholar]
- 9. Mehta B, Ramanathan M, Weinstock-Guttman B. Vitamin D and multiple sclerosis: can vitamin D prevent disease progression? Expert Rev Neurother. 2011;11:469–471 [DOI] [PubMed] [Google Scholar]
- 10. Weinstock-Guttman B, Zivadinov R, Qu J, Cookfair D, Duan X, Bang E, et al. Vitamin D metabolites are associated with clinical and MRI outcomes in multiple sclerosis patients. J Neurol Neurosurg Psychiatry. 2011;82:189–195 [DOI] [PubMed] [Google Scholar]
- 11. Seo EG, Norman AW. Three-fold induction of renal 25-hydroxyvitamin D3-24-hydroxylase activity and increased serum 24,25-dihydroxyvitamin D3 levels are correlated with the healing process after chick tibial fracture. J Bone Miner Res. 1997;12:598–606 [DOI] [PubMed] [Google Scholar]
- 12. Birkenhäger-Frenkel DH, Pols HA, Zeelenberg J, et al. Effects of 24R,25-dihydroxyvitamin D3 in combination with 1α-hydroxyvitamin D3 in predialysis renal insufficiency: biochemistry and histomorphometry of cancellous bone. J Bone Miner Res. 1995;10:197–204 [DOI] [PubMed] [Google Scholar]
- 13. D'Ippolito G, Schiller PC, Perez-Stable C, Balkan W, Roos BA, Howard GA. Cooperative actions of hepatocyte growth factor and 1,25-dihydroxyvitamin D3 in osteoblastic differentiation of human vertebral bone marrow stromal cells. Bone. 2002;31:269–275 [DOI] [PubMed] [Google Scholar]
- 14. Howard GA, Turner RT, Sherrard DJ, Baylink DJ. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981;256:7738–7740 [PubMed] [Google Scholar]
- 15. Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J Bone Miner Res. 2011;26:1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhawan P, Christakos S. Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase) transcription by glucocorticoids: cooperative effects of the glucocorticoid receptor, C/EBPβ, and the vitamin D receptor in 24(OH)ase transcription. J Cell Biochem. 2010;110:1314–1323 [DOI] [PubMed] [Google Scholar]
- 18. Hidalgo AA, Deeb KK, Pike JW, Johnson CS, Trump DL. Dexamethasone enhances 1α,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J Biol Chem. 2011;286:36228–36237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hidalgo AA, Trump DL, Johnson CS. Glucocorticoid regulation of the vitamin D receptor. J Steroid Biochem Mol Biol. 2010;121:372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann NY Acad Sci. 2002;966:73–81 [DOI] [PubMed] [Google Scholar]
- 21. De Nijs RN. Glucocorticoid-induced osteoporosis: a review on pathophysiology and treatment options. Minerva Med. 2008;99:23–43 [PubMed] [Google Scholar]
- 22. D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122 [DOI] [PubMed] [Google Scholar]
- 23. Curtis KM, Gomez LA, Rios C, et al. EF1α and RPL13a represent normalization genes suitable for RT-qPCR analysis of bone marrow derived mesenchymal stem cells. BMC Mol Biol. 2010;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linsley C, Wu B, Tawil B. The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Eng Part A. 2013;19:1416–1423 [DOI] [PubMed] [Google Scholar]
- 25. Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994;39:941–947 [DOI] [PubMed] [Google Scholar]
- 26. van Driel M, Koedam M, Buurman CJ, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1α-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419 [DOI] [PubMed] [Google Scholar]
- 27. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Studer D, Lischer S, Jochum W, Ehrbar M, Zenobi-Wong M, Maniura-Weber K. Ribosomal protein L13a as a reference gene for human bone marrow-derived mesenchymal stromal cells during expansion, adipo-, chondro-, and osteogenesis. Tissue Eng Part C Methods. 2012;18:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). J Biol Chem. 1995;270:9420–9428 [DOI] [PubMed] [Google Scholar]
- 30. Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629 [DOI] [PubMed] [Google Scholar]
- 31. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247 [DOI] [PubMed] [Google Scholar]
- 32. Grzelak A, Rychlik B, Bartosz G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radic Biol Med. 2001;30:1418–1425 [DOI] [PubMed] [Google Scholar]
- 33. Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33 [DOI] [PubMed] [Google Scholar]
- 34. Ornoy A, Goodwin D, Noff D, Edelstein S. 24,25-Dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature. 1978;276:517–519 [DOI] [PubMed] [Google Scholar]
- 35. Norman AW, Henry HL, Malluche HH. 24R,25-Dihydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 are both indispensable for calcium and phosphorus homeostasis. Life Sci. 1980;27:229–237 [DOI] [PubMed] [Google Scholar]
- 36. Seo EG, Einhorn TA, Norman AW. 24R,25-Dihydroxyvitamin D3: an essential vitamin D3 metabolite for both normal bone integrity and healing of tibial fracture in chicks. Endocrinology. 1997;138:3864–3872 [DOI] [PubMed] [Google Scholar]
- 37. Norman AW, Okamura WH, Bishop JE, Henry HL. Update on biological actions of 1α,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3. Mol Cell Endocrinol. 2002;197:1–13 [DOI] [PubMed] [Google Scholar]
- 38. Henry HL, Norman AW. Vitamin D: two dihydroxylated metabolites are required for normal chicken egg hatchability. Science. 1978;201:835–837 [DOI] [PubMed] [Google Scholar]
- 39. Matsumoto T, Ezawa I, Morita K, Kawanobe Y, Ogata E. Effect of vitamin D metabolites on bone metabolism in a rat model of postmenopausal osteoporosis. J Nutr Sci Vitaminol (Tokyo). 1985;31 Suppl:S61-5 [DOI] [PubMed] [Google Scholar]
- 40. Endres B, Kato S, DeLuca HF. Metabolism of 1α,25-dihydroxyvitamin D(3) in vitamin D receptor-ablated mice in vivo. Biochemistry. 2000;39:2123–2129 [DOI] [PubMed] [Google Scholar]
- 41. Sömjen D, Sömjen GJ, Harell A, Mechanic GL, Binderman I. Partial characterization of a specific high affinity binding macromolecule for 24R,25 dihydroxyvitamin D3 in differentiating skeletal mesenchyme. Biochem Biophys Res Commun. 1982;106:644–651 [DOI] [PubMed] [Google Scholar]
- 42. Sömjen D, Sömjen GJ, Weisman Y, Binderman I. Evidence for 24,25-dihydroxycholecalciferol receptors in long bones of newborn rats. Biochem J. 1982;204:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kato A, Seo EG, Einhorn TA, Bishop JE, Norman AW. Studies on 24R,25-dihydroxyvitamin D3: evidence for a nonnuclear membrane receptor in the chick tibial fracture-healing callus. Bone. 1998;23:141–146 [DOI] [PubMed] [Google Scholar]
- 44. Seo EG, Kato A, Norman AW. Evidence for a 24R,25(OH)2-vitamin D3 receptor/binding protein in a membrane fraction isolated from a chick tibial fracture-healing callus. Biochem Biophys Res Commun. 1996;225:203–208 [DOI] [PubMed] [Google Scholar]
- 45. Pedrozo HA, Schwartz Z, Rimes S, et al. Physiological importance of the 1,25(OH)2D3 membrane receptor and evidence for a membrane receptor specific for 24,25(OH)2D3. J Bone Miner Res. 1999;14:856–867 [DOI] [PubMed] [Google Scholar]
- 46. St-Arnaud R. CYP24A1-deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J Steroid Biochem Mol Biol. 2010;121:254–256 [DOI] [PubMed] [Google Scholar]
- 47. Larsson D, Nemere I, Aksnes L, Sundell K. Environmental salinity regulates receptor expression, cellular effects, and circulating levels of two antagonizing hormones, 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3, in rainbow trout. Endocrinology. 2003;144:559–566 [DOI] [PubMed] [Google Scholar]
- 48. van Driel M, Koedam M, Buurman CJ, et al. Evidence that both 1α,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006;99:922–935 [DOI] [PubMed] [Google Scholar]
- 49. Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286 [DOI] [PubMed] [Google Scholar]
- 50. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312 [PubMed] [Google Scholar]
- 51. Alm JJ, Heino TJ, Hentunen TA, Väänänen HK, Aro HT. Transient 100 nM dexamethasone treatment reduces inter- and intraindividual variations in osteoblastic differentiation of bone marrow-derived human mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:658–666 [DOI] [PubMed] [Google Scholar]
- 52. Atkins GJ, Anderson PH, Findlay DM, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528 [DOI] [PubMed] [Google Scholar]
- 53. Matilainen JM, Malinen M, Turunen MM, Carlberg C, Väisänen S. The number of vitamin D receptor binding sites defines the different vitamin D responsiveness of the CYP24 gene in malignant and normal mammary cells. J Biol Chem. 2010;285:24174–24183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim MS, Fujiki R, Kitagawa H, Kato S. 1α,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol Cell Endocrinol. 2007;265–266:168–173 [DOI] [PubMed] [Google Scholar]
- 55. Ellfolk M, Norlin M, Gyllensten K, Wikvall K. Regulation of human vitamin D3 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol Pharmacol. 2009;75:1392–1399 [DOI] [PubMed] [Google Scholar]
- 56. Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci USA. 1989;86:4455–4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mahonen A, Jukkola A, Risteli L, Risteli J, Mäenpää PH. Type I procollagen synthesis is regulated by steroids and related hormones in human osteosarcoma cells. J Cell Biochem. 1998;68:151–163 [PubMed] [Google Scholar]
- 58. Fujisawa R, Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci (Landmark Ed). 2012;17:1891–1903 [DOI] [PubMed] [Google Scholar]
- 59. Nemere I, Wilson C, Jensen W, Steinbeck M, Rohe B, Farach-Carson MC. Mechanism of 24,25-dihydroxyvitamin D3-mediated inhibition of rapid, 1,25-dihydroxyvitamin D3-induced responses: role of reactive oxygen species. J Cell Biochem. 2006;99:1572–1581 [DOI] [PubMed] [Google Scholar]
- 60. Li XY, Boudjelal M, Xiao JH, et al. 1,25-Dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol Endocrinol. 1999;13:1686–1694 [DOI] [PubMed] [Google Scholar]
- 61. Mizwicki MT, Norman AW. Two key proteins of the vitamin D endocrine system come into crystal clear focus: comparison of the X-ray structures of the nuclear receptor for 1α,25(OH)2 vitamin D3, the plasma vitamin D binding protein, and their ligands. J Bone Miner Res. 2003;18:795–806 [DOI] [PubMed] [Google Scholar]