Abstract
The small subunit of U2AF, which functions in 3′ splice site recognition, is more highly conserved than its heterodimeric partner yet is less thoroughly investigated. Remarkably, we find that the small subunit of Schizosaccharomyces pombe U2AF (U2AFSM) can be replaced in vivo by its human counterpart, demonstrating that the conservation extends to function. Precursor mRNAs accumulate in S. pombe following U2AFSM depletion in a time frame consistent with a role in splicing. A comprehensive mutational analysis reveals that all three conserved domains are required for viability. Notably, however, a tryptophan in the pseudo-RNA recognition motif implicated in a key contact with the large subunit by crystallographic data is dispensable whereas amino acids implicated in RNA recognition are critical. Mutagenesis of the two zinc-binding domains demonstrates that they are neither equivalent nor redundant. Finally, two- and three-hybrid analyses indicate that mutations with effects on large-subunit interactions are rare whereas virtually all alleles tested diminished RNA binding by the heterodimer. In addition to demonstrating extraordinary conservation of U2AF small-subunit function, these results provide new insights into the roles of individual domains and residues.
The heterodimeric splicing factor U2AF (U2 snRNP auxiliary factor) recognizes the 3′ splice site in the earliest biochemically defined complex formed on premessenger RNAs (reviewed in reference 34). Human U2AF, initially identified as an activity necessary for the recruitment of U2 snRNP to the branchpoint (41), consists of a 65-kDa subunit (58) and a 35-kDa subunit (60). The large subunit of U2AF contacts the polypyrimidine tract, while the small subunit recognizes the 3′ AG (27, 55, 58, 64). Orthologs of both subunits are found in Drosophila melanogaster (21, 37), Caenorhabditis elegans (62, 63), Schizosaccharomyces pombe (33, 50), and Arabidopsis thaliana (8). While a poorly conserved ortholog of the U2AF large subunit, Mud2p, has been identified in Saccharomyces cerevisiae, the genome does not contain an open reading frame that resembles the small subunit (1). The absence of a U2AF small-subunit orthologue in S. cerevisiae versus its strong conservation in S. pombe (62% identity and 78% similarity with human U2AF35 over the first three domains; see Fig. 3) provides an impetus to understand better the function of this enigmatic splicing factor.
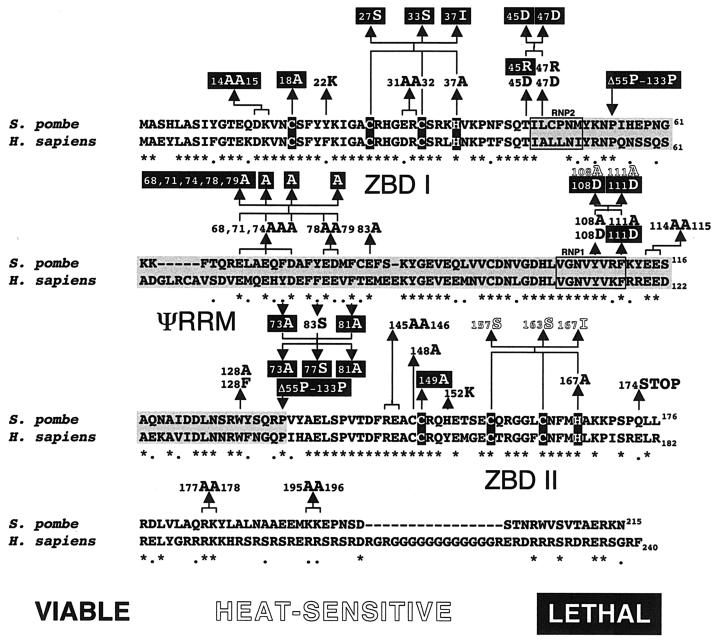
FIG. 3.
Sequence alignment of S. pombe U2AFSM and human U2AF35, indicating the locations of mutations analyzed in this study. The alignment was produced using the ClustalW program with minor manual refinement. *, amino acid identity; •, amino acid similarity. The putative zinc-coordinating residues are highlighted in white characters on a black background for each ZBD. The ΨRRM is highlighted in black characters on a gray background with the RNP2-like (amino acids 46 to 51) and RNP1-like (amino acids 104 to 111) peptides boxed. On the basis of the results of our structure-function studies (see text) together with those obtained in a structure-based alignment of several RRM-RNA complexes (49), we shifted the position of the RNP2-like peptide by one amino acid relative to the results shown in Fig. 2 of reference 24. The single and multiple amino acid substitutions analyzed here (with their phenotypic consequences denoted according to the key) are indicated above the S. pombe sequence.
Because human U2AF35 is present in a stable complex with U2AF65, it was quite surprising when early studies provided evidence that the small subunit is dispensable for splicing in vitro (57). Subsequently, however, reconstitution experiments demonstrated that the small subunit is required for only a subset of splicing events in HeLa nuclear extracts whereas the large subunit appeared to be critical for excision of all introns tested (see, e.g., references 13, 15, 27, 61, 65). The small subunit of U2AF may also be dispensable for some splicing events in vivo, as none of the pre-mRNAs tested showed splicing defects in C. elegans after RNA interference-mediated depletion (T. Blumenthal, personal communication) or in a Drosophila mutant that expresses only 1/10 of the normal level of this splicing factor (37); however, the small-subunit mutation did influence an alternative splicing decision (30).
Like its heterodimeric partner, the small subunit of U2AF is a modular protein. At its core is a pseudo-RNA recognition motif (ΨRRM) similar to those found in several other splicing factors, including the large subunit of U2AF (31). The ΨRRM is flanked by two highly conserved regions homologous to members of the Nup475 family of zinc-binding proteins, which contain the consensus sequence YKTELCX8CX5CX3H (zinc ligands determined by nuclear magnetic resonance analysis are underlined) (53). The most divergent domain of the U2AF small subunit is the C terminus: in metazoan orthologs, this region contains different combinations of RS dipeptides and runs of glycines (60, 62), while in S. pombe it is shorter and lacks recognizable features (see Fig. 3).
Until recently, little was known about the interactions of the small subunit with other splicing factors. A major breakthrough was provided by X-ray crystallographic analysis of a “minimal heterodimer” consisting of the ΨRRM of human U2AF35 complexed with a proline-rich peptide from the large subunit (24). The structure revealed that the two proteins form a tight junction via reciprocal “tongue-in-groove” interactions involving tryptophan residues. Two-hybrid data indicated that human U2AF35 binds SR proteins (54, 56) and S. pombe U2AFSM interacts with SKIP (for “Ski-interacting protein”; also known as Prp45p/SNW1/NcoA-62/Bx42/Cwf13p) (5), a transcriptional coactivator-corepressor recently shown to function in splicing (reference 2 and references therein). Finally, far-Western blot analysis suggests that both the large and small subunits of human U2AF interact with spliceosome-associated protein 155 (SAP155) (12). However, the relevance of these interactions to splicing in vivo remains to be demonstrated.
Structure-function analyses of the small subunit of U2AF have been hindered by the difficulty of purifying large quantities except in the presence of the large subunit or a fragment thereof (24, 40) and by the lack of a facile genetic system in which to assess the effects of amino acid substitutions. To close this gap in our understanding, we have undertaken a comprehensive mutational analysis of the S. pombe small subunit. Three important conclusions emerged from this study. First, this protein is essential for life, most likely due to its role in splicing. Second, all three conserved domains are critical for U2AF function in vivo whereas the variable C terminus is dispensable. Finally, both zinc-binding domains (ZBDs), as well as the ΨRRM, contribute to RNA binding by the heterodimer. Because human U2AF35 can substitute for the S. pombe protein in vivo, these conclusions are relevant to splicing in human cells.
MATERIALS AND METHODS
Plasmid construction and mutagenesis.
The cDNA sequence of uaf2+ (50) was used to design an oligonucleotide probe (probe 1) (Table 1) for cloning a 5.1-kb XbaI fragment of genomic DNA identified by Southern blot analysis. To generate a uaf2+ null allele, we used oligonucleotides 2 and 3 (Table 1) to introduce PstI and SalI sites upstream and downstream of the open reading frame as originally reported (50) via mutagenesis with commercially available reagents (Amersham Corp., Arlington Heights, Ill.). The gene disruption plasmid uaf2::LEU2/pBSK+ was generated by successively subcloning the ∼2.0-kb SalI-KpnI fragment carrying downstream flanking sequences, the ∼2.2-kb HindIII LEU2 fragment from pIRT2 (17), and the ∼2.1-kb PstI fragment carrying upstream flanking sequences into pBSK+.
TABLE 1.
Probe, oligonucleotides, and primers used in these studies
| Probe, oligonucleotide, or primer and function | Designation | Sequence |
|---|---|---|
| Probe for genomic Southern blot analysis | ||
| 1 | spU2AF23.oligo.1 | 5′ gcgttcagcagttacactgacccatcg 3′ |
| Oligonucleotides for uaf2+ phosphorothioate-based site-directed mutagenesis | ||
| 2 | U2AF23.3.mut.cjw | 5′ catgttctgcagatatatcgtac 3′ |
| 3 | U2AF23.4.mut.cjw | 5′ gacccatcgattcgtcgactcactaggcgtaatcaggaacatcgtaagggtaggcggcggcttgggctcctttttcatttc 3′ |
| 4 | U2AF23.N-ZN CCCH/SSSI.8 | 5′ gagcaagataaagtcaattcctcattctattataaaataggagcttctagacatggggaacgctcctctcgtaaaattg tcaaaccaaattttagtcaaacg 3′ |
| 5 | U2AF23.N-ZN CCH/SSI.9 | 5′ gcaacacgaaacttcggaatctcagcggggtggcctttctaattttatgattgccaaaaaaccaagtcc 3′ |
| 6 | U2AF23.10.Q174STOP | 5′ gccaaaaaaccaagtccttagcttttacgcg 3′ |
| 7 | U2AF23.E31A/R32A.15.mut | 5′ gtagacatggggctgcttgctctcgtaaacatgtc 3′ |
| 8 | U2AF23.E78A/D79A.16.mut | 5′ gatgcattctacgccgctatgttttgtgagttttc 3′ |
| 9 | U2AF23.E114A/E115A.17.mut | 5′ gattcaagtatgctgcttctgcccaaaatgctattg 3′ |
| 10 | U2AF23.R145A/E146A.18.mut | 5′ gtaactgattttgctgctgcatgttgccggcaacacg 3′ |
| 11 | U2AF23.R177A/D178A.19.mut | 5′ cctcagcttttagctgctttggtactagcacaaagaaag 3′ |
| 12 | U2AF23.K195A/K196A.20.mut | 5′ gcagaagaaatggccgctgagcccaatagtgattccacg 3′ |
| Oligonucleotides for uaf2+ overlap extension PCR mutagenesis | ||
| 13 | U2AF23.BamHI.5′.29 | 5′ cgcggatccagatcttaatagattatcattaagatcaatc 3′ |
| 14 | U2AF23.BamHI.3′.30 | 5′ cgcggatcccatatgtttctaatcgatctcctttag 3′ |
| 15 | 23.C18A.pcr.mut.1.31 | 5′ ggtactgagcaagataaagtcaatgcctcattctattataaaatagg 3′ |
| 16 | 23.C18A.pcr.mut.2.32 | 5′ attgactttatcttgctcagtacc 3′ |
| 17 | 23.H37A.pcr.mut.1.33 | 5′ ggggaacgctgctctcgtaaagccgtcaaaccaaattttagtc 3′ |
| 18 | 23.H37A.pcr.mut.2.34 | 5′ tttacgagagcagcgttccccatg 3′ |
| 19 | 23.Y108D/F111D.mut1.35 | 5′ ggagatcacttagtcggaaatgttgacgtaagagacaagtatgaagagtctgcc 3′ |
| 20 | 23.Y108A/F111A.mut1.36 | 5′ ggagatcacttagtcggaaatgttgccgtaagagccaagtatgaagagtctgcc 3′ |
| 21 | 23.Y108-/F111-.mut2.37 | 5′ aacatttccgactaagtgatctcc 3′ |
| 22 | 23.C149A.pcrmut.1.38 | 5′ gtaactgattttcgcgaggcatgtgctcggcaacacgaaacttcgg 3′ |
| 23 | 23.C149A.pcrmut.2.39 | 5′ acatgcctcgcgaaaatcagttac 3′ |
| 24 | 23.C167A.pcrmut.1.40 | 5′ cggggtggcctttgtaattttatggctgccaaaaaaccaagtcctc 3′ |
| 25 | 23.C167A.pcrmut2.41 | 5′ cataaaattacaaaggccaccccg 3′ |
| 26 | 23D14A/K15Amut1.42 | 5′ gcaagtatttatggtactgagcaagctgctgtcaattgctcattctattataaaata 3′ |
| 27 | 23D14A/K15Amut2.43 | 5′ ttgctcagtaccataaatacttgc 3′ |
| 28 | 23.S18C.pcrmut.2.42 | 5′ ggtactgagcaagataaagtcaattgctcattctattataaaatagg 3′ |
| 29 | 23dP55-V134pcrmut.43 | 5′ cgatcttatgtcccaacatgtataaaaacccagtctacgccgagttatctcc 3′ |
| 30 | 23dP55-V134pcrmut.44 | 5′ tgggtttttatacatgttgggacataagatcg 3′ |
| 31 | 23.FYF/ASA.1 | 5′ cgcagcgtgaacttgcagaacaagctgatgcattctccgaggacatggcttgtgagttttcaaaatacgg 3′ |
| 32 | 23.FYF/ASA.2 | 5′ ttgttctgcaagttcacgctgcg 3′ |
| 33 | 23.Y108D.1.52 | 5′ ggagatcacttagtcggaaatgttgacgtaagattcaagtatgaagagtctgcc 3′ |
| 34 | 23.Y108A.1.53 | 5′ ggagatcacttagtcggaaatgttgccgtaagattcaagtatgaagagtctgcc 3′ |
| 35 | 23.F111D.1.54 | 5′ ggagatcacttagtcggaaatgtttatgtaagagacaagtatgaagagtctgcc 3′ |
| 36 | 23.F111A.1.55 | 5′ ggagatcacttagtcggaaatgtttatgtaagagccaagtatgaagagtctgcc 3′ |
| 37 | 23T45DL47D.1.56 | 5′ cgtaaacatgtcaaaccaaattttagtcaagacatcttatgtcccaacatgtataaaaacccaattc 3′ |
| 38 | 23.T45D.1.58 | 5′ cgtaaacatgtcaaaccaaattttagtcaagacatcttatgtcccaacatgtataaaaacccaattc 3′ |
| 39 | 23.T45R.1.60 | 5′ cgtaaacatgtcaaaccaaattttagtcaacgtatcttatgtcccaacatgtataaaaacccaattc 3′ |
| 40 | 23.L47D.1.61 | 5′ cgtaaacatgtcaaaccaaattttagtcaaacgatcgactgtcccaacatgtataaaaacccaattc 3′ |
| 41 | 23.L47R.1.63 | 5′ cgtaaacatgtcaaaccaaattttagtcaaacgatccgttgtcccaacatgtataaaaacccaattc 3′ |
| 42 | 23T45-L47-.2.64 | 5′ ttgactaaaatttggtttgacatgtttacg 3′ |
| 43 | 23.EED/AAA.1.65 | 5′ ggcaaaaagttcacgcagcgtgcccttgcagcccaatttgctgcattctacgaggacatgttttg 3′ |
| 44 | 23.EED/AAA.2.66 | 5′ cacgctgcgtgaactttttgcc 3′ |
| 45 | 23.E83A.1.67 | 5′ gcattctacgaggacatgttttgtgccttttcaaaatacggtgaagttgaac 3′ |
| 46 | 23.E83A.2.68 | 5′ cacaaaacatgtcctcgtagaatgc 3′ |
| 47 | 23.EEDED/5A.1.70 | 5′ ggcaaaaagttcacgcagcgtgcccttgcagcccaatttgctgcattctacgccgctatgttttgtgagttttcaaaatacgg 3′ |
| 48 | 23.F73A.1.71 | 5′ cgcagcgtgaacttgcagaacaagctgatgcattctacgaggacatg 3′ |
| 49 | 23.Y77S.1.72 | 5′ cttgcagaacaatttgatgcattctccgaggacatgttttgtgagttttc 3′ |
| 50 | 23.Y77S.2.73 | 5′ agaatgcatcaaattgttctgcaag 3′ |
| 51 | 23.F81A.1.74 | 5′ caatttgatgcattctacgaggacatggcttgtgagttttcaaaatacggtgaag 3′ |
| 52 | 23.F81A.2.75 | 5′ catgtcctcgtagaatgcatcaaattg 3′ |
| 53 | 23.W128A.1.76 | 5′ gctattgatgatttaaattcccgagcctattcccaaagaccagtctacgc 3′ |
| 54 | 23.W128F.177 | 5′ gctattgatgatttaaattcccgattttattcccaaagaccagtctacgc 3′ |
| 55 | 23.W128-.2.78 | 5′ tcgggaatttaaatcatcaatagc 3′ |
| 56 | 23.C148A.1.80 | 5′ ctgtaactgattttcgcgaggcagcttgccggcaacacgaaacttcg 3′ |
| 57 | 23.C148-.2.81 | 5′ tgcctcgcgaaaatcagttacag 3′ |
| Oligonucleotides for uaf1+ overlap extension PCR mutagenesis | ||
| 60 | prp2.W135A.1 | 5′ cagtggaaaaggaagcgctctttagccgacattaaaccacctggttatg 3′ |
| 61 | prp2.W135F.1 | 5′ cagtggaaaaggaagcgctctttatttgacattaaaccacctggttatg 3′ |
| 62 | prp2.W135-.2 | 5′ taaagagcgcttccttttccactg 3′ |
| 63 | prp2.pcr.NcoI.5′ | 5′ tcattcactaccatggatttgtcttccaga 3′ |
| Primers for making two-hybrid and modified RNA three-hybrid constructs | ||
| 64 | U2AF23.5.PCR | 5′ ccatatacatatggcaagtcatttggc 3′ |
| 65 | U2AF23.11.F | 5′ atataaaaccatggcaagtcatttggcaag 3′ |
| 66 | U2AF23.12.R | 5′ ctatggatccttaatttttgcgttcagcagttac 3′ |
| 67 | U2AF23.13.PCR | 5′ ggtatcctaggataaattaaaaacgcaagtc 3′ |
| 68 | U2AF23.RI.5′ | 5′ gccggaattcgcaagtcatttggcaagtatttatg 3′ |
| 69 | U2AF23.RI.3′ | 5′ gccggaattcttaatttttgcgttcagcagttacactgac 3′ |
| 70 | U2AF23.NotI.5′.27 | 5′ aaggaaaaaagcggccgcaatggcaagtcatttggcaagtatttatgg 3′ |
| 71 | U2AF23.NotI.3′.28 | 5′ ttccttttttgcggccgcttaatttttgcgttcagcagttacac 3′ |
| 72 | U2AF35.RI.5′ | 5′ gccggaattcgcggagtatctggcctccatc 3′ |
| 73 | U2AF35.RI.3′ | 5′ gccggaattctcagaatcgcccagatctttcacg 3′ |
| 74 | psr2.5.NcoI | 5′ acatgccatggcctcggagactagattgtttgttggc 3′ |
| 75 | psr2BamHI3′ | 5′ cgcggatccttaccattcagcagcgacctgtcc 3′ |
| 76 | SRp55.NcoI.5′ | 5′ acatgccatgggaccgcgcgtctacataggacgc 3′ |
| 77 | SRp55.BamHI.3′ | 5′ cgcggatccttaatctctggaactcgacctgg 3′ |
| 78 | ASF/SF2.NcoI.5′ | 5′ acatgccatgggttcgggaggtggtgtgattcgtg 3′ |
| 79 | ASF2/SF2.BamHI.3′ | 5′ cgcggatccttatgtacgagagcgagatctgc 3′ |
| 80 | ID4.05c_aXmaIS | 5′ ccgggaatctctattctttaggtacctggttat 3′ |
| 81 | ID4.05c_aClaIAS | 5′ cgataaccaggtacctaaagaatagagatt 3′ |
| Primers for RT-PCR splicing assays | ||
| 82 | cdc16-2.exon2 | 5′ ttattggataaatgccttgaagtcc 3′ |
| 83 | cdc16-2.exon3 | 5′ tcggaaaataaacatttgtgcaatc 3′ |
Mutant uaf2 alleles were generated by either site-directed mutagenesis on single-stranded DNA (the N-terminal CCH/SSI, C-terminal CCH/SSI, Q174STOP, and charged-to-alanine mutations [with the exception of D14A and K15A]) with oligonucleotides 2 to 12 (Table 1) or overlap extension PCR with Platinum pfx DNA polymerase (Invitrogen Corp., Carlsbad, Calif.) and the flanking primers 13 and 14 in combination with the appropriate internal primers (primers 13 to 57) listed in Table 1. All mutant alleles were subcloned into the shuttle vector pURT2 and confirmed by DNA sequencing (Cleveland Genomics). The plasmid uaf2+/pREP82 was constructed by inserting a PCR fragment generated using primers 64 and 67 into the NdeI and BamHI sites of pREP82 (6). The corresponding large-subunit plasmid uaf1+/pREP81 was constructed by transferring the NdeI-BamHI fragment from pREP2-Prp2 (35) to pREP81.
U2AFSM two-hybrid constructs were prepared by PCR amplification using primers 65 and 66, cleavage with NcoI and BamHI, and subcloning into pACT2 (BD Sciences Clontech, Palo Alto, Calif.). The Uaf1p (U2AFLG) coding region was amplified using primers prp-Nde and prp-Bam (35), and the resulting fragment was inserted into pAS2-1. To analyze heterodimer formation and SR protein interactions, we amplified the U2AFSM coding region with primers 68 and 69 and inserted the PCR fragment into the EcoRI site of pBRIDGE (BD Sciences Clontech). The analogous construct for human small subunit was made by amplifying the cDNA from pREP2-hU2AF35 (35) with primers 72 and 73 and insertion into pBRIDGE. Constructs to express Srp2p, SRp55, and ASF/SF2 were made by amplifying cDNAs (kindly provided by J. Bruzik and J. Bokar, Case Western Reserve University) with the appropriate pairs of primers 74 to 79 (Table 1).
For RNA three-hybrid analyses, we modified the protein expression vector by inserting a 980-bp StuI fragment containing the MET2 promoter and PGK1 terminator from pBRIDGE into the blunted NotI sites of pACT2. The uaf1+ coding region was amplified with primer 63 (Table 1) and prp-Bam and inserted between the NcoI and BamHI sites, while uaf2+ wild-type and mutant alleles were amplified from the pURT2 constructs (described above) with primers 70 and 71 and inserted into the NotI site. To express the sequence of a fragment from the intron within open reading frame SPAC1D4.05c (hereafter designated I-1D4) in the modified RNA three-hybrid system, the 5′ phosphorylated oligonucleotides 80 and 81 were annealed and inserted into the XmaI and ClaI sites of p4130 (kindly provided by J. Woolford, Carnegie Mellon University; described in reference 10) to generate the plasmid ID4/pIII MS2-1B,H,C.
S. pombe manipulations.
To disrupt one copy of the uaf2+ locus, the recipient diploid strain SpDS3 (ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-d18/ura4-d18) (35) was transformed with 10 μg of a gel-purified linear XhoI-NdeI fragment from U2AF23::LEU2/pBSK+. Screening for stable integrants yielded five candidates, of which only one demonstrated a pattern consistent with integration at the uaf2+ locus (Fig. 1). Correct integration in the SpCW1 strain was confirmed by a second Southern blotting using SalI to cleave both inside and outside the integration boundaries. To determine whether U2AFSM is essential for life in S. pombe, we used random spore analysis (32). Complementation assays and plasmid recovery were performed as previously described (35). Briefly, SpCW1 was transformed with plasmids containing the wild-type uaf2+ gene (UAF2-pURT2 and UAF2-pREP2), the human U2AF35 cDNA (U2AF35-pREP2), or the corresponding empty vectors (pURT2 and pREP2). The untransformed recipient strain, as well as the five plasmid-bearing strains, were sporulated and tested for growth on defined media as described in the footnotes to Table 2.
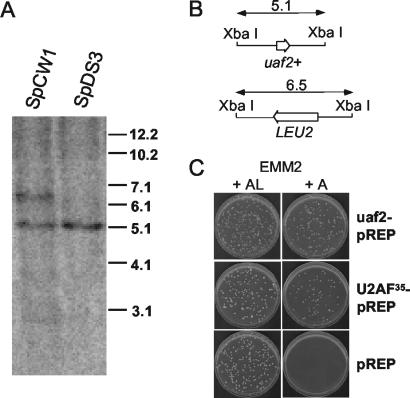
FIG. 1.
Analysis of a diploid S. pombe strain heterozygous for the uaf2::LEU2 deletion-replacement allele. (A) Genomic Southern blot of DNA from SpCW1 (see Materials and Methods) and its parent strain, SpDS3. The probe was pBSK+, containing the 5.1-kb XbaI fragment labeled by random priming. In SpCW1, replacement of one copy of the uaf2+ gene with LEU2 increases the size of the endogenous XbaI fragment (5.1 kb) by 1.4 kb, producing a 6.5-kb fragment containing the uaf2::LEU2 null allele. The second allele of uaf2+ remains a 5.1-kb fragment. In the parental diploid SpDS3 (3), both copies migrate at 5.1 kb. (B) Schematic representations of the wild-type and deletion-replacement alleles of uaf2+. (C) Plate assay demonstrating complementation of the fission yeast small-subunit null allele by plasmids expressing either S. pombe or human U2AF35. Empty vector was used as a negative control. A, adenine; L, leucine. See the legend to Table 2 for details.
TABLE 2.
Results of random spore and plasmid complementation analyses on an S. pombe strain heterozygous for gene disruption at the uaf2+ locusa
| Diploid strain sporulated | No. of haploids analyzed | No. of colonies on EMM2 + AUb | No. of colonies on EMM2 + ALc | No. of colonies on EMM2 + Ad | No. of colonies on YEAe |
|---|---|---|---|---|---|
| uaf2::LEU2f | 90 | 0 | 0 | 0 | 90 |
| uaf2::LEU2/UAF2-pURT2 | 90 | 52g | 90 | 52g | 90 |
| uaf2::LEU2/UAF2-pREP2 | 90 | 40g | 90 | 40g | 90 |
| uaf2::LEU2/U2AF35-pREP2 | 90 | 46g | 90 | 46g | 90 |
| uaf2::LEU2/pURT2 | 90 | 0 | 90 | 0 | 90 |
| uaf2::LEU2/pREP2 | 90 | 0 | 90 | 0 | 90 |
Spores resulting from plasmid transformations were initially plated on EMM2 + AL minimal medium (32) to select for growth of plasmid-containing haploids; these cells were then tested for growth on the media defined in the following footnotes.
Minimal medium supplemented with adenine and uracil (each at 100 mg/liter). Cells harboring the disrupted allele will grow on this medium only when the gene product is not essential for life or when the plasmidborne gene can complement the gene disruption. Cells that do not contain the uaf2+::LEU2 allele will not grow due to lack of complementation for the leu1-32 mutation.
Minimal medium supplemented with adenine and leucine (each at 100 mg/liter). Cells harboring the disrupted allele will grow on this medium only when the gene product is not essential for life or when the plasmidborne gene can complement the gene disruption. Cells that do not contain the uaf2+::LEU2 allele will still grow due to the presence of leucine in the medium. Prototrophy for uracil is provided by the plasmidborne ura4+ gene.
Minimal medium supplemented with adenine at 100 mg/liter. Cells harboring the disrupted allele will grow on this medium only when the gene product is not essential for life or when the plasmidborne gene can complement the gene disruption. Cells that do not contain the uaf2+LEU2 replacement will not grow due to auxotrophy for leucine. Prototrophy for uracil is provided by the plasmidborne ura4+ gene.
Rich medium (YEA) (35). Growth on this medium allows confirmation that the cells selected for analysis are haploid by color assay (adenine auxotrophs are pink or red).
Spores derived from the untransformed SpCW1 diploid were harvested from rich medium (YEA) and tested for growth on the minimal medium plates. These cells are unable to grow on EMM2 + AU medium due to auxotrophy for leucine, EMM2 + AL medium due to auxotrophy for uracil, or EMM2 + A medium due to auxotrophy for both leucine and uracil.
The number of spores able to grow on EMM2+AU and EMM2+A is expected to be 45, and the variation observed here is typical (see, e.g., references 3 and 32). The numbers in the AU and A columns are identical due to replica plating.
Metabolic depletions (3) employed 20 μM thiamine. Splicing of the second intron of cdc16+ was assayed using semiquantitative (22-cycle) reverse transcription-PCR (RT-PCR) (36) with primers 82 and 83.
Two-hybrid and modified RNA three-hybrid interaction analyses.
Two-hybrid plasmids encoding binding domain and activation domain fusions were cotransformed into S. cerevisiae strain PJ69-4A (MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ) (20) as described previously except that dimethyl sulfoxide was added to the final incubation step. For three-hybrid assays, the constructs described above were cotransformed into the L40 S. cerevisiae strain (MATa ura3-52 leu2-3,112 his3Δ200 trp1Δ1 ade2 LYS2::(lexAop)-HIS3 ura3::(lexAop)-lacZ) (44), kindly provided by J. Woolford. Duplicate transformants were grown to mid-log phase and replica plated onto media containing various amounts of 3-AT (3-amino-triazole).
RESULTS
Human U2AF35 can substitute for fission yeast U2AFSM in vivo.
To pave the way for using genetic complementation to assay the functions of mutant or heterologous U2AF small subunits, we first constructed a diploid strain of S. pombe in which one copy of the gene (designated uaf2+, following the nomenclature adopted for C. elegans U2AF) (63) was replaced with a nutritional marker. A transformant carrying the deletion-replacement allele correctly integrated at one of the chromosomal loci was identified by Southern blotting (Fig. 1A and B). The results of random spore analysis indicate that the protein encoded by this locus is required for viability in S. pombe, as no Leu+ spores survived (Fig. 1C and Table 2). This result is consistent with earlier work showing that the Drosophila and C. elegans orthologs (dmU2AF38 and Uaf2, respectively) are essential for life (37, 63) and with the previous demonstration by Romfo et al. that disruption of the U2AF large-subunit gene in S. pombe (initially designated prp2+ by Potashkin et al. [33] but here renamed uaf1+) is lethal (35).
Spores carrying the uaf2::LEU2 null allele are rescued by plasmidborne wild-type uaf2+ either expressed from its own promoter (Table 2) or inserted between transcriptional control elements derived from the thiamine-repressible nmt1 (for “no message on thiamine”) gene (Fig. 1C and Table 2). To test whether the nonconserved C terminus of U2AFSM has a required function in vivo, we made the Q174STOP mutation, which eliminates the entire domain (see Fig. 3). The truncated protein supports wild-type growth at all temperatures examined (18, 30, and 37°C). Although the C-terminal RS domain of Drosophila U2AF38 is also dispensable, this is no longer true when the large subunit also lacks an RS domain (39).
Because the C terminus is the only region that diverges significantly between fission yeast and human U2AF35, this result prompted us to test whether the two proteins are functionally conserved. Remarkably, expression of the human U2AF35 cDNA under nmt1 control compensates for the absence of the native uaf2+ gene (Fig. 1C and Table 2). This result contrasts with the earlier finding by Romfo et al. that expressing human U2AF large subunit did not reverse the lethality of the S. pombe large-subunit deletion (35). Functional conservation parallels sequence conservation, as the small subunits display more extensive amino acid similarity than do the large subunits (33, 50) (see Fig. 3; see also Fig. 1 of reference 35).
Metabolic depletion of S. pombe U2AFSM produces a splicing defect in vivo.
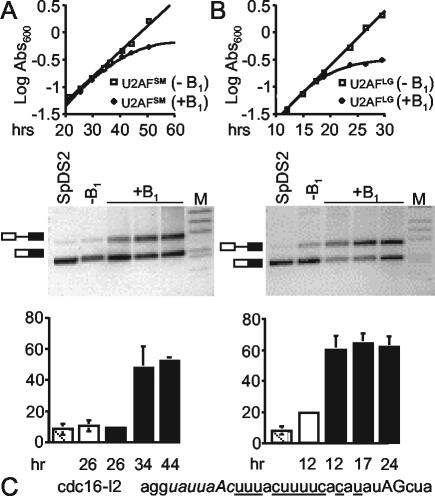
To determine whether U2AFSM is required for splicing in S. pombe, we used an attenuated version of the nmt promoter, which does not produce detectable levels of mRNA under repressing conditions (6, 11). Figure 2A (top panel) shows that growth became noticeably impaired approximately 36 h after thiamine addition (compared to the results seen with same strain grown without the vitamin) and had nearly ceased after 50 h. To determine whether the growth defect could be due to impaired pre-mRNA splicing, we assayed removal of the second intron of cdc16+ (cdc16-I2) at the time points indicated by semiquantitative RT-PCR (36). As shown in Fig. 2A (middle panel), a comparable accumulation of precursor relative to the results seen with mature RNA is observed at 34 and 44 h after thiamine addition. Quantitation (Fig. 2A, bottom panel) demonstrates that the splicing defect is significant compared to the results seen with either the same strain growing logarithmically in the absence of thiamine or an otherwise isogenic haploid strain with wild-type uaf2+ in the chromosome and no plasmid.
FIG. 2.
Effects of metabolically depleting either subunit of S. pombe U2AF. (A) Growth and splicing data for Uaf2p (U2AFSM). (Top panel) Growth curves in the presence (+B1) or absence (−B1) of thiamine for a haploid strain in which the uaf2+ gene disruption is complemented by a plasmid carrying wild-type uaf2+ expressed from the thiamine-repressible nmt1 promoter. Abs600, absorbance at 600 nm. (Middle panel) RT-PCR splicing assays on cdc16-I2 in cells harboring uaf2+ under thiamine control. Total RNA was isolated from the depletion strain at the time points indicated (in hours) after addition of B1 and at the first time point in the absence of B1. Total RNA was also extracted from the control haploid strain SpDS2 (4) at an A600 of 1.0. Mock RT-PCRs lacking reverse transcriptase indicated no contamination with genomic DNA (data not shown). (Bottom panel) Quantitation of the splicing assays depicted in the middle panel expressed as percentages of precursor accumulation [precursor RNA/(precursor mRNA + mature mRNA) × 100]. The levels of precursor and mature message were determined using Bio-Rad Quantity One gel documentation software (version 4.1.1). Error bars indicate standard deviations for three splicing assays. (B) Results are presented as described for panel A except that the data are for a strain in which Uaf1p (U2AFLG) was undergoing metabolic depletion. (C) Sequence of the cdc16-I2 pre-mRNA from the branchpoint (italic characters) to three bases downstream of the 3′ AG (uppercase characters). Designations for pyrimidines between the branchpoint and 3′ splice site are underlined.
To compare the contributions of the large and small subunits to splicing in vivo, we metabolically depleted U2AFLG and examined the effect on splicing of cdc16-I2. Figure 2B (top panel) shows that addition of thiamine to a strain in which the sole copy of uaf1+ is under nmt control results in a decreased rate of growth after approximately 19 h, with a plateau after approximately 24 h. Analysis of splicing by RT-PCR (Fig. 2B, middle and bottom panels) demonstrates that as seen with the small-subunit depletion, cdc16-I2 precursor accumulates prior to an observable growth defect, consistent with a cause-and-effect relationship. Because growth reproducibly slowed sooner during large-subunit depletions, we tested whether the effects of depleting the small subunit might be an indirect effect of destabilizing the large subunit. Counter to this possibility, the results of Western blot analysis with antibody directed against the S. pombe large subunit (35) demonstrated that U2AFLG levels remain constant over the course of the U2AFSM metabolic depletion (data not shown). Thus, the small subunit of U2AF is not essential to the stability of the large subunit, in accord with nuclear magnetic resonance data indicating that the large subunit does not undergo gross structural rearrangements upon binding to its partner whereas the small-subunit ΨRRM adopts a stable, defined structure only in the context of the heterodimer (23).
A single charged-to-alanine mutation in the small subunit of S. pombe U2AF is lethal.
To identify residues and domains required for U2AF small-subunit function in vivo, phenotypes were determined for 38 independent mutant alleles (Fig. 3). We first targeted all seven consecutive pairs of charged amino acids that are conserved between the fission yeast and human proteins in a charged-to-alanine scanning mutagenesis strategy (51). Among these mutations, only the [D14A, K15A] substitution located just upstream from the proposed metal ligands in the amino-terminal ZBD was lethal (Fig. 3). In contrast, [R145A, E146A], which affects a similar pair of amino acids in the second ZBD, was viable. Because charged-to-alanine mutagenesis was devised to target residues that contact other macromolecules (51) and because the small subunit of U2AF has multiple binding partners (see the introduction), we were surprised that six of the seven alleles examined displayed normal growth at standard, high, and low temperatures (Fig. 3). To exclude the possibility that these mutations produce dominant lethality (for an explanation, see reference 4), we recovered plasmids and sequenced them; all mutations were genetically stable (data not shown). Thus, we conclude that the small subunit of U2AF is resistant to charged-to-alanine mutational inactivation.
The ΨRRM is critical for U2AFSM function in vivo.
To assess the importance of the ΨRRM, we constructed a deletion that removes approximately 90% of the domain (ΔP55-P133) (Fig. 3). As expected on the basis of evidence that interactions between the large and small subunits are critical in Drosophila melanogaster (38), the ΨRRM deletion is lethal in S. pombe (Fig. 3). Because the two subunits of human U2AF engage in an extensive molecular embrace centered on reciprocal aromatic-aliphatic stacking interactions involving tryptophans (24), we were surprised that replacing the corresponding S. pombe U2AFSM tryptophan, W128, with either phenylalanine or alanine produces no discernible effect on growth (Fig. 3). These and all other mutant phenotypes were tested multiple times with independent transformants, and recovered plasmids were shown to have the correct sequences. Further reinforcing the view that W128F and W128A do not dramatically reduce the interaction with U2AFLG, the mutants show signals indistinguishable from the wild type in the yeast two-hybrid system (Fig. 4). Finally, to completely convince ourselves that mutating W128 produces no functional consequences, we performed a competitive growth assay (59) in which a strain carrying the plasmidborne W128A allele was grown together with an isogenic wild-type strain at 30 and 37°C. We then isolated plasmids at various time points up to 60 generations and determined the fractions of mutant and wild-type sequences present. The small (<4%) decline in the level of the mutant alleles in the population was within the error of the measurements. Thus, we conclude that the presence of an aromatic residue at position 128 is not crucial for U2AFSM function in vivo.
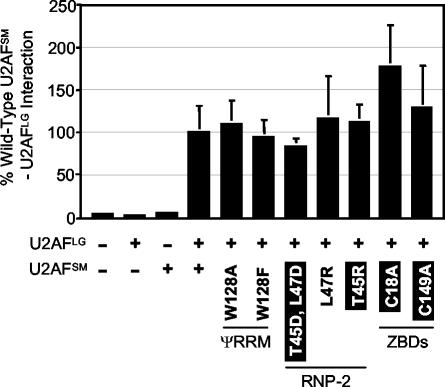
FIG. 4.
Two-hybrid analyses of protein-protein interactions between wild-type U2AFLG and wild-type and mutant U2AFSM. The figure shows the results of β-galactosidase assays, with the data expressed as percentages of the wild-type U2AFSM-U2AFLG interactions. For reference, the phenotypic consequences for S. pombe of these alleles are indicated according to the key for Fig. 3. Error bars indicate standard deviations (n = 3).
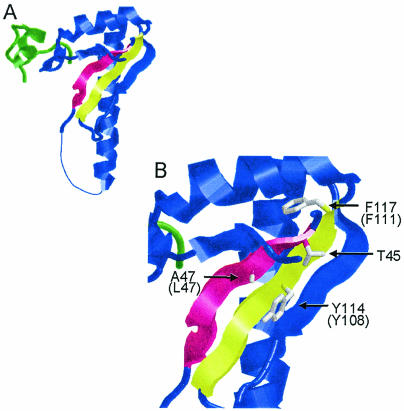
Despite lacking the large-subunit RRMs, which contribute the majority of RNA binding energy for intact human U2AF (19, 58), the minimal heterodimer was able to bind weakly to an adenovirus-major late RNA fragment containing a polypyrimidine tract and a 3′ splice site (24). To explain this finding, these authors proposed a model in which two residues in and near an RNP2-like hexapeptide and one residue within an RNP1-like octapeptide in the small-subunit ΨRRM interact with RNA. To determine whether the corresponding amino acids in U2AFSM are functionally important in S. pombe, we mutated the amino acids indicated on the ribbon diagram in Fig. 5B. Individual replacements of T45 and L47 with aspartic acid are viable; however, simultaneous replacement of both residues is lethal. Replacement of T45 with arginine is also lethal, while an arginine at L47 (which is an alanine in the human ortholog) allows normal growth (Fig. 3). Despite the conservation of Y108, replacing it with either a small nonpolar residue (Y108A) or a charged residue (Y108D) is tolerated (Fig. 3). This amino acid is analogous to aromatic residues in other RRM proteins that stack with the heterocyclic rings of the nucleotide bases (49). Because the mutations we introduced are incompatible with such a function, our data suggest that Y108 contacts are not crucial for the contribution of U2AFSM to RNA binding by the heterodimer.
FIG. 5.
(A) Three-dimensional ribbon diagram of the domain of the human U2AF minimal heterodimer. (B) Close-up image of the U2AF35 ΨRRM region proposed to contact RNA. The β-sheet containing the RNP1-like peptide is colored yellow, and the β-sheet containing the RNP2-like peptide is shown in red. Amino acids corresponding to those mutated in the present study are shown as stick figures to allow the orientations of side chains to be visualized. Numbers and identities of the amino acids in the small subunit of S. pombe U2AF are given in parentheses where they differ from the human ortholog. The images were prepared using RasMol software (version 2.7.2.1) (42) and the coordinates supplied by Kielkopf et al. (24).
A second, generally aromatic, residue that participates in RNA binding via intercalation in canonical RRMs is oriented in the opposite direction in ΨRRMs, rendering an interaction with RNA unlikely (49) (Fig. 5B). This residue is nevertheless functionally important (as determined on the basis of mutagenic studies of other ΨRRMs, including the one in Mud2p) (1). In the small subunit of S. pombe U2AF, mutating F111 to alanine is tolerated but a less conservative substitution with aspartic acid (F111D) is not (Fig. 3). As expected, the [Y108D, F111D] double mutant is lethal whereas changing the same amino acids to alanine produces a temperature-sensitive strain (Fig. 3).
The ΨRRM contributes to RNA binding by the S. pombe U2AF heterodimer.
We next tested whether the ΨRRM of U2AFSM contributes to RNA binding. To circumvent difficulties with purification and to facilitate examination of multiple mutant proteins, we carried out these studies in a modified RNA three-hybrid system (44) engineered to allow production of both subunits of S. pombe U2AF (Materials and Methods). Initial experiments showed that Uaf1p (S. pombe U2AFLG) alone had a lower apparent affinity for an RNA fragment containing the heterologous β-globin 3′ splice site (55) compared to the results seen when it was combined with Uaf2p (S. pombe U2AFSM [data not shown]), in accord with what was observed in vitro for human, Drosophila, and C. elegans U2AF (27, 38, 55, 64). Expression of the small subunit alone produces no signal above background (data not shown). A search of introns annotated by the S. pombe Genome Project (http://www.sanger.ac.uk/Projects/S_pombe/) identified five 3′ splice sites similar in length and pyrimidine content to the β-globin 3′ splice site. Of these, I-1D4 displays the strongest interaction with the S. pombe U2AF heterodimer (Fig. 6 and data not shown).
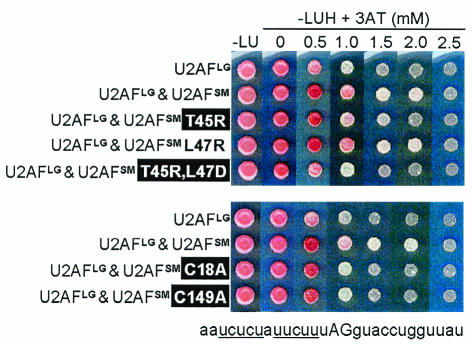
FIG. 6.
Plate assays to test RNA binding in the modified RNA three-hybrid system. The strength of the RNA-protein interaction is reflected by growth in the presence of increasing amounts of 3-AT (9, 44). (Top panel) Effects of mutations in or near the RNP2-like motif of the U2AFSM ΨRRM. (Bottom panel) Effects of mutations in putative zinc ligands of U2AFSM. Phenotypes for S. pombe are indicated as described for Fig. 3. The sequence of the test RNA (I-1D4), which corresponds to the 3′ end of an S. pombe intron (from just downstream of the branchpoint to 10 bases beyond the 3′ AG), is shown at the bottom. All assays were repeated with essentially identical results. L, leucine; U, uracil; H, histidine.
After calibration, we used the modified three-hybrid system to test whether the lethality of substitutions in or near the RNP2-like peptide might reflect diminished RNA binding by S. pombe U2AF. The results indicate that the viable L47R mutation promotes wild-type levels of interaction with the I-1D4 RNA and that the lethal substitution mutations, T45R and [T45D, L47D], decrease affinity for the target RNA (Fig. 6, top panel). Western blot analysis using antibodies directed against the hemagglutinin tag demonstrated that the mutant proteins accumulate to high levels in S. cerevisiae (data not shown). Thus, we conclude that the essential nature of the RNP2-like peptide in the U2AF small subunit is most likely due to its role in RNA binding. Consistent with their involvement in RNA binding, two-hybrid analyses indicate that two of the mutations in this region have no discernible effect on heterodimer formation; even with the [T45D, L47D] double mutation, the reduction is not significant (Fig. 4). Mutations in the amino acid of the RNP1-like peptide proposed by Kielkopf et al. (24) to participate in RNA binding (Y108) did not confer a phenotype in S. pombe and thus were not tested in the modified three-hybrid system.
The small subunit of S. pombe U2AF interacts with an SR protein.
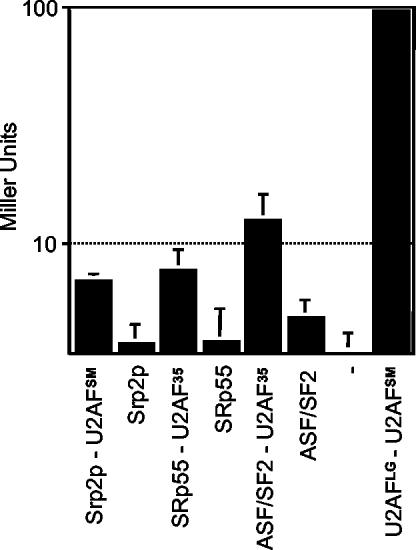
To determine whether the small subunit of S. pombe U2AF interacts with SR proteins, we carried out yeast two-hybrid assays with both known members of this family in fission yeast; for reference, parallel analyses were carried out with human proteins. When β-galactosidase activity was used to quantitate interactions, similar results were observed for several combinations, including human U2AF35-SRp55 and U2AF35-ASF/SF2 and S. pombe U2AFSM-Srp2p (Fig. 7). In contrast, only background levels of activity were observed for U2AFSM-Srp1p (data not shown). The SR protein-U2AFSM interactions are reproducible and yet quite weak relative to the interaction between the large and small subunits of U2AF (7 to 9%), as previously observed (56).
FIG. 7.
Two-hybrid analyses of protein-protein interactions between small-subunit orthologs and SR proteins from S. pombe and humans. The figure shows the results of β-galactosidase assays (expressed in Miller units on a logarithmic histogram). The protein combination assayed in each case is indicated beneath the histogram. The y axis is adjusted to the background activity observed in the host strain harboring empty bait and prey vectors. Error bars indicate standard deviations (n = 3).
On the basis of their crystallographic data, Kielkopf et al. (24) proposed that the charged face of the unusually long amphipathic α-helix of the small-subunit ΨRRM (Fig. 5A) could serve as a docking site for SR proteins via their basic RS domains. Because two of the five amino acids with a conserved negative charge between the human and S. pombe U2AF small subunits had been targeted by the phenotypically silent [E78A, D79A] mutation, we constructed a block change of the remaining three residues to alanine. The [E68A, E71A, D74A] mutation also has no discernible effect on growth, even at high temperature (Fig. 3). In contrast, an extreme quintuple charged-to-alanine mutation [E68A, E71A, D74A, E78A, D79A] did not support life (Fig. 3). This mutant could not be analyzed in the yeast two-hybrid system, unfortunately, as the protein was not stably produced in S. cerevisiae (data not shown).
Kielkopf et al. (24) also proposed that three conserved aromatic residues located on the hydrophobic face of the amphipathic helix might form a solvent-exposed π-stacked array suitable for interacting with another protein. In contrast to this model, we found that mutating the middle aromatic amino acid to serine (Y77S) does not affect growth. However, alanine substitutions at either of the outside amino acids in the proposed array (F73 and F81) as well as a triple mutation are lethal, indicating that this region plays a critical role in U2AFSM structure or function in vivo.
Both ZBDs of S. pombe U2AFSM are required for viability.
Despite strong conservation of the ZBDs of the U2AF small subunit (24) (Fig. 3), a human heterodimer consisting of full-length large subunit and only the central ΨRRM of the small subunit was reported to support splicing in a HeLa cell extract depleted of U2AF (16). To test whether the ZBDs are required for small-subunit function in vivo, we mutated the amino acids proposed to coordinate zinc. First, the N-terminal cysteine of the upstream ZBD was replaced with alanine (C18A). The lethal phenotype demonstrates the importance of this residue and, by inference, of the domain (Fig. 3). In contrast, the analogous substitution (C148A) within the carboxy-terminal ZBD (ZBD II) confers wild-type growth. The unexpected viability of C148A prompted us to make an alanine substitution at the cysteine just downstream, C149, which is also conserved between the fission yeast and human proteins (Fig. 3). In similarity to the results seen with C18A, this substitution is lethal, suggesting that C149 rather than C148 may actually be the first zinc ligand in the second metal-binding domain. In contrast, replacing the terminal coordinating histidine residue with alanine in either ZBD allows wild-type growth (Fig. 3).
In an effort to identify other residues important for the function of ZBDs I and II in vivo, we next mutated tyrosine 22 and histidine 152 to lysine (Fig. 3). Neither substitution produces a detectable phenotype, in contrast to the results seen with a Y-to-K substitution at the analogous position of the second ZBD of Nup475, which reduced its affinity for metal ions (53). Finally, we made two triple substitutions, [C27S, C33S, H37I] and [C157S, C163S, H167I], which change the last three coordinating residues of each ZBD. A histidine-to-isoleucine substitution had previously been found to diminish the function of Zfs1p, an S. pombe protein that harbors tandem copies of the YKTELCX8CX5CX3H zinc-binding motif (22). While the triple substitution in ZBD I is lethal, the same mutation in ZBD II supports growth (Fig. 3), demonstrating that the two ZBDs are not equivalent. Although viable, the ZBD II triple mutant grows very slowly even at 30°C, is completely unable to grow at 37°C, and displays an aberrant morphology (unpublished data).
The ZBDs also contribute to RNA binding.
To determine whether the lethality associated with mutating coordinating cysteines within each ZBD might be due to effects on RNA binding, we used the modified three-hybrid system. As illustrated in Fig. 6 (bottom panel), both the C18A and the C149A mutations reduce binding to the level observed with the large subunit alone. Western blot analysis demonstrates that these mutant proteins accumulate to high levels in S. cerevisiae (data not shown), indicating that the RNA binding defects do not simply reflect protein instability. The inability of the ZBD mutants to promote RNA binding is also not due to a defect in heterodimerization, as two-hybrid analyses demonstrate that the C18A and C149A mutations result in heterodimers with U2AFLG at wild-type levels (Fig. 4). Taken together, our reverse genetic analysis in S. pombe and RNA three-hybrid data demonstrate that the two ZBDs are independently required for RNA binding by the U2AF heterodimer.
DISCUSSION
The small subunit of U2AF functions in splicing in vivo.
Our extensive mutational analysis of the small subunit of fission yeast U2AF yielded several important conclusions about the function and evolution of this protein. First, U2AFSM is essential for life in S. pombe, in accord with the indispensability of its counterparts in D. melanogaster (37) and C. elegans (63). Second, metabolic depletion experiments provide evidence that the essential nature of the small subunit is due to its role in splicing. Third, mutational analyses indicate that the ΨRRM is required for life; however, while several residues implicated in RNA binding are critical, surprisingly, a tryptophan proposed to provide a key contact with the large subunit is not. Fourth, the effects of mutations in the two ZBDs indicate that each performs a nonredundant, indispensable role that most likely reflects participation in RNA binding. Because the small subunit of human U2AF can functionally replace its fission yeast orthologue, these results have implications for splicing in more complex eukaryotes.
In light of in vitro data indicating that the requirement for the small subunit of human U2AF correlates inversely with the strength of the polypyrimidine tract (reviewed in reference 28), we were somewhat surprised to find that the substrate used for our metabolic depletion experiments was highly dependent on the small subunit of S. pombe U2AF. As illustrated in Fig. 2C, the 3′ splice site of cdc16-I2 has a fairly strong polypyrimidine tract well above average for S. pombe (unpublished data). In an effort to reconcile our observation that depleting either subunit of S. pombe U2AF produced similar effects on cdc16-I2 splicing with studies of U2AF-depleted HeLa cell extracts, which show that substrates with strong polypyrimidine tracts can be spliced after addition of U2AF65 alone (reviewed in reference 28), we are carrying out splicing assays on a diverse panel of S. pombe pre-mRNAs.
Evolution of the U2AF small subunit.
Our genetic complementation data demonstrate that the core function(s) of the U2AF small subunit has been preserved since S. pombe split from animals and plants 1 to 1.2 billion years ago (45, 52). Although S. cerevisiae and S. pombe diverged comparatively recently (330 to 420 million years ago) (45), budding yeast has apparently shed the U2AF small subunit and minimized the requirement for the large subunit (1). S. pombe has also retained other trans-acting factors implicated in the formation of early 3′ splicing complexes, including canonical SR proteins (14, 26). It is tempting to speculate that the reduced intron number and virtual absence of multiintronic precursors in budding yeast (46) compared to the results seen with fission yeast (52) might account for the divergence of early 3′ splice site recognition factors. Because both the small subunit of U2AF and SR proteins are part of multigene families in metazoans (8, 25, 29, 47), we hypothesize that this expanded repertoire makes possible the complex regulation of splicing required for multicellular life.
While an interaction between the small subunit of U2AF and SR proteins is conserved between S. pombe and metazoans, the domain most recently proposed to mediate this interaction (24) is refractory to mutations. Although several mutations that significantly reduce the net negative charge support life, however, we cannot exclude the possibility that the most severe mutations, which diminish protein stability, might affect function when produced at normal levels. Alternatively, it is conceivable that our mutations disrupt a nonessential interaction: to our knowledge, the binding of the small subunit of U2AF to SR proteins has not yet been demonstrated to be essential in any organism.
The ΨRRM: a protein interaction domain that also functions in RNA binding.
The ΨRRM has also been designated the PUMP (for “PUF60, U2AF, Mud2p protein-protein interaction”) domain on the basis of its ability to mediate exceptionally strong homo- or heterodimeric contacts between proteins (31). Our data demonstrate that this domain also contributes to RNA binding, as suggested earlier by Kielkopf et al. (24). In fact, the effects of mutations in U2AFSM validate certain predictions of their model, which was based on structural data for the canonical HuD RRM (49). First, substituting arginine for threonine at position 45 is lethal, possibly due to the large guanidino side chain interfering with RNA binding. Second, although the leucine at position 47 is refractory to single substitutions, we reasoned that it might nevertheless contribute to RNA binding, as RRMs make multiple contacts with RNA. In consistency with this idea, combining the viable L47D and T45D mutations produces intra-allelic synergistic lethality in S. pombe and reduces RNA binding in three-hybrid assays. In contrast, single substitutions of the amino acid within the RNP1-like peptide predicted by Kielkopf et al. (24) to contact RNA demonstrate that it is not critical for life. However, a double RNP1 mutant displays conditional lethality, thus providing a valuable reagent to characterize further the role of U2AFSM in vivo.
In light of the crystallographic data implicating W128 in a key contact with the large subunit, we were surprised that mutating this amino acid to alanine produces no discernible effect either in S. pombe or in the two-hybrid system. In the context of the minimal heterodimer, the analogous mutation in human U2AF35 ΨRRM reduced its affinity for the U2AF65 fragment from the nanomolar to the millimolar range (24). The lack of a phenotype for W128 seems unlikely to reflect differences between the fission yeast and other orthologs, as the two subunits are tightly complexed in S. pombe (18) and deletion analyses indicate that the regions make contact are the same as those in other species (40, 50, 60). Moreover, recently published sequence data indicate that this amino acid in the small subunit of U2AF is not invariably aromatic, as originally believed (48). While even a small aliphatic residue is tolerated in fission yeast U2AFSM, intriguingly, the aromatic character of the U2AFLG tryptophan implicated in the tongue-in-groove interaction appears to be more critical, as a phenylalanine substitution is permissible but replacement with alanine is lethal (unpublished data). Our finding that the presence of a single tryptophan is apparently sufficient to mediate the interaction between the large and small subunits of U2AF in vivo may be related to recent structural data for an SF1-branchpoint bridging protein (BBP) peptide complexed with the ΨRRM-PUMP domain of the large subunit of U2AF. In this interface, a tryptophan from BBP is inserted into a hydrophobic binding pocket on the large subunit of U2AF in a manner strikingly reminiscent of that seen with the U2AF subunit interface; however, the reciprocal tryptophan is absent (43).
The ZBDs of the U2AF small subunit are essential to its function in vivo and are required for maximal RNA binding by the heterodimer.
Our mutagenesis data demonstrate that the two ZBDs of the U2AF small subunit are independently required for its function in vivo. Taken at face value, these observations appear incompatible with a report that a heterodimer incorporating only the ΨRRM of the small subunit can reconstitute a U2AF-deficient splicing extract in vitro (16). While it is possible that the difference reflects a functional distinction between U2AF small subunits in S. pombe and larger eukaryotes, the high degree of sequence identity and (especially) our finding of functional conservation argue against this explanation. As noted by Guth et al. (16), the level of recombinant U2AF required to reconstitute activity in oligo(dT)-depleted nuclear splicing extracts exceeds the endogenous level of U2AF. Thus, a surplus of exogenously added U2AF heterodimer containing only the ΨRRM of the small subunit may drive spliceosome formation in the absence of the additional binding energy afforded by ZBD I and ZBD II.
Our finding that mutations in the ΨRRM or in either ZBD of U2AFSM inhibit its contribution to RNA binding by the heterodimer is reminiscent of earlier data showing that multiple domains of the BBP are required for high-affinity RNA binding (7). More importantly, these data may provide an explanation for the intriguing observation that the small subunit of U2AF cross-links to an unexpectedly long stretch of RNA extending into the 3′ exon (27, 55). It will be interesting to learn whether (as for BBP) (7) one domain makes sequence-specific contacts with the 3′ AG while the others contribute to affinity but not specificity.
In summary, we have carried out the first comprehensive analysis of the U2AF small subunit in vivo, providing a wealth of new insights into the role of this multifaceted splicing factor.
Acknowledgments
We thank Joseph Bokar, James Bruzik, and John Woolford for materials and Nicole Sample for expert technical assistance. We are grateful to Jonatha Gott, Michael Harris, Hua Lou, David McPheeters, Helen Salz, and members of our laboratory for critical comments on the manuscript.
This research was supported by a grant to J.A.W. from the National Institutes of Health.
REFERENCES
- 1.Abovich, N., X. Liao, and M. Rosbash. 1994. The yeast MUD2 protein: an interaction with PRP11 defines a bridge between commitment complex and U2 snRNP addition. Genes Dev. 8:843-854. [DOI] [PubMed] [Google Scholar]
- 2.Albers, M., A. Diment, M. Muraru, C. S. Russell, and J. D. Beggs. 2003. Identification and characterization of Prp45p and Prp46p, essential pre-mRNA splicing factors. RNA 9:138-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althoff, S. M., S. W. Stevens, and J. A. Wise. 1994. The Srp54 GTPase is essential for protein export in the fission yeast Schizosaccharomyces pombe. Mol. Cell. Biol. 14:7839-7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez, C. J., C. M. Romfo, R. W. VanHoy, G. L. Porter, and J. A. Wise. 1996. Mutational analysis of U1 function in Schizosaccharomyces pombe: pre-mRNAs differ in the extent and nature of their requirements for this snRNA. RNA 2:404-418. [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrozkova, M., F. Puta, L. Fukova, M. Skruzny, J. Brabek, and P. Folk. 2001. The fission yeast ortholog of the coregulator SKIP interacts with the small subunit of U2AF. Biochem. Biophys. Res. Commun. 284:1148-1154. [DOI] [PubMed] [Google Scholar]
- 6.Basi, G., E. Schmid, and K. Maundrell. 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123:131-136. [DOI] [PubMed] [Google Scholar]
- 7.Berglund, J. A., M. L. Fleming, and M. Rosbash. 1998. The KH domain of the branchpoint sequence binding protein determines specificity for the pre-mRNA branchpoint sequence. Nucleic Acids Res. 4:998-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domon, C., Z. J. Lorkovic, J. Valcarcel, and W. Filipowicz. 1998. Multiple forms of the U2 small nuclear ribonucleoprotein auxiliary factor U2AF subunits expressed in higher plants. J. Biol. Chem. 273:34603-34610. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann, C. R., B. Kraemer, M. Wickens, and J. Kimble. 2002. GLD-3, a bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev. Cell 3:697-710. [DOI] [PubMed] [Google Scholar]
- 10.Fewell, S. W., and J. R. Woolford. 1999. Ribosomal protein S14 of Saccharomyces cerevisiae regulates its expression by binding to RPS14B pre-mRNA and to 18S rRNA. Mol. Cell. Biol. 19:826-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsburg, S. 1993. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21:2955-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gozani, O., J. Potashkin, and R. Reed. 1998. A potential role for U2AF-SAP 155 interactions in recruiting U2 snRNP to the branch site. Mol. Cell. Biol. 18:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graveley, B., K. J. Hertel, and T. Maniatis. 2001. The role of U2AF35 and U2AF65 in enhancer-dependent splicing. RNA 7:806-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross, T., K. Richert, C. Mierke, M. Lutzelberger, and N. F. Kaufer. 1998. Identification and characterization of srp1, a gene of fission yeast encoding a RNA binding domain and a RS domain typical of SR splicing factors. Nucleic Acids Res. 26:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guth, S., C. Martínez, R. K. Gaur, and J. Valcárcel. 1999. Evidence for substrate-specific requirement of the splicing factor U2AF35 and for its function after polypyrimidine tract recognition by U2AF65. Mol. Cell. Biol. 19:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guth, S., T. Ø. Tange, E. Kellenberger, and J. Valcárcel. 2001. Dual function for U2AF35 in AG-dependent pre-mRNA splicing. Mol. Cell. Biol. 21:7673-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindley, J., G. Phear, M. Stein, and D. Beach. 1987. suc1+ encodes a predicted 13-kd protein that is essential for cell viability and is directly involved in the division cycle of Schizosaccharomyces pombe. Mol. Cell. Biol. 7:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, T., J. Vilardell, and C. Query. 2002. Pre-spliceosomal formation in S. pombe requires a stable complex of SF1-U2AF59-U2AF23. EMBO J. 21:5516-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, T., Y. Muto, M. R. Green, and S. Yokoyama. 1999. Solution structures of the first and second RNA-binding domains of human U2 small nuclear ribonucleoprotein particle auxiliary factor (U2AF65). EMBO J. 18:453-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaar, R., S. E. Roche, E. L. Beall, M. R. Green, and D. C. Rio. 1993. The conserved pre-mRNA splicing factor U2AF from Drosophila: requirement for viability. Science 262:569-573. [DOI] [PubMed] [Google Scholar]
- 22.Kanoh, J., S. Asako, and M. Yamamoto. 1995. Schizosaccharomyces pombe zfs1+ encoding a zinc-finger protein functions in the mating pheromone recognition pathway. Mol. Biol. Cell 6:1185-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellenberger, E., G. Stier, and M. Sattler. 2002. Induced folding of the U2AF35 RRM upon binding to U2AF65. FEBS Lett. 528:171-176. [DOI] [PubMed] [Google Scholar]
- 24.Kielkopf, C. L., N. A. Rodionova, M. R. Green, and S. K. Burley. 2001. A novel peptide recognition mode revealed by the X-ray structure of a core U2AF35/U2AF65 heterodimer. Cell 106:595-605. [DOI] [PubMed] [Google Scholar]
- 25.Lorkovic, Z. J., and A. Barta. 2002. Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 30:623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutzelberger, M., T. Gross, and N. Kaufer. 1998. Srp2, an SR protein family member of fission yeast: in vivo characterization of its modular domains. Nucleic Acids Res. 27:2618-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merendino, L., S. Guth, D. Bilboa, C. Martinez, and J. Valcarcel. 1999. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature 402:838-841. [DOI] [PubMed] [Google Scholar]
- 28.Moore, M. 2000. Intron recognition comes of AGe. Nature 7:14-16. [DOI] [PubMed] [Google Scholar]
- 29.Mount, S. M., and H. K. Salz. 2000. Pre-messenger RNA processing factors in the Drosophila genome. J. Cell Biol. 150:F37-F44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagengast, A. A., S. M. Stitzinger, C. H. Tseng, S. M. Mount, and H. K. Salz. 2003. Sex-lethal splicing autoregulation in vivo: interactions between SEX-LETHAL, the U1 snRNP and U2AF underlie male exon skipping. Development 130:463-471. [DOI] [PubMed] [Google Scholar]
- 31.Page-McCaw, P., K. Amonlirdviman, and P. Sharp. 1999. PUF60: a novel U2AF65-related splicing activity. RNA 5:1548-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter, G., P. Brennwald, and J. A. Wise. 1990. U1 small nuclear RNA from Schizosaccharomyces pombe has unique and conserved features and is encoded by an essential single-copy gene. Mol. Cell. Biol. 10:2874-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potashkin, J., K. Naik, and K. Wentz-Hunter. 1993. U2AF homolog required for splicing in vivo. Science 262:573-575. [DOI] [PubMed] [Google Scholar]
- 34.Reed, R. 2000. Mechanisms of fidelity in pre-mRNA splicing. Curr. Opin. Cell Biol. 12:340-345. [DOI] [PubMed] [Google Scholar]
- 35.Romfo, C., S. Lakhe-Reddy, and J. A. Wise. 1999. Molecular genetic analysis of U2AF59 in Schizosaccharomyces pombe: differential sensitivity of introns to mutational inactivation. RNA 5:49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romfo, C. M., C. J. Alvarez, W. J. van Heeckeren, C. J. Webb, and J. A. Wise. 2000. Evidence for splice site pairing via intron definition in Schizosaccharomyces pombe. Mol. Cell. Biol. 20:7955-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudner, D., K. Kannar, S. Breger, and D. Rio. 1996. Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc. Natl. Acad. Sci. USA 93:10333-10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudner, D. Z., K. S. Breger, R. Kanaar, M. D. Adams, and D. C. Rio. 1998. RNA binding activity of heterodimeric splicing factor U2AF: at least one RS domain is required for high-affinity binding. Mol. Cell. Biol. 18:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudner, D. Z., K. S. Breger, and D. Rio. 1998. Molecular genetic analysis of the heterodimeric splicing factor U2AF: the RS domain on either the large or small Drosophila subunit is dispensable in vivo. Genes Dev. 12:1010-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudner, D. Z., R. Kanaar, K. S. Breger, and D. C. Rio. 1998. Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol. Cell. Biol. 18:1765-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruskin, B., P. D. Zamore, and M. R. Green. 1988. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell 52:207-219. [DOI] [PubMed] [Google Scholar]
- 42.Sayle, R. A., and E. J. Milner-White. 1995. RasMol: biomolecular graphics for all. Trends Biochem. Sci. 9:374-376. [DOI] [PubMed] [Google Scholar]
- 43.Selenko, P., G. Gregorovic, R. Sprangers, G. Stier, Z. Rhani, A. Kramer, and M. Sattler. 2003. Structural basis for the molecular recognition between human splicing factors U2AF65 and SF1/mBBP. Mol. Cell 11:965-976. [DOI] [PubMed] [Google Scholar]
- 44.SenGupta, D. J., B. Zhang, B. Kraemer, P. Pochart, S. Fields, and M. Wickens. 1996. A three-hybrid system to detect RNA-protein interactions in vivo. Proc. Natl. Acad. Sci. USA 93:8496-8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sipiczki, M. 4 August 2000, posting date. Where does fission yeast sit on the tree of life? Genome Biol. 1: 1011.1-1011.4. [Online.] http://genomebiology.com/2000/1/2/REVIEWS/1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spingola, M., L. Grate, D. Haussler, and M. Ares, Jr. 1999. Genome-wide bioinformatic and molecular analysis of introns in Saccharomyces cerevisiae. RNA 5:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tupler, R., G. Perini, and M. R. Green. 2001. Expressing the human genome. Nature 409:832-833. [DOI] [PubMed] [Google Scholar]
- 48.Vasquez, M., C. Atorrasagasti, N. Bercovich, R. Volcovich, and M. J. Levin. 2003. Unique features of the Trypanosoma cruzi U2AF35 splicing factor. Mol. Biochem. Parasitol. 128:77-81. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X., and T. M. Tanaka Hall. 2001. Structural basis for recognition of AU-rich element RNA by the HuD protein. Nat. Struct. Biol. 8:141-145. [DOI] [PubMed] [Google Scholar]
- 50.Wentz-Hunter, K., and J. Potashkin. 1996. The small subunit of the splicing factor U2AF is conserved in fission yeast. Nucleic Acids Res. 24:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wertman, K. F., D. G. Drubin, and D. Botstein. 1992. Systematic mutational analysis of the yeast ACT1 gene. Genetics 132:337-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, R. Lyne, A. Stewart, J. Sgouros, N. Peat, J. Hayles, S. Baker, D. Basham, S. Bowman, K. Brooks, D. Brown, S. Brown, T. Chillingworth, C. Churcher, M. Collins, R. Connor, A. Cronin, P. Davis, T. Feltwell, A. Fraser, S. Gentles, A. Goble, N. Hamlin, D. Harris, J. Hidalgo, G. Hodgson, S. Holroyd, T. Hornsby, S. Howarth, E. J. Huckle, S. Hunt, K. Jagels, K. James, L. Jones, M. Jones, S. Leather, S. McDonald, J. McLean, P. Mooney, S. Moule, K. Mungall, L. Murphy, D. Niblett, C. Odell, K. Oliver, S. O'Neil, D. Pearson, M. A. Quail, E. Rabbinowitsch, K. Rutherford, S. Rutter, D. Saunders, K. Seeger, S. Sharp, J. Skelton, M. Simmonds, R. Squares, S. Squares, K. Stevens, K. Taylor, R. G. Taylor, A. Tivey, S. Walsh, T. Warren, S. Whitehead, J. Woodward, G. Volckaert, R. Aert, J. Robben, B. Grymonprez, I. Weltjens, E. Vanstreels, M. Rieger, M. Schaefer, S. Mueller-Auer, C. Gabel, M. Fuchs, C. Fritzc, E. Holzer, D. Moest, H. Hilbert, K. Borzym, I. Langer, A. Beck, H. Lehrach, R. Reinhardt, T. M. Pohl, P. Eger, W. Zimmermann, H. Wedler, R. Wambutt, B. Purnelle, A. Goffeau, E. Cadieu, S. Dreano, S. Gloux, S. Lelaure, V. Mottier, F. Galibert, S. J. Aves, Z. Xiang, C. Hunt, K. Moore, S. M. Hurst, M. Lucas, M. Rochet, C. Gaillardin, V. A. Tallada, A. Garzon, G. Thode, R. R. Daga, L. Cruzado, J. Jimenez, M. Sanchez, F. del Rey, J. Benito, A. Domınguez, J. L. Revuelta, S. Moreno, J. Armstrong, S. L. Forsburg, L. Cerrutti, T. Lowe, W. R. McCombie, I. Paulsen, J. Potashkin, G. V. Shpakovski, D. Ussery, B. G. Barrell, and P. Nurse. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]
- 53.Worthington, M., B. Amann, D. Nathans, and J. Berg. 1996. Metal binding properties and secondary structure of the zinc-binding domain of Nup475. Proc. Natl. Acad. Sci. USA 93:13754-13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, J., and T. Maniatis. 1993. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75:1061-1070. [DOI] [PubMed] [Google Scholar]
- 55.Wu, S., C. Romfo, T. Nilsen, and M. Green. 1999. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature 402:832-835. [DOI] [PubMed] [Google Scholar]
- 56.Yeakley, J. M., H. Tronchere, J. Olesen, J. A. Dyck, H. Y. Wang, and X. D. Fu. 1999. Phosphorylation regulates in vivo interaction and molecular targeting of serine/arginine-rich pre-mRNA splicing factors. J. Cell Biol. 145:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamore, P. D., and M. R. Green. 1991. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 10:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamore, P. D., J. Patton, and M. Green. 1992. Cloning and domain structure of the mammalian splicing factor U2AF. Nature 355:609-614. [DOI] [PubMed] [Google Scholar]
- 59.Zarrinpar, A., Park, S.-H., and W. A. Lim. 2003. Optimization of specificity in a cellular protein interaction network by negative selection. Nature 426:676-680. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, M., P. D. Zamore, M. Carmo-Fonseca, A. I. Lamond, and M. R. Green. 1992. Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc. Natl. Acad. Sci. USA 89:8769-8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu, J., and A. R. Krainer. 2000. Pre-mRNA splicing in the absence of an SR protein RS domain. Genes Dev. 14:3166-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zorio, D. A., K. Lea, and T. Blumenthal. 1997. Cloning of Caenorhabditis U2AF65: an alternatively spliced RNA containing a novel exon. Mol. Cell. Biol. 17:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zorio, D. A., and T. Blumenthal. 1999. U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans. RNA 5:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorio, D. A., and T. Blumenthal. 1999. Both subunits of U2AF recognize the 3′ splice site in Caenorhabditis elegans. Nature 402:835-838. [DOI] [PubMed] [Google Scholar]
- 65.Zuo, P., and T. Maniatis. 1996. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 10:1356-1368. [DOI] [PubMed] [Google Scholar]