Abstract
Dopamine neurons in the ventral tegmental area (VTA) govern reward and motivation and dysregulated dopaminergic transmission may account for anhedonia and other symptoms of depression. Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine kinase that regulates a broad range of brain functions through phosphorylation of a myriad of substrates, including tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis. We investigated whether and how Cdk5 activity in VTA dopamine neurons regulated depression-related behaviors in mice. Using the Cre/LoxP system to selectively delete Cdk5 in the VTA or in midbrain dopamine neurons in Cdk5loxP/loxP mice, we showed that Cdk5 loss of function in the VTA induced anxiety- and depressive-like behaviors that were associated with decreases in TH phosphorylation at Ser31 and Ser40 in the VTA and dopamine release in its target region, the nucleus accumbens. The decreased phosphorylation of TH at Ser31 was a direct effect of Cdk5 deletion, whereas decreased phosphorylation of TH at Ser40 was likely caused by impaired cAMP/protein kinase A (PKA) signaling, because Cdk5 deletion decreased cAMP and phosphorylated cAMP response element-binding protein (p-CREB) levels in the VTA. Using Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technology, we showed that selectively increasing cAMP levels in VTA dopamine neurons increased phosphorylation of TH at Ser40 and CREB at Ser133 and reversed behavioral deficits induced by Cdk5 deletion. The results suggest that Cdk5 in the VTA regulates cAMP/PKA signaling, dopaminergic neurotransmission, and depression-related behaviors.
Keywords: cAMP, Cdk5, depression, dopamine, DREADD, tyrosine hydroxylase
Introduction
The mesolimbic dopamine system governs the rewarding effects of food, sex, and social interaction, whereas poor appetite, anhedonia, lack of motivation, decreased sex drive, and social withdrawal are core symptoms of depression (Nestler and Carlezon, 2006). Patients with Parkinson's disease show high incidence (30–50%) of comorbid depression (Burn, 2002). Deep brain stimulation of the nucleus accumbens (NAc) produces an acute relief of depressive symptoms in depressed patients (Schlaepfer et al., 2008). The D2 dopamine receptor and its signaling partners have been implicated in depressive-like behaviors (Bibb, 2005; Park et al., 2005). Distinct neuroadaptations in the ventral tegmental area (VTA)-NAc pathway confer susceptibility and resistance to social defeat stress, an animal model of depression (Berton et al., 2006; Krishnan et al., 2007). Optogenetic stimulation and inhibition of VTA dopamine neurons modulate depression-related behaviors (Chaudhury et al., 2013; Tye et al., 2013). Dysfunction of dopaminergic transmission may underlie the manifestation of certain depressive symptoms.
Cyclin-dependent kinase 5 (Cdk5) is a serine/threonine kinase that regulates many functions, including brain development, synaptic plasticity, cognition, learning, and memory, and dysfunction of Cdk5 has been implicated in neurodegenerative and neuropsychiatric diseases (Barnett and Bibb, 2011; Su and Tsai, 2011; Cheung and Ip, 2012). Recent studies have shown that forebrain Cdk5 activity regulates anxiety- and depressive-like behaviors. Mice with forebrain-specific deletion of Cdk5 in pyramidal neurons exhibit hyperactivity and reduced anxiety- and depressive-like behavior (Su et al., 2013). Microinjection of a Cdk5 inhibitor into the dentate gyrus of rat hippocampus blocked depressive-like behavior induced by chronic mild stress (Zhu et al., 2012). A prominent function of Cdk5 is to modulate dopamine signaling in the brain. Cdk5 phosphorylates dopamine cyclic-AMP regulated phosphoprotein (DARPP-32), which allows DARPP-32 to become a protein kinase A (PKA) inhibitor and therefore a protein phosphatase 1 (PP1) activator (Bibb et al., 1999). Cdk5 also directly phosphorylates the N-terminal region of tyrosine hydroxylase (TH) at Ser31, the catalytic enzyme for dopamine synthesis (Kansy et al., 2004; Moy and Tsai, 2004). Forebrain-specific Cdk5 knock-out mice exhibit enhanced neuronal excitability of NAc neurons, psychomotor-activating effects of cocaine, and food motivation (Benavides et al., 2007). Genetic deletion of the Cdk5 cofactor p35 in mice produces attention-deficit/hyperactivity disorder (ADHD) phenotype (Drerup et al., 2010) and ADHD is associated with perturbed dopaminergic neurotransmission (Tripp and Wickens, 2009). Because Cdk5 has intimate interaction with dopamine signaling system, Cdk5 activity in dopamine neurons may play a role in regulating anhedonia and other depression-related behaviors.
Here, we used a Cre/LoxP system to delete Cdk5 in the VTA or in dopamine neurons and examined its impact on depression-related behavior. To selectively delete Cdk5 in the VTA, adeno-associated viral vectors (AAVs) carrying Cre recombinase were microinjected into the VTA of Cdk5loxP/loxP mice. Cdk5 was also conditionally deleted in dopamine neurons by breeding dopamine transporter (DAT)-Cre mice with Cdk5loxP/loxP mice. We examined the effects of loss of function of Cdk5 in the VTA on depression-related behaviors, TH phosphorylation, and dopamine release. Our results suggest that Cdk5 in the VTA regulates dopaminergic neurotransmission and depression-related behaviors.
Materials and Methods
Animals
Mice were given ad libitum access to food and water and housed 4–5 per cage in a temperature-controlled (23 ± 1°C) and humidity-controlled (40–60%) room with a 12 h light-dark cycle. Animal maintenance and use were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Homozygous Cdk5-floxed mice (Cdk5loxP/loxP, Jax stock #014156), Cdk5wt/wt mice (Jax stock #000664), heterozygous DAT-Cre+/− mice (Jax stock #006660) and ROSA26-flox-STOP-flox-lacZ (R26R) reporter mice (Jax stock #003474) were obtained from The Jackson Laboratory. All of the mouse lines were maintained on the same C57BL/6 background. By crossing Cdk5loxP/loxP mice with DAT-Cre+/− mice, we have generated a compound, DAT-Cre+/−/Cdk5loxP/wt mouse line. We then crossed this compound, DAT-Cre+/−/Cdk5loxP/wt mice with Cdk5loxP/loxP mice to create dopamine neuron-specific Cdk5 conditional knock-out (cKO) mice (DAT-Cre+/−/Cdk5loxP/loxP). DAT-Cre−/−/Cdk5loxP/wt or DAT-Cre−/−/Cdk5loxP/loxP mice were used as experimental controls. All experiments were performed on adult male mice (8–14 weeks of age at the beginning of the experiments).
Animal surgery and intra-VTA AAV microinjection
AAV2-Cre-GFP and AAV2-GFP were obtained from the Penn Vector Core of University of Pennsylvania (Philadelphia, PA); AAV8-hSyn-DIO-rM3D-Gs-mCherry was obtained from University of North Carolina Vector Core Facilities (Chapel Hill, NC). Mice were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed in a stereotaxic device (David Kopf Instruments). AAVs (0.3 μl per side) were bilaterally microinjected into the VTA via injector cannulae (33-gauge; Plastics One), which were connected through C313C connectors to 2 μl Hamilton microsyringes. The stereotaxic coordinates for VTA were as follows: anteroposterior, −3.0 mm; mediolateral, ± 1.0 mm; dorsoventral, −4.8 mm at a 7° angle (Paxinos and Franklin, 2001). The injection rate was 0.3 μl over 5 min and the injectors were kept in place for an additional 5 min to ensure adequate AAV diffusion from the injector tip. After the surgery, animals received subcutaneous injections of analgesic (buprenorphine, 0.05 mg/kg) 3 times daily for 2 d.
Chronic unpredictable mild stress paradigm
Mice were subjected to various chronic unpredictable mild stressors for 1 week (sub-CUS) or 5 weeks (CUS) based on published studies (Willner et al., 1987; Koo and Duman, 2008; Zhong et al., 2014). The stressors included restraint (1 h in a soft, flexible plastic cone, DecapiCone; Braintree Scientific), inversion of day/night light cycle, cold (in a cold room at 4°C for 1 h), 45° tilted cage (overnight), cage rotation (20 min), rat bedding (odor, 3 h), wet bedding (250 ml of water added into cage, overnight) and no bedding (overnight), low-intensity stroboscopic illumination (10 Hz, overnight), food and water deprivation (overnight), and overcrowding (overnight). On average, two stressors were administered per day. The timeline of the stressor exposure is described in Table 1. For stressed animals, cages were changed after “wet bedding,” “no bedding,” and “rat bedding” stressors. Nonstressed controls were handled only for cage changes and behavioral tests.
Table 1.
Experimental schedule for the sub-CUS or CUS procedure in mice
| Week | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| 1 | Cold | Restraint | Light inversion | Cage rotation | Cold | Restraint | Light inversion |
| Wet bedding | No bedding | Cage tilt | Strobe | Food and water deprivation | Overcrowding | Wet bedding | |
| 2 | Cold | Cage rotation | Restraint | Rat bedding | Light inversion | Cage rotation | Cold |
| Cage tilt | Food and water deprivation | Wet bedding | Strobe | No bedding | Food and water deprivation | Wet bedding | |
| 3 | Rat bedding | Restraint | Cage rotation | Light inversion | Cold | Cage tilt | Light inversion |
| Strobe | Light inversion | No bedding | Food and water deprivation | Wet bedding | Strobe | Overcrowding | |
| 4 | Cold | Restraint | Cage rotation | Rat bedding | Cold | Restraint | Cage rotation |
| No bedding | Food and water deprivation | Strobe | Light inversion | Cage tilt | Wet bedding | No bedding | |
| 5 | Cold | Cage rotation | Light inversion | Cold | Cage rotation | Light inversion | Restraint |
| Food and water deprivation | Strobe | Wet bedding | Cage tilt | No bedding | Overcrowding | Cage tilt |
Sub-CUS, Week 1; CUS, week 1 to week 5.
Behavior
Open field test.
For the open field test (OFT), mice were placed individually in one corner of the open field (50 cm length × 45 cm wide × 30 cm deep box) and allowed to freely explore the arena during a 20 min test session. Locomotor activities were recorded using an automated video-tracking system (Mobile Datum). Total distance traveled and time spent in the center of the box during first 5 min were calculated. Time in center (center time) was defined as the amount of time that was spent in the central 25 cm × 22.5 cm area of the open field.
Sucrose preference test.
For the sucrose preference test (SPT), mice were individually housed and trained to drink from two drinking bottles for 48 h. One bottle contained 1% sucrose (in tap water) and the other contained tap water. The SPT was performed after the OFT (see Figs. 2, 5, 11). During the SPT, mice were deprived of food and water for 8 h and the consumption of sucrose solution and water over the next 16 h was measured. The sucrose preference was calculated as sucrose solution consumed divided by the total amount of solution consumed (i.e., a percentage).
Figure 2.
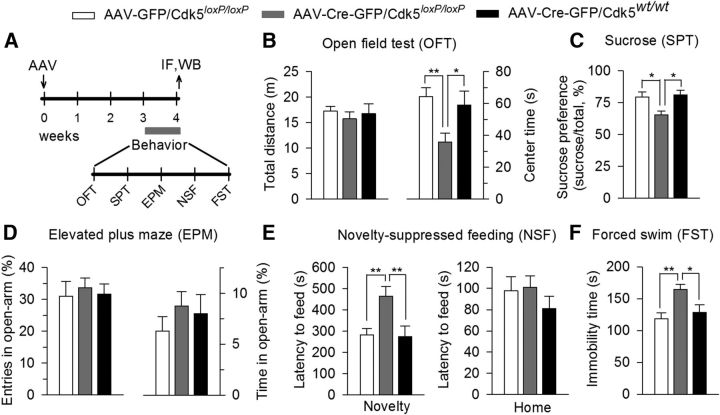
AAV2-Cre-GFP-mediated VTA-specific deletion of Cdk5 produced anxiety- and depressive-like behaviors in Cdk5loxP/loxP mice. Cdk5loxP/loxP mice received bilateral intra-VTA injection of AAV2-Cre-GFP. As control groups, Cdk5loxP/loxP mice received AAV2-GFP injection or Cdk5wt/wt mice received AAV2-Cre-GFP injection. A, Timeline for the AAV microinjection, behavioral tests, immunofluorescence labeling (IF), and Western blotting (WB). B, VTA-specific deletion of Cdk5 (Cdk5loxP/loxP mice received bilateral intra-VTA injection of AAV2-Cre-GFP) significantly decreased the center time (p < 0.01) without affecting total distance traveled in the OFT (p > 0.05). C, Cdk5 deletion significantly decreased sucrose preference (p < 0.01). D, Cdk5 deletion did not affect the entries into the open arms (p > 0.05) and the time spent in the open arms (p > 0.05) in the EPM test. E, Cdk5 deletion increased the latency to feed in the novel environment (Novelty) in the NSF test (p < 0.01), but did not significantly affect the latency to feed in the home cage (Home; p > 0.05). F, Cdk5 deletion increased the immobility time in FST (p < 0.01). The p values for Tukey's post hoc test results are shown on the top (*p < 0.05, **p < 0.01; n = 10–13 mice/group) for AAV, AAV2-Cre-GFP, and AAV2-GFP.
Figure 5.
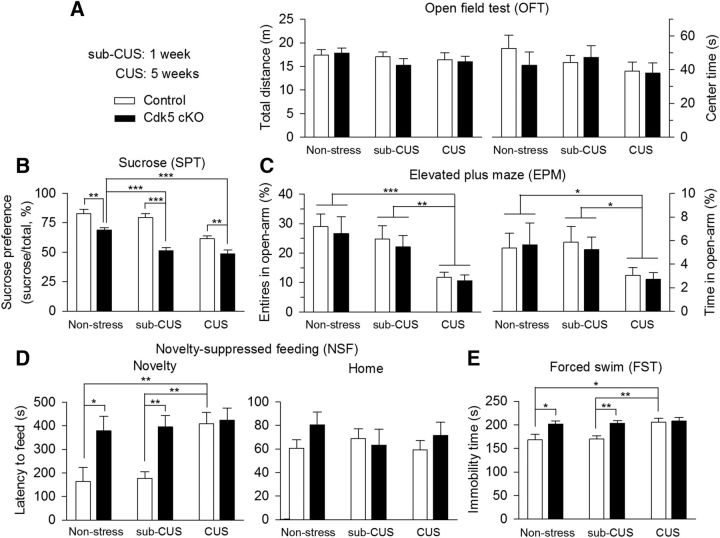
Cdk5 cKO mice exhibited anxiety- and depressive-like behaviors. Control and Cdk5 cKO mice were subjected to various and repeated unpredictable mild stressors for a period of 1 week (sub-CUS) or 5 weeks (CUS) or were not subjected to any stressors (nonstressed). A, In the OFT neither stress nor Cdk5 cKO altered the total distance traveled (p > 0.05) or center time (p > 0.05). B, In nonstressed groups, Cdk5 cKO mice showed significant decrease in the SPT compared with wild-type control mice (**p < 0.01). In sub-CUS groups, Cdk5 cKO mice exhibited significant decrease in the SPT compared with control mice (***p < 0.001). In the CUS groups, Cdk5 cKO mice showed significant decrease in the sucrose preference compared with wild-type control mice (**p < 0.01). CUS (***p < 0.001), but not sub-CUS (p > 0.05), significantly decreased sucrose preference in wild-type control mice. However, both sub-CUS (***p < 0.001) and CUS (***p < 0.001) further decreased sucrose preference in Cdk5 cKO mice. C, In the EPM test, CUS, but not sub-CUS, significantly decreased the number of entries in the open arms (nonstress vs CUS, ***p < 0.001; sub-CUS vs CUS, **p < 0.01) and the time spent in the open arms (nonstress vs CUS, *p < 0.05; sub-CUS vs CUS, *p < 0.05). However, Cdk5 cKO had no significant effects on the number of entries into the open arms (p > 0.05) and the time spent in the open arms (p > 0.05). D, In nonstressed groups, Cdk5 cKO mice showed a significant increase in the latency to feed in the novel environment compared with wild-type control mice (*p < 0.05). In the sub-CUS groups, Cdk5 cKO mice also showed significant increase in the latency to feed compared with control mice (**p < 0.01), whereas CUS significantly increased the latency to feed in control mice (**p < 0.01), but did not produce further increases in the latency to feed in Cdk5 cKO mice (p > 0.05) in the NSF test. Neither stress (p > 0.05) nor Cdk5 cKO (p > 0.05) affected the latency to feed in the home cage. E, In nonstressed groups, Cdk5 cKO mice showed significant increase in the immobility time compared with wild-type control mice (*p < 0.05). In the sub-CUS groups, Cdk5 cKO mice also showed significant increases in immobility time compared with control mice (**p < 0.01), whereas CUS significantly increased immobility time in control mice (*p < 0.05) but did not produce further increases in immobility time in Cdk5 cKO mice (p > 0.05). The p values for Tukey's post hoc test results are shown on the top (*p < 0.05, **p < 0.01; ***p < 0.001; n = 10–12 mice/group).
Figure 11.
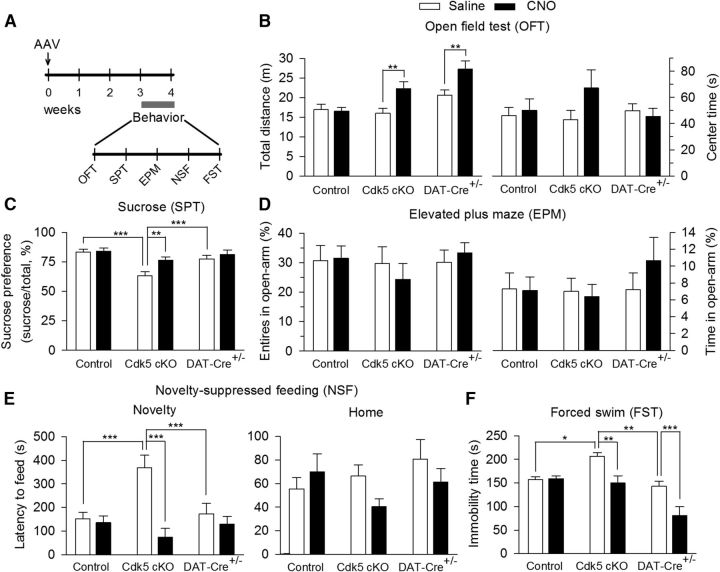
CNO-induced activation of the DREADD receptor rM3D-Gs in VTA dopamine neurons reversed anxiety- and depressive-like behaviors induced by Cdk5 cKO. A, Timeline for the AAV microinjection and behavioral tests. B, In the OFT CNO, but not intraperitoneal saline injection, increased the total traveled distance in Cdk5 cKO (**p < 0.01) and DAT-Cre+/−/Cdk5wt/wt (**p < 0.01) mice without affecting the total traveled distance in control mice (p > 0.05). Neither genotype (p > 0.05) nor CNO injection (p > 0.05) altered the center time in the open field. C, The sucrose preference was significantly decreased in Cdk5 cKO mice compared with that in control (***p < 0.001) and DAT-Cre+/−/Cdk5wt/wt (***p < 0.001) mice and this decrease was reversed by CNO injection (**p < 0.01). In addition, CNO injection did not significantly affect sucrose preference in DAT-Cre+/−/Cdk5wt/wt mice (p > 0.05). D, Neither genotype nor CNO injection significantly changed the number of entries into the open arms (p > 0.05) and the time spent in the open arms (p > 0.05) in the EPM test. E, In the NSF test, the latency to feed in the novel environment was significantly increased in Cdk5 cKO mice compared with that in control (***p < 0.001) and DAT-Cre+/−/Cdk5wt/wt (***p < 0.001) mice and this increase was reversed by CNO injection (***p < 0.001). In addition, CNO injection did not significantly affect the latency to feed in DAT-Cre+/−/Cdk5wt/wt mice (p > 0.05). In contrast, neither genotype (p > 0.05) nor CNO injection (p > 0.05) affected the latency to feed in the home cage. F, In the FST, the immobility time was significantly increased in Cdk5 cKO mice compared with that in control (*p < 0.05) and DAT-Cre+/−/Cdk5wt/wt (**p < 0.01) mice and CNO injection reversed Cdk5 cKO-induced increase in the immobility time (**p < 0.01). However, CNO injection did significantly decrease the immobility time in DAT-Cre+/−/Cdk5wt/wt mice (***p < 0.001; control: DAT-Cre−/−/ Cdk5loxP/loxP or DAT-Cre−/−/Cdk5loxP/wt; Cdk5 cKO: DAT-Cre+/−/Cdk5loxP/loxP; DAT-Cre+/−: DAT-Cre+/−/Cdk5wt/wt; n = 9–10 mice/group).
Elevated plus maze.
The elevated plus maze (EPM) apparatus (Stoelting) comprises 2 open arms (35 × 5 cm) across from each other and perpendicular to 2 closed arms (35 × 5 × 15 cm) that are connected by a center platform (5 × 5 cm). It was elevated 40 cm above the floor. Mice were placed in the center platform facing a closed arm and allowed to freely explore the maze for 5 min. The location of the mice was tracked with the automated video-tracking system. The number of entries and time spent in open arms were analyzed.
Novelty-suppressed feeding.
The novelty-suppressed feeding (NSF) test was performed similar to a published protocol (Santarelli et al., 2003). Mice were deprived of food for 24 h before being placed in a novel environment (a plastic box 45 cm long × 35 cm wide × 20 cm deep) where 5 food pellets (regular chow) were placed on a piece of white filter paper (11 cm in diameter) in the center of the box. A mouse was placed in one corner of the box and the latency to feed was measured. Feeding was defined as biting the food using forepaws, not simply sniffing or touching the food. Immediately after the test, the animal was transferred to the home cage and the latency to feed in the home cage was measured to serve as controls.
Forced swim test.
For the forced swim test (FST), mice were placed individually into glass cylinders (13 cm diameter, 25 cm tall) filled to a depth of 18 cm with water (25 ± 1°C). The mice were placed in the cylinders for 6 min. The time spent immobile during the last 4 min was scored. Immobility was defined as the cessation of all movements (e.g., climbing, swimming) except those necessary for the mouse to keep its head above water (i.e., floating).
Western blotting
Experimental procedure was conducted as we have described previously (Pan et al., 2011; Yu et al., 2013). Mice were anesthetized with isoflurane and rapidly decapitated. Brains were sliced in a 1 mm matrix (Zivic Instruments) and the VTA was bilaterally punched out using 18 gauge blunt needles and immediately homogenized in 0.2 ml of lysis buffer, pH 7.6, containing the following (in mm): 50 Tris-acetate, 50 NaF, 10 EDTA, and 10 EGTA, along with 0.01% Triton X-100, protease inhibitors (Research Product International), and protein phosphatase inhibitors I and II (Sigma-Aldrich). After centrifugation at 1000 × g for 10 min, total protein concentration of the supernatant was assayed using a Bio-Rad protein assay kit. The concentration of protein was calibrated to 1 or 2 μg/μl with 2× loading buffer containing 0.1 m Tris-HCl, pH 6.8, 4% (w/v) SDS, 20% (v/v) glycerol, 10% (v/v) 2-mercaptoethanol, and 0.04% (w/v) bromophenol blue. Samples were separated in SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in blocking buffer containing 5% (w/v) milk and 0.1% (v/v) Tween 20 in Tris-buffered saline (TBS-T) for ∼1–2 h at room temperature and incubated overnight at 4°C with antibodies against Cdk5 (mouse, 1:1000; Millipore), p35/25 (1:200; Santa Cruz Biotechnology), p-TH-Ser31 (1:200; R&D Systems), p-TH-Ser40 (1:300; Millipore), TH (1:500; Santa Cruz Biotechnology), phosphorylated cAMP response element-binding protein (p-CREB)-Ser133 (1:200; Cell Signaling Technology), CREB (1:200; Cell Signaling Technology), or GAPDH (1:1000; Cell Signaling Technology) antibodies. Membranes were washed 3 times with TBS-T and then probed with anti-mouse IgG, HRP-linked antibody (1:5000; Bio-Rad) or anti-rabbit IgG, HRP-linked antibody (1:3000; Cell Signaling Technology) for 2 h at room temperature before being developed using an ECL immunoblotting detection system (Thermo Scientific). Immunoreactivity of Western blots was quantified by densitometry using ImageJ software and normalized to GAPDH.
cAMP ELISA
The VTA sample collection was similar to the process described in the “Western Blotting” section. The VTA was dissected out bilaterally, frozen in liquid nitrogen, homogenized in ice-cold hydrochloric acid (0.1 m), and centrifuged at 13,000 × g for 50 min at 4°C. cAMP in the supernatant was determined by direct cAMP ELISA kit (Enzo Life Sciences). The optical density was read at 405 nm using an ELX800 Universal Microplate Reader (Bio-TEK Instruments). Finally, the cAMP concentration was normalized to total protein, which was assayed using a Bio-Rad protein assay kit.
Immunohistochemistry
Mice were anesthetized with isoflurane and transcardially perfused with 0.1 m sodium PBS, followed by 4% paraformaldehyde in 4% sucrose-PBS, pH 7.4. After perfusion, the brain was removed and postfixed in the same fixative for 4 h at 4°C and was then dehydrated in increasing concentrations of sucrose (20% and 30%) in 0.1 m PBS at 4°C and frozen on dry ice. Coronal VTA sections were made at 20 μm thickness with a cryostat.
DAB staining.
VTA Sections were incubated with antibodies against TH (1:300; Santa Cruz Biotechnology) or p-CREB-Ser133 (1:200; Cell Signaling Technology) at 4°C for 48 h. After 3 washes with PBS, sections were then incubated with goat anti-rabbit IgG-HRP (1:100; Bio-Rad) for 2 h at room temperature. Immunoreactivity was visualized with 0.05% DAB–0.004% H2O2 in 0.1 m ammonium phosphate buffer (APB) for 10 min, after which the reaction was stopped with APB for 5 min and then rinsed in PBS, dehydrated, and coverslipped. Sections were imaged with a Nikon Eclipse 80i microscope and analyzed by ImageJ software. TH+ cells or p-CREB+ cells in the VTA were counted from the left and right side of the brain in 2 sections from each animal at ∼3.3 mm and 3.4 mm posterior to bregma. The coordinates were determined by comparing the brain structures in the immunohistological sections with those in mouse stereotaxic atlas (Paxinos and Franklin, 2001). The number of TH+ cells or p-CREB+ cells was normalized to the VTA area, which was determined with the aid of the same stereotaxic atlas.
Immunofluorescence staining.
VTA sections were incubated with antibodies against β-galactosidase (chicken, 1:1000; Abcam), Cdk5 (rabbit, 1:100; Santa Cruz Biotechnology), and/or TH (mouse or rabbit, 1:300; Santa Cruz Biotechnology) at 4°C for 48 h. Based on different host species of first antibodies, VTA sections were incubated in the different second antibodies: anti-chicken IgG Alexa Fluor 568 conjugate (1:1000; Life Technologies), anti-rabbit IgG-Texas red conjugate (1:200; Santa Cruz Biotechnology), or anti-mouse IgG-FITC conjugate (1:200; Santa Cruz Biotechnology) for 4 h at room temperature in the dark. The sections were analyzed using a Nikon Eclipse TE-2000U confocal microscope.
Slice preparation and fast-scan cyclic voltammetry
Mice were anesthetized by isoflurane inhalation and decapitated. Striatal slices containing the NAc (250 μm) were prepared as described previously (Wang et al., 2010). Slices were prepared at 4–6°C in a solution containing the following (in mm): 220 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgSO4, 26 NaHCO3, 10 glucose, and 1 sodium ascorbate, and transferred to and stored in artificial CSF (ACSF) containing the following (in mm): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose at room temperature. All solutions were saturated with 95% O2 and 5% CO2.
To detect dopamine release with fast-scan cyclic voltammetry (FSCV), a glass-encased cylindrical carbon fiber (7 μm diameter; Goodfellow) microelectrode with an exposed final length of 100–150 μm was lowered into the NAc shell at approximate locations: anteroposterior, 0.9–1.7 mm; mediolateral, ± 0.5 mm; dorsoventral, −4.4 mm. The coordinates were determined by visual inspection of the slice with a mouse brain atlas (Paxinos and Franklin, 2001). The microelectrode was filled with the solution containing 150 mm KCl. Triangular waveforms (holding at −0.4 V, from −0.4 V to 1.3 V at 400 V/s) were applied every 100 ms using Demon software (Yorgason et al., 2011) and a Chem-Clamp Potentiostat (Dagan). A stimulating electrode was placed at ∼100 μm from the carbon fiber microelectrode and dopamine release was evoked by a single electrical stimulus pulse (250 μA, 0.1 ms duration) or a 5-pulse train stimuli at 10, 30, and 50 Hz. After the recordings, electrodes were calibrated in 0.1–1 μm dopamine in the ACSF. Recordings were performed at 32 ± 1°C using an automatic temperature controller (Warner Instrument).
Chemicals
Clozapine-N-oxide (CNO) was purchased from Enzo Life Sciences. All other common chemicals were purchased from Sigma-Aldrich.
Data analysis and statistics
All results are expressed as mean ± SEM. Results were analyzed with Student's t test or one-way or two-way ANOVA followed by Tukey's post hoc analysis. Results were considered to be significant at p < 0.05.
Results
VTA-specific deletion of Cdk5 produced depressive-like behaviors
To assess the role of Cdk5 in the VTA in modulating depression-related behavior, we deleted the Cdk5 gene locally using AAV type 2 vector expressing green fluorescent protein (GFP)-tagged Cre recombinase (AAV2-Cre-GFP). To evaluate the effectiveness of the Cre recombinase in the AAV, we first injected AAV2-Cre-GFP or a control vector (AAV2-GFP) stereotaxically into the VTA (Fig. 1A) of R26R LacZ (gene encoding β-galactosidase) reporter mice carrying the reporter cassette in the ROSA 26 locus. After recovery for 3 weeks, immunofluorescence labeling showed that β-galactosidase was expressed in nearly all cells infected with AAV2-Cre-GFP (Fig. 1B). The fusion of Cre with GFP in the AAV2-Cre-GFP vector allowed Cre-GFP to be predominantly localized in the nucleus (Fig. 1B,C). Approximately 85% of dopamine neurons in the VTA were infected by the AAVs, as shown by coexpression of TH and GFP (Fig. 1C–E). Approximately 25% of GFP neurons were not colabeled with TH, suggesting that a substantial number of nondopamine neurons were also infected by the AAVs (Fig. 1C,D). These results indicate that the AAV2-delivered Cre recombinase is effective and can be used to delete Cdk5 in the VTA.
Figure 1.
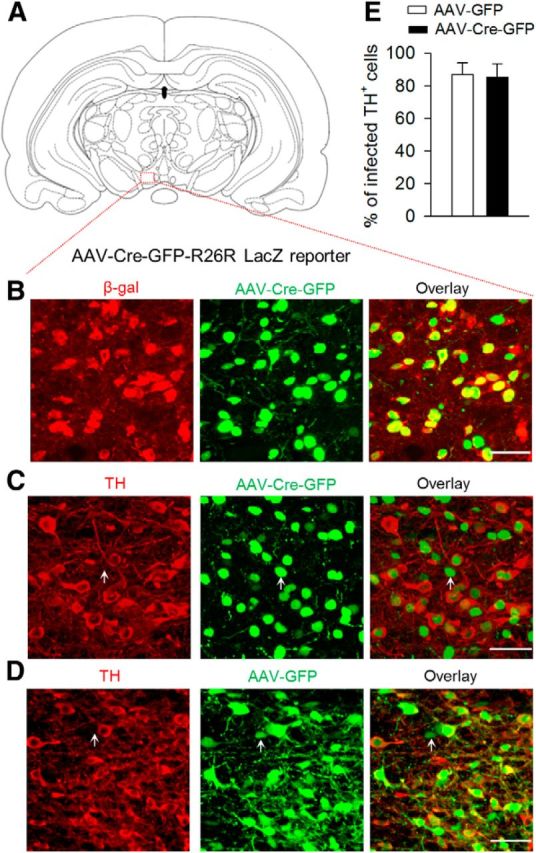
Injection of AAV2-Cre-GFP into the VTA of R26R LacZ reporter mice induced the expression of β-galactosidase. A, Location of VTA injection. B, Immunofluorescence labeling showed that β-galactosidase (β-gal, red) was expressed in AAV2-Cre-GFP-infected neurons (green). C, D, AAV2-Cre-GFP (C) or AAV2-GFP (D; green) was expressed in TH+ (dopamine neuron marker, red) dopamine neurons in the VTA. In addition, a substantial number of TH− nondopamine neurons were also infected by the AAVs (indicated by arrow). Scale bar, 50 μm. E, Summarized data showed that AAV2-Cre-GFP or AAV2-GFP infected ∼85% of TH+ dopamine neurons in the VTA (n = 3 mice/group). Error bars indicate SEM.
We then injected AAV2-Cre-GFP or AAV2-GFP into the VTA bilaterally in Cdk5-floxed mice homozygous for the loxP-Cdk5-loxP sequence (Cdk5loxP/loxP). In an additional control experiment, AAV2-Cre-GFP was bilaterally injected into the VTA of Cdk5wt/wt mice. The timeline of the AAV injection, behavioral tests, immunofluorescence labeling, and Western blotting is shown in Figure 2A. Three weeks after the AAV injection, we investigated whether Cdk5 deletion in the VTA affected depression- and anxiety-related behaviors. The OFT was used to determine whether VTA-specific deletion of Cdk5 affected locomotor activity and anxiety-related behavior. Reduced activity in the center of an open field has been correlated with anxiety- and depressive-like behaviors in rodents (El Yacoubi et al., 2003). We analyzed the total distance traveled and time spent in center (center time) in the OFT. A one-way ANOVA revealed that VTA-specific deletion of Cdk5 did not affect locomotor activity, as shown by the total distance traveled (F(2,33) = 0.37, p = 0.69), but significantly decreased the center time (F(2,33) = 5.83, p = 0.007; Fig. 2B). We then performed the SPT to assess anhedonia, a core symptom of depression (Duman, 2007). VTA-specific deletion of Cdk5 significantly decreased the sucrose preference (F(2,33) = 6.14, p = 0.005; Fig. 2C). We used the EPM test to evaluate anxiety-related behavior. A decrease in number of entries and the time spent in the open arms indicates anxiety-like behavior (Rodgers and Dalvi, 1997; Komada et al., 2008). However, neither the entries into the open arms (F(2,33) = 0.14, p = 0.87) nor the time spent in the open arms (F(2,33) = 0.75, p = 0.48) was affected by VTA-specific deletion of Cdk5 (Fig. 2D). The NSF test is another test for depressive- and anxiety-like behavior (Santarelli et al., 2003). In the NSF test, a fasting mouse faces the choice between eating the food in the center of an open field and avoiding the novel environment. An increase in the latency to feed in the novel environment indicates anxiety-like behavior. VTA-specific deletion of Cdk5 significantly increased the latency to feed in the novel environment (F(2,33) = 7.01, p = 0.003), but did not alter the latency to feed in the home cage (F(2,33) = 0.68, p = 0.51; Fig. 2E), suggesting that the Cdk5-deletion-induced change in the latency to feed in the novel environment in the NSF test cannot be explained by possible changes in appetite. Finally, we performed the FST, which has been used for screening behavioral effects of antidepressants (Porsolt et al., 1977). An increase in the immobility time in the FST suggests behavioral despair and depression. VTA-specific deletion of Cdk5 significantly increased the immobility time in the FST (F(2,33) = 6.64, p = 0.004; Fig. 2F). Therefore, VTA-specific deletion of Cdk5 induces depressive-like behaviors in most of the behavioral tests.
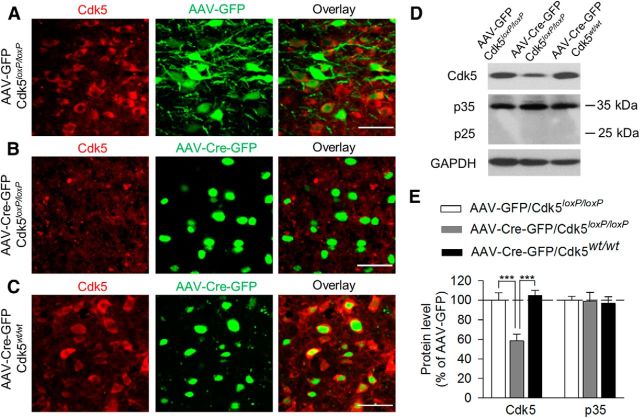
After the behavioral tests, mice were euthanized and Cdk5 protein expression was determined using immunofluorescence labeling and Western blotting. We found that Cdk5 was expressed in the VTA in Cdk5loxP/loxP mice that received intra-VTA injection of AAV2-GFP or in Cdk5wt/wt mice that received intra-VTA injection of AAV2-Cre-GFP. However, Cdk5 was lost in the VTA in Cdk5loxP/loxP mice that received intra-VTA injection of AAV2-Cre-GFP (Fig. 3A–C). Western blotting analysis of VTA tissue samples showed that Cdk5 levels in the VTA were significantly decreased in Cdk5loxP/loxP mice that received AAV2-Cre-GFP injection compared with the other two groups (F(2,15) = 15.65, p < 0.001; Fig. 3D,E). VTA-specific deletion of Cdk5 did not alter the protein levels of Cdk5 cofactor p35 significantly (F(2,15) = 0.06, p = 0.94; Fig. 3D,E). p25, a truncated form of p35 (Patrick et al., 1999), was not detectable in VTA tissue samples (Fig. 3D). These results confirmed that AAV2-Cre-GFP was effective in deleting Cdk5 in the VTA and suggested that VTA-specific deletion of Cdk5 induced depressive-like behaviors.
Figure 3.
AAV2-Cre-GFP-mediated VTA-specific deletion of Cdk5 in the VTA. After the behavioral tests, Cdk5 protein levels in the VTA were determined using immunofluorescence labeling (A–C) and Western blotting (D,E). Timeline for the AAV microinjection, behavioral tests, immunofluorescence labeling, and Western blotting is shown in Figure 2A. A–C, Immunofluorescence labeling showed that Cdk5 was expressed in the VTA in Cdk5loxP/loxP mice that received intra-VTA injection of AAV2-GFP (A) or in Cdk5wt/wt mice that received intra-VTA injection of AAV2-Cre-GFP (C). However, Cdk5 was lost in the VTA in Cdk5loxP/loxP mice that received intra-VTA injection of AAV2-Cre-GFP (B). Scale bar, 50 μm. D, E, Representative (D) and summarized (E) data of Western blots for Cdk5, p35/25, and GAPDH showing that VTA-specific deletion of Cdk5 significantly decreased protein levels of Cdk5 in the VTA (p < 0.001) without affecting the protein levels of Cdk5 cofactor p35 (p > 0.05). Immunoreactivity was normalized to GAPDH and is presented as a percentage of that of Cdk5loxP/loxP mice with AAV2-GFP injection group. The p values for Tukey's post hoc test results are shown on the top (***p < 0.001; n = 6 mice/group).
Dopamine neuron-specific conditional knock-out of Cdk5 induced depressive-like behaviors
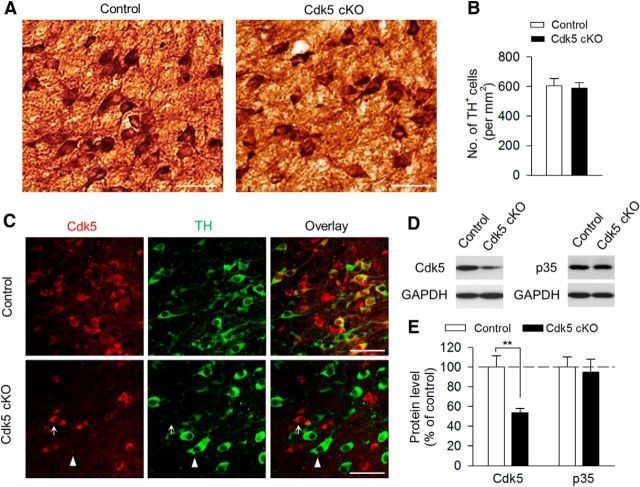
The VTA consists of mainly dopamine and GABAergic neurons (Johnson and North, 1992), so we next sought to determine the role of Cdk5 specifically in VTA dopamine neurons in regulating depression-related behavior. To delete Cdk5 selectively from dopamine neurons, we used DAT-Cre+/− mice that express Cre recombinase controlled by DAT promoter (Bäckman et al., 2006). We generated dopamine neuron-specific Cdk5 knock-out mice by crossing homozygous Cdk5loxP/loxP mice with heterozygous DAT-Cre+/− mice. Among the offspring generated, DAT-Cre+/−/Cdk5loxP/loxP mice were used as the Cdk5 conditional knock-out (Cdk5 cKO) group. DAT-Cre−/−/Cdk5loxP/loxP and DAT-Cre−/−/Cdk5loxP/wt mice were used as the control groups. The control and Cdk5 cKO mice were grossly normal. Immunohistochemical staining with TH did not detect any overt alterations in the gross morphology and total number of TH+ dopamine neurons in Cdk5 cKO mice compared with those in control mice (t(22) = 0.29, p = 0.78; Fig. 4A,B). In control mice, Cdk5 was expressed in both TH+ dopamine neurons and TH− nondopamine neurons. In Cdk5 cKO mice, Cdk5 and TH labeling were nonoverlapping and Cdk5 was expressed in TH− neurons but was absent in TH+ neurons (Fig. 4C). Western blotting analysis of VTA tissue samples showed that Cdk5 protein levels were significantly decreased in Cdk5 cKO mice (t(10) = 3.68, p = 0.004; Fig. 4D,E), whereas p35 protein levels were not significantly changed (t(10) = 0.31, p = 0.77; Fig. 4D,E). These data confirmed the effectiveness and specificity of Cdk5 deletion in dopamine neurons.
Figure 4.
Generation of dopamine neuron-specific Cdk5 cKO mice. A, DAB staining for TH of VTA sections of control and Cdk5 cKO mice. Scale bar, 50 μm. B, Summarized data showing that the total numbers of TH+ dopamine neurons were not significantly different between control and Cdk5 cKO mice (p > 0.05; n = 12 sections from 3 mice/group). C, Immunofluorescence staining for Cdk5 (red) and TH (green) of VTA sections of control and Cdk5 cKO mice. Cdk5 was expressed in both TH+ and TH− neurons in the VTA in control mice (top). Cdk5 was absent in TH+ dopamine neurons (indicated by arrowhead), but was expressed in TH− neurons (indicated by arrow) in Cdk5 cKO mice (bottom; n = 3 mice/group). Scale bar, 50 μm. D, E, Representative (D) and summarized (E) data of Western blots showing that Cdk5 levels in the VTA were significantly decreased in Cdk5 cKO mice compared with those in control mice (**p < 0.01; n = 6 animals/group). However, p35 protein levels were not significantly different between these two genotypes (p > 0.05). Immunoreactivity was normalized to GAPDH and is presented as a percentage of that of control mice.
Next, we investigated whether conditional knock-out of Cdk5 in dopamine neurons affected depression-related behaviors. Behavioral tests shown in Figure 2 were performed in animals without prior stress exposure and such baseline responses do not reflect stress vulnerability, which could be more relevant to pathophysiology of depression. We therefore subjected control and Cdk5 cKO mice to sub-CUS and CUS (Willner et al., 1987; Koo and Duman, 2008). Control and Cdk5 cKO mice were exposed to a variety of mild stressors in an unpredictable manner for 1 week (sub-CUS) or 5 weeks (CUS; Table 1) or were not subjected to any stressors (nonstressed). Two-way ANOVA showed that neither stress nor Cdk5 cKO altered locomotor activity, as shown by the total distance traveled (stress: F(2,59) = 0.86, p = 0.43; Cdk5 cKO: F(1,59) = 0.38, p = 0.54; stress × Cdk5 cKO interaction: F(2,59) = 0.44, p = 0.65) and center time (stress: F(2,59) = 1.10, p = 0.34; Cdk5 cKO: F(1,59) = 0.26, p = 0.61; stress × Cdk5 cKO interaction: F(2,59) = 0.51, p = 0.60) in the OFT (Fig. 5A). In the SPT, stress and Cdk5 cKO had significant main effects on the sucrose preference (stress: F(2,59) = 23.25, p < 0.001; Cdk5 cKO: F(1,59) = 53.80, p < 0.001, stress × Cdk5 cKO interaction: F(2,59) = 4.02, p = 0.02; Fig. 5B). Tukey's post hoc tests indicated that, in nonstressed groups, Cdk5 cKO mice exhibited a significant decrease in the baseline sucrose preference compared with wild-type control mice (p = 0.006). CUS (p < 0.001), but not sub-CUS (p = 0.74), significantly decreased the sucrose preference in wild-type control mice, whereas both sub-CUS (p < 0.001) and CUS (p < 0.001) further decreased the sucrose preference in Cdk5 cKO mice. These results indicate that Cdk5 cKO mice exhibited increased vulnerability to stress.
In the EPM test, stress, but not Cdk5 cKO, had significant main effects on the number of entries into the open arms (stress: F(2,59) = 9.88, p < 0.001; Cdk5 cKO: F(1,59) = 0.40, p = 0.53; stress × Cdk5 cKO interaction: F(2,59) = 0.02, p = 0.98) and the time spent in the open arms (stress: F(2,59) = 4.26, p = 0.02; Cdk5 cKO: F(1,59) = 0.08, p = 0.78; stress × Cdk5 cKO interaction: F(2,59) = 0.09, p = 0.91; Fig. 5C). In addition, CUS, but not sub-CUS, significantly decreased the number of entries in the open arms (nonstress vs CUS, p < 0.001; nonstress vs sub-CUS, p = 0.51) and the time spent in the open arms (nonstress vs CUS, p = 0.04; nonstress vs sub-CUS, p = 1.00). In the NSF test, stress and Cdk5 cKO had significant main effects on the latency to feed in the novel environment (stress: F(2,59) = 5.47, p = 0.007; Cdk5 cKO: F(1,59) = 15.73, p < 0.001; stress × Cdk5 cKO interaction: F(2,59) = 3.20, p = 0.04; Fig. 5D). Tukey's post hoc tests indicated that, in nonstressed groups, Cdk5 cKO mice showed significant increase in the latency to feed compared with wild-type control mice (p = 0.02). In sub-CUS groups, Cdk5 cKO mice also exhibited significant increase in the latency to feed compared with control mice (p = 0.002). However, CUS significantly increased the latency to feed in control mice (p = 0.001) but did not produce further increase in the latency to feed in Cdk5 cKO mice (p = 0.87). In contrast, neither stress nor Cdk5 cKO affected the latency to feed in the home cage (stress: F(2,59) = 0.45, p = 0.64; Cdk5 cKO: F(1,59) = 1.21, p = 0.28; stress × Cdk5 cKO interaction: F(2,59) = 0.83, p = 0.44; Fig. 5D), suggesting that changes in the latency to feed in the novel environment in the NSF test cannot be explained by possible changes in appetite. In the FST, stress and Cdk5 cKO had main effects on the immobility time (stress: F(2,59) = 5.99, p = 0.004; Cdk5 cKO: F(1,59) = 16.19, p < 0.001; stress × Cdk5 cKO interaction: F(2,59) = 3.27, p = 0.04; Fig. 5E). In nonstressed groups, Cdk5 cKO mice showed significant increase in the immobility time compared with wild-type control mice (p = 0.03). In sub-CUS groups, Cdk5 cKO mice also showed significant increase in the immobility time compared with control mice (p = 0.002), whereas CUS significantly increased the immobility time in control mice (p = 0.02), but did not produce further increase in the immobility time in Cdk5 cKO mice (p = 0.80). Together, these results suggest that Cdk5 cKO increased stress vulnerability in the SPT, but not in the FST or NSF tests.
VTA- or dopamine neuron-specific deletion of Cdk5 decreased the phosphorylation of TH and CREB
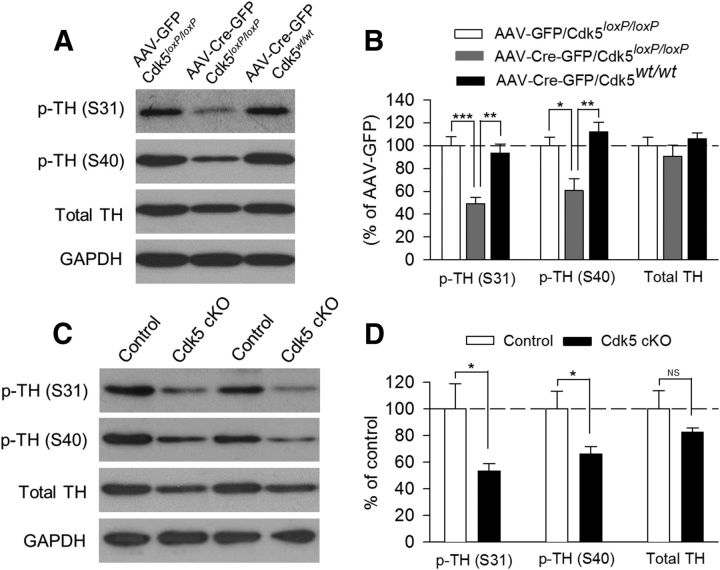
To find the molecular mechanisms responsible for the depressive-like behavior induced by Cdk5 deletion, we investigated whether Cdk5 substrates were altered by Cdk5 deletion in the VTA (AAV2-Cre-GFP) or dopamine neurons (Cdk5 cKO). Cdk5 interacts with a large number of substrates (Barnett and Bibb, 2011; Su and Tsai, 2011). One of the Cdk5 substrates is TH, the rate-limiting enzyme for dopamine synthesis (Dunkley et al., 2004). Cdk5 phosphorylates TH at serine residue Ser31 (Kansy et al., 2004; Moy and Tsai, 2004), but not at Ser40 (Kansy et al., 2004). Western blotting was performed to determine p-TH at Ser31 and Ser40 and total TH in VTA tissue samples. In the first set of experiments, we examined the effects of VTA-specific deletion of Cdk5 with AAV2-Cre-GFP on p-TH and TH protein levels. VTA tissue samples were prepared from Cdk5loxP/loxP mice that received intra-VTA injection of AAV2-Cre-GFP or AAV2-GFP and from Cdk5wt/wt mice that received intra-VTA injection of AAV2-Cre-GFP (Fig. 6A,B). There was a significant decrease in the protein level of p-TH (Ser31) in Cdk5loxP/loxP mice that received AAV2-Cre-GFP injection compared with the other two groups (F(2,12) = 15.00, p < 0.001). The phosphorylation of TH at Ser40 in the VTA was also decreased after VTA-specific deletion of Cdk5 (F(2,12) = 9.54, p = 0.003) and the total TH protein levels were not significantly changed (F(2,12) = 0.99, p = 0.40; Fig. 6A,B). In the second set of experiments, we performed Western blotting for p-TH at Ser31 and Ser40 and total TH in VTA tissue samples prepared from Cdk5 cKO and control mice (Fig. 6C,D). There was a significant decrease in the protein level of p-TH (Ser31) in Cdk5 cKO mice compared with that of control mice (t(10) = 2.37, p = 0.04). p-TH (Ser40) levels in the VTA was also decreased in Cdk5 cKO mice (t(10) = 2.39, p = 0.04). Cdk5 cKO had a modest effect on the total TH protein level that did not reach statistical significance (t(10) = 1.27, p = 0.23; Fig. 6C,D).
Figure 6.
VTA- or dopamine neuron-specific deletion of Cdk5 decreased p-TH levels in the VTA. A, Representative Western blots for p-TH (Ser31), p-TH (Ser40), total TH, and GAPDH in VTA homogenates prepared from Cdk5loxP/loxP mice that received intra-VTA injection of AAV2-Cre-GFP or AAV2-GFP and from Cdk5wt/wt mice that received intra-VTA injection of AAV2-Cre-GFP. B, Summarized data showing that the protein levels of p-TH (Ser31; p < 0.001) and p-TH (Ser40; p < 0.01) were significantly decreased in Cdk5loxP/loxP mice that received AAV2-Cre-GFP injection compared with the other two groups. The total TH protein level was not significantly changed (p > 0.05). The p values for Tukey's post hoc test results are shown on the top (*p < 0.05, **p < 0.01; ***p < 0.001; n = 5 mice/group). Immunoreactivity was normalized to GAPDH and is presented as a percentage of that of Cdk5loxP/loxP mice with AAV2-GFP injection group (AAV-GFP). C, Representative Western blots for p-TH (Ser31), p-TH (Ser40), total TH, and GAPDH in VTA homogenates prepared from control and Cdk5 cKO mice. D, Summarized data showing that the protein levels of p-TH (Ser31) and p-TH (Ser40) were significantly decreased in Cdk5 cKO mice compared with those in control mice (*p < 0.05). The total TH protein level was not significantly changed in Cdk5 cKO mice (p > 0.05; n = 6 mice/group). Immunoreactivity was normalized to GAPDH and is presented as a percentage of that of control mice.
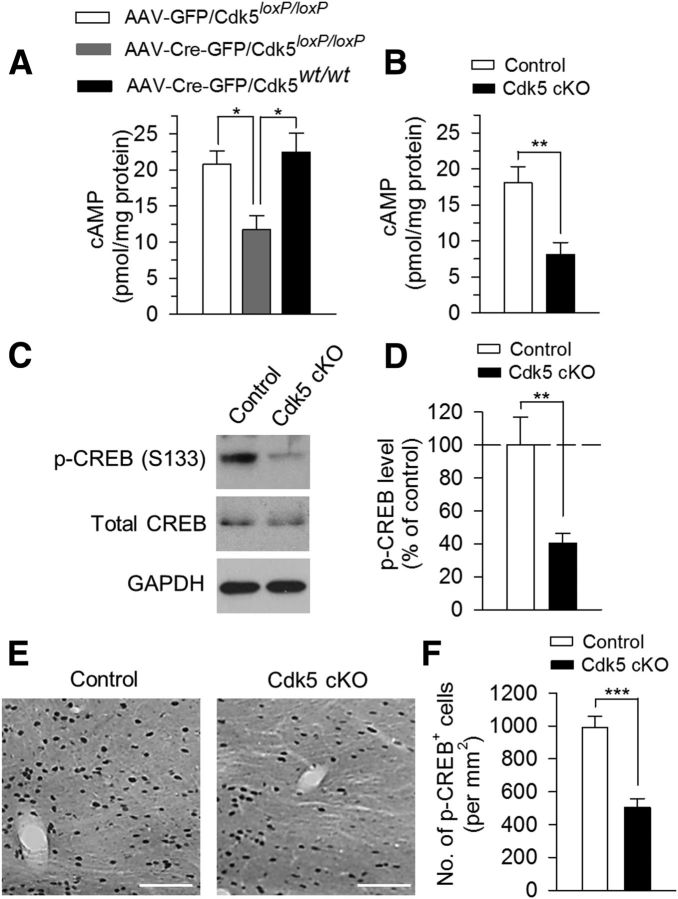
What might be the mechanism for Cdk5 deletion-induced decrease in p-TH (Ser40) levels? TH phosphorylation at Ser40 is known to be controlled by PKA (Haycock, 1996; Salvatore et al., 2001; Kansy et al., 2004). We measured cAMP levels in VTA tissue samples using an ELISA. We found that cAMP levels were significantly decreased in Cdk5loxP/loxP mice that received AAV2-Cre-GFP injection compared with those in Cdk5loxP/loxP mice that received AAV2-GFP injection or Cdk5wt/wt mice that received AAV2-Cre-GFP injection (F(2,12) = 7.20, p = 0.009; Fig. 7A). Similarly, cAMP levels were also significantly decreased in Cdk5 cKO mice compared with those in control mice (t(10) = 3.59, p = 0.005; Fig. 7B). cAMP/PKA phosphorylates and activates the transcription factor CREB at Ser133 (Silva et al., 1998). CREB in the NAc has been linked to mood regulation (Park et al., 2005; Wallace et al., 2009). Western blotting analysis showed that p-CREB (Ser133) in the VTA was significantly decreased in Cdk5 cKO mice (t(12) = 3.34, p = 0.006; Fig. 7C,D). In addition, immunohistochemical staining of midbrain VTA sections with p-CREB showed that p-CREB+ cells in the VTA were significantly decreased in Cdk5 cKO mice (t(30) = 5.48, p < 0.001; Fig. 7E,F).
Figure 7.
VTA- or dopamine neuron-specific deletion of Cdk5 disrupted cAMP signaling in the VTA. A, ELISA showing that cAMP levels were significantly decreased in Cdk5loxP/loxP mice that received AAV2-Cre-GFP injection compared with those in Cdk5loxP/loxP mice that received AAV2-GFP injection or Cdk5wt/wt mice that received AAV2-Cre-GFP injection (p < 0.01). The p values for Tukey's post hoc test results are shown on the top (*p < 0.05; n = 5 mice/group). B, ELISA showing that cAMP levels in the VTA were significantly decreased in Cdk5 cKO mice compared with those in control mice (*p < 0.01; n = 6 mice/group). C, D, Representative (C) and summarized (D) data of Western blots showing that p-CREB (Ser133) levels in the VTA were significantly decreased in Cdk5 cKO mice compared with those in control mice (**p < 0.01; n = 7 mice/group). Immunoreactivity was normalized to GAPDH and is presented as a percentage of that of control mice. E, F, Representative (E) and summarized (F) data of DAB staining showing that p-CREB+ cells in the VTA were significantly decreased in Cdk5 cKO mice compared with those in control mice (***p < 0.001; n = 16 sections from 4 mice/group). Scale bar, 100 μm.
Cdk5 cKO decreased dopamine responses in the NAc
Phosphorylation of TH at Ser40 increases the catalytic activity of TH, and thereby dopamine synthesis, and phosphorylation of TH at Ser31 also increases the catalytic activity of TH, but to a much less extent than for Ser40 phosphorylation (Dunkley et al., 2004). The decreases in p-TH at Ser31 and Ser40 imply that dopamine synthesis and release was decreased in Cdk5 cKO mice. To test this, we measured dopamine concentration in the shell of NAc slices directly using FSCV (Wightman, 2006; Yorgason et al., 2011). Dopamine is synthesized by dopamine neurons in the VTA and is axonally transported for release in the NAc. Dopamine release was evoked by electrical stimulation and detected by carbon-fiber microelectrode placed in the NAc shell. We examined dopamine responses evoked by a single electrical stimulus pulse or a train of stimuli (5 pulses) at 10, 30, and 50 Hz at fixed intensity (250 μA, 0.1 ms duration; Fig. 8). The recordings were made with the investigator blinded to the genotypes of the mice. The average peak concentration of dopamine in the NAc shell was decreased in Cdk5 cKO mice at single pulse and 10, 30, and 50 Hz 5-pulse stimulation (1 pulse: t(25) = 2.26, p = 0.03; 10 Hz: t(25) = 3.62, p = 0.001; 30 Hz: t(25) = 3.31, p = 0.003; 50 Hz: t(25) = 2.56, p = 0.02; Fig. 8B). The decay time constant (τ) of falling phase of dopamine transients is a function of dopamine uptake rate (Yorgason et al., 2011). The decay time constants of the dopamine responses were not significantly different between control and Cdk5 cKO mice at all stimulation frequencies (1 pulse: t25 = 0.66, p = 0.51; 10 Hz: t25 = 1.01, p = 0.32; 30 Hz: t25 = 0.67, p = 0.51; 50 Hz: t25 = 0.17, p = 0.86; Fig. 8C), suggesting that dopamine clearance in the NAc is not altered by Cdk5 cKO. Together, these results suggest that Cdk5 cKO in dopamine neurons decreased the phosphorylation of TH (Ser31) and TH (Ser40), which in turn decreased dopamine synthesis in the VTA and dopamine release in its target region, the NAc.
Figure 8.
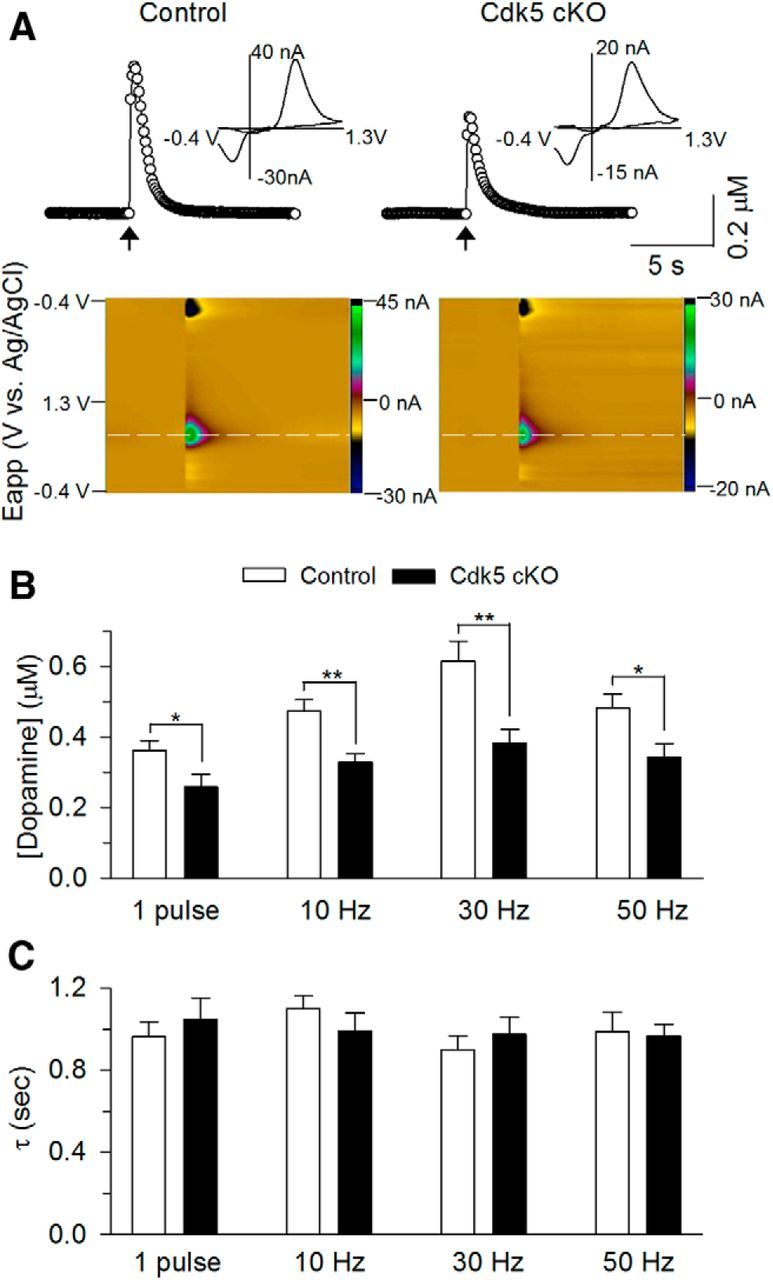
Cdk5 cKO decreased dopamine release in the NAc shell. A, Concentration trace (top) and color plot (bottom) for dopamine release triggered by electrical stimulation of the NAc shell in control and Cdk5 cKO mice. Top, Representative traces showing the concentration of dopamine (μm) over time in response to 5-pulse electrical stimulation at 30 Hz (indicated by arrow). Inset shows characteristic dopamine voltammogram. Bottom, Corresponding color plot depicting the voltammetric data with time on the x-axis, applied scan potential (Eapp) on the y-axis, and background-subtracted faradaic current shown on the z-axis in pseudocolor. Dopamine can be identified by an oxidation peak (green) at +0.6 V and a smaller reduction peak (black) at −0.2 V. B, Summarized data showeingthe average peak concentration of dopamine in the NAc Shell in response to single pulse and 5-pulse stimulation (10, 30, and 50 Hz; indicated by arrow). Dopamine release was significantly decreased in Cdk5 cKO mice at single pulse (*p < 0.05), 10 Hz (**p < 0.01), 30 Hz (**p < 0.01), and 50 Hz (*p < 0.05) stimulation compared with those in control mice. C, Summarized data showing no difference in the decay time constant (τ) of the dopamine responses between control and Cdk5 cKO mice at all stimulation frequencies (p > 0.05; n = 14 from 5 control mice; n = 13 from 5 Cdk5 cKO mice).
It has been shown that bath application of the Cdk5 inhibitor roscovitine enhanced dopamine response in striatal slices, as detected by amperometry (Chergui et al., 2004). Cdk5 inhibitors may increase dopamine responses by enhancing P/Q-type calcium channels (Tomizawa et al., 2002; Yan et al., 2002) and presynaptic neurotransmitter release (Kim and Ryan, 2010). Moreover, roscovitine inhibits dopamine transporter activity directly and independently of Cdk5 (Price et al., 2009) and dopamine uptake inhibitors are known to enhance dopamine response in the striatum (Yorgason et al., 2011).
Selective elevation of cAMP levels in VTA dopamine neurons reversed depressive-like behaviors induced by Cdk5 cKO
If a decrease in cAMP signaling contributes to depressive-like behaviors induced by Cdk5 cKO, activation of cAMP signaling in dopamine neurons should rescue these behavioral deficits. We used the Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technology to selectively elevate cAMP levels in VTA dopamine neurons and examined its impact on depression-related behaviors. DREADDs are mutated muscarinic G-protein-coupled receptors that are exclusively activated by pharmacologically inert ligand CNO, but not natural neurotransmitters (Armbruster et al., 2007). By expressing Gs-coupled DREADD (rM3D-Gs; Guettier et al., 2009; Farrell et al., 2013) selectively in dopamine neurons in the VTA, one can inducibly and reversibly increase cAMP levels in the VTA with cell specificity. Cre-inducible AAVs carrying a “double-floxed” inverted open reading frame (AAV8-hSyn-DIO-rM3D-Gs-mCherry, referred as rM3D-Gs) were bilaterally microinjected into the VTA of Cdk5 cKO and control mice. Three weeks after the AAV injections, we examined the expression of the AAVs in midbrain sections. In Cdk5 cKO mice, the AAVs, as indicated by mCherry, were expressed in ∼85% of TH+ dopamine neurons (green), but were not expressed in TH− neurons in the VTA. The AAVs were largely limited to VTA and were seldom expressed in the neighboring substantia nigra (Fig. 9). In control mice (DAT-Cre−/−/Cdk5loxP/wt or DAT-Cre−/−/Cdk5loxP/loxP), the AAVs (mCherry) were not expressed because of the lack of Cre-recombinase in these mice (data not shown).
Figure 9.
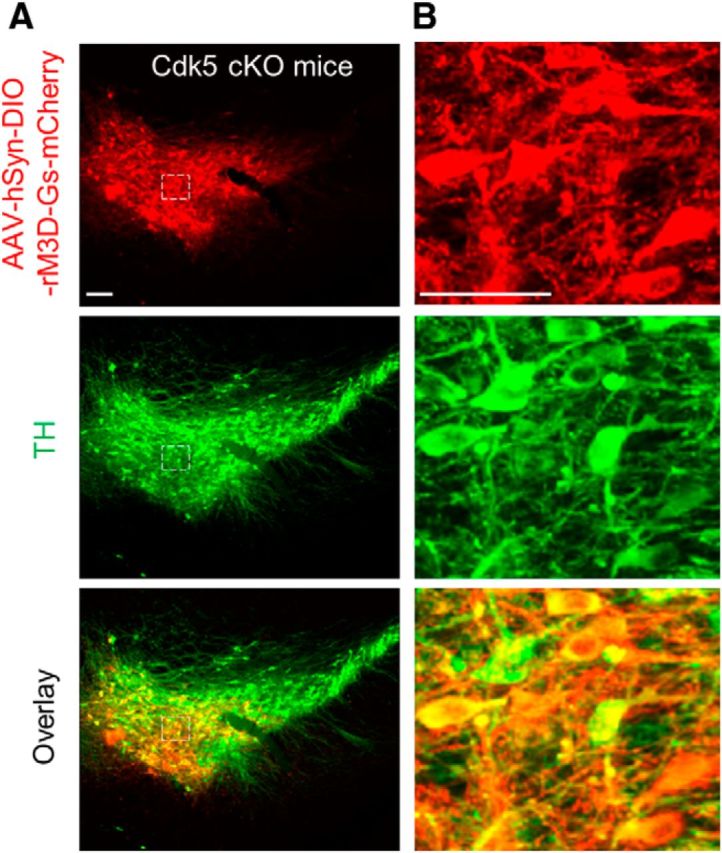
The DREADD receptor rM3D-Gs was expressed in midbrain dopamine neurons in the Cdk5 cKO (DAT-Cre+/−/Cdk5loxP/loxP) mice. A, Immunofluorescence labeling for AAV8-hSyn-DIO-rM3D-Gs-mCherry (red) and TH (green) in the midbrain VTA sections. Scale bar, 100 μm. B, High-power view of AAV8-hSyn-DIO-rM3D-Gs-mCherry and TH staining in the dashed boxes shown in A. AAVs were expressed in ∼85% of VTA dopamine neurons in the VTA. Scale bar, 50 μm. n = 3 mice/group.
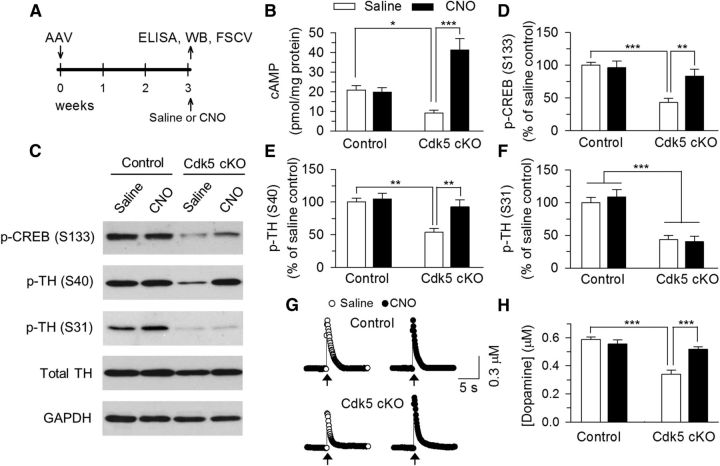
Having confirmed the expression of rM3D-Gs in VTA dopamine neurons, we next examined the effectiveness of intraperitoneal injection of CNO in activating rM3D-Gs in the VTA. The timeline of the AAV injection, ELISA, Western blotting, and FSCV is shown in Figure 10A. Previous studies have shown that intraperitoneal injection of CNO rapidly (<15 min) activates DREADDs in the brain, reaches the peak after ∼30–40 min, and the effect of CNO lasts several hours (Alexander et al., 2009; Rogan and Roth, 2011). We therefore chose to inject saline (0.9% NaCl) or CNO (2 mg/kg in saline) 30 min before euthanizing the mice and collecting VTA tissue samples. We measured cAMP levels in VTA samples using ELISA. Two-way ANOVA analysis revealed that CNO injection, but not genotype, had significant main effects on cAMP levels in the VTA (genotype: F(1,21) = 1.66, p = 0.21; CNO: F(1,21) = 17.56, p < 0.001; genotype × CNO interaction: F(1,21) = 19.96, p < 0.001; Fig. 10B). Tukey's post hoc tests showed that cAMP levels in the VTA were significantly decreased in Cdk5 cKO mice compared with control mice (p = 0.039), CNO did not cause cAMP accumulation in control mice that did not express rM3D-Gs due to lack of Cre-recombinase, but greatly enhanced cAMP levels in Cdk5 cKO mice that expressed rM3D-Gs selectively in dopamine neurons (p < 0.001).
Figure 10.
DREADD receptor rM3D-Gs-mediated activation of cAMP/PKA signaling in the VTA of Cdk5 cKO mice. A, Timeline for the AAV injection, ELISA, Western blotting (WB), and FSCV. B, ELISA showing that cAMP levels in the VTA were significantly decreased in Cdk5 cKO mice compared with those in control mice (*p < 0.05) and CNO greatly enhanced cAMP levels in Cdk5 cKO mice (*p < 0.001). C–F, Representative (C) and summarized (D, E) data of Western blots showing that p-CREB (Ser133; D) and p-TH (Ser40; E) levels in the VTA were significantly decreased in Cdk5 cKO mice compared with those in control mice (***p < 0.001, **p < 0.01) and these decreases were reversed by intraperitoneal injection of CNO (**p < 0.01). In addition, p-CREB (Ser133) and p-TH (Ser40) levels were not significantly different between control and Cdk5 cKO mice that received CNO injections (p > 0.05). In contrast, p-TH (Ser31) levels in the VTA were decreased in Cdk5 cKO mice (***p < 0.001) and CNO injection did not significantly affect p-TH (Ser31) levels (p > 0.05; F). n = 5 mice/group. Immunoreactivity was normalized to GAPDH and is presented as a percentage of that of control mice with saline injection (saline control). G, Representative traces of FSCV showing the micromolar concentration of dopamine over time in the NAc Shell in response to 5-pulse electrical stimulation at 30 Hz (indicated by arrow). H, Summarized data of FSCV showing that the average peak concentration of dopamine was significantly decreased in Cdk5 cKO mice compared with those in control mice (***p < 0.001) and these decreases were reversed by CNO (***p < 0.001). n = 9–11 from 4 mice/group.
We therefore investigated whether CNO increased p-CREB (Ser133), p-TH (Ser40), and p-TH (Ser31) in the VTA. Two-way ANOVA analysis revealed that Cdk5 cKO and CNO injection had significant main effects on p-CREB (Ser133; Cdk5 cKO: F(1,16) = 17.03, p < 0.001; CNO: F(1,16) = 4.58, p = 0.04; Cdk5 cKO × CNO interaction: F(1,16) = 6.69, p = 0.02; Fig. 10C,D) and p-TH (Ser40) levels (Cdk5 cKO: F(1,16) = 12.97, p = 0.002; CNO: F(1,16) = 7.21, p = 0.02; Cdk5 cKO × CNO interaction: F(1,16) = 4.54, p = 0.04; Fig. 10C,E) in the VTA. Tukey's post hoc tests showed that the levels of p-CREB (Ser133) and p-TH (Ser40) were significantly decreased in Cdk5 cKO mice compared with those in control mice (p-CREB: p < 0.001; p-TH: p = 0.001), and these decreases were reversed by CNO (p-CREB: p = 0.004; p-TH: p = 0.004). Importantly, p-CREB (Ser133) and p-TH (Ser40) levels were not significantly different between control and Cdk5 cKO mice that received CNO injections (p-CREB: p = 0.29; p-TH: p = 0.31). In contrast, Cdk5 cKO decreased p-TH (Ser31) levels in the VTA (F(1,16) = 54.22, p < 0.001), but CNO did not affect p-TH (Ser31) levels (F(1,16) = 0.11, p = 0.75; Fig. 10C,F). These results suggest that activation of rM3D-Gs by CNO increases cAMP levels and rescues Cdk5 cKO-induced deficits in CREB phosphorylation at Ser133 and TH phosphorylation at Ser40.
To determine whether changes in p-TH (Ser40) by CNO affected dopamine release, we measured dopamine concentration directly in the shell of NAc slices prepared from these 4 groups of mice 30 min after intraperitoneal injections of saline or CNO (2 mg/kg). We examined dopamine responses evoked by 5 pulses at 30 Hz, which is known to induce maximal or near maximal dopamine release in the NAc (Fig. 8). Two-way ANOVA revealed that Cdk5 cKO and CNO injection had significant main effects on dopamine concentration in the NAc (Cdk5 cKO: F(1,38) = 26.54, p < 0.001; CNO: F(1,38) = 5.01, p = 0.03; Cdk5 cKO × CNO interaction: F(1,38) = 12.56, p = 0.001; Fig. 10G,H). Tukey's post hoc tests showed that the dopamine concentration was significantly decreased in Cdk5 cKO mice compared with those in control mice (p < 0.001) and these decreases were reversed by CNO (p < 0.001).
We next determined whether activation of rM3D-Gs by CNO in VTA dopamine neurons could rescue depressive-like behaviors induced by Cdk5 cKO. Control (DAT-Cre−/−/ Cdk5loxP/loxP or DAT-Cre−/−/Cdk5loxP/wt) and Cdk5 cKO (DAT-Cre+/−/Cdk5loxP/loxP) mice received bilateral intra-VTA injections of rM3D-Gs as shown in Figure 10. We added an additional control group (DAT-Cre+/−/Cdk5wt/wt) of mice that received bilateral intra-VTA injections of rM3D-Gs. The timeline of the AAV injection and behavioral tests is shown in Figure 11A. Three weeks after the AAV injections, mice received intraperitoneal injection of saline or CNO (2 mg/kg) 30 min before each of the behavioral tests except for the SPT, during which mice received CNO injection every 8 h 2 times because the behavioral effects of CNO persist for several hours (Alexander et al., 2009). In the OFT 2-way ANOVA showed that genotype and CNO injection had significant main effects on locomotor activity, as indicated by the total traveled distance (genotype: F(2,49) = 11.44, p < 0.001; CNO: F(1,49) = 10.82, p = 0.002; genotype × CNO interaction: F(2,49) = 3.25, p = 0.04; Fig. 11B). Tukey's post hoc tests showed that CNO injection, but not saline injection, increased locomotor activity in Cdk5 cKO (p = 0.006) and DAT-Cre+/−/Cdk5wt/wt (p = 0.004) mice without affecting the locomotor activity in control mice that did not express rM3D-Gs (p = 0.85). Neither genotype nor CNO injection had significant effects on the center time in the open field (genotype: F(2,49) = 0.87, p = 0.43; CNO: F(1,49) = 0.88, p = 0.35; genotype × CNO interaction: F(2,49) = 1.05, p = 0.36; Fig. 11B). In the SPT, genotype and CNO injection had main effects on the sucrose preference (genotype: F(2,49) = 15.17, p < 0.001; CNO: F(1,49) = 8.01, p = 0.007; genotype × CNO interaction: F(2,49) = 3.21, p = 0.04; Fig. 11C). Tukey's post hoc tests showed that the sucrose preference was significantly decreased in Cdk5 cKO mice compared with control (p < 0.001) and DAT-Cre+/−/Cdk5wt/wt (p < 0.001) mice, and this decrease was reversed by CNO injection (p = 0.004). In addition, CNO injection did not significantly affect sucrose preference in DAT-Cre+/−/Cdk5wt/wt mice (p = 0.28). In the EPM test, neither genotype nor CNO injection had significant effects on the number of entries into the open arms (genotype: F(2,49) = 0.57, p = 0.57; CNO: F(1,49) = 0.01, p = 0.90; genotype × CNO interaction: F(2,49) = 0.45, p = 0.64) and the time spent in the open arms (genotype: F(2,49) = 0.73, p = 0.49; CNO: F(1,49) = 0.31, p = 0.58; genotype × CNO interaction: F(2,49) = 0.66, p = 0.52; Fig. 11D). In the NSF test, genotype and CNO injection had main effects on the latency to feed in the novel environment (genotype: F(2,49) = 3.26, p = 0.04; CNO: F(1,49) = 18.68, p < 0.001; genotype × CNO interaction: F(2,49) = 10.41, p < 0.001; Fig. 11E). Tukey's post hoc tests showed that the latency to feed was significantly increased in Cdk5 cKO mice compared with that in control (p < 0.001) and DAT-Cre+/−/Cdk5wt/wt (p < 0.001) mice, and this increase was reversed by CNO injection (p < 0.001). Furthermore, CNO injection did not significantly affect the latency to feed in DAT-Cre+/−/Cdk5wt/wt mice (p = 0.36). In contrast, neither genotype nor CNO injection affected the latency to feed in the home cage (genotype: F(2,49) = 1.11, p = 0.34; CNO: F(1,49) = 1.12, p = 0.30; genotype × CNO interaction: F(2,49) = 1.63, p = 0.21; Fig. 11E). In the FST, genotype and CNO injection had main effects on the immobility time (genotype: F(2,49) = 15.39, p < 0.001; CNO: F(1,49) = 14.98, p < 0.001; genotype × CNO interaction: F(2,49) = 4.19, p = 0.02; Fig. 11F). Tukey's post hoc tests showed that the immobility time was significantly increased in Cdk5 cKO mice compared with that in control (p = 0.02) and DAT-Cre+/−/Cdk5wt/wt (p = 0.002) mice, and CNO treatment reversed Cdk5 cKO-induced increase in the immobility time (p = 0.002). However, CNO did significantly decrease the immobility time in DAT-Cre+/−/Cdk5wt/wt mice (p < 0.001). Because CNO increased the locomotor activity in the OFT (Fig. 11B), the decrease in the immobility time in the FST might be explained by the secondary effect of increased locomotor activity. Therefore, activating rM3D-Gs in VTA dopamine neurons by CNO reversed Cdk5 cKO-induced behavioral deficits in the SPT and NSF tests and CNO decreased the immobility time in the FST in wild-type DAT-Cre+/−/Cdk5wt/wt mice and Cdk5 cKO (DAT-Cre+/−/Cdk5loxP/loxP) mice.
Discussion
We showed that Cdk5 deletion in the VTA and in midbrain dopamine neurons induced depressive-like behaviors that were accompanied by decreases in TH phosphorylation at Ser31 and Ser40 in the VTA and dopamine release in the NAc. Cdk5 deletion in the VTA decreased cAMP levels in the VTA, and selectively increased cAMP in VTA dopamine neurons with DREADD technology reversed Cdk5-deletion-induced deficits in TH phosphorylation, dopamine release, and depressive-like behaviors.
We used AAV-Cre-GFP and DAT-Cre driver to delete Cdk5 in Cdk5loxP/loxP mice. The AAV-Cre-GFP approach minimized potential effects of Cdk5 deletion on neuronal development. However, this approach could not discriminate between dopamine and nondopamine neurons. The Cdk5 cKO mice allowed us to delete Cdk5 selectively in dopamine neurons. Cdk5 deletion using these two approaches produced very similar depression-related behavioral phenotypes, although subtle difference in the OFT was found: mice in which Cdk5 was deleted by AAV2-Cre-GFP exhibited a significant decrease in the center time (Fig. 2B), whereas Cdk5 cKO mice displayed normal center time (Fig. 5A). It is unclear whether this difference can be attributed to different methods of obtaining Cdk5 deletion. We subjected control and Cdk5 cKO to the CUS paradigm and found that Cdk5 cKO mice exhibited increased stress vulnerability in the SPT, but not in the FST or NSF test (Fig. 5). Therefore, Cdk5 cKO mice exhibited increased level of anhedonia in response to chronic stress, a common feature for depression. Together, these two approaches complemented each other and provided evidence that Cdk5 activity in VTA dopamine neurons modulates depression-related behaviors. However, there are a number of important caveats and limitations to consider when interpreting the behavioral results. As discussed in a recent review (Nestler and Hyman, 2010), the FST is not a behavioral model for depression, but rather a method for screening antidepressant; sucrose preference is a good measure of anhedonia, but anhedonia is not unique to depression and is observed in other psychiatric diseases; and the NSF test may represent a better model of anxiety than depression.
Recent studies have shown that Cdk5 activity in the hippocampus regulates anxiety- and depressive-like behavior. Microinjection of a Cdk5 inhibitor into the dentate gyrus of rat hippocampus blocked depressive-like behavior induced by chronic mild stress, whereas overexpression of p35, an activator of Cdk5, in the dentate gyrus blocked the behavioral effect of certain antidepressants (Zhu et al., 2012). Mice with forebrain-specific deletion of Cdk5 in pyramidal neurons exhibit hyperactivity and increased center time in the OFT increased exploration in the light chamber in the light/dark exploration test, and decreased immobility time in the FST, suggesting reduced anxiety and depression (Su et al., 2013). Species and regional differences might explain the differential modulation of depression-related behaviors by Cdk5 deletion in the hippocampus and VTA.
TH, the rate-limiting enzyme in dopamine synthesis, is a known Cdk5 substrate (Kansy et al., 2004; Moy and Tsai, 2004). Cdk5 phosphorylates TH at Ser31 (Kansy et al., 2004; Moy and Tsai, 2004), but not at Ser40 (Kansy et al., 2004). We found that p-TH (Ser31) levels in the VTA were decreased in Cdk5 cKO mice, which can be explained by the absence of Cdk5-mediated phosphorylation of TH (Ser31). Interestingly, p-TH (Ser40) levels in the VTA were also significantly decreased in Cdk5 cKO mice. Considering that p-TH (Ser40) is phosphorylated by PKA (Haycock, 1996; Salvatore et al., 2001), we investigated whether the Cdk5 cKO-induced decrease in p-TH (Ser40) levels was caused by impaired cAMP/PKA signaling. Indeed, ELISA showed that the cAMP level was decreased in the VTA tissue of Cdk5 cKO mice. The expression of p-CREB, a direct downstream target of cAMP/PKA (Silva et al., 1998), was decreased in the VTA in Cdk5 cKO mice. cAMP is hydrolyzed and inactivated by phosphodiesterases (PDEs; Conti et al., 2003). Proteomic screening revealed that hippocampus-specific Cdk5 knock-out results in an increase in PDE protein levels and an impairment of cAMP/PKA signaling (Guan et al., 2011), so it is therefore likely that similar mechanisms operate in the VTA in Cdk5 cKO mice, leading to a decrease in phosphorylation of TH (Ser40). It should be pointed out that many molecules other than the cAMP pathway are altered after Cdk5 deletion, including receptors, channels, and trafficking molecules (Hawasli et al., 2007; Guan et al., 2011). Other pathways may also be involved in biomedical and behavioral changes induced by Cdk5 deletion. Although evaluating other substrates may provide additional information about the number of collected TH+ cells and cell types affected by Cdk5 cKO, it is likely that the changes in substrates of cAMP/PKA and Cdk5 mainly originate from dopamine neurons.
TH phosphorylation at Ser40 increases dopamine synthesis, whereas TH phosphorylation at Ser31 had a modest effect on dopamine synthesis (Dunkley et al., 2004). Cdk5 cKO decreased TH phosphorylation at both serine sites, which in turn led to a decrease in dopamine synthesis and release. Indeed, FSCV recordings showed that dopamine release in the NAc shell was significantly decreased in Cdk5 cKO mice. By expressing the DREADD receptor rM3D-Gs in VTA dopamine neurons, we were able to elevate cAMP selectively with cell-type specificity and minimal invasiveness. We found that stimulation of rM3D-Gs with CNO increased cAMP, p-CREB, and p-TH (Ser40) levels, but not p-TH (Ser31) levels, in the VTA and normalized dopamine responses in the NAc in Cdk5 cKO mice. These results suggest that TH phosphorylation at Ser40 plays a predominant role in dopamine synthesis (Dunkley et al., 2004) or, alternatively, that cAMP-induced TH phosphorylation at Ser40 can maintain dopamine synthesis by overcoming Cdk5 cKO-induced decrease in TH phosphorylation at Ser31.
These results indicate that Cdk5 positively modulates cAMP/PKA and dopamine synthesis in the VTA. In contrast, Cdk5 exerts opposite modulation of cAMP/PKA and dopamine signaling in the striatum. Phosphorylation of DARPP-32 at Thr75 by Cdk5 inhibits PKA and activates PP1 (Bibb et al., 1999; Nishi et al., 2000), whereas phosphorylation of DARPP-32 at Thr34 by D1 dopamine receptor-mediated activation of PKA inhibits PP1 (Nishi et al., 1997; Svenningsson et al., 1998; Bibb et al., 1999). Therefore, Cdk5 negatively regulates D1 dopamine receptor/PKA signaling in the striatum. In support of this idea, pharmacological inhibition or genetic deletion of Cdk5 in the striatum enhances psychomotor activating and rewarding effects of cocaine and cocaine-seeking behavior (Bibb et al., 2001; Benavides et al., 2007; Taylor et al., 2007). DARPP-32 was not expressed in the VTA (Ouimet et al., 1992) and the feedback loop in the striatum does not exist in the VTA, which may explain the differential modulation of cAMP/PKA and dopamine signaling between the VTA and striatum.
We have shown that Cdk5 deletion decreased cAMP, p-CREB, and p-TH levels in the VTA and dopamine release in the NAc and induced depressive-like behaviors, whereas activation of rM3D-Gs by CNO reversed most of the biomedical and behavioral changes induced by Cdk5 cKO. Although our experimental data are correlative rather than causative, we speculate that the deficits in TH phosphorylation and dopaminergic transmission might underlie Cdk5 deletion-induced depressive-like behaviors. In addition to TH, Cdk5 phosphorylates a large number of substrates (Barnett and Bibb, 2011; Su and Tsai, 2011). For example, Cdk5 phosphorylates N-type voltage-gated calcium channels, resulting in facilitation of neurotransmitter release via an increase in the number of docked vesicles at the synaptic cleft (Su et al., 2012). Cdk5 cKO may decrease dopamine release by negatively modifying presynaptic N-type calcium channels. Our studies do not exclude the possibility that other Cdk5 targets could be responsible for the behavioral deficits induced by Cdk5 deletion in the VTA.
Perturbed dopaminergic transmission might play a role in the pathophysiology of depression. In patients with untreated Parkinson's disease, higher depression scores were associated with lower dopamine synthesis capacity in the striatum, as determined by positron emission tomography of 18F-fluorodopa uptake (Joutsa et al., 2013). The dopamine agonist pramipexole produced antidepressant effects in patients who failed to respond to standard antidepressant treatments (Franco-Chaves et al., 2013). Long-lasting changes in action potential firing pattern, gene regulation, and protein expression occur in the VTA-NAc pathway in several animal models of depression and antidepressant treatment (Shirayama et al., 2004; Berton et al., 2006; Krishnan et al., 2007; Chartoff et al., 2009; Wilkinson et al., 2009; Cao et al., 2010). Optogenetic inhibition of VTA dopamine neurons induces depressive-like behaviors, whereas chronic mild stress induces a depression-like phenotype that is reversed by phasic activation of VTA dopamine neurons (Tye et al., 2013). Conversely, phasic activation of VTA neurons projecting to the NAc induces susceptibility to social-defeat stress and optogenetic inhibition of the VTA-NAc projection induces resilience (Chaudhury et al., 2013). We have shown that Cdk5 loss of function in the VTA decreased TH phosphorylation and dopamine synthesis and release and induced depressive-like behaviors. Together, these results strongly support the idea that the mesolimbic dopamine system is critically involved in mood regulation and its dysfunction underlies the manifestation of depressive-like behaviors.
Footnotes
This work was supported by the National Institutes of Health (Grants R21 MH095921 and RO1 DA035217) and the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin. We thank Ming-Hu Han (Icahn School of Medicine at Mount Sinai) for critical reading of the manuscript.
The authors declare no competing financial interests.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Barnett DG, Bibb JA. The role of Cdk5 in cognition and neuropsychiatric and neurological pathology. Brain Res Bull. 2011;85:9–13. doi: 10.1016/j.brainresbull.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR, Bibb JA. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bibb JA. Decoding dopamine signaling. Cell. 2005;122:153–155. doi: 10.1016/j.cell.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Burn DJ. Depression in Parkinson's disease. Eur J Neurol. 2002;9:44–54. doi: 10.1046/j.1468-1331.9.s3.6.x. [DOI] [PubMed] [Google Scholar]
- Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci U S A. 2004;101:2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012;22:169–175. doi: 10.1016/j.tcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Drerup JM, Hayashi K, Cui H, Mettlach GL, Long MA, Marvin M, Sun X, Goldberg MS, Lutter M, Bibb JA. Attention-deficit/hyperactivity phenotype in mice lacking the cyclin-dependent kinase 5 cofactor p35. Biol Psychiatry. 2010;68:1163–1171. doi: 10.1016/j.biopsych.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. A silver bullet for the treatment of depression? Neuron. 2007;55:679–681. doi: 10.1016/j.neuron.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ, Lee HM, Sciaky N, Simmons A, Nonneman RJ, Huang XP, Hufeisen SJ, Guettier JM, Moy SS, Wess J, Caron MG, Calakos N, Roth BL. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology. 2013;38:854–862. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Chaves JA, Mateus CF, Luckenbaugh DA, Martinez PE, Mallinger AG, Zarate CA., Jr Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study. J Affect Disord. 2013;149:319–325. doi: 10.1016/j.jad.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Su SC, Gao J, Joseph N, Xie Z, Zhou Y, Durak O, Zhang L, Zhu JJ, Clauser KR, Carr SA, Tsai LH. Cdk5 is required for memory function and hippocampal plasticity via the cAMP signaling pathway. PLoS One. 2011;6:e25735. doi: 10.1371/journal.pone.0025735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, Roth BL, Wess J. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW. Short- and long-term regulation of tyrosine hydroxylase in chromaffin cells by VIP and PACAP. Ann N Y Acad Sci. 1996;805:219–230. doi: 10.1111/j.1749-6632.1996.tb17485.x. discussion 230–231. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Rinne JO, Eskola O, Kaasinen V. Reduced striatal dopamine synthesis capacity is associated with symptoms of depression in patients with de novo unmedicated Parkinson's disease. J Parkinsons Dis. 2013;3:325–329. doi: 10.3233/JPD-130205. [DOI] [PubMed] [Google Scholar]
- Kansy JW, Daubner SC, Nishi A, Sotogaku N, Lloyd MD, Nguyen C, Lu L, Haycock JW, Hope BT, Fitzpatrick PF, Bibb JA. Identification of tyrosine hydroxylase as a physiological substrate for Cdk5. J Neurochem. 2004;91:374–384. doi: 10.1111/j.1471-4159.2004.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;pii:1088. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Moy LY, Tsai LH. Cyclin-dependent kinase 5 phosphorylates serine 31 of tyrosine hydroxylase and regulates its stability. J Biol Chem. 2004;279:54487–54493. doi: 10.1074/jbc.M406636200. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci U S A. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman-Rakic P, Rakic P, Greengard P. Immunocytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. J Comp Neurol. 1992;323:209–218. doi: 10.1002/cne.903230206. [DOI] [PubMed] [Google Scholar]
- Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011;31:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Nguyen MD, Fischer A, Luke MP, Affar el B, Dieffenbach PB, Tseng HC, Shi Y, Tsai LH. Par-4 links dopamine signaling and depression. Cell. 2005;122:275–287. doi: 10.1016/j.cell.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 2001. [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Price DA, Sorkin A, Zahniser NR. Cyclin-dependent kinase 5 inhibitors: inhibition of dopamine transporter activity. Mol Pharmacol. 2009;76:812–823. doi: 10.1124/mol.109.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/S0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Su SC, Seo J, Pan JQ, Samuels BA, Rudenko A, Ericsson M, Neve RL, Yue DT, Tsai LH. Regulation of N-type voltage-gated calcium channels and presynaptic function by cyclin-dependent kinase 5. Neuron. 2012;75:675–687. doi: 10.1016/j.neuron.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Rudenko A, Cho S, Tsai LH. Forebrain-specific deletion of Cdk5 in pyramidal neurons results in mania-like behavior and cognitive impairment. Neurobiol Learn Mem. 2013;105:54–62. doi: 10.1016/j.nlm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience. 1998;84:223–228. doi: 10.1016/S0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci U S A. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K, Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, Liu QS. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology. 2010;35:2249–2261. doi: 10.1038/npp.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM. Detection technologies. Probing cellular chemistry in biological systems with microelectrodes. Science. 2006;311:1570–1574. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Yan Z, Chi P, Bibb JA, Ryan TA, Greengard P. Roscovitine: a novel regulator of P/Q-type calcium channels and transmitter release in central neurons. J Physiol. 2002;540:761–770. doi: 10.1113/jphysiol.2001.013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Zhong P, Liu X, Sun D, Gao HQ, Liu QS. Metabotropic glutamate receptor I (mGluR1) antagonism impairs cocaine-induced conditioned place preference via inhibition of protein synthesis. Neuropsychopharmacology. 2013;38:1308–1321. doi: 10.1038/npp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Pan B, Liu XJ, Long JZ, Zhang HT, Cravatt BF, Liu QS. Monoacylglycerol lipase inhibition blocks chronic mild stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.24. doi: 10.1038/npp.2014.24. Advance online publication. Retrieved Jan. 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WL, Shi HS, Wang SJ, Xu CM, Jiang WG, Wang X, Wu P, Li QQ, Ding ZB, Lu L. Increased Cdk5/p35 activity in the dentate gyrus mediates depressive-like behaviour in rats. Int J Neuropsychopharmacol. 2012;15:795–809. doi: 10.1017/S1461145711000915. [DOI] [PubMed] [Google Scholar]