Abstract
The prevalence of childhood-onset type 2 diabetes mellitus has increased dramatically over the past two or three decades in Japan, but epidemiological and clinical data remain limited. This survey was conducted to elucidate the current use of antidiabetic medications and the efficacy, safety and problems associated with the use of these agents. Clinical data on 259 children (younger than 18 yr of age; 121 boys and 138 girls) with type 2 diabetes treated at 42 medical centers throughout Japan between June and September 2003 were analyzed. Sixty-nine percent of all the type 2 diabetic patients (78% of the boys, 63% of the girls) were obese (percent overweight ≥ 20%) at the time of diagnosis. Overall, 172 subjects (66%) were treated using anti-hyperglycemic agents, including α-glucosidase inhibitors (α-GI), insulin, metformin and sulfonylureas (SUs). Many patients who were initially treated with a single medication eventually required insulin alone or in combination with an additional agent, suggesting that their diabetic control had deteriorated during the course of treatment. The HbA1c level of the 14 subjects who received only metformin decreased significantly without an improvement in obesity. Three cases with adverse events were reported, but causal relations with anti-hyperglycemic agents were not clear. In conclusion, mainly α-GI, insulin and metformin have been prescribed for childhood-onset type 2 diabetes patients in Japan. The results of this survey suggest that metformin is safe and effective for the treatment of type 2 diabetes with obesity in children and adolescents.
Keywords: type 2 diabetes mellitus, anti-hyperglycemic agents, metformin, obesity, children and adolescents
Introduction
The prevalence of childhood-onset type 2 diabetes mellitus has increased dramatically over the past two or three decades in Japan (1). Several nation-wide surveys have been conducted, but epidemiological and clinical data remain limited (2).
It is well known that life style modification and weight loss are the most important interventions that may realistically be expected to prevent disease progression and complications in type 2 diabetes mellitus in adults (3, 4). If the treatment goals with nutrition education and exercise are not met, pharmacologic therapy is indicated for children with type 2 diabetes (5). Indeed, many pediatricians realize the necessity of medical therapy for type 2 diabetes in children, and research on oral anti-hyperglycemic agents for children and adolescents is warranted.
The UK Prospective Diabetes Study (UKPDS) reported that intensive blood-glucose control with sulfonylureas (SUs) or insulin substantially reduced the risk of complications but not macrovascular disease in adult type 2 diabetes patients (6). The UKPDS also reported that, since intensive glucose control with metformin appears to decrease the risk of diabetes-related endpoints in overweight diabetic patients, and is associated with less weight gain and fewer hypoglycemic attacks than are insulin and SU, it may be the first-line pharmacological therapy of choice for these patients (7). Metformin has been recommended as a first choice for the treatment of type 2 diabetes in children and adolescents by the American Diabetes Association (ADA) (5). Metformin improves glycemic control by reducing hepatic glucose production, increasing insulin sensitivity, and reducing intestinal glucose absorption, without increasing insulin secretion (8, 9). In the U.S., metformin was approved by the FDA in 2000 for the treatment of diabetes in children based on results from a randomized controlled trial (10).
In Japan, no oral anti-hyperglycemic agents, including metformin, have been approved for children with type 2 diabetes by the Ministry of Health, Labour and Welfare. Furthermore, information on the efficacy, safety and problems associated with the use of anti-hyperglycemic agents for children is insufficient.
This survey was conducted to elucidate the current use of antidiabetic medication and the efficacy, safety and problems associated with these agents in children and adolescents with type 2 diabetes mellitus in Japan.
Subjects and Methods
A questionnaire survey targeted councilors of the Japanese Society for Pediatric Endocrinology (JSPE) and members of the Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT) in February 2003, in advance of our main survey of current medical treatments for childhood-onset type 2 diabetes mellitus. Eighty-three (67%) of 124 members answered the questionnaire. Seventy-two doctors currently managed childhood-onset type 2 diabetes patients, and the total number was estimated to be 530 patients. Seventy doctors agreed to participate in the main survey. Clinical data sheets were sent to the 70 members of the JSPE and JSGIT, who signed up to participate in this survey, in June 2003. Clinical data on 259 children (younger than 18 yr of age; 121 males and 138 females) with type 2 diabetes treated at 42 medical centers throughout Japan were received between June 2003 and September 2003, and were then analyzed.
The percent overweight was determined on the basis of the Japanese standard body weights for height by age and sex: (actual body weight – standard weight)/standard weight × 100 (%) (11).
The study was approved by the institutional ethics review board of Tokyo Women’s Medical University and informed consent was obtained from the patients and/or their parents.
Statistical Analysis
The Mann-Whitney U, χ2 and Fisher exact probability tests were used for statistical analysis. P values <0.05 were considered statistically significant.
Results
The ages of the 259 recruited patients were 11.9 ± 2.1 yr (6–16 yr) at diagnosis and 14.4 ± 2.0 yr (9–17 yr) at survey. Their HbA1c were 8.8 ± 2.8% (4.4–18.4%) at diagnosis and 7.0 ± 2.2% (4.1–14.2%) at survey. The percent overweight values at diagnosis ranged from –21.0% to 166.2%. Sixty-nine percent (n=174) of 253 patients had a family history of diabetes: father, mother, siblings and/or grandparents.
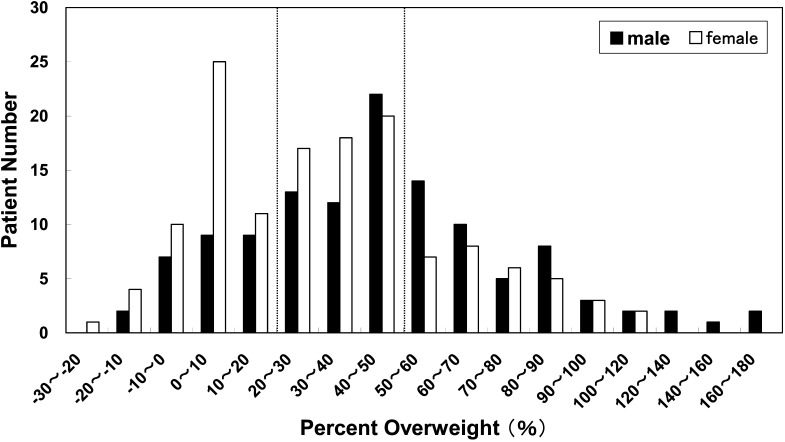
Figure 1 shows the distribution of percent overweight at diagnosis of type 2 diabetes. Seventy-eight percent (n=93) of the boys and 63% (n=86) of the girls were obese (percent overweight ≥20%) at the time of diagnosis. Thirty-nine percent (n=47) of the boys and 23% (n=31) of the girls had percent overweight values ≥50%.
Fig. 1.
The percent overweight distribution of patients at the time of type 2 diabetes mellitus diagnosis. The percent overweight was determined on the basis of the Japanese standard body weights for height by age and sex: (actual body weight – standard weight)/ standard weight × 100 (%). Seventy-eight % (n=93) of the boys and 63% (n=86) of the girls were obese (percent overweight ≥20%) at the time of diagnosis.
One-hundred and seventy subjects (66%) were diagnosed through school-age screening for glycosuria. HbA1c levels of patients identified by school-age glycosuria screening were 8.4 ± 2.6% and most were asymptomatic. Eighty-nine patients were found by means other than school-age screening, and their HbA1c levels were 9.5 ± 3.0%. HbA1c levels of patients identified by school-age glycosuria screening were significantly lower than those of the others (P<0.005).
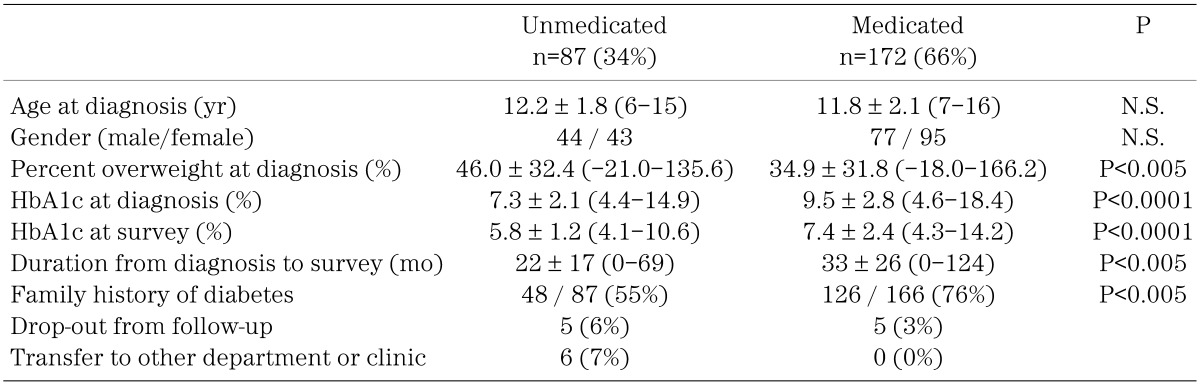
Eighty-seven (34%) of 259 subjects were treated without medication by adjusting their diet and physical activities. One-hundred and seventy-two subjects (66%) were treated using anti-hyperglycemic agents, including α-glucosidase inhibiter (α-GI), insulin, metformin, sulfonylureas (SUs) and nateglinide. Table 2 shows clinical characteristics of medicated and unmedicated subjects with type 2 diabetes. Percent overweight at diagnosis was higher in unmedicated (46.0 ± 32.4%) than in medicated (34.9 ± 31.8%) patients (P<0.005). HbA1c levels of unmedicated patients were lower than those of medicated patients at both diagnosis (7.3 ± 2.1% vs. 9.5 ± 2.8%, P<0.0001) and survey (5.9 ± 1.2% vs. 7.4 ± 2.4%, P<0.0001). The frequency of a family history of diabetes was significantly higher in medicated (75.9%) than in unmedicated (55.2%) patients (P<0.005).
Table 2. Clinical characteristics of medicated or unmedicated patients with type 2 diabetes mellitus.
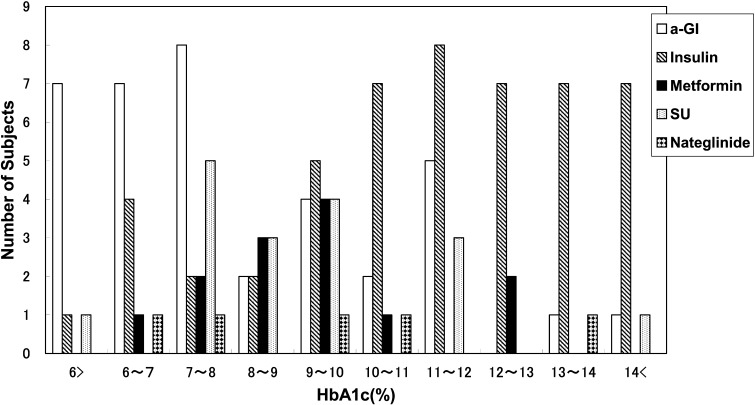
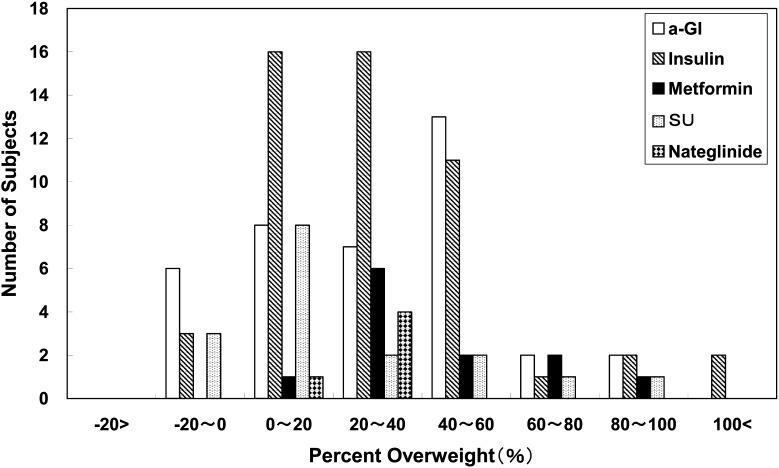
α-GI was the most commonly used anti-hyperglycemic agent, followed by insulin, metformin, SUs, nateglinide and a combination of α-GI and metformin (Table 3). Figure 2 shows the distribution of HbA1c at the time of diagnosis according to the prescribed medication. α-GI was prescribed mainly for children with a lower HbA1c level (8.1 ± 2.5%), while insulin was prescribed mainly for subjects with a higher HbA1c level (11.3 ± 2.6%) at the time of diagnosis (Table 3, Fig. 2). Metformin and SUs were given mainly to subjects with moderate levels of HbA1c, while metformin was prescribed mainly for subjects with a higher percent overweight (49.9 ± 29.9%) in contrast to SUs in subjects with lower percent overweight (21.4 ± 28.1%) at the time of diagnosis (Table 3, Fig. 3).
Table 3. Age, HbA1c and percent overweight at the time of diagnosis according to prescribed medications.
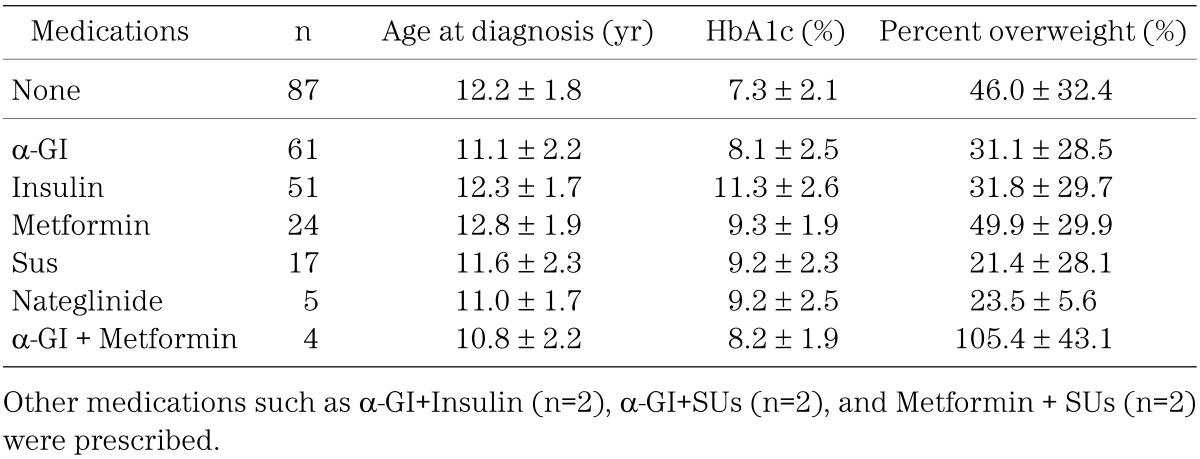
Fig. 2.
Distribution of HbA1c at the time of diagnosis according to prescribed medication. Mainly α-GI was prescribed for children with a lower HbA1c level, while mainly insulin was prescribed for children with a higher HbA1c level. Metformin and SUs were mainly prescribed for children with a moderate HbA1c level.
Fig. 3.
Distribution of percent overweight at the time of diagnosis according to prescribed medication. Metformin was prescribed mainly for subjects with a higher percent overweight, while SUs was given to those with a lower percent overweight at the time of diagnosis.
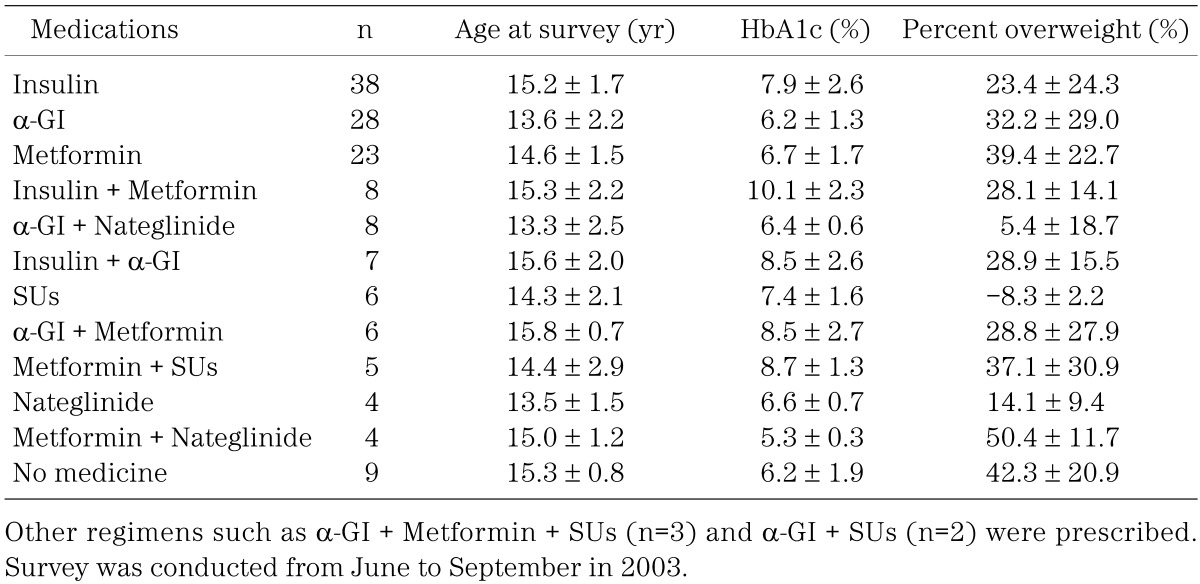
Table 4 shows age, HbA1c and percent overweight at the time of survey according to the prescribed medication. Many patients who were initially treated with a single agent eventually required combination therapy with an additional medication.
Table 4. Age, HbA1c and percent overweight at the time of survey according to prescribed medications.
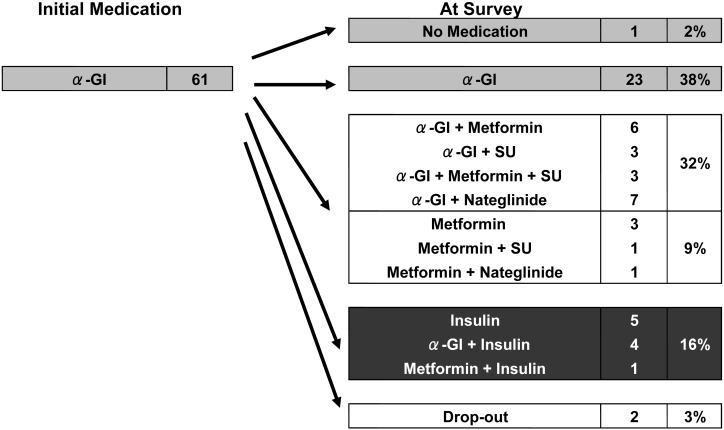
Twenty-three (38%) of 61 patients who started with α-GI alone remained on monotherapy with α-GI. However, 10 (16%) of 61 patients who started with α-GI were given insulin therapy, 14 (23%) were also given metformin, 9 (15%) had SUs added to their treatments, and for 8 (13%) nateglinide was added (Fig. 4). The addition of another agent to α-GI treatment for 59% of patients suggests that their diabetic control had not changed for the better or had deteriorated during the course of treatment.
Fig. 4.
Changes in medication during treatment in cases initially treated with α-GI. Twenty-three (38%) of 61 patients who started on α-GI alone remained on α-GI monotherapy. However, 10 (16%) of 61 patients who started on α-GI were also given insulin therapy, metformin was added in 14 (23%), cases SUs in 9 (15%), and nateglinide in 8 (13%).
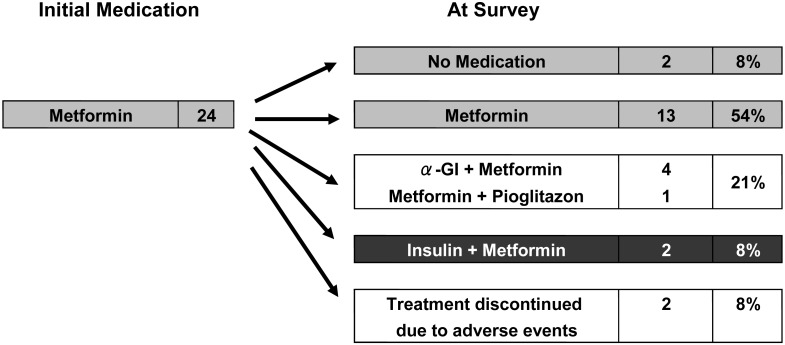
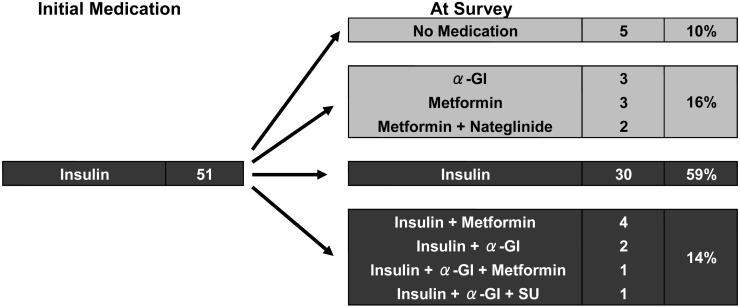
Two (8%) of 24 patients who started with metformin alone were given insulin therapy (Fig. 5). On the other hand, 13 (25%) of 51 patients who started with insulin alone were able to discontinue insulin therapy, suggesting recovery of endogenous insulin secretion (Fig. 6).
Fig. 5.
Changes in medication during treatment in cases initially treated with metformin. Two (8%) of 24 patients started on metformin alone, and insulin therapy was later added.
Fig. 6.
Changes in medication during treatment in cases initially treated with insulin. Thirteen (25%) of 51 patients who started on insulin alone were able to discontinue insulin therapy, suggesting the recovery of endogenous insulin secretion.
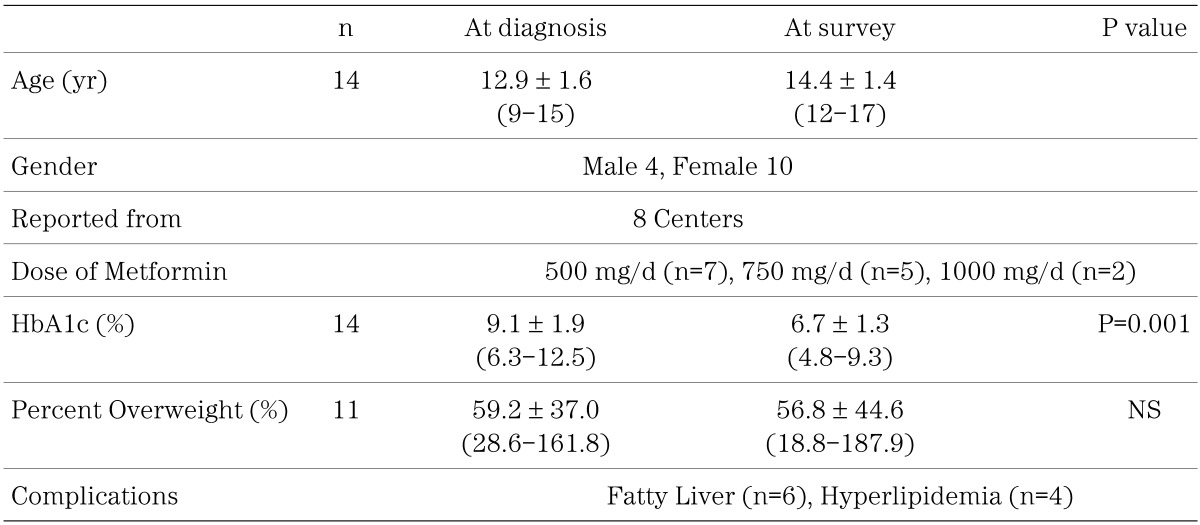
Fourteen subjects from 8 centers were treated with metformin alone (Table 5). Regarding the metformin dosage, 7 of the 14 received 500 mg or less, 5 were given 750 mg, and 2 subjects received 1000 mg. The HbA1c level of the 14 subjects who received only metformin decreased significantly from 9.1 ± 1.9% to 6.7 ± 1.3% (P=0.001) without a significant improvement in obesity (Table 5).
Table 5. Changes in HbA1c and percent overweight in 14 type 2 diabetes patients treated with metformin alone.
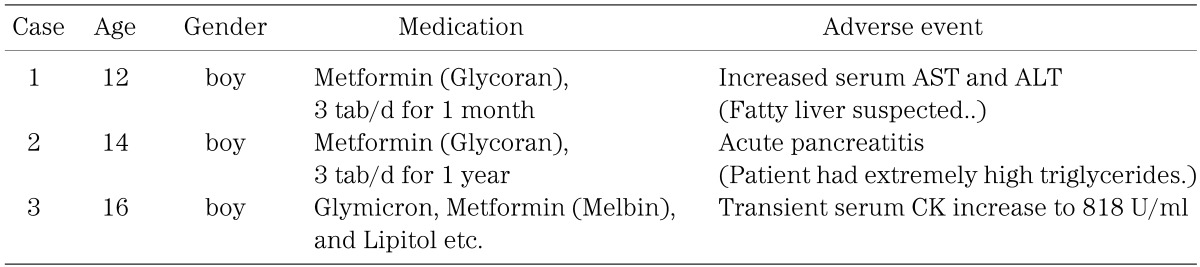
Three cases with adverse events were reported (Table 6), but causal relations with metformin were not clear. There were no reports of lactic acidosis among these patients.
Table 6. Three cases with adverse events reported in this survey.
Discussion
School-age screening for glycosuria has proven useful for finding diabetes mellitus in Japan, but a follow-up system for the diabetic patients identified has not yet been established in many regions of Japan. Furthermore, the precise number of children and adolescents with childhood-onset type 2 diabetes in Japan is unknown. The nation-wide survey in 1999 by Ohki et al. recruited 812 patients less than 18 yr of age with type 2 diabetes mellitus in Japan (2). They estimated that the prevalence of type 2 diabetes in Japan was 3.25: 100,000 children. In 2000, 1,019 patients with type 2 diabetes and 3,740 with type 1 diabetes were registered with the official fund for chronic diseases in children and adolescents in Japan (12). In our survey, about 530 patients with type 2 diabetes were estimated to be under the management of pediatric endocrinologists in 2003 in Japan and clinical records of 259 patients were obtained and analyzed. The present survey may represent one half to one quarter of the population with childhood-onset type 2 diabetes in Japan. This survey revealed the clinical characteristics of childhood-onset type 2 diabetes and current medical treatment for type 2 diabetes by pediatric endocrinologists in Japan.
This survey also revealed that 78% of the boys and 63% of the girls were obese (percent overweight ≥20%) at the time of their diagnosis. Obesity was confirmed to be one of the main factors associated with a childhood-onset type 2 diabetes in Japan. It was notable that the percent overweight distribution was similar between male and female patients except for a high frequency in non-obese female patients with 0–10% of percent overweight (Fig. 1). No individual in this non-obese female population (n=25) had positive GADAb titers. The frequency of a family history of diabetes in this non-obese female population was slightly higher (76%) than that of the obese population (68%), suggesting that this non-obese female population may be associated with stronger genetic factors including MODY.
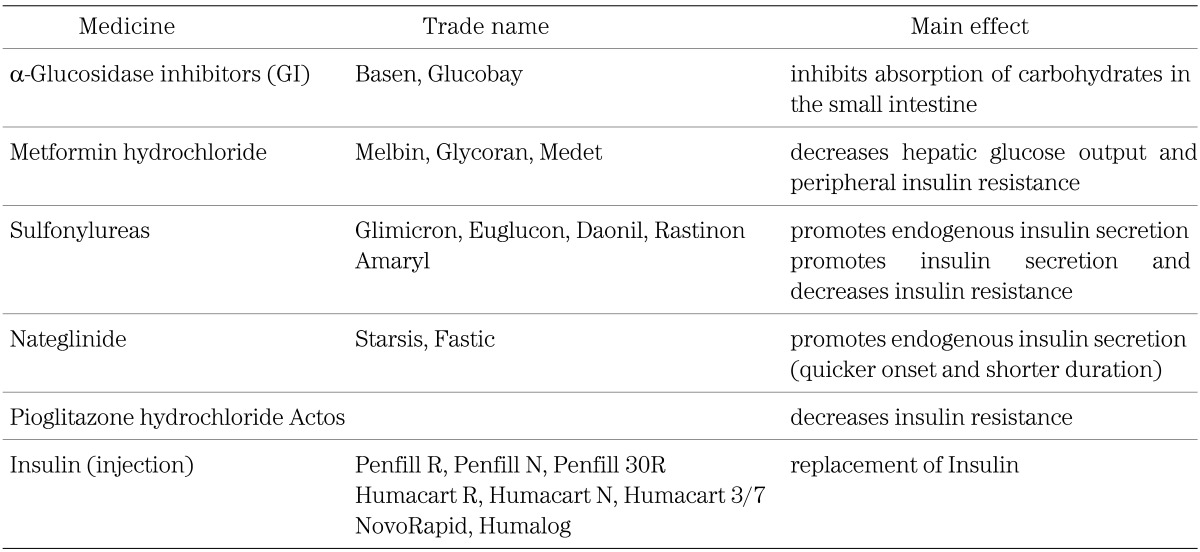
In total, 172 subjects (66%) were treated with anti-hyperglycemic agents. The frequency of anti-hyperglycemic agent usage has doubled, as compared to the approximately one third of patients who were on medication in the survey of 1999 by Ohki et al. (2). Mainly α-GI, insulin and metformin are currently prescribed for childhood-onset type 2 diabetes patients. α-GI is used mainly for children with a lower HbA1c level, in contrast to insulin which is mostly prescribed for subjects with a higher HbA1c level at the time of diagnosis (Table 3, Fig. 2). Metformin and SUs were prescribed mainly for subjects with moderate levels of HbA1c, metformin mainly for those with a higher percent overweight in contrast to SUs given to subjects with a lower percent overweight at the time of diagnosis (Table 3, Fig. 3). The choice of these medications in this survey is considered to be reasonable, given the functional mechanism of each agent (Table 1).
Table 1. Prescribed medication for 172 type 2 diabetes patients in this survey.
After the start of treatment with a single medication, combination therapy with an additional agent increased in many patients, suggesting that their diabetic control had not changed for the better or had deteriorated during the course of treatment. Further study to clarify the clinical characteristics of patients with poor diabetic control during the course of treatment may be necessary.
Medication for children with type 2 diabetes has not been approved by the Ministry of Health, Labour and Welfare. Anti-hyperglycemic agents except for insulin are all off-label pharmaceuticals for children with diabetes in Japan. This situation may not be unusual worldwide.
The American Diabetes Association Consensus Statement in 2000 was, “Of note is the fact that efficacy and safety data are not available for children nor are any of the oral drugs FDA approved for use in children. … If treatment goals with nutrition education and exercise are not met, pharmacologic therapy is indicated. The first oral agent used should be metformin (5)”. In the U.S., metformin was approved in 2000 by the FDA for use in children (older than 10 years of age, maximum dose: 2000 mg/day), based on the results of a randomized controlled trial (10).
In the UKPDS report, metformin similarly improved HbA1c level as did SUs and insulin therapy but did not induce weight gain (7). In meta-analysis metformin lowers blood glucose and HbA1c significantly, compared with placebo, and the body weight is significantly lower after metformin treatment compared with SU treatment because of an increase in body weight after SU treatment (13). Our survey reveals that the HbA1c level of the 14 subjects who received only metformin decreased significantly without a remarkable change of percent overweight (Table 5).
Lactic acidosis is a life-threatening condition characterized by low arterial pH (<7.35) and elevated arterial lactate levels (>5.0 mEq/l). Lactic acidosis is precipitated by phenformin, a biguanide formerly used to control hyperglycemia in type 2 diabetes. Metformin is now the only biguanide in general use, since it has a 10–20-fold lower risk of lactic acidosis than phenformin, and is regarded as a safe drug provided it is not used in at-risk patients, such as those with renal failure (14). There is no evidence to date that metformin therapy is associated with an increased risk of lactic acidosis or with increased levels of lactate compared with other antihyperglycemic treatments if the drugs are prescribed under study conditions, taking into account contraindications (15). Three cases with adverse events were reported (Table 6), but causal relations with metformin were not clear, and there were no reports of lactic acidosis among 51 patients treated with metformin in this survey. These results provide very useful and important information for us.
In conclusion, mainly α-GI, insulin and metformin have been prescribed for childhood-onset type 2 diabetes patients in Japan. This survey suggests that metformin is safe and effective for the treatment of type 2 diabetes with obesity in children. Additional studies on the efficacy and safety associated with a higher dosage (1000–1500 mg) of metformin in Japanese children may be required. Furthermore, we hope that a medication for children with type 2 diabetes will be approved by the Ministry of Health, Labour and Welfare in the near future.
Acknowledgments
This work was supported in part by grants from the Ministry of Health, Labour and Welfare of Japan.
We appreciate the following doctors who collaborated on this survey: Kohtaro Asayama, University of Occupational & Environmental Health; Yoshiya Ito, Asahikawa Medical College; Hiroaki Inomata, Teikyou University Ichihara Hospital; Tatsuhiko Urakami, Nihon University; Machiko Endo, Hakodate Goryokaku Hospital; Yukashi Ohki, Nippon Medical School; Nobuhide Ohyama, Ohyama Pediatric Clinic; Taisuke Okada, Kochi University; Kazumichi Onigata, Gunma University; Jiro Kagawa, Fujieda City General Hospital; Masayuki Kaji, Shizuoka Children’s Hospital; Tomoyuki Kawamura, Osaka City University; Toru Kikuchi, Niigata University; Nobuyuki Kikuchi, Yokohama City University Medical Center; Akihiko Kinugasa, Kyoto Prefectual University of Medicine; Kaichi Kida, Ehime University; Kazutaka Konishi, Abuyama Kodomo Clinic; Susumu Konda, Konda Kodomo Clinic; Kaori Sasaki, Tokyo Women’s Medical University; Osamu Shinohara, Tokai University; Kazuhiko Jinno, Hiroshima General Hospital of West JR; Toshihiro Tajima, Hokkaido University; Reiko Horikawa, National Center for Child Health and Development; Hiroyuki Tanaka, Okayama University; Masakuni Tokuda, Tokuda Kodomo Clinic; Yoshikazu Nishi, Hiroshima Red Cross Hospital; Soroku Nishiyama, Kumamoto University; Keiichi Hanaki, Tottori University; Masao Hayashi, National East Saga Hospital; Hirohiko Higashino, Kansai Medical University; Humi Arai, Shinshu University; Shinichiro Miyakawa, Hiroshima University; Ryuichi Kuromaru, Kyusyu University; Shigeki Miyamoto, Chiba Children’s Hospital; You Mogi, National Defence Medical College; Tetsuo Taguchi, Niigata Prefectual Shinhotta Hospital; Ichiro Yokota, University of Tokushima.
References
- 1.Kitagawa T, Owada M, Urakami T, Yamanouchi K. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr 1998; 37: 111–5 doi: 10.1177/000992289803700208 [DOI] [PubMed] [Google Scholar]
- 2.Ohki Y, Ohkawa T, Kishi M, Owada M, Sasaki N, Matsuura N. The survey on the childhood (<18 years of age)-onset type 2 diabetes mellitus in Japan. The report of Health, Welfare and Labor Ministry in Japan, 2001. (in Japanese)
- 3.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, life style, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345: 790–7 doi: 10.1056/NEJMoa010492 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403 doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Type 2 diabetes in children and adolescents (Consensus Statement). Diabetes Care 2000; 23: 381–9 doi: 10.2337/diacare.23.3.381 [DOI] [PubMed] [Google Scholar]
- 6.UKPDS Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–53 doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 7.UKPDS Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–65 doi: 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 8.Gerich JE. Drug therapy: oral hypoglycemic agents. N Engl J Med 1989; 321: 1231–45 doi: 10.1056/NEJM198911023211805 [DOI] [PubMed] [Google Scholar]
- 9.Bailey CJ. Biguanides and NIDDM (Review). Diabetes Care 1992; 15: 755–72 doi: 10.2337/diacare.15.6.755 [DOI] [PubMed] [Google Scholar]
- 10.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002; 25: 89–94 doi: 10.2337/diacare.25.1.89 [DOI] [PubMed] [Google Scholar]
- 11.Murata M. 2. Assessment of obesity 1) Growth curve and corpulence degree. Japanese Journal of Pediatrics (Syonika Rinsho). 2003; 56: 2315–26 (in Japanese). [Google Scholar]
- 12.Kato T. Chronic disease in children and adolescents. The Journal of Child Health 2004; 63: 489–94 (in Japanese). [Google Scholar]
- 13.Johansen K. Efficacy of metformin in the treatment of NIDDM. Meta-analysis. Diabetes Care 1999; 22: 33–7 doi: 10.2337/diacare.22.1.33 [DOI] [PubMed] [Google Scholar]
- 14.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334: 574–9 doi: 10.1056/NEJM199602293340906 [DOI] [PubMed] [Google Scholar]
- 15.Saltpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med 2003; 163: 2594–602 doi: 10.1001/archinte.163.21.2594 [DOI] [PubMed] [Google Scholar]