Abstract
The aim of this study was to compare the therapeutic effects of rhGH administered either by subcutaneous needle-injection (pens) or subcutaneous needle-free jet-injection (VISION®). Furthermore, a survey was carried out after using VISION® for 12 mo. A needle-free injection group consisting of 18 subjects (11 males and 7 females, mean age 5.87 ± 2.05 yr at the start of hGH therapy) who have not used pen injectors to date, were allowed to use VISION® in their third to fifth years of GH therapy. In addition, a group of 8 subjects who had been using pen injectors at our clinic (6 males and 2 females, mean age 6.54 ± 2.78 at the start of GH therapy) was monitored as a control. The results indicate that there are no significant differences between the mean growth rates, growth rate SD scores or height SD scores when comparing injection devices. Furthermore, the survey of VISION® revealed that 70% of the subjects found it slightly or not painful at or after injection, 70% found VISION® very easy or easy to use, and 80% found the weight of the device appropriate. All subjects expressed a desire to continue using VISION® in the future. Our results suggest that there are no problems with the effectiveness of hGH treatment with VISION®, a needle-free jet-injection device and that VISION® is an effective device for children who have an aversion to needle injection.
Keywords: GH deficiency, GH therapy, Needle-free jet-injection device
Introduction
Syringe materials have changed from glass to plastic, while the shape of the syringe itself has not changed much. The biggest change, has been in the size of the needle. In particular, needles have become thinner, reaching the present size of 32 gauge.
On the other hand, new syringe design has been sought. In order to eliminate the fear, pain and infection of needle injection, needle-free injection systems have been designed and put into clinical use.
Needle-free injection devices used in Japan include the Twin-Jector EZ and EZ II for human growth hormone use, SYRIJET for dental surgery/surgical management, and ShimaJET, recently developed and commercialized by a Japanese company for insulin use.
Several studies have compared needle-free injectors to conventional syringes with needles. So far, the results have been conflicting: the needle-free injector has been reported to be both less painful and more readily accepted by the patients and their families than conventional needle injection (1, 2), while other reports found no differences between needle-free injection and conventional syringes (3).
In 1993 we investigated the use of a needle-free injection system for GH therapy and reported that it was beneficial in improving patients’ adherence to treatment regimes and that no differences were found in the therapeutic effects, compared with needle injection (4). In our clinic, we carried out GH therapy using a needle-free injector for children who feared needle injection. However, due to a product recall in November 2000, an alternative needle-free injection system, VISION®, was imported directly from ANTARES PHARMA Co., U.S.A. and used for GH therapy.
We have collected data from 18 subjects using the VISION® needle-free injector and 8 subjects using conventional needle pen-injectors, and compared the data. Furthermore, a survey of the VISION® users was carried out. The results are reported below.
Subjects and Methods
The needle-free injection (VISION®) manufactured by Antares Pharma Co. is shown in Fig. 1. The needle-free syringe was exchanged with a new one, once a week.
Fig. 1.
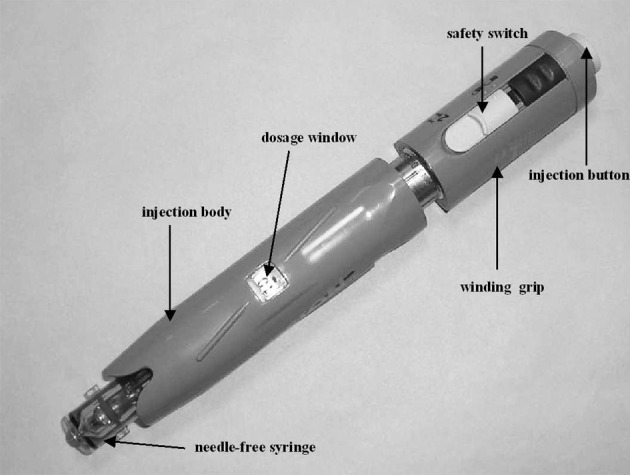
Needle-free injection device, VISION®.
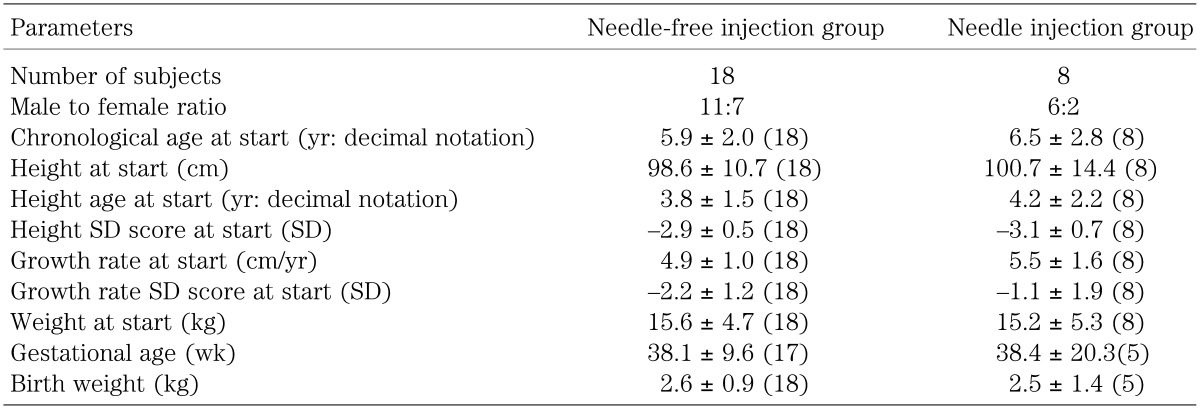
A needle-free injection group consisting of 18 subjects (11 males and 7 females, mean age 5.87 ± 2.05 yr at the start of hGH therapy) who have not used needle injectors to date, were allowed to use VISION® in their third to fifth years of GH therapy. In addition, a group of 8 subjects who had been using needle injectors at our clinic (6 males and 2 females, mean age 6.54 ± 2.78 at the start of GH therapy) was monitored as a control (Table 1). All subjects were pediatric patients with GH deficiency (GHD).
Table 1. Patient background of needle-free injection group and needle injection group.
Growth rates, growth rate SD scores and height SD scores from the needle-free injection group were compared with the control group and evaluated. Numerical values are shown as mean ± SD. Using the t-test, probabilities of not more than 5% were determined as significant difference.
A survey was carried out after using VISION® for 12 mo (Table 2), in which the operation and effectiveness of the device were evaluated.
Table 2. Survey contents.

Written consent to participate in this survey was obtained from the parents of the patients.
Results
For each parameter, as shown in Table 1, no differences were observed in the backgrounds of the needle and needle-free groups of patients.
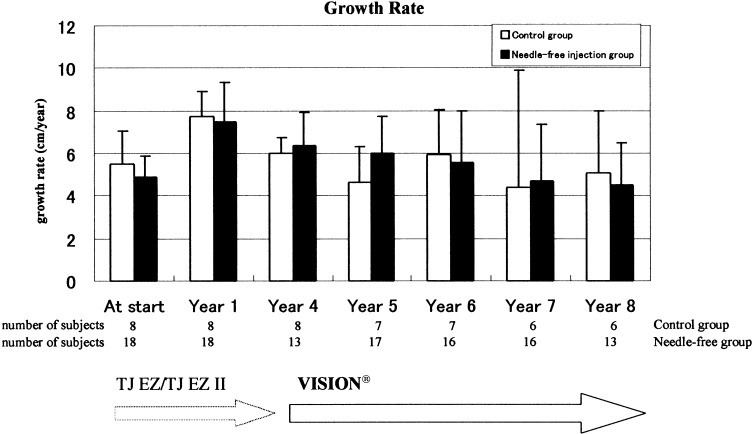
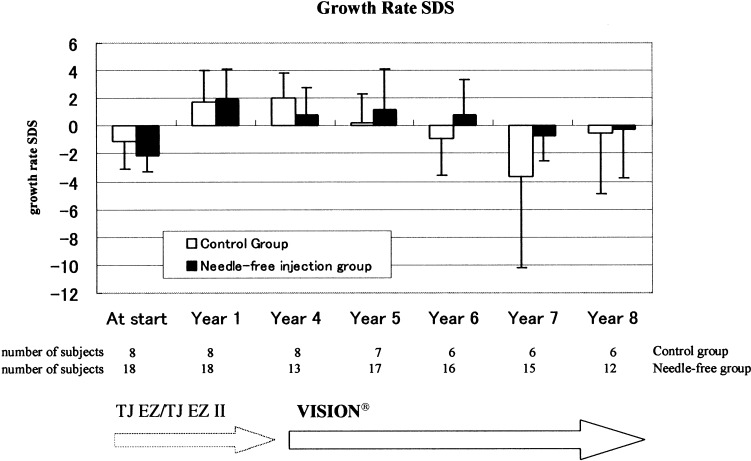
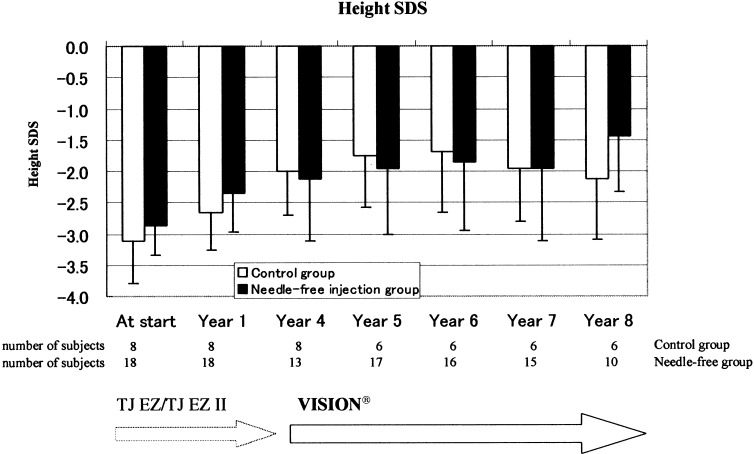
Figure 2 shows growth rates. The growth rates for both groups over 8 yr from the start of GH treatment were compared, with no significant differences observed between the needle and the needle-free groups. Figures 3 and 4 show growth rate SD scores and height SD scores, respectively. No differences were observed between the two groups in either the growth rate SD scores or the height SD scores.
Fig. 2.
Mean (SD) growth rates during duration of treatment by needle-free injection or traditional needle injection.
Fig. 3.
Mean (SD) growth rate SD scores during duration of treatment by needle-free injection or traditional needle injection.
Fig. 4.
Mean (SD) height SD scores during duration of treatment by needle-free injection or traditional needle injection.
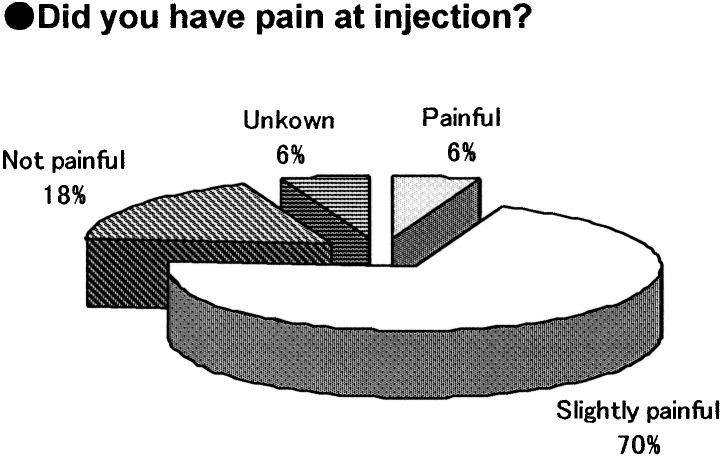
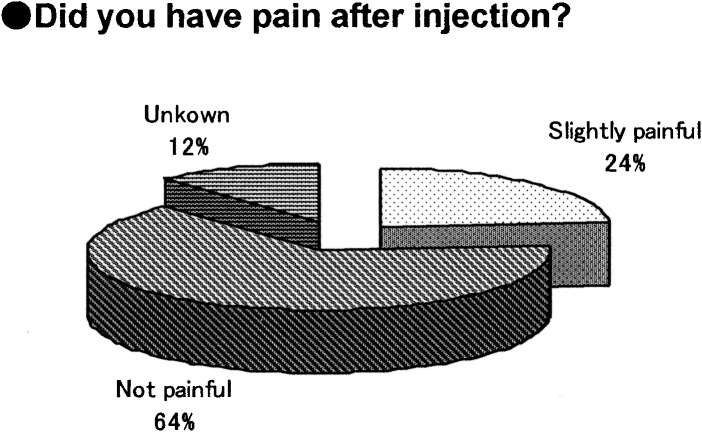
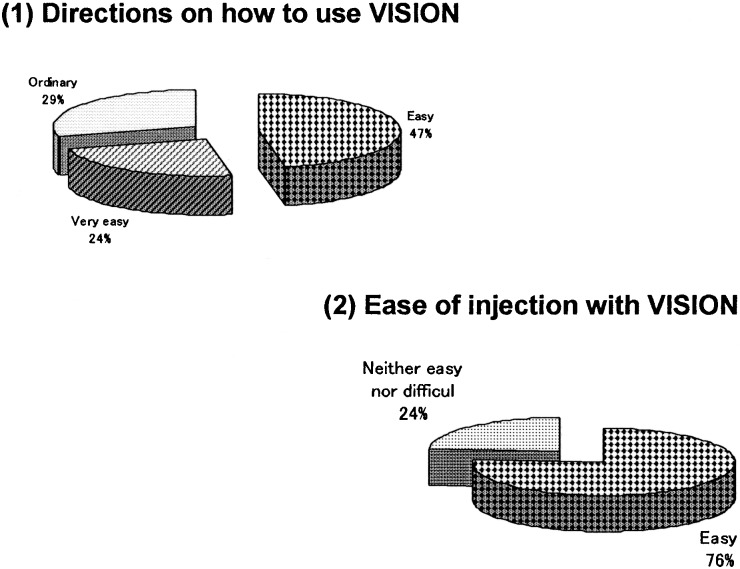
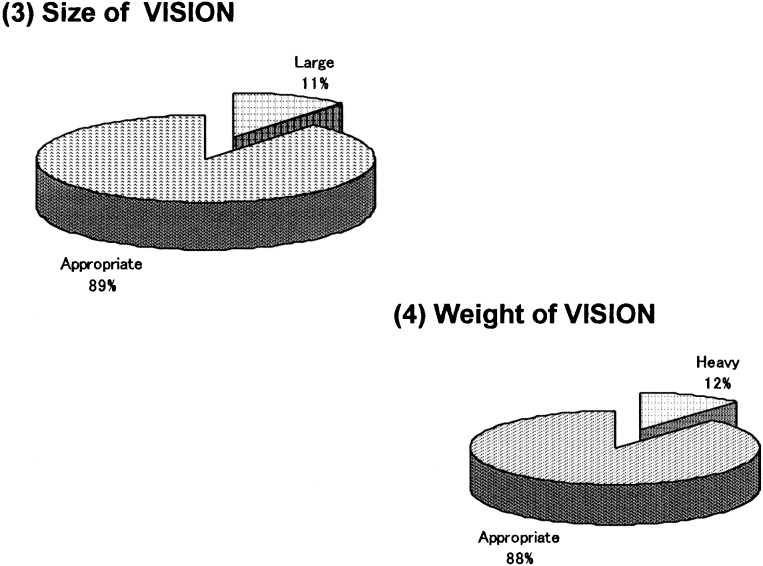
The survey was given to the 18 subjects who had experienced needle-free injection using Twin-Jector EZ, Twin-Jector EZ II and/or VISION®. Answers were obtained from 17 subjects (6 girls and 11 boys). The response rate was 94.4%. Figures 5, 6, 7, 8,9 show pain at injection, pain after injection, how to use, size/weight and desire to continue use, respectively. At injection, 70% felt slight or no pain. After injection, approximately 70% felt no pain. In comparison with the previous needle-free injectors, Twin-Jector EZ and/or Twin-Jector EZ II, about 70% responded that the directions for use of VISION were easy or very easy to understand. They also found that VISION® itself was easy to use. The subjects found the VISION injector to be appropriately sized and easy to handle. In addition, 80% or more answered that VISION® was not heavy. All subjects expressed a desire to continue using VISION in the future.
Fig. 5.
Responses to the descriptive questionnaire after using the needle-free injection (VISION®) for 12 mo.
Fig. 6.
Responses to the descriptive questionnaire after using the needle-free injection (VISION®) for 12 mo.
Fig. 7.
Responses to the descriptive questionnaire after using the needle-free injection (VISION®) for 12 mo.
Fig. 8.
Responses to the descriptive questionnaire after using the needle-free injection (VISION®) for 12 mo.
Fig. 9.
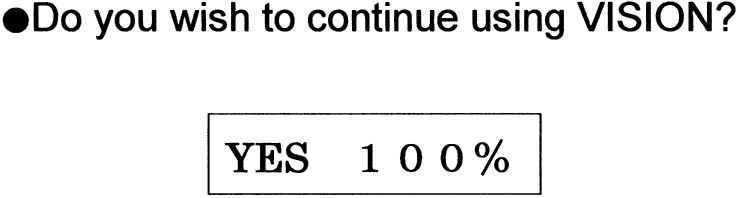
Responses to the descriptive questionnaire after using the needle-free injection (VISION®) for 12 mo.
Regarding breakage, in 4.5 yr of use by 18 subjects, a total of 3 syringes were broken by 2 subjects at our clinic. All incidents occurred at the initial stage of using VISION® and could have been caused by inappropriate operation.
Discussion
GH deficiency was treated by intramuscular injection of GH extracted from human pituitary glands in the early 1960s. Since then, there have been many advances in treatment encompassing administration, the injection product and the injection device.
In our clinic, we use a pen needle device for the treatment of GH. But the needle-free alternative is an effective solution for children who have an aversion to needle injection, and it is useful for increasing the dose of GH treatment.
In the United States, VISION® has been approved for subcutaneous self-administration of insulin by insulin-dependent diabetes mellitus patients. In Europe, VISION® (ZomaJect 2) has been used for subcutaneous self-injection of growth hormone.
Several studies have been carried out comparing pharmacokinetic and pharmacodynamic characteristics of needle-free systems with traditional needle injection (5,6,7,8,9). These studies support the use of jet injection as a viable alternative to the traditional injection pens with needles.
This study was designed to compare the therapeutic effects of rhGH administered by subcutaneous needle-injection (pens) with subcutaneous needle-free jet-injection (VISION®). The results showed that no significant differences were found in the growth rates, growth rate SD scores or height SD scores, when comparing injection devices. Furthermore, the survey of VISION® revealed that 70% found it slightly or not painful at or after injection, 70% found VISION® very easy or easy to use, and 80% found the weight of the device appropriate.
As for complaints on using VISION®, only 3 syringes were damaged by 2 subjects in 4.5 yr of use of VISION®. All breakages occurred at the initial stage of using VISION® and could have been caused by inappropriate handling. Subsequently, there was no breakage observed up to the end of study. There were no other complaints. Therefore, it was concluded that there are no problems regarding safety and operationality for hGH treatment with VISION®, a needle-free injector.
The results indicate that there are no problems regarding effectiveness of the VISION® needle-free injector for hGH treatment. The results also indicate that VISION® is an effective device for children who have an aversion to needle injection.
References
- 1.Theinz GE, Sizonenko PC. Risks of jet injection of insulin in children. Eur J Pediartr 1991;150: 544–6 [DOI] [PubMed] [Google Scholar]
- 2.Jovanovic-Peterson L, Sparks S, Palmer JP, Peterson CM. Jet-injection insulin is associated with decrease antibody production and postprandial glucose variability when compared with needle-injection insulin in gestational diabetic women. Diabetes Care 1993;16(11): 1479–84 doi: 10.2337/diacare.16.11.1479 [DOI] [PubMed] [Google Scholar]
- 3.Schneider U, Birnbacher R, Schober E. Painfulness of needle and jet injection in children with diabetes mellitus. Eur J Pediatr 1994;153(6): 409–10 doi: 10.1007/BF01983402 [DOI] [PubMed] [Google Scholar]
- 4.Igarashi Y, Okuno A, Ito Y, Watanabe A, Komatsu K, Tanaka H, et al Usefulness of Jet Valve-type needle–less injector for growth hormone therapy. Clinical Report 1993;27(15): 5951–61(in Japanese) [Google Scholar]
- 5.Agerso H, Moller-Pedersen J, Cappi S, Thomann P, Jesussek B, Senderovitz T. Pharmacokinetics and pharmacodynamics of new formulation of recombinant human growth hormone administered by ZomaJet 2 Vision, a new needle-free device, compared to subcutaneous administration using a conventional syringe. J Clin Pharmacol 2002;42: 1262–8 doi: 10.1177/009127002762491361 [DOI] [PubMed] [Google Scholar]
- 6.Dorr HG, Zabransky S, Keller E, Otten BJ, Partsh CJ, Nyman L, et al. Are needle-free injection a useful alternative for growth hormone therapy in children? Safety and pharmacokinetics of growth hormone delivered by a new needle-free injection device compared to a fine gauge needle. J Pediatr Endocrinol Metab 2003;16(3): 383–92 doi: 10.1515/JPEM.2003.16.3.383 [DOI] [PubMed] [Google Scholar]
- 7.Houdijk ECAM, Herdes E, Delemarre-Van de Waal HA. Pharmacokinetics and pharmacodynamic of recombinant human growth hormone by subcutaneous jet- or needle-injection in patients with growth hormone deficiency. Acta Pediatr 1997;86: 1301–7. doi: 10.1111/j.1651-2227.1997.tb14902.x [DOI] [PubMed] [Google Scholar]
- 8.Murray FT, Silverstein JH, Frye K, Gertner JH, Johnson SB, Diamond T, et al. Clinical testing and patient satisfaction with use of a new needle-free device in children. Today’s Therapeutic Trends 2000;18: 305–12 [Google Scholar]
- 9.Verhagen A, Ebels JT, Dogterom AA, Jonkman JHG. Pharmacokinetics and pharmacodynamics of single dose of recombinant human growth hormone after subcutaneous administration by jet-injection: comparison with conventional needle-injection. Eur J Clin Pharmacol 1995;49: 69–72 doi: 10.1007/BF00192361 [DOI] [PubMed] [Google Scholar]