Abstract
Auxological data are the gold standard indexes of the therapeutic conditions in patients with CYP21 deficiency over long-term periods, whereas urinary pregnanetriol (PT) for 24 h has been used as an index for short-term periods. We previously reported that the range of 1.2–2.1 mg/m2/day of PT for 24 h (24-h PT) could be used as an index of optimal control in patients with CYP21 deficiency. The purpose of this study was to analyze the range of PT in the first morning urine samples (morning PT) as an index of optimal control in patients with CYP21 deficiency. First, the therapeutic periods of 15 participants (aged 2 yr and 5 mo to 17 yr and 4 mo) were classified into excessive, good or poor control periods using auxological data and Cushing-like symptoms, and 24-h PT levels were analyzed in each period, retrospectively. The 95% confidence intervals for the means of 24-h PT levels in the excessive, good and poor control periods were 0.24–2.24 (n=25), 2.88–4.92 (n=114) and 13.26–21.28 (n=72) mg/gCr, respectively. Subsequently, 24-h PT and morning PT levels collected on the same day were analyzed for 14 participants (aged 9 mo to 29 yr and 8 mo). There was a significant correlation between the above two PT levels (n=25, p<0.0001). When the 24-h PT range of the good control period, 2.88–4.92 mg/gCr, was adjusted by the correlation, the ideal morning PT range became 2.15–3.34 mg/gCr. In conclusion, a morning PT in the range of 2.2–3.3 mg/gCr can be used as an index of optimal control in patients with CYP21 deficiency.
Keywords: CYP21 deficiency, urinary pregnanetriol, first morning urine sample
Introduction
CYP21 deficiency is an autosomal recessive disease caused by mutations in the CYP21A2 gene (1). About 90% of cases of congenital adrenal hyperplasia are due to mutations in the CYP21A2 gene (1), and its incidence is about 1 in 20,000 live births in Japan (2). The aims of treatment for CYP21 deficiency are to supply glucocorticoids and mineralocorticoids and to suppress excessive secretions of androgen and ACTH. The major goals of the treatment are to maintain normal growth and to allow normal sexual maturation in order for patients to achieve normal adult height and fertility. Glucocorticoids have a narrow optimal therapeutic dose, and overtreatment leads to Cushing-like symptoms. Conversely, undertreatment leads to increased height velocity, advanced bone age, and hyperpigmentation due to excesses of androgen and ACTH. Therefore, careful monitoring of clinical, auxological and biochemical indexes has been used to judge therapeutic conditions.
Auxological data are the gold standard indexes of therapeutic conditions in patients with CYP21 deficiency over long-term periods [months to a year] (3,4,5). Urinary pregnanetriol for 24 h [24-h PT] (5,6,7,8) and serum 17OH-progesterone [17OHP] (6,7,8,9,10) are used as indexes for short-term periods (hours to days). Previous studies have shown that a single random measurement of 17OHP is not reliable, because the level fluctuates widely depending on the circadian rhythm of ACTH (9, 10). However, the 24-h PT presumably can be used as a more stable index than 17OHP.
Therefore, we previously analyzed an ideal 24-h PT range based on auxological data and reported that the range of 1.2–2.1 mg/m2/day could be used as an index of optimal control in patients with CYP21 deficiency (11). The first morning urine sample can be obtained easily compared with collection of urine for 24 h. However, the utility of PT in the first morning urine samples (morning PT) as an index of therapeutic condition has never been reported. The purpose of this study was to determine the ideal range of morning PT as an index of optimal control for CYP21 deficiency.
Participants
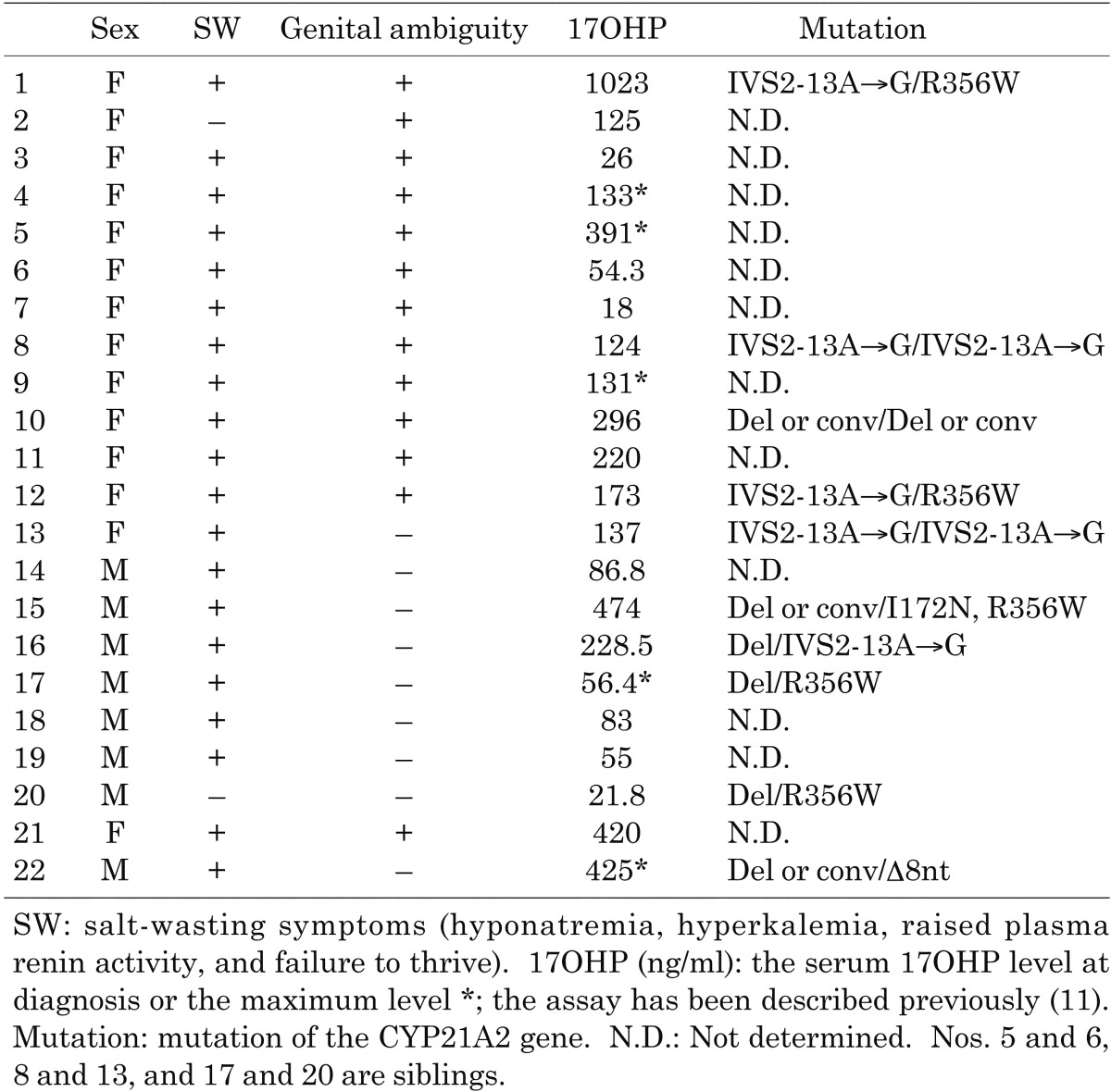
Twenty-two participants with CYP21 deficiency (8 males and 14 females, aged 9 mo to 29 yr and 8 mo) who were followed up in Tokyo Metropolitan Kiyose Children’s Hospital were studied after obtaining informed consent. All participants were diagnosed with a salt-wasting or simple virilizing form in the neonatal period; and their characteristics are shown in Table 1. No patients with a non-classical form were included in this study. As replacement therapy, oral hydrocortisone was used for all participants, and fludrocortisone was concurrently administered to all participants except for two who had a simple virilizing form (Table 1; participants 2 and 20). Hydrocortisone was replaced with dexamethasone in participant 10 when she was 15 years old. The CYP21A2 gene analyses were performed for 10 participants after obtaining informed consent from the participants and/or their parents, and the mutations were identified for these 10 participants.
Table 1. Participants.
Methods
Ideal PT range in 24-h urine samples
Twenty-four h urine samples were collected from 15 participants (Table 1; participants 1–15, aged 2 yr and 5 mo to 17 yr and 4 mo) at their homes or at our hospital every 1–3 mo for PT measurements. The creatinine index was used to judge the accuracy of urine collection, and only samples with a creatinine index of 10–25 mg/kg/day were analyzed retrospectively. The participants and samples were the same as those used in our previous study (11).
In this study, PT levels were corrected with urine creatinine and were analyzed in the following 3 control periods. These 3 periods were defined using auxological data, which is the gold standard index of the therapeutic conditions in patients with CYP21 deficiency, and Cushing-like symptoms, as described previously (11). PT measurements from the first and last months of each period were excluded from the analysis.
Excessive control period: When the participants had Cushing-like symptoms (moon face, striae, etc.), they were classified as being in an excessive control period.
When the increases in the standard deviation scores for body weight were used in advance to determine this period, the PT levels in this period (29 samples from 5 participants) were distributed widely, overlapped with the PT levels in the other 2 periods, and the increases in the body weights of 3 of the 5 participants did not reflect overtreatment (11). Thus, an overtreatment state was defined using Cushing-like symptoms instead of increase in body weight in our previous study (11).
Good control period: When the maximal change in the standard deviation scores for height (HT SDS) was less than ± 0.2/yr during the prepubertal period (Tanner stage 1), the period was classified as a good control period. These periods ranged from 12–30 mo in length, and they may reflect appropriate treatment.
Poor control period: When the change in HT SDS was more than 0.4/yr during the prepubertal period, the period was classified as a poor control period. These periods ranged from 5–23 mo in length and were characterized by continuous increases in HT SDS. These periods may reflect undertreatment.
Ideal PT range in the first morning urine samples
Twenty-four h and first morning urine samples were collected from 14 participants (Table 1; participants 9–22, aged 9 mo to 29 yr and 8 mo) on the same day at our hospital for PT measurements. The first morning urine samples were obtained between 7 a.m. and 9 a.m. before the first doses of medication and they were from the first urinations since the participants went to bed the night before. The urine samples were collected using an indwelling urethral catheter in participant 17 because of his age (9 mo old). Urine from between midnight and 6 a.m. was used as the first morning urine sample in this participant. Two sets of 24-h and first morning urine samples were collected from 11 participants, and one set collected from 3 participants.
The correlation between the 24-h PT and morning PT levels was analyzed. Based on the results, ideal morning PT levels were calculated.
Assays
Urinary PT was measured by gas chromatography-mass spectrometry using an HP-6690 mass selective detector (Biomedical Laboratories) with a sensitivity of 0.05 mg/L. The intra- and interassay coefficients of variation were 3.78 and 5.66%, respectively, at physiological concentrations.
Statistics
Comparisons between PT levels in the 3 control periods were performed using the Kruskal-Wallis test. The 95% confidence intervals for the means (95% CI) of the PT levels in each period were calculated. Regression analysis was used to determine correlation between 24-h PT and morning PT levels.
Results
The ideal PT range in 24-h urine samples is 2.88–4.92 mg/gCr
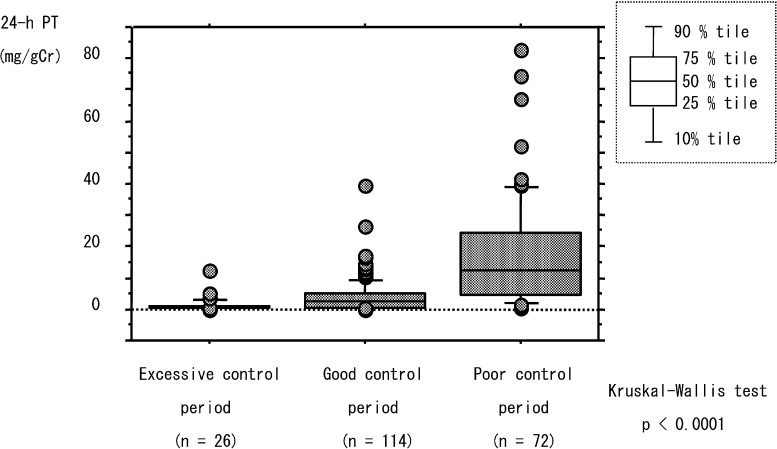
The 24-h PT levels were distributed in the ranges of 0.08–12.6 (26 samples from 5 participants aged 12 yr and 10 mo to 17 yr and 4 mo), 0.02–39.71 (114 samples from 10 participants aged 2 yr and 6 mo to 9 yr and 7 mo) and 0.27–82.93 (72 samples from 7 participants aged 2 yr and 5 mo to 7 yr and 3 mo) mg/gCr in the excessive, good and poor control periods, respectively (Fig. 1). The 95% CIs of the PT levels were 0.24–2.24, 2.88–4.92 and 13.26–21.28 mg/gCr in the above 3 periods, respectively, and there was no overlapping range among the levels.
Fig. 1.
24-h PT levels in the 3 control periods. The 24-h PT levels were distributed in the ranges of 0.08–12.6, 0.02–39.71 and 0.27–82.93 mg/gCr in the excessive, good and poor control periods, respectively. Comparisons between the ranges of the 3 periods were performed using the Kruskal-Wallis test.
The ideal PT range in the first morning urine samples is 2.15–3.34 mg/gCr
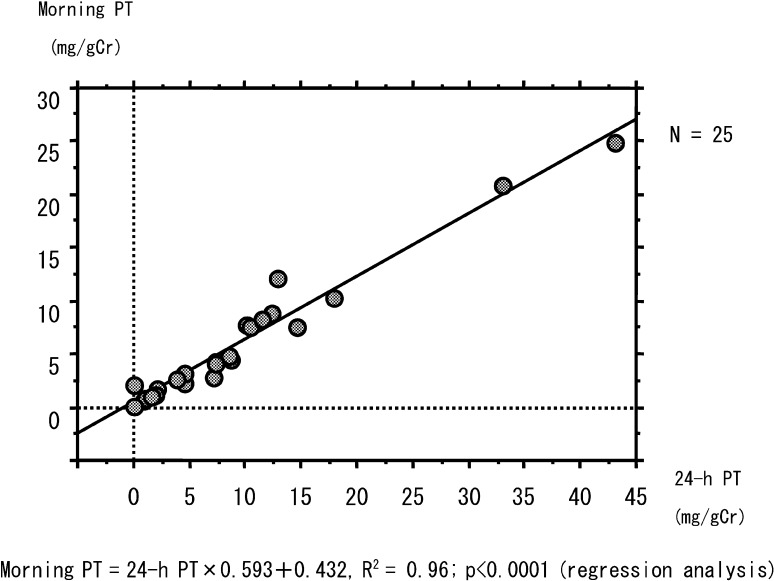
There was a significant correlation between the 24-h PT and morning PT levels (25 samples from 14 participants aged 9 mo to 29 yr and 8 mo, p<0.0001, R2=0.96; Fig. 2). When limited sets of urine samples without outliers (24-h PT<15 mg/gCr, n=22, or 24-h PT<10 mg/gCr, n=16) were analyzed in the same manner, there was similar significant correlation (p<0.0001). When the 95% CI of the 24-h PT levels in the good control period (2.88–4.92 mg/gCr) were adjusted by the correlation, the morning PT levels were 2.15–3.34 mg/gCr.
Fig. 2.
Correlation between 24-h PT and morning PT levels. There was a significant correlation between 24-h PT and morning PT levels using regression analysis (p<0.0001, R2=0.96).
Discussion
In this study, morning PT levels were compared with 24-h PT levels, and an ideal morning PT range as an index of optimal control was calculated. This is the first report in which an ideal morning PT range has been analyzed in patients with CYP21 deficiency.
Morning PT levels in the range of 2.15–3.34 mg/gCr can be used as an index of optimal control of CYP21 deficiency; this range was calculated from 24-h PT levels in the good control period by using the significant correlation between the 24-h PT and morning PT levels. Morning PT levels can be used easily and frequently to check the therapeutic conditions in patients with CYP21 deficiency.
No samples from the infantile or pubertal periods have been used to analyze the 24-h PT (11). Therefore, the usefulness of the range of morning PT in this study was not proven during these periods. This is a limitation of our study, and further studies concerning long-term auxological data are required to confirm the value of this range in these periods.
In conclusion, morning PT is a useful index of the therapeutic conditions in patients with CYP21 deficiency. The range of 2.2–3.3 mg/gCr can be used as an index of optimal control.
References
- 1.Paul MS. The adrenal cortex. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Wiliams textbook of endocrinology. 10th ed, Philadelphia: WB Saunders; 2002. p.491–551. [Google Scholar]
- 2.Tajima T, Fujieda K, Nakae J, Toyoura T, Shimozawa K, Kusuda S, et al. Molecular basis of nonclassical steroid 21-hydroxylase deficiency detected by neonatal mass screening in Japan. J Clin Endocrinol Metab 1997;82: 2350–6 [DOI] [PubMed] [Google Scholar]
- 3.Appan S, Hindmarsh PC, Brook CGD. Monitoring treatment in congenital adrenal hyperplasia. Arch Dis Child 1989;64: 1235–9 doi: 10.1136/adc.64.9.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einaudi S, Lala R, Corrias A, Matarazzo P, Pagliardini S, Sanctis C. Auxological and biochemical parameters in assessing treatment of infants and toddlers with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Pediatr Endocrinol 1993;6: 173–8 [PubMed] [Google Scholar]
- 5.Bailey CC, Komrower GM, Palmer M. Management of congenital adrenal hyperplasia. Arch Dis Child 1978;53: 132–5 doi: 10.1136/adc.53.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knorr D, Bidlingmaier F, Holler W, Kuhnle U. Diagnosis of homozygosity and heterozygosity in congenital adrenal hyperplasia and control of treatment. J Steroid Biochem 1983;19: 645–53 doi: 10.1016/0022-4731(83)90231-5 [DOI] [PubMed] [Google Scholar]
- 7.Erhardt E, Solyom J. homoki J, Juricskay S, Soltesz G. Correlation of blood-spot 17-hydroxyprogesterone daily profiles and urinaly steroid profiles in congenital adrenal hyperplasia. J Pediatr Endocrinol Metab 2000;13: 205–10 doi: 10.1515/JPEM.2000.13.2.205 [DOI] [PubMed] [Google Scholar]
- 8.Hughes IE, Winter JSD. The relationships between serum concentrations of 17OH-progesterone and other serum and urinaly steroids in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 1978;46: 98–104 doi: 10.1210/jcem-46-1-98 [DOI] [PubMed] [Google Scholar]
- 9.Charmandari E, Matthews DR, Johnston A, Brook CGD, Hindmarsh PC. Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: Is current replacement therapy satisfactory? J Pediatr Endocrinol Metab 2001;86: 4679–85 [DOI] [PubMed] [Google Scholar]
- 10.Young MC, Robinson JA, Read GF, Riad-Fahmy D, Hughes IA. 17OH-progesterone rhythms in congenital adrenal hyperplasia. Arch Dis Child 1988;63: 617–23 doi: 10.1136/adc.63.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izawa M, Aso K, Ariyasu D, Hasegawa Y. Pregnanetriol in the range of 1.2–2.1 mg/m2/day as an index of optimal control in CYP21A2 deficiency. Clin Pediatr Endocrinol 2007;16: 45–52. doi: 10.1297/cpe.16.45 [DOI] [PMC free article] [PubMed] [Google Scholar]