Abstract
The treatment for hypophosphatemic rickets in children includes phosphate and vitamin D preparations. In children, this regimen significantly improves symptoms, while a treatment for adult patients has not been established. We therefore investigated the clinical courses of 15 adult patients who discontinued therapy when final height was achieved in order to assess the necessity of treatment in adulthood. Thirteen patients developed symptomatic complications, including bone fractures. Among the 13, the 10 patients who restarted therapy all showed clinical improvement, and no side effects of treatment were observed. This study shows that there are some patients with hypophosphatemic osteomalacia who need continuous treatment during adulthood.
Keywords: hypophosphatemic osteomalacia, rickets
Introduction
Hypophosphatemic rickets/osteomalacia without hypercalciuria is characterized by defects in proximal renal tubular phosphate reabsorption and impaired regulation of renal vitamin D metabolism (1, 2). Mutations in the PHEX gene are responsible for the majority of cases of the hereditary form of this disorder (2).
There is a well-established treatment for children. Administration of phosphate and vitamin D preparations improve growth failure (3) and lower extremity deformities, which are the main clinical manifestations of affected children (4). This therapy is generally continued until final height is achieved.
On the other hand, there is some controversy over the treatment for adults. At present, the long term prognoses of untreated adults are not well known. The complications for adults reported in this disorder so far include joint pain, osteoarthritis, enthesopathy and fractures (5,6,7,8,9). Furthermore, the issue of whether all affected adults benefit from therapy has not been fully assessed.
The purpose of this study was to evaluate the necessity of treatment in adult patients by investigating the symptoms of untreated adult patients and their clinical responses to therapy.
Subjects
Forty-three patients with hypophosphatemic rickets and osteomalacia received care at Tokyo Metropolitan Kiyose Children’s Hospital between January 1973 and October 2006. Among the 43 patients, 31 were older than 20 yr at the time of the study (October 2006). All adults were diagnosed in childhood, and diagnosis of hypophosphatemic rickets was based on medical history, physical examination, radiographic signs of rickets, hypophosphatemia due to low renal tubular phosphate reabsorption and elevated serum alkaline phosphatase activity. Patients with hypercalciuria were excluded.
Fifteen adults (4 men and 11 women; aged 22–45 yr) out of the 31 adults with hypophosphatemic osteomalacia were included in this study. The eligibility criteria included previous treatment with phosphate and vitamin D preparations (1,25 dihydroxycalciferol or 1α-hydroxyvitamin D3), cessation of both phosphate and vitamin D preparations when final height was achieved and follow up for longer than 6 months beyond cessation of treatment. Four of the 15 started with 1,25 dihydroxycalciferol and later switched to 1α-hydroxyvitamin D3, and eleven took 1α-hydroxyvitamin D3 throughout treatment. Final height was defined as the height when the height velocity was less than 1 cm/yr. The reasons for the 16 adults who were excluded were as follows: thirteen could not be followed up with after discontinuation of treatment when final height was achieved, and three adults who continued taking vitamin D, but stopped taking oral phosphate, were transferred to other hospitals and their clinical courses were unknown.
The therapy used for adults at our institution was phosphate, which included 0.7 g PO4 per 1 g neutral phosphate, and 1α-hydroxyvitamin D3. The dose of phosphate and vitamin D was adjusted to fulfill the following criteria. 1. The difference between basal serum inorganic phosphorus (iP) and maximum serum iP after per oral use of phosphate was to be 1.0 mg/dl or above (maximum serum iP is usually obtained 60 min after oral phosphate intake). 2. The maximum intact PTH concentration was not to exceed the threefold of the upper limit of the normal range. 3. Hypercalciuria (urine calcium/creatinine over 0.3) and hypercalcemia (serum calcium level over 10.3 mg/dl) were to be avoided. The typical starting dose for an adult (50 kg) based on these rules was 4.0–6.0 g phosphate per day divided into 4 doses and 1.5–2.5 µg 1α-hydroxyvitamin D3 per day. While patients received this therapy, we continued to see them, monitor their serum and urine markers every 3 to 4 mo and adjust the doses of the medications.
Mutational analyses of the PHEX gene were carried out in eight of the fifteen patients who consented for the genetic test, and mutations were found in seven patients (data not shown).
Methods
The hospital records were reviewed retrospectively to analyze the clinical courses of all 15 patients. The clinical events in this paper are based on patient complaints except in the case of fractures, which were radiologically diagnosed.
Results
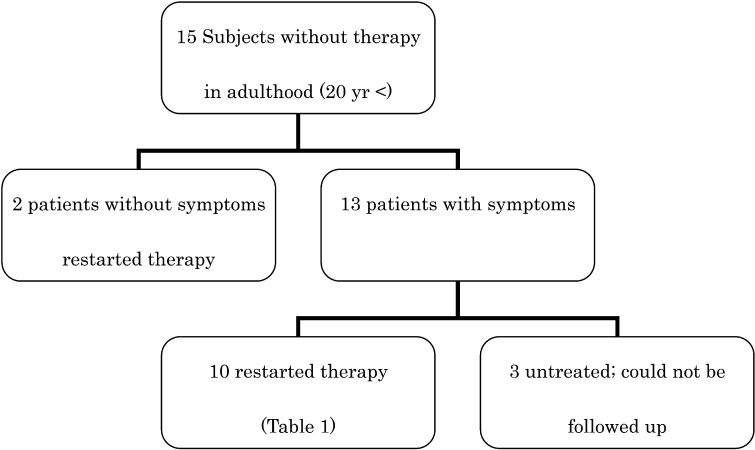
All 15 patients were asymptomatic at the time of cessation of treatment. Two resumed therapy, although they had no symptoms for 3 yr and 10 yr, respectively. They have not complained of any symptoms since resumption of treatment (Fig. 1).
Fig. 1.
Clinical courses of the 15 subjects.
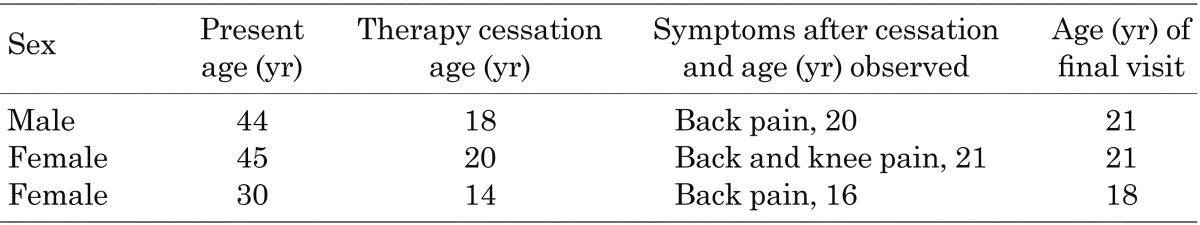
The remaining 13 patients became symptomatic 2 to 10 yr after cessation. Among these patients, all 10 patients who restarted taking phosphate and vitamin D showed improvement in symptoms during the subsequent treatment. Their pretreatment serum calcium and intact PTH levels were within the normal ranges for Japanese adults (data not shown). No adverse effects that required cessation of therapy were observed. The details for these patients are shown in Table 1. Fractures were observed in the femur, ankles, pubic bone and costa. All fractures were considered to be pathologic because they occurred as a result of routine activities or minor trauma. Joint and bone pain were predominantly seen in knees and ankles. The clinical courses of the three symptomatic and untreated patients were unknown (Table 2).
Table 1. Clinical features of the 10 symptomatic patients who became symptomatic after cessation and restarted therapy.
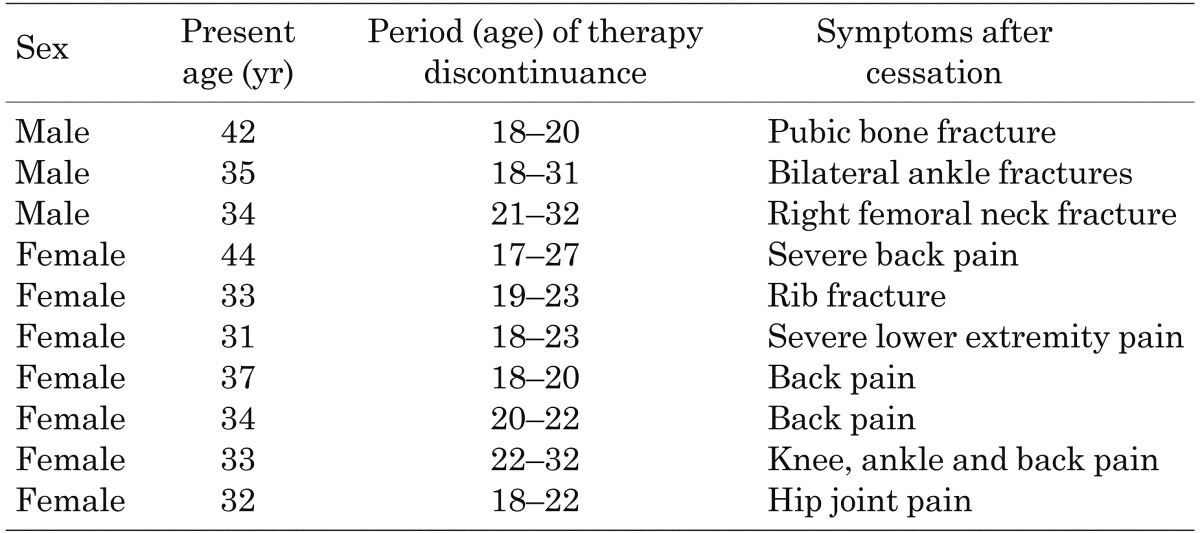
Table 2. Three patients who became symptomatic after cessation but did not restart therapy.
Discussion
This study implies that continuous treatment during adulthood prevents severe complications of hypophosphatemic osteomalacia. The majority of the untreated adult patients became symptomatic within 2 to 10 yr. No clear interpretations of the differences in the period until symptom recurrence could be made, although individual severity of the disease, previous treatment, dietary calcium and phosphate intake and amount of daily exercise were possible factors. The symptoms were improved by treatment with phosphate and vitamin D preparations, and none of the patients who restarted the treatment redeveloped serious symptoms. Similar results were obtained in a previous trial by Sullivan et al. In their study, 87% of adult patients (n=16) with bone or joint pain showed improvement in symptoms as a result of therapy (11).
Among the thirteen patients who had symptoms after cessation of therapy, six experienced significant problems, such as pathologic fractures and severe articular pain, which caused difficulties in everyday life. Dental abscesses, calcification of entheses and spinal stenosis, which have been described as common adult complications in previous reports (5, 8), were not experienced in the individuals in the present study. This may have been due to the relatively young age of the participants in this study.
At least four points remain controversial. First, the results of the present study may reflect a selection bias since the most severely affected patients are those most likely to seek medical treatment. In other words, patients who could not be followed up with were likely those least affected and did not need therapy in adulthood. Second, the optimal duration of therapy for adults remains unknown. Considering that the pathology of the disease persists throughout life, it is reasonable to propose that lifelong therapy is necessary, at least for those who develop symptoms when therapy is stopped. Third, there is concern about the side effects of long-term therapy. In a previous study, treatment of adults was not recommended due to a high probability of side effects (11). In the present study, adjustment of the medication by monitoring urinary calcium and serum PTH, as described above, prevented progression to nephrocalcinosis and tertiary hyperparathyroidism. Future studies are needed to evaluate the possible adverse effects of the therapy when it is continued for longer periods. Finally, since some patients should continue treatment during adulthood, how to identify these patients is still in question. This remains to be solved.
In conclusion, the results of the present study indicate that some patients with hypophosphatemic rickets should continue treatment during adulthood.
References
- 1.de Beur SMJ, Levine MA. Molecular pathogenesis of hypophosphatemic rickets. J Clin Endocrinol Metab 2002;87: 2467–73 [DOI] [PubMed] [Google Scholar]
- 2.Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nature Genetics 1995;11: 130–6 doi: 10.1038/ng1095-130 [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto J, Koto S, Hasegawa Y. Final height of Japanese patients with X-linked hypophosphatemic rickets: effect of vitamin D and phosphate therapy. Endocr J 2000;47: 163–7 doi: 10.1507/endocrj.47.163 [DOI] [PubMed] [Google Scholar]
- 4.Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med 1991;325: 1843–8 doi: 10.1056/NEJM199112263252604 [DOI] [PubMed] [Google Scholar]
- 5.Reid IR, Hardy DC, Murphy WM, Teitelbaum SL, Bergfeld MA, Whyte MP. X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine 1989;68: 336–52 doi: 10.1097/00005792-198911000-00002 [DOI] [PubMed] [Google Scholar]
- 6.de Menezes Filho H, de Castro LC, Damiani D. Hypophosphatemic rickets and osteomalacia. Arq Bras Endocrinol Metabol 2006;50: 802–13 doi: 10.1590/S0004-27302006000400025 [DOI] [PubMed] [Google Scholar]
- 7.Berndt M, Ehrich JH, Lazovic D, Zimmermann J, Hillmann G, Kayser C, et al. Clinical course of hypophosphatemic rickets in 23 adults. Clin Nephrol 1996;45: 33–41 [PubMed] [Google Scholar]
- 8.Tenenhouse HS, Econs MJ. Mendelian hypophosphatemias. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editors. The metabolic & molecular bases of inherited disease. New York: McGraw-Hill; 2001. p.5039–67. [Google Scholar]
- 9.Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP. X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology 1989;171: 403–14 [DOI] [PubMed] [Google Scholar]
- 10.Sullivan W, Carpenter T, Glorieux F, Travers R, Insogna K. A prospective trial of phosphate and 1,25-dihydroxyvitamin D3 therapy in symptomatic adults with X-Linked hypophosphatemic rickets. J Clin Endocrinol Metab 1992;75: 879–85 [DOI] [PubMed] [Google Scholar]
- 11.Stickler GB, Morgenstern BZ. Hypophosphatemic rickets: final height and clinical symptoms in adults. Lancet 1989;2: 902–5 doi: 10.1016/S0140-6736(89)91559-6 [DOI] [PubMed] [Google Scholar]