Abstract
We report vitamin D toxicity in an infant following consecutive administration use of a large dose of vitamin D, causing symptomatic hypercalcemia, which was successfully managed with injectable calcitonin. However, the child developed bilateral medullary nephrocalcinosis which persisted 42 mon after the initial episode.
Keywords: vitamin D deficiency, vitamin D toxicity, infants, nephrocalcinosis
Introduction
Despite plenty of sunshine available in the tropical regions, vitamin D deficiency (VDD) is widely prevalent among Asian Indians (1, 2). Increasing awareness about the wide prevalence of hypovitaminosis among Asian Indians has lead to widespread use of vitamin D therapy in subjects with clinical features suggestive of hypocalcaemia including bone pain, muscle cramps, etc. in India. Such supplementation of vitamin D is perceived to be safe by general physicians for Asian Indians who have high background prevalence of VDD. However, in western countries, there have been reports of vitamin D toxicity (VDT) following its inadvertent use (3, 4), and serious side effects are especially common in the pediatric age group (5). There has been no report of vitamin D therapy leading to symptoms of vitamin D intoxication in India. We report the details of a patient, an Asian Indian infant, with VDT leading to nephrocalcinosis following intramuscular vitamin D therapy.
Case Report
An 8-month-old male child of Asian-Indian origin was brought to the emergency unit with complaints of persistent vomiting and refusal to feed for two weeks. A detailed history of the patient revealed that child had visited a local physician due to abnormal twitching of the extremities a month ago and was found to have serum calcium of 7.8 mg/dl. The patient was advised a single dose, intramuscular injection of vitamin D containing 600,000 units of cholecalciferol and 500 mg calcium carbonate daily.
However, the child’s parents did not consult the treating physician again and continued the same treatment for two more weeks (one injection of vitamin D every week; in total three injections were given over 3 wk). One week after the third injection of vitamin D, the child developed vomiting and visited the local physician. On investigation he was found to have serum calcium of 11.5 mg/dl. A provisional diagnosis of vitamin D toxicity was considered and the patient was referred to the endocrine services for further management. The child was born (birth weight 2400 g) after full term pregnancy by Cesarean section (for breech presentation) to non-consanguineous vegetarian parents. He was exclusively breast fed till the age of four months, after that breast feed was supplemented with cow’s milk (by bottle) and at the age of 6 mon, semi-solid home foods (mainly cereals) were introduced.
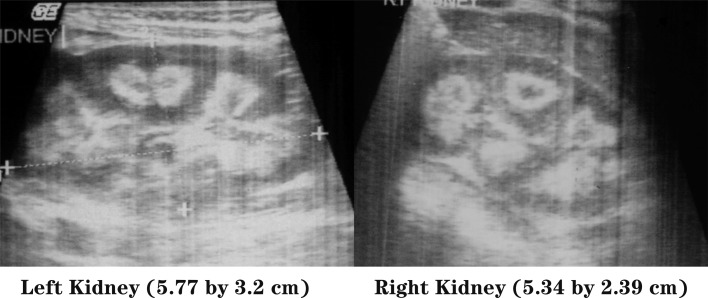
Physical examination revealed a child of sickly appearance, who was dehydrated and afebrile. Further investigations revealed normal leukocyte count, urine microscopic examination, renal functions (serum creatinine 0.4 mg/dl), electrolytes (serum sodium 139 meq/l, serum potassium 4.7 meq/l) and liver function test (SGPT 34 IU/l, serum alkaline phosphatase 219 IU/l; normal range for age <625 IU/l) and chest X-rays. Serum calcium was found to be elevated (11.5 mg/dl, with normal range of 8.0–10.5 mg/dl) with normal serum phosphorus of 5.0 mg/dl (3.5–5.5 mg/dl) for the age. Serum was stored for 25-hydroxy vitamin D and intact PTH estimation. The patient was managed with intravenous normal saline. His vomiting and dehydration improved after 24 h. However his serum calcium continued to show a rising trend of 12.5 mg/dl next day and 14.5 mg/dl after two days. Calcitonin injection (Salmon calcitonin, Zydus Cadila Pharma, dose 4 IU/kg subcutaneously) resulted in a prompt decrease in serum calcium within 24 h. The patient’s dehydration improved and he became cheerful. Calcitonin was continued on an alternate day basis. Ultrasound neck and tetrafosmin parathyroid scans showed no abnormalities. Echocardiography did not show aortic stenosis. Hand X-rays showed a dense line at the margin of the metaphysis of the lower ends of both the radius and the ulna (Fig. 2). Twenty-four-hour urinary calcium excretion (collected before starting injection of calcitonin) was 57 mg with a body weight of 7.00 kg (normal, less than 4 mg/kg/day) with a calcium: creatinine ratio of 0.83 (normal ratio of 0.2 to 0.7 during infancy). Ultrasound examinations of the abdomen showed medullary nephrocalcinosis in both kidneys (Fig. 1). Serum calcium, phosphorus and alkaline phosphatase values of the parents were normal. Reports of Vitamin D (radio-immunoassay; Diasorin, USA) and serum intact PTH (immuno-radiometric assay; Diasorin, USA) showed 25 hydroxy vitamin D of >100 ng/ml (normal range of 9–37 ng/ml) and undetectable PTH (normal range of 7–53 pg/ml). A final diagnosis of VDT was made and the patient was scheduled for a month of calcitonin injection every other day. His serum calcium decreased progressively and normalized in 3 mon. The patient otherwise showed absolutely normal growth and developmental milestones. A follow up visit 42 mon later revealed normal serum calcium, urinary calcium/creatinine ratio. However, ultrasonography demonstrated no reduction in the nephrocalcinosis (Fig. 3).
Fig. 2.
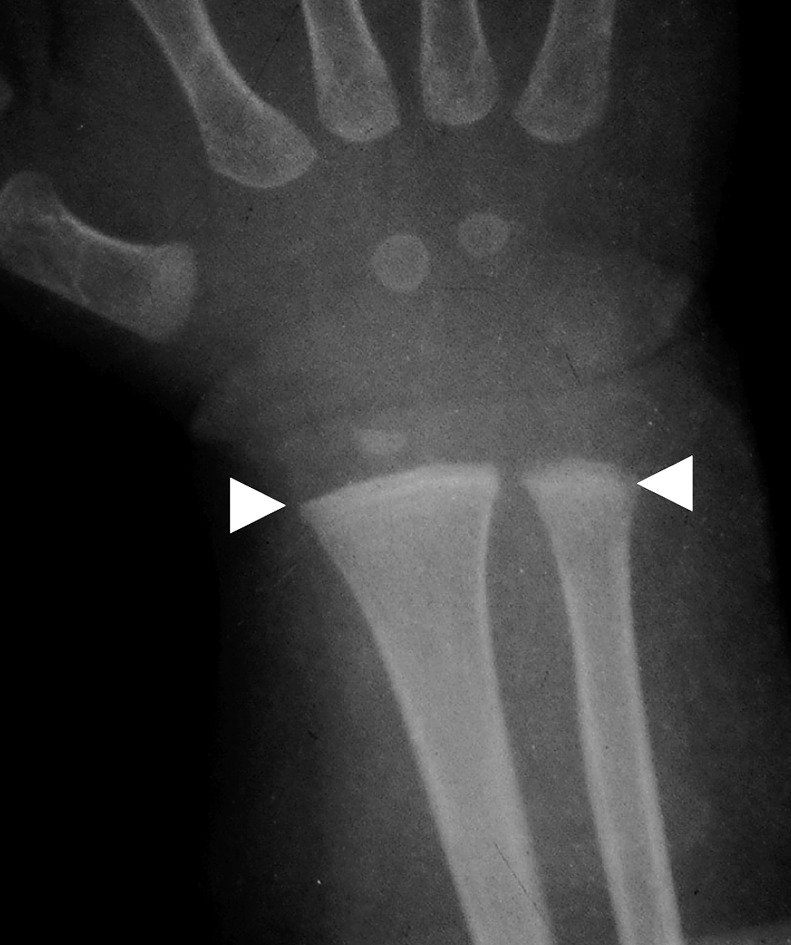
Hand X-rays showing dense lines at the end of radius and ulna (white arrowheads).
Fig. 1.
Ultrasonography of kidneys showing medullary nephrocalcinosis.
Fig. 3.
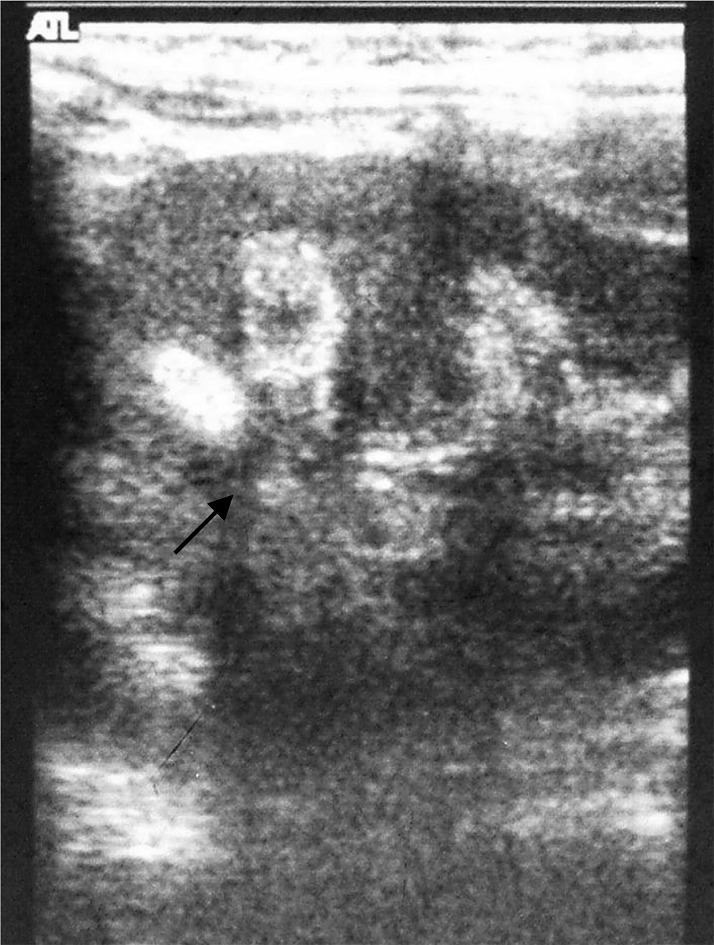
Ultrasonography of left kidney (size 7.2 by 3.9 cm) 42 mon after initial episodes showing persistence of medullary nephrocalcinosis with acoustic shadow (black arrow).
Discussion
There have been several reports of VDT in children, mostly from vitamin D sufficient regions (3,4,5). The present report for the first time highlights the possibility and clinical features of VDT in an Asian Indian Infant who was prescribed a large dose of vitamin D (total 1800,000 units).
VDD was considered to be rare among Indians because of plenty of sunshine (6). However, recent studies have shown that serum 25(OH) levels are subnormal in Asian Indians (1, 2). Such VDD has been reported in all age groups; i.e. neonate to adults. The paradox of wide prevalence of VDD in a sun-drenched country like India is related to skin pigmentation, atmospheric pollution, and limited exposure of body surface area to sunshine (1, 2).
Vitamin D is beneficial and safe when used correctly at its recommended doses. Guidelines issued by the American Academy of Pediatrics (AAP) and the National Academy of Sciences suggest that all breastfeeding infants and non breastfeeding infants who drink <500 mL of milk per day should receive 200 IU of vitamin D per day (7). The amount of vitamin D necessary to cause hypervitaminosis varies widely among individuals. Continued ingestion of 50,000 units or more daily by a person with normal parathyroid function and sensitivity to vitamin D may result in poisoning (8). VDT occurs over days of ingesting thousands of international units of vitamin D; therefore, it is unlikely that patients will show overt manifestations of VDT with continued ingestion of vitamin D at doses available in multivitamin pills (5).
Early symptoms of VDT include anorexia, nausea, vomiting, weakness, lethargy, constipation, and non-specific aches and pains. Renal function can become impaired as a result of nephrocalcinosis. The long term effects of VDT are severe and at times, like nephrocalcinosis, irreversible. Nephrocalcinosis should it occur, may lead to permanent renal impairment. Treatment of VDT includes immediate removal of the exogenous source, intravenous fluid hydration, loop diuretics (Thiazides promote calcium retention), glucocorticoids, and a low-calcium diet. In severe cases, calcitonin, oral alendronate, and pamidronate have also been used successfully (3, 9, 10).
Nephrocalcinosis is an uncommon disease in childhood. Vitamin D toxicity has been seen as one of the important but uncommon causes of iatrogenic nephrocalcinosis. In a retrospective survey of 152 children and adolescents with nephrocalcinosis, prophylactic bolus administration of vitamin D was adjudged to be a cause of nephrocalcinosis in 9% cases (11).
Resolution of medullary nephrocalcinosis in children has not been well documented. There are some reports of reversibility especially in cases with furosemide-induced nephrocalcinosis and nephrocalcinosis occurring after treatment in X-linked hypophosphatemic rickets (12, 13). Resolution of vitamin D induced nephrocalcinosis has not been reported in the literature. In one study, none of the five children with vitamin D induced nephrocalcinosis showed resolution over the years (14). Similarly, resolution was not seen even after follow-up of one to thirteen years (15). Nephrocalcinosis in a pre-term infant was found to have affected renal function on long-term follow-up (16). In a study by Mantan et al., nephrocalcinosis was associated with a decline in the glomerular filtration rate (GFR) from 82.0 (42–114) ml/min per 1.73 m2 body surface area to 70.8 (21.3–126.5) ml/min per 1.73 m2 body surface area (P=0.001), after median follow-up of 35 mon. No resolution of nephrocalcinosis was seen in our case, even at 42 mon after the initial episode; however renal function remained within the normal range for the infant’s age.
Growing awareness of VDD among physicians has increased the use of vitamin D preparations. However, we often prescribe vitamin D without extra precautions. Physicians dealing with children need to be especially cautious when choosing from various preparations of commercially available vitamin D. The treatment schedule and possible toxic effects should be clearly explained to the parents, especially when an injectable preparation of vitamin D is being prescribed. At the same time, parents should be clearly advised against unsupervised or un-prescribed supplements of vitamin D in attempt to make bones stronger.
References
- 1.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr 2000; 72: 472–5 [DOI] [PubMed] [Google Scholar]
- 2.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr 2005; 81: 1060–4 [DOI] [PubMed] [Google Scholar]
- 3.Gurkan F, Davutoglu M, Bosnak M, Ece A, Dikici B, Bilici M, et al. Pamidronate treatment in acute vitamin D intoxication. J Endocrinol Invest 2004; 27: 680–2 [DOI] [PubMed] [Google Scholar]
- 4.Atabek ME, Pirgon O, Sert A. Oral alendronate therapy for severe vitamin D intoxication of the infant with nephrocalcinosis. J Pediatr Endocrinol Metab 2006; 19: 169–72. doi: 10.1515/JPEM.2006.19.2.169 [DOI] [PubMed] [Google Scholar]
- 5.Barrueto F, Helena HWF, Howland MA, Hoffman RS, Nelson LS. Acute vitamin D intoxication in a child. Pediatrics 2005; 116: e453–6. doi: 10.1542/peds.2004-2580 [DOI] [PubMed] [Google Scholar]
- 6.Stanbury SW. Vitamin-D deficiency in Asians. Lancet 1973; 25: 446. doi: 10.1016/S0140-6736(73)92311-8 [DOI] [PubMed] [Google Scholar]
- 7.Gartner LM, Greer FR, American Academy of Pediatrics, Section on Breastfeeding and Committee on Nutrition.Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics 2003; 111: 908–10. doi: 10.1542/peds.111.4.908 [DOI] [PubMed] [Google Scholar]
- 8.Marcus R. Agents affecting calcification and bone turn over. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Goodman Gilman A, editors. Goodman & Gilman’s The Pharmaceutical basis of therapeutics. 9TH ed. New York: McGraw-Hill 1996. p.1519–53. [Google Scholar]
- 9.Velin P, DuPont D. Severe acute hypercalcemia in a child caused by accidental vitamin D poisoning. Pediatrie 1988; 43: 753–6 [PubMed] [Google Scholar]
- 10.Hatun S, Cizmecioglu F. Use of alendronate in the treatment of vitamin D intoxication in infants. Turk J Pediatr 2005; 47: 373–5 [PubMed] [Google Scholar]
- 11.Ronnefarth G, Misselwitz J. Nephrocalcinosis in children: a retrospective survey. Members of the Arbeitsgemeinschaft fur padiatrische Nephrologie. Pediatr Nephrol 2000; 14: 1016–21. doi: 10.1007/s004670050065 [DOI] [PubMed] [Google Scholar]
- 12.Auron A, Alon US. Resolution of medullary nephrocalcinosis in children with metabolic bone disorders. Pediatr Nephrol 2005; 20: 1143–5. doi: 10.1007/s00467-005-1899-4 [DOI] [PubMed] [Google Scholar]
- 13.Seikaly MG, Baum M. Thiazide diuretics arrest the progression of nephrocalcinosis in children with X-linked hypophosphatemia. Pediatrics 2001; 108: E6. doi: 10.1542/peds.108.1.e6 [DOI] [PubMed] [Google Scholar]
- 14.Lin MT, Tsau YK, Tsai WY, Tsai WS, Lu FL, Hsiao PH, et al. Nephrocalcinosis in childhood. Acta Paediatr Taiwan 1999; 40: 27–30 [PubMed] [Google Scholar]
- 15.Navarro M, Acevedo C, Espinosa L, Pena A, Picazo ML, Larrauri M. Vitamin D3 poisoning and irreversible sequela. An Esp Pediatr 1985; 22: 99–106 [PubMed] [Google Scholar]
- 16.Kist-van Holthe JE, van Zwieten PH, Schell-Feith EA, Zonderland HM, Holscher HC, Wolterbeek R, et al. Is nephrocalcinosis in preterm neonates harmful for long-term blood pressure and renal function? Pediatrics 2007; 119: 468–75. doi: 10.1542/peds.2006-2639 [DOI] [PubMed] [Google Scholar]
- 17.Mantan M, Bagga A, Virdi VS, Menon S, Hari P. Etiology of nephrocalcinosis in northern Indian children. Pediatr Nephrol 2007; 22: 829–33. doi: 10.1007/s00467-006-0425-7 [DOI] [PubMed] [Google Scholar]