Abstract
Persistent hyperinsulinemic hypoglycemia of infancy (PHHI) is often resistant to medical therapy and is normally treated by subtotal pancreatectomy to avoid neurological complications. However, many problems after surgery, such as recurrence of hypoglycemia and diabetes mellitus, remain to be solved. This report concerns a case of PHHI that was resistant to octreotide or diazoxide alone but was successfully controlled with subcutaneous injection of octreotide in combination with nocturnal glucose infusion through central venous catheter. The patient exhibited natural remission of hyperinsulinism with age, and all treatment was ceased at the age of 4 yr. Growth and neurological development of the patient have been normal. This combined therapy can be a therapeutic option as a substitute for surgical solutions.
Keywords: hyperinsulinism, Infantile hypoglycemia, nocturnal glucose infusion, octreotide
Introduction
PHHI (persistent hyperinsulinemic hypoglycemia of infancy) is characterized by hypoglycemia caused by inappropriate secretion of insulin during the neonatal or infantile period. Diazoxide and octreotide have been used for treatment of PHHI (1). However, neither is effective in some cases. In cases of PHHI resistant to these medical treatments, subtotal pancreatectomy has long been the main solution for avoidance of the neurological complications caused by profound hypoglycemic attack. However, there are many recent reports of long-term complications for it, such as development of diabetes mellitus by adolescence. Moreover, repeated surgical intervention is required in some cases because of recurrent hypoglycemia (2,3,4,5). On the other hand, spontaneous attenuation of insulin secretion has also been reported during the natural course of PHHI (5).
This report concerns a case of PHHI in which surgical treatment was avoided by combined therapy with subcutaneous injection of octreotide and nocturnal glucose infusion through a central venous catheter (CVC).
Case Report
A female patient, delivered at 40 wk and weighing 4,343 g, was presented with seizure caused by hyperinsulinemic hypoglycemia on the day of birth. Her blood tests showed no specific findings other than excessive secretion of insulin (insulin 23.4 µU/ml, glucose 38 mg/dl, insulin/glucose ratio: 0.62). Serum ketones at the time of diagnosis were 24 µmol/l for AcAc and 29 µmol/l for 3-OHBA. She required continuous glucose infusion (5–12.5 mg/kg/min) through a percutaneous central venous catheter to maintain a normal blood glucose (BG) level. Subcutaneous octreotide injection was initiated when she was 40 d of age. After developing Kawasaki disease at 2 mo of age, her BG level improved transiently and remained stable without any treatment for over a month. However, control of her BG level worsened again and subcutaneous injection of octreotide of up to 21 µg/kg/day (6 times a day) was reinitiated at the age of 3 mo. Although there was a partial response to octreotide treatment, the BG level remained unstable, and asymptomatic hypoglycemia (under 50 mg/dl) was observed at least two times daily. Thus, up to 14 mg/kg/day of diazoxide (3 times a day) was introduced in parallel with octreotide treatment but was discontinued 3 wk later because of an increased frequency of hypoglycemic attack and occurrence of side effects including edema and hirsuties. She was referred to our hospital at the age of 4 mo. At presentation, her height was 69.5 cm (+ 3.1 SD) and her weight was 8,730 g (+ 2.7 SD). Her neurological development was normal. The results of blood tests, including endocrinological examinations, were normal. Serum UN, creatinine, AST, ALT, T-Bil and NH3 were 6.0 mg/dl, 0.1 mg/dl, 25 U/l, 13 U/l, 0.3 mg/dl and 49 μg/dl, respectively. The results of blood gas analysis for pH, HCO3– and BE were 7.33, 20.7 mEq/l and – 4.4 mEq/l, respectively. The serum level of TSH, FT3, FT4, GH and cortisol were 3.0 µU/ml, 4.35 pg/ml, 1.5 ng/dl, 4.6 ng/ml and 1.3 µg/dl, respectively. Her mother was diagnosed with gestational impairment of glucose tolerance. There was no family history of hypoglycemia. Genetic analysis, which was performed after obtaining informed consent, revealed a compound heterozygote of the SUR1 gene, i.e., a nonsense mutation in the paternal allele (exon 25 Arg 998→stop codon), and an unknown variant in the maternal allele (exon 12 Val 560→Met).
Results
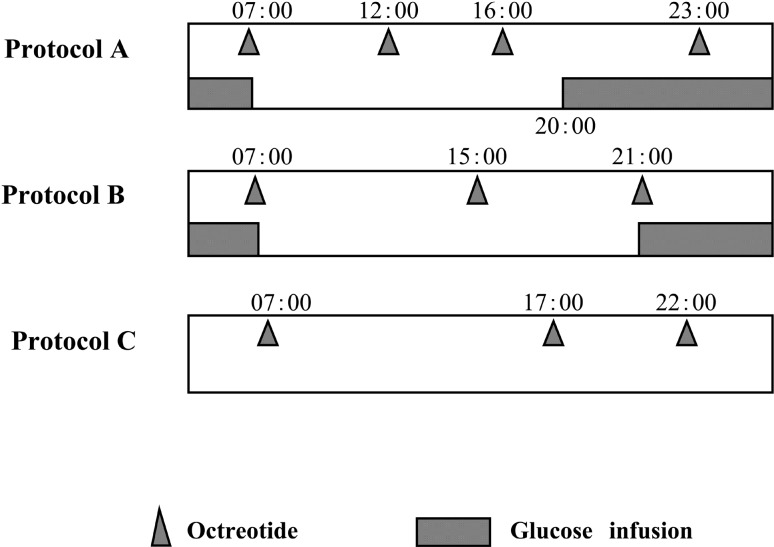
As medical treatment with octreotide and diazoxide was ineffective, subtotal pancreatectomy was planned and a CVC was inserted prior to the surgery. Constant supply of glucose through the CVC (2.0 mg/kg/min) resulted in normal maintenance of the BG profile with the octreotide treatment (21.0 µg/kg/day, 6 times a day). Cyclic glucose infusion (3.4 mg/kg/min × 16 h) with subcutaneous octreotide injection (14.0 µg/kg/day) was subsequently attempted at the age of 6 mo. Surgical intervention was aborted because the patient’s condition remained stable with this medical treatment for a month. At the age of 7 mo, the daily therapeutic protocol was fixed to injection of octreotide (14.0 µg/kg/day) and nocturnal glucose infusion (4.5 mg/kg/min × 11 h; Fig. 1 protocol A). Because protocol A maintained good control of her condition, informed consent was obtained from the patient’s parents for trial of administration of the combined therapy at home by them, and she was then discharged from our hospital. The dose of octreotide and quantity of glucose infusion were reduced in a step-by-step manner (Fig. 1). The next protocol introduced at the age of 1 yr and 2 mo consisted of octreotide injection (11.0 µg/kg/day) and nocturnal glucose infusion (3.5 mg/kg/min × 10 h; protocol B). When the patient was 2 yr and 7 mo of age, nocturnal glucose infusion was discontinued; treatment was continued with octreotide only (6.5 µg/kg/d; protocol C). The dose and frequency of octreotide injection were then gradually reduced. At the end of protocol C, overnight fasting test without octreotide injection was performed in the hospital. Finally, octreotide treatment was completely ceased at the age of 4 yr and 2 mo. At this time, the patient experienced no neurological complications, and her height and weight were 103.3 cm (+ 0.7 SD), weight 17.5 kg (+ 0.9 SD), respectively.
Fig. 1.
Three sequential protocols of treatment with octreotide and glucose infusion. The schedule and doses of octreotide injection and glucose infusion described were reduced in a step-by-step manner. All treatment was ceased at the age of 4 yr and 2 mo.
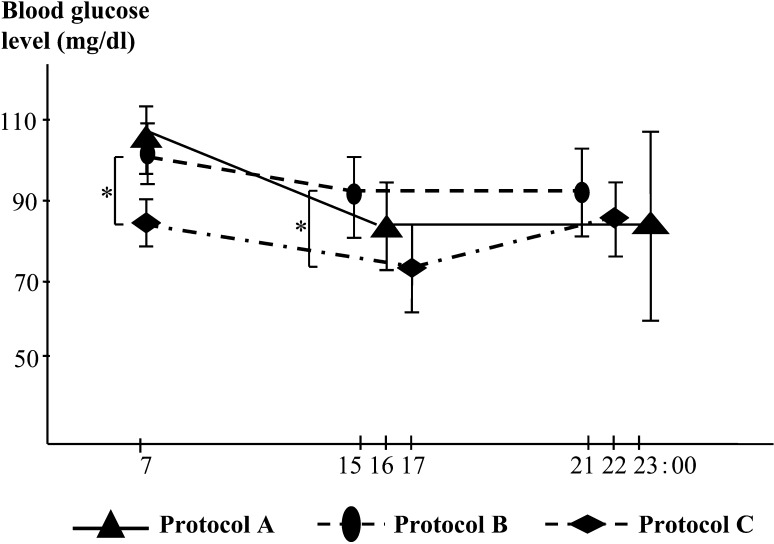
The patient’s daily profile of BG levels during protocols A to C is shown in Fig. 2. BG level was measured just before each octreotide injection. The mean monthly values of BG for each protocol are presented in the figure. In protocol A, the mean BG levels for each time of day were 105.0 ± 9.1 mg/dl for 07:00, 85.0 ± 12.2 for 16:00, and 88.0 ± 25.0 for 23:00. In protocol B, they were 101.8 mg/dl ± 7.8 for 7:00, 93.4 ± 11.2 for 15:00, and 91.9 ± 11.9 for 21:00. In protocol C, they were 84.2 ± 5.8 mg/dl for 7:00, 71.5 ± 12.3 for 17:00, and 86.5 ± 10.2 for 22:00. Although there was a statistically significant decrease in BG level between protocols B and C, as shown in Fig. 2, the patient did not exhibit hypoglycemia during protocols A to C. The average morning BG level during the month after treatment cessation was stable at 92.4 ± 13.4 mg/dl.
Fig. 2.
Daily profile of blood glucose levels before each octreotide injection in protocols A to C. *P<0.05.
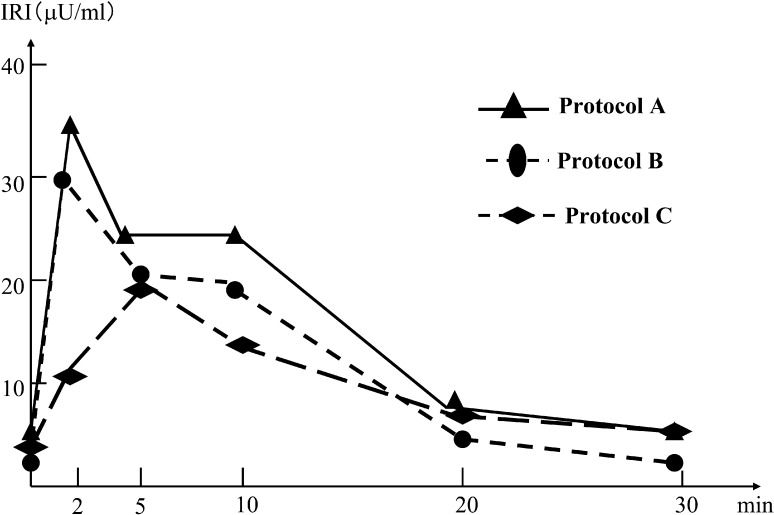
The results of glucagon stimulation tests for protocols A to C are shown in Fig. 3. In each protocol, a dose of 30 µg/kg glucagon was administered intravenously four hours after octreotide injection. The dose of octreotide corresponded to each protocol. The serum insulin level was measured at 0, 2, 5, 10, 20, and 30 min after glucagon infusion. Basal serum insulin levels were under 8 µU/ml in all protocols. The peak levels of the insulin concentration in protocols A to C decreased gradually, and appearance of the peak level was delayed in protocol C compared with those of protocols A and B. As a result, we confirmed that excessive secretion of insulin spontaneously improved during our therapy.
Fig. 3.
Insulin levels in glucagon stimulation tests for protocols A to C. A dose of 30 µg/kg glucagon was intravenously administered 4 h after octreotide injection. The doses of octreotide correspond to each therapeutic protocol.
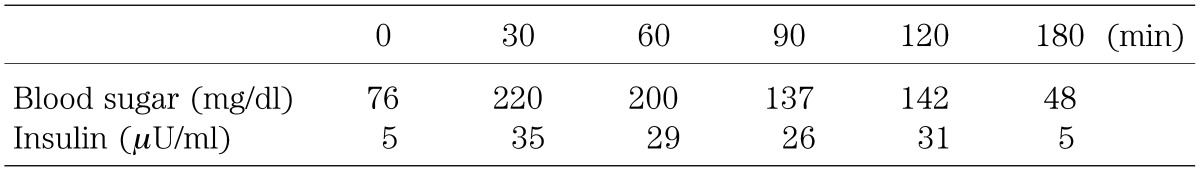
The results of an oral glucose tolerance test (OGTT; 1.75 g/kg glucose) performed 3 mo after cessation of octreotide treatment are shown in Table 1. The fasting blood glucose level was normal while the blood glucose levels at 1 and 2 h after glucose administration were 200 mg/dl and 142 mg/dl, respectively, which was defined as impaired glucose tolerance. Although the hemoglobin A1c level at that time was 5.1%, which was within the normal range, the results of the OGTT suggest that the patient has the potential to develop diabetes in the future.
Table 1. Results of oral glucose tolerance test (1.75 g/kg) 3 mo after treatment cessation.
Discussion
In PHHI, severe hypoglycemia in neonatal or infantile periods can cause irreversible neurological damage. A retrospective study of 90 PHHI patients showed that mental retardation existed in 21% of the patients and that epilepsy existed in 18% of the patients (6). Another study from Germany investigated the long-term outcome of 114 patients with PHHI and suggested that psychomotor or mental retardation existed in 44% of the patients and that epilepsy existed in 25% of the patients (4). Surgical intervention has been the treatment of choice in many cases of PHHI in order to avoid these types of neurological complication.
Surgical approaches and their outcomes are significantly different between the two histopathological subtypes, diffuse and focal types of PHHI. Focal types are curable by resection of the focal lesions, while diffuse types require subtotal pancreatectomy if medical treatments are ineffective. In addition, patients still have the potential of recurrent or persistent hypoglycemia even after they receive subtotal pancreatectomy (7).
Recently, preoperative localization of the focal lesion has been possible by arterial calcium stimulation/venous sampling (ASVS), intrahepatic pancreatic portal venous sampling (PVS) or Positron Emission Tomography (PET) using 18F-Fluoro-L-DOPA (8, 9). Among these methods, PET might be the most practical because ASVS and PVS are more invasive and less available.
Subtotal pancreatectomy can result in many long-term complications, such as diabetes mellitus and exocrine failure. Because of decreased glucagon reserves, it is more difficult to maintain normoglycemia in such cases than in other types of diabetes mellitus. It has been suggested that many non-operated cases in clinical remission also develop diabetes; however, pancreatectomy can hasten its onset (2, 3). In another report, six out of seven patients who underwent near-total pancreatectomy had defective linear growth that was mainly caused by impaired insulin secretion, and compared with control children with type 1 diabetes mellitus, the pancreatectomized children showed poorer linear growth (10).
PHHI is a self-limiting disease; many patients controlled medically without surgery and many of those who remain symptomatic after surgery undergo clinical remission later in childhood. However, previous reports have shown that the response of insulin secretion to glucose and lack of insulin suppressibility are not fully improved even after clinical remission (3, 5).
Based on these reports, immediate application of subtotal pancreatectomy in cases of PHHI should be avoided. Screening for the focal or diffuse type of PHHI should first be conducted. In the cases of diffuse type, medical control should be attempted before subtotal pancreatectomy.
In our case, screening for a focal lesion was not conducted because 18F-Fluoro-L-DOPA PET was not available at our facility at that time. Medical treatment with diazoxide or octreotide alone was ineffective, and pancreatectomy was planned in order to avoid neurological complications until a CVC was inserted. However, the patient’s BG level was successfully controlled by a combined therapy of octreotide and glucose infusion through the CVC, without the need for surgical treatment. The patient’s growth and neurological development have been maintained normally. Clinical remission of hyperinsulinemia was observed with age and was also demonstrated by the results of glucagon stimulation tests. In addition, the result of OGTT after cessation of treatment showed that the patient had a blunted and slightly excessive response, suggesting that she may develop diabetes in the future. This result might be due to the natural course of her disease and might not be the side effect of octreotide because octreotide has a short-lived inhibitory effect on insulin secretion. In fact, long-term inhibition of insulin secretion after cessation of octretide has not been reported.
One of the most controversial issues regarding the use of octreotide in children is the growth inhibition caused by suppression of growth-related hormones. In our case, the patient’s growth curve remained normal during the 40 mo of octreotide treatment. Although more data is needed to assess the risk of growth inhibition from octreotide treatment, there are many case reports demonstrating normal growth rates during octreotide treatment.
Another complication of combined therapy is the risk of CVC problems, such as occlusion, leakage, or infection. In such cases, additional octreotide or glucose infusion through a peripheral line could be a solution. It is also very important that the patients and families are able to cope with the injections and appropriate management of the CVC.
Genetic mutation of the SUR gene involving the K+- ATP channel of β cells has been found in many cases of PHHI (11,12,13,14,15). In general, mutation of the SUR1 gene is inherited in an autosomal recessive manner. On the other hand, loss of the maternal allele of chromosome 11 in the focal lesion and SUR1 mutation of the paternal allele have been found in some cases of focal types of PHHI (16). In our case, mutation from the paternal allele was confirmed, while a rare variant was found in the maternal allele. It is not clear whether this variant is a missence mutation or a rare polymorphism. Thus, if the maternal variant is polymorphism, it is possible that the patient had a focal type of PHHI or that another SUR1 mutation exists in the maternal allele in a region not analyzed.
We conclude that combined therapy with a daytime injection of octreotide and nocturnal glucose infusion can be an alternative therapeutic option to subtotal pancreatectomy and that it can allow time to wait for natural remission in patients with PHHI.
Acknowledgements
We wish to thank for Dr. Tanizawa of Yamaguchi University for genetic analysis of the patient.
References
- 1.Hasegawa T, Tanaka T, Kanzaki S, Sugihara S, Yakotani S, Tanaka H, et al. Diagnosis and therapeutic guideline of hyperinsulinemic hypoglycemia. J Jpn Pediatric Soc 2006; 110: 1472–4 [Google Scholar]
- 2.Cade A, Walters M, Puntis JWL, Arthur RJ, Stringer MD. Pancreatic exocrine and endocrine function after pancreatectomy for persistent hyperinsulinemic hypoglycemia of infancy. Arch Dis Child 1998; 79: 435–9 doi: 10.1136/adc.79.5.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser B, Hirsch H, Landau H. Persistent hyperinsulinemic hyperglycemia of infancy. Long-term octreotide treatment without pancreatectomy. J Pediatr 1993; 123: 644–50 doi: 10.1016/S0022-3476(05)80970-9 [DOI] [PubMed] [Google Scholar]
- 4.Meissner T, Wendel U, Burgard P, Schaetzle S, Mayatepek E. Long-term follow-up of 114 patients with congenital hyperinsulinism. Eur J Endocrinol 2003; 149: 43–51 doi: 10.1530/eje.0.1490043 [DOI] [PubMed] [Google Scholar]
- 5.Leibowitz G, Glaser B, Higazi AA, Salameh M, Cerasi E, Landau H. Hyperinsulinemic hypoglycemia of infancy (nesidioblastosis) in clinical remission. High incidence of diabetes mellitus and persistent β-cell dysfunction at long-term follow-up. J Clin Endoclinol Metab 1995; 80: 386–92 [DOI] [PubMed] [Google Scholar]
- 6.Menni F, Lonlay P, Sevin C, Touati G, Peigne C, Barbier V, et al. Neurologic Outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycemia. Pediatrics 2001; 107: 476–9 doi: 10.1542/peds.107.3.476 [DOI] [PubMed] [Google Scholar]
- 7.Fekete CN, De Lonlay P, Jaubert F, Rahier J, Brunelle F, Saudubray JM. The surgical management of congenital hyperinsulinemic hypoglycemia in infancy. J Ped Surg 2004; 39: 267–9 doi: 10.1016/j.jpedsurg.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 8.Hussain K, Seppanen M, Nanto-Salnen K, Adzick NS, Stanley CA, Thornton P, et al. The diagnosis of ectopic focal hyperinsulinism of infancy with [18F]-dopa positron emission tomography. J Clin Endocrinol Metab 2006; 91: 2839–42 doi: 10.1210/jc.2006-0455 [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro MJ, De Lonlay P, Delzescaux T, Boddaert N, Jaubert F, Bourgeois S, et al. Characterization of hyperinsulinism in infancy assessed with PET and 18F-Fluoro-L-DOPA. J Nuc Med 2005; 46: 560–6 [PubMed] [Google Scholar]
- 10.Soliman AT, Alsaimi I, Darwish A, Asfour MG. Growth and endocrine function after near total pancreatectomy for hyperinsulinemic hypoglycemia. Arc Dis Child 1996; 74: 379–85. doi: 10.1136/adc.74.5.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 1995; 268: 423–6 doi: 10.1126/science.7716547 [DOI] [PubMed] [Google Scholar]
- 12.Shyng SL, Ferrigi T, Shepard JB, Nestorowicz A, Glaser B, Permutt MA, et al. Functional analyses of novel mutations in the sulfonylurea receptor 1 associated with persistent hyperinsulinec hypoglycemia of infancy. Diabetes 1998; 47: 1145–51 doi: 10.2337/diabetes.47.7.1145 [DOI] [PubMed] [Google Scholar]
- 13.Glaser B, Chiu KC, Anker R, Nestorowicz A, Landau H, Ben-Bassat H, et al. Familial hyperinsulinism maps to chromosome 11p14-15.1, 30cM centromeric to the insulin gene. Nat Genet 1994; 7: 185–8 doi: 10.1038/ng0694-185 [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Ye Y, Lighter E. Mutations of the pancreatic islet inward rectifier also lead to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet 1996; 5: 1809–12 doi: 10.1093/hmg/5.11.1809 [DOI] [PubMed] [Google Scholar]
- 15.Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of KATP: an inward rectifier subunit plus the sulphonylurea receptor. Science 1995; 270: 1166–70 doi: 10.1126/science.270.5239.1166 [DOI] [PubMed] [Google Scholar]
- 16.Dekelbab BH, Sperling MA. Hyperinsulinemic hypoglycemia in infancy: the challenge continues. Diabetes Metab Res Rev 2004; 20: 189–95 doi: 10.1002/dmrr.448 [DOI] [PubMed] [Google Scholar]