Abstract
It is still in doubt whether the standard-dose growth hormone (GH) used in Japan (0.5 IU/kg/week, 0.167 mg/kg/week) for growth hormone deficiency is effective for achieving significant adult height improvement in non-growth hormone deficient (non-GHD) short children. We compared the growth of GH-treated non-GHD short children with that of untreated short children to examine the effect of standard-dose GH treatment on non-GHD short children. GH treatment with recombinant human growth hormone (rhGH) was started before the age of 11 yr in 64 boys and 76 girls with non-GHD short stature registered at the Foundation for Growth Science who have now reached their adult height. In 119 untreated boys and 127 untreated girls whose height standard deviation score (SDS) was below –2 SD at the age of 6 yr, height growth was followed until 17 yr. Height SDS was significantly lower before GH treatment in the GH-treated group than at the age of 6 yr in the untreated group, in both sexes. Adult height and adult height SDS were significantly greater in the untreated group than in the GH-treated group, in both sexes, although the change in height SDS did not differ significantly. Height SDS was significantly lower before GH treatment in the GH-treated group than at the age of 6 yr in the untreated group, so 57 boys and 57 girls whose height SDS at the age of 6 yr in the untreated group closely matched the height SDS before GH treatment in the GH-treated group were chosen for comparison. Height SDS did not differ significantly between the GH-treated group before GH treatment and the untreated group at the age of 6 yr, nor were there differences between these subgroups in adult height, adult height SDS, or height SDS change, in either sex. The effect of GH treatment is reported to be dose-dependent and doses over 0.23 mg/kg/week are reported to be necessary to improve adult height in non-GHD short children. Currently, the GH dose is fixed at 0.175 mg/kg/week in Japan, and we expected to find, and indeed concluded, that ordinary GH treatment in Japanese, non-GHD short children does not improve adult height.
Keywords: non-GHD short children, GH treatment, GH dosage
Introduction
The effectiveness of growth hormone (GH) treatment on adult height in non-growth hormone deficient (non-GHD) children is controversial. A recent double-blind, placebo-controlled study clearly demonstrated that children with idiopathic short stature treated with high-dose GH are significantly taller than placebo-treated short children when they reach adult height (1). It is still unclear whether the standard dose used in Japan has any significant efficacy on increasing the adult height of non-GHD short children.
It is known that approximately 60% of non-GHD short children show a tendency toward delayed puberty and reach an adult height within the normal range (> –2 SD) without any treatment (2). Therefore, the normalization of adult height following GH treatment does not prove the efficacy of GH treatment in these patients, unless we establish this through comparison of the resulting adult heights with the adult heights of untreated non-GHD short children. In Japan, since it is now practically impossible to study the efficacy of GH treatment on adult height in non-GHD short children in a controlled study, we compared data on file at the Foundation for Growth Science and epidemiological data in Akita Prefecture (2).
The Foundation for Growth Science was founded in 1977 and has been monitoring the use of GH by its registration system, which includes judgment of eligibility to start and to continue GH treatment, and a system for reporting adverse events. The data on the application sheets were utilized as they are the largest database of GH treatment in Japan. The epidemiological data in Akita Prefecture was collected through the collaborative activity of pediatric endocrinologists and the local committee of education.
Subjects and Methods
The following two groups were compared:
1) GH-treated group: 64 boys and 76 girls for whom data were on file at the Foundation for Growth Science and had started growth hormone (GH) treatment with recombinant growth hormone (rhGH) at a dose of 0.5 IU (0.167 mg)/kg/week before the age of 11 yr and thereafter reached their adult height (as defined below). They were diagnosed as having non-GHD short stature because their peak GH values were below 10 ng/ml in two GH provocation tests but over 10 ng/ml in at least one test. Their tallest height after their height velocity became less than 2 cm/yr was defined as their adult height. The age at adult height estimation was 16.89 ± 1.01 yr and 15.34 ± 0.93 yr in boys and girls, respectively.
2) Untreated group: 119 boys and 127 girls whose height SDS was below –2 SD at 6 years of age were followed until 17 yr old. Height at the age of 17 yr was defined as adult height in these patients.
In the second comparison, height SDS at start of GH treatment in the GH-treated group and at the age of 6 yr in the untreated group were matched and 57 boys and 57 girls were selected from the both groups.
The height standard of the national survey of 1990 was used for the calculation of height SDS. Student’s t-test was used for comparisons of the two groups. The significance level was set at 0.05.
Results
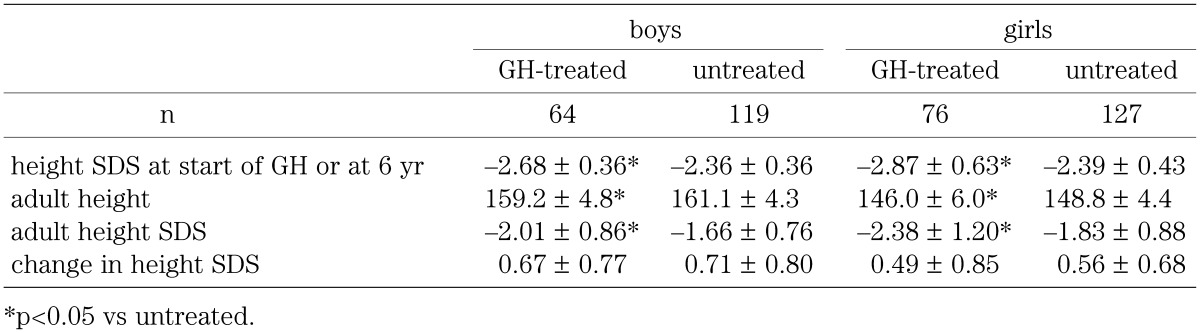
In the GH treated group, the age at the start of GH was 10.7 ± 0.9 yr and 9.6 ± 0.9 yr, and the duration of GH treatment was 6.2 ± 1.0 yr and 5.7 ± 1.0 yr for boys and girls, respectively. Table 1 shows the height SDS before GH treatment in the GH-treated group and the height SDS at the age of 6 yr in the untreated group, and adult height and adult height SDS in both sexes, as well as the change in height SDS in the GH-treated group between before GH treatment and after attaining adult height and in the untreated group between the ages of 6 yr and 17 yr. In both sexes, the height SDS was significantly smaller in the GH-treated group before GH treatment than in the untreated group at the age of 6 yr, and adult height and adult height SDS were significantly smaller in the GH-treated group than in the untreated group. Change in height SDS, however, did not differ significantly: gains in height SDS were observed not only in the GH-treated group but also in the untreated group.
Table 1. Comparison of clinical characteristics between GH-treated and untreated groups of non-GHD short children.
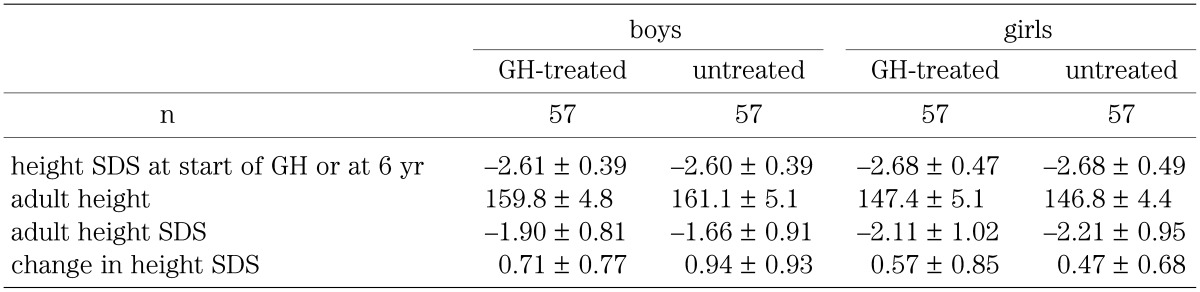
Because there was a significant difference between height SDS in the GH-treated group before GH treatment and height SDS in the untreated group at the age of 6 yr, 57 boys and 57 girls whose height SDS at the age of 6 yr in the untreated group closely matched the height SDS of patients in the GH-treated group before GH treatment were chosen and compared with the GH-treated group. Table 2 shows height SDS in the GH-treated group before GH treatment and in the untreated group at the age of 6 yr, adult height, and adult height SDS in both sexes. The changes in height SDS in the GH-treated group between before GH treatment and adult height and in the untreated group between the ages of 6 yr and 17 yr are also shown. No significant differences were found in height SDS between the GH-treated group before GH treatment and the untreated group at the age of 6 yr, or in adult height or adult height SDS, nor did the change in height SDS differ significantly between the GH-treated group and the untreated group.
Table 2. Comparison of clinical characteristics between the GH-treated and untreated groups of non-GHD short children, when GH-treated short children were matched with untreated short children whose height SDS at 6 yr of age was similar to the height SDS of GH-treated short children before treatment.
Discussion
Table 3 shows data on GH treatment outcomes in non-GHD short children from six important studies conducted in Europe and the United States (1, 3,4,5,6,7), which have used recombinant hGH. Evaluation reveals some problems with these reports: 1) ages at the start of treatment and durations of therapy differ; 2) therapeutic doses differ; and 3) therapeutic effects cannot be evaluated, since most reports did not include untreated controls. Many reports, like the studies cited in Table 3, evaluate the effect of GH treatment by comparing the measured outcome with predicted adult height (PAH) instead of by comparison with controls. Despite these problems, it is clear that in all the studies higher doses than the Japanese standard dose were used and the change in height SDS from the start of GH treatment to adult height was greater in these studies than that of this study. The change in height SDS reported by the studies varied from 0.8 to 1.85 SD (Table 3). The studies employing higher doses (5,6,7) of GH achieved more improvement.
Table 3. Adult height after GH treatement in non-GHD short children in Europe and the United States.
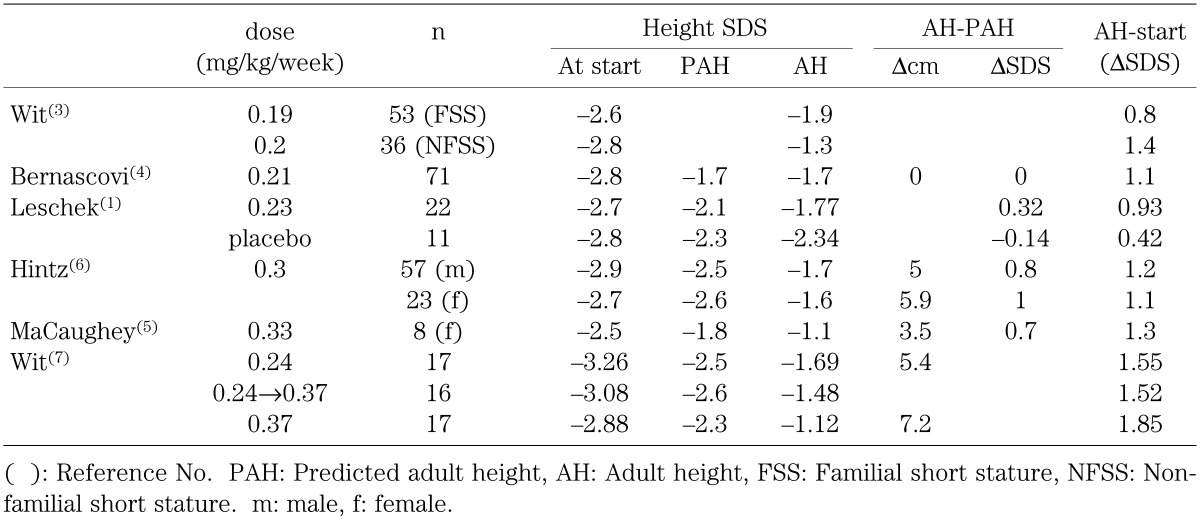
Leschek et al. (1) conducted a randomized, double-blind, placebo-controlled trial. Sixty-eight non-GHD short children received either GH (0.23 mg/kg/week) or placebo. Adult height was finally evaluated in 22 GH-treated children and 11 placebo-treated children and was significantly greater in the GH-treated group than in the placebo-treated group by 0.51 SDS. However the injection frequency was three times per week in both groups. When these resuits are compared with those of Wit et al. (7) who used daily injections of a similar dose of GH, the effect on adult height was greater in daily injection.
McCaughey (5) compared GH effects with untreated controls, though few in number. At a dose of 30 IU/m2/week (about 0.33 mg/kg/week), adult height was 7.5 cm taller on average in GH-treated children after a mean treatment period of 6.2 yr than in the untreated control group. Hintz et al. (6) estimated the effect of GH treatment on the adult height of non-GHD short children at a dose of 0.3 mg/kg/week for 5.5–6.0 yr, the difference between predicted adult height before treatment and achieved adult height was 9.2 cm greater in boys and 5.7 cm in girls than the corresponding difference in the untreated historical controls.
Wit et al. (7) compared the adult height of 50 GH-treated patients with idiopathic short stature. Patients were treated with GH at 0.24 mg/kg/week, 0.24 mg/kg/week for the first year and at 0.37 mg/kg/week thereafter, or 0.37 mg/kg/week, and their mean height SDS increased by 1.55, 1.52, and 1.85 SD, respectively. They concluded that the effect of GH treatment on adult height was dose-dependent and that regimens increasing dosage from the second year were less efficacious.
One study in Japan found that after 4.2 yr of GH treatment at a dose of 0.5 IU/kg/week (0.167 mg/kg/week), the average adult height of 9 GH-treated non-GHD short boys, 154.2 cm, was significantly shorter than the adult height of 18 untreated short boys, 162.0 cm (8). Bone age at the onset of puberty did not differ significantly between the groups, but pubertal height gain was significantly greater in the untreated boys. Yet the approximately 1-yr bone age difference at the start of treatment, though it was not statistically significant, might cause to early epiphyseal closure and hence lower the adult height in the GH-treated group rather than GH treatment itself. Another study by the same group (Kawai et al. (9)) compared adult height in 11 GH-treated non-GHD short girls treated at the same dose, 0.5 IU/kg/week for 4.2 yr on average, with 11 untreated short girls, and found no significant difference.
The long-term effect of GH treatment is usually evaluated in terms of adult height. Since adult height, however, depends heavily on height SDS at the start of GH treatment (10), change in height SDS from the start of GH treatment to adult height is a more precise evaluation. In our study, the tallest height after the timing of less than 2 cm/yr of height velocity was defined as adult height, and adult height was taller in untreated short children than in GH-treated non-GHD short children. But this is because the pre-treatment height SDS of the GH-treated non-GHD short children was significantly lower than the baseline height SDS of the untreated short children. Adjusted for pre-treatment height SDS, adult height SDS was not significantly different between the GH-treated patients and the untreated controls. In no situation did the change in height SDS in the two groups differ significantly. It can only be concluded that, in this study, GH treatment in non-GHD short children was ineffective in improving adult height.
In GHD, there is a significant positive correlation between age at onset of puberty and age at the start of GH treatment (11,12,13). Increasing evidence points to an important role for GH in gonadal function through induction of local production of IGF-I in the ovary or in the testis and by increase of gonadotropin-dependent gonadal functions such as sex steroid production and ovulation (13,14,15,16). These facts demonstrate that GH accelerates pubertal onset and that the resulting relatively early pubertal development adversely effects decompensates the catch-up growth observed for the first few years following initiation of GH treatment. The lack of improvement in adult height in GH-treated non-GHD short children is attributable mainly to the early induction of puberty by GH treatment and the relatively poor response to GH in non-GHD short children, even though GH treatment improves growth velocity during the first two or three years (10). It is well known that adult height shows a strong positive correlation with height at the onset of puberty in GH-treated short children (11). Therefore, to achieve normal adult height in GH-treated short children, height must be normalized at onset of puberty.
In this study, puberty was not analyzed since the onset of the puberty is often not correctly evaluated and in our experience tends to be judged later than its actual occurrence. Therefore, the prepubertal height SDS and adult height SDS were analyzed. The GH dosage used in Japan now (0.175 mg/kg/week) induces catch-up growth for only a few years, but in non-GHD short children it seems insufficient to normalize height by the onset of puberty and to increase height SDS to an extent exceeding natural improvement in untreated short children.
Natural improvement of height SDS in short children has been reported to range between 0.4 and 0.7 SD (1, 2, 17), as it did in our study. To prove that GH treatment is effective in non-GHD short children, it is necessary to achieve a gain in height SDS greater than 1 SD through GH treatment. Leschek et al. (1) report 0.93 SD improvement on average by GH at a dose of 0.23 mg/kg/week injected thrice a week, significantly greater than the natural improvement in placebo-treated short children (0.42 SD on average). Other studies employing higher doses reported greater than 1 SD improvement (5,6,7).
There may be a small possibility that the real adult height is different from the adult height defined in this study. It is possible that both GH treated and untreated groups will grow after the adult height of this definition. GH-treated non-GHD short children will grow after the growth velocity drops below 2 cm/yr, but such growth is around 5 mm in our experience. Untreated non-GHD short children have a tendency of delayed puberty. Boys with delayed puberty will grow after 17 yr, but the growth is less than 1 cm with a few exceptions (18). Therefore, these minimal differences in adult height do not essentially change our conclusion.
The above findings and considerations demonstrate that the effect of GH treatment is dose-dependent and suggest that doses over 0.23 mg/kg/week are necessary to achieve meaningful improvements in adult height in non-GHD short children. We conclude that GH treatment in non-GHD short children in Japan does not improve the adult height of treated children because the GH dose currently being administered, 0.175 mg/kg/week, is insufficient.
Acknowledgement
This study was supported by the Grants from the Foundation for Growth Science and from the Ministry of Health, Labour and Welfare.
References
- 1.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2004; 89: 3140–8 doi: doi: 10.1210/jc.2003-031457 [DOI] [PubMed] [Google Scholar]
- 2.Komatsu K, Okamura T, Takada G, Miyashita M, Ohno T, Tanaka T. Analysis of natural growth of children with short stature at prepuberty or at final height. I. Follow-up of healthy children at Akita Prefecture. J Jpn Pediatr Soc 1997; 101: 610–6 [Google Scholar]
- 3.Wit JM. Growth hormone treatment of idioapthic short stature in KIGS. In: Ranake MB, Wilton P, editors. Growth hormone therapy in KIGS-10 years’ experience. Heidelberg, 1999, p.225–43. [Google Scholar]
- 4.Bernasconi S, Street ME, Volta C, Mazzardo G. Final height in non-growth hormone deficient children treated with growth hormone. Clin Endocrinol (Oxf) 1997; 47: 261–6 doi: 10.1046/j.1365-2265.1997.2751082.x [DOI] [PubMed] [Google Scholar]
- 5.McCaughey ES, Mulligan J, Voss LD, Betts PR. Randomized trial of growth hormone in short normal girls. Lancet 1998; 351: 940–4 [DOI] [PubMed] [Google Scholar]
- 6.Hintz R, Attie KM, Baptista J, Roche A. Effect of growth hormone treatment on adult height of children with idiopathic short stature. N Engl J Med 1999; 340: 502–7 doi: 10.1056/NEJM199902183400702 [DOI] [PubMed] [Google Scholar]
- 7.Wit JM, Rekers-Mombarg LT, Cutler GB, Crowe B, Beck TJ, Roberts K, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: evidence for a dose effect. J Pediatr 2005; 146: 45–53 doi: 10.1016/j.jpeds.2004.08.055 [DOI] [PubMed] [Google Scholar]
- 8.Kawai M, Momoi T, Yorifuji T, Yamanaka C, Sakai H, Furusho K. Unfavorable effects of growth hormone therapy on the final height of boys with short stature not caused by growth hormone deficiency. J Pediatr 1997; 130: 205–9 doi: 10.1016/S0022-3476(97)70344-5 [DOI] [PubMed] [Google Scholar]
- 9.Kawai M, Momoi T, Yorifuji T, Muroi J, Uematsu A, Yamanaka C, et al. Growth hormone treatment does not improve the final height of girls with short stature not caused by growth hormone deficiency. Clin Pediatr Endocrinol 1998; 7: 93–8. doi: 10.1297/cpe.7.93 [DOI] [Google Scholar]
- 10.Tanaka T, Cohen P, Clayton P, Laron Z, Hintz RL, Sizonenko PC. Diagnosis and management of growth hormone deficiency in childhood and adolescence. Part 2: growth hormone treatment in growth hormone deficient children. GH & IGF Research 2002; 12: 323–41 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Yoshizawa A, Tanae A, Hibi I, Shizume K. Relationships between puberty and growth at adolescence in growth-hormone-deficient males: effect of growth hormone and of associated gonadal suppression therapy. Horm Res 1990; 33(suppl 4): 102–5 doi: 10.1159/000181593 [DOI] [PubMed] [Google Scholar]
- 12.Price DA, Shalet SM, Clayton PE. Management of idiopathic growth hormone deficient patients during puberty. Acta Paediatr Scand 1998; 347(Suppl): 44–51 [PubMed] [Google Scholar]
- 13.Tanaka T. Pubertal aspects of idiopathic growth hormone deficiency. In: Tanake MB, Gunnarsson R, editors. Progress in growth hormone therapy-5 years of KIGS. Mannheim: J&J Verlag; 1994: p.112–28. [Google Scholar]
- 14.Tres LL, Smith EP, Van Wyk JJ, Kierszenbaum AL. Immunoreactive sites and accumulation of somatomedin-C in rat Sertoli-spermatogenic cell cocultures. Exp Cell Res 1986; 16: 33–50. doi: 10.1016/0014-4827(86)90424-6 [DOI] [PubMed] [Google Scholar]
- 15.Davoren JB, Hsueh AJW. Growth hormone increases ovarian levels of immunoreactive somatomedin C/insulin-like growth factor I in vivo. Endocrinology 1986; 118: 888–90 doi: 10.1210/endo-118-2-888 [DOI] [PubMed] [Google Scholar]
- 16.Adashi EY, Resnick CE, D’Ercole AJ, Svoboda NE, Van Wyke JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev 1985; 6: 400–20 doi: 10.1210/edrv-6-3-400 [DOI] [PubMed] [Google Scholar]
- 17.Wit JM, Rekers-Mombarg LT. Final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab 2002; 87: 604–11 doi: 10.1210/jc.87.2.604 [DOI] [PubMed] [Google Scholar]
- 18.Okamura T, Takada G, Miyashita M, Ohno T, Watanabe T, Tanaka T. Growth of school children in Akita Prefecture. Clin Pediatr Endocrinol 1994; 3 (suppl 5): 155–7. doi: 10.1297/cpe.3.Supple5_155 [DOI] [Google Scholar]


