Abstract
Much epidemiological evidence has linked low birth weight with late cardiovascular risk. In order to investigate the effect of intrauterine growth restriction (IUGR) on early atherosclerosis in the fetus, we measured aortic wall thickness (abdominal aortic intima-media thickness: aIMT) by ultrasonography in 15 neonates with IUGR and in 31 neonates considered to be appropriate for gestational age (AGA). Furthermore, we evaluated the relationship between aIMT, serum insulin-like growth factor-I (IGF-I) and low-density lipoprotein (LDL) particle size to investigate the possible effect of these atherosclerosis-related factors on the early atherosclerosis process. The results showed that the mean aIMT was significantly greater in the IUGR neonates than in the AGA neonates (least squares mean ± SE, 537 ± 24.8 vs. 471 ± 17.0 µm, p=0.037). The serum IGF-I levels were lower in the IUGR neonates than in the AGA neonates (27.9 ± 4.3 vs. 42.7 ± 2.9 ng/ml, p=0.009). A significant negative correlation was observed between aIMT and IGF-I in the IUGR neonates (r=–0.646, p=0.009); however, a positive correlation was observed between aIMT and IGF-I (r=0.416, p=0.020) in the AGA neonates. There appeared to be no relationship between aIMT and LDL particle diameter. Atherogenic small, dense LDL was not detected in the IUGR infants. In conclusion, neonates with IUGR have significant aortic thickening with decreased IGF-I, suggesting that prenatal events might predispose them to later cardiovascular risk.
Keywords: atherosclerosis, aortic wall thickening, IUGR, IGF-I, LDL particle
Introduction
Much recent research has addressed the role that the fetal environment might play in adult health. In many, but not all studies, low birth weight seems to be associated with an increase in cardiovascular risk similar to that seen with major environmental risk factors, such as cigarette smoking or hypertension (1). Small or disproportionate newborns have increased rates of coronary heart disease, high blood pressure, high cholesterol levels and abnormal glucose metabolism (2). The mechanisms whereby slow intrauterine growth confers vascular risk have not been clearly established, particularly in regard to whether fetal events might result in any arterial abnormalities in newborn babies. Recently, two studies demonstrated aortic wall thickness by ultrasound-based examination in newborns with intrauterine growth restriction (IUGR) (3, 4). Although the mechanisms underlying the current data have not been fully elucidated, IUGR may be associated with increased sympathetic tone, dyslipidaemia characterised by raised concentrations of atherogenic lipoproteins and reduced concentrations of insulin-like growth factor I (IGF-I), all of which might contribute to arterial wall thickening (3, 4).
The main established endocrine regulators of fetal growth include insulin and the IGF system (5, 6). Thus, although the fetus may be largely growth hormone (GH) independent, local tissue production of IGF-I is critical for normal intrauterine growth. In postnatal life, alterations in the GH/IGF-I axis are associated with cardiovascular disease (CVD), and growth hormone hypersecretion as well as GH deficiency are characterized by an increased prevalence of atherogenic CVD (7, 8).
In terms of the etiologic role of lipid levels in the onset of early atherosclerosis, postmortem studies have demonstrated that maternal hypercholesterolemia is associated with increased fatty-streak formation in fetal aortas (9). Small, dense low-density lipoprotein (SDLDL) particles are potent atherogenic lipoproteins that are considered to be a metabolic marker of insulin resistance (10, 11), although the pathological significance of SDLDL in the fetal process of atherosclerosis is unknown.
In the present study, we examined the utility of ultrasound-based measurement of the distal segment of the dorsal abdominal aortic intima-media thickness (aIMT) in newborns as a sensitive marker of atherosclerosis risk (12). Additionally, we aimed to investigate the relationship between aIMT, serum IGF-I and LDL particle size in newborns with IUGR.
Subjects and Methods
The study subjects were 46 neonates, including those with low birth weights, who were hospitalized at the Center for Perinatal Medicine, Dokkyo Medical University, between January and April 2008. They were split into two groups as follows: 15 IUGR neonates aged 29–38 wk and weighing 860–2,158 g, and 31 appropriate for gestational age (AGA) neonates aged 23–40 wk and weighing 672–3,202 g. The neonates were categorized into the IUGR and AGA groups in accordance with the recommendations of the Japan Pediatric Society and Japan Society of Obstetrics and Gynecology issued in 1994 (13). Neonates with a birth weight that was less than the 10th percentile of the standard birth weight curve for Japanese neonates were classified as IUGR neonates instead of small for gestational age (SGA). In this paper, neonates with IUGR mean only neonates whose birth weight was less than the 10th percentile as mentioned in the report of Skilton et al. (3), which was the first report to show an increased aIMT in neonates with IUGR. Neonates with a birth weight that was within the 10th–90th percentiles were classified as AGA neonates. In addition, the 15 IUGR infants were classified as either symmetric (n=3) or asymmetric IUGR (n=12). Neonates at gestational ages of 22–40 wk, neonates from twin pregnancies and neonates with a maternal medical history of pregnancy-induced hypertension syndrome, hyperemesis gravida, maternal diabetes, prenatal administration of steroids or perinatal alcohol intake or smoking were included in the present study; neonates considered to be large for gestational age, those with congenital malformations and extremely low birth weight infants with serious respiratory and cardiovascular failure were excluded from the study. Twenty-four cases, excluding 22 twin pregnancy cases to avoid redundancy in the data, were included from analysis of maternal characteristics, placentas and umbilical cords.
All of the data for the neonates and their mothers shown in Table 1 were collected at the Center for Perinatal Medicine during history taking and medical examinations. The data for the neonates were based on records routinely made by pediatricians and nurses immediately after birth. Measurement of blood pressure in the neonates was conducted with a Dinamap Pro 100 (GE Medical Systems), and blood glucose in whole blood was measured with an Antsence II blood glucose meter (Horiba). The maternal characteristic, placenta and umbilical cord data were recorded by obstetricians and nurses during outpatient visits to the Department of Obstetrics and during hospitalization in the Obstetric Ward. In regard to appetite of the mothers (whether or not they recently had an appetite), intake of over-the-counter supplements containing folic acid (whether or not mothers took related supplements) and smoking or drinking (whether or not mothers had a history of smoking and drinking), all date was collected by history taking, and Table 1 shows the percentages of mothers who answered “Yes” in the IUGR and AGA groups. The height, weight and body mass index (BMI) of each mother was obtained before pregnancy. The weight gain during pregnancy of each mother was obtained by comparing the mother’s weight before pregnancy with that at hospitalization. The lowest hemoglobin (Hb) level before birth was selected as the maternal Hb level. The placental and umbilical diameters were measured by midwives using a ruler. The largest umbilical diameter from vertical and horizontal measurements was used. Maternal blood pressure was measured immediately before birth.
Table 1. Characteristics of newborns, their mothers and placental parameters.
Informed signed consent was obtained from both parents prior to participation in this study.
Ultrasound studies
One physician performed ultrasonography on the neonates in an incubator at a constant temperature at random immediately after birth, before looking at the clinical and examination data of the neonates. A Sonos 7500 (Philips, USA) high-resolution ultrasonography computer equipped with a vascular preset for aIMT prepared previously using an 11 MHz linear-type probe or 12 MHz sector-type probe was used for the measurements. Clear images were obtained by adjusting the focus and gain in a supine position (Fig. 1). Longitudinal images of the vessels, excluding vascular bifurcations, 0.5–1 cm wide were obtained by moving the ultrasonic probe over the abdominal aorta distally in the possible range. This site was selected based on a report showing frequent occurrence of pathological vascular changes in the distal abdominal aorta in infants (14). The focus of the far wall (dorsal side of the aorta wall) was adjusted to obtain the clearest images possible. The aIMT was measured by selecting diastolic static images in synchronization with a cardiograph by using a built-in manual measuring device in the ultrasonograph. It was measured at three arbitrary sites for the far wall in the image. Mean aIMT was defined as the average of the measurements.
Fig. 1.
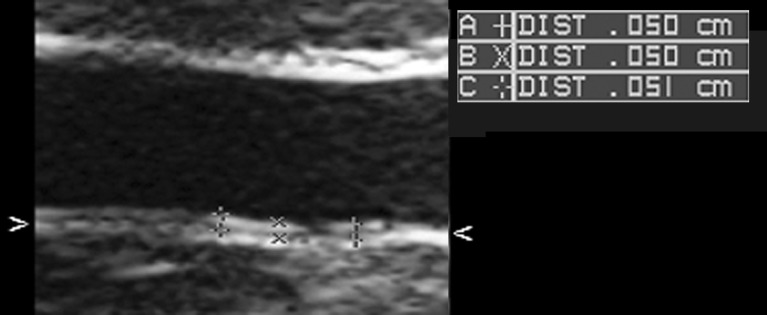
Images obteined from scanning of the far wall of the distal abdominal aorta near the aortic bifurcation. The intima-media complex was measured using these images. The mean aIMT based on these images is 503 µm.
Measurement of LDL particle size
Samples were collected from neonates before the first intravenous dextrose infusion or before milk feeding. The samples were centrifuged for 15 min, and the obtained serum was stored at –40°C until analysis. The serum IGF-I level was measured using the immunoradiometric assay (IRMA) method (Mitsubishi Kagaku Iatron, Inc., Japan). The lowest detectable IGF-I level was 6.3 ng/ml, and the intra- and interassay coefficients of variation’s were 3.3% and 8.2%, respectively.
The LDL particle diameter in neonatal serum was measured and was determined, according to the method described by Krauss et al. (15), by gradient gel electrophoresis using 2.5 to 16% polyacrylamide. After the gels were equilibrated at 150 V for 20 min, electrophoresis was performed for each gel; each gel contained a serum sample diluted 1:1 with sample buffer (consisting of 31% sucrose, 0.06% EDTA-2Na and 0.01% BPP) to a volume of 10 µl. Each gel also contained thyroglobulin, apoferritin and latex beads as reference standards of known diameter. The gels were then electrophoresed at 150 V for 19 h and were stained for lipid with Oil red O and incubated at 55°C for 24 h; the gels were also stained for protein with Coomassie Brilliant Blue for 15 min. They were then destained with ethanol and immersed in acetic acid. The destained gels were then scanned with an image scanner (Epson GT-6500: Seiko Epson Corporation, Nagano, Japan) and analysed using an image processing and analysis program for Macintosh (Image J 1.38x: National Institutes of Health, Bethesda, MD, USA). The migration distances were subsequently determined, and the LDL particle diameter was then calculated by comparing the mobility of the sample with the mobility of the three calibrated standards on each gel. Small, dense LDL was defined as LDL with a particle diameter <25.5 nm based on the criteria proposed by Austin et al. (16). Measurement of LDL particle diameter has previously been reported in children (17). We defined LDL with a particle diameter <25.5 nm the same as in adults.
Statistical methods
The data for the IUGR neonates and AGA neonates were first compared using analysis of covariance (ANCOVA; covariance was gestational age). The Student’s independent sample t-test was then used for gestational age, and the χ2 test was used to determine rates. The results are presented as the least squares mean (standard error), mean value (standard deviation) and frequency (%), respectively. The relationships between aIMT, gestational age, serum IGF-I level and LDL particle diameter were examined with Pearson’s correlation coefficient. All data were considered to be statistically significant at p<0.05. Dr. SPSS II for Windows version 11.0 (SPSS Inc.) was used for all analyses.
Results
Table 1 shows the data collected from the neonates and their mothers. The values for items related to malnutrition and low birth weight, such as birth weight, birth height, weight gain during pregnancy, placental weight and placental diameter (18), were significantly lower in the IUGR neonates than in the AGA neonates.
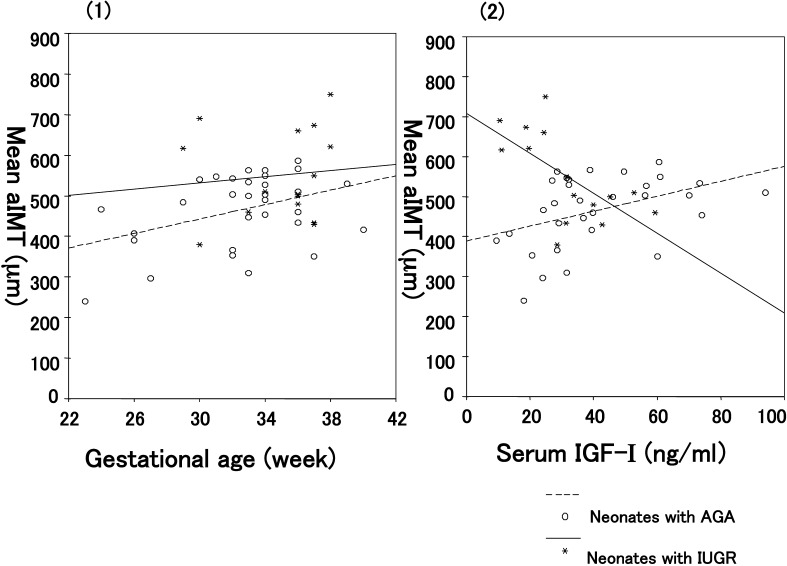
Mean aIMT was higher in the IUGR neonates than in the AGA neonates (least squares mean ± SE, 537 ± 24.8 vs. 471 ± 17.0 µm, p=0.037) as shown in Table 1 and Fig. 2-(1). Regarding IGF-I, the serum IGF-I levels were significantly lower in the IUGR neonates than in the AGA neonates (27.9 ± 4.3 vs. 42.7 ± 2.9 ng/ml, p=0.009), and a significant negative correlation was observed between the mean aIMT and serum IGF-I level in the IUGR neonates (r=–0.646, p=0.009; Table 2, Fig. 2-(2)). The mean aIMTs between the asymmetric (n=3) and symmetric (n=12) IUGR infants were not significantly different (563 ± 33.2 vs. 495 ± 66.8, p=0.38).
Fig. 2.
(1) Relationship between mean aIMT and gestational age in the IUGR and AGA infants (least squares mean ± SE, 537 ± 24.8 vs. 471 ± 17.0 µm, p=0.037; analysis of covariance). (2) Relationship between mean aIMT and serum IGF-I levels in the IUGR (r=–0.646, p=0.009) and AGA neonates (r=0.416, p=0.020).
Table 2. Relationship between mean aortic intima-media thickness and gestational age and hematological factors.
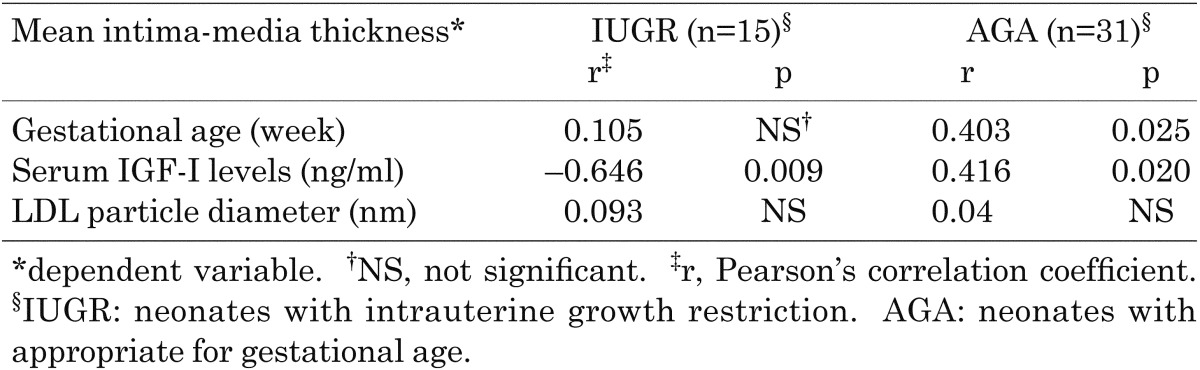
The mean aIMT of the AGA neonates exhibited positive correlation with the gestational age (r=0.403, p=0.025) and significant positive correlation with the serum IGF-I level (r=0.416, p=0.020; Table 2).
There was no significant difference in LDL particle diameter between the IUGR and AGA neonates (Table 1). Atherogenic SDLDL was not detected in IUGR infants. Furthermore, there were no significant correlations between aIMT and LDL particle diameter (Table 2) or between LDL particle diameter and the serum IGF-I level (date not shown).
Discussion
As with the major cardiovascular risk factors, impaired growth in utero is associated with functional (endothelial dysfunction) and structural (increased wall thickness) changes in the arterial vasculature consistent with early atherosclerosis (19,20,21). Studying the vasculature of neonates or young children may provide strong evidence concerning whether intrauterine factors per se can influence the vasculature. Carotid intima-media thickness has been measured in children as young as 2 yr of age (22). However, it stands to reason that because the abdominal aorta is the site of the earliest fatty streaks (14), it may present a more useful indicator of early atherosclerosis in children. Indeed, it has recently been demonstrated that noninvasive measurement of the abdominal aortic wall thickness using high-resolution ultrasonography is a better indicator of early atherosclerosis in children than carotid intima-media thickness (12).
The thickness of the intrauterine aortic wall may indicate that these intrauterine factors contribute to postnatal atherosclerosis. Although there remains no evidence that these changes in the aortic wall in neonates occur as either the precursors of arterial plaques or in association with an increased risk of cardiovascular events in adulthood, autopsy studies in children have shown that atherosclerotic lesions begin to develop first in the intima of the aorta (14), and a fetal postmortem study has revealed that fatty streaks from fetal aortas contain both native and oxidized LDL and macrophages; thus, fatty streaks share a morphology similar to early atherosclerotic lesions (9). Therefore, it is probable that intrauterine factors program predisposition towards development of postnatal atherosclerosis, which leads to adult CVD.
In the present study, we found that the IUGR neonates exhibit significantly larger aIMTs with decreased serum IGF-I levels compared with the AGA infants. In addition, the mean aIMT correlated negatively with the serum IGF-I level. These results were consistent with the findings of two previous studies. Skilton et al. (3) initially observed that aIMT was significantly higher in neonates with IUGR than in those without IUGR, and Koklu et al. (4) subsequently found a higher aIMT with a decreased concentration of IGF-I in IUGR neonates.
There are several potential mechanisms by which IGF-I could modify atherosclerotic processes, either locally or in a systemic manner (23). In agreement with our findings for the IUGR neonates, Colao et al. (24) found that the IGF-I level determines the carotid intima-media thickness in both patients with IGF-I deficiency and healthy controls. IGF-I plays an important role with regard to the endothelium and increases in nitric oxide production (25). Decreased nitric oxide activity is associated with intimal thickening (26). Decrease of the IGF-I concentration resulting from GH deficiency is well-characterized by the increased prevalence of atherogenic CVD (8, 27).
We also found that the mean aIMT increased with gestational age in both the AGA and IUGR neonates; aortic wall thickening in accordance with gestational age is considered to be a physiological phenomenon involved in development of the fetal aorta. In terms of the relationship between aIMT and IGF-I, the inverse relationship found in the IUGR infants was not found in the AGA infants. The reason for this was unclear in the present study, But we speculate that humoral factors other than IGF-I, such as leptin (28, 29), which is related to the nutritional state of the fetus, may be relevant to our finding that aIMT was not increased despite the low IGF-I concentrations in the AGA infants. Further evaluation in a larger number of cases is necessary to confirm this finding and to clarify this issue.
An etiologic role for lipid levels in the onset of early atherosclerosis is supported by postmortem studies. Maternal hypercholesterolemia is well known to be associated with increased fatty-streak formation in fetal aortas, possibly in association with the fetal cholesterol levels (9). The increase in fatty streaks associated with maternal hypercholesterolemia is most marked in the fetal abdominal aorta and aortic arch, whereas there are negligible differences in the thoracic aorta (9, 30). In the present study, there was no apparent relationship between aIMT and LDL particle diameter, and atherogenic SDLDL was not detected in the IUGR infants. Furthermore, there was no relationship between the IGF-I level and LDL particle size. Therefore, it seems that altered LDL metabolism is not of a pathological significance in the fetal process of atherosclerosis.
The causes of IUGR have been categorized into fetal, placental and maternal factors. In regard to differences in maternal characteristics between IUGR and AGA in terms of pregnancy, placental weight, maternal age, BMI, lifestyle, such as smoking or drinking, and the prevalence of diabetes, no significant differences were found for any of the characteristics except for placental weight and diameter, which were smaller in the IUGR mothers. Weight gain during pregnancy was also lower in the mothers of the IUGR infants, and this suggests that there might be possible relationship between the maternal nutritional state and fetal IGF-I concentration. In other studies, it has been reported that the infants of diabetic mothers and mothers who smoke during pregnancy have thicker aortic walls (31).
In conclusion, we have found that aortic wall thickness, an early marker of atherosclerosis in children, is increased in neonates with IUGR in association with a decreased IGF-I concentration. These suggest that prenatal events could predispose them to cardiovascular risk in later life. The results reported herein should be confirmed in larger study cohort because the sample size of the present study cohort was small.
Acknowledgements
I wish to thank Mrs. Yasuyo Kawai for her expert assistance with the laboratory studies. A summary of this article was presented in October 2008 at the 42nd Annual Scientific Meeting of the Japanese Society for Pediatric Endocrinology in Yonago, Japan.
References
- 1.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;2: 577–80 doi: 10.1016/S0140-6736(89)90710-1 [DOI] [PubMed] [Google Scholar]
- 2.Alberry M, Soothill P. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 2007;92: F62–7 doi: 10.1136/adc.2005.082297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet 2005;365: 1484–6 doi: 10.1016/S0140-6736(05)66419-7 [DOI] [PubMed] [Google Scholar]
- 4.Koklu E, Ozturk MA, Kurtoglu S, Akcakus M, Yikilmaz A, Gunes T. Aortic intima-media thickness, serum IGF-I, IGFBP-3, and leptin levels in intrauterine growth-restricted newborns of healthy mothers. Pediatr Res 2007;62: 704–9 doi: 10.1203/PDR.0b013e318157caaa [DOI] [PubMed] [Google Scholar]
- 5.Christou H, Connors JM, Ziotopoulou M, Hatzidakis V, Papathanassoglou E, Ringer SA, et al. Cord blood leptin and insulin-like growth factor levels are independent predictors of fetal growth. J Clin Endocrinol Metab 2001;86: 935–8 [DOI] [PubMed] [Google Scholar]
- 6.Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab 2005;86: 84–90 doi: 10.1016/j.ymgme.2005.07.028 [DOI] [PubMed] [Google Scholar]
- 7.Lombardi G, Colao A, Marzullo P, Ferone D, Longobardi S, Esposito V, et al. Is growth hormone bad for your heart? Cardiovascular impact of GH deficiency and of acromegaly. J Endocrinol 1997;155: S33–7 [PubMed] [Google Scholar]
- 8.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 2002;106: 939–44 doi: 10.1161/01.CIR.0000027563.44593.CC [DOI] [PubMed] [Google Scholar]
- 9.Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest 1997;100: 2680–90 doi: 10.1172/JCI119813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima M, Kanno H, Yamazaki Y, Koyama S, Kanazawa S, Arisaka O. Association of low-density lipoprotein particle size distribution and cardiovascular risk factors in children. Acta Paediatr 2005; 94: 281–6 doi: 10.1111/j.1651-2227.2005.tb03070.x [DOI] [PubMed] [Google Scholar]
- 11.Stan S, Levy E, Delvin EE, Hanley JA, Lamarche B, O'Loughlin J, et al. Distribution of LDL particle size in a population-based sample of children and adolescents and relationship with other cardiovascular risk factors. Clin Chem 2005;51: 1192–200 doi: 10.1373/clinchem.2004.046771 [DOI] [PubMed] [Google Scholar]
- 12.Jarvisalo MJ, Jartti L, Nanto-Salonen K, Irjala K, Ronnemaa T, Hartiala JJ, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation 2001;104: 2943–7 doi: 10.1161/hc4901.100522 [DOI] [PubMed] [Google Scholar]
- 13.Ogawa A, Iwamura T, Kuriya N, Nishida H, Takeuchi H, Takada M, et al. Birth size standards by gestational age for Japanese neonates. Acta Neonatologica Japonica 1998;34: 624–32 [Google Scholar]
- 14.McGill HC, Jr, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW, et al. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY research group. Pathobiological determinants of atherosclerosis in youth. Arterioscler Thromb Vasc Biol 2000;20: 836–45 doi: 10.1161/01.ATV.20.3.836 [DOI] [PubMed] [Google Scholar]
- 15.Krauss RM. Heterogeneity of plasma low-density lipoproteins and atherosclerosis risk. Curr Opin Lipidol 1994;5: 339–49 doi: 10.1097/00041433-199410000-00005 [DOI] [PubMed] [Google Scholar]
- 16.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988;260: 1917–21 doi: 10.1001/jama.1988.03410130125037 [DOI] [PubMed] [Google Scholar]
- 17.Arisaka O, Kojima M, Yamazaki Y, Kanazawa S, Koyama S, Shimura N, et al. Relationship between the presence of small, dense low-density lipoprotein and plasma lipid phenotypes in Japanese children. J Atheroscler Thromb 2004;11: 220–3 doi: 10.5551/jat.11.220 [DOI] [PubMed] [Google Scholar]
- 18.Klaus MH, Fanaroff AA. Classification and physical examination of the newborn infant. In: Routhgate WM, Pittard WB 3rd, editors. Care of the high-risk neonate, 5th edition. Philadelphia: WB Saunders; 2001. p.100–29. [Google Scholar]
- 19.Leeson CP, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, et al. Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation 1997;96: 2233–8 doi: 10.1161/01.CIR.96.7.2233 [DOI] [PubMed] [Google Scholar]
- 20.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation 2001;103: 1264–8 doi: 10.1161/01.CIR.103.9.1264 [DOI] [PubMed] [Google Scholar]
- 21.Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE, Bots ML. Birth weight and carotid intima-media thickness: new perspectives from the atherosclerosis risk in young adults (ARYA) study. Ann Epidemiol 2004;14: 8–16 doi: 10.1016/S1047-2797(03)00068-1 [DOI] [PubMed] [Google Scholar]
- 22.Pauciullo P, Iannuzzi A, Sartorio R, Irace C, Covetti G, Di Costanzo A, et al. Increased intima-media thickness of the common carotid artery in hypercholesterolemic children. Arterioscler Thromb 1994;14: 1075–9 doi: 10.1161/01.ATV.14.7.1075 [DOI] [PubMed] [Google Scholar]
- 23.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res 2000;86: 125–30 doi: 10.1161/01.RES.86.2.125 [DOI] [PubMed] [Google Scholar]
- 24.Colao A, Di Somma C, Filippella M, Rota F, Pivonello R, Orio F, et al. Insulin-like growth factor-1 deficiency determines increased intima-media thickness at common carotid arteries in adult patients with growth hormone deficiency. Clin Endocrinol (Oxf) 2004;61: 360–6 doi: 10.1111/j.1365-2265.2004.02105.x [DOI] [PubMed] [Google Scholar]
- 25.Walsh MF, Barazi M, Pete G, Muniyappa R, Dunbar JC, Sowers JR. Insulin-like growth factor I diminishes in vivo and in vitro vascular contractility: role of vascular nitric oxide. Endocrinology 1996;137: 1798–803 [DOI] [PubMed] [Google Scholar]
- 26.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986;320: 454–6 doi: 10.1038/320454a0 [DOI] [PubMed] [Google Scholar]
- 27.Colao A. The GH/IGF axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf) 2008;69: 347–58 doi: 10.1111/j.1365-2265.2008.03292.x [DOI] [PubMed] [Google Scholar]
- 28.Varvarigou A, Mantzoros CS, Beratis NG. Cord blood leptin concentrations in relation to intrauterine growth. Clin Endocrinol (Oxf) 1999;50: 177–83 doi: 10.1046/j.1365-2265.1999.00630.x [DOI] [PubMed] [Google Scholar]
- 29.Beltowski J. Leptin and atherosclerosis. Atherosclerosis 2006;189: 47–60 doi: 10.1016/j.atherosclerosis.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: fate of early lesions in children (FELIC) study. Lancet 1999;354: 1234–41 doi: 10.1016/S0140-6736(99)02131-5 [DOI] [PubMed] [Google Scholar]
- 31.Gunes T, Koklu E, Yikilmaz A, Ozturk MA, Akcakus M, Kurtoglu S, et al. Influence of maternal smoking on neonatal aortic intima-media thickness, serum IGF-I and IGFBP-3 levels. Eur J Pediatr 2007;166: 1039–44 doi: 10.1007/s00431-006-0376-9 [DOI] [PubMed] [Google Scholar]