Abstract
The present study was designed to determine the plasma level of orexin and its relationship with other metabolic and anthropometric markers in obese children. Forty-seven obese Japanese children, consisting of 31 boys and 16 girls, were enrolled in the study. Their ages were 10.4 ± 0.5 (mean ± s.e.m.) yr, and their percentage overweight was 42.9 ± 1.9%. Blood was drawn after an overnight fast. The age-matched control group consisted of 26 nonobese children, 13 boys and 13 girls. Plasma orexin-A concentration was higher in obese children (17.0 ± 0.4 pg/ml; p<0.001) than in the control children (13.5 ± 1.1 pg/ml). Similarly, plasma leptin concentration was higher in obese children (12.0 ± 1.0 ng/ml; p<0.001) than in the control children (5.2 ± 0.4 ng/ml). There was a highly significant positive correlation between the two parameters in the obese children (r=0.49, p<0.001). Plasma orexin-A level was correlated significantly with waist-to-hip ratio, while leptin level was correlated with percentage overweight, waist circumference and percentage body fat in the obese children. These results suggest that high plasma orexin-A level parallels the leptin level in obese children.
Keywords: orexin (hypocretin), leptin, obesity, child and adolescents, immunoassay
Introduction
Orexins-A and -B, which are also called hypocretins, have recently been identified as novel hypothalamic neuropeptides (1). They strongly activate the appetite of animals through action in the lateral hypothalamus, and have been implicated in the central regulation of metabolic rate and glucose homeostasis. On the other hand, leptin plays a central role in hypothalamic regulation of food intake. It inhibits the orexinergic pathway of acceleration of food intake in the lateral hypothalamic area.
Both orexin-A and -B are derived from a common precursor preproorexin, which consists of 131 amino acid residues, by proteolytic processing (2). Orexin-A and -B consist of 33 and 28 amino acids, and their molecular weight are 3561 and 2899, respectively. There are two types of orexin receptors, both of which are coupled with G-protein. The affinity of orexin 1 receptor (OX1R) is 50 times higher to orexin-A than to orexin-B, while orexin 2 receptor (OX2R) shows equal affinity to both types of ligands.
Orexinergic neurons are known to project to multiple neuronal systems (3), and the physiological role of orexin in the central nervous system (CNS) has been elucidated at least partly. The gene expression of orexin is regulated by blood glucose and feeding (4, 5). Moreover, orexin regulates the sleep-wake cycle and other behaviors such as grooming through a dopaminergic pathway in CNS. Depletion of orexin in CNS plays a central role in the pathogenesis of narcolepsy in both animals and man (6, 7).
The orexin gene is expressed in the testis (8), ovary, intestine (9), pancreas (10), and adrenals (11). Although orexin-A occurs in blood in a substantial amount, little is known about the action mechanism of orexin-A in peripheral tissues. Orexin-A, but not orexin-B, permeates the blood brain barrier (12). Orexin-A, when administered intra-peritoneally to the rat, stimulates insulin secretion by direct action on the pancreatic islets (10). We have already demonstrated that orexin-A stimulates catecholamine synthesis in bovine adrenal medullary cells via the OX1R route (13).
To our knowledge, plasma orexin concentration has been measured in only adult studies. The present study was designed to determine the plasma level of orexin and its relationship with other metabolic and anthropometric markers in obese children.
Materials and Methods
Subjects
Forty-seven obese Japanese children, consisting of 31 boys and 16 girls, who visited the Clinic for Obese Children at either the University of Yamanashi or the University of Occupational and Environmental Health, were consecutively enrolled in the study (Table 1). According to the criteria for obesity in childhood adopted by the Ministry of Health, Labor and Welfare in Japan, a child was considered to be obese when the body weight exceeded 120% of the standard body weight, which is defined as the mean body weight corresponding to the height for that age obtained from the national statistics for Japanese school children in 1990. The ages of the subjects were 10.4 ± 0.5 (mean ± s.e.m.) yr, and their percentage overweight was 42.9 ± 1.9%. They had no endocrine, metabolic or kidney diseases. Blood was drawn after an overnight fast and they were subjected to anthropometric measurements including height, body weight, waist circumference, hip circumference, and triceps and subscapular skinfold thicknesses.
Table 1. Anthropometric data in control and obese children.
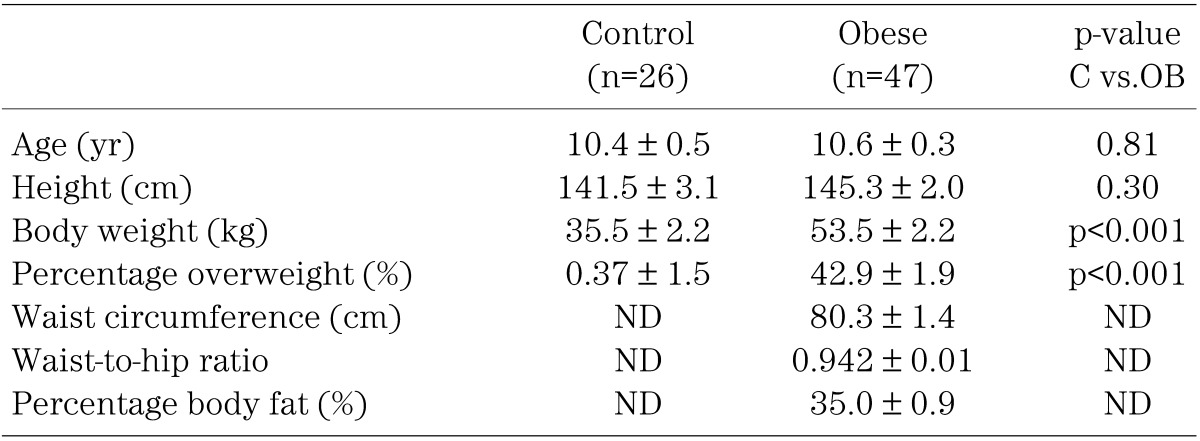
The age-matched control group for measuring orexin and leptin (mean age, 10.4 ± 0.3 yr) consisted of 26 nonobese children, 13 boys and 13 girls (Table 1). Their percentage overweight was 0.37 ± 1.5%. Blood was drawn after an overnight fast. Since there were no significant sex-related differences among the anthropometric and clinical laboratory data in either the obese or control children, the boys and girls were combined as one group each for the obese and nonobese children.
The Human Study Committee of the University of Yamanashi and the University of Occupational and Environmental Health approved this study. Informed consent was obtained either from each subject or from his or her parents as appropriate.
Anthropometric measurements
Anthropometric measurements were performed, as described previously (14), by the medical staff at both clinics. In brief, height was measured to the nearest 0.1 cm and body weight to the nearest 0.1 kg using a stadiometer. The waist circumference was measured at the level of the umbilicus, and hip circumference at the level of maximum extension of the buttocks, to the nearest 0.1 cm. Skinfold thickness was measured to the nearest 0.1 cm using the skinfold calipers at triceps (halfway between the acromion and the olecranon) and subscapular (1 cm below the inferior angle of the scapula). The percentage overweight was calculated using a small programmed calculator (Pocket Growth Checker GEN-185, Sumitomo Pharmaceuticals Co., Osaka, Japan). The percentage body fat, based on the sum of triceps and subscapular skinfold thicknesses, was obtained using Brozek’s equation (15), after body density was calculated according to Nagamine’s formula (16).
Biochemical measurements
Orexin-A in human plasma was assayed by a radioimmunoassay kit (Peninsula Laboartory Inc., Belmont, CA, USA). Leptin in human plasma was assayed by an enzyme-linked immunosorbent assay kit (IBL Co., Ltd., Fujioka, Gunma, Japan). The sera were stored frozen at –80°C until measurement. Clinical blood biochemical data were obtained from the clinical laboratories of both the University Hospital of Yamanashi and the University Hospital of Occupational and Environmental Health. Low-density lipoprotein-cholesterol (LDL-C) was calculated using the Friedewald equation [LDL-C = TC – HDL-C – TG/ 5] (17).
Statistics
Data are presented as the means and s.e.m. The statistical significance between means was estimated by an unpaired t-test. Differences were considered statistically significant at p<0.05. Pearson’s correlation coefficients were calculated by least-squares linear regression analysis. The statistical analyses were performed using SPSS version 11.0.1J (SPSS Japan Inc., Tokyo, Japan).
Results
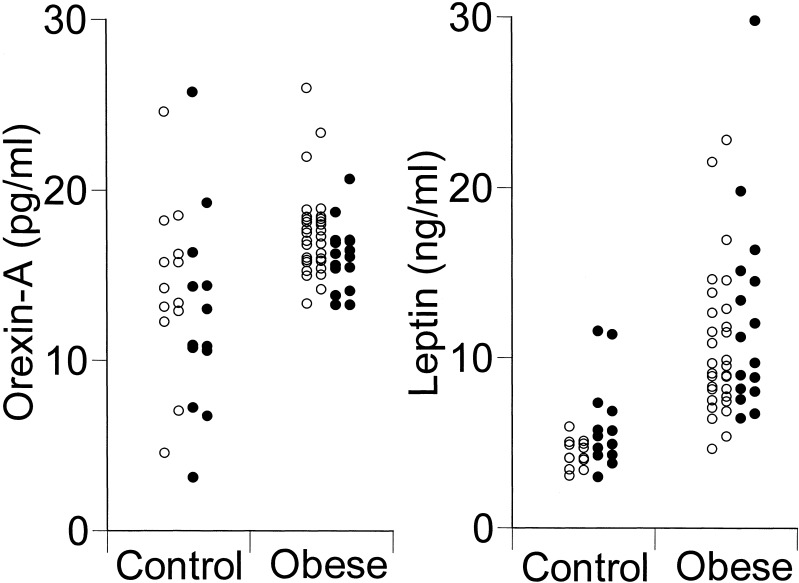
Table 2 summarizes the data on clinical blood biochemistry for obese children. Plasma orexin-A concentration was higher in obese children (17.0 ± 0.4 pg/ml; p<0.001) than in the control children (13.5 ± 1.1 pg/ml) (Fig. 1). Similarly, plasma leptin concentration was higher in obese children (12.0 ± 1.0 ng/ml; p<0.001) than in the control children (5.2 ± 0.4 ng/ml) (Fig. 1).
Table 2. Data on clinical blood biochemistry for obese children (n=47).

Fig. 1.
Plasma levels of orexin-A and leptin in obese and control children. Open circles indicate boys and closed circles girls. There is no significant sex-related difference in the plasma concentration of either orexin or leptin. Obese vs. control: p<0.001 for both indices.
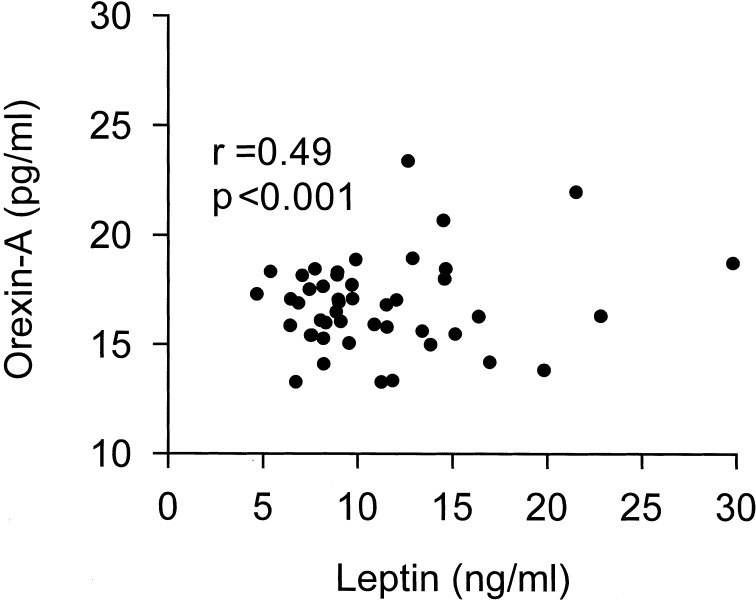
Table 3 summarizes relationships between the anthropometric indices and the plasma level of orexin-A or leptin in obese children. The plasma orexin-A level was correlated significantly with the waist-to-hip ratio only, while the leptin level was correlated with percentage overweight, waist circumference and percentage body fat. The relationship between plasma levels of orexin-A and leptin is shown in Fig. 2. There was a highly significant positive correlation between the two parameters. None of the data on clinical blood biochemistry shown in Table 2 was correlated with the plasma level of orexin-A or leptin.
Table 3. Correlation between anthropometric indices and plasma level of orexin-A or leptin in obese children (n=47).
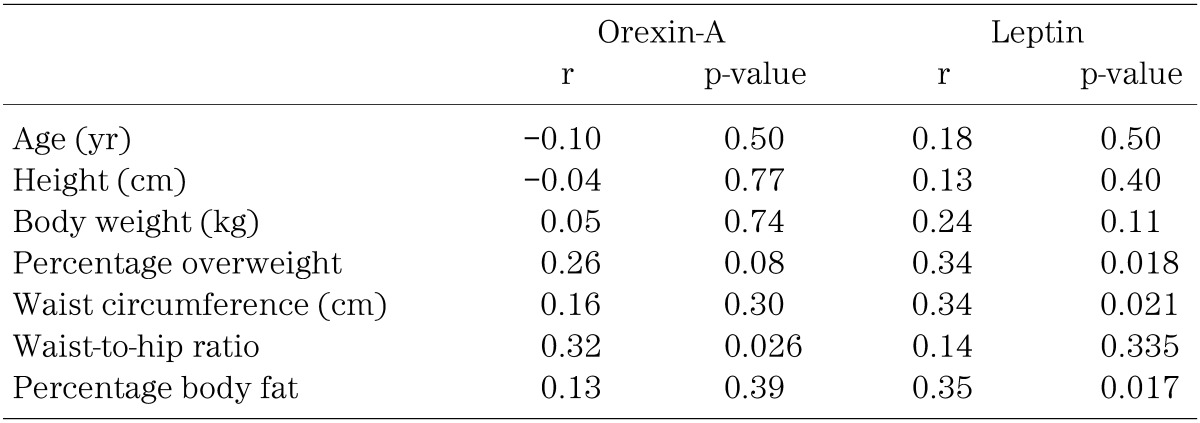
Fig. 2.
Correlation between plasma levels of orexin-A and leptin in obese children (n=47).
Discussion
Plasma levels of both orexin-A and leptin were higher in the obese than the control children, and orexin-A was correlated positively with leptin. However, both parameters were correlated with only limited numbers of anthropometric indices, and with no other data of clinical blood biochemistry.
Arihara et al. (18) reported that plasma immunoreactive orexin-A level was 6.91 ± 0.85 pg/ml in healthy adults after extraction of orexin-A by Sep Pak chromatography from acidified plasma. On the other hand, Komaki et al. (19) reported that the basal level of orexin-A was 29.9 ± 1.6 pg/ml without extraction, using the same commercial kit as ours. They also reported that the plasma orexin-A level increased to 38.9–47.9 pg/ml during fasting for 7–10 days in the treatment of psychosomatic diseases in nonobese women (19). The reported level by Arihara et al. (18) was lower, and those by Komaki et al. (19) were higher than ours. In our study, orexin-A was not correlated with the age of the subjects. Matsumura et al. reported (20) in subjects, whose ages ranged from 23 to 79 yr, that plasma orexin-A level was higher in the elderly adults than in the younger ones. However, whether the difference in plasma concentrations between our study and other studies is dependent on the ages of the subjects needs to be studied further.
The high level of orexin, which promotes appetite, in obese children, observed here, implies that their appetite is not suppressed regardless of their obesity. The high plasma level of orexin-A appeared to parallel the level of leptin in the present study. If the orexinergic effect parallels that of leptin in the hypothalamic regulation of appetite, this association can, at least partly, explain the apparent leptin resistance in the present obese children. However, this is unlikely because leptin is known to inhibit the orexinergic pathway of acceleration of food intake in the lateral hypothalamic area. Adam et al. reported (21) that the plasma orexin level was inversely correlated with body mass index in the range of 20–59 kg/m2 in adults. Conversely, Matsumura et al. (22) reported that plasma orexin-A level was positively correlated with either body mass index or fat mass in lean and normal weight adults with chronic obstructive pulmonary diseases.
Patients with narcolepsy show a low concentration of orexin-A in cerebrospinal fluid (23, 24), however, their plasma levels of orexin were reported to be either low (23) or normal (24). Around half of the orexinergic neurons in the hypothalamus are under the inhibitory regulation of leptin. Thus, orexin is secreted by fasting in order to conserve energy in the central nervous system. Whether the peripheral mechanism related to orexin is also regulated by leptin or not is unknown. In our previous study in bovine adrenal cells (13), orexin appeared to accelerate energy metabolism rather than to conserve energy. This dual action of orexin in central and peripheral mechanisms needs to be studied further.
In the present study, plasma orexin-A level was correlated significantly with the waist-to-hip ratio but not with waist circumference. On the other hand, the opposite was the case with leptin. The clinical implication of these observations needs to be studied further. Lissner et al. (25) reported in Swedish women that hip circumference showed a health-promoting effect, and that the effect was independent of the adverse health effect of waist circumference. They suggested that waist-to-hip ratio and waist circumference had mutually different implications in health and longevity of men. Thus, the correlation profile of the two indices may be different from each other.
Acknowledgement
This work was supported in part by Health Science Research Grants (Research on Children and Families) from Ministry of Health, Labor and Welfare, Japan, and also in part by Grant-in-Aid #14570787 from Japan Society for the Promotion of Science.
References
- 1.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92: 573–85 doi: 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 2.Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawa M, et al. Structure and function of human prepro-orexin gene. J Biol Chem 1999; 274: 17771–6 doi: 10.1074/jbc.274.25.17771 [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998; 18: 9996–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, et al. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes 1999; 48: 2132–7 doi: 10.2337/diabetes.48.11.2132 [DOI] [PubMed] [Google Scholar]
- 5.Griffond B, Risold PY, Jacquemard C, Colard C, Fellmann D. Insulin-induced hypoglycemia increases preprohypocretin (orexin) mRNA in the rat lateral hypothalamic area. Neurosci Lett 1999; 262: 77–80 doi: 10.1016/S0304-3940(98)00976-8 [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999; 98(3): 365–76 doi: 10.1016/S0092-8674(00)81965-0 [DOI] [PubMed] [Google Scholar]
- 7.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197): 39–40 doi: 10.1016/S0140-6736(99)05582-8 [DOI] [PubMed] [Google Scholar]
- 8.Mitsuma T, Hirooka Y, Kayama M, Mori Y, Yokoi Y, Rhue N, et al. Radioimmunoassay for orexin A. Life Sci 2000; 66: 897–904 doi: 10.1016/S0024-3205(99)00673-6 [DOI] [PubMed] [Google Scholar]
- 9.Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron 1999; 24: 941–51 doi: 10.1016/S0896-6273(00)81041-7 [DOI] [PubMed] [Google Scholar]
- 10.Nowak KW, Mackowiak P, Switonska MM, Fabis M, Malendowicz LK. Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life-Sci 2000; 66: 449–54 doi: 10.1016/S0024-3205(99)00611-6 [DOI] [PubMed] [Google Scholar]
- 11.Lopez M, Senaris R, Gallego R, Garcia Caballero T, Lago F, Seoane L. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology 1999; 140: 5991–4 doi: 10.1210/endo.140.12.7287 [DOI] [PubMed] [Google Scholar]
- 12.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther 1999; 289: 219–23 [PubMed] [Google Scholar]
- 13.Kawada Y, Ueno S, Asayama K, Tsutsui M, Utunomiya K, Toyohira Y, et al. Stimulation of catecholamine synthesis by orexin-A in bovine adrenal medullary cells through orexin receptor 1. Biochem Pharmacol 2003; 66: 141–7 doi: 10.1016/S0006-2952(03)00236-3 [DOI] [PubMed] [Google Scholar]
- 14.Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, et al. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children: Restoration of serum level after treatment. Obes Res 2003; 11: 1072–9 doi: 10.1038/oby.2003.147 [DOI] [PubMed] [Google Scholar]
- 15.Brozek J, Grande J, Anderson T, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci 1963; 110: 113–40 doi: 10.1111/j.1749-6632.1963.tb17079.x [DOI] [PubMed] [Google Scholar]
- 16.Nagamine S, Suzuki S. Anthropometry and body composition of Japanese young men and women. Hum Biol. 1964; 36: 8–15 [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499 [PubMed] [Google Scholar]
- 18.Arihara Z, Takahashi K, Murakami O, Totsune K, Sone M, Satoh F, et al. Immunoreactive orexin-A in human plasma. Peptides 2001; 22: 139–42 doi: 10.1016/S0196-9781(00)00369-7 [DOI] [PubMed] [Google Scholar]
- 19.Komaki G, Matsumoto Y, Nishikata H, Kawai K, Nozaki T, Takii M, et al. Orexin-A and leptin inversely in fasting non-obese subjects. Eur J Endcrinol 2001; 144: 645–51 doi: 10.1530/eje.0.1440645 [DOI] [PubMed] [Google Scholar]
- 20.Matsumura T, Nakayama M, Nomura A, Naito A, Kamahara K, Kadono K, et al. Age-related changes in plasma orexin-A concentration. Exp Gerontol 2002; 37: 1127–30 doi: 10.1016/S0531-5565(02)00092-X [DOI] [PubMed] [Google Scholar]
- 21.Adam JA, Menheere PPCA, van Dielen FMH, Soeters PB, Buurman WA, Greve JWM. Decreased plasma orexin-A l evels in obese individuals. Int J Obes 2002; 26: 274–6. doi: 10.1038/sj.ijo.0801868 [DOI] [PubMed] [Google Scholar]
- 22.Matsumura T, Nakayama M, Satoh H, Naito A, Kamahara K, Sekizawa K. Plasma orexin-A levels and body composition in COPD. Chest 2003; 123: 1060–5 doi: 10.1378/chest.123.4.1060 [DOI] [PubMed] [Google Scholar]
- 23.Higuchi S, Usui A, Murasaki M, Matsushita S, Nishioka N, Yoshino A, et al. Plasma orexin-A is lower in patients with narcolepsy. Neurosci Lett 2002; 318: 61–4 doi: 10.1016/S0304-3940(01)02476-4 [DOI] [PubMed] [Google Scholar]
- 24.Dalal MA, Schuld A, Haack M, Uhr M, Geisler P, Eisenshr I, et al. Normal plasma level of orexin-A (hypocretin-1) in narcoleptic patients. Neurology 2001; 56: 1749–51 doi: 10.1212/WNL.56.12.1749 [DOI] [PubMed] [Google Scholar]
- 25.Lissner L, Bjorkelund C, Heitmann BL, Seidell JC, Bengtsson C. Larger hip cericumference independentlu predicts health and longevity in Swedish female cohort. Obes Res 2001; 9: 644–6 doi: 10.1038/oby.2001.85 [DOI] [PubMed] [Google Scholar]