Abstract
Serum levels of free insulin-like growth factor (IGF)-I were measured by immunoradiometric assay (IRMA) in fasting sera of 137 normal boys and 120 normal girls aged from 8 to 15 yr to study relationships between free IGF-I levels and ages, total IGF-I, IGF binding protein (IGFBP)-1, IGFBP-3, and acid-labile subunit (ALS) levels. In both sexes, serum free IGF-I levels and the ratios of free IGF-I to total IGF-I were significantly higher in the pubertal age groups than in the prepubertal age groups. Serum levels of free IGF-I showed a significant positive correlation with those of total IGF-I, IGFBP-3 and ALS, while they showed a significant negative correlation with those of IGFBP-1. These observations suggest that increase in serum free IGF-I levels during puberty is caused by a dramatic increase in total IGF-I, rather than IGFBP-3, and a decrease in IGFBP-1. Also, high free IGF-I levels may play an important role in pubertal growth spurt.
Keywords: free insulin-like growth factor (IGF)-I, total IGF-I, IGF binding protein (IGFBP)-1, IGFBP-3, acid-labile subunit (ALS)
Introduction
Most of the circulating insulin-like growth factor (IGF)-I is bound to IGF-binding proteins (IGFBPs), and small amounts of IGF-I are present in the free form, which is called free IGF-I (1, 2). Since the free form is more freely transferred to the tissues, free IGF-I is suggested to have more potent biological action than the complex forms of IGF-I (3,4,5,6,7,8). Two different methods are currently used for the estimation of free IGF-I levels. One is ultrafiltration by centrifugation (UF) (9) and the other one is direct immunoradiometric assay (IRMA) (10). There is a difference between these two methods: IRMA measures free plus readily dissociable IGF-I, while UF measures only free IGF-I (11). Recently specific immunoradiometric assays (IRMA) have been developed, and have demonstrated that serum free IGF-I levels are age- and sex-dependent (7, 8, 12, 13). Serum free IGF-I levels have also been demonstrated to show a significant circadian variation that exhibits a nocturnal decrease and an increase in the morning in healthy children (14).
In the present study, we measured serum free IGF-I levels in fasting sera of 137 normal boys and 120 normal girls aged from 8 to 15 yr to study their age- and sex-related changes, and relationships between free IGF-I levels and total IGF-I, lGFBP-1, lGFBP-3, and acid labile subunit (ALS) levels.
Subjects and Methods
Subjects
The subjects were 137 healthy Japanese boys and 120 healthy girls aged from 8 to 15 yr. Children with short stature, obesity and endocrinological abnormalities were excluded. The serum samples were obtained for screening of hyperlipemia in 1993 and had been frozen. Using the serum samples without abnormal biochemical data, we measured free IGF-I, total IGF-I, IGFBP-1, IGFBP-3 and ALS levels in 1996 and 1997. The serum samples had been obtained after overnight fasting, which is very useful for minimizing the effects of diurnal changes in free IGF-I and IGFBP-1 levels (14, 15).
Measurements
Free IGF-I, total IGF-I, IGFBP-1, IGFBP-3 and ALS levels were determined in the same serum samples. Serum free IGF-I levels were measured by an IRMA kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA); the sensitivity is 0.03 ng/mL, the coefficient of variation in the intra-assay was 3.3–10.3% and the coefficient of variation in the inter-assay was 7.7–10.7%. Serum total IGF-I levels were measured by radioimmunoassay (RIA) as reported previously (16). Serum IGFBP-1 levels were measured by an immunoenzymometric assay (IEMA) kit (Medix Biochemica Ab, Kauniainen, Finland), serum IGFBP-3 levels measured by an RIA kit (Eiken Chemical Co., Ltd., Tokyo, Japan), and serum ALS levels were also measured by an RIA kit (Bioclone Australia Pty., Ltd., Marrickville, New South Wales, Australia).
Statistical analysis
Since free IGF-I, total IGF-I, IGFBP-1, IGFBP-3 and ALS levels showed a normal distribution of the data that had been converted logarithmically, we analyzed the data in the logarithmic state. Differences in the hormone levels and ratios among the age groups were tested by analysis of variance. Correlation between the different variables was estimated by linear regression analysis. P-values less than 0.05 were considered to be statistically significant.
Results
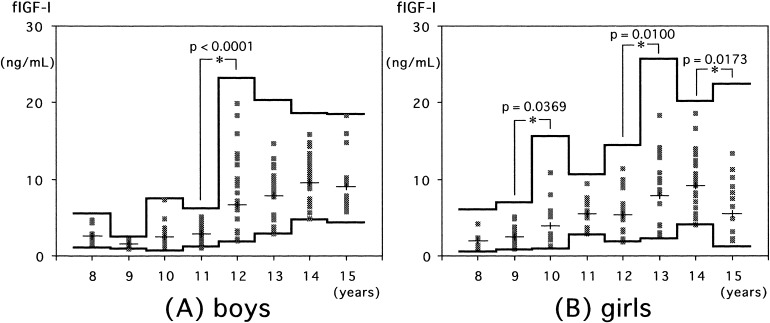
In normal children, serum free IGF-I increased with age in both sexes (Fig. 1). In boys, a statistically significant increment in free IGF-I levels over the consecutive age group was observed at 12 yr (Fig. 1A). In girls, statistically significant increments in free IGF-I levels occurred at 10 and 13 yr, and a statistically significant decrement in free IGF-I levels was observed at 15 yr (Fig. 1B).
Fig. 1.
Serum free IGF-I levels in normal boys (A) and girls (B). Individual values are shown by the dots. The means and means ± 2SDs (logarithmic converted data) in each age group are shown by the lines.
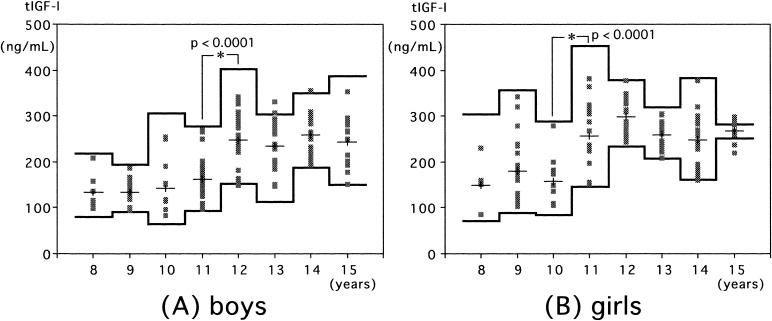
Serum total IGF-I also increased with age in both sexes (Fig. 2). A statistically significant increment in total IGF-I levels was observed at 12 yr in boys (Fig. 2A) and at 11 yr in girls (Fig. 2B).
Fig. 2.
Serum total IGF-I levels in normal boys (A) and girls (B). Individual values are shown by the dots. The means and means ± 2SDs (logarithmic converted data) in each age group are shown by the lines.
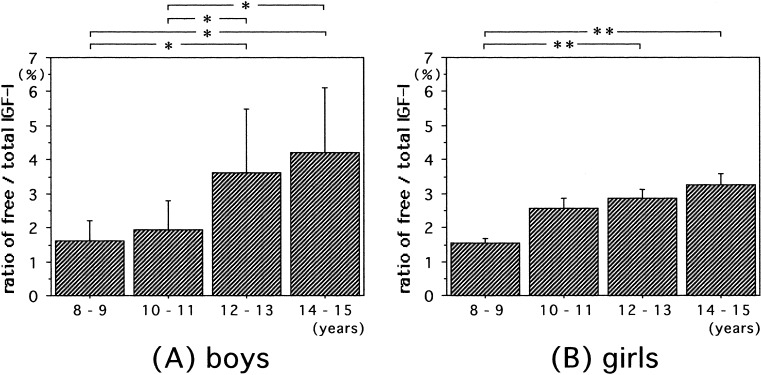
The ratios of free to total IGF-I were significantly higher in boys aged 12–13 and 14–15 yr than in boys aged 8–9 and 10–11 yr (Fig. 3A). The free to total IGF-I ratios were significantly higher in girls aged 12–13 and of 14–15 yr than in girls aged 8–9 yr (Fig. 3B).
Fig. 3.
Ratios of serum free to total IGF-I in normal boys (A) and girls (B). The columns indicate the means. The vertical bars indicate 1SD. *; p<0.0001, **; p<0.0005.
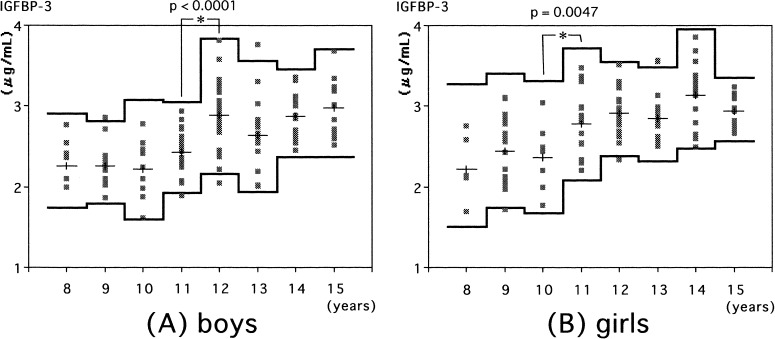
Serum IGFBP-3 increased with age in both sexes (Fig. 4). A statistically significant increment in IGFBP-3 levels was observed at 12 yr in boys (Fig. 4A) and at 11 yr in girls (Fig. 4B).
Fig. 4.
Serum IGFBP-3 levels in normal boys (A) and girls (B). Individual values are shown by the dots. The means and means ± 2SDs (logarithmic converted data) in each age group are shown by the lines.
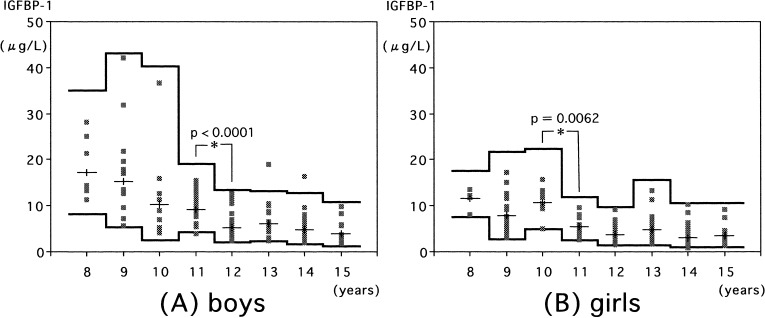
Serum IGFBP-1 gradually decreased with age in both sexes (Fig. 5). A statistically significant decrement in IGFBP-1 levels was observed at 12 yr in boys (Fig. 5A) and at 11 yr in girls (Fig. 5B).
Fig. 5.
Serum IGFBP-1 levels in normal boys (A) and girls (B). Individual values are shown by the dots. The means and means ± 2SDs (logarithmic converted data) in each age group are shown by the lines.
Serum free IGF-I positively correlated with total IGF-I, IGFBP-3 and ALS, and negatively correlated with IGFBP-1 (Table 1).
Table 1. Correlation between fIGF-I and other parameters.
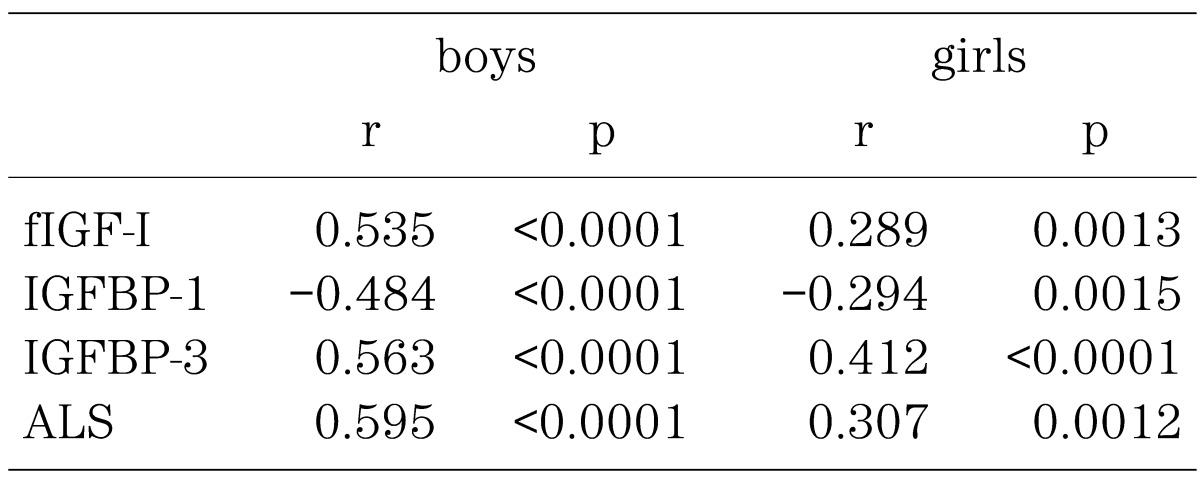
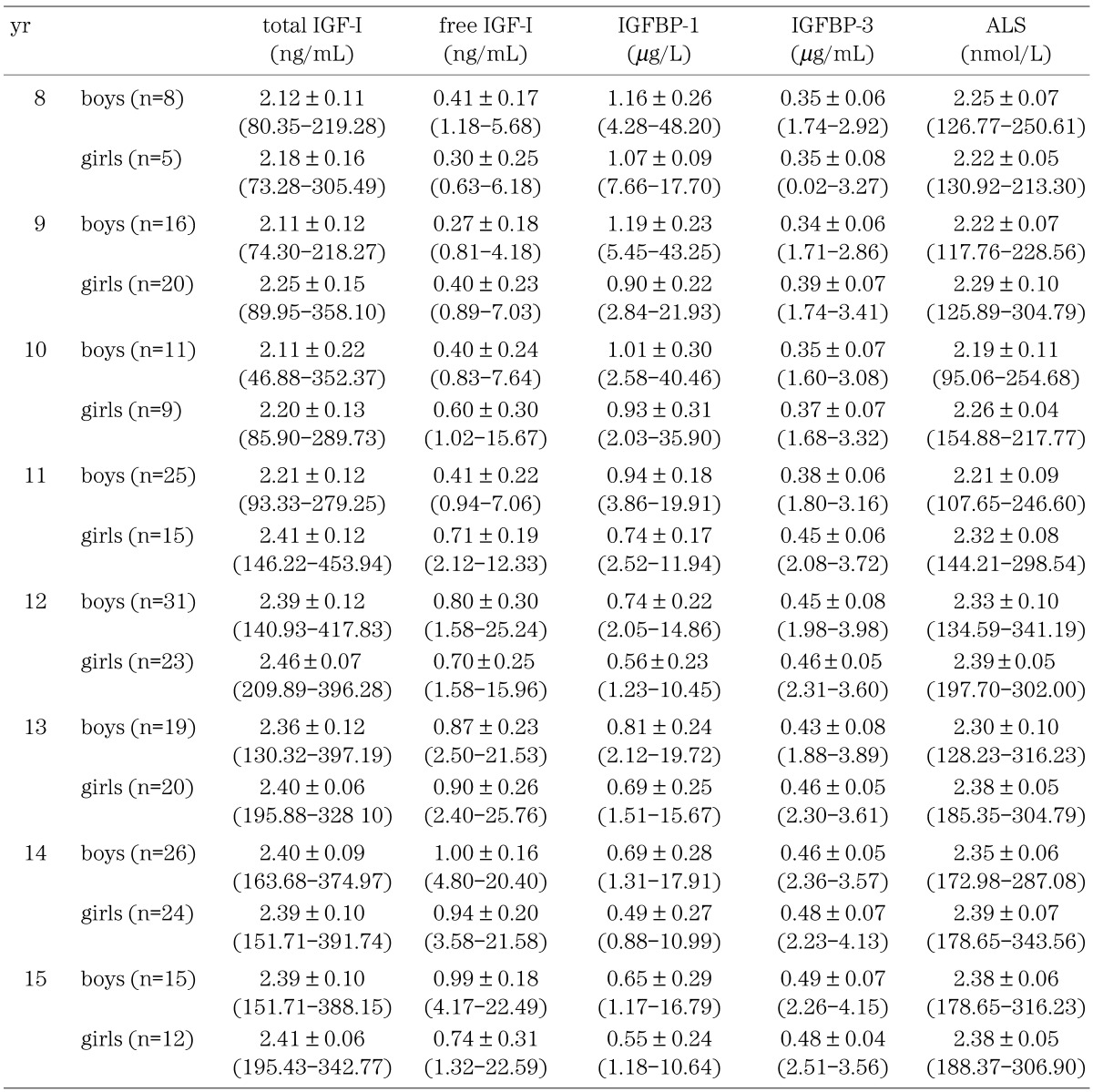
Table 2 shows the levels of free IGF-I, total IGF-I, IGFBP-1, IGFBP-3 and ALS in the present study; Mean ± SD in logarithmically converted data and –2SD to +2SD in raw data.
Table 2. Mean ± SD (logarithmic converted data) and –2SD to +2SD (raw data) of total IGF-I, free IGF-I, IGFBP-1, IGFBP-3 and ALS.
Discussion
Although serum free IGF-I levels in normal children, in patients with growth hormone deficiency, and in patients with precocious puberty have been previously reported (5,6,7,8, 12, 13), they were determined in non-fasting samples. Since Heuck et al. reported circadian variation in serum free ultrafiltrable IGF-I concentrations in healthy children (14), fasting samples obtained at similar times of day would be preferable to assess age- and sex-dependent changes in the free IGF-I levels. In the present study, we determined the free IGF-I levels in the fasting samples obtained in the morning. We observed significantly higher ratios of free to total IGF-I in pubertal age groups than in prepubertal age groups, while Yamada et al. and Kawai et al. did not observe any increase in these ratios (12, 13). This difference might conceivably be caused by the difference in the time of serum sampling.
We observed significant increases in the mean free and total IGF-I levels at the age of 12 yr in boys, which is close to the average age of peak pubertal height velocity in Japanese boys, and in the mean free IGF-I levels at the age of 10 yr and in the mean total IGF-I levels at the age of 11 yr in girls, which are close to average age of peak pubertal height velocity in Japanese girls. Although we also observed a significant increase in mean free IGF-I levels at the age of 13 yr in girls, it seems that this apparent increase was caused by the relatively low free IGF-I levels in 12-yr-old girls in the present study. Furthermore, a significant decrement in free IGF-I levels was observed in 15-yr-old girls, which is over the average age of peak pubertal height velocity in Japanese girls.
High ratios of free to total IGF-I were reported in sera samples from early infancy and normal pregnancy, and were attributed to increased IGFBP-3 proteolytic activity (7, 17). In present study, we observed that free and total IGF-I and IGFBP-3 levels increased, while IGFBP-1 levels decreased during the pubertal period. Pubertal decrease in IGFBP-1 is ascribed to increased insulin secretion during puberty (18). It is known that IGF-I also binds to IGFBP-1 (11); and it is also well known that growth hormone (GH) secretion increases during puberty. IGF-I, IGFBP-3 and ALS are GH dependent factors (13, 16, 19, 20) and free IGF-I is also assumed to be GH dependent (Table 1), since serum free IGF-I levels have been found to be decreased in patients with growth hormone deficiency (GHD) (5, 8, 13). We think that the high free IGF-I levels and the high ratio of free to total IGF-I during the pubertal period are attributable to the more dramatic increase in total IGF-I levels than that in IGFBP-3, caused by increased GH secretion, and to the decrease in IGFBP-1 along with pubertal maturation caused by increased insulin secretion during puberty.
Since free IGF-I is GH dependent and a more potent biological stimulator to bone than GH itself, we hypothesize that increased IGF-I levels during puberty have an important role in the pubertal growth spurt, although we didn’t have any auxological data in this study. Sex steroid hormones mainly regulate pubertal growth, since patients suffering from both precocious puberty and GHD develop pubertal growth spurt without GH treatment (21). However, GH also contributes to the pubertal growth spurt, since GH secretion increases during puberty and the pubertal growth spurt in GHD is small without GH treatment (22).
The high ratios of free to total IGF-I might also play a role in the pubertal growth spurt, although the ratios further increased in late puberty when growth velocity decreases. Although the ratios further increased in late puberty when growth velocity decreased in this study, the situation is the same as the relation between sex steroid hormones and growth velocity during puberty. Sex steroid hormones increase along with pubertal maturation and reach an adult level, but the growth velocity decreases in the late pubertal period. The decrease in growth velocity in the late puberty is mainly regulated by bone maturation toward epiphyseal fusion and not by growth factors or sex steroid hormones.
In conclusion, increased free IGF-I levels during puberty were caused by dramatic increase in total IGF-I levels, rather than that in IGFBP-3, and by a decrease in IGFBP-1. Also, IGF-I levels may play some role in the pubertal growth spurt. These findings strengthen further the concept that pubertal increase in GH secretion serves a role in generating the growth spurt.
References
- 1.Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in health man: estimates of half lives and production rates. Acta Endocrinol (Copenh) 1989; 121: 753–8. doi: [DOI] [PubMed] [Google Scholar]
- 2.Jones JI, Clemmons DR. Insulin-like growth factors, and their binding proteins: biological actions. Endocr Rev 1995; 16: 3–34 [DOI] [PubMed] [Google Scholar]
- 3.Daughaday WH, Ward AP, Goldberg AC, Trivedi B, Kapadia M. Characterization of somatomedin binding in human serum by ultracentrifugation and gel filtration. J Clin Endocrinol Metab 1982; 55: 916–21 doi: 10.1210/jcem-55-5-916 [DOI] [PubMed] [Google Scholar]
- 4.Juul A, Dalgaard P, Blum WF, Bang P, Hall K, Michaelsen KF, et al. Serum levels of insulin-like growth factor (IGF) binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinolo Metab 1995; 80: 2534–42 [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa Y, Hasegawa T, Takada M, Tsuchiya Y. Plasma free insulin-like growth factor I concentrations in growth hormone deficiency in children and adolescents. Eur J Endocrinol 1996; 134: 184–9 doi: 10.1530/eje.0.1340184 [DOI] [PubMed] [Google Scholar]
- 6.Juul A, Flyvbjerg A, Frystyk J, Müller J, Skakkebæk NE. Serum concentrations of free and total insulin-like growth factor-I, IGF binding proteins -1 and -3 and IGFBP-3 protease activity in boys with normal or precocious puberty. Clin Endocrinol 1996; 44: 515–23 doi: 10.1046/j.1365-2265.1996.711531.x [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa Y, Hasegawa T, Fujii K, Konii H, Anzo M, Aso T, et al. High ratios of free to total Insulin-like growth factor-I in early infancy. J Clin Endocrinol Metab 1997; 82: 156–8 doi: 10.1210/jcem.82.1.3679 [DOI] [PubMed] [Google Scholar]
- 8.Juul A, Holm K, Kastrup KW, Pedersen SA, Michaelsen KF, Schike T, et al. Free insulin-like growth factor-I levels in 1430 healthy children and adults, and its diagnostic value in patients suspected of growth hormone deficiency. J Clin Endocrinol Metab 1997; 82: 2497–502 [DOI] [PubMed] [Google Scholar]
- 9.Frystyk J, Skjærbæk C, Dinesen B, Ørskov H. Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS lett 1994; 348: 185–91 doi: 10.1016/0014-5793(94)00602-4 [DOI] [PubMed] [Google Scholar]
- 10.Lee PDK, Powell DR, Baker B, Liu F, Mathew G, Levitsky I, et al. Characterization of a direct, non-extraction immunoradiometric assayfor free IGF-I. 76th Annu Meet Endocr Soc: Anaheim; 1994. Abstract.
- 11.Frystyk J, Ivarsen P, Stoving RK, Dall R, Bek T, Hagen C, et al. Determination of free insulin-like growth factor-I in human serum: comparison of ultrafiltration and direct immunoradiometric assay. Growth Horm IGF Res 200111(2): 117–27Erratum in: Growth Horm IGF Res 2001 Jun;11(3):189-9 doi: 10.1054/ghir.2001.0197 [DOI] [PubMed] [Google Scholar]
- 12.Yamada M, Hasegawa T, Hasegawa Y. Increase in free Insulin-like growth factor-I levels in precocious and normal puberty. Endocr J 1998; 45: 407–12 doi: 10.1507/endocrj.45.407 [DOI] [PubMed] [Google Scholar]
- 13.Kawai N, Kanzaki S, Takano-Watou S, Tada C, Yamanaka Y, Miyata T, et al. Serum free insulin-like growth factor I (IGF-I), total IGF-I, and IGF-binding protein-3 concentrations in normal children and children with growth hormone deficiency. J Clin Endocrinol Metab 1999; 84: 82–9 [DOI] [PubMed] [Google Scholar]
- 14.Heuck C, Skjærbæk C, Ørskov H, Wolthers OD. Circadian variation in serum free ultrafiltrable insulin-like growth factor I concentrations in healthy children. Pediatr Res 1999; 45: 733–6 doi: 10.1203/00006450-199905010-00021 [DOI] [PubMed] [Google Scholar]
- 15.Yasunaga T, Tanaka T, Katsumata N, Tanae A, Hibi I. Diurnal variation of serum insulin-like growth factor binding protein-1 in three boys and fasting insulin-like growth factor binding protein-1 levels in normal children. Clin Pediatr Endocrinol 1996; 5 (Suppl 8): 99–102. doi: 10.1297/cpe.5.Supple8_99 [DOI] [Google Scholar]
- 16.Suwa S, Katsumata N, Maesaka H, Tokuhiro E, Yokoya S. Serum insulin-like growth factor I (somatomedin-C) level in normal subjects from infancy to adulthood, pituitary dwarfs and normal variant short children. Endocrinol Japon 1988; 35: 857–64 doi: 10.1507/endocrj1954.35.857 [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa T, Hasegawa Y, Takada M, Tsuchiya Y. The free form of insulin-like growth factor I increases in circulation during normal human pregnancy. J Clin Endocrinol Metab 1995; 80: 3284–6 [DOI] [PubMed] [Google Scholar]
- 18.Caprio S. Insulin: the other anabolic hormone of puberty. Acta Paediatr 1999; (Suppl 433): 84–7 doi: 10.1111/j.1651-2227.1999.tb14410.x [DOI] [PubMed] [Google Scholar]
- 19.Blum WF, Ranke MB, Kietzmann K, Gauggel E, Zeisel HJ, Bierich JR. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab 1990; 70: 1292–8 doi: 10.1210/jcem-70-5-1292 [DOI] [PubMed] [Google Scholar]
- 20.Nimura A, Katsumata N, Horikawa R, Tanae A, Tanaka T. Acid-labile subunit (ALS) measurements in children. Endocr J 2000; 47: S111–4 doi: 10.1507/endocrj.47.SupplMarch_S111 [DOI] [PubMed] [Google Scholar]
- 21.Attie KM, Ramirez NR, Conte FA, Kaplan SL, Grumbach MM. The pubertal growth spurt in eight patients with true precocious puberty and growth hormone deficiency: evidence for a direct role of sex steroids. J Clin Endocrinol Metab 1990; 71: 975–94 doi: 10.1210/jcem-71-4-975 [DOI] [PubMed] [Google Scholar]
- 22.Aynsley-Green A, Zachmann M, Prader A. Interaction of the therapeutic effects of growth hormone and testosterone on growth in hypopituitarism. J Pediatr 1976; 89: 992–9 doi: 10.1016/S0022-3476(76)80619-1 [DOI] [PubMed] [Google Scholar]