Abstract
Background
Nausea, retching and vomiting are very commonly experienced by women in early pregnancy. There are considerable physical and psychological effects on women who experience these symptoms. This is an update of a review of interventions for nausea and vomiting in early pregnancy previously published in 2003.
Objectives
To assess the effectiveness and safety of all interventions for nausea, vomiting and retching in early pregnancy, up to 20 weeks’ gestation.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 May 2010).
Selection criteria
All randomised controlled trials of any intervention for nausea, vomiting and retching in early pregnancy. We excluded trials of interventions for hyperemesis gravidarum which are covered by another review. We also excluded quasi-randomised trials and trials using a crossover design.
Data collection and analysis
Four review authors, in pairs, reviewed the eligibility of trials and independently evaluated the risk of bias and extracted the data for included trials.
Main results
Twenty-seven trials, with 4041 women, met the inclusion criteria. These trials covered many interventions, including acupressure, acustimulation, acupuncture, ginger, vitamin B6 and several antiemetic drugs. We identified no studies of dietary or other lifestyle interventions. Evidence regarding the effectiveness of P6 acupressure, auricular (ear) acupressure and acustimulation of the P6 point was limited. Acupuncture (P6 or traditional) showed no significant benefit to women in pregnancy. The use of ginger products may be helpful to women, but the evidence of effectiveness was limited and not consistent. There was only limited evidence from trials to support the use of pharmacological agents including vitamin B6, and anti-emetic drugs to relieve mild or moderate nausea and vomiting. There was little information on maternal and fetal adverse outcomes and on psychological, social or economic outcomes. We were unable to pool findings from studies for most outcomes due to heterogeneity in study participants, interventions, comparison groups, and outcomes measured or reported. The methodological quality of the included studies was mixed.
Authors’ conclusions
Given the high prevalence of nausea and vomiting in early pregnancy, health professionals need to provide clear guidance to women, based on systematically reviewed evidence. There is a lack of high-quality evidence to support that advice. The difficulties in interpreting the results of the studies included in this review highlight the need for specific, consistent and clearly justified outcomes and approaches to measurement in research studies.
Medical Subject Headings (MeSH): Acupuncture Therapy [methods], Antiemetics [therapeutic use], Ginger [chemistry], Morning Sickness [etiology; therapy], Nausea [etiology; *therapy], Phytotherapy [methods], Pregnancy Complications [*therapy], Randomized Controlled Trials as Topic, Treatment Outcome, Vitamin B 6 [therapeutic use], Vitamin B Complex [therapeutic use], Vomiting [etiology; *therapy]
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Nausea and vomiting are commonly experienced by women in early pregnancy. Prevalence rates of between 50% and 80% are reported for nausea, and rates of 50% for vomiting and retching (Miller 2002; Woolhouse 2006). Retching (or dry heaving, without expulsion of the stomach’s contents) has been described as a distinct symptom that is increasingly measured separately to vomiting and nausea (Lacasse 2008; O’Brien 1996; Zhou 2001).
The misnomer ‘morning sickness’, which is colloquially used to describe nausea, vomiting and retching of pregnancy, belies the fact that symptoms can occur at any time of the day. Pregnant women experience nausea, vomiting and retching mostly in the first trimester, between six and 12 weeks, but this can continue to 20 weeks and persists after this time for up to 20% of women (Jewell 2003; Miller 2002).
Hyperemesis gravidarum, which is characterised by severe and persistent vomiting, is less common, affecting between 0.30% and 3% of pregnant women (Eliakim 2000; Jewell 2003; Miller 2002). Hyperemesis gravidarum is defined in different ways, though a widely used definition describes it as “intractable vomiting associated with weight loss of more than 5% of prepregnancy weight, dehydration and electrolyte imbalances which may lead to hospitalization” (Miller 2002). Ketosis is also commonly included as a consequence of hyperemesis gravidarum (Kousen 1993; Quinlan 2003). Including inpatient hospitalisation in the definition of hyperemesis gravidarum is problematic (Swallow 2002) as some instances may be alleviated or controlled by outpatient interventions (Bsat 2003). Within the operational definitions of hyperemesis gravidarum, there is generally a focus on the effects of the vomiting (dehydration, ketosis, weight loss). The lack of a standard definition has implications for the measurement of outcomes in controlled studies.
It is important to exclude pathological causes of nausea and vomiting before concluding that this is specific to pregnancy. Pregnant women being treated for nausea, vomiting and retching of pregnancy should have the other pathological causes of nausea and vomiting (such as peptic ulcers, cholecystitis, gastroenteritis, appendicitis, hepatitis, genito-urinary (e.g. pyelonephritis), metabolic and neurological disorders) considered and excluded before a diagnosis of nausea, vomiting and retching of pregnancy is given (Davis 2004; Koch 2002; Quinlan 2003).
Thought to be associated with rising levels of human chorionic gonadotropin (hCG) or estrogens, the causes of nausea, vomiting and retching of pregnancy remain unknown (Goodwin 2002). Vestibular, gastrointestinal, olfactory and behavioural factors may influence the woman’s response to the hormonal changes (Goodwin 2002). Social, psychological and cultural influencing factors have also been studied (Buckwalter 2002; O’Brien 1999). The number of previous pregnancies and the number of fetuses both seem to affect the risk of nausea and vomiting of pregnancy (Einarson 2007; Louik 2006). Conditions with higher levels of hCG (multiple pregnancies and molar pregnancies (hydatidiform mole)) have been associated with more prevalent and more severe nausea and vomiting of pregnancy. Based on observational studies, nausea, vomiting and retching in the first trimester were thought to be associated with a decreased risk of miscarriage, preterm delivery, low birthweight, stillbirth and fetal and perinatal mortality (Czeizel 2004; Weigel 1989) although a later study challenged these claims (Louik 2006).
There are considerable physical and psychological effects on women who experience these symptoms, with altered family, social or occupational functioning (Attard 2002; Chou 2003; Chou 2008; O’Brien 1992; O’Brien 1997; Swallow 2004). Nausea and vomiting affect women’s daily activities and their relationships (Atanackovic 2001; Attard 2002; Magee 2002b). The distress and functional limitations caused by nausea without vomiting are increasingly acknowledged (Davis 2004). Women have reported that they would like their symptoms and ensuing distress acknowledged to a greater degree by health professionals (Locock 2008). Studies have also highlighted the economic burden on women and society, mainly due to lost productivity and healthcare costs (Attard 2002; Piwko 2007).
Women are commonly offered advice about the (usually) self-limiting nature of the condition and advised to avoid foods, smells, activities or situations that they find nauseating and to eat small frequent meals of dry, bland foodstuffs (Davis 2004; Ornstein 1995). Many remedies are suggested for nausea and vomiting in early pregnancy, including pharmaceutical and non-pharmaceutical interventions.
Pharmaceutical treatments include anticholinergics, antihistamines, dopamine antagonists, vitamins (B6 and B12), H3 antagonists or combinations of these substances (Koren 2002a; Kousen 1993; Magee 2002a; Quinlan 2003). The teratogenic effects (ability to disturb the growth or development of the embryo or fetus) of pharmaceutical medications (such as thalidomide) used in the past to control these symptoms have led to caution about prescribing and taking medications in the first trimester. This has led to the under-use of drugs that have been found to be safe and effective, for example, Bendecitin/Diclectin (doxylamine and pyridoxine) (Koren 2002a; Ornstein 1995). This drug was withdrawn from the US market because of the legal costs associated with its defence, despite its record of safety and a lack of legal rulings against it (Brent 2002; Koren 2002a; Ornstein 1995).
Because of concern about pharmaceuticals in early pregnancy and the general rise in the use of complementary and alternative therapies, non-pharmaceutical treatments are increasingly used to treat nausea and vomiting in pregnancy, because they may be perceived as ‘natural’ and therefore safe or lower risk than medications. These include herbal remedies (ginger, chamomile, peppermint, raspberry leaf), acupressure, acustimulation bands and acupuncture, relaxation, autogenic feedback training, homeopathic remedies (nux vomica, pulsatilla), massage, hypnotherapy, dietary interventions, activity interventions, emotional support, psychological interventions and behavioural interventions/modifications (Aikins Murphy 1998; Davis 2004; Jewell 2003; Niebyl 2002; Wilkinson 2000).
Studies report that healthcare professionals frequently recommend non-pharmaceutical treatments (Bayles 2007; Westfall 2004) and women frequently use them (Ernst 2002b; Tiran 2002). Alongside this growth in their use, there are concerns about the efficacy and safety of non-pharmaceutical treatments (Ernst 2002a; Ernst 2002b; Tiran 2002; Tiran 2003), as they are less rigorously regulated than pharmaceutical remedies. In addition, women and professionals are more likely to underestimate their possible risks (Tiran 2002; Tiran 2003).
OBJECTIVES
To assess the effectiveness and safety of all interventions for nausea, vomiting and retching in early pregnancy, up to 20 weeks’ gestation.
METHODS
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials of any intervention for nausea, vomiting and retching in early pregnancy. However, we excluded trials of interventions for hyperemesis gravidarum, which are being covered by another Cochrane review, the protocol for which is currently being prepared. We have not included quasi-randomised trials and trials using a crossover design. We have included studies reported in abstracts only, provided that there was sufficient information in the abstract, or available from the author, to allow us to assess eligibility and risk of bias.
Types of participants
Women experiencing nausea, vomiting and/or retching in pregnancy (but not hyperemesis gravidarum), where recruitment to a trial took place up to 20 weeks’ gestation.
Types of interventions
We included all interventions for nausea, vomiting and/or retching.
Types of outcome measures
Primary outcomes
Symptomatic relief
Reduction or cessation in nausea, vomiting and/or retching. We examined outcomes measured by all commonly used instruments, including the following.
Pregnancy-Unique Quantification of Emesis and Nausea (PUQE), comprising three subscales covering nausea, vomiting and retching during the past 12 hours, measured using a five-point Likert scale; possible range three to 15, representing no symptoms to maximal symptoms; the cut-off point for severe symptoms is 13. This scale was developed by clinician-researchers at the Canadian Motherisk Program (Koren 2002a) studying nausea and vomiting in pregnancy and validated using the Rhodes Index (see next paragraph) and independent variables (Koren 2002b; Koren 2005; Lacasse 2008).
The Rhodes Index of Nausea, Vomiting and Retching (three subscales: nausea, vomiting and retching, eight items, measures levels and distress caused by these; possible score range is eight to 40 representing no symptoms to maximal symptoms; the cut-off point for severe symptoms is 33. Originally created by Rhodes (Rhodes 1984) to measure the nausea and vomiting symptoms associated with chemotherapy, this index has been validated in studies of nausea and vomiting of pregnancy (O’Brien 1996; Zhou 2001).
McGill Nausea Questionnaire: measures nausea only. This questionnaire includes a qualitative measure (sets of verbal, affective and other descriptors of nausea); a nausea rating index (nine sets of words ranked in order of increasing severity); an overall nausea index; and a visual analogue scale (no nausea to extreme nausea, 10 cm scale). Developed by Melzack for cancer chemotherapy and validated for use in studies of nausea and vomiting in pregnancy (Lacroix 2000; Melzack 1985).
Nausea and Vomiting of Pregnancy Instrument: includes three questions, one each about nausea, vomiting and retching in the past week; possible range is zero to15; the cut-off point for severe symptoms is 8. Reliability and validity have been adequately described (Swallow 2002; Swallow 2005).
Visual analogue scales (graded 0 to 10) to record severity of nausea (Can Gurkan 2008; Pongrojpaw 2007; Vutyavanich 1995).
The primary outcome of reduction in symptoms, encompasses non-worsening of symptoms (including up to those of hyperemesis gravidarum).
Adverse maternal and fetal/neonatal outcomes
Adverse fetal/neonatal outcomes
Fetal or neonatal death. This includes spontaneous abortion, stillbirth (death of a fetus of at least 500 g weight or before 20 weeks’ gestation); neonatal death (death of a baby born alive, within 28 days of birth).
Congenital abnormalities (an abnormality of prenatal origin, including structural, genetic and/or chromosomal abnormalities and biochemical defects, but not including minor malformations that do not require medical treatment) (South Australian Health Commission 1999; Zhou 1999).
Low birthweight (less than 2.5 kg).
Early preterm birth (before 34 weeks’ gestation).
Adverse maternal outcomes
Pregnancy complications (antepartum haemorrhage, hypertension, pre-eclampsia (hypertension ≥ 140/90 mm Hg (millimetres of mercury), proteinuria ≥ 0.3 g/L from the 20th week of pregnancy).
Secondary outcomes
Quality of life
Quality of life outcomes encompass emotional, psychological, physical well-being; women’s assessment of the pregnancy experience; women’s ability to cope with the pregnancy. Measured using the General Health Questionnaire (GHQ) and other generic Quality of Life (QoL) and other well-being (mental health) and coping measures (Attard 2002; Chou 2003; Lacasse 2008; Swallow 2004; Swallow 2005) and a validated pregnancy-specific Quality of Life instrument (Magee 2002b).
Economic costs
Direct financial costs to women (purchase of treatments).
Productivity costs (time off work).
Healthcare system costs (provision of services, consultation time, staff time) (Attard 2002; Koren 2005; Piwko 2007).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (28 May 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Because of the non-pharmaceutical interventions which are recommended for nausea and vomiting in early pregnancy, we also liaised with the Cochrane Complementary Medicine Field to identify any other trials.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager software (RevMan 2008) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in order to assess whether the process was truly random. We assessed the methods as:
adequate (e.g. random-number table; computer random number generator);
inadequate (odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We have described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We have also provided information relating to whether the intended blinding was effective if this was available. We have noted where there had been partial blinding (e.g. where it was not feasible to blind participants, but where outcome assessment was carried out without knowledge of group assignment).
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition/exclusion where reported, and any re-inclusions in analyses which we undertook.
We assessed the methods as:
adequate (e.g. where there was little or no missing data and where reasons for missing data were balanced across groups);
inadequate (e.g. where missing data were likely to be related to outcomes or not balanced across groups or where high levels of missing data were likely to introduce serious bias or make the interpretation of results difficult);
unclear (e.g. where there was insufficient reporting of attrition or exclusions to permit a judgement to be made).
(5) Selective reporting bias
We have described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We assessed the methods as:
adequate (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported);
inadequate (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We have described for each included study any important concerns we have about other possible sources of bias. Potential examples would include where there was a risk of bias related to the specific study design, where a trial stopped early due to some data-dependent process, or where there was extreme baseline imbalance.
We assessed whether each study was free of other issues that could put it at risk of bias and assessed each as:
adequate;
inadequate;
unclear.
(7) Overall risk of bias
We have made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We have explored the impact of the level of bias through undertaking sensitivity analyses, see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way between trials. We have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We have not included any crossover trials. We did not identify any cluster-randomised trials on this topic. If we had identified such trials, and they were otherwise eligible for inclusion, we would have included and analysed them with individually randomised trials using the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009).
Dealing with missing data
For included studies, we have noted levels of attrition. We have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
We have analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there is sufficient information in the trial report, we have attempted to restore them to the correct group.
For all outcomes we have carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we have attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We visually examined the forest plots for each analysis to look for obvious heterogeneity and used the I2 and T2 statistics to quantify heterogeneity among the trials. If we identified moderate or substantial heterogeneity (I2 greater than 50% and T2 greater than zero) we used a random-effects model in meta-analyses and have indicated the values of I2 and T2 and the P value for the Chi2 test for heterogeneity. For outcomes where there are high levels of heterogeneity we would advise caution in the interpretation of results.
Assessment of reporting biases
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and we thought that the missing data might introduce serious bias, we explored the impact of including such studies in the overall assessment of results by sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed-effect meta-analysis for combining data where trials examined the same intervention, and we judged the trials’ populations and methods were sufficiently similar. Where we suspected clinical or methodological heterogeneity among studies sufficient to suggest that treatment effects might differ between trials, we used random-effects meta-analysis.
If we identified substantial heterogeneity in a fixed-effect meta-analysis, we repeated the analysis using a random-effects method.
Subgroup analysis and investigation of heterogeneity
We planned separate subgroup analyses by type of intervention, where comparability of trials and data allowed.
We planned to use the following primary outcomes in subgroup analysis.
Symptomatic relief (reduction or cessation of nausea, vomiting and/or retching);
adverse fetal and neonatal outcomes;
adverse maternal outcomes.
For fixed- and random-effects meta-analyses we planned to assess differences between subgroups by inspection of the subgroups’ confidence intervals; non-overlapping confidence intervals indicating a statistically significant difference in treatment effect between the subgroups. In this version of the review data were not available to carry out planned subgroup analysis.
Sensitivity analysis
We planned to perform sensitivity analyses where appropriate, for example where there was risk of bias associated with the quality of some of the included trials, or to explore the effects of fixed-effect or random-effects analyses for outcomes with statistical heterogeneity. However, as studies examined a variety of interventions we were able to pool only very limited data from a small number of studies. In updates of the review, if more data become available we will carry out planned sensitivity analyses.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search strategy identified 66 reports representing 55 studies (some of the studies resulted in more than one publication). Of these 55 studies, 27 met the inclusion criteria for the review, we excluded 22, four are awaiting further assessment, and two studies have not been completed yet.
Included studies
The included studies examined a range of interventions. Acupressure is a noninvasive variation of acupuncture which involves the application of constant pressure to specific points or areas. P6 (or Neigun point) acupressure is proposed to treat symptoms of nausea and vomiting (O’Brien 1996). The P6 point is located on the medial aspect of the forearm, at a specific point near the wrist. The effectiveness of acupressure to the P6 acupressure point was examined in five studies; in four of these the use of acupressure wrist bands was compared with placebo (Belluomini 1994; Norheim 2001; O’Brien 1996; Werntoft 2001), and in one with vitamin B6 (Jamigorn 2007) (in this study women in both groups also received a placebo intervention). One study examined the use of acustimulation to the P6 acupressure point (Rosen 2003). Another study compared auricular (on the ear) acupressure to placebo (Puangsricharern 2008). Two trials compared acupuncture with sham acupuncture (Knight 2001; Smith 2002); in one of these (Smith 2002) separate groups received traditional and P6 acupuncture.
The use of ginger (prepared as syrup or capsules) to relieve nausea was examined in nine studies; in four of these ginger was compared with a placebo preparation (Keating 2002; Ozgoli 2009; Vutyavanich 2001; Willetts 2003), in one with an anti-emetic (dimenhydrinate) (Pongrojpaw 2007), and in four studies the comparison group received vitamin B6 (Chittumma 2007; Ensiyeh 2009; Smith 2004; Sripramote 2003).
In two studies the intervention group received vitamin B6 (pyridoxine), which was compared with placebo preparations (Sahakian 1991; Vutyavanich 1995).
One study examined the use of moxibustion compared with traditional Chinese herbs (Fan 1995).
Six studies examined the use of antiemetic drugs: five compared placebo tablets with active treatment (fluphenazine (Price 1964), hydroxyzine hydrochloride (Erez 1971), Bendectin (Bentyl, Decapryn and pyridoxine) (Geiger 1959) or Debendox (a mixture of dicyclomine, doxylamine and pyridoxine) (McGuiness 1971), or thiethylperazine (Newlinds 1964)); one study (Bsat 2003) looked at the effectiveness of three different anti-emetics (metoclopramide with vitamin B6, prochlorperazine and promethazine).
All of the studies recruited women with symptoms of nausea (with or without vomiting) although we specifically excluded studies focusing on women with hyperemesis gravidarum. The severity of symptoms was not always made clear, and it is possible that some of the included studies may have recruited some women with more severe symptoms. One study included separate data for those women with the most severe nausea and vomiting (Rosen 2003), though not in a form that allowed us to analyse these separately as part of subgroup analysis.
The stage of pregnancy at which women were recruited to studies varied, although predominantly women were recruited during the first trimester (less than 12 weeks’ gestation). In one study (Fan 1995) women with gestational ages of more than eight weeks were included, but the upper limit was not specified; one study recruited women up to 20 weeks (McGuiness 1971), one up to 24 weeks (O’Brien 1996) and one up to 36 weeks (Price 1964). Although most of the women in these trials were in the first trimester and therefore, we did not wish to exclude the studies; separate figures were not provided on those women with nausea later in pregnancy, and so we were not able to exclude these women from the analyses. All of the studies collected outcome data on persistence of nausea symptoms or relief from nausea. Nevertheless, pooling data from studies was complicated by the variability in the way outcome data were collected and reported. The Rhodes Index of Nausea, Vomiting and Retching was used in nine studies (Belluomini 1994; Chittumma 2007; Jamigorn 2007; O’Brien 1996; Puangsricharern 2008; Rosen 2003; Smith 2002; Smith 2004; Willetts 2003). Not all studies collected or reported data on all dimensions (duration, frequency, distress) of the three subscales (nausea, vomiting, retching) included in the index. Eight studies collected ordinal data (Bsat 2003; Erez 1971; Fan 1995; Geiger 1959; Knight 2001; McGuiness 1971; Newlinds 1964; Price 1964). In these studies women were asked, for example, to rate symptoms on a five-point Likert-type scale or to describe the relief from symptoms on a three-point scale; we have converted some of the data from studies using such scales into binary data for incorporating them into the review. In 11 studies a visual analogue scale (VAS) was used (Keating 2002; Knight 2001 (for overall effectiveness rating); Ensiyeh 2009; Norheim 2001; Ozgoli 2009; Pongrojpaw 2007; Sahakian 1991; Sripramote 2003; Vutyavanich 1995; Vutyavanich 2001; Werntoft 2001). The wording on each VAS differed slightly, though in most cases women were asked to rate their symptoms on a 10 cm (or 100 mm) line, with 0 representing no symptom(s) (for example, no nausea) and 10 representing the worst symptom(s) (for example, the worst possible nausea). No authors provided details of validity or reliability testing of the VAS used.
Several studies reported the number of vomiting episodes recorded by women each day (Bsat 2003; Ensiyeh 2009; Keating 2002; Ozgoli 2009; Pongrojpaw 2007; Sahakian 1991; Sripramote 2003; Vutyavanich 1995; Vutyavanich 2001; Werntoft 2001), in addition to those above that used the Rhodes Index, which also measures frequency of vomiting. One study measured the use of rescue medication (Jamigorn 2007), and two others the use of over-the-counter and prescribed medication (Puangsricharern 2008; Rosen 2003).
In this review we chose to describe outcomes relating to women’s experience of nausea and vomiting at approximately three days after the start of treatment, as many of the studies provided data at this time point. We judged that this was a clinically meaningful point as most medication and other interventions would be expected to have achieved some effect within this timeframe. Where this information was not available, we chose the closest time point to three days that was reported. In the Characteristics of included studies tables, we have set out the time points when outcome data on symptoms were collected and reported in relation to the commencement of treatment. This information is important, as for many women symptoms are likely to resolve over time with or without treatment, particularly as the pregnancy progresses beyond the first trimester. In studies where outcome data were collected weekly over three or four weeks (e.g. Smith 2002; Smith 2004) we considered that differences between groups would be more difficult to detect at later follow-up points, and for these studies we have used symptom data from the earlier assessments (e.g. after seven days) in the data and analyses tables.
As well as symptomatic relief, our primary outcomes also included maternal and fetal/neonatal adverse effects. Five studies reported adverse fetal outcomes (Ensiyeh 2009; Erez 1971; Smith 2002; Vutyavanich 2001; Willetts 2003). Adverse maternal outcomes (such as preterm labour or spontaneous abortion) were reported for five studies (Ensiyeh 2009; Smith 2002; Smith 2004; Vutyavanich 2001; Willetts 2003). Worsening of symptoms was reported in two studies (Bsat 2003; Rosen 2003). Three studies reported on maternal weight loss/gain, which we had not prespecified as a maternal outcome (Jamigorn 2007; Keating 2002; Rosen 2003); this could be viewed as being related to symptom control, but is presented with the secondary outcomes in the results section. In addition, seven studies described the side effects of treatment such as headache, heartburn or sleepiness (Chittumma 2007; Erez 1971; Knight 2001; McGuiness 1971; Pongrojpaw 2007; Sripramote 2003; Willetts 2003).
Our secondary outcomes included quality of life of women during pregnancy, and economic costs (directly to women, productivity costs, and costs to the healthcare system). Two studies (Smith 2002; Smith 2004) measured Quality of LIfe using the MOS 36 Short Form Health Survey (SF36). One study (Knight 2001) used the Hospital Anxiety and Depression Scale. No studies measured economic costs.
See the Characteristics of included studies tables for more information on participants, interventions and outcomes measured.
Studies awaiting further assessment and ongoing studies
Four studies are awaiting further assessment; in all four cases studies were reported in brief abstracts, and our initial attempts to contact authors, or to identify subsequent publications have not been successful (Adamczak 2007; Biswas 2006; Hsu 2003; Mamo 1995). If we identify further reports from these studies we will reassess eligibility.
Two studies are ongoing. One multicentre trial (Nguyen 2008) examining the use of Diclectin (Debendox) for nausea and vomiting in pregnancy is planned to end in late 2009. We hope to include results from this study, if available, in updates of this review. Another study (Wibowo 2009) is comparing the effectiveness of different doses of vitamin B6 (“high” and “low” doses, which are undefined in the trial record).
Excluded studies
After assessment of study eligibility we excluded 22 studies identified by the search strategy. The main reason we excluded studies was because they were not randomised trials, or they used a crossover design. Six studies used quasi-randomised designs, for example allocation according to day of the week, or alternate allocation (Baum 1963; Can Gurkan 2008; Diggory 1962; Dundee 1988; Fitzgerald 1955; Winters 1961); such studies are at high risk of bias, and were therefore not included in the review. In three studies it was not clear to us that there was any sort of random allocation to groups (Conklin 1958; Lask 1953; Steele 2001). Seven studies used a crossover design (Bayreuther 1994; Cartwright 1951; De Aloysio 1992; Evans 1993; Hyde 1989; King 1955; Wheatley 1977); such designs are not usually appropriate during pregnancy when symptoms may not be stable over time. We excluded three studies as they focused on women with hyperemesis gravidarum, a group that we had decided to exclude from the review (Heazell 2006; Higgins 2009; Kadan 2009). Two of these studies are ongoing (Higgins 2009; Kadan 2009). We excluded one study because it was reported in a trial registry, and we found no evidence that the study had taken place; we carried out a search of databases to look for any publications from the study without success (Luz 1987). One study did not focus on the relief of nausea, but rather on hypocorticalism in pregnancy (Ferruti 1982); and finally, one trial record describes a study which looked at pre-emptive treatment (before any symptoms appear) with a combination of pyridoxine hydrochloride and doxylamine succinate (Diclectin) in a subsequent pregnancy for women who had experienced severe symptoms of nausea/vomiting of pregnancy (or hyperemesis gravidarum) in a previous pregnancy (Koren 2006).
Risk of bias in included studies
Allocation
Sequence generation
In eight of the included studies the method used to generate the randomisation sequence was not described or was not clear (Erez 1971; Fan 1995; Geiger 1959; McGuiness 1971; Ozgoli 2009; Pongrojpaw 2007; Price 1964; Werntoft 2001). In the study by Belluomini 1994 the trial was described as having a balanced block design, but it was not clear how the sequence order was generated or what the block size was. All the remaining studies were assessed as having adequate methods to generate the randomisation sequence: four studies used external randomisation services (Jamigorn 2007; Smith 2002; Smith 2004; Willetts 2003), five studies used computer-generated sequences (Bsat 2003; Keating 2002; Knight 2001; O’Brien 1996; Rosen 2003) (although the small block size in the Knight 2001 study (four) may have meant the sequence could be anticipated); and the remaining seven studies reported the use of tables of random numbers (Chittumma 2007; Ensiyeh 2009; Puangsricharern 2008; Sahakian 1991; Sripramote 2003; Vutyavanich 1995; Vutyavanich 2001).
Allocation concealment
In 12 studies the methods used to conceal the study group allocation were not described or were not clear (Belluomini 1994; Bsat 2003; Ensiyeh 2009; Erez 1971; Fan 1995; Newlinds 1964; Norheim 2001; Ozgoli 2009; Pongrojpaw 2007; Puangsricharern 2008; Sahakian 1991; Werntoft 2001). In the remaining studies we judged that the methods were adequate; four studies used an external randomisation service (Jamigorn 2007; Smith 2002; Smith 2004; Willetts 2003); five used sealed opaque sequentially numbered envelopes (Chittumma 2007; Knight 2001; Rosen 2003; Sripramote 2003; Vutyavanich 2001); one (O’Brien 1996) used numbered sealed envelopes, without stating if they were opaque or not; and in five placebo controlled trials, coded drug boxes or containers were used (Geiger 1959; Keating 2002; McGuiness 1971; Price 1964; Vutyavanich 1995).
Blinding
Most of the studies included in the review were placebo controlled. In three studies the routes of treatment administration (oral, injection etc) were different and double/multiple placebo control was not attempted (Bsat 2003; Chittumma 2007; Fan 1995). In two studies, where there were more than two active intervention arms, the type of treatment was blinded but the control condition (no intervention) was not (O’Brien 1996; Werntoft 2001). In all studies, all symptomatic outcomes were self-assessed by women, whether recorded by women themselves or a researcher.
The success of blinding was not reported in most trials. Where the treatment involved acupressure, acustimulation, or acupuncture, blinding may not have been convincing to women or clinical staff. In one acupuncture trial (Knight 2001), the author reported that there was no attempt to blind clinical staff, but women were described as being blind to group allocation. In five studies (Chittumma 2007; Knight 2001; Norheim 2001; Smith 2002; Smith 2004), the authors examined whether blinding was actually effective. It was reported in these studies that blinding may not always have been effective. We will return to this issue in the discussion.
Incomplete outcome data
The amount of missing outcome data in most of these studies was generally low, with attrition levels below 10%; in these studies most women were available to follow up, although there were missing data for some outcomes. There were higher rates of attrition in the studies by Pongrojpaw 2007 (11%), Willetts 2003 (17.5%), Rosen 2003 (18.6%), Knight 2001 (20%) and Newlinds 1964 (20%). In four studies attrition was greater than 20% (Sahakian 1991 (20.2%, attrition per group not stated); Smith 2002 (24% by week four of a four-week study), Smith 2004 (29.3% by day 21) and Belluomini 1994 (33%). The reasons for attrition varied and eight studies stated that women were lost to follow up for reasons that may have related to study outcomes (e.g. because they developed more severe symptoms, did not comply with taking study medication, or had adverse events) which may have put these studies at particularly high risk of bias (Belluomini 1994; Bsat 2003; Jamigorn 2007; Keating 2002; Knight 2001; Newlinds 1964; O’Brien 1996; Rosen 2003). In one study (Erez 1971), there was no attrition at three weeks, but for the later follow-up data on pregnancy outcome, there was high attrition (24%), as these women had given birth elsewhere. The reasons for this were not given, so it is possible that women were referred for high-risk deliveries or other adverse events; again, there may be a high risk of bias in this study. In one study (McGuiness 1971), the number of women randomised was not clear, making it impossible for us to assess attrition. In another study (Werntoft 2001), the approximate number of questionnaires (n = 80) given out was stated, and the study stopped when 20 per group returned them, but it is not known how many per group had been given out, and therefore, attrition cannot be accurately measured. Intention-to-treat (ITT) analysis was reported for two studies (Jamigorn 2007 (drop-outs counted as treatment failures); Knight 2001), and Vutyavanich 2001 included the three placebo drop-out participants in the results, assuming relief equal to best improvement in the placebo group.
Selective reporting
Although most of the studies provided some data on nausea, or relief from nausea, information on other outcomes was sparse. Not all subscales were reported for instruments such as the Rhodes Index (Belluomini 1994). Data from only selected time points were presented in some studies (Belluomini 1994; Keating 2002). In one study, results were presented using the number of assessments of outcomes (280 assessments for 35 participants in the control group and 256 assessments for 32 participants in treatment group), rather than the number of participants (Ozgoli 2009). Statements in the text about results were not always backed up with numerical results (e.g. Belluomini 1994 (re results from days eight to 10);Bsat 2003 (re drug use and compliance)).
As stated above (Included studies), few studies described side effects from treatment or adverse events for mothers or babies.
In six studies we had difficulty interpreting outcome data as they were presented only, or largely, in graphical form (Bsat 2003; Jamigorn 2007; Norheim 2001; O’Brien 1996; Rosen 2003; Willetts 2003).
Some studies (Pongrojpaw 2007; Sahakian 1991; Smith 2002) provided a large amount of outcome data, for example, mean scores on several dimensions of scales recorded over several days. Interpreting such data is not simple, and increases the risk of spurious statistically significant findings.
Other potential sources of bias
Three studies reported drug company involvement (provision of drugs and placebo, funding, or other sources of support) (Keating 2002; McGuiness 1971; Willetts 2003). One study stopped early; in this trial it was stated that approximately 80 women were randomised, but the study was ended when 20 women in each of three groups had returned their data collection forms (Werntoft 2001). In the Price 1964 trial, some baseline imbalance between study groups in terms of gestational age at recruitment was reported, and in the Puangsricharern 2008 study there were differences in baseline demographic characteristics, with the control group participants having higher education and income levels than the treatment group. In one study (Geiger 1959), two women were included in both the treatment and control groups, as they received medication on two separate occasions when they visited the clinic during the study period. In several studies (for example, Jamigorn 2007 and Rosen 2003), women were free to take other medication which may have had a bearing on outcomes; without information on what other medication women were using, it is difficult to interpret these data. In the Bsat 2003 study, women in one of the intervention groups received vitamin B6 as well as the main intervention (an anti-emetic). Therefore, it is possible that the vitamin supplement had some independent or interaction effect on outcomes.
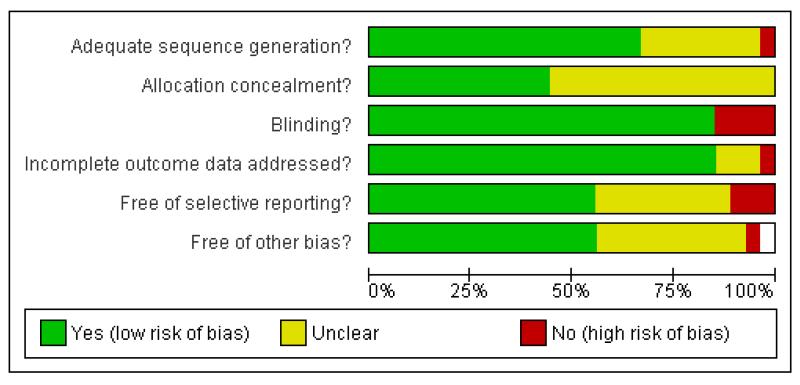
Figure 1 and Figure 2 show the summary and graph of methodological quality, respectively. These highlight that, across studies, there is a lack of clarity on many ‘risk of bias’ criteria, particularly in relation to sequence generation and allocation concealment, selective reporting and other possible sources of bias.
Figure 1.
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Figure 2.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
Interventions for nausea and vomiting in early pregnancy: 27 studies with 4041 women
Primary outcomes
The primary outcomes for this review were as follows.
Symptomatic relief (specifically a reduction or cessation in nausea, retching and/or vomiting).
- Adverse maternal and fetal/neonatal outcomes.
- Adverse maternal outcomes included pregnancy complications (antepartum haemorrhage, hypertension, preeclampsia) .
- Adverse fetal/neonatal outcomes included fetal or neonatal death, congenital abnormalities, low birthweight or early preterm birth.
Symptomatic relief
P6 Acupressure versus placebo (four studies with 408 women)
Four studies compared P6 acupressure to placebo, and we have included data from three of these in the data tables. None of these studies showed evidence of a statistically significant effect for acupressure. Results from one study (Norheim 2001) favoured P6 acupressure for improving (i.e. reducing) the intensity of symptoms, but the difference between groups was not statistically significant (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.44 to 1.39). After three days of treatment there was no strong evidence that, compared with placebo, the treatment improved nausea in the Werntoft 2001 trial (mean difference (MD) 0.10, 95% CI −1.49 to 1.69). Using scores averaged over one to three days, results from the Belluomini 1994 study did not show that acupressure improved scores on the nausea and vomiting subscales or on the total Rhodes Index score (for nausea MD 0.39, 95% CI −0.80 to 1.58, for vomiting MD 0.26, 95% CI −1.06 to 1.58, for total Rhodes score MD 1.17, 95% CI −1.52 to 3.86).
One further study (O’Brien 1996) compared P6 acupressure and placebo, but data from this study were not in a form that allowed us to enter them into RevMan tables. The authors reported no statistically significant differences between treatment and placebo groups for symptom relief.
P6 Acupressure versus vitamin B6 (one study with 66 women)
Jamigorn 2007 compared P6 acupressure with vitamin B6 and results showed no statistically significant difference between the two interventions for improvement of nausea on day three (data obtained from authors) (MD 0.20, 95% CI −2.24 to 2.64). The authors also report on the use of rescue medication (which may be a proxy measure for lack of symptom relief); results favoured P6 acupressure (MD −2.2, 95% CI −3.98 to −0.42). Jamigorn 2007 also compared P6 acupressure and vitamin B6 in terms of satisfaction with the intervention (which could be considered as a proxy for its effectiveness); results suggest that women were more satisfied with acupressure but evidence of a difference between groups did not reach statistical significance (MD 0.40, 95% CI −0.04 to 0.84).
Auricular acupressure versus placebo (one study with 91 women)
One study compared auricular acupressure (administered by participants by pressing on magnetic balls taped to an acupressure point on the ear) with placebo (no treatment) (Puangsricharern 2008). The authors report that they were using mean total Rhodes Index score and total number of vomiting episodes from days four to six to measure treatment effect. They subsequently concluded that there were no significant differences between groups (though average Rhodes scores across these days were not directly reported). The treatment started on day three (for the acupressure group) and the results for the total Rhodes score at day six (three days after treatment started) appear to favour the treatment group, although scores were lower in this group at baseline so results are difficult to interpret (MD −3.60, 95% CI −6.62 to −0.58 Analysis 3.1). There were no differences between groups for the number of anti-emetic drugs used (MD - 0.10, 95% CI −0.37 to 0.17).
Acustimulation versus placebo (one study with 230 women)
Rosen 2003 compared low-level nerve stimulation therapy over the volar aspect of the wrist at the P6 point with placebo. In this study, nausea symptoms were recorded over three weeks, with weekly assessments of changes from baseline. The author reported the “time-averaged” change in the Rhodes Index total experience scale over the entire three-week study period, and suggested that there was more improvement over time in the active treatment group (change score 6.48 (95% CI 5.31 to 7.66) versus 4.65 (95% CI 3.67 to 5.63) in the placebo group (data not shown in analysis tables). In this study, both groups experienced improved scores over the evaluation period, and data (presented in graphical form in the study report) were not simple to interpret. Results for women in the Rosen 2003 study with mild to moderate symptoms were described in an abstract by De Veciana 2001, and in another brief abstract results were reported for those women with severe symptoms (Miller 2001). However, neither abstract provided usable data for subgroup analysis.
Acupuncture versus placebo (two studies with 648 women)
One trial compared traditional acupuncture, P6 acupuncture, sham acupuncture and no treatment (Smith 2002). The data tables show three comparisons: between both traditional and P6 acupuncture and sham acupuncture, and between traditional and P6 acupuncture. None of the results show significant differences (Analysis 5.1 to Analysis 7.3) for relief from nausea, dry retching and vomiting. Knight 2001 also compared acupuncture to placebo but the data were not in a form that allowed us to enter them in RevMan 2008 tables; the authors used median scores because of the skewness of the data. They report no statistically significant differences between the control and intervention groups for symptom relief.
Moxibustion versus Chinese drugs (one study with 302 women)
Fan 1995 reported that in a study comparing moxibustion with Chinese drugs, symptoms for all women in both groups either “improved” or were “cured”.
Ginger versus placebo (four studies with 283 women)
Ginger was compared with placebo in four studies (Keating 2002; Ozgoli 2009; Vutyavanich 2001; Willetts 2003), although one study did not provide data on symptomatic relief in a way which we could use (Willetts 2003). In a small study (n = 26) (Keating 2002), results favoured ginger over placebo for improving nausea by day nine (RR 0.29, 95% CI 0.10 to 0.82). Results also favoured ginger for stopping vomiting at day six (RR 0.42, 95% CI 0.18 to 0.98). In the study by Vutyavanich 2001 (n = 70), results suggested that improvement in nausea symptoms was greater in the placebo group over four days of treatment (MD 1.20, 95% CI 0.22 to 2.18), but when ITT analysis was carried out (to include three missing patients in the placebo group counted as treatment failures), the evidence of a difference between groups was no longer statistically significant (MD 0.60 95% CI −0.51 to 1.71).
Ozgoli 2009 also compared ginger with placebo and presented the results on nausea intensity using the total number of nausea-intensity assessments per group (assessments were carried out twice daily over four days for each participant, resulting in a total of 280 assessments for treatment group and 256 assessments for control group). Apart from those results which are not easily interpreted, and have not been included in our analysis, improvements in nausea intensity are reported in percentages per group (from which numbers have been calculated and analysed in this review). Data on overall improvements appear to have been gathered during interview (by an unblinded researcher) on day five, rather than by comparing scores over time, but this is unclear. These results show a statistical difference between groups, favouring the treatment group (RR 1.48, 95% CI 1.07 to 2.04) on “nausea intensity improvement”. The authors also report a reduction in the incidence of vomiting following treatment of 50% in the intervention group compared with 9% in the control group, although the original data on post-treatment vomiting are not reported, and are not included in our analysis tables.
Ginger versus vitamin B6 (four studies with 624 women)
Four trials compared ginger and vitamin B6 (Chittumma 2007; Ensiyeh 2009; Smith 2004; Sripramote 2003).
In the two trials comparing ginger to vitamin B6 that had comparable outcomes reported (Chittumma 2007; Sripramote 2003), no statistically significant difference was found between groups (SMD −0.00, 95% CI −0.25 to 0.25, I2 = 0%) for symptom scores on day three. Results from the Chittumma 2007 study favoured ginger compared with vitamin B6 using the Rhodes Index to measure symptom relief, while in the Sripramote 2003 trial results favoured vitamin B6, using a 10 cm VAS to measure level of nausea; but neither of these results was statistically significant. Post-treatment number of vomiting episodes on day three was similar in the two intervention groups in the Sripramote 2003 trial (MD 0.00, 95% CI −0.60 to 0.60). Ensiyeh 2009 and Smith 2004 present results on improvement in symptoms and pooled results show no statistically significant difference between groups for the number of women reporting no relief (RR 0.84, 95% CI 0.47 to 1.52 (random-effects)) although there was moderate heterogeneity for this outcome and results should be interpreted with caution (heterogeneity: T2 = 0.11, I2 = 52%. P = 0.15).
Ginger versus Dimenhydrinate (one study with 170 women)
One study (Pongrojpaw 2007) compared ginger and dimenhydrinate, but the results for symptomatic relief were not easily interpreted and therefore, data have not been added to data tables in RevMan 2008.
Vitamin B6 versus placebo (two studies with 416 women)
In two studies comparing vitamin B6 with placebo (Sahakian 1991; Vutyavanich 1995), results favoured vitamin B6 for reduction in nausea after three days (MD 0.92, 95% CI 0.40 to 1.44). Comparing the number of patients vomiting post-treatment, there was no strong evidence that vitamin B6 reduced vomiting (average RR 0.76, 95% CI 0.35 to 1.66). As there was high heterogeneity for this outcome we used a random-effects model and results should be interpreted with caution (heterogeneity: I2 = 77%, T2 = 0.25, P = 0.04).
Anti-emetic medication versus placebo (six studies with 803 women)
There were six studies of anti-emetic medications. A range of anti-emetics (Hydroxyzine, Debendox (Bendectin) Thiethylperazine and Fluphenazine-Pyridoxine) were compared with placebos, and in one study, three anti-emetic medications were compared. One study (Erez 1971) compared Hydroxyzine to placebo, with the results favouring Hydroxyzine for relief of nausea (RR 0.23 95% CI 0.15 to 0.36).
Bsat 2003 compared three drug regimens: Pyridoxine-metoclopromide, Prochlorperazine and Promethazine. Results were reported in graphs and we have not entered estimated figures into data tables. Approximately 65%, 38% and 40% of women in each group, respectively, responded that they felt better on the third day of treatment. The authors conclude that their results favour Pyridoxine-metoclopromide over the other two regimens.
Two studies (Geiger 1959 and McGuiness 1971) compared Debendox (Bendectin) with placebo, and results for nausea relief favoured the intervention group. However there was high heterogeneity when results from these two studies were combined, and the time point at which outcome data were collected was not clear in the McGuiness 1971 study, and so in the analyses we have provided subtotals only. In the McGuiness 1971 study, while fewer women in the Debendox group had no relief in symptoms, the difference between groups was not statistically significant (RR 0.65, 95% CI 0.36 to 1.17). In the Geiger 1959 study, only three of 52 women receiving Debendox reported no improvement in symptoms compared with 20/57 for controls.
Thiethylperazine was compared with placebo in one study and women in the placebo group were less likely to experience symptom relief (RR 0.49 95% CI 0.31 to 0.78) (Newlinds 1964). Finally, fluphenazine-pyridoxine seemed to improve symptoms compared with placebo in one trial, but results did not reach statistical significance (RR 0.52, 95% CI 0.27 to 1.01) (Price 1964); this is an antipsychotic drug (from the piperazine class of phenothiazines).
Adverse maternal and fetal/neonatal outcomes
Adverse maternal and fetal outcomes were reported for some studies across several comparisons.
Acupressure versus vitamin B6
Weight gain was reported by Jamigorn 2007 and results favoured the acupressure group (MD 0.70, 95% CI 0.24 to 1.16), with higher weight gain in this group.
Acupressure versus placebo
Norheim 2001 reported that 63% of participants in the acupressure group and 90% in the placebo group reported problems (including pain, numbness, soreness and hand-swelling) using the wristband. Three women (two in the treatment group, one in the placebo group) said they felt more sick during the study period.
Acustimulation versus placebo
Rosen 2003 reported on weight gain, dehydration and ketonuria. There was significantly more weight gain and less dehydration in the treatment group (MD 1.70, 95% CI 0.23 to 3.17; RR 0.24, 95% CI 0.07 to 0.83 respectively) but there was no significant difference for ketonuria at the end of the trial period (RR 0.48, 95% CI 0.15 to 1.55). The authors report that there was no significant difference between groups on entry to the trial for ketonuria, though those most likely to withdraw from the study had ketonuria at entry (but at non-significant level).
GInger versus placebo
Vutyavanich 2001 reported on the rates of spontaneous abortion, with no significant difference between groups (RR 0.36, 95% CI 0.04 to 3.33). Similarly, for delivery by caesarean section, there was no difference between groups (RR 1.64, 95% CI 0.51 to 5.29). The authors reported that there were no congenital abnormalities in either group. As with the other studies reporting such fetal outcomes, this study did not have sufficient power to show differences between groups; we will return to this in the discussion.
Willetts 2003 compared fetal adverse outcomes (such as stillbirth, neonatal death, preterm delivery, congenital abnormalities) with expected numbers based on data at one hospital in Sydney. The results were not clearly presented by randomisation group, but were shown for the overall number who completed the main study, with descriptive text about the number in the ginger group. The authors concluded that those exposed to ginger did not appear to be at greater risk of fetal abnormalities.
Also in a study of ginger versus placebo, Keating 2002 reported weight change measured at the four week follow-up visit, but data were not presented in a usable form; the authors commented that most women in both groups maintained or gained weight.
Ginger versus vitamin B6
Smith 2004 reported on outcomes including spontaneous abortion, stillbirth, heartburn, congenital abnormality, antepartum haemorrhage/abruption or placenta praevia, pregnancy-induced hypertension, pre-eclampsia and preterm birth. There were no neonatal deaths in either group and no significant differences between the groups (Analysis 9.4 to Analysis 9.10). Similarly in Ensiyeh 2009, no significant differences were found in the maternal and fetal outcomes reported (spontaneous abortions, caesarean delivery, congenital anomaly of the baby (Analysis 9.4, Analysis 9.6, Analysis 9.15). The authors report that “all were discharged in good condition”, though elsewhere they say that data collection and follow up took 12 weeks; women were recruited to the trial at 17 weeks’ gestation or less, implying a longer follow-up time. Chittumma 2007 reported on arrhythmia and headache, with no evidence of a difference in effect between groups (Analysis 9.12; Analysis 9.11). Two studies (Chittumma 2007; Sripramote 2003) report results for heartburn, with no significant effect (RR 2.35, 95% CI 0.93 to 5.93, heterogeneity: I2 = 3%, P = 0.31). Chittumma 2007 reported on drowsiness, with neither ginger nor vitamin B6 favoured (RR 0.65, 95% CI 0.27 to 1.56). Sripramote 2003 reported on sedation, with no strong evidence for either intervention (RR 0.81, 95% CI 0.47 to 1.39).
Ginger versus dimenhydrinate
Pongrojpaw 2007 reported on the side effects of drowsiness and heartburn. More people in the dimenhydrinate group experienced drowsiness, while more in the ginger group experienced heartburn, but evidence of differences between groups was not statistically significant (drowsiness: RR 0.08, 95% CI 0.03 to 0.18; heartburn: RR 1.44, 95% CI 0.65 to 3.20).
Antiemetic drugs
In the trials of anti-emetic drugs, fetal outcome was recorded only by Erez 1971. In that study, of the 79 cases available for follow up in the hydroxyzine group, there were four spontaneous abortions (three in the first trimester and one in the second trimester) and one perinatal death. In the 36 cases available for follow up from the placebo group, there were two first trimester spontaneous abortions (spontaneous abortions: RR 0.91, 95% CI 0.17 to 4.75; perinatal mortality: RR 1.39, 95% CI 0.06 to 33.26). In the text, the authors report that slight drowsiness was reported by 7% (n = 7) of the treatment group, but no other adverse effects were reported, and there were no hospitalisations in either group.
Bsat 2003 reported a non-significant difference in hospitalisation across the three groups receiving pyridoxine-metoclopramide, prochlorperazine and promethazine. They comment that subsequent pregnancy courses were similar and only one neonatal anomaly was seen (a cardiac defect in the prochlorperazine group). McGuiness 1971 stated that side effects were reported by 12 patients in the Debendox group (including drowsiness for three patients, feeling weak for two, tiredness for two) compared to six adverse effects reported in the placebo group (including tiredness, sleepiness, depression and constipation). Newlinds 1964 reported that side effects occurred in 12 of the 93 patients who received thiethylperazine and 10 of the 87 in the placebo group. These adverse effects included drowsiness (four treatment, three placebo), aggravation of nausea (two treatment, three placebo), “cerebral stimulation”, described as mild in the text, and included restlessness (two in treatment group, none in placebo). Price 1964 reported that there were no side effects in the fluphenazine-pyridoxine group and one patient in the placebo group reported drowsiness. Geiger 1959 reported that one patient in the Bendectin group reported listlessness; no other adverse effects were reported.
Secondary outcomes
The secondary outcomes for this review were:
quality of life (emotional, psychological and physical well-being, women’s assessment of the pregnancy experience, women’s coping with the pregnancy);
economic costs (direct financial costs to women, productivity costs and/or health system costs).
Only three studies reported quality of life (and related) results (Knight 2001; Smith 2002; Smith 2004).
Knight 2001 used the Hospital Anxiety and Depression Scale (HADS) and reported median scores for the intervention and control groups, but the data were not in a form that allowed us to enter them in RevMan 2008 tables. The authors report that for both anxiety and depression scores, there was no evidence for a group effect or a group-time effect, but there was for a time effect (in favour of acupuncture). However, both scores dropped over the course of the study for both groups. The median rating of global effectiveness was the same for both groups.
Smith 2002 and Smith 2004 used the MOS 36 Short Form Health Survey. Smith 2002 reported the change in mean scores on the SF36 Form (Quality of Life) for the four groups receiving traditional acupuncture, P6 acupuncture, sham acupuncture and no treatment, respectively. They report eight sets of results for three time points and highlight in the text that there was a group effect on the social function and mental health SF36 domains, favouring traditional acupuncture in both cases. Smith 2004 also reported changes in mean scores across eight domains of the SF-36, with a significant difference, favouring ginger, found only in two domains: social function and physical role function.
No study reported economic data of any sort.
DISCUSSION
Summary of main results
Nausea and vomiting in early pregnancy are common. Symptoms are generally self-limiting, are not usually life threatening and, provided women do not have very severe vomiting, do not often lead to serious complications. Nevertheless, early pregnancy nausea and vomiting may be extremely distressing to women, and may disrupt their physical and social functioning. In this context, and in view of concerns about the possible teratogenic effects of pharmacological agents, non-pharmacological approaches to symptom control have become increasingly popular and have been recommended in clinical practice guidelines (NICE 2008).
In this review we found little strong or consistent evidence that non-pharmacological therapies are effective in reducing symptoms. Evidence regarding the effectiveness of P6 acupressure (including acustimulation at this point) was limited. There was some evidence of the effectiveness of auricular acupressure, though further larger studies are required to confirm this. Acupuncture (P6 or traditional) showed no significant benefit to women with nausea and vomiting in early pregnancy. The use of preparations containing ginger may be helpful to women, but in this review the evidence of effectiveness was limited, and not consistent.
We also found only limited evidence from trials to support the use of pharmacological agents including vitamin B6, antihistamines, and other anti-emetic drugs to relieve mild or moderate nausea and vomiting (a related Cochrane review is examining their use in women with more severe symptoms). There were no studies of dietary or other lifestyle interventions identified.
Overall completeness and applicability of evidence
We attempted to be as inclusive as possible in the search strategy and have included studies reported in languages other than English. Nevertheless, the literature included in the review was predominantly reported in European and North American journals and this may have introduced some bias and limited the applicability of results.
Interpreting the findings of the studies included in the review was not simple. Some of the studies were more than 50 years old and during the time period covered by this research, the attitudes of women and clinical staff towards symptoms and towards symptom relief may have changed. Most of the studies examining nonpharmacological approaches have been published more recently, yet there is very little conclusive evidence on the efficacy of complementary or alternative therapies.
The main focus of the review was on the effectiveness of interventions to relieve symptoms. However, our prespecified outcomes also included the impact of interventions on the well-being of mothers and babies. Although there may be a perception that complementary and alternative approaches are not ‘invasive’, their safety has not been adequately evaluated. Few studies reported pregnancy outcomes, adverse effects from treatments, or adverse events. It may not be safe to assume that because negative outcomes were not reported that they did not occur. In those studies (mainly those focusing on pharmacological interventions) that did report data on side effects and adverse events, none had the statistical power to provide convincing evidence regarding relatively rare adverse outcomes.
The studies reviewed here contained very little information on the psychological, social or economic impact of nausea on pregnant women. The scales used tended to focus on the experience of symptoms; but very little data were presented on other aspects of quality of life such as the impact of nausea on family and social functioning, or on relationships. Many women experience symptoms whilst attempting to care for young children or whilst attending work; none of the studies reported on outcomes relating to the impact of interventions on the ability to perform work, on sickness absence from work, or on the economic impact of symptoms.
Some of the interventions examined in the review, such as ginger or acupressure wrist bands, may be transferable to clinical contexts other than those in which they were tested as they may be relatively low cost (although studies did not provide information on this) and acceptable to women and staff. Other interventions may require special equipment not generally available in antenatal care settings (e.g. acustimulation or acupuncture) and staff may need particular skills and training; even if these interventions had been proven effective, they may not be easily transferable between care settings.
Quality of the evidence
We were unable to pool findings from studies for most review outcomes due to heterogeneity in study participants (e.g. stage of pregnancy and severity of symptoms), interventions (and co-interventions), comparison groups, and outcomes measured or reported. For this reason, most of the results were derived from single studies with findings that have not been replicated elsewhere. Where results from more than one study were pooled, inconsistencies in findings between studies was reflected in high levels of statistical heterogeneity for some outcomes; we have indicated in the results section those outcomes affected by high heterogeneity and advise caution in interpreting those results.
The methodological quality of the included studies was mixed. Some studies had high rates of attrition, poor allocation concealment and other methodological problems which put them at high risk of bias. Lack of effective blinding may also have introduced bias; although many of the included studies were described as being double blind or keeping women blind to group allocation, we had concerns about the effectiveness of blinding. Sham acupressure, acupuncture or acustimulation may not be convincing to women. Some of the trials which investigated the effectiveness of blinding provided some evidence that women may have had some idea of group allocation (Chittumma 2007; Knight 2001; Norheim 2001; Smith 2002; Smith 2004). The lack of blinding or unconvincing blinding may be particularly relevant where the main outcome is women’s subjective, self-reported symptoms. We had intended to carry out sensitivity analysis whereby we would exclude from the analyses those studies at high risk of bias to see what impact this would have on findings; however, we did not do this because we were unable to pool data for most interventions and outcomes, and results were derived from single trials.
Lack of clear information on how studies were conducted and in reporting results means that some findings may be difficult to interpret. Few of the studies provided clear information on whether or not women were using other over-the-counter remedies or prescribed medications to control symptoms. This information would have been very helpful in understanding results. One study reported the use of “rescue” medication (Jamigorn 2007). In other studies the treatment effect may have been underestimated if women in control groups were more likely than those in intervention groups to use other treatments.
The effectiveness of vitamin B6 was difficult to interpret. In some studies, vitamin B6 was the active intervention, in others it was the control condition, and in at least one study it was given in addition to one of the interventions (Bsat 2003); in this study it was not clear whether the results obtained for the anti-emetic plus B6 group were attributable to the anti-emetic alone, vitamin B6 alone or both acting together.
The way in which outcomes were measured and reported in studies varied considerably. Some studies used the validated instruments described under Primary outcomes. Other studies used ordinal data such as three- or five-point scales. In these cases, in order to include data in the analysis tables, we converted the data into binary form by choosing cut-off points. We attempted to be consistent in choice of cut off, opting for no relief versus improvement in symptoms, but we acknowledge that the choice may have impacted the results. There was also variation in the way continuous data were collected, with some studies using visual analogue scales or validated scales. Eight studies in the review used the Rhodes Index. This was originally created to measure the nausea and vomiting symptoms of chemotherapy (Rhodes 1984), and has been validated for use in studying these symptoms in pregnancy (Zhou 2001). However, the use of Rhodes Index of Nausea, Vomiting and Retching, for example, was not consistent in studies; some trials used shortened forms or did not collect or report data on all subscales. Further, as we mentioned earlier, in some trials data were collected repeatedly and a great deal of (not always consistent) data were presented. In this review we have tried to present findings for a time point approximately three days after the start of treatment, but this was not always possible. The lack of consistency in the way outcome data were measured and reported should be kept in mind when interpreting results.
The use of pregnancy-specific nausea and vomiting measurement instruments in future studies may facilitate better outcome measurement. As described in Primary outcomes, the Pregnancy Unique Quantification of Emesis (and nausea) (PUQE) has been has been developed by clinician-researchers at the Canadian Motherisk Program. This is a three-item (plus a global question) instrument. The clinician-researchers had been using the Rhodes Index and stated that they found it to be detailed but cumbersome and time-consuming (Koren 2002b). They also noted the strong correlations between the severity of a physical symptom and the stress caused by that symptom. Also nausea was measured twice (duration and number of bouts (frequency) of nausea). They also felt that, based on their experience, frequency of nausea was more difficult for women to define. The PUQE has been validated against four independent criteria (Koren 2005) and with an established Quality of LIfe instrument (Lacasse 2008) and it has been used in studies that were not included in this review (for example Koren 2006). Other pregnancy-specific instruments have been developed (Magee 2002b; Swallow 2002) but these have not been used in published randomised controlled trials identified within this review).
Potential biases in the review process
We acknowledge that there was the potential for bias at all stages in the reviewing process. We attempted to minimise bias in a number of ways; for example, two review authors independently carried out data extraction and assessed risk of bias. However, we acknowledge that such assessments involve subjective judgments, and another review team may not have agreed with all of our decisions. A further possible source of bias (discussed above) was the choice of time points for symptom assessment and the cut off points chosen to convert ordinal into binary data for entry into RevMan 2008. Again, we attempted to minimise bias by discussing such issues and attempting to be consistent across studies and outcomes.
Agreements and disagreements with other studies or reviews
Current clinical practice guidelines suggest that acupressure and ginger may be useful in the relief of symptoms of nausea and vomiting (NICE 2008). Our results suggest that the evidence underpinning such recommendations is inconsistent and relatively weak.
There are several other non-Cochrane reviews and overviews of interventions for nausea and vomiting in pregnancy in the literature (Aikins Murphy 1998; Bryer 2005; Davis 2004; Jewell 2003; Kousen 1993; Magee 2002a; Magee 2006; McParlin 2008; Niebyl 2002; Quinlan 2003; Wilkinson 2000). These reviews present partial evidence to back up their conclusions. Bryer 2005 reviews the same three studies of ginger included in this review (Keating 2002; Smith 2004; Vutyavanich 2001), and comments on the variety of doses and preparations used and the lack of safety reporting. Nonetheless, drawing on an observational study of teratogeny, Bryer 2005 concludes that “ginger is a safe and effective treatment option for nausea and comparable with vitamin B6 in effectiveness”. Our review found limited and inconsistent evidence of such effectiveness. Davis 2004 proposes “an evidence based review” and describes briefly the findings of some trials of both pharmaceutical and non-pharmaceutical treatments, but does not comment on the quality of studies and concludes that treatment has been “poorly refined”. Magee 2002a offers an “evidence-based approach of safety and effectiveness” of pharmacological therapies, and reproduces a forest plot of various treatments from a previous review (Mazzotta 2000). The authors conclude that evidence from controlled trials has shown that Bendectin/Diclectin, antihistamine blockers and phenothiazines as a group are safe and effective for treatment. The current review would not support that simplistic conclusion, based on the quality and consistency of evidence. A more recent review (Magee 2006) does comment on the quality of the trials reviewed and the lack of consistent outcome measurement, as was also found in the current review. In a more recent review, McParlin 2008 suggests that “ginger and acupressure are two alternative therapies that have the most promising results from clinical trials”, citing one clinical trial, one set of practice guidelines, one observational study on safety, and the previous Cochrane review on this topic (Jewell 2003a). However, Jewell 2003a had concluded more cautiously that the single trial of ginger was encouraging, though its active ingredient is not known and that the evidence on acupressure was mixed.
Some reviews include crossover studies, which is problematic as symptoms generally improve over time. For example, Ernst 2000 includes one trial which studied ginger for nausea and vomiting across different groups (postoperative sickness, seasickness, etc). The included trial was a crossover study with 30 patients; nonetheless these study results are pooled with two other studies and found to collectively favour ginger over placebo.
AUTHORS’ CONCLUSIONS
Implications for practice
Women will continue to seek treatments for the often distressing symptoms of nausea and vomiting in pregnancy. They may take over-the-counter and complementary therapies, based on anecdotal or peer advice. There are many sources of advice for women on the Internet, including peer fora. Wilkinson 2000 found a lack of consensus about safety of herbal treatments (including ginger) for nausea and vomiting in pregnancy in 300 non-medical sources identified in a literature review. This highlights the necessity of health professionals providing clear guidance to women, based on systematically reviewed evidence. On the basis of this review, high-quality consistent evidence is lacking to support the accuracy or appropriateness of that advice. Current guidelines and other reviews often offer incomplete evidence, without comment on the quality of evidence. Health professionals’ decisions about treatments should take account of the lack of clear and consistent evidence found in this review and acknowledge that it is not possible at present to identify, with confidence, safe and effective interventions for nausea and vomiting in early pregnancy.
Implications for research
The difficulties in interpreting the results of the studies included in this review highlight the need for specific and clearly justified outcomes in research on interventions for nausea and vomiting in pregnancy. The range of instruments used to measure these symptoms (including those not developed for this patient group) also suggest the need for a consistent and appropriate approach to measurement, which may be addressed by the PUQE scale described above. There is also a need to systematically measure quality of life and adverse maternal and fetal and neonatal outcomes, to ensure that studies are of most usefulness to health professionals and women seeking safe and effective treatments. We did not identify any studies of dietary or behavioural interventions. Dietary and behavioural strategies (eating low fat, small, frequent meals) were often recommended to all participants (in both treatment and placebo groups) within the studies in this review. Only one study (Ozgoli 2009) measured adherence to dietary advice. The effectiveness of dietary and other behavioural strategies also needs to be evaluated in good quality trials.
PLAIN LANGUAGE SUMMARY.
Interventions for nausea and vomiting in early pregnancy
Nausea, retching or dry heaving, and vomiting in early pregnancy are very common and can be very distressing for women. Many treatments are available to women with “morning sickness”, including drugs and complementary and alternative therapies. This review aimed to examine if these treatments have been found to be effective and safe because of the concern that taking medications may adversely affect the development of the fetus.
This review found a lack of high-quality evidence to back up any advice on which interventions to use. We examined 27 randomised controlled trials which included 4041 women in early pregnancy. These studies examined the effectiveness of many treatments including acupressure to the acupuncture point on the wrist (P6), acustimulation, acupuncture, ginger, vitamin B6 and several conventional drugs that are used to reduce nausea or vomiting. Some studies showed a benefit in improving nausea and vomiting symptoms for women, but generally effects were inconsistent and limited. Studies were carried out in a way that meant they were at high risk of bias, and therefore, it was difficult to draw firm conclusions. Most studies had different ways of measuring the symptoms of nausea and vomiting and therefore, we could not look at these findings together. Few studies reported maternal and fetal adverse outcomes and there was very little information on the effectiveness of treatments for improving women’s quality of life.
ACKNOWLEDGEMENTS
As part of the pre-publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
The University of Liverpool, UK.
External sources
-
Health Research Board, Ireland.
Cochrane Fellowship held by Anne Matthews 2007-9
National Institute for Health Research, UK.
TD is supported by a NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | A randomised blinded study. | |
| Participants | 90 pregnant women, with gestation of 12 weeks or less by the completion of the study. Exclusion criteria were diagnosed hyperemesis gravidarum, diseases that cause nausea and emesis, and current use of anti-emetic medication | |
| Interventions | Treatment group received acupressure using an acupressure point (Nei Guan PC-6); placebo point (on palmar surface of the hand, proximal to the head of the fifth metacarpal joint) used for the sham control group. Applied for 10 minutes 4 times per day | |
| Outcomes | Nausea and vomiting were measured using the Rhodes Index of Nausea and Vomiting Form-2 (scale range of 0-32, 3 subscales: nausea (duration, frequency and distress), vomiting (amount, frequency and distress) and retching (frequency and distress) Outcomes were measured each evening for 10 consecutive days; data from the first 3 days were used as pre-treatment data; data from days 5-7 were used to measure treatment effect |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Women were assigned by a randomised block design to P6 acupressure or sham acupressure group. How this was done is not described. Probably adequate, though size of blocks not stated |
| Allocation concealment? | Unclear | Not described. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Subjects and their referring practitioners were blind to the group assignment (though this presupposes a lack of knowledge of acupressure). Subjects self-reported outcomes |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 30 of 90 participants did not complete the study (16 treatment and 14 control). Failure to return forms, very incomplete forms or loss to follow up explained attrition of 13 treatment and 12 control participants. The remaining attrition (3 treatment and 2 control) was explained as prescribing anti-emetics, abdominal surgery and voluntary dropout Though this high attrition rate might introduce bias, it is similar between groups |
| Free of selective reporting? | No | Retching subscale results not reported. Subscale results for nausea and vomiting presented averaged for days 1-3 and 5-7; data from days 8, 9, 10 were not presented; it was reported in text that data from these days “demonstrated no significant differences between the treatment and placebo groups because nausea and vomiting had improved over time” (average gestation 8.5 weeks +/− 1. 4 weeks) |
| Free of other bias? | Yes | Internal reliability of Rhodes Index reported for day 2 of pre-treatment (r = 0.88); not explained why this day was chosen. Unlikely to introduce bias |
| Methods | Prospective randomised comparison of 3 drug regimens. | |
| Participants | 169 women with singleton pregnancies in first trimester presenting to their obstetrical provider with nausea and/or vomiting | |
| Interventions | 3 “commonly prescribed pharmaceutical regimens in the outpatient management of nausea and vomiting in pregnancy”, to “mirror local practices” Group A: 50 mg intramuscular injection of pyridoxine, with metoclopramide 10 mg orally every 6 hours as needed Group B: prochlorperazine as needed 25 mg rectal suppositories every 12 hours or 10 mg tablets orally every 6 hours as needed Group C: promethazine 25 mg orally every 6 hours as needed. |
|
| Outcomes | Change in symptoms: scores 1-5 on a scale which comprised: much worse, worse, same, better, much better; recorded by participants on third day of treatment. Responses then divided into 2 subgroups:-those who answered 1-3 (same-worse) and those who answered 4-5 (better) Women also recorded the number of emesis episodes the day before and on the third day of treatment; dry heaves (retching) were counted as nausea, but not vomiting episodes Worsening of symptoms was evaluated and patient admission for hydration or inpatient management was considered on an individual basis Hospitalisation for the specific management of nausea or vomiting was noted Patients also recorded their “medication compliance”. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Patients were divided into 3 groups based on a computer-generated randomisation |
| Allocation concealment? | Unclear | Not described. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
No | Not blinded. The authors raise the possibility of the “placebo effect” of the intramuscular route. The authors conclude that it would have been ideal to randomise the patients in a double blind fashion and possibly to establish a placebo group |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 12 patients lost to follow up (3, 5 and 4 from groups A, B and C respectively). 1 patient from Group A withdrew from the study with side effects (acute dystonic reactions, thought to be secondary to metoclopramide) |
| Free of selective reporting? | No | Results for “subjective response” presented only in graphical format; not usable Emesis frequency only reported. States that “drug usage and compliance was comparable between all three groups”, but no description of amount of each drug used (most were on an “as required” basis) |
| Free of other bias? | No | 2 drugs were given to Group A; this treatment was found to be most effective; it is not possible to identify whether 1 or both agents were effective. The authors note that combining 2 agents that may also both work independently may raise questions of fairness - this was done to mirror local practices Unclear who and where drugs were administered (e.g. IM injections on an “as required” basis). Study was done in out-patient setting |
| Methods | Randomised double-blind controlled trial. | |
| Participants | 126 pregnant women at 16 weeks’ gestation or less who had nausea and vomiting, required anti-emetics, had no medical conditions, and were not hospitalised | |
| Interventions | Treatment group: 2, 325 mg capsules of ginger or placebo group: 2, 12.5 mg identical capsules Capsules taken 3 times daily before meals for 4 days. |
|
| Outcomes | Primary outcome: change of nausea vomiting scores (mean of post-treatment minus baseline scores). Symptoms recorded at baseline and each day during treatment The 3 physical symptoms of Rhodes’s score were measured (episodes of nausea, duration of nausea and number of vomits); range lowest score of 3 to maximum of 15 Secondary outcomes measured: occurrence of side effects such as heartburn, arrhythmia, headache and sedation |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Women were randomly allocated. The randomisation of patients was done using a table of random numbers with blocks of 4 to receive ginger or vitamin B6. When using blocks of 4, it may be possible to predict sequence |
| Allocation concealment? | Yes | The treatment code was concealed by placing the patient’s assignments in sequence in sealed opaque envelopes that were drawn in ascending consecutive order |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | The codes were kept strictly confidential for blinding the physician and subjects and were broken at the end of the study. Women self-reported outcomes 4 cases in the ginger group identified what they were taking, none in the B6 group did |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 2 cases in the ginger group and 1 case in the vitamin B6 group were lost to follow up |
| Free of selective reporting? | Yes | All outcomes reported. |
| Free of other bias? | Unclear | 1 person in the ginger group and 4 in the B6 group took other medications (common cold, headache); 3 of the ginger group and 4 of the B6 group took other ginger products during the trial. At the end of the trial, the use of other antiemetics was reported by 7 of 12 patients (5.7%); unclear what this means The authors chose a study period of 4 days because previous studies showed that the effect of ginger was evident within a few days of treatment and too long a period would result in a higher rate of subject noncompliance and loss to follow up |
| Methods | Double-blind randomised controlled trial. | |
| Participants | Pregnant women with nausea, with or without vomiting, who first attended the antenatal clinic at or before 17 weeks’ gestation. Women were excluded if they had other diseases that might cause nausea and vomiting, had mental health problems, had taken tablets in the previous week that might have aggravated nausea or vomiting symptoms, refused to participate or were unable to return 1 week later for follow up During the study, 80 women were eligible and 70 agreed to participate, 35 randomised to each group |
|
| Interventions | Ginger 1 g/day or vitamin B6 40 mg/day for 4 days (for both groups: 2 capsules daily, after breakfast and dinner) | |
| Outcomes | Severity of nausea using a VAS, number of episodes of vomiting recorded, 3 times daily during treatment for 4 days (average daily scores and mean nausea score calculated over the 4 days of treatment). At 7 day follow up treatment response was assessed using a 5-point Likert scale (much worse, worse, same, better, much better). Median change in severity of nausea and number of vomiting episodes compared by group Secondary outcomes also measured were: side effects and adverse effects on pregnancy outcomes such as abortion, preterm birth, congenital anomaly, perinatal death and mode of birth Compliance was assessed by pill count and by asking women if they took the drugs |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | 35 women were randomised to the ginger group and 35 to the vitamin B6 group, using a table of random numbers |
| Allocation concealment? | Unclear | No details given. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Double-blind; women self-reported outcomes. |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 1 woman randomised to vitamin B6 group did not return to the clinic, so she was excluded from the study. Results presented by intention to treat, after excluding the 1 woman |
| Free of selective reporting? | Unclear | Only changes in scores and number of vomiting episodes presented, as well as frequency of improvement in symptoms (by category much worse to much better) The authors stated that data collection and follow up took 12 weeks; also stated that pregnancy outcomes including preterm birth, perinatal death, congenital anomaly, mode of delivery were assessed, which could not have been concluded within 12 weeks. Median changes in scores presented only Compliance/adherence to treatment is not recorded. |
| Free of other bias? | Yes | Power analysis was said to be used to determine the sample size, resulting in 31 per group to achieve a power of 0.80 with an alpha of 0.05; however effect size (presumably for primary outcome) needed for the calculation is not stated. Not likely to introduce bias |
| Methods | Double blind study/evaluation. | |
| Participants | 150 pregnant women in the first 2 months of pregnancy, reporting recurrent nausea and had vomited at least 3 times per week over the previous 2 weeks | |
| Interventions | Hydroxyzine Hydrochloride 25 mg capsules twice daily orally (morning and 2 pm) or identical capsules of placebo for 3 weeks | |
| Outcomes | Effectiveness of the medication graded subjectively by the patient as follows: complete relief, partial relief, no relief. Evaluation of effectiveness of drug and side effects was made 3 weeks after starting the medication Side effects were evaluated (not stated how, by whom). “Fetal wastage” and fetal anomalies checked. |
|
| Notes | Initially no attempt was made to eliminate causes of recurrent vomiting other than pregnancy Comments that spontaneous remission or psychological factors may have played a role and this was evident from the fact that 22% of the placebo group had some response |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | By random selection from the available preparations, 100 patients received Hydroxyzine and 50 patients received the placebo |
| Allocation concealment? | Unclear | Not described. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Neither the physicians involved not the patient recipients knew which was the active drug and which the placebo |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | No missing data for follow up at 3 weeks (primary outcome of symptom relief); obstetrical outcome reported for 115 (of 150); 21 of treatment group and 14 of control group could not be evaluated as they delivered elsewhere |
| Free of selective reporting? | Yes | All outcomes reported. |
| Free of other bias? | Unclear | Blind evaluation of drug efficacy and any side effects was made 3 weeks after the institution of the medication There was a 2:1 ratio of treatment: control participants. |
| Methods | Randomised comparison/observation. | |
| Participants | 302 patients with pregnant vomiting, with menstruation suspended for more than 2 months (maximum not stated) | |
| Interventions | Patients were treated according to differentiation of symptoms and signs and types of syndromes (1. deficiency of both the spleen and the stomach, 2. incoordination between the liver and the stomach) 2 treatment groups: Moxibustion group (specified points). Chinese drug group (specified herbs). |
|
| Outcomes | Criteria for evaluating the therapeutic effect: cured, improved, ineffective. Not described who measured these. “Cure” defined as disappearance of symptoms after treatment for 1 week, but outcome measurement time-point(s) not specified | |
| Notes | Gestation unclear; more than 8 weeks since last menstrual period | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Patients were randomly divided into 2 groups; no further details |
| Allocation concealment? | Unclear | No detail. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
No | Not attempted; different modes of treatment. |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | All 151 per group reported on (total of 302). |
| Free of selective reporting? | Yes | Reported as % cured/improved. States that all had improvement or cure |
| Free of other bias? | Unclear | Not stated who recorded outcome. Concluding statement: “the therapeutic effect of moxibustion is superior to that of Chinese drug therapy. It is also simple and easy to be performed, and it an ideal therapy” Unclear about study design: first paragraph states: “In the past several years, the author has cooperated with some gynaecologists from this and other hospitals to treat pregnant vomiting with moxibustion therapy and achieved significant therapeutic effect. It is introduced as follows. General data. 302 and two [sic] patients with pregnancy vomiting were randomly divided into two groups, 151 cases in each group” |
| Methods | Within a series of studies; a double-blind comparative experiment, a controlled double blind study | |
| Participants | 100 ward (not explained) and private patients. | |
| Interventions | Bendectin (10 mg each of Bentyl, Decapryn and Pyridoxine), 2 tablets nightly, an additional tablet in the morning as required Placebo (not described). |
|
| Outcomes | Relief from nausea and vomiting: complete, partial or no relief No description of how or when outcomes were measured. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No description about randomisation. |
| Allocation concealment? | Unclear | No description about allocation concealment; tablets were in envelopes with an identification number |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Neither the physician nor the patient was aware of the identity of the tablet administered |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 1 patient in treatment group received medication for 1 day, otherwise all patients’ outcomes reported in results |
| Free of selective reporting? | Yes | Outcomes reported. |
| Free of other bias? | Unclear | 2 patients were included in both the treatment and control groups as they received medication on 2 separate occasions during the study Not stated how or when outcomes were recorded. |
| Methods | A single blind randomised study. | |
| Participants | 66 pregnant women with mild to moderate nausea and vomiting between 6 and 12 weeks’ gestation, in the outpatient setting | |
| Interventions | The patients in the acupressure group were advised to apply Sea-Bands on P6 point and identical looking tablets were used as placebo in the same regimen as vitamin B6. Those in vitamin B6 group were advised to apply Sea-Bands on the dummy point and 50 mg tablets of vitamin B6 were prescribed every 12 hours for 5 days | |
| Outcomes | Primary outcome: self-recorded nausea and vomiting according to Rhodes Index of Nausea and Vomiting form 2 (8 item, 5-point LIkert-type instrument). Women evaluated their symptoms twice daily for 7 days Secondary outcomes: weight gain and medication use- use of the rescue drug (oral dimenhydrinate 50 mg every 6 hours when required) |
|
| Notes | The authors state that the Rhodes Index was “translated into Thai and tested for validity and reliability by experts” but provide no other details on this | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomisation was done into 2 groups (acupressure and vitamin B6 groups) by an independent remote researcher who had no prior knowledge of the patients by using a block of 4 technique |
| Allocation concealment? | Yes | Sequential sealed envelopes picked by independent, remote researcher |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | “A single blind randomised study.” A possible concern is whether since the study was conducted in Thailand, participants might have known where the P6 point was and therefore, would have known when they were using ‘placebo’ acupressure on the point that was not P6 |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 6 patients did not complete the study (3 in each group). The attrition of the 6 people was explained as follows: “one patient from the withdrawal [sic] group was lost to follow-up”, 1 had irritation from the acupressure band, 2 patients lost their acupressure devices, 2 patients had incomplete forms ITT analysis was performed, counting all withdrawals as treatment failures |
| Free of selective reporting? | Unclear | All outcomes reported but change in Rhodes score only presented graphically; results for weight change and rescue drug use presented fully |
| Free of other bias? | Unclear | The initial Rhodes Index score in the B6 group was higher than acupressure group; stated to be not significant, sample size 33 per group The authors acknowledge that it is possible that the rescue drug provided a large reduction of the symptoms but that it was not possible to exclude it for ethical reasons. They state that the use of the rescue drug did not differ by group (although they also report 0.6+/−1.6 tablets vs 2.8 +/−4.7 tablets P > 0.05 in acupressure and vitamin B6 groups respectively) They also state that the improvement of nausea and vomiting in the present study may be spontaneous due to a placebo effect, the additional medications used, or either of the treatments |
| Methods | Double blind placebo-controlled randomised clinical trial. | |
| Participants | Pregnant women in first trimester attending obstetric visit. 26 women were enrolled with 14 patients in the intervention group and 12 in the placebo group | |
| Interventions | Intervention group: tablespoon of syrup 250 mg ginger, honey and water Placebo: water, honey, lemon oil. 1 tablespoon mixed in 4-8 ounces of cold water 4 times/day. |
|
| Outcomes | Each subject kept a daily diary for first 2 weeks to record the number of syrup drinks ingested and the degree of nausea and vomiting Degree of nausea and vomiting “A numerical scale of 1 through 10 was used to quantify the level of nausea, number of vomiting episodes and the patient’s perspective of her daily functioning related to her symptoms”. No information about the scale Outcomes reported: point improvement on the nausea scale; number of vomiting episodes; maintenance/gain in weight. |
|
| Notes | A statistical analysis was not applied to the results because of the small numbers | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Subjects were randomised to the placebo or the study group by computer-generated numbers matching the numbers on identical-appearing bottles of ginger or placebo syrup |
| Allocation concealment? | Unclear | Nothing stated about allocation concealment. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Described as “double blind”. Participants: 1 woman withdrew - did not like the taste - “no other patient indicated that she could distinguish the taste of ginger” No further information about blinding of study personnel “double-blinded”; outcomes self-reported by participants |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Attrition from the placebo group - 1 woman did not take the study drink as her nausea resolved, 2 women stopped the study on days 7 and 11 because of no improvement and they were prescribed antiemetics In the ginger group, 1 woman stopped the study at day 5 as she could not tolerate the taste of the drink; another woman stopped the study on day 10 when her symptoms resolved Results are reported for days 6, 9, 14, for groups with varying sizes, linked with this attrition |
| Free of selective reporting? | No | Reported 4 point, 2 point improvement in nausea; reported vomiting on day 6 was not pre-specified and seems arbitrary Patient’s perspective on her daily functioning - not reported Weight gain/loss reported in results - not specified as an outcome |
| Free of other bias? | Unclear | The ginger syrup is prepared and sold by New Chapter Inc (Brattleboro, Vt). The company also prepared the placebo syrup and provided both syrups free of charge |
| Methods | Subject- and observer-masked RCT. | |
| Participants | 55 pregnant women, gestation between 6 and 10 weeks. | |
| Interventions | Acupuncture - fully described. Sham with a cocktail stick. 15 minute treatments, twice in the first week and once weekly for 2 weeks, minimum number of treatments was 3 |
|
| Outcomes | Primary outcomes measured using a 100 mm visual analogue scale - marked no nausea to nausea worst imaginable. Completed the scale daily to represent the worst experience of nausea in the previous 24 hours. Also recorded number of times they vomited in past 24 hours; plus adverse effects Hospital Anxiety and Depression Scale as a secondary measure - at baseline and immediately after the last treatment Overall effectiveness - within 2 weeks completion of treatment, using 5-point LIkert-type: much worse (1) to very much better or cured (5) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Participants randomly assigned to 2 groups. Computer-generated random numbers, stratified for severity of nausea, randomisation in blocks of 4. It may be possible to predict randomisation sequence in small blocks |
| Allocation concealment? | Yes | By opening opaque sequentially numbered envelopes containing codes |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Women described as masked to allocation (1 woman in each group thought that they had received sham acupuncture, all others thought they had had acupuncture); acupuncturist knew treatment (not feasible not to); outcome assessors blinded to group allocation |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Acupuncture group: 5 people dropped out for no reason and 1 was admitted for hyperemesis Sham acupuncture group: 1 withdrew consent before treatment; 2 dropped-out for no reason, 1 had a missed abortion and 1 was admitted for hyperemesis. ITT analysis performed |
| Free of selective reporting? | Yes | Number of vomiting episodes not reported; state that women failed to record systematically data on vomiting Median scores reported only (data not normally distributed; failed Mauchly’s test of sphericity). Median rating of 4 (range 3-5) for global effectiveness for both groups, reported by the authors as “indicating an overall level of satisfaction with the treatment”, implying satisfaction with sham treatment also |
| Free of other bias? | Yes | Authors state limitation of availability of acupuncturist, variable times between treatments for some women. Sham procedure might have placebo effects (A-delta fibres stimulated) |
| Methods | Double-blind comparison. Tablets supplied in bottles serially numbered from 1 to 100 and each contained 28 tablets, either the active product or lactose | |
| Participants | Pregnant women who complained of nausea and vomiting in the first trimester; no women admitted to the trial after 20 weeks; 1 woman entered the trial twice with 2 pregnancies. General practice setting. Results reported for 41 women in intervention group, 40 women in control group. It was not stated how many women entered the trial | |
| Interventions | Intervention group: Debenox (a mixture of dicyclomine, doxylamine and pyridoxine) 2 tablets at bedtime each night (dose not stated) for 14 consecutive nights Control group: inert dummy tablets of identical appearance |
|
| Outcomes | Change in grade of nausea or vomiting at the second visit compared to the first (time between visits not specified). Symptoms were graded according to severity between Grade 0 and 4 (no explanation or validation information given, no sources cited) Grade 0: no nausea or vomiting (only applicable on the second visit); Grade 1: slight nausea only which is acknowledged only on questioning; Grade 2: more severe nausea complained of by the patient spontaneously; Grade 3: vomiting once or twice a day; Grade 4: more severe vomiting 3 or more times a day. Side effects were also reported - though these were not mentioned as being measured |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | No | Nothing stated about randomisation. |
| Allocation concealment? | Yes | Implied “A sealed code was available to us in case of emergency but this was not broken throughout the course of the trial, since no untoward reactions occurred” |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | The trial was described as a “double blind” comparison. Appears women, doctors and independent observer collecting data were blinded The control group received “inert dummy tablets of identical appearance”. “The tablets were supplied in bottles serially numbered from 1 to 100 and each contained 28 tablets, either the active product or lactose. A sealed code was available to us in case of emergency but this was not broken throughout the course of the trial, since no untoward reactions occurred.” “The data was [sic] collected by an independent observer with training in medical statistics who assessed the results ‘blind’ and commented impartially on the outcome of the study.” |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
No | No information of number of women who entered the trial so it is not possible to establish of there was any attrition; no missing data information at all |
| Free of selective reporting? | Yes | Reports on pre-specified outcome. |
| Free of other bias? | Unclear | Within the introduction the authors state that Debenox had been in use for many years in their practice and that “the absence of untoward side actions with ‘Debenox’, in particular teratogenesis, has been amply demonstrated by the passage of 12 years” “Thanks are due to Dr J. P. Birkett, Merrell Division, Richardson-Merrell Limited for supplies of inert and active tablets, statistical aid and secretarial help.” |
| Methods | Clinical trial. | |
| Participants | 225 pregnant women in the first and 2nd trimesters of pregnancy | |
| Interventions | Thiethylperazine 30 mg daily. Placebo. |
|
| Outcomes | Therapeutic response: good, fair, poor. No information on time-point of evaluation(s) | |
| Notes | The patients were not told they were taking part in a trial. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Staff reported to be blind to group allocation - not described how |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | The staff of the clinic did not know which of the 2 apparently similar tables the patient would receive Double-blind trial. |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | High attrition: 45 (20% of 225, 19 from treatment group, 26 from placebo group) were not included in the analysis because of failure to return for assessment, transfer to another hospital or failure to take the tablets (breakdown by reason not given). Results about therapeutic response reported for 180 patients (but 8 from treatment group and 8 from placebo group were not classified “because of equivocal evidence, intercurrent illness or abortion”), results for fetal outcome reported for 147 patients |
| Free of selective reporting? | Yes | All outcomes reported. |
| Free of other bias? | Yes | |
| Methods | Randomised controlled trial. | |
| Participants | 97 pregnant women, 6-12 weeks’ gestation, with nausea for at least 1 week before trial entry 139 women responded to the study invitation, 97 women took part (symptoms disappeared, too ill, too late in pregnancy) |
|
| Interventions | Acupressure group: wristbands (with button/knob on the inside) day and night on Neigun point of both arms Placebo group: wristband (with felt patch in stead of button) identical on the outside to acupressure band 4 day run-in, 4 day intervention, 4 day follow up. |
|
| Outcomes | Symptoms of nausea and vomiting recorded daily - 3 recordings What problems they had: no problems, nausea, vomiting. How many hours they had suffered. Every evening an overall evaluation of their symptoms on a Visual Analogue Scale (0-5 no problems to worst thinkable level of nausea and vomiting) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Block randomisation in blocks of 20. |
| Allocation concealment? | Unclear | Not stated. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | A study assistant instructed the women in the use of the wristbands (investigators blinded) |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | All 97 participants reported on in results: 13 women who did not complete all of the daily forms were assigned values equivalent to the last reported value on the outcome variables |
| Free of selective reporting? | Unclear | Unclear about numbers per group in results tables (%s presented) |
| Free of other bias? | Yes | The authors highlight potential selection, information and performance of intervention bias - but these appear to be no greater than for other similar studies |
| Methods | Randomised controlled trial. | |
| Participants | 161 women with symptoms of nausea with or without vomiting and retching during pregnancy. Gestational age for most (78.6%) women was < 12 weeks, maximum gestation 24 weeks (not stated for how many) | |
| Interventions | P6 (Neiguan) acupressure group - band applied for 5 days, removed morning of day 6 Placebo acupressure group (acupressure band inappropriately placed) Control group - no treatment. 7-day study. |
|
| Outcomes | Symptoms of nausea and vomiting, using Rhodes Inventory of Nausea and Vomiting (Form 2), measuring prevalence and amount of distress caused by symptoms over 12 hour period, recorded twice daily from entry to the study to 6 days later | |
| Notes | Gestation up to 23.6 weeks; no raw usable data provided. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Use of a process of block randomisation (size of blocks not specified), computer generated |
| Allocation concealment? | Yes | The blocks of group assignments were computer generated and placed in numbered sealed envelopes before the study began. Participants were given numbers that corresponded with their envelope numbers and this was determined by the order in which they entered the study |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Only participants randomised to the control group were aware of their group assignment |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 12 participants withdrew at various times during the study (5 lost of interest in evaluating their symptoms during the study, 3 for disappointment at being assigned to control group, 2 were hospitalised for severe symptoms and 4 refused to discontinue the intervention at the appropriate time) |
| Free of selective reporting? | Unclear | Results reported graphically only, as mean squares. |
| Free of other bias? | Yes | |
| Methods | Single-blind RCT. | |
| Participants | 70 pregnant women under 20 weeks’ gestational age, without any surgical or medical history, without a history of smoking or drug use, and with mild or moderate nausea with or without vomiting were recruited to the study | |
| Interventions | Treatment group: 1 g ginger daily (as 4, 250 mg ginger capsules, 1 capsule morning, noon, afternoon and night) for 4 days Control group: placebo capsules, similar in appearance to ginger capsules, containing only lactose, for 4 days |
|
| Outcomes | Nausea severity and intensity on 0-10 VAS twice daily; number of vomiting episodes daily; general changes to nausea and vomiting recorded during interview with researcher after 4 days of treatment Adherence to dietary advice was also recorded and assessed by interview after day 4 The incidence of unspecified “complications” was also recorded |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomly assigned, no details given. |
| Allocation concealment? | Unclear | Stated that the experimental group was matched with the control group regarding demographic and obstetrical characteristics. The results section states that matching groups on these characteristics did not reveal any significant differences between the two, so maybe matching relates to comparisons after allocation |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
No | Described as single blinded study. Participants were blinded to the content of the capsules. Researchers not blinded. Researchers were involved in aspects of outcome assessment- no blinding |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 67 women completed the study; 3 from the experimental group failed to complete the after-treatment questionnaire |
| Free of selective reporting? | Unclear | Results reported unclearly; daily scores/results not presented, presented by number of assessments, not participants. Overall percentage improvement by group then reported in the text; unclear if this is based on overall assessment on day 5, or assessment of change in scores |
| Methods | Randomised controlled trial. | |
| Participants | 170 pregnant women less than 16 weeks’ gestation, with symptoms of nausea and vomiting | |
| Interventions | Group A: received 1 ginger capsule (0.5 g ginger powder) twice daily Group B: identical capsule of 50 mg dimenhydrinate twice daily |
|
| Outcomes | Primary outcome: improvement in nausea and vomiting symptoms. Degree of nausea measured using a 10 cm visual analogue scale to grade the severity of nausea over past 24 hours on first visit; on the following 7 days of treatment recordings were made twice daily in the morning and evening Number of episodes of vomiting recorded daily. Secondary outcomes: occurrence of side effects such as drowsiness, heartburn, palpitation and mouth dryness |
|
| Notes | Results difficult to interpret. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomly allocated - no detail. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Identical capsules used; no other details; blinding implied. |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 11% attrition: 8 women from ginger group and 11 women from dimenhydrinate group were lost to follow up (no further details given) |
| Free of selective reporting? | Unclear | Unclear reporting of results; many tests. Explained tests due to high variation in pre-intervention scores |
| Free of other bias? | Yes | |
| Methods | A double-blind placebo-controlled study. | |
| Participants | 78 patients complaining of nausea or vomiting in pregnancy, gestation was over 20 weeks for some participants: 13-24 weeks’ gestation: 8 in treatment group, 4 in placebo. 25-36 weeks: 6 in placebo group. Not specified: 1 in placebo group. |
|
| Interventions | Treatment group: fluphenazine 1 mg (repeat action tablet) plus 50 mg pyridoxine Placebo: identically appearing placebo tablets. |
|
| Outcomes | Intensity of nausea and vomiting graded by women at outset and at the conclusion of 1 week of therapy. 6-point scale (0-6) ranged from no nausea or vomiting (0) to vomiting more than 3 times/day (6). Initial symptoms: 1-2 classified as mild, 3-4 as moderate and 5-6 as severe. Effectiveness was measured by deducting the post-treatment score from the initial score - therapeutic response for each category of initial symptoms is expressed as excellent, good or poor by proper assignment of numerical values (based on their initial score) | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Investigator bias is eliminated by the provision of a numbered series of bottles in which drug and identically appearing placebo tablets are randomly distributed; no details of how randomised |
| Allocation concealment? | Unclear | Not described - patients were given 1 of successively numbered bottles in the series |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Blinding implied - identities of the coded tablets revealed after outcome data collected |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | All participants included in reported results. |
| Free of selective reporting? | Yes | Outcomes reported according to classification. |
| Free of other bias? | Yes | Later gestation of some participants in placebo group. |
| Methods | RCT. | |
| Participants | 98 pregnant women with symptoms of nausea and vomiting, of not more than 14 weeks’ gestation were recruited; exclusion criteria: women with molar pregnancy, multifetal pregnancy, blighted ovum, hyperemesis gravidarum, or current use of anti-emetic medication | |
| Interventions | Treatment group: auricular acupuncture, using round magnetic balls as ear pellets. These were placed with adhesive tape at the auricles of both ears (on auricular point at inner surface of auricle at the concha ridge zone, according to the meridians of Traditional Chinese Medicine). Women in this group were instructed to start pressing the magnets for 30 seconds 4 times a day (before meals and at bedtime), starting on the third day until the 6th day. First 2 days used as control days Control group: no treatment, except oral anti-emetic drugs (as below) Both groups were allowed to take 1 tablet of 50mg dimenhydrinate every 6 hours as required if they could not tolerate their symptoms; remaining tablets were counted at end of 1 week of the study |
|
| Outcomes | Frequency, duration and distress of nausea and vomiting and retching symptoms was measured using the Rhodes Index (range 0-32, 8 5-point self-report items); completed every morning for 6 days. Scores from day 4-6 used to measure treatment effect | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Patients were randomised using a random table of numbers. |
| Allocation concealment? | Unclear | No details stated. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
No | No blinding. Did not use placebo on ear due to stated limited area on the ear and possibility of affecting the treatment point; self-reporting of outcomes |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 91 patients completed the study. 7 patients lost to follow up, 4 in the treatment group, 3 in the control group. No explanation given |
| Free of selective reporting? | Yes | All outcomes reported. Stated in results section that no one in treatment group experienced any adverse effect from acupressure and satisfaction with treatment is also reported as (% of treatment group satisfied). Adverse effects and satisfaction not stated as outcomes to be measured |
| Free of other bias? | Unclear | Differences between groups on education, income and occupation within baseline characteristics reported (women in control group were more educated, higher income and a higher percentage were housewives, in the occupational category) |
| Methods | RCT. | |
| Participants | 230 pregnant women with symptoms of mild to severe nausea and vomiting between 6 and 12 weeks’ gestation | |
| Interventions | Nerve stimulation therapy at the P6 acupuncture point, via a wristband Placebo: identical but non-stimulating device. |
|
| Outcomes | Primary outcomes - assessment of nausea and vomiting, self-recorded symptoms according to the Rhodes Index of Nausea, Vomiting and Retching; data collected on 12 days of the 21 day study, days 1-7, 9, 11, 13, 17 and 21 Secondary outcomes - weight gain or loss, change in urinary ketones and specific gravity and medication use |
|
| Notes | Some results (changes in scores over time) in graphical form only Includes participants with mild to severe symptoms; does not present result separately for each group. Miller 2001 presents results for participants with severe symptoms only (73 of the 193 total). De Veciana 2001 [abstract] reports mild/moderate vs severe- not reported in Rosen 2003 [main/only full paper]. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated list. |
| Allocation concealment? | Yes | Sequentially numbered opaque, sealed envelopes. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Participants were blinded (to preserve blinding, patients were told that they may or may not feel a tingling sensation), study personnel and those assessing outcomes were not blinded. Because of the subjects’ different responses to active and sham devices, it was not possible to blind study personnel. Unlikely to introduce bias, as self-reporting |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Unclear | 187 women completed the trial; high attrition - 43 patients did not complete the study (18.6%), 22 in treatment group and 21 in the control group. Patients who withdrew were more likely to be multiparous and to have ketonuria. 3 patients from each group withdrew due to adverse events, only 1 attributable to the device. Patients were excluded if they completed fewer than 9 form sets (from total of 23). 4 patients in the treatment group and 1 patient in the control group were “non-compliant” |
| Free of selective reporting? | Yes | Results not reported by subgroup (based on severity) in the full paper available for this study |
| Free of other bias? | Yes | |
| Methods | Randomised, double-blind placebo-controlled study. | |
| Participants | 74 pregnant women consented to participate; 59 women completed the protocol | |
| Interventions | Treatment group: vitamin B6 - 25 mg tablets every 8 hours for 72 hours Placebo: identical appearing tablets to be taken using the same regimen |
|
| Outcomes | Severity of nausea: marked on 10 cm unmarked Visual Analogue Scale: 0 as no nausea and 10 as worst possible nausea; recorded by women 4 times daily (am, noon, pm, bedtime) for the 3 days of treatment Number of episodes of emesis per 24 hours recorded daily for 3 days |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised by a table of numbers. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | All individuals involved in the study except for the pharmacist were blinded to the nature of the medication It is not clear at what stage group allocation became known. |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Unclear | 20.2% drop-out rate high, not clear which group attrition was in |
| Free of selective reporting? | Unclear | After data collection but before data analysis, the authors say that they arbitrarily divided the patients into 2 subgroups according to the severity of their nausea - patients with a nausea score of greater than 7 were in the severe nausea group and those with scores less than or equal to 7 were categorised in the mild to moderate subgroup and these 2 groups were then compared. As the results showed that there was a significant improvement in the severe nausea subgroup who received the intervention, bias in the arbitrary post hoc cut-off for severity subgroup bias cannot be ruled out Unclear reporting - average of averages, mean change from baseline (standard error of the difference in the means) etc |
| Free of other bias? | Yes | |
| Methods | RCT. | |
| Participants | 593 women less than 14 weeks’ gestation with symptoms of nausea or vomiting | |
| Interventions | Traditional acupuncture group (traditional diagnosis and then acupuncture to selected points) P6 acupuncture group (Pericardium point on wrist only). Sham acupuncture group (to points near true points). No acupuncture (control) group (general advice and phoned and asked about their wellbeing). The authors state that to reduce disappointment when women were allocated to the control group, a standardised information sheet was made available about advice on diet, lifestyle and the use of vitamin B6 during the 4-week study period. Not stated if all women got this advice (including about vitamin B6) Treatment was administered weekly for 4 weeks from all 3 acupuncture groups. Very detailed descriptions given |
|
| Outcomes | Primary outcomes: nausea, vomiting, dry retching at days 7, 14, 21 and 26 (measured by the Rhodes Index of Nausea and Vomiting Form 2) and health status on days 1, 14 and 28 (measured by MOS 36 Short Form Health Survey (SF36)) Pregnancy outcomes: perinatal outcome, congenital abnormalities, pregnancy complications and infant outcomes |
|
| Notes | Related 2 articles report pregnancy outcomes and placebo response and effect of time and related abstract reports women’s experiences of nausea (data collected prior to randomisation from 253 women) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomisation sequence in variable balanced blocks. |
| Allocation concealment? | Yes | Centralised, external telephone randomisation service. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Women in acupuncture and sham acupuncture groups were blinded - asked to guess which group they were in (84% guessed correctly that they were in Traditional Acupuncture group, 77% P6 Acupuncture and 41% Sham Acupuncture) (reported unclearly in Table 4 in Smith & Crowther 2002). They were asked why they guessed what they did and this was also related by the study investigators to their beliefs about acupuncture. Authors acknowledge that the trial was advertised in the media, which sensationalised the trial, for example, under the heading “morning sickness cure” |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 24% attrition by week 4; number of forms (nausea and vomiting and SF36) not completed and number of pregnancy losses per group stated described in detail |
| Free of selective reporting? | Unclear | Many results reported (mean differences not reported). |
| Free of other bias? | Unclear | Vitamin B6 advice given to control group not clear whether to others also |
| Methods | Randomised controlled equivalence trial. | |
| Participants | 291 women with nausea or vomiting, less than 16 weeks’ pregnant | |
| Interventions | Ginger 1.05 g daily (1 capsule of ginger 350 mg 3 times a day) Vitamin B6 daily (1 capsule vitamin B6 25 mg 3 times a day). Treatment was for 3 weeks to test whether ginger and vitamin B6 were equivalent in treating symptoms |
|
| Outcomes | Equivalence and examined any change in nausea and vomiting scores, measured at days 7, 14 and 21, measured using the Rhodes Index of Nausea and Vomiting Form 2. They recorded baseline for 3 days before randomisation Health status measured using the MOS 36 Short Form Health Survey, recorded at baseline and day 21 Secondary outcomes: occurrence of any side effects and adverse pregnancy outcomes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Women were randomly assigned by logging on to the service at a Trials Unit; the computer-generated randomisation schedule used balanced variable blocks and was prepared by a researcher not involved in the trial |
| Allocation concealment? | Yes | Centralised system as above, capsules contained in opaque envelopes |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Identical capsules. Data entry personnel blinded. Data available on blinding from women - 40% unsure about which group they belonged to (76% from ginger group and 65% of B6 group correctly guessed which group they were in) |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Loss to follow up in ginger group (n = 146) was 26 and vitamin B6 group (n = 145) was 30 |
| Free of selective reporting? | Yes | Does not appear to be any. |
| Free of other bias? | Yes | |
| Methods | Double-blind RCT. | |
| Participants | 138 women with nausea and vomiting of pregnancy at or before 16 weeks’ gestation | |
| Interventions | Ginger 500 mg orally (1 capsule) 3 times daily for 3 days. Vitamin B6 10 mg daily (1 capsule), identical to ginger capsule 3 times daily for 3 days (used as a positive control for ethical reasons) |
|
| Outcomes | Primary outcomes: improvement in nausea symptoms, measured using 10 cm Visual Analogue Scale (0 as no nausea to 10 as nausea as bad as it could be) Number of vomiting episodes also recorded. Other secondary outcomes: occurrence of side effects such as drowsiness, palpitations, heartburn and mouth dryness |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | A pharmacist not responsible for patient care used a table of random numbers to prepare the treatment assignment by randomisation with a block of 4 to receive ginger or vitamin B6 |
| Allocation concealment? | Yes | The treatment code was concealed by placing the patient’s assignment in sequence in sealed opaque envelopes that were drawn in ascending consecutive order |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Codes were kept concealed until the end of the study. |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 4 cases in the ginger and 6 cases in the vitamin B6 group did not return for follow up; 64 evaluable in each group |
| Free of selective reporting? | Yes | No selective reporting apparent. |
| Free of other bias? | Yes | |
| Methods | Randomised double-blind placebo-controlled trial. | |
| Participants | 342 pregnant women at </= 17 weeks’ gestation. | |
| Interventions | Oral pyridoxine (vitamin B6) received 20 10 mg tablets to be taken every 8 hours (6-8 am, 2-4 pm, 10 pm-12 md) for 5 days Placebo: identical-looking tablets to be taken in the same regime |
|
| Outcomes | Primary outcome: change in the secondary outcome severity of nausea; measured in a Visual Analogue Scale in centimetres (10 cm 0 as no nausea to 10 as nausea as bad as it could be). Average daily nausea scores calculated and then mean nausea score over 5 days Secondary outcome: change in the number of vomiting episodes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Patients were then randomised into 2 groups by a table of random numbers |
| Allocation concealment? | Yes | A list that revealed drug codes was kept by the research assistant and was not accessible to the physicians |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Pyridoxine and placebo tablets were prepared by the hospital pharmacy and packed similarly in envelopes in an envelope containing 20 tablets each. Neither the patients nor the physicians knew the identity of the drugs administered. A list that revealed drug codes was kept by the research assistant and was not accessible to the physicians |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | All included in analysis except 6 who did not return for follow up (2 in placebo group, 4 in treatment group) |
| Free of selective reporting? | Yes | All outcomes reported. |
| Free of other bias? | Yes | No other sources of bias apparent. |
| Methods | Randomised double-masked placebo-controlled trial. | |
| Participants | 70 women with nausea and vomiting of pregnancy before 17 weeks’ gestation (88 eligible for inclusion, 70 agreed to participate) | |
| Interventions | Ginger 1 g daily orally (250 mg capsules 4 times per day, 3 after meals and 1 before bed); capsules prepared from ginger roots (preparation described) Placebo - identical capsules. Treatment was for 1 week. |
|
| Outcomes | Primary outcome: improvement in nausea symptoms. Severity of nausea recorded a 10 cm visual analogue scale 0 as no nausea, 10 as nausea as bad as could be; twice daily (noon and evening) for 4 days. Average daily scores and mean score over 4 days calculated Number of vomiting episodes daily recorded. At follow up 1 week after treatment overall severity was measured using a 5-point Likert scale (much worse, worse, same, better, much better) Occurrence of side effects and adverse effects on pregnancy outcomes also recorded: such as abortion, preterm birth, congenital anomaly, perinatal death and mode of delivery |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | A research nurse who was not responsible for patient care used a table of random numbers to prepare the treatment assignment |
| Allocation concealment? | Yes | The treatment codes were kept in sequence in a sealed black envelope that could not be read through. As each subject entered the trial, she received the next envelope in the sequence which determined her assignment |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Neither the patients nor the physicians knew the identity of the drugs administered |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Low number of women lost to follow up (3, all in placebo group, included in the analysis, assuming symptom relief equal to the best improvement in the placebo group) |
| Free of selective reporting? | Yes | All outcomes reported. |
| Free of other bias? | Yes | No other sources of bias identified. |
| Methods | Randomised placebo-controlled pilot study. | |
| Participants | Pregnant women with normal pregnancy and nausea and vomiting of pregnancy Results presented for 60 women - 80 envelopes had been distributed by the time 20 women in each group, totally 60 women or 75% had returned the envelopes (12 of the 20 explained, 8 missing, unknown group) No clear gestational criteria set, 1 woman after 6 weeks’, 1 after 16 weeks’ gestation and most (n = 34) entered the study after 9-11 weeks’ gestation; there was a statistically significant difference in mean gestational age by group (control group highest) |
|
| Interventions | Acupressure at the P6 (Neigun) point, using wristbands with a button; worn daily for 2 weeks, only removing it when showering Acupressure at a placebo point, wristband with a button, applied at upper side of wrist; worn daily for 2 weeks Control group - no acupressure. |
|
| Outcomes | 100 mm visual analogue scale with anchors at each end to indicate the extremes of the sensation under study (no nausea to extreme nausea). Recorded before treatment, on day 1, after 3 days, after 6 days and after 14 days Incidence of vomiting also reported - not described as an outcome of interest |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Only description: women drew an envelope from a box, envelopes had the same appearance but different contents |
| Allocation concealment? | Unclear | Only description: women drew an envelope from a box, envelopes had the same appearance but different contents |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Women did not open the envelope until they got home; blinding possible only if in 1 of 2 acupressure (P6 or placebo) groups; this then presumes no prior knowledge of acupressure (not stated) |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Unclear | Study stopped when 20 completed questionnaires per group were received - states about 80 envelopes were given out - not clear how many per group were given out. From the 20 not completed, 12 are explained: 6 questionnaires from the P6 and placebo groups were excluded due to incompleteness, 4 women found the wristband too tight to use and 2 women had miscarriages. 8 women did not respond and it was not possible to identify which group they belonged to (implies did not know how many in each group were given out) |
| Free of selective reporting? | Yes | All outcomes reported. |
| Free of other bias? | Yes | |
| Methods | Double-blind randomised placebo-controlled trial. | |
| Participants | 120 pregnant women less than 20 weeks’ gestation who had experienced morning sickness for at least 1 week with no relief through dietary changes | |
| Interventions | 125 mg ginger extract (EV.EXT35, equivalent to 1.5 g dried ginger) Placebo, containing soya bean oil in identical wax-sealed capsules 4 times/day (8 am, 12 noon, 4 pm, 8 pm) 4 days. |
|
| Outcomes | Primary outcomes: nausea experience. Secondary outcomes: other 8 scores. Nausea, vomiting, retching as measured by the Rhodes Index of Nausea, Vomiting and Retching (RINVR) (8-item 5-point Likert-type tool, measuring frequent, duration and distress caused by symptoms) Recorded 1 hour after capsule was taken, for baseline day and 4 days of treatment Side effects and adverse events also reported. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | The random allocation sequence was generated by Eurovita Pty Ltd Denmark using random blocks of 6 and was placed in sealed envelopes and posted to the researchers |
| Allocation concealment? | Yes | Sealed envelopes. |
| Blinding? Change in grade of nausea or vomiting at second visit compared to first |
Yes | Participants, those administering the treatment and those assessing the outcomes were all blinded to the group assignment |
| Incomplete outcome data addressed? Change in grade of nausea or vomiting at second visit compared to first |
Yes | 21 women excluded from final analysis due to insufficient data: 12 for adverse events (details given) and 9 for non-compliance |
| Free of selective reporting? | Unclear | Mostly report the primary outcome (nausea experience), little reported on vomiting and retching Results displayed in graphs only, no raw (usable) data. |
| Free of other bias? | Unclear | Eurovita funded the study, generated the allocation sequence and manufactured the ginger extract 9EV.EXT35) Stated in Discussion that treatment continued for ginger group for 8 days and placebo took ginger for 4 days and all were given 2 weeks’ supply following the end of the trial. Only the data for 4 days was analysed, hence the findings of the follow-up assessment (for the 81 women who completed the main study) should be viewed with caution. No direct attempt can be made to infer cause or association between the findings and the use of ginger over the 8-day period of the principal study |
IM: intramuscular
ITT: intention to treat
RCT: randomised controlled trial
VAS: Visual Analogue Scale
vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Baum 1963 | Quasi-randomised, alternate allocation of patients to groups |
| Bayreuther 1994 | Crossover design. |
| Can Gurkan 2008 | Planned as a randomised study, but not carried out as planned (patients on each day placed in same group) |
| Cartwright 1951 | Crossover design. |
| Conklin 1958 | Not randomised, patients “arbitrarily allocated” to groups. |
| De Aloysio 1992 | Crossover design. |
| Diggory 1962 | Quasi-randomised “each patient in sequence was allocated”; control group reallocated if not improving |
| Dundee 1988 | Not an RCT; women allocated to groups by day of the week; non-responders replaced in treatment group |
| Evans 1993 | Crossover design. |
| Ferruti 1982 | This is a study of hypocorticalism in pregnancy. |
| Fitzgerald 1955 | Not an RCT; alternate allocation of patients. |
| Heazell 2006 | Severe symptoms, in-patient; hyperemesis gravidarum implied (severe symptoms plus ketonuria) |
| Higgins 2009 | Hyperemesis gravidarum (day care versus in-patient management; trial registry record only, no data) |
| Hyde 1989 | Crossover design. |
| Kadan 2009 | Hyperemesis gravidarum as specified condition of participants (RCT, with crossover if first drug allocated not effective: thiamine 100mg IV or promethazine 25mg IV; started February 2009, trial registry record only, ongoing at May 2010) |
| King 1955 | Type of crossover design. |
| Koren 2006 | Pre-emptive treatment; not treating symptoms. |
| Lask 1953 | Not an RCT. |
| Luz 1987 | No data available; this is a communication of a planned trial - a search identified no further publications from this study |
| Steele 2001 | Quasi-experimental design posttest-only and post-test repeated measure |
| Wheatley 1977 | Crossover design. |
| Winters 1961 | Quasi randomised trial - “test material and placebo were strictly alternated” |
IV: intravenous
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective randomised trial. |
| Participants | 110 pregnant women with gestation of 8 to 14 weeks. |
| Interventions | Solumedrol dose pack (tapered) versus phenergan. |
| Outcomes | Weight, number of episodes of emesis per day, pregnancy outcome |
| Notes | The authors report that the solumedrol group had significantly fewer emesis episodes that the phenergan group on days 3, 7, 14 Abstract only; no full text reference/article retrieved; unable to contact authors. Insufficient detail to include this study |
| Methods | A single-masked randomised controlled trial. |
| Participants | 78 pregnant women with gestation of 6 to 16 weeks (63 completed the study) |
| Interventions | Ginger extract (dose not stated) versus doxylamine succinate plus pyridoxine |
| Outcomes | Severity and frequency of nausea; subjective feeling of well-being; type of delivery; neonatal complications and abnormality |
| Notes | The authors state that there was no statistical difference between groups on outcomes measured, though sample size was inadequate. They state that the tolerability and compliance of ginger were more satisfactory with no adverse event in mother or fetus, but they do not give any further details about this Abstract only - no full text reference/article retrieved; unable to contact authors. Insufficient detail to include this study |
| Methods | Prospective double-blinded randomised controlled trial. |
| Participants | 77 pregnant women attending the ED. |
| Interventions | P6 Acupressure versus sham acupressure, via a wristband. |
| Outcomes | Nausea severity (using the McGill Nausea Questionnaire), measured at baseline, 30 and 60 minutes Subsequent anti-emetic administration, length of ED stay. |
| Notes | The authors report that no differences between groups were reported at any time point Abstract only - no full text reference/article retrieved; unable to contact authors. Insufficient detail to include this study |
| Methods | Prospective randomised trial. |
| Participants | 38 pregnant women in first trimester presenting with severe pregnancy vomiting (not stated if hyperemesis gravidarum); title of abstract states “early pregnancy nausea and vomiting” |
| Interventions | Acupressure via sea-band device on both wrists versus control (counselled and dietary advice) |
| Outcomes | Anti-emetic drug use, hospitalisation. |
| Notes | The authors only report higher levels of anti-emetic medication usage for the control group (37%) than the acupressure group (11%) and that there was no significant difference in hospitalisation. However they do not state the denominators for the groups Abstract only - no full text reference/article retrieved; unable to contact authors. Insufficient detail to include this study |
ED: emergency department
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The efficacy of Diclectin for nausea and vomiting in pregnancy |
| Methods | Double-blind, multicenter, randomised, placebo-controlled trial |
| Participants | Pregnant females, 18 years and older, gestation of pregnancy 7-14 weeks at first dose, suffering from NVP Pregnancy Unique Quantification of Emesis (PUQE) score >/= 6 |
| Interventions | Treatment group: Diclectin (Doxylamine succinate 10 mg/pyridoxine hydrochloride 10 mg Placebo. |
| Outcomes | Symptoms of nausea and vomiting will be measured daily using a standardised (unspecified) questionnaire |
| Starting date | January 2008 (planned end date August 2009); no results available at May 2010 |
| Contact information | Hoang Nguyen nvp.Pregnancy@premier-research.com |
| Notes |
| Trial name or title | Vitamin B6 concentration and treatment in nausea and vomiting of pregnancy |
| Methods | Randomised, double-blind controlled study. |
| Participants | Females with nausea and vomiting of pregnancy, aged 15-40 years; other medical conditions causing nausea and vomiting of pregnancy outruled. Gestation of pregnancy not specified |
| Interventions | High does of Vitamin B6 versus low dose of vitamin B6 (doses not stated) |
| Outcomes | Primary outcomes: nausea and vomiting score (2 week timeframe) Secondary outcome: vitamin B6 concentration in nausea and vomiting of pregnancy (2 week timeframe) |
| Starting date | January 2008 (estimated study completion date: October 2008); no results available at May 2010 |
| Contact information | Noroyono Wibowo MD PhD, Indonesia, wibowonoroyono@yahoo.com |
| Notes |
ED: emergency department
DATA AND ANALYSES
Comparison 1. P6 Acupressure versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No improvement in intensity of symptoms (while using wristbands) reported | 1 | 97 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.44, 1.39] |
| 2 Mean nausea score after day 3 using VAS | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [−1.49, 1.69] |
| 3 Mean nausea score days 1-3 (average) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [−0.80, 1.58] |
| 4 Mean emesis scores days 1-3 (average) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [−1.06, 1.58] |
| 5 Mean total scores (Rhodes Index) days 1-3 (average) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [−1.52, 3.86] |
Comparison 2. P6 Acupressure versus vitamin B6.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea scores on day 3 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−2.24, 2.64] |
| 2 Poor symptom relief/amount of rescue medication (number of tablets) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | −2.20 [−3.98, −0.42] |
| 3 Satisfaction rating of intervention by participants | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [−0.04, 0.84] |
| 4 Weight gain from entry date to end of the trial (kg) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [0.24, 1.16] |
Comparison 3. Auricular acupressure versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea/vomiting score (combined Rhodes Index score) on day 6 (3 days after treatment started) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | −3.60 [−6.62, −0.58] |
| 2 Number of anti-emetic drugs used on day 6 (3 days after treatment started) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−0.37, 0.17] |
Comparison 4. Acustimulation therapy at P6 point versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (in lbs) over 3 week period | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | 1.7 [0.23, 3.17] |
| 2 Dehydration: occurrences reported | 1 | 187 | Risk Ratio (M-H, Fixed, 95% CI) | 0.24 [0.07, 0.83] |
| 3 Ketonuria at the end of the trial | 1 | 187 | Risk Ratio (M-H, Fixed, 95% CI) | 0.48 [0.15, 1.55] |
Comparison 5. Traditional acupuncture versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean nausea score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.70 [−1.36, −0.04] |
| 2 Mean dry retching score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.20 [−0.57, 0.17] |
| 3 Mean vomiting score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−0.58, 0.38] |
Comparison 6. P6 Acupuncture versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean nausea score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [1.00, 0.40] |
| 2 Mean dry retching score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [−0.30, 0.50] |
| 3 Mean vomiting score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−0.78, 0.18] |
Comparison 7. Traditional acupuncture versus P6 acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean nausea score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.40 [−1.12, 0.32] |
| 2 Mean dry retching score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−0.65, 0.05] |
| 3 Mean vomiting score on day 7 | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−0.26, 0.66] |
Comparison 8. Ginger versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in nausea (mean change score) over 4 days of treatment: women available to follow up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.22, 2.18] |
| 2 Improvement in nausea (mean change score) over 4 days of treatment: ITT analysis | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [−0.51, 1.71] |
| 3 Little improvement in nausea | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 0.29 [0.10, 0.82] |
| 4 Number of women continuing vomiting at day 6 | 1 | 22 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 [0.18, 0.98] |
| 5 Spontaneous abortion | 1 | 67 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.04, 3.33] |
| 6 Caesarean delivery | 1 | 67 | Risk Ratio (M-H, Fixed, 95% CI) | 1.64 [0.51, 5.29] |
| 7 Improvement in nausea intensity after treatment (day 5) | 1 | 67 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [1.07, 2.04] |
Comparison 9. Ginger versus vitamin B6.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea vomiting score day 3 | 2 | 251 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.00 [−0.25, 0.25] |
| 1.1 Rhodes Index | 1 | 123 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.09 [−0.44, 0.27] |
| 1.2 10 cm VAS | 1 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [−0.27, 0.43] |
| 2 Post-treatment number of vomiting episodes: day 3 | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3 No improvement in symptoms | 2 | 360 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.47, 1.52] |
| 4 Spontaneous abortion | 2 | 360 | Risk Ratio (M-H, Fixed, 95% CI) | 0.49 [0.17, 1.42] |
| 5 Stillbirth | 1 | 291 | Risk Ratio (M-H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 6 Congenital abnormality | 1 | 291 | Risk Ratio (M-H, Fixed, 95% CI) | 0.50 [0.13, 1.95] |
| 7 Antepartum haemorrhage/abruption, placenta praevia | 1 | 291 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.29, 3.36] |
| 8 Pregnancy-induced hypertension | 1 | 291 | Risk Ratio (M-H, Fixed, 95% CI) | 1.24 [0.34, 4.53] |
| 9 Pre-eclampisa | 1 | 291 | Risk Ratio (M-H, Fixed, 95% CI) | 1.49 [0.43, 5.17] |
| 10 Preterm birth | 1 | 291 | Risk Ratio (M-H, Fixed, 95% CI) | 1.66 [0.40, 6.80] |
| 11 Arrhythmia | 1 | 123 | Risk Ratio (M-H, Fixed, 95% CI) | 3.05 [0.13, 73.40] |
| 12 Headache | 1 | 123 | Risk Ratio (M-H, Fixed, 95% CI) | 0.20 [0.01, 4.15] |
| 13 Heartburn | 2 | 251 | Risk Ratio (M-H, Fixed, 95% CI) | 2.35 [0.93, 5.93] |
| 14 Sedation or drowsiness | 2 | 251 | Risk Ratio (M-H, Fixed, 95% CI) | 0.75 [0.48, 1.19] |
| 15 Caesarean delivery | 1 | 69 | Odds Ratio (M-H, Fixed, 95% CI) | 0.60 [0.15, 2.36] |
Comparison 10. Ginger versus dimenhydrinate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Drowsiness | 1 | 170 | Risk Ratio (M-H, Fixed, 95% CI) | 0.08 [0.03, 0.18] |
| 2 Heartburn | 1 | 170 | Risk Ratio (M-H, Fixed, 95% CI) | 1.44 [0.65, 3.20] |
Comparison 11. Vitamin B6 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean reduction in nausea score after 3 days | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [0.40, 1.44] |
| 2 Number of patients with emesis post-therapy | 2 | 392 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.35, 1.66] |
Comparison 12. Hydroxyzine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No relief from nausea | 1 | 150 | Risk Ratio (M-H, Fixed, 95% CI) | 0.23 [0.15, 0.36] |
| 2 Spontaneous abortion (1st or 2nd trimester) | 1 | 115 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.17, 4.75] |
| 3 Perinatal mortality | 1 | 115 | Risk Ratio (M-H, Fixed, 95% CI) | 1.39 [0.06, 33.26] |
Comparison 13. Debendox (Bendectin) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No improvement of symptoms | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only |
Comparison 14. Thiethylperazine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor relief from symptoms | 1 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 0.49 [0.31, 0.78] |
Comparison 15. Fluphenazine-pyridoxine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor response to treatment | 1 | 78 | Risk Ratio (M-H, Fixed, 95% CI) | 0.52 [0.27, 1.01] |
HISTORY
Protocol first published: Issue 1, 2009
Review first published: Issue 9, 2010
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We did not include a Summary of Findings table as we had planned, as there were insufficient comparisons that included multiple studies.
Footnotes
DECLARATIONS OF INTEREST: Anne Matthews and Mary Doyle are qualified reflexologists. They are both also qualified midwives.
References to studies included in this review
- Belluomini 1994.Belluomini J, Litt RC, Lee KA, Katz M. Acupressure for nausea and vomiting of pregnancy: a randomized, blinded study. Obstetrics & Gynecology. 1994;84:245–8. [PubMed] [Google Scholar]
- Bsat 2003.Bsat F, Bayer-Zwirello L, Seubert D, Hoffman D. Randomized study of three common outpatient treatments for nausea and vomiting of pregnancy [abstract] American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S181. [Google Scholar]; * Bsat FA, Hoffman DE, Seubert DE. Comparison of three outpatient regimens in the management of nausea and vomiting in pregnancy. Journal of Perinatology. 2003;23:531–5. doi: 10.1038/sj.jp.7210986. [DOI] [PubMed] [Google Scholar]
- Chittumma 2007.Chittumma P, Kaewkiattikun K, Wiriyasiriwach B. Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: a randomized double-blind controlled trial. Journal of the Medical Association of Thailand. 2007;90(1):15–20. [PubMed] [Google Scholar]
- Ensiyeh 2009.Ensiyeh J, Sakineh MAC. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomised controlled trial. Midwifery. 2009;25(6):649–53. doi: 10.1016/j.midw.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Erez 1971.Erez S, Schifrin BS, Dirim O. Double-blind evaluation of hydroxyzine as an antiemetic in pregnancy. Journal of Reproductive Medicine. 1971;7:57–9. [PubMed] [Google Scholar]
- Fan 1995.Fan Y. Observation on the therapeutic effect of moxibustion for treatment of pregnant vomiting. World Journal of Acupuncture and Moxibustion. 1995;5(4):31–3. [Google Scholar]
- Geiger 1959.Geiger CJ, Fahrenbach DM, Healey FJ. Bendectin in the treatment of nausea and vomiting in pregnancy. Obstetrics & Gynecology. 1959;14:688–90. [PubMed] [Google Scholar]
- Jamigorn 2007.Jamigorn M, Phupong V. Acupressure and vitamin B6 to relieve nausea and vomiting in pregnancy: a randomized study. Archives of Gynecology and Obstetrics. 2007;276(3):245–9. doi: 10.1007/s00404-007-0336-2. [DOI] [PubMed] [Google Scholar]
- Keating 2002.Keating A, Chez RA. Ginger syrup as an antiemetic in early pregnancy. Alternative Therapies in Health & Medicine. 2002;8(5):89–91. [PubMed] [Google Scholar]
- Knight 2001.Knight B, Mudge C, Openshaw S, White A, Hart A. Effect of acupuncture on nausea of pregnancy: a randomized, controlled trial. Obstetrics & Gynecology. 2001;97:184–8. doi: 10.1016/s0029-7844(00)01152-2. [DOI] [PubMed] [Google Scholar]
- McGuiness 1971.McGuiness BW, Taylor Binns D. ‘Debendox’ in pregnancy sickness. Journal of the Royal College of General Practitioners. 1971;21:500–3. [PMC free article] [PubMed] [Google Scholar]
- Newlinds 1964.Newlinds JS. Nausea and vomiting in pregnancy: a trial of thiethylperazine. Medical Journal of Australia. 1964;51:234–6. [PubMed] [Google Scholar]
- Norheim 2001.Norheim AJ, Pedersen EJ, Fonnebo V, Berge L. Acupressure against morning sickness [Akupressur mot svangerskapskvalme.] Tidsskrift for Den Norske Laegeforening. 2001;121(23):2712–5. [PubMed] [Google Scholar]; * Norheim AJ, Pedersen EJ, Fonnebo V, Berge L. Acupressure treatment of morning sickness in pregnancy A randomised, double-blind, placebo-controlled study. Scandinavian Journal of Primary Health Care. 2001;19(1):43–7. doi: 10.1080/028134301300034666. [DOI] [PubMed] [Google Scholar]
- O’Brien 1996.O’Brien B, Relyea MJ, Taerum T. Efficacy of P6 acupressure in the treatment of nausea and vomiting during pregnancy. American Journal of Obstetrics and Gynecology. 1996;174:708–15. doi: 10.1016/s0002-9378(96)70454-4. [DOI] [PubMed] [Google Scholar]
- Ozgoli 2009.Ozgoli G, Goli M, Simbar M. Effects of ginger capsules on pregnancy, nausea, and vomiting. Journal of Alternative and Complementary Medicine. 2009;15(3):243–6. doi: 10.1089/acm.2008.0406. [DOI] [PubMed] [Google Scholar]
- Pongrojpaw 2007*.Pongrojpaw D. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea vomiting in pregnancy. American Journal of Obstetrics and Gynecology. 2006;195(6 Suppl 1):S92. [Google Scholar]; Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. Journal of the Medical Association of Thailand. 2007;90(9):1703–9. [PubMed] [Google Scholar]
- Price 1964.Price JJ, Barry MC. A double blind study of fluphenazine with pyridoxine. Pennsylvania Medical Journal. 1964;67:37–40. [PubMed] [Google Scholar]
- Puangsricharern 2008.Puangsricharern A, Mahasukhon S. Effectiveness of auricular acupressure in the treatment of nausea and vomiting in early pregnancy. Journal of the Medical Association of Thailand. 2008;91(11):1633–8. [PubMed] [Google Scholar]
- Rosen 2003.De Veciana M, Stewart L, Miller H, Slotnick R, Rebarber A, Rosen T. Multicenter randomized controlled trial of nerve stimulation therapy for the relief of nausea and vomiting in pregnancy [abstract] American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S182. doi: 10.1016/s0029-7844(03)00375-2. [DOI] [PubMed] [Google Scholar]; Miller H, De Veciana M, Stewart L, Rebarber A, Rosen T, Slotnick R. A multicenter randomized controlled trial of nerve stimulation therapy: subanalysis of severe nausea and vomiting symptoms [abstract] American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S188. doi: 10.1016/s0029-7844(03)00375-2. [DOI] [PubMed] [Google Scholar]; * Rosen T, De Veciana M, Miller HS, Stewart L, Rebarber A, Slotnick RN. A randomized controlled trial of nerve stimulation for relief of nausea and vomiting in pregnancy. Obstetrics & Gynecology. 2003;102(1):129–35. doi: 10.1016/s0029-7844(03)00375-2. [DOI] [PubMed] [Google Scholar]
- Sahakian 1991*.Sahakian V, Rouse D, Sipes S, Rose N, Niebyl J. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: a randomized, double-blind placebo-controlled study. Obstetrics & Gynecology. 1991;78:33–6. [PubMed] [Google Scholar]; Sahakian V, Rouse DJ, Rose NB, Niebyl JR. Vitamin B6 for nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology. 1991;164:322. [PubMed] [Google Scholar]
- Smith 2002.Smith C, Crowther C. The placebo response and effect of time in a trial of acupuncture to treat nausea and vomiting in early pregnancy. Complementary Therapies in Medicine. 2002;10(4):210–6. doi: 10.1016/s0965-2299(02)00072-9. [DOI] [PubMed] [Google Scholar]; * Smith C, Crowther C, Beilby J. Acupuncture to treat nausea and vomiting in early pregnancy: a randomized controlled trial. Birth. 2002;29(1):1–9. doi: 10.1046/j.1523-536x.2002.00149.x. [DOI] [PubMed] [Google Scholar]; Smith C, Crowther C, Beilby J. Pregnancy outcome following women’s participation in a randomised controlled trial of acupuncture to treat nausea and vomiting in early pregnancy. Complementary Therapies in Medicine. 2002;10:78–83. doi: 10.1054/ctim.2002.0523. [DOI] [PubMed] [Google Scholar]; Smith C, Crowther C, Beilby J. Women’s experiences of nausea and vomiting in early pregnancy. 2nd Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30-April 4; Australia: Alice Springs; 1998. p. 142. [Google Scholar]
- Smith 2004*.Smith C, Crowther C, Willson K, Hotham N, McMillian V. A randomized controlled trial of ginger to treat nausea and vomiting in pregnancy. Obstetrics & Gynecology. 2004;103(4):639–45. doi: 10.1097/01.AOG.0000118307.19798.ec. [DOI] [PubMed] [Google Scholar]; Smith CA, Hotham N, MacMillan V, Crowther CA. Comparison of ginger and vitamin B6 for treating nausea and vomiting in early pregnancy: a randomised controlled trial. Perinatal Society of Australia and New Zealand 7th Annual Congress; Tasmania, Australia. 2003 March 9-12.2003. p. P47. [Google Scholar]
- Sripramote 2003.Sripramote M, Lekhyananda N. A randomized comparison of ginger and vitamin b6 in the treatment of nausea and vomiting in pregnancy. Journal of the Medical Association of Thailand. 2003;86:846–53. [PubMed] [Google Scholar]
- Vutyavanich 1995.Vutyavanich T, Wongtra-ngan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology. 1995;173:881–4. doi: 10.1016/0002-9378(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Vutyavanich 2001.Kraisarin T, Vutyavanich T. The effectiveness of ginger for the treatment of nausea and vomiting of pregnancy. Thai Journal of Obstetrics and Gynaecology. 2000;12(4):340. [Google Scholar]; * Vutyavanich T, Kraisarin T, Ruangsri RA. Ginger for nausea and vomiting in pregnancy: randomized double masked, placebo controlled trial. Obstetrics & Gynecology. 2001;97:577–88. doi: 10.1016/s0029-7844(00)01228-x. [DOI] [PubMed] [Google Scholar]
- Werntoft 2001.Werntoft E, Dykes AK. Effect of acupressure on nausea and vomiting during pregnancy. Journal of Reproductive Medicine. 2001;46(9):835–9. [PubMed] [Google Scholar]
- Willetts 2003.Willetts KE, Ekangaki A, Eden JA. Effect of a ginger extract on pregnancy-induced nausea: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2003;43:139–44. doi: 10.1046/j.0004-8666.2003.00039.x. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Baum 1963.Baum G, Boxer EI, Davidson JH, Lawrence N, Lewis JBR, Morgan DJR, et al. Meclozine and pyridoxine in pregnancy sickness. Practitioner. 1963;190:251–3. [Google Scholar]
- Bayreuther 1994.Bayreuther J, Lewith GT, Pickering R. A double-blind cross-over study to evaluate the effectiveness of acupressure at pericardium 6 (P6) in the treatment of early morning sickness (EMS) Complementary Therapies in Medicine. 1994;2:70–6. [Google Scholar]
- Can Gurkan 2008.Can Gurkan O, Arslan H. Effect of acupressure on nausea and vomiting during pregnancy. Complementary Therapies in Clinical Practice. 2008;14(1):46–52. doi: 10.1016/j.ctcp.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Cartwright 1951.Cartwright EW. Dramamine in nausea and vomiting of pregnancy. Western Journal of Surgery. 1951;59:216–34. [PubMed] [Google Scholar]
- Conklin 1958.Conklin FJ, Nesbitt REL. Buclizine hydrochloride for nausea and vomiting of pregnancy. Obstetrics & Gynecology. 1958;11:214–9. [PubMed] [Google Scholar]
- De Aloysio 1992.De Aloysio D, Penacchioni P. Morning sickness control in early pregnancy by Neiguan point acupressure. Obstetrics & Gynecology. 1992;80:852–4. [PubMed] [Google Scholar]
- Diggory 1962.Diggory PLC, Tomkinson JS. Nausea and vomiting in pregnancy. A trial of meclozine dihydrochloride with and without pyridoxine. Lancet. 1962;2:370–2. doi: 10.1016/s0140-6736(62)90228-3. [DOI] [PubMed] [Google Scholar]
- Dundee 1988.Dundee JW, Sourial FBR, Ghaly RG, Bell PF. P6 acupressure reduces morning sickness. Journal of the Royal Society of Medicine. 1988;81:456–7. doi: 10.1177/014107688808100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans 1993.Evans AT, Samuels SN, Marshall C, Bertolucci LE. Suppression of pregnancy-induced nausea and vomiting with sensory afferent stimulation. Journal of Reproductive Medicine. 1993;38(8):603–6. [PubMed] [Google Scholar]
- Ferruti 1982.Ferruti MM, Speranza R. Use of the combination of adrenal cortex extract, pyridoxal-5-phosphate, cyanocobalamin and ascorbic acid in the treatment of hypocorticism in pregnancy [Italian] [Sulla utilita dell’associazione di estratto corticosurrenale, piridossal–5–fosfato, cianocobalamina e acido ascorbico nel trattamento degli stati di ipocorticalismo in gravidanza.] Minerva Ginecologica. 1982;34:619–23. [PubMed] [Google Scholar]
- Fitzgerald 1955.Fitzgerald JPB. The effect of promethazine in nausea and vomiting of pregnancy. New Zealand Medical Journal. 1955;54:215–8. [PubMed] [Google Scholar]
- Heazell 2006.Heazell A, Thorneycroft J, Walton V, Etherington I. Acupressure for the in-patient treatment of nausea and vomiting in early pregnancy: a randomized control trial. American Journal of Obstetrics and Gynecology. 2006;194(3):815–20. doi: 10.1016/j.ajog.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Higgins 2009.Higgins JR. [accessed 5 January 2009];Day care versus inpatient management of nausea and vomiting of pregnancy - D.I.M. trial. Current Controlled Trials. http://controlled-trials.com/
- Hyde 1989.Hyde E. Acupressure therapy for morning sickness. A controlled clinical trial. Journal of Nurse Midwifery. 1989;34:171–8. doi: 10.1016/0091-2182(89)90077-3. [DOI] [PubMed] [Google Scholar]
- Kadan 2009.Kadan Y. [accessed 31 July 2009];Does thiamine help vomiting and nausea in pregnancy? ClinicalTrials.gov. http://clinicaltrials.gov.
- King 1955.King AG. The treatment of pregnancy nausea with a pill. Obstetrics & Gynecology. 1955;6:332–8. [PubMed] [Google Scholar]
- Koren 2006.Koren G. [accessed 21 March 2006];Pre-emptive treatment of severe nausea and vomiting of pregnancy (ongoing trial) ClinicalTrials.gov. http://clinicaltrials.gov/
- Lask 1953.Lask S. Treatment of nausea and vomiting of pregnancy with antihistamines. BMJ. 1953;1:652–3. doi: 10.1136/bmj.1.4811.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz 1987.Luz NP, Gonzales MAG. Effect of bromopride on nausea and vomiting during pregnancy. 1987. Personal communication.
- Steele 2001.Steele NM, French J, Gatherer-Boyles J, Newman S, Leclaire S. Effect of acupressure by sea-bands on nausea and vomiting of pregnancy. Journal of Obstetric, Gynecologic and Neonatal Nursing. 2001;30(1):61–70. [PubMed] [Google Scholar]
- Wheatley 1977.Wheatley D. Treatment of pregnancy sickness. British Journal of Obstetrics and Gynaecology. 1977;84:444–7. doi: 10.1111/j.1471-0528.1977.tb12620.x. [DOI] [PubMed] [Google Scholar]
- Winters 1961.Winters HS. Antiemetics in nausea and vomiting of pregnancy. Obstetrics & Gynecology. 1961;18:753–6. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Adamczak 2007.Adamczak J, Kasdaglis J, Rinehart B, Antebi Y, Wolf E, Terrone D. A prospective randomized trial of solumedrol dose pack vs. phenergan for the treatment of symptomatic nausea and vomiting in pregnancy. American Journal of Obstetrics and Gynecology. 2007;197(6 Suppl 1):S88. Abstract no: 277. [Google Scholar]
- Biswas 2006.Biswas SC, Bal R, Kamilya GS, Mukherjee J, Hazra A, Narender S, et al. A single-masked, randomized, controlled trial of ginger extract in the treatment of nausea and vomiting of pregnancy [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 Jan 6-9; India: Cochin, Kerala State; 2006. p. 53. [Google Scholar]
- Hsu 2003.Hsu E, Pei V, Shofer FS, Abbuhl SB. A prospective randomized controlled trial of acupressure vs sham for pregnancy-related nausea and vomiting in the emergency department. Academic Emergency Medicine. 2003;10(5):437. [Google Scholar]
- Mamo 1995.Mamo J, Mamo D, Pace M, Felice D. Evaluation of sea-band acupressure device for early pregnancy nausea and vomiting. 27th British Congress of Obstetrics And Gynaecology; Dublin, Ireland. 1995 July 4-7.1995. p. 283. [Google Scholar]
References to ongoing studies
- Nguyen 2008.Nguyen H. [accessed 20 February 2008];The efficacy of diclectin for nausea and vomiting of pregnancy. ClinicalTrials.gov. http://clinicaltrials.gov/
- Wibowo 2009.Wibowo N. [accessed 4 January 2009];B6 treatment for nausea and vomiting in pregnancy. ClinicalTrials.gov. http://clinicaltrials.gov/
Additional references
- Aikins Murphy 1998.Aikins Murphy P. Alternative therapies for nausea and vomiting of pregnancy. Obstetrics & Gynecology. 1998;91(1):149–55. doi: 10.1016/s0029-7844(97)00582-6. [DOI] [PubMed] [Google Scholar]
- Atanackovic 2001.Atanackovic G, Wolpin J, Koren G. Determinants of the need for hospital care among women with nausea and vomiting of pregnancy. Clinical & Investigative Medicine. 2001;24(2):90. [PubMed] [Google Scholar]
- Attard 2002.Attard CL, Kohli MA, Coleman S, Bradley C, Hux M, Atanackovic G, et al. The burden of illness of severe nausea and vomiting of pregnancy in the United States. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S220–S227. doi: 10.1067/mob.2002.122605. [DOI] [PubMed] [Google Scholar]
- Bayles 2007.Bayles BP. Herbal and other complementary medicine use by Texas midwives. Journal of Midwifery and Women’s Health. 2007;52(5):473–8. doi: 10.1016/j.jmwh.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Brent 2002.Brent R. Medical, social, and legal implications of treating nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S262–S266. doi: 10.1067/mob.2002.122603. [DOI] [PubMed] [Google Scholar]
- Bryer 2005.Bryer E. A literature review of the effectiveness of ginger in alleviating mild-to-moderate nausea and vomiting of pregnancy. Journal of Midwifery and Women’s Health. 2005;50(1):e1–e3. doi: 10.1016/j.jmwh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Bsat 2003.Bsat FA, Hoffman DE, Seubert DE. Comparison of three outpatient regimens in the management of nausea and vomiting in pregnancy. Journal of Perinatology. 2003;23(7):531. doi: 10.1038/sj.jp.7210986. [DOI] [PubMed] [Google Scholar]
- Buckwalter 2002.Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S210–S214. doi: 10.1067/mob.2002.122600. [DOI] [PubMed] [Google Scholar]
- Chou 2003.Chou FH, Lin LL, Cooney AT, Walker LO, Riggs MW. Psychosocial factors related to nausea, vomiting, and fatigue in early pregnancy. Journal of Nursing Scholarship. 2003;35(2):119–25. doi: 10.1111/j.1547-5069.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- Chou 2008.Chou FH, Avant KC, Kuo SH, Fetzer SJ. Relationships between nausea and vomiting, perceived stress, social support, pregnancy planning, and psychosocial adaptation in a sample of mothers: a questionnaire survey. International Journal of Nursing Studies. 2008;45(8):1185–91. doi: 10.1016/j.ijnurstu.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Czeizel 2004.Czeizel AE, Puho E. Association between severe nausea and vomiting in pregnancy and lower rate of preterm births. Paediatric and Perinatal Epidemiology. 2004;18(4):253–9. doi: 10.1111/j.1365-3016.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- Davis 2004.Davis M. Nausea and vomiting of pregnancy: an evidence-based review. Journal of Perinatal & Neonatal Nursing. 2004;18(4):312–28. doi: 10.1097/00005237-200410000-00002. [DOI] [PubMed] [Google Scholar]
- De Veciana 2001.De Veciana M, Stewart L, Miller H, Slotnick R, Rebarber A, Rosen T. Multicenter randomized controlled trial of nerve stimulation therapy for the relief of nausea and vomiting in pregnancy. American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S182. doi: 10.1016/s0029-7844(03)00375-2. [DOI] [PubMed] [Google Scholar]
- Einarson 2007.Einarson TR, Navioz Y, Maltepe C, Einarson A, Koren G. Existence and severity of nausea and vomiting in pregnancy (NVP) with different partners. Journal of Obstetrics and Gynaecology. 2007;27(4):360–2. doi: 10.1080/01443610701327362. [DOI] [PubMed] [Google Scholar]
- Eliakim 2000.Eliakim R, Abulafia O, Sherer DM. Hyperemesis gravidarum: a current review. American Journal of Perinatology. 2000;17(4):207–18. doi: 10.1055/s-2000-9424. [DOI] [PubMed] [Google Scholar]
- Ernst 2000.Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. British Journal of Anaesthesia. 2000;84(3):367–71. doi: 10.1093/oxfordjournals.bja.a013442. [DOI] [PubMed] [Google Scholar]
- Ernst 2002a.Ernst E, Schmidt K. Health risks over the Internet: advice offered by “medical herbalists” to a pregnant woman. Wiener Medizinische Wochenschrift. 2002;152(7-8):190–2. doi: 10.1046/j.1563-258x.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- Ernst 2002b.Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG: an international journal of obstetrics and gynaecology. 2002;109:227–35. doi: 10.1111/j.1471-0528.2002.t01-1-01009.x. [DOI] [PubMed] [Google Scholar]
- Goodwin 2002.Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S184–S189. doi: 10.1067/mob.2002.122592. [DOI] [PubMed] [Google Scholar]
- Higgins 2009.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; [updated September 2009]. 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Jewell 2003.Jewell D. Nausea and vomiting in early pregnancy. American Family Physician. 2003;68(1):143–4. [PubMed] [Google Scholar]
- Koch 2002.Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S198–S203. doi: 10.1067/mob.2002.122598. [DOI] [PubMed] [Google Scholar]
- Koren 2002a.Koren G, Levichek Z. The teratogenicity of drugs for nausea and vomiting of pregnancy: perceived versus true risk. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S248–S252. doi: 10.1067/mob.2002.122601. [DOI] [PubMed] [Google Scholar]
- Koren 2002b.Koren G, Boskovic R, Hard M, Maltepe C, Navioz Y, Einarson A. Motherisk - PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S228–S231. doi: 10.1067/mob.2002.123054. [DOI] [PubMed] [Google Scholar]
- Koren 2005.Koren G, Piwko C, Ahn E, Boskovic R, Maltepe C, Einarson A. Validation studies of the Pregnancy Unique-Quantification of Emesis (PUQE) scores. Journal of Obstetrics and Gynaecology. 2005;25(3):241–4. doi: 10.1080/01443610500060651. [DOI] [PubMed] [Google Scholar]
- Kousen 1993.Kousen M. Treatment of nausea and vomiting in pregnancy. American Family Physician. 1993;48(7):1279. [PubMed] [Google Scholar]
- Lacasse 2008.Lacasse A, Rey E, Ferreira E, Morin C, Berard A. Validity of a modified Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) scoring index to assess severity of nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology. 2008;198(1):71.e1–71.e7. doi: 10.1016/j.ajog.2007.05.051. [DOI] [PubMed] [Google Scholar]
- Lacroix 2000.Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. American Journal of Obstetrics and Gynecology. 2000;182(4):931–7. doi: 10.1016/s0002-9378(00)70349-8. [DOI] [PubMed] [Google Scholar]
- Locock 2008.Locock L, Alexander J, Rozmovits L. Women’s responses to nausea and vomiting in pregnancy. Midwifery. 2008;24:143–52. doi: 10.1016/j.midw.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Louik 2006.Louik C, Hernandez-Diaz S, Werler MM, Mitchell AA. Nausea and vomiting in pregnancy: maternal characteristics and risk factors. Paediatric and Perinatal Epidemiology. 2006;20(4):270–8. doi: 10.1111/j.1365-3016.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- Magee 2002a.Magee LA, Mazzotta P, Koren G. Evidence-based view of safety and effectiveness of pharmacologic therapy for nausea and vomiting of pregnancy (NVP) American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S256–S261. doi: 10.1067/mob.2002.122596. [DOI] [PubMed] [Google Scholar]
- Magee 2002b.Magee LA, Chandra K, Mazzotta P, Stewart D, Koren G, Guyatt GH. Development of a health-related quality of life instrument for nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology. 2002;186(5):S232–S238. doi: 10.1067/mob.2002.122604. [DOI] [PubMed] [Google Scholar]
- Magee 2006.Magee L, Shrim A, Koren G. Diagnosis and management of nausea and vomiting in pregnancy. Fetal and Maternal Medicine Review. 2006;17(1):45–67. [Google Scholar]
- Mazzotta 2000.Mazzotta P, Magee LA. A risk-benefit assessment of pharmacological and nonpharmacological treatment for nausea and vomiting of pregnancy. Drugs. 2000;59:781–800. doi: 10.2165/00003495-200059040-00005. [DOI] [PubMed] [Google Scholar]
- McParlin 2008.McParlin C, Graham RH, Robson SC. Caring for women with nausea and vomiting in pregnancy: new approaches. British Journal of Midwifery. 2008;16(5):280–5. [Google Scholar]
- Melzack 1985.Melzack R, Rosberger Z, Hollingsworth ML, Thirlwell M. New approaches to measuring nausea. Canadian Medical Association Journal. 1985;133(8):755–8. [PMC free article] [PubMed] [Google Scholar]
- Miller 2001.Miller H, De Veciana M, Stewart L, Rebarber A, Rosen T, Slotnick R. A multicenter randomized controlled trial of nerve stimulation therapy: subanalysis of severe nausea and vomiting symptoms [abstract] American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S188. doi: 10.1016/s0029-7844(03)00375-2. [DOI] [PubMed] [Google Scholar]
- Miller 2002.Miller F. Nausea and vomiting in pregnancy: the problem of perception--is it really a disease? American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S182–S183. doi: 10.1067/mob.2002.122594. [DOI] [PubMed] [Google Scholar]
- NICE 2008.National Collaborating Centre for Women’s and Children’s Health . Clinical guideline 62. National Institute for Clinical Excellence (NICE); London: Mar, 2008. Antenatal care: routine care for the healthy pregnant woman. [Google Scholar]
- Niebyl 2002.Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S253–5. doi: 10.1067/mob.2002.122595. [DOI] [PubMed] [Google Scholar]
- O’Brien 1992.O’Brien B, Naber S. Nausea and vomiting during pregnancy: effects on the quality of women’s lives. Birth. 1992;19:138–43. doi: 10.1111/j.1523-536x.1992.tb00671.x. [DOI] [PubMed] [Google Scholar]
- O’Brien 1996.O’Brien B, Relyea MJ, Taerum T. Efficacy of P6 acupressure in the treatment of nausea and vomiting during pregnancy. American Journal of Obstetrics and Gynecology. 1996;174(2):708–15. doi: 10.1016/s0002-9378(96)70454-4. [DOI] [PubMed] [Google Scholar]
- O’Brien 1997.O’Brien B, Relyea J. Diary reports of nausea and vomiting during pregnancy. Clinical Nursing Research. 1997;6(3):239. doi: 10.1177/105477389700600305. [DOI] [PubMed] [Google Scholar]
- O’Brien 1999.O’Brien B, Relyea MJ. Use of Indigenous explanations and remedies to further understand nausea and vomiting during pregnancy. Health Care for Women International. 1999;20(1):49–61. doi: 10.1080/073993399245953. [DOI] [PubMed] [Google Scholar]
- Ornstein 1995.Ornstein M, Einarson A, Koren G. Bendectin/diclectin for morning sickness: a Canadian follow-up of an American tragedy. Reproductive Toxicology. 1995;9(1):1–6. doi: 10.1016/0890-6238(94)00050-7. [DOI] [PubMed] [Google Scholar]
- Piwko 2007.Piwko C, Ungar WJ, Einarson TR, Wolpin J, Koren G. The weekly cost of nausea and vomiting of pregnancy for women calling the Toronto Motherisk Program. Current Medical Research and Opinion. 2007;23(4):833–40. doi: 10.1185/030079907x178739. [DOI] [PubMed] [Google Scholar]
- Pongrojpaw 2007.Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. Journal of the Medical Association of Thailand. 2007;90(9):1703–9. [PubMed] [Google Scholar]
- Quinlan 2003.Quinlan JD, Hill DA. Nausea and vomiting of pregnancy. American Family Physician. 2003;68(1):121–8. [PubMed] [Google Scholar]
- RevMan 2008.The Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Rhodes 1984.Rhodes V, Watson P, Johnson M. Development of reliable and valid measures of nausea and vomiting. Cancer Nursing. 1984;7:33–41. [PubMed] [Google Scholar]
- South Australian Health Commission 1999.South Australian Health Commission . South Australian Health Commission (Pregnancy Outcome Statistics) Regulations. South Australian Health Commission; 1999. [Google Scholar]
- Swallow 2002.Swallow BL, Lindow SW, Masson EA, Hay DM. Development of an instrument to measure nausea and vomiting in pregnancy. Journal of Obstetrics and Gynaecology. 2002;22(5):481–5. doi: 10.1080/0144361021000003582. [DOI] [PubMed] [Google Scholar]
- Swallow 2004.Swallow BL, Lindow SW, Masson EA, Hay DM. Psychological health in early pregnancy: relationship with nausea and vomiting. Journal of Obstetrics and Gynaecology. 2004;24(1):28–32. doi: 10.1080/01443610310001620251. [DOI] [PubMed] [Google Scholar]
- Swallow 2005.Swallow BL, Lindow SW, Masson EA, Hay DM. Women with nausea and vomiting in pregnancy demonstrate worse health and are adversely affected by odours. Journal of Obstetrics and Gynaecology. 2005;25(6):544–9. doi: 10.1080/01443610500230783. [DOI] [PubMed] [Google Scholar]
- Tiran 2002.Tiran D. Nausea and vomiting in pregnancy: safety and efficacy of self-administered complementary therapies. Complementary Therapies in Nursing and Midwifery. 2002;8(4):191–6. doi: 10.1054/ctnm.2002.0651. [DOI] [PubMed] [Google Scholar]
- Tiran 2003.Tiran D. The use of herbs by pregnant and childbearing women: a risk-benefit assessment. Complementary Therapies in Nursing & Midwifery. 2003;9(4):176–81. doi: 10.1016/S1353-6117(03)00045-3. [DOI] [PubMed] [Google Scholar]
- Vutyavanich 1995.Vutyavanich T, Wongtra-ngan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology. 1995;173(3 Pt 1):881–4. doi: 10.1016/0002-9378(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Weigel 1989.Weigel MM, Weigel RM. Nausea and vomiting of early pregnancy and pregnancy outcomes. An epidemiological study. British Journal of Obstetrics and Gynaecology. 1989;96:1304–11. doi: 10.1111/j.1471-0528.1989.tb03228.x. [DOI] [PubMed] [Google Scholar]
- Westfall 2004.Westfall RE. Use of anti-emetic herbs in pregnancy: women’s choices, and the question of safety and efficacy. Complementary therapies in nursing & midwifery. 2004;10(1):30–6. doi: 10.1016/S1353-6117(03)00057-X. [DOI] [PubMed] [Google Scholar]
- Wilkinson 2000.Wilkinson JM. What do we know about herbal morning sickness treatments? A literature survey. Midwifery. 2000;16(3):224–8. doi: 10.1054/midw.1999.0209. [DOI] [PubMed] [Google Scholar]
- Woolhouse 2006.Woolhouse M. Complementary medicine for pregnancy complications. Australian Family Physician. 2007;35(9):695. [PubMed] [Google Scholar]
- Zhou 1999.Zhou Q, O’Brien B, Relyea J. Severity of nausea and vomiting during pregnancy: what does it predict? Birth. 1999;26(2):108–14. doi: 10.1046/j.1523-536x.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- Zhou 2001.Zhou Q, O’Brien B, Soeken K. Rhodes Index of Nausea and Vomiting--Form 2 in pregnant women. A confirmatory factor analysis. Nursing Research. 2001;50(4):251–7. doi: 10.1097/00006199-200107000-00009. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Jewell 2003a.Jewell D, Young G. Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Systematic Reviews. 2003;(Issue 4) doi: 10.1002/14651858.CD000145. [Art. No.: CD000145. DOI: 10.1002/14651858.CD000145.pub2] [DOI] [PubMed] [Google Scholar]
- *.Indicates the major publication for the study