Abstract
A fungal survey that targeted the tribe Arachnopezizeae (Hyaloscyphaceae, Helotiales) was conducted in Korea. One variety belonging to Proliferodiscus and one species belonging to Rodwayella are recorded for the first time from Korea. Proliferodiscus inspersus var. magniascus differs from P. earoleucus by its amyloid reaction in ascal apices and larger asci. Rodwayella citrinula is distinguished from other members of the genus by small ascospores and its occurrence on grasses. The descriptions, illustrations and cultural characteristics of the species are provided.
Keywords: Arachnopezizeae, Hyaloscyphaceae, Mycobiota, Taxonomy
Hyaloscyphaceae Nannf. (Helotiales, Ascomycetes) is a group of fungi with worldwide distribution [1]. Its members are mainly characterized by small apothecia clothed with variously shaped marginal and lateral hairs [2] and play a role as decomposers of various organic substrates in the ecosystem such as decaying woods, dead branches, and even fallen leaves. Due to its mini-fruit bodies that are often less than 1 mm in diameter, the diversity of Hyaloscyphaceae in Korea has been poorly investigated and most of fungal surveys in Korea have been mainly concentrated on edible and poisonous mushrooms, and plant pathogenic fungi causing serious economic losses in crop production. Members of Hyaloscyphaceae in Korea have been occasionally reported in studies of other fungal groups [3, 4, 5, 6, 7, 8, 9, 10, 11], but floral survey of Hyaloscyphaceae in Korea is largely limited. Recently, Han et al. [12, 13] described five new species belonging to the genera Amicodiaca, Psilachnum, and Lachnum under the tribes Hyaloscypheae and Lachneae according to the classification system suggested by Nannfeldt [2] in which the family is subdivided into three tribes, namely, Arachnopezizeae, Hyaloscypheae, and Lachneae. To date, a total of 26 species in 14 genera have been reported in Korea.
From 2006 to 2011, an intensive fungal survey of Hyaloscyphaceae had been conducted in Korea. In this study, we focus on species belonging to the tribe Arachnopezizeae, which is considered as a distinct group in Hyaloscyphaceae due to the presence of subiculum beneath the apothecia [14]. In Korea, only three species of Arachnopezizeae have been reported, namely, Arachnopeziza aurelia (Pers.) Fuckel [10], A. aurata Fuckel (as A. nivea Lorton) [5] and Proliferodiscus earoleucus (Berk. & Broome) J. H. Haines & Dumont [11]. Here, we provide descriptions and illustrations of two previously unrecorded taxa of the tribe Arachnopezizeae in Korea, Proliferodiscus inspersus var. magniascus and Rodwayella citrinula.
Specimens were collected from various sites in Korea. Apothecia collected on woody substrates were normally air-dried at room temperature, whereas those collected on herbaceous substrates were pressed with clean papers and dried on the laboratory bench. All of the collected materials deposited in Korea University herbarium (KUS). For obtaining a pure culture, fresh apothecia were attached to the inside of the lid of a Petri dish to facilitate ejection of their ascospores on 50% potato dextrose agar (PDA) containing 200 µg/mL of streptomycin sulfate (S6501-100G; Sigma-Aldrich, St. Louis, MO, USA). The discharge of ascospores was confirmed by using an SZ40 stereomicroscope (Olympus, Tokyo, Japan), and germinated ones were then carefully transferred to fresh PDA and incubated for 10 wk at 25℃ under a 12-hr fluorescent light/12-hr dark cycle. The obtained cultures deposited in Korean Agricultural Culture Collection (KACC), Suwon, Korea.
Fresh materials were primarily mounted in distilled water for confirmation of the natural colors of their microstructures. Dried materials were revived in 3~10% aqueous KOH. Amyloid reactions were tested by using Melzer's reagent (MLZ) or Lugol's solution (IKI). Line drawings were made with the aid of an Olympus BX50 microscope equipped with an Olympus U-DA drawing tube. Measurements were made in distilled water, Congo red, lacto-cotton blue, IKI, or MLZ; they are reported as follows: minimum - maximum (length) × minimum - maximum (width) [mean length ± standard deviation × mean width ± standard deviation, Q (l/w ratio) = average ± standard deviation]. The following descriptions are arranged in alphabetical order by scientific name. The cultural characteristics and the pictures of fresh apothecia are also given for those species where the data are available. New records from Korea are marked with an asterisk (*) and a key for classification of the tribe Arachnopezizeae in Korea is provided.
Arachnopeziza aurata Fuckel, Jb. Nassau. Ver. Naturk. 23~24: 304 (1870)
≡ Belonidium auratum (Fuckel) Sacc., Syll. Fung. (Abellini) 8: 499 (1889).
≡ Gorgoniceps aurata (Fuckel) Höhn., Sber. Akad. Wiss. Wien, Math. -Naturw. Kl., Abt. 1 132: 116 (1923).
≡ Arachnopezizella aurata (Fuckel) Kirschst., Ann. Mycol. 36: 397 (1938).
= Peziza rhabdosperma Berk. & Broome, Ann. Mag. Nat. Hist., Ser. 4, 17: 143 (1876).
≡ Tapesia rhabdosperma (Berk. & Broome) W. Phillips, Man. Br. Discomyc. (Lond.): 280 (1887).
≡ rhabdospermum (Berk. & Broome) Sacc., Syll. Fung. (Abellini) 11: 417 (1895).
≡ Belonium rhabdospermum (Berk. & Broome) Boud., Hist. Classif. Disc. Eur. 118 (1907).
= Arachnopeziza nivea Lorton, Bull. Soc. Myc. Fr. 30: 224 (1914).
≡ Tapesia nivea (Lorton) Sacc., Syll. Fung. 24: 1199 (1928).
= Peziza phlegmacea Ellis, Bull. Torrey Club 9: 19 (1882).
≡ Tapesia phlegmacea (Ellis) Ellis ex Sacc. pro syno., Syll. Fung. 8: 500 (1889).
≡ Belonidium phlegmacea (Ellis) Sacc., Syll. Fung. 11: 417 (1895).
= Trichobelonium virgeneum Rick apud Rehm et Rick, Brotéria 5: 224 (1906).
= Arachnopeziza filamentosa Torrend, Brotéria, Ser. Bot. 11: 102 (1913).
= Arachnopeziza arctostaphyli Cash, Mycologia 28: 247 (1936).
Specimens examined
Korea: Jeju, Muljangohri, on damp rotting wood, 33o24'34'' N, 126o36'23'' E, alt. 850 m, 26 Apr 2002, KUS-F50440; Hongcheon, Experimental Forest of Kangwon National University, alt. 210 m, 37o44'29'' N 127o49'51'' E, 27 Apr 2002, KUS-F50469; Jeju, Mt. Halla National Park, Seongpanak, 33o23'5'' N, 126o37'10'' E, alt. 750 m, 27 Apr 2002, KUS-F50470; Pocheon, National Arboretum, alt. 50 m, 37o41'42'' N, 127o9'47'' E, 8 May 2002, KUS-F50493; Namjeju, Hangyeong-Andeok Gotjawal, 33o17'44" N, 126o19'24" E, alt. 190 m, 7 May 2005, KUS-F51009; Seogwipo, Seogwipo Recreation Forest, alt. 730 m, 33o18'52" N, 126o28'24" E, 8 May 2006, KUS-F51018; Jeju, Mt. Halla National Park, Seongpanak, 33o23'5" N, 126o37'12"E, alt. 750 m, 9 May 2006, KUS-F51037 and KUS-F51038; Hongcheon, Hwajeon-ri, 37o36'56'' N, 127o45'20'' E, alt. 210 m, 16 Jun 2006, KUS-F51082; Gangneung, Daegwanryeong Recreation Forest, 37o42'39'' N, 128o47'48'' E, alt. 200 m, 9 May 2007, KUS-F51550; Wonju, Mt. Chiak National Park, Guryongsa Temple, 37o23'28'' N, 128o3'11'' E, alt. 450 m, 21 May 2007, KUS-F51577; Yangyang, Mt. Seorak National Park, Jujeongol Valley, 38o5'14'' N, 128o25'49'' E, alt. 540 m, 5 Jul 2007, KUS-F51648; Jeju, Mt. Halla National Park, Mulchatoreum, 33o23'40'' N, 126o37'58'' E, alt. 740 m, 15 Apr 2008, KUS-F52014; Seogwipo, Seogwipo Recreation Forest, 33o18'50" N, 126o27'56" E, alt. 720 m, 16 Apr 2008, KUS-F52022; Hongcheon, Yeonhwasa Temple, 37o48'28'' N, 127o51'2'' E, alt. 310 m, 28 Apr 2008, KUS-F52038; Hamyang, Jirisan Recreation Forest, 35o20'58'' N, 127o38'38'' E, alt. 590 m, 5 May 2008, KUS-F52040; Cheolwon, Bokjusan Recreation Forest, 38o8'32'' N, 127o28'44'' E, alt. 580 m, 16 May 2008, KUS-F52056; Wonju, Mt. Chiak National Park, Chiaksan Recreation Forest, 37o25'26'' N, 128o1'2'' E, alt. 340 m, 16 Apr 2009, KUS-F52415; Daejeon, Maninsan Recreation Forest, 36o11'54'' N, 127o27'02'' E, alt. 330m, 22 Apr 2009, KUS-F52427; Namyangju, Chukryeongsan Recreation Forest, 37o44'30'' N, 127o18'23'' E, alt. 160 m, 6 May 2009, KUS-F52453; Chuncheon, Seo-myeon, Deokduwon-ri, 37o51'53" N, 127o39'34" E, alt. 180 m, 24 Apr 2010, KUS-F52657.
Cultural characteristics
Colonies slow growing; 30 mm in diameter after 6 wk; flat; margins irregular; aerial hyphae cottony, weakly sulcate, pinkish white, becoming light gray toward the center; submerged mycelium grayish brown; reverse concolorous; light yellow pigment diffused from the colony margin into the surrounding agar; no sporulation observed (KACC43877).
Notes
The white apothecia developed on subiculum, multiseptate cylindric ascospores, and filiform paraphyses are consistent with the previously reported characteristics of A. aurata [15, 16, 17, 18]. This fungus is one of the most common and worldwide-distributed species of Arachnopezizeae. In Korea, this fungus was previously reported as A. nivea Lorton by Cho [5]. Arachnopeziza aurata has long been supposed to be a species complex since some morphological characteristics (e.g., the thickness of ectal excipulum and the size of ascospores) are highly variable within the species [16]. Korf [16] hypothesized that these variants may correlate with its substrate specificity. Our specimens also showed a wide range of spore length variation (ranging from 38 to 68 µm), but the relationship with their substrates could not be confirmed because the woods where the apothecia occurred were found in rotten condition resulting in their identification difficult. The variation in hymenial color was also observed within the specimens. It seems to be induced by surrounding environmental factors, especially sunlight; apothecia collected on substrates lying in deep shade or buried under soil tended to be whitish, whereas those collected from substrates exposed to sunlight tended to be yellowish.
Arachnopeziza aurelia (Pers.) Fuckel, Jb. Nassau. Ver. Naturk. 23~24: 303 (1870)
≡ Peziza aurelia Pers., Mycol. Eur. (Erlanga) 1: 270 (1822).
≡ Belonidium aurelia (Pers.) De Not., Comm. Soc. Crittogam. Ital. 1: 381 (1864).
≡ Polynema aurelium (Pers.) Fuckel, Symnol. Myc. Nachtr. I, 49 (1871).
≡ Lachnella aurelia (Pers.) Quél., Enchir. Fung. 315 (1886).
≡ Tapesia aurelia (Pers.) W. Phillips, Man. Br. Discomyc. (Lond.): 280 (1887).
= Patellaria bicolor Curr., Trans. Linn. Soc. Lond. 24: 494 (1864).
= Tapesia fulgens Hazsl., Verh. Zool. -Bot. Ges. Wien 37: 163 (1887).
≡ Belonidium fulgens (Hazsl.) Sacc., Syll. Fung. 8: 500 (1889).
≡ Arachnopeziza fulgens (Hazsl.) Boud., Hist. Classif. Disc. Eur. 126 (1907).
= Peziza wauchii Grev., Scottish Cryptogamic Flora, Tab. 139 (1825).
≡ Stictis wauchii (Grev.) Berk., Outl. Brit. Fung. (Lond.): 375 (1860).
Specimen examined
Korea: Cheongju, near Sangdang Mountain Fortress, on fallen leaves, branches and fruits of Quercus acutissima, 36o21'41'' N, 127o13'32'' E, alt. 460 m, 11 Apr 2007, KUS-F51520.
Notes
This species is easily recognized by its conspicuously bright colored apothecia, obvious subiculum, and short ascospores. It closely resembles A. candidofulva (Schwein.) Korf in general appearance, but is distinguished by its 3-septate ascospores [16]. Arachnopeziza obtusiloba (Schwein.) Korf is also similar in having 3-septate ascospores, but is characterized by whitish apothecia and larger ascospores (16.4~20 µm long) [16]. Arachnopeziza hiemalis Yei Z. Wang is distinguished from A. aurelia by its much larger asci (115~150 × 11~12 µm) and ascospores (20~28 × 4.5~5.5 µm) [19]. It was first reported in Korea by Park et al. in 1985 [10].
Proliferodiscus earoleucus (Berk. & Broome) J. H. Haines & Dumont, Mycologia 75: 539 (1983)
≡ Peziza earoleuca Berk. & Broome, J. Linn. Soc., Bot. 14: 105 (1873) [1875].
Specimen examined
Korea: Hamyang, Jirisan Recreation Forest, on dead branches of Pinus sp., 35o20'58'' N, 127o38'35'' E, alt. 580 m. 13 Jul 2001, KUS-F50016.
Note
This species was first reported in Korea by Raitviir and Shin [11]. Proliferodiscus earoleucus is distinguished from other members of the genus by its stipitate apothecia and lack of amyloid reaction in ascal apices [20]. This species has been regarded as a synonym of P. inspersus until the inconsistent amyloid reactions of asci were noticed by Haines and Dumont [20].
*Proliferodiscus inspersus var. magniascus W. Y. Zhuang, Fungi and Lichens of Shennongjia. Mycol. Lichenol. Exped. Shennongjia (Beijing): 103 (1989) (Figs. 1 and 2)
Fig. 1.

Proliferodiscus inspersus var. magniascus (KUS-F52660). A, Apothecia occurred on rotting wood; B, Proliferating apothecium (scale bars: A = 1 mm, B = 0.5 mm); C, Colony (KACC45526) incubated on potato dextrose agar at room temperature for 6 wk.
Fig. 2.
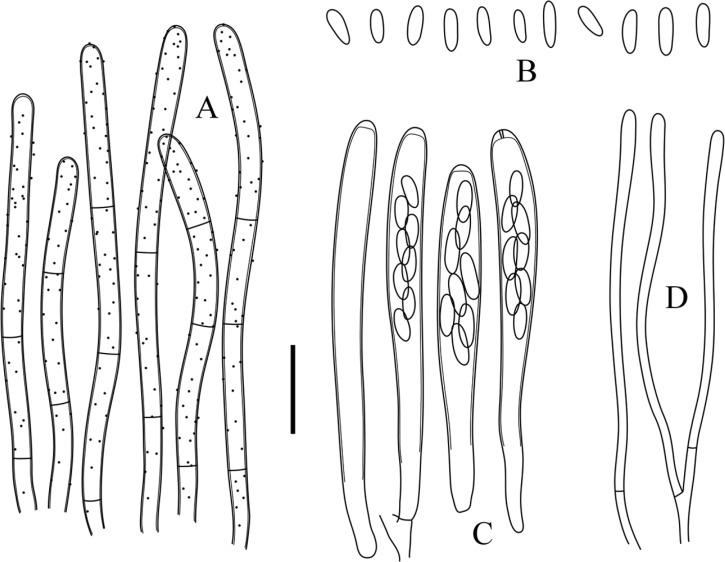
Proliferodiscus inspersus var. magniascus (KUS-F52660). A, Thick-walled marginal hairs; B, Ellipsoid to ovoid ascospores; C, Cylindric-clavate asci with apical apices blued in Melzer's reagent; D, Cylindric paraphyses with slightly swollen apex (scale bar = 10 µm).
Apothecia scattered to densely gregarious, sessile or seated on a short and stout stipe, sometimes confluent into compound fruitbodies; disc up to 1mm in diameter, plano-concave, pale yellowish to orange when fresh, turning pale grayish yellow when dry; receptacle saucer-shaped to shallow cupulate, externally densely covered with grayish white hairs when fresh, partly turning greenish gray when dry; subiculum hyphae hyaline to subhyaline, frequently branched, agglutinated, 2~4.5 µm wide; ectal excipulum composed of prismatic thick-walled cells; hairs flexuous, straight at the margin, becoming curved and curled toward the base, thick-walled, hyaline to grayish, totally bearing amorphous granules, 44~75 µm long, 2~3.5 µm wide; asci arising from simple septa, cylindric-clavate, 8-spored, hyaline, ascal pore blued in MLZ and IKI without KOH pretreatment, 36~50 × 3~4.5 µm ([42.1 ± 3.95] × [3.5 ± 0.46] µm, n = 35); ascospores ellipsoid to ovoid, irregularly biseriate, hyaline, aseptate, aguttulate, sometimes containing minute polar lipid globules in CB mountant, 3.7~5.3 × 1~1.5 µm ([4.4 ± 0.49] × [1.2 ± 0.17] µm, Q = 3.82 ± 0.56, n = 50); paraphyses cylindric, slightly swollen upward, apex 1~1.5 µm wide, forked, 1~2-septate near the base, slightly exceeding the asci by 2~5 µm.
Specimen examined
Korea: Hongcheon, near Yeonhwasa temple, on damp rotting branches, 37°48'29'' N, 127°51'3'' E, alt. 300 m, 29 Apr 2010, KUS-F52660.
Cultural characteristics
Colonies slow growing; 30~33 mm in diameter after 6 weeks incubation on PDA; slightly convex; margins uneven; aerial hyphae cottony, sulcate, white to yellowish white; submerged mycelium invisible from above; reverse dark brown; golden yellow exudates sparsely produced on the surface of the colonies; dark brown pigment diffused from the colony margin into the surrounding agar; no sporulation observed (KACC45526) (Fig. 1C).
Notes
The proliferating apothecia surrounded by subiculum-like hyphae and thick-walled hairs are typical features of the genus Proliferodiscus [20]. The stipitate apothecia and large asci are in agreement with the description of P. inspersus var. magniascus W. Y. Zhuang [21]. This fungus is similar to P. earoleucus (Berk. & Broome) J. H. Haines & Dumont which was previously reported from Korea by Raitviir and Shin [11], however, the amyloid reactions of ascal apices are different [20]. Proliferodiscus inspersus var. magniascus has amyloid asci whereas P. earoleucus has inamyloid asci. Proliferodiscus inspersus var. inspersus (Berk. & M. A. Curtis) J. H. Haines & Dumont is distinguished from this taxon by its much smaller asci (28~38 × 2~3.2 vs. 36~50 × 3~4.5 µm) [20, 21]. This is the first record of P. inspersus var. magniascus from Korea. Except for the original record from China [21], this species has not been reported in other countries, suggesting its restricted distribution in Eastern Asia. Although the genus Proliferodiscus is currently assigned to Arachnopezizeae [20], its taxonomic position has been constantly debated; for example, some molecular phylogenetic studies have shown that a species of Proliferodiscus was clustered with members of Lachneae [22, 23].
*Rodwayella citrinula (P. Karst.) Spooner, in Dennis, Fungi of the Hebrides (Kew): 383 (1986) (Figs. 3 and 4)
Fig. 3.

Rodwayella citrinula (KUS-F52675). A, Apothecia growing on damp rotting leaves of Carex sp.; B, Yellow discoid apothecia (scale bars: A= 5mm, B = 1mm); C, Colony (KACC45530) incubated on potato dextrose agar at room temperature for 6 wk.
Fig. 4.
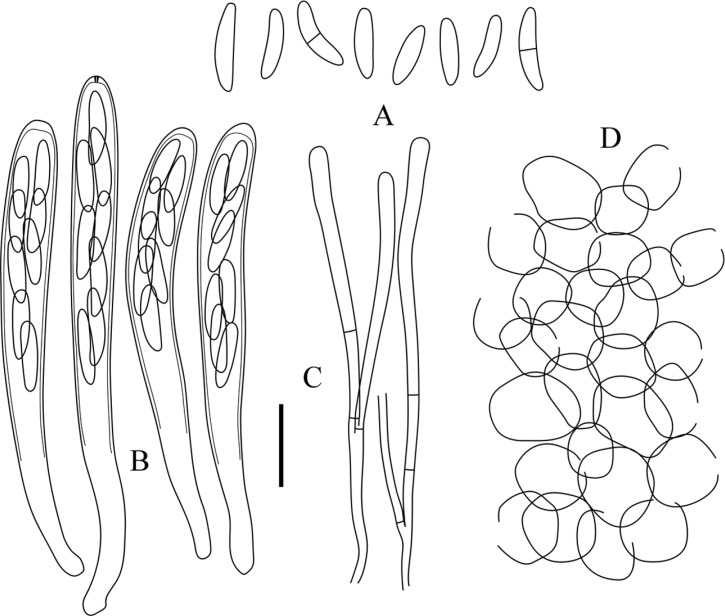
Rodwayella citrinula (KUS-F52443). A, Elliptic-fusoid ascospores, 0~1-septate; B, Cylindric-clavate asci, apical pore blued in Melzer's reagent; C, Paraphyses with clavate apex; D, Ectal excipulum composed of globose cells (scale bar = 10 µm).
≡ Helotium citrinulum P. Karst., Not. Soc. Fauna Fl. Fenn. 11: 238 (1871).
≡ Pezizella citrinula (P. Karst.) Sacc., Syll. Fung. (Abellini) 8: 288 (1889).
≡ Hymenoscyphus citrinulus (P. Karst.) J. Schröt., in Cohn, Krypt. -Fl. Schlesien (Breslau) 3.2: 71 (1893) [1908].
= Helotium flexuosum Massee, Br. Fung. -Fl. 4: 263 (1895).
≡ Calycina flexuosa (Massee) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 448 (1898).
= Mollisiella citrinula Boud, Hist, Class. Discom. Eu. 142 (1907).
= Helotium citrinulum Seaveri Rehm. Ascom. 1634; Ann. Myc. 4: 67 (1967).
Apothecia superficial, gregarious, subiculum-like hyphae sparsely developed below the apothecia, sessile to very shortly stipitate; disc 0.5~1 mm in diameter, flat to slightly convex, smooth to pruinose, pale ocher or mustard yellow when fresh, turning brownish yellow to dark orange when dry; subiculum hyphae thin-walled, 2~3 µm wide, parallel to interwoven; receptacle saucer-shaped, lighter than hymenium, lemon to yellowish white, glabrous or finely pubrulent; ectal excipulum composed of parallel or interwoven hyaline hyphae, thin-walled, 1.8~2 µm wide; excipulum near the bases composed of textura angularis to globulosa, individual cells thin-walled, smooth, 6~15 µm in diameter; asci arising from croziers, cylindric to cylindric-clavate, 8-spored, apical pore blued in MLZ and IKI, 39~61 × 4.6~6 µm ([48 ± 6.21] × [5.2 ± 0.41] µm, n = 50); ascospores elliptic-fusoid to elliptic-clavate, straight or slightly bent, hyaline, smooth, aseptate, becoming 1-septate at maturity, sometimes constricted at the medium septum, irregularly biseriate, occupied upper 1/2~3/5 of the ascal length, 7.7~12.5 × 1.5~2.2 µm ([9.5 ± 1.34] × [1.9 ± 0.18] µm, Q = 5.12 ± 0.62, n = 90); paraphyses cylindric with slightly swollen apex, 2.5~3 µm wide at the apex, 1~2 µm wide below, hyaline, 1~4-septate, forked, slightly exceeding the asci.
Specimens examined
Korea: Danyang, Darian valley, Sobaeksan National Park, on damp rotting leaves of Carex sp., 36°57'46'' N, 128°25'24'' E, alt. 390 m, 28 Apr 2009, KUS-F52443; Muju, Gucheondong valley, Deokyusan National Park, on previous year's dead sheath of Carex ligulata var. austrokoreensis Ohwi, 35°52'59" N, 127°46'39" E, alt. 680 m, 19 May 2010, KUS-F52675.
Cultural characteristics
Colonies slow growing; 46~55 mm in diameter after 6 wk; flat; margins irregular; aerial hyphae cottony, weakly sulcate, white, becoming light yellowish toward the center; submerged mycelium brown to chestnut; reverse concolorous; yellow pigment diffused from the colony margin into the surrounding agar; no sporulation observed (KACC45530) (Fig. 3C).
Notes
The yellowish apothecia seated on subicular hyphae, elliptic-fusoid ascospores that are slightly curved and its substrate preference on grasses are mostly in agreement with the descriptions of R. citrinula (= Helotium citrinulum) [15, 17]. It is noticeable that ascospores of the Korean specimen are somewhat longer (7.7~12.5 vs. 6~9 µm long) than the previous reports [15, 17]. Among the three hitherto known species of the genus, R. citrinula is the only species found growing on herbaceous stems [24] although R. myricae has been once collected from a dead rachis of Pteridium aquilinum in Australia [18]. Rodwayella myricae and R. sessilis are distinguished from this species by their broad ellipsoid ascospores (ca. 5~7 µm wide) and larger asci, 80~95 × 10~12 µm [24] and 89~100 × 11~15 µm [18], respectively. Although Rodwayella spp. are devoid of properly differentiated hairs, Spooner [18] and Spooner and Dennis [24] suggested that this genus should be assigned to Hyaloscyphaceae based on the presence of subiculum and their ascal structures which are similar to those of other members of Hyaloscyphaceae. The taxonomic placement of the genus remains uncertain, but it is beyond the scope of the present study to deal with the accurate placement of the genus. This is the first record of this species and genus from Korea. Rodwayella citrinula has previously been reported in European countries [15] and North America [17], but not in Asia.
Key to all known species of Arachnopezizeae in Korea
Apothecia developed on subiculum or subiculum-like hyphae. Ectal excipulum composed of hyaline cells.
1. Apothecia without differentiated hairs ... Rodwayella
Apothecia sessile, yellowish to lemon, growing on herbaceous stems ... R. citrinula
1'. Apothecia with differentiated hairs ... 2
2. Apothecia often compounded, hairs thick-walled, coarsely warted ... Proliferodiscus
Ascal apices inamyloid, asci shorter than 40 µm ... P. earolecus
Ascal apices amyloid, asci longer than 40 µm ... P. inspersus var. magniascus
2'. Independent apothecia scattered or gregarious, ascospores septate ... Arachnopeziza
Apothecia whitish, ascospores cylindric multiseptate ... A. aurata
Apothecia yellow to orange, ascospores ellipsoid, 0~3-septate ... A. aurelia
ACKNOWLEDGEMENTS
This work was supported by a research grant from a research program for agricultural science and technology of National Institute of Horticultural and Herbal Science (PJ008418), Rural Development Administration, Republic of Korea.
References
- 1.Kirk PM, Cannon PF, Minter DW, Stalpers JA. Dictionary of the fungi. 10th ed. Wallingford: CABI Publishing; 2008. [Google Scholar]
- 2.Nannfeldt JA. Studien über die morphologie und systematik der nichtlichenisierten inoperculaten Discomyceten. Nova Acta Regiae Soc Sci Ups. 1932;8:1–368. [Google Scholar]
- 3.Cho DH. Notes on the Korean Ascomycetes (III) Korean J Plant Resour. 1997;10:265–270. [Google Scholar]
- 4.Cho DH. Notes on the Korean Ascomycetes (VI) Korean J Plant Resour. 1998;11:126–131. [Google Scholar]
- 5.Cho DH. Developmental distribution on fungi in Mt. Jiri areas. (I. On unrecorded species in fungi) Korean J Plant Resour. 1999;12:62–68. [Google Scholar]
- 6.Cho DH. Notes on the Korean Ascomycetes (VIII) Plant Resour. 2001;4:107–110. [Google Scholar]
- 7.Cho DH, Kang CK, Park HJ. Notes on the Korean Ascomycetes (V) Korean J Plant Resour. 1997;10:369–374. [Google Scholar]
- 8.Jung HS. Fungal flora of Ullung Island (VI). On ascomycetous, auriculariaceous, and gasteromycetous fungi. Korean J Mycol. 1995;23:1–9. [Google Scholar]
- 9.Korea Forest Research Institute. Biodiversity in forest ecosystem. Research report. Seoul: Korea Forest Research Institute; 1995. pp. 89–102. [Google Scholar]
- 10.Park PJ, Lee JY, Otani Y. Taxonomical studies on Discomycetes in Korea (I) Korean J Mycol. 1985;13:27–40. [Google Scholar]
- 11.Raitviir A, Shin HD. New and interesting inoperculate discomycetes from Korea. Mycotaxon. 2003;85:331–340. [Google Scholar]
- 12.Han JG, Park MJ, Shin HD. Psilachnum staphyleae, a new member of foliicolous Hyaloscyphaceae from Korea. Mycotaxon. 2009;110:219–224. [Google Scholar]
- 13.Han JG, Hosoya T, Shin HD. Amicodisca castaneae sp. nov. (Hyaloscyphaceae, Helotiales) on Japanese chestnut bur. Mycotaxon. 2011;118:89–94. [Google Scholar]
- 14.Raitviir A. Synopsis of the Hyaloscyphaceae. Scripta Mycol. 1970;1:1–115. [Google Scholar]
- 15.Dennis RWG. A revision of the British Helotiaceae in the herbarium of the Royal Botanic Gardens, Kew, with notes on related European species. CMI Mycol Pap. 1956;62:1–216. [Google Scholar]
- 16.Korf RP. A monograph of the Arachnopezizeae. Lloydia. 1951;14:129–180. [Google Scholar]
- 17.Seaver FJ. The North American cup-fungi (inoperculates) Monticello: Lubrecht & Cramer; 1978. [Google Scholar]
- 18.Spooner BM. Helotiales of Australasia: Geoglossaceae, Orbiliaceae, Sclerotiniaceae, Hyaloscyphaceae. Bibl Mycol. 1987;116:1–711. [Google Scholar]
- 19.Wang YZ. A new species of Arachnopeziza from Taiwan. Mycotaxon. 2009;108:485–489. [Google Scholar]
- 20.Haines JH, Dumont KP. Studies in the Hyaloscyphaceae II: Proliferodiscus, a new genus of Arachnopezizoideae. Mycologia. 1983;75:535–543. [Google Scholar]
- 21.Zhuang WY. Some common discomycetes in Shennongjia, Hubei Province. In: Anon, editor. Fungi and lichens of Shennongjia. Beijing: World Publishing Co.; 1989. pp. 98–106. [Google Scholar]
- 22.Abeln ECA, De Pagter MA, Verkley GJM. Phylogeny of Pezicula, Dermea and Neofabraea inferred from partial sequences of the nuclear ribosomal RNA gene cluster. Mycologia. 2000;92:685–693. [Google Scholar]
- 23.Cantrell SA, Hanlin RT. Phylogenetic relationships in the family Hyaloscyphaceae inferred from sequences of ITS regions, 5.8S ribosomal DNA and morphological characters. Mycologia. 1997;89:745–755. [Google Scholar]
- 24.Spooner BM, Dennis RWG. New or interesting ascomycetes from the Highlands and Islands. Sydowia. 1985;38:294–316. [Google Scholar]