SUMMARY
Inflammatory responses, like all biological cascades, are shaped by a delicate balance between positive and negative feedback loops. It is now clear that in addition to positive and negative checkpoints, the inflammatory cascade rather unexpectedly boasts an additional checkpoint, a family of chemicals that actively promote resolution and tissue repair without compromising host defence. Indeed the resolution phase of inflammation is just as actively orchestrated and carefully choreographed as its induction and inhibition. In this review we explore the immunological consequences of these omega-3-derived specialized pro-resolving mediators (SPMs) and discuss their place within what is currently understood of the role of the arachidonic acid-derived prostaglandins, lipoxins and their natural C15-epimers. We propose that treatment of inflammation should not be restricted to the use of inhibitors of the acute cascade (antagonism) but broadened to take account of the enormous therapeutic potential of inducers (agonists) of the resolution phase of inflammation.
INTRODUCTION
Infection and tissue injury drive the acute inflammatory response, which, in its simplest form, is characterised by the sequential release of mediators (including histamine, bradykinin and 5-hydroxytryptophan (5HT)), resulting in the immediate influx of polymorphonuclear leukocytes (PMNs) followed by phagocytosis via monocytes-macrophages, leading to leukocyte clearance and resolution. Indeed, for the past 40 years research has focused on identifying factors that initiate and perpetuate inflammation with the objective of developing anti-inflammatory drugs to alleviate diseases driven by on-going or dysregulated inflammation. More recently, emphasis has shifted to the other end of the inflammatory spectrum, i.e. resolution, in order to understand how immune-mediated inflammatory responses are terminated. The concept that antagonists which limit the duration of a biological cascade are generated at the same time that a cascade is induced is very familiar in other self-limiting pathways of interest to immunologists such the complement and coagulation cascades. However, homeostasis takes an unexpected twist in the inflammation cascade. Events at the onset of acute inflammation establish biosynthetic circuits for a series of chemical mediators that later not only serve as antagonists but also serve as agonists; in other words they don’t just inhibit the inflammatory cascade, they actively dismantle it leading to the restoration of tissue homeostasis and function. Anti-inflammation and pro-resolution are therefore not equivalent. The agonists that actively promote resolution (an emerging family of pro-resolving lipid mediators including lipoxins, resolvins and protectins) are fundamentally different from the antagonists that limit the duration and magnitude of the inflammatory response at both the molecular and cellular levels (Ryan and Godson, 2010; Serhan, 2007). In this review we will explore the pathways, cells and molecules involved in curbing inflammation and which begin the process of tissue repair. Advances in this area will help shed light on why inflammation persists and provide drug development opportunities based upon stimulating endogenous pro-resolution mediators and their pathways which act as agonists along with the more traditional antagonists which are currently in clinical use.
WHAT IS RESOLUTION AND WHO ARE THE MAIN PLAYERS?
The mediators and cell types involved in the active resolution of acute inflammatory responses are emerging as important determinants of immune systems status and function. Inflammation does not switch off in a passive manner, but involves a program of unique pathways (Figure 1), mediators and cell subtypes (Serhan, 2007). It is important to note that the cells can’t move without specific instructions - marching orders - that in the acute inflammatory response come in the form of chemical gradients of mediators (vide infra). Hence, the focus on identification and decoding of the mediators of the terminating events in the resolution of inflammation are of considerable interest. At the tissue and cellular level, resolution of inflammation has been defined, in broad terms, by the rate of polymorphonuclear cell (PMN) clearance to the point when they are absent from the site of primary tissue injury. The key steps in this process include i) clearance of the inciting stimuli, ii) catabolism of local survival signals and silencing of intracellular pro-inflammatory signalling pathways, iii) normalisation of chemokine gradients, apoptosis of PMNs, iv) their efferocytosis by tissue and monocyte-derived macrophages and v) either incorporation of these myeloid cells into the local population or their recirculation via lymph or blood. Lipid mediators (LM) including eicosanoids and the more recently discovered omega-3 derived ‘specialized pro-resolution mediators’ represent the key signaling molecules in this process (Figures 1 and 2), which regulate the inflammatory profile and promote the return of affected tissues to homeostasis (Serhan, 2010; Serhan et al., 2008).
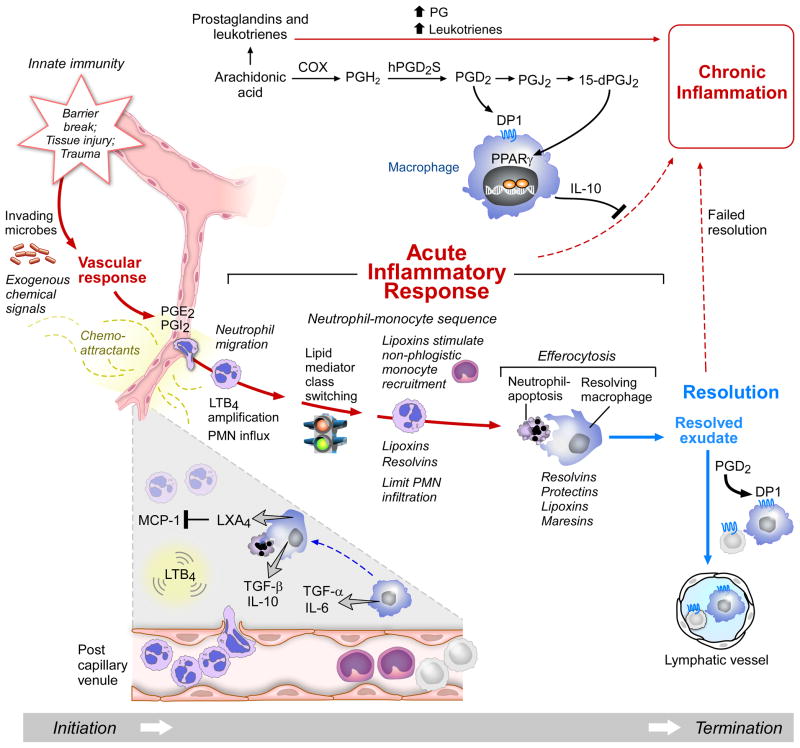
Figure 1. Acute Inflammatory Response and the Role of Lipid Mediators in Resolution or its Failure.
Initiation of the acute response starts with changes in blood flow stimulated by PGE2 and PGI2, and LTB4, which is produced from arachidonic acid, stimulates PMN recruitment. Excess prostaglandins and leukotrienes contribute to chronic inflammation. Cyclooxygenase (COX) production of PGD2 via human PGD2 synthase (hPGD2s) activates its receptor DPI, a GPCR that stimulates IL-10 and anti-inflammatory cytokine, which blocks the path to chronic inflammation. PGD2 can be converted to PGJ2 and 15-dPGJ2 to products that activate PPARγ to activate resolution (see text for details).
Lipid mediator class switching is the temporal switch in inflammatory exudates that activates lipoxin production. LXA4 regulates MCP-1 and non-phlogistic monocyte recruitment and stops LTB4-stimulated PMN influx. Lipoxins and resolvins limit PMN further influx to the site and stimulate efferocytosis and the clearance of cellular debris by resolving macrophages. Resolvins, protectins, lipoxins and maresins (SPM) stimulate and enhance efferocytosis and promote resolution. Loss of any of the cell types receptors or chemical mediators can in theory lead to failed resolution that may underlie the persistent inflammation recognized as chronic inflammation associated with many diseases.
Figure 2. Time Course of Self-Limited Inflammatory Response and the Resolution n-3 Metabolome.
Upper panel: The ideal outcome for an acute challenge: edema, followed by PMN influx, then return to baseline and non-phlogistic recruitment of monocytes and macrophages for resolution. The temporal biosynthesis of SPM is initiated by leukocyte congregation within the exudate. In resolution phase, specific miRNA are regulated and some of these are controlled by resolvins in recent studies (see Li et al., 2013 and refs. within).
Lower panel: Resolving exudate. The E-series resolvins are biosynthesized from EPA, initiated by oxygen insertion at the 18th carbon position to produce RvE1 via an epoxide intermediate, RvE2 and RvE3. D-series resolvin biosynthesis is initiated with oxygen insertion at the 17th carbon position of n-3 DHA. The intermediate 17S-HpDHA is precursor to both protectin and D-series resolvins. Human and mouse macrophages also convert DHA to maresins via oxygen insertion initiated at the 14th carbon to biosynthesize maresin 1 (MaR1) via a recently identified 13S,14S-epoxy-intermediate that also is bioactive. The biosynthesis steps in human leukocytes and in mice are reviewed in Serhan (2007), and stereochemical assignments with confirmation of their potent pro-resolving actions are reviewed in Serhan and Petasis (2011). Apoptotic PMN resolution phase microparticles resolving macrophages produce SPM (Dalli and Serhan, 2012). By definition, each SPM enhances efferocytosis and resolution in vivo in animal models; see text for details. Also, some SPM stimulate the biosynthesis of other families of SPM. For example, RvE1 stimulates LXA4 production in mouse lung (Levy and Serhan, 2014).
The presence of specialized regulatory or pro-resolution cell subsets including phagocytosing monocyte-derived macrophages and myeloid-derived suppressor cells, T regulatory cells (Tregs), for instance, is also instrumental in helping switch off inflammation in a non-phlogistic manner (D’Alessio et al., 2009). Resolution and clearance of cellular debris from mucosal surfaces involves the essential polyunsaturated fatty acids (PUFA)-derived mediators including SPM that accomplish this as well as activate anti-microbial mechanisms in mucosal epithelial cells (Colgan et al., 2013). Functionally, pro-resolving mediators and cell subtypes serve to act not merely as inflammatory brakes, but also facilitate the return of the site to homeostasis in the process of catabasis: the return from disease state (Serhan, 2007; Serhan and Chiang, 2013). Failure of resolution has classically been discussed in terms of either an insufficiency or inadequacy leading to chronic inflammation, excess tissue damage, dysregulation of tissue healing including fibrosis and has been implicated in multiple disease states (Serhan, 2007) including the development of auto-immunity (for reviews, see Tabas and Glass, 2013).
Resolvins, Protectins and Maresins: Specialized Pro-Resolving Mediators (SPMs)
Cells within multi-cellular responses require chemical gradients of signals that instruct each to move and/or stop as needed (Figure 1). In an acute inflammatory response many chemical signals are produced. Some are from microbial origins while others are biosynthesized by the host in response to tissue injury and invasion (Majno and Joris, 2004). Among the chemical signals at the site of an acute inflammatory response, those that originate from host essential fatty acids are of particular interest because of their nutritional regulation of the response (Zhang and Spite, 2012) and the potential to design small molecule mimetics of these molecules (Serhan, 2007).
Chemical mediators biosynthesized from arachidonic acid include prostaglandins and leukotriene B4, which are involved in the initiating steps that permit white blood cells to leave the post-capillary venules, i.e. diapedesis (Samuelsson et al., 1987; Serhan, 2007). More recent research has focused on elucidating chemical mediators and mechanisms involved in endogenous anti-inflammation and its resolution (Levy et al., 2002; Serhan et al., 2000). A systems approach with LC-MS-MS-based lipidomics, in vivo animal models, exudate cell trafficking and functional assessment with isolated human cells has uncovered bioactive products identified within the resolution phase of acute sterile inflammation (illustrated in top panel of Figure 1) that activate pro-resolving mechanisms (Hong et al., 2003; Serhan et al., 2000; Serhan et al., 2002).
Focusing on self-limited resolving exudates also has permitted a direct assessment of the host’s responses that enable the return to homeostasis. Key bioassays that have proven critical in the initial studies focused on human neutrophil (PMN) transmigration across endothelial cells and epithelial cells (Colgan et al., 2013; Serhan et al., 2000) and the phagocytosis of cellular debris and dead PMN (Majno and Joris, 2004). Attention focused on these cellular responses because neutrophils are among the first responders to injury and microbial invasion. The hypothesis that endogenous chemical mediators are produced via cell-cell interactions within developing inflammatory exudates (i.e. pus) that control the size, magnitude and duration of the exudate turned out to be the case in animal models (Schwab et al., 2007; Serhan et al., 2002). This approach has proven to be highly relevant to human disease in that anti-PMN therapy (Takano et al., 1998) has been shown to limit tissue damage and uncontrolled inflammation.
The historical milestones in resolution of inflammation from observation of the gross cardinal signs of inflammation (Figure 1) to active resolution to new therapeutics first in humans are an exciting new area of investigation. In ancient medical texts of the 11th and 12th century, the notion of treating inflammation with “resolvents” as “mollificants” to help resolve disease was already present. This concept of stimulating resolution of inflammation was apparently lost until the chemical structures of endogenous mediators that stimulate resolution were finally elucidated (Serhan et al., 2002). From this line of investigation the resolvin, protectin and maresin pathways were elucidated and their functions in activating resolution of inflammation uncovered. Within exudates resolving to homeostasis, the fundamental cellular processes proved predictive of the actions of SPM in more complex animal disease models because the cessation of PMN entry into tissue and removal of dead PMN as well as cellular debris are central to all organs of the body and many human disease pathologies. This is what makes pro-resolving different from simple anti-inflammation or blocking mediators and natural mechanisms in the host. It is now apparent that a broad range of endogenous and exogenous agents belong to the immunoresolvents, pharmacologic agents or local endogenous mediators that stimulate resolution of inflammation in animal models and hopefully in human diseases (Dalli et al., 2013a).
Of interest, the n-3 essential fatty acids EPA and DHA were found to be substrates for the biosynthesis of potent anti-inflammatory pro-resolving endogenous mediators within inflammatory resolving exudates (Hong et al., 2003; Serhan et al., 2002). Identification of omega-3 fatty acids as nutrients that activate pro-resolving mechanisms in inflammation has opened new areas of investigation, since uncontrolled inflammation is now widely appreciated to be a unifying theme and the fundamental basis for many widely occurring diseases (Figure 1). It is worth noting that there already exists a large body of literature addressing the anti-inflammatory potential of EPA and DHA (for a recent review, see Calder, 2013). Yet, the molecular basis by which these essential fatty acids exert anti-inflammatory responses has remained the subject of intense discussion. DHA and EPA have many known critical functions in mammalian biology, and neither EPA nor DHA is produced by humans to any great extent and therefore must be absorbed within the diet (Calder, 2013). One area where the function of DHA and its mechanism is clear is the role of DHA in the retina. It has been argued that DHA is an ancient molecule with functional roles in the brain and eye imposed via evolutionary pressure (Crawford et al., 2013). Thus, uncovering special chemical mediators biosynthesized from n-3 fatty acids EPA, DHA and DPA during self-limited inflammatory responses in the resolution phase of mouse exudates with functions on individual human leukocytes has many implications. For further mechanistic details on the biosynthesis of the SPM (resolvins, protectins and maresins), see recent reviews covering their biosynthesis (Serhan, 2007), actions and total organic synthesis (Serhan and Petasis, 2011). In this review we focus on the SPM production in resolution and activities on phagocytes and disease models relevant to acute inflammation and the innate response.
RESOLVINS AND PROTECTINS
Specialized pro-resolving mediators (SPMs, Figures 1 and 2), derived from different polyunsaturated fatty acid (omega-3 EPA and DHA) substrates, exert diverse biological effects on immune function including the ability to counter-regulate mediators that trigger leukocyte trafficking (Figures 1 and 2). Of the essential polyunsaturated fatty acids, namely those that are not biosynthesized de novo to any great extent in humans, the omega-3 fatty acids such as DHA and EPA are supplied in the diet (De Caterina, 2011). Marine oils are rich in both DHA and EPA; in humans, DHA is concentrated in neural tissues including brain and retina and is enriched in human milk, plasma and sperm, where their physical properties in the membrane bilayers govern membrane fluidity (Calder, 2013; Crawford et al., 2013). Within the immune system, PMN influx or chemotaxis occurs along chemical gradients that are either from exogenous sources (degradation products of microbial proteins and lipids (e.g. LPS) or endogenous chemoattractants from the host (chemokines, e.g. IL-8 and arachidonic acid-derived leukotriene B4 (see Figure 1, left side)). These endogenous and exogenous factors also stimulate the non-phlogistic recruitment of monocytes, which positively affect the efferocytosis of apoptotic granulocytes (Figures 1 and 2). Thus, SPMs have overlapping pro-resolving actions most likely arising from the ability to bind and act as agonists on alternate SPM receptors (e.g. RvD1 on the lipoxin A4 (LXA4) receptor (Wang et al., 2011)). However, despite common actions, the source of different families of specialized pro-resolution mediators in inflammation appears diverse. Recent evidence suggests that RvE1 and RvE2 are biosynthesized by PMNs via the 5-LOX pathway (Oh et al., 2011), whilst mouse eosinophils are responsible for generation of 12/15-LOX-derived mediators PD1 and the recently discovered RvE3 (Isobe et al., 2012; Yamada et al., 2011). Deficiency of these cell types in tissues during the resolution phase may lead to impaired biosynthesis with deleterious consequences (Yamada et al., 2011). The same may be the case for the essential PUFAs (n-3 polyunsaturated fatty acids, i.e. EPA and DHA) at the inflammatory site.
Functionally, SPMs enhance bacterial clearance by stimulating mucosal production of bactericidal peptides (Colgan et al., 2013), enhancing bacterial phagocytosis by PMNs and macrophages and working synergistically with antibiotics to enhance their therapeutic action and hence bacterial clearance (Chiang et al., 2012). Importantly, despite encouraging resolution they do not compromise host immune competence (Serhan, 2007; Spite et al., 2009). Several of the pro-resolving mediators actually increase survival from infections in mouse models of disease (bacterial and viral infections) (Table 1). For instance, resolvin E1 (RvE1) administered prior to a murine model of aspiration pneumonia (hydrochloric acid with subsequent Escherichia coli challenge) is associated with a reduction in pro-inflammatory cytokines, decreased pulmonary PMN accumulation, enhanced bacterial clearance and improved survival (Seki et al., 2010). Mechanistically, RvE1, at concentrations as low as 1nM, enhances macrophage phagocytosis (Hong et al., 2008) as well as NADPH oxidase-mediated reactive oxygen species generation. Resolvins of the D-series appear equally efficacious as RvE1 (Dalli et al., 2013a). Resolvin D1 (RvD1) treatment prior to LPS-induced acute lung injury improves pathological indices and survival rates (Wang et al., 2011) and may be equally effective in central nervous system inflammation (Xu et al., 2013). The common mechanism appears to be suppression of NF-kB activation in a partly PPARγ-dependent manner, with associated reduction in downstream signalling and alterations in transcriptomics (Arita et al., 2005; Liao et al., 2012).
Table 1.
Resolvins and SPM in Resolution of Infections and Increasing Survival in Murine Infections
| Lipoxins, Resolvins and Protectins | Increase Survival | Disease | Shorten Resolution | Reference |
|---|---|---|---|---|
| LXA4 | √ | Bacterial infections | Walker et al. (2011) | |
| 15-epi- LXA4 | √ | Lung injury | El Kebir et al. (2009a) | |
| RvE1 | √ | Colitis | + | Arita et al. (2005) |
| √ | E. coli peritonitis | + | Chiang et al. (2012) | |
| √ | Candida yeast | + | Haas-Stapleton et al. (2007) | |
| √ | Acid-induced lung injury | + | Levy and Serhan (2014) | |
| RvD1, RvD5, PD1 | √ | E. coli peritonitis infection | + | Chiang et al. (2012) |
| RvD1 | √ | Acute lung injury | + | Wang et al. (2011) |
| RvD2 | √ | Cecal ligation and puncture sepsis | + | Spite et al. (2009) |
| √ | Burn wound sepsis | + |
Bohr et al. (2013) Kurihara et al. (2013) |
|
| Protectins | √ | H1N1 viral infections | Morita et al. (2013) |
Resolvin D2 (RvD2), but not its isomer trans-RvD2, improves survival in murine polymicrobial sepsis (cecal ligation and puncture (CLP)) (Table 1). Its actions are multifaceted, targeting the modulation of leukocyte-endothelial interactions directly (adhesion receptor expression) and via stimulation of endothelial nitric oxide (NO) production, altering the cytokine and eicosanoid profiles (reduced IL-17, IL-10, prostaglandin [PG]E2 and LTB4), enhancing bacterial phagocytosis and intraphagosomal vacuolar production of reactive oxygen species (ROS) (Spite et al., 2009). Recently the ability of RvD2 to restore directionality to neutrophil migration and prevent sepsis-induced immune dysfunction and thus increase survival from a secondary septic challenge post-burn injury has been demonstrated (Kurihara et al., 2013). Resolvin D3 appears later in the resolution phase of peritonitis, displaying potent actions limiting PMN infiltration and stimulating macrophage uptake of apoptotic cells (Dalli et al., 2013a). Hence, despite being inherently anti-inflammatory and pro-resolution in nature, SPMs exert diverse actions in host defense and pathogen interactions (Table 1) in a manner that is, paradoxically, conducive to optimal bacterial clearance and not immunosuppressive.
Maresins: Macrophage Mediators In Resolving Inflammation and Organ Regeneration
From study of resolution phase macrophages, a new pathway able to produce potent mediators from DHA has been uncovered and coined maresins. The stereochemical assignments of maresin 1 (MaR1) and its related isomers have been established (Serhan et al., 2012) as well as the key epoxide intermediate (Figure 2, lower panel) in the maresin biosynthetic pathway (Dalli et al., 2013b). MaR1 is produced by human macrophages from endogenous DHA and is the newest pathway and family of mediators identified. In these recent studies, MaR1 was obtained and matched to synthetic 7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid prepared by total organic synthesis. MaR1 alcohol groups and Z/E geometry of conjugated double bonds were assigned using isomers also prepared by total organic synthesis and confirmed using NMR. MaR1’s potent defining actions were confirmed with synthetic MaR1, namely limiting neutrophil (PMN) infiltration in murine peritonitis (ng/mouse range) as well as enhancing human macrophage uptake of apoptotic PMNs. Macrophage-derived microparticles contribute to the production of MaR1 and SPM in that they stimulate efferocytosis (Figure 2) as well as provide substrates and pathway intermediates in SPM biosynthesis to recipient cell types (Dalli and Serhan, 2012).
The appearance of MaR1 in the inflammatory response can be in the later stages with the entry of resolution phase macrophages that release maresins. MaR1 appears slightly more potent than Resolvin D1 (RvD1) in stimulating human macrophage efferocytosis, an action not shared by prostaglandins or leukotriene B4. MaR1 and RvE1 each accelerate tissue regeneration in planaria, increasing the rate of head reappearance. With surgery or injury of planaria (when cut in half), MaR1 is biosynthesized from deuterium-labeled (d5)-DHA indicating that this pathway is conserved in evolution from flatworm to humans (Serhan et al., 2012). Blocking this pathway delays organ regeneration, and MaR1 rescues this primordial response of the organism. MaR1 dose-dependently inhibited TRPV1 currents in neurons, blocked capsaicin-induced inward currents (IC50 ≈ 0.5 nM) and reduced both inflammatory and chemotherapy-induced neuropathic pain in mice. MaR1’s potent anti-inflammatory and pro-resolving actions were recently confirmed in murine colitis, where it is protective in both DSS and TNBS models of colitis (Marcon et al., 2013). Hence, MaR1 has multiple potent actions in regulating inflammation-resolution, tissue regeneration and resolving pain. These findings also suggest that shared chemical signals occur in biological processes as diverse as cellular trafficking in the resolution of inflammation to tissue regeneration in worms.
In addition to the assignments of SPM stereochemistry of the endogenous bioactive products (i.e. RvE1, RvD1, RvD2, RvD3, etc.) and structure function with materials prepared by organic synthesis (Serhan and Petasis, 2011), other groups have focused on the synthesis of these molecules that are now commercially available and expanded their roles and mechanisms in a range of systems relevant to human biology. Recently, the total organic synthesis of resolvins and MaR1 has also been achieved by Rodriguez and Spur (2012). Kobayashi et al. also reported on the total organic synthesis of protectin D1 and E-series resolvins (Ogawa and Kobayashi, 2011). Total organic synthesis of 18-HEPE, a precursor from 18-HpEPE of E-series resolvins (see Figure 2), has also been reported (Krishnamurthy et al., 2011), confirming the earlier biogenic synthesis of this previously undescribed structure and precursor (Serhan et al., 2000). Importantly, the stereoselective actions of each SPM have proven highly effective in regulating human PMN and monocytes in microfluidic chambers (Jones et al., 2012; Kasuga et al., 2008; Oh et al., 2012). Together, these clearly establish the direct actions of these compounds on human cells, where they can activate specific G-protein coupled receptors and hence their potential for therapeutic use in man.
Resolvins and lipoxin A4 are present in human breast milk in high amounts (Weiss et al., 2013), in fish such as salmon (Raatz et al., 2011), and are produced in humans following supplementation with omega-3 (EPA, DHA) in peripheral blood at amounts where they are found to be bioactive in experimental systems (Mas et al., 2012). Also, they are produced in humans following strenuous exercise (Markworth et al., 2013) and in human adipose tissue (Claria et al., 2013).
LEUKOTRIENES AND LIPOXINS: Arachidonic acid-derived mediators
Alongside omega-3-derived SPMs, arachidonic acid-derived lipoxins and their carbon 15 position epimers (Figures 1 and 3) also exert beneficial actions on inflammation and resolution (Chiang et al., 2005). Although the LX were first identified and biosynthesized from isolated human leukocytes and eosinophils (Samuelsson et al., 1987), their functions in inflammation in vivo remained to be established. For instance, in a rabbit model of periodontal disease, transgenic over expression of the 15-LOX type I is protective of inflammation-induced bone loss and dermal inflammation via increased production of LXA4 monitored using LC-MS-MS-based identification (Serhan et al., 2003). Also, treatment with LXA4 following inhaled LPS-induced lung injury has been demonstrated to limit inflammation (Jin et al., 2007) and to reduce pro- and anti-inflammatory cytokine production, enhance macrophage recruitment, reduce blood bacterial load and improve mortality in a rat CLP model (Walker et al., 2011). In the latter study, macrophage recruitment was increased without impairing phagocytic function, and systemic inflammation reduced without increasing bacterial spread, mirroring the actions of resolvins, namely RvE1 and RvD2 (Seki et al., 2010; Spite et al., 2009; and see refs. in Table 1).
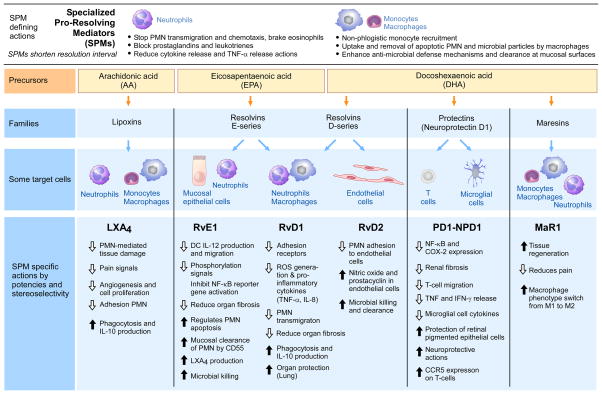
Figure 3. SPM Actions and Target Cell Type.
The defining actions of SPM in the innate response of interest in immunology. The actions to stimulate termination and resolution. The precursors essential polyunsaturated fatty acids (AA, EPA, DHA) are converted by leukocytes to separate chemically distinct families of mediators that stimulate active resolution responses of isolated cell types and tissues in vivo in animal models. Some of the specific target cell types and representative potent actions of the SPM members from each family of related structures are listed. All of these actions of SPMs are stereoselective and in the picogram-nanogram range potency. See Serhan and Chiang (2013) and text for further details.
A similar paradoxical relationship between an attenuated innate immune response (PMN trafficking to the infected site) versus increased host-pathogen interaction (as determined by survival) has been demonstrated in both wild-type mice treated with MK 886 (a 5-lipoxygenase [5-LOX] inhibitor) and in 5-LOX-deficient mice (Benjamim et al., 2005). This effect could be partially replicated if antagonists of cysteinyl-leukotrienes (cysteinyl-LTs, a family including LTC – F4) were given, but crucially not on antagonism of the classically pro-inflammatory leukotriene B4 (LTB4). This elegantly demonstrates the hierarchical, multi-faceted and often opposing effects of eicosanoids within infection and sepsis. In this setting it appears that the prevention of the cysteinyl-LT’s deleterious effects on the vasculature (and hence host haemodynamics) assumes primacy as the main cause of benefit in 5-LOX antagonism (or deletion). In contrast, selective LTB4 inhibition prior to and post cecal ligation and puncture (CLP) in a model of sepsis appears to have little effect on vascular tone and permeability, but may have blunted the innate immune response (specifically neutrophil trafficking), exacerbating the infective insult (Lammermann et al., 2013; Rios-Santos et al., 2003). Hence the 5-LOX-derived mediators play critical roles in the physiologic response of the host to infection.
The complex interplay between AA-derived LM in systemic inflammation has also been highlighted in recent results showing that flavocoxid, a dual cyclooxygenase (COX)-2 and 5-LOX inhibitor, reduces the expression of NF-kB, COX-2 and 5-LOX with improved survival in a murine-CLP model (Bitto et al., 2012). Plasma IL-10 and counter-intuitively LXA4 concentrations were increased whilst TNF-α, IL-6, LTB4 and PGE2 were decreased. The authors suggest that LXA4 is biosynthesized in mice via another biosynthetic route. For example, initiation via the combined mouse lipoxygenase that carries both 12-LOX and 15-LOX activities in murine systems (a type of 15-LOX found in humans) when both COX and 5-LOX are inhibited. Whether the improvement in outcome is due to enhanced pro-resolution effects driven by increased LXA4, decreased cytokine storm (TNF-α and IL-6), augmentation of the immune response via reducing PGE2 and 5-LOX-derived LTs (discussed below), selective shunting of AA down the COX or LOX pathways (Chen et al., 1994), or a combination of all the above is unclear.
Both biologically active ATL and aspirin-triggered resolvins (Serhan, 2007) trigger resolution (Figures 1 and 3) by inhibiting leukocyte trafficking in a NO-dependent manner in both murine (Paul-Clark et al., 2004) and human inflammation (Morris et al., 2009) and by down-regulating superoxide production in neutrophils along with macrophage inflammatory peptide 2 and IL-1β production (Serhan, 2007). The importance of ATL and LXA4 has previously been demonstrated in severe inflammation: the absence of LXA4 leading to un-bridled inflammation and elevated mortality in animal models of infection due to DC hypersensitivity. Along these lines, certain parasites can stimulate supraphysiologic amounts of LXA4 as part of their highly evolved mechanism to evade the host response (Bannenberg et al., 2004; Serhan, 2007). Indeed, administration of 15-lipoxygenase to mice stimulates endogenous LXA4 and is anti-inflammatory, and certain parasites carry their own 15-lipoxygenase to presumably act on host substrates. Moreover, during Mycobacterium tuberculosis infection, 5-lipoxyganse-derived LXA4 is elevated, dampening T helper-1 (Th1) cell cytokine responses essential for bacterial clearance. Thus, not only are lipoxins used by infectious agents to parry host defence responses, but inhibitors of their biosynthesis could prove useful in clearing such recalcitrant infections as tuberculosis (Bafica et al., 2005).
Prostaglandins (PGs) AND RESOLUTION: It’s time and place that counts!
PGs derived from COX metabolism of arachidonic acid regulate the changes in blood flow required for leukocytes to exit the post-capillary venules (Figure 1) in the initiation phase as well as mediate the class switching of lipid mediators (Levy et al., 2001) required for termination of the acute response (Figure 2, upper panel) and can exert immuno-suppressive effects through upregulation of intracellular cAMP (Aronoff et al., 2006; Luo et al., 2004). PGE2 and PGI2, for example, reduce the ability of inflammatory leukocytes to phagocytose and kill microorganisms (Aronoff et al., 2004; Rossi et al., 1998; Weinberg et al., 1985), as well as inhibit the production of downstream pro-inflammatory mediators (Aronoff et al., 2007; Takayama et al., 2002; Xu et al., 2008). In contrast, these PGs enhance the production of IL-10 (Harizi et al., 2002). Indeed, overexpression of PGE2 has been reported in a number of conditions associated with increased susceptibility to infection, including cancer (Starczewski et al., 1984), aging (Hayek et al., 1997) and cystic fibrosis (Medjane et al., 2005; Strandvik et al., 1996). Interestingly, PGE2, generated during the onset phase of inflammation, also indirectly elicits pro-resolution actions by switching on the transcription of enzymes required for the biosynthesis of other classes of bioactive lipids that are potent pro-resolution mediators, such as LXs (Levy et al., 2001), resolvins (Rvs) and protectins (PDs) (Hong et al., 2003; Marcheselli et al., 2003; Serhan et al., 2000; Serhan et al., 2002).
While PGD2 can elicit immuno-modulatory and anti-inflammatory effects in the same manner as described for PGE2 and PGI2 via ligation to DP1 (Figure 1, upper panel), it can also act independently of DP1 and DP2 receptor activation when non-enzymatically dehydrated into biologically active PGs of the J2 series (e.g. PGJ2, Δ12,14-PGJ2 and 15-deoxy-Δ12,14-PGJ2 [15d-PGJ2]) (Diab et al., 2002). These cyclopentenone PGs, as they are so called, form covalent attachments with reactive sulphydryl groups on intracellular regulatory proteins, enabling them to modulate their function (Kim et al., 2006; Oliva et al., 2003; Renedo et al., 2007). 15d-PGJ2 for example, via ligation to the nuclear receptor PPAR-γ (Khan, 1995), decreases pro-inflammatory cytokine release and modifies gene expression (Ricote et al., 1998) as well as directly inhibits the actions of IκB kinase (IKK), the kinase responsible for the activation of NF-κB (Cernuda-Morollon et al., 2001; Straus et al., 2000). Independent of PPAR-γ, 15d-PGJ2 can preferentially inhibit monocyte rather than neutrophil trafficking through differential regulation of cell-adhesion molecule and chemokine expression (Gilroy et al., 2003; Pasceri et al., 2000); regulate macrophage activation and pro-inflammatory gene expression (Lawrence, 2002); and induce leukocyte apoptosis through a caspase-dependent mechanism (Bishop-Bailey and Hla, 1999; Gilroy et al., 2003; Lawrence et al., 2001). Moreover, it has been illustrated that PGD2-derived compounds act as endogenous braking signals for lymphocytes to stimulate resolution (Trivedi et al., 2006).
It is now clear that the inflammatory programme contains key checkpoints as well as temporal and spatial switches that regulate its onset, maintenance and resolution. It turns out that the resolution phase of inflammation is just as actively orchestrated and carefully choreographed as its induction and maintenance. A key finding in recent years is that the biosynthetic pathways required for inflammation to resolve often require the prior production of lipid mediators that are produced locally during the initiation of acute inflammatory response. In particular, PGE2 and PGD2, which are responsible for inflammation induction, subsequently stimulate anti-inflammatory circuits by inducing 15-LOX in neutrophils. This class switch in eicosanoid production, from LTs and PGs to lipoxins (Levy et al., 2001), provides temporal and spatial check points during the inflammatory cascade (the lipid mediator class switch is illustrated in Figure 1).
Whereas the anti-inflammatory prostanoids (e.g. PGD2 and PGE2) can limit the extent of inflammation, they and their metabolites can also stimulate active tissue remodelling and repair; in other words, class switching may not always be required for resolution to be initiated. Within a single pathway of the eicosanoid family initiated by COX-2 and subsequently regulated by the enzyme hematopoietic PGD2 synthase (hPGD2S), pro-resolving mediators PGD2 and 15d-PGJ2 are produced at sufficient amounts in vivo (Figure 1, upper panel) to drive the resolution of acute inflammation (Rajakariar et al., 2007).
Abandoned for over 15 years primarily due to one null randomized-controlled trial (Bernard et al., 1997), the concept of COX-inhibition and hence lipid mediator manipulation in severe inflammatory states is re-gaining traction (Aronoff, 2012; Eisen, 2012). A key factor in this has been the recent publication of multiple observational data sets relating predominantly aspirin and statin administration, but also non-steroidal anti-inflammatory agents (NSAIDs), to clinical benefit. This information, coupled with a more advanced appreciation of how inflammatory resolution-pathways, and local-acting lipid mediators in particular, may impact on immune competence, necessitates a careful clinical re-appraisal of validity of these drugs.
CELLS OF THE RESOLUTION CASCADE
Inflammation does not occur in isolation, but in distinct organs with quite different anatomical locations and physiological functions. The architecture of organs is very closely adapted to their function. Tissue-resident stromal cells such as fibroblasts, endothelial and epithelial cells define the microanatomy and architecture of organs and provide the appropriate microenvironment in which specialized immune functions can occur. In addition to their landscaping properties these stromal cells are not just passive players in immune-mediated inflammatory responses but play an active role in governing the persistence of inflammatory disease, as well as enabling immunological memory to become established in a site-specific manner (Hammerschmidt et al., 2008). The response of the immune system to tissue damage involves a carefully choreographed series of cellular interactions between immune and non-immune cells (Figure 1). Immune cells require stromal cells in order to home to and survive within the site of inflammation. Given that all inflammatory reactions take place within a defined background of specialised stromal cells, understanding the biology of both hematopoietic and non-hematopoietic cells is important in understanding how inflammatory cell infiltrates become established and persistent in chronic immune-mediated inflammatory diseases.
Several specialized cell phenotypes have been implicated in the resolution of inflammation (Figure 1). Monocyte–derived macrophages in particular exhibit a wide-range of phenotypic plasticity, the origins of which lie in specific tissue- and hematopoietic cell-derived stimuli (Sica and Mantovani, 2012). These lineage sub-types may exert a wide range of effects both pro and anti-inflammatory, beneficial and detrimental, in diverse disease states (Mantovani et al., 2004) and in response to different pathogens (Mege et al., 2011). Our group has recently described a previously unknown resolution-phase macrophage denoted (rM) that possesses a hybrid phenotype of alternative activation, mannose receptor expression and synthesis of IL-10 and arginase 1 (classically M2, anti-inflammatory (Gordon, 2003)) with high COX2 and iNOS expression (classically M1, pro-inflammatory) (Bystrom et al., 2008). This rM phenotype, inducible by elevating intra-cellular cAMP, is vital in encouraging innate-type lymphocyte repopulation of inflamed cavities (Bystrom et al., 2008), a key step in restoring tissue homeostasis and controlling responses to secondary infective challenge (Rajakariar et al., 2008). Transcriptomic analysis of rM has suggested a potential role in antigen processing and presentation, T- and B-lymphocyte recruitment, termination of inflammatory cell trafficking and clearance but also highlighted the naivety of attempting to rigidly define and categorize such plastic cells in a dynamic environment (Stables et al., 2011).
Medeiros and colleagues (Medeiros et al., 2009) have shown that the efferocytosis of apoptotic cells by activated macrophages causes suppression of Fcγ receptor-mediated phagocytosis and bacterial killing by an autocrine and/or paracrine PGE2 mechanism in the lung. E-prostanoid 2 (EP2) receptor-mediated elevation of intracellular cAMP appeared to be the primary pathway with apoptotic cell-induced functional impairment, being reversed with aspirin or indomethacin pre-treatment and direct EP2-receptor antagonists (Medeiros et al., 2009). Other authors have implicated TGF-β (Freire-de-Lima et al., 2006), potentially via PGE2 again (directly or indirectly (Diaz et al., 1989)) or 15-LOX-derived LXA4 (Freire-de-Lima et al., 2006), and an IFN-γ activated NO mechanism (Ren et al., 2008) as the soluble immunosuppressive mediators involved in the effect of apoptotic cells on phagocytes and their resultant function. This heterogeneity indicates that the exact pathway of apoptotic cell immune regulation is pleiotropic and may depend on the type and activation state of the efferocytosing phagocyte (Dalli and Serhan, 2012; Medeiros et al., 2009; Ryan and Godson, 2010).
More recent as yet unpublished results have highlighted that these cells are phenotypically similar to myeloid-derived suppressor cells (MDSC). In mice, MDSC’s represent a heterogeneous group of GR-1+CD11b+ cells, comprised of both granulocytic and monocytic subsets - the precursors of neutrophils, macrophages and dendritic cells (DC) (Gabrilovich and Nagaraj, 2009). They are notable for their potent ability to suppress T-cell proliferation and responses, in particular Th1 cells, through production of nitric oxide and reactive oxygen species (especially peroxynitrite), and for their expression upon activation of immune suppressive inducible nitric oxide synthase, arginase and a range of both pro and anti-inflammatory cytokines (Gabrilovich and Nagaraj, 2009). They may also induce the de novo development of T-regulatory cells (Treg) (Huang et al., 2006).
Like MDSC, regulatory T-cells (Treg) have an increasingly appreciated role in inflammatory resolution (Venet et al., 2008). These CD4+ T cells are characterized by high surface expression of CD25 (IL-2Rα chain), the T-cell inhibitory receptor CTL-associated antigen 4 (CTLA-4), the glucocorticoid-induced TNF receptor (GITR) and the transcriptional regulator forkhead box p3 (Foxp3+), the latter correlating with Treg cell suppressive activity (Campbell and Ziegler, 2007). Treg cells may be subdivided into natural and induced (or adaptive) Treg cells. The former arises during the normal process of maturation in the thymus, the latter developing from conventional CD4+CD25− T-cells in the periphery under specific stimulatory conditions elicited by infection and collateral tissue damage (Bluestone and Abbas, 2003). At the site of inflammation, T-reg cells exert three major immunomodulatory effects: direct killing of cytotoxic cells, inhibition of pro-inflammatory cytokine secretion by cytotoxic cells (especially IL-2 and TNF-α) and secretion of anti-inflammatory cytokines (notably TGF-β and IL-10) (Kessel et al., 2009). Classically studied in the setting of chronic infection, the potential for Treg cell numbers, activation and functional capacity to influence the outcome of pathogen-host interactions is well established (Belkaid and Rouse, 2005). Their role in the resolution of organ-specific autoimmune inflammation and ability to attenuate subsequent reactions has recently been described (Rosenblum et al., 2011), while in LPS-induced acute lung injury depletion of Treg cells has been associated with worse outcomes (D’Alessio et al., 2009) as was abolition of their chemotactic gradient (Wang et al., 2012),
Implications for treatment: Resolution of inflammation without immune suppression?
It is now increasingly clear that the inflammatory cascade has a number of tactically placed controllers and checkpoints that limit the magnitude and duration of its response. However, the identification, isolation and molecular dissection of the new families of specialized pro-resolving mediators described in this review (Figure 3) will encourage a move away from focusing on the development of yet more anti-inflammatory, potentially “resolution toxic” drugs towards those that are “resolution friendly” replacement therapies (Chiang et al., 2008; Gilroy et al., 2004; Schwab et al., 2007). While the total organic synthesis of the natural SPM is challenging and costly, requiring many steps, the syntheses as well as those of biologically and chemically stable mimetics have been achieved (O’Sullivan et al., 2007; Serhan and Petasis, 2011). Along these lines, a resolvin E1 mimetic that has performed well in Phase I and 2 clinical trials in patients with ocular inflammation is currently in Phase III clinical trial1. Other SPMs, including PD1 and MaR1, are also in clinical development programs with the goal of stimulating the endogenous resolution programs of inflammation.
Although the SPMs and pro-resolving prostaglandins are attractive new therapeutic targets they have some inherent pharmacological limitations that will need to be overcome before they can be easily adopted for clinical use. For example, time of administration in the inflammatory cascade may turn out to be critical, particularly since the tight spatial and temporal regulation of prostaglandins may be difficult to recapitulate in humans, where timing the start of an inflammatory insult can be challenging. It remains unclear whether the SPMs and pro-resolving prostaglandins will have any therapeutic efficacy in chronic persistent inflammation as opposed to acute, potentially resolving inflammatory conditions such as sepsis. Equally, while the differential role of lipid mediators in host defence is becoming increasingly appreciated, it must be emphasised that while certain families including lipoxins and resolvins can each prime leukocytes for bacterial killing, while others, namely prostaglandins, impair this process. Inspired from research on tuberculosis infection (Bafica et al., 2005), it may be possible, therefore, to tailor lipid mediators, mimetic and/or inhibitors of their biosynthetic pathways in specific cell types to tackle certain classes of bacterial infections.
Developing new pro-resolving agents will mean producing stable, cost-effective pharmacological agents that limit leukocyte (e.g. PMN) traffic, encourage PMN apoptosis at sites of inflammation and tissue injury as well as clearance, and help restore the stromal microenvironment to its pre-inflammatory state. This is likely to benefit patients with defects in the resolution of a range of diseases not only including inflammation, but also infection and possibly cancer. But such an approach will require a change in philosophy away from inhibition and depletion strategies towards activation and replacement. It will also require a search for defects in the resolution of inflammation in clinical settings: leaky resolution as the cause of persistent disease rather than the lack of sufficient anti-inflammatory therapy. Perhaps only then will the next generation of compounds, namely immunoresolvents, with the potency and proresolving mechanisms of aspirin and steroids, but without the crippling side effects of bleeding and immune suppression, emerge.
Acknowledgments
C.N.S. thanks Mary Halm Small for expert assistance in manuscript preparation and support from National Institutes of Health grants P01GM095467 and R01GM38765 (C.N.S.). D.W.G. is a Wellcome Trust Funded Senior Research Fellow. Our apologies to the many investigators and collaborators we have been unable to cite in this review because of space limitations.
Abbreviations
- SPM
specialized pro-resolving mediators
- 17R-HDHA
17R-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid
- AT
aspirin-triggered
- AT-RvD3
4S,11R,17R-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- LT
leukotriene
- LTB4
leukotriene B4, 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid
- LXA4
lipoxin A4, 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid
- LXB4
lipoxin B4, 5S,14R,15S-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid
- MaR1
maresin 1, 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid
- PD1-NPD1
protectin D1-neuroprotectin D1, 10R,17S-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid
- PG
prostaglandin
- PGE2
Prostaglandin E2, 9-oxo-11R,15S-dihydroxy-5Z,13E-prostadienoic acid
- Rv
resolvin
- RvD1
resolvin D1, 7S, 8R,17S-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvD2
resolvin D2, 7S, 16R, 17S-trihydroxy-4Z, 8E, 10Z, 12E, 14E,19Z- docosahexaenoic acid
- RvD3
resolvin D3, 4S,11R,17S-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid
- RvD5
Resolvin D5, 7S,17S-dihydroxy-4Z,8E,10Z,13Z,15E,19Z-docosahexaenoic acid
- RvE1
resolvin E1, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid
- RvE2
resolvin E2, 5S,18R-trihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid
Footnotes
http://www.anidapharma.com/science.html; http://www.auventx.com/auven/products/RX10045.php; http://www.resolvyx.com/news-pubs/releases/050108.asp; http://www.resolvyx.com
Disclosure: C.N.S. is inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. C.N.S. was scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. C.N.S.’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM. Cyclooxygenase inhibition in sepsis: is there life after death? Mediators Inflamm. 2012;2012:696897. doi: 10.1155/2012/696897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006;26:827–833. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, Moore BB, Peebles RS, Faccioli LH, Peters-Golden M. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol. 2007;178:1628–1634. doi: 10.4049/jimmunol.178.3.1628. [DOI] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Aliberti J, Hong S, Sher A, Serhan CN. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis. J Exp Med. 2004;199:515–523. doi: 10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- Benjamim CF, Canetti C, Cunha FQ, Kunkel SL, Peters-Golden M. Opposing and hierarchical roles of leukotrienes in local innate immune versus vascular responses in a model of sepsis. J Immunol. 2005;174:1616–1620. doi: 10.4049/jimmunol.174.3.1616. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336:912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- Bitto A, Minutoli L, David A, Irrera N, Rinaldi M, Venuti FS, Squadrito F, Altavilla D. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Critical care. 2012;16:R32. doi: 10.1186/1364-8535-16-R32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-Deoxy-Delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J Biol Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Nguyen BT, Madenci A, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141–C1149. doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Ehrentraut SF, Glover LE, Kominsky DJ, Campbell EL. Contributions of neutrophils to resolution of mucosal inflammation. Immunol Res. 2013;55:75–82. doi: 10.1007/s12026-012-8350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MA, Broadhurst CL, Guest M, Nagar A, Wang Y, Ghebremeskel K, Schmidt WF. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot Essent Fatty Acids. 2013;88:5–13. doi: 10.1016/j.plefa.2012.08.005. [DOI] [PubMed] [Google Scholar]
- D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013a;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013b;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Diaz A, Varga J, Jimenez SA. Transforming growth factor-beta stimulation of lung fibroblast prostaglandin E2 production. J Biol Chem. 1989;264:11554–11557. [PubMed] [Google Scholar]
- Eisen DP. Manifold beneficial effects of acetyl salicylic acid and nonsteroidal anti-inflammatory drugs on sepsis. Intensive care medicine. 2012;38:1249–1257. doi: 10.1007/s00134-012-2570-8. [DOI] [PubMed] [Google Scholar]
- El Kebir D, József L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12-14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. FASEB J. 2003;17:2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Haas-Stapleton EH, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator Resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159:2445–2451. [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S-W, Zhang L, Lian Q-Q, Liu D, Wu P, Yao S-L, Ye D-Y. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth Analg. 2007;104:369–377. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, Irimia D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci U S A. 2012;109:20560–20565. doi: 10.1073/pnas.1210269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel A, Bamberger E, Masalha M, Toubi E. The role of T regulatory cells in human sepsis. J Autoimmun. 2009;32:211–215. doi: 10.1016/j.jaut.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Khan MM. Regulation of IL-4 and IL-5 secretion by histamine and PGE2. Adv Exp Med Biol. 1995;383:35–42. doi: 10.1007/978-1-4615-1891-4_5. [DOI] [PubMed] [Google Scholar]
- Kim EH, Na HK, Surh YJ. Upregulation of VEGF by 15-deoxy-Delta12,14-prostaglandin J2 via heme oxygenase-1 and ERK1/2 signaling in MCF-7 cells. Ann N Y Acad Sci. 2006;1090:375–384. doi: 10.1196/annals.1378.041. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy VR, Dougherty A, Haller CA, Chaikof EL. Total synthesis and bioactivity of 18R-hydroxy eicosapentaenoic acid. J Org Chem. 2011;76:5433–5437. doi: 10.1021/jo2002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, Tompkins RG, Fagan SP, Irimia D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013;27:2270–2281. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. Modulation of inflammation in vivo through induction of the heat shock response, effects on NF-kappaB activation. Inflamm Res. 2002;51:108–109. doi: 10.1007/BF02684012. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:27.21–27.26. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dalli J, Chiang N, Baron RM, Quintana C, Serhan CN. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by microRNA and proresolving mediators. Immunity. 2013;39:885–898. doi: 10.1016/j.immuni.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Jones SM, Phare SM, Coffey MJ, Peters-Golden M, Brock TG. Protein kinase A inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J Biol Chem. 2004;279:41512–41520. doi: 10.1074/jbc.M312568200. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. 2. New York: Oxford University Press; 2004. [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol. 2013;191:4288–4298. doi: 10.4049/jimmunol.1202743. [DOI] [PubMed] [Google Scholar]
- Markworth JF, Vella LD, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, Maddipati KR, Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1281–R1296. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. The Journal of experimental medicine. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjane S, Raymond B, Wu Y, Touqui L. Impact of CFTR DeltaF508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L816–824. doi: 10.1152/ajplung.00466.2004. [DOI] [PubMed] [Google Scholar]
- Mege JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis. 2011;24:230–234. doi: 10.1097/QCO.0b013e328344b73e. [DOI] [PubMed] [Google Scholar]
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- O’Sullivan TP, Vallin KS, Shah ST, Fakhry J, Maderna P, Scannell M, Sampaio AL, Perretti M, Godson C, Guiry PJ. Aromatic lipoxin A4 and lipoxin B4 analogues display potent biological activities. Journal of medicinal chemistry. 2007;50:5894–5902. doi: 10.1021/jm060270d. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Kobayashi Y. Total synthesis of the antiinflammatory and proresolving protectin D1. Tetrahedron Lett. 2011;52:3001–3004. [Google Scholar]
- Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva JL, Perez-Sala D, Castrillo A, Martinez N, Canada FJ, Bosca L, Rojas JM. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J2 binds to and activates H-Ras. Proc Natl Acad Sci U S A. 2003;100:4772–4777. doi: 10.1073/pnas.0735842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatz SK, Golovko MY, Brose SA, Rosenberger TA, Burr GS, Wolters WR, Picklo MJ., Sr Baking reduces prostaglandin, resolvin, and hydroxy-fatty acid content of farm-raised Atlantic salmon (Salmo salar) J Agric Food Chem. 2011;59:11278–11286. doi: 10.1021/jf202576k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan GJ, Fitzgerald D, Yaqoob MM, Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakariar R, Lawrence T, Bystrom J, Hilliard M, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol. 2008;181:3277–3284. doi: 10.4049/jimmunol.181.5.3277. [DOI] [PubMed] [Google Scholar]
- Renedo M, Gayarre J, Garcia-Dominguez CA, Perez-Rodriguez A, Prieto A, Canada FJ, Rojas JM, Perez-Sala D. Modification and activation of Ras proteins by electrophilic prostanoids with different structure are site-selective. Biochemistry. 2007;46:6607–6616. doi: 10.1021/bi602389p. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Rios-Santos F, Benjamim CF, Zavery D, Ferreira SH, de Cunha FQ. A critical role of leukotriene B4 in neutrophil migration to infectious focus in cecal ligaton and puncture sepsis. Shock. 2003;19:61–65. doi: 10.1097/00024382-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Rodriguez AR, Spur BW. Total synthesis of the macrophage derived anti-inflammatory lipid mediator Maresin 1. Tetrahedron Lett. 2012;53:4169–4172. [Google Scholar]
- Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AG, McCutcheon JC, Roy N, Chilvers ER, Haslett C, Dransfield I. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J Immunol. 1998;160:3562–3568. [PubMed] [Google Scholar]
- Ryan A, Godson C. Lipoxins: regulators of resolution. Curr Opin Pharmacol. 2010;10:166–172. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. Journal of immunology. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13:632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation-resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczewski M, Voigtmann R, Peskar BA, Peskar BM. Plasma levels of 15-keto-13,14-dihydro-prostaglandin E2 in patients with bronchogenic carcinoma. Prostaglandins Leukot Med. 1984;13:249–258. doi: 10.1016/0262-1746(84)90037-4. [DOI] [PubMed] [Google Scholar]
- Strandvik B, Svensson E, Seyberth HW. Prostanoid biosynthesis in patients with cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 1996;55:419–425. doi: 10.1016/s0952-3278(96)90125-8. [DOI] [PubMed] [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- Trivedi SG, Newson J, Rajakariar R, Jacques TS, Hannon R, Kanaoka Y, Eguchi N, Colville-Nash P, Gilroy DW. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc Natl Acad Sci U S A. 2006;103:5179–5184. doi: 10.1073/pnas.0507175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin A4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther. 2011;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao L, Lv J, Yin Q, Liang X, Chu Y, He R. BLT1-dependent alveolar recruitment of CD4(+)CD25(+) Foxp3(+) regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med. 2012;186:989–998. doi: 10.1164/rccm.201202-0261OC. [DOI] [PubMed] [Google Scholar]
- Weinberg DA, Weston LK, Kaplan JE. Influence of prostaglandin I2 on fibronectin-mediated phagocytosis in vivo and in vitro. J Leukoc Biol. 1985;37:151–159. doi: 10.1002/jlb.37.2.151. [DOI] [PubMed] [Google Scholar]
- Weiss GA, Troxler H, Klinke G, Rogler D, Braegger C, Hersberger M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013;12:89. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MX, Tan BC, Zhou W, Wei T, Lai WH, Tan JW, Dong JH. Resolvin d1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS Neurosci Ther. 2013;19:235–243. doi: 10.1111/cns.12069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL, Jr, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute periotonitis by producing proresolving mediators in mice. FASEB J. 2011;25:561–568. doi: 10.1096/fj.10-170027. [DOI] [PubMed] [Google Scholar]
- Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]