Abstract
PSA-TRICOM (PROSTVAC) is a novel vector-based vaccine designed to generate a robust immune response against prostate-specific antigen (PSA)–expressing tumor cells. The purpose of this report is to present an overview of both published studies and new data in the evaluation of immune responses to the PSA-TRICOM vaccine platform, currently in phase III testing. Of 104 patients tested for T-cell responses, 57% (59/104) demonstrated a ≥ 2-fold increase in PSA-specific T cells 4 weeks after vaccine (median 5-fold increase) compared with pre-vaccine, and 68% (19/28) of patients tested mounted post-vaccine immune responses to tumor-associated antigens not present in the vaccine (antigen-spreading). The PSA-specific immune responses observed 28 days after vaccine (i.e., likely memory cells) are quantitatively similar to the levels of circulating T cells specific for influenza seen in the same patients. Measurements of systemic immune response to PSA may underestimate the true therapeutic immune response (as this does not account for cells that have trafficked to the tumor) and does not include antigen-spreading. Furthermore, while the entire PSA gene is the vaccine, only one epitope of PSA is evaluated in the T-cell responses. Since this therapeutic vaccine is directed at generating a cellular/Th1 immune response (T-cell costimulatory molecules and use of a viral vector), it is not surprising that < 0.6% of patients (2/349) tested have evidence of PSA antibody-induction following vaccine. This suggests that post-vaccine PSA kinetics were not affected by PSA antibodies. An ongoing phase III study will evaluate the systemic immune responses and correlation with clinical outcomes.
Keywords: antigen cascade, antigen spreading, cancer vaccine, clinical trial
Introduction
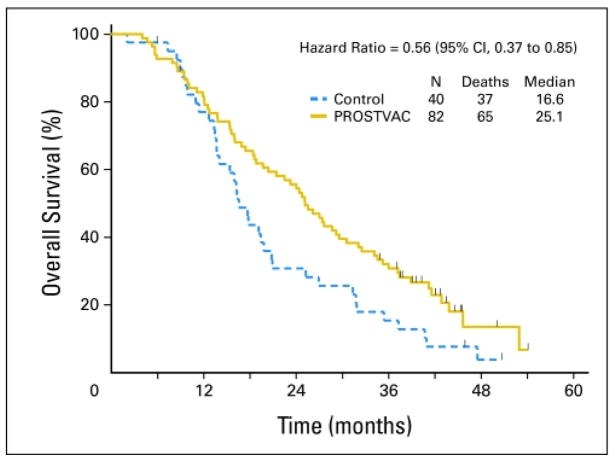
PROSTVAC is a vector-based therapeutic cancer vaccine composed of a series of poxviral vectors (vaccinia during the initial priming vaccine and fowlpox for all boosts) engineered to express prostate-specific antigen (PSA) and a triad of human T-cell costimulatory molecules (B7.1, ICAM-1, and LFA-3, designated TRICOM) (1). The vaccine is designed to break immunologic tolerance to PSA and initiate a robust immune response against prostate cancer cells. Early studies of PROSTVAC demonstrated the safety and immunologic activity of this approach in men with advanced prostate cancer (2–6), and a multicenter randomized phase II study showed preliminary evidence of improved overall survival (OS) (Fig. 1) (7). Based on these findings, an international randomized placebo-controlled phase III study is currently underway (8). This registration endpoint study is designed to confirm an association between the use of PROSTVAC and improved OS in men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer.
Figure 1.
Kaplan-Meier curve of overall survival. Solid gold line: PROSTVAC arm. Dashed blue line: control arm. Vertical ticks: censoring times. Estimated median overall survival: 25.1 months (PROSTVAC arm) vs. 16.6 months (control arm). Used with permission (7).
Findings of a small phase II study (n = 32) suggested that patients who mounted the highest (≥ 6-fold) increase in PSA-specific T cells, as measured by ELISPOT assay pre- and post-vaccine, had improved OS compared with patients who did not mount as great an increase in PSA-specific T cells (9). This hypothesis-generating finding is consistent with the putative mechanism suggested by preclinical models: an increase in tumor-specific CD8+ T cells.
Preclinical studies of poxviral-vector vaccines containing tumor-associated antigens (TAAs) and encoding TRICOM in self-antigen murine models have shown that the vaccine’s ability to treat tumors is completely abrogated by the depletion of CD8+ T cells, and partially abrogated by the depletion of CD4+ T cells and natural killer (NK) cells (10). Thus, we have sought to understand the impact of this vaccine primarily by analyzing compiled raw data from multiple trials (some previously published), focusing on the vaccine’s ability to generate tumor-specific T cells. This information will directly expand our understanding of the vaccine’s potential mechanism of action, and provide data for the first time to suggest that using PSA kinetics following this PSA-based vaccine is not confounded by the generation of a PSA-antibody response.
Materials and Methods
Collection of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs; apheresis for some patients) were collected prior to and 4 weeks after vaccinations (around day 28). PBMCs were isolated by Ficoll (Amersham Biosciences; Piscataway, NJ) density gradient separations, washed 3 times, and cryopreserved in liquid nitrogen at a concentration of 1–2 × 107 cells/mL until assayed.
ELISPOT
A modification of the procedure described by Gulley et al. (11) was performed, using K562/A*0201 as antigen-presenting cells (APCs), as previously reported (12). We used T-cell responses to influenza MP 58–66 and HIV peptides as positive and negative controls, respectively, and analyzed T-cell responses to the TAAs PSA, mucin 1 (MUC1), prostatic acid phosphatase (PAP), prostate-specific membrane antigen (PSMA), prostate stem cell antigen (PSCA), brachyury, AN07, XAGE-1, and PAGE-4. A positive response was defined as a ≥ 2-fold increase in TAA-specific T cells following vaccination. Additionally, the same frozen aliquot of PBMCs from a healthy donor was used in all assays with the positive and negative controls to confirm that there was no deviation above 20% of spots from assay to assay. The post-vaccine immune responses are noted as the maximal post-vaccine immune response.
Flow cytometry analysis
Multicolor flow cytometry analysis was performed on cryopreserved PBMCs by staining for 30 minutes at 4°C with CD3-V450, CD8-FITC or APC, HLA-DR-PerCPCy5.5, CD25-PECy7, CD45RA-PerCP-Cy5.5, CD62L-FITC, CD127-V450, CCR7-PE-Cy7, Tim-3-AF700, CD4-APC-Cy7, CTLA-4-FITC, and FOXP3-APC (BD Biosciences, San Jose, CA). For NK cells, CD3-V450, CD16-APC-Cy7, and CD56-PE-Cy7 were used. For MDSCs, CD33-PE, CD11b-APC-Cy7, HLA-DR-PerCP-Cy5.5, CD14-V450 and CD15-APC were used. 1×105 cells were acquired on an LSRII (BD Biosciences; San Jose, CA), and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). The appropriate isotype controls were used, and dead cells were excluded from the analysis.
Analysis of anti-PSA antibodies
Anti-PSA antibodies in the serum of patients pre- and post-vaccine were assessed by ELISA, as described by Madan et al. (13). Patient sera and normal human serum were diluted starting at 1:50. We performed 3 serial dilutions of 1:5 and a final dilution of 1:6250. We used purified mouse anti-PSA immunoglobulin (IgG1) antibody (Fitzgerald Industries, Concord, MA) as positive control for PSA binding. MOPC-21 (IgG1 antibody; Sigma-Aldrich, St Louis, MO) was used as isotype-matched control.
Cytokine detection
Serum samples pre- and post-vaccine were screened for IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p70, IL-13, and TNF-α using a multiplex cytokine/chemokine kit (Meso Scale Discovery, Gaithersburg, MD).
Statistical Methods
The Kaplan-Meier method was used to analyze overall survival and the log-rank test was used to compare strata. Distributions of paired data were tested against zero with the Wilcoxon signed-rank test. Spearman’s correlation coefficient was used to show the association between 2 variables.
Results
Induction of PSA-specific T cells (compiled data)
To date, the NCI has conducted 7 clinical trials of poxviral vaccines encoding PSA, some of which have yielded data on immune responses. All of these trials were prospectively designed to evaluate immune response, and all have previously been published (Supplemental Figure 1) (5, 9, 11, 13–17). Of the 104 patients tested, 57% (59/104) had a ≥ 2-fold increase in PSA-specific T cells following vaccine, as measured by IFN-γ ELISPOT assay (Table 1A). Of these 59 patients, the median number of PSA-specific T cells 28 days post-vaccine was 30/million PBMCs (Table 1B). By comparison, the median baseline level of influenza-specific T cells in those same 59 patients was 33.3/million PBMCs. These levels of influenza-specific T cells are similar to what we have observed in multiple studies.
Table 1.
PSA-specific T cells induced after vaccination with poxviral vaccines encoding PSA
| A. | ||
|---|---|---|
| Disease state | % of patients with PSA+ ELISPOT (≥ 2-fold increase) | Trial (NCT #) (ref) |
| localized | 72.0% (18/25) | NCT00005916 (11, 15) |
| bCRPC | 62.5% (5/8) | NCT00020254 (14) |
| bCRPC | 25.0% (1/4) | NCT00450463 (17) |
| mCRPC | 48.6% (17/35) | NCT00060528 (9) |
| mCRPC | 11.1% (1/9) | NCT00113984 (13) |
| mCRPC | 73.9% (17/23) | NCT00045227 (16) |
| Total | 56.7% (59/104) | |
| B. | ||
|---|---|---|
| Median | Min. – Max. | |
| Baseline PSA-specific T cells* | 5.00 | 5.00 – 20.00 |
| Maximum post-vaccine PSA-specific T cells* | 30.00 | 10.00 – 202.51 |
| Fold increase in PSA-specific T cells post-vaccine | 5.00 | 2.00 – 19.33 |
| Flu-specific T cells* | 33.33 | 6.67 – 343.29 |
Localized: localized prostate cancer; bCRPC: biochemically progressive (non-metastatic) castration-resistant prostate cancer; mCRPC: metastatic CRPC
spots/million
Levels of circulating PSA-specific T cells in patients whose PSA-specific T cells increased ≥ 2-fold following vaccine (57% or 59/104 evaluated patients), and comparison with baseline levels of circulating influenza matrix protein-specific T cells in these same patients. Of 193 post-vaccine ELISPOTs, 60% (115/193) had a 2-fold increase in PSA-specific T cells compared with baseline, with 31/59 patients having more than 1 post-vaccine ELISPOT.
To determine evidence of cross-priming and generation of an immune response to TAAs not found in the vaccine (a phenomenon known as antigen-cascade or antigen-spreading (18)), we tested patients for TAA-specific immune responses to non-vaccine (i.e., non-PSA) antigens in 4 separate clinical trials (11, 13, 15, 16). Of the 28 patients tested, 68% (19/28) showed evidence of antigen-cascade post-vaccination (Table 2). Eight of the 28 patients had only one additional cascade antigen tested (MUC-1 along with PSA), whereas 20 had 2 to 4 cascade antigens tested. Of those 20, 16 had a ≥ 2-fold increase in TAA-specific T cells to at least 1 cascade antigen, and 9/16 had a positive response to at least 2 cascade antigens (Supplemental Table 1).
Table 2.
Evidence of antigen spreading by induction of antitumor T-cell responses to antigens not found in the vaccine
Vaccine effects on the number and the function of regulatory T cells (compiled and new data)
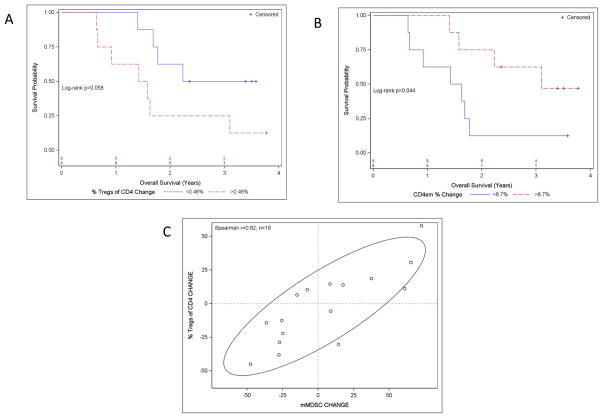
An analysis of alterations in the number and the function of regulatory T cells (Tregs) in patients treated with PSA-TRICOM on 2 phase II studies indicated that the number and the suppressive function of Tregs decreased in some patients post-vaccination (19). Additionally, there were trends indicating an association between decreased function of Tregs following vaccination (P = 0.029; r = −0.45) and improved OS. Tregs expressing CTLA-4 were more suppressive. Those patients who lived longer than predicted as estimated by the Halabi nomogram (20) had an improved effector T cell:CTLA-4+ Treg ratio post vaccine (P = 0.029). Additional immune studies are reported here for the first time. Preliminary data from one study (21) suggested a significant decrease in Tregs relative to CD4+ T cells within the tumor following vaccination. In another of these studies (9), patients showed a trend demonstrating that a decrease in the percentage of Tregs post-vaccine was associated with longer OS (log-rank P = 0.058) (Fig. 2A). In addition, an increase in the frequency of the CD4 effector memory T-cell subsets showed a trend towards an association with longer OS (log-rank P = 0.044) (Fig. 2B). Interestingly, we also found a direct correlation between the change in Treg percentages and the change in the frequency of monocytic myeloid-derived suppressor cells (mMDSCs) (Spearman r = 0.82) (Fig. 2C).
Figure 2.
Actuarial analysis and associations of changes in the frequencies of immune-cell subsets vs. overall survival. We analyzed PBMCs of 16/32 patients in the phase II trial of PROSTVAC pre- vs. post-vaccine for immune-cell subset phenotypes, as described in Materials and Methods. For actuarial analysis, the data were dichotomized at the median and log-rank test was used to compare strata. Due to the very small number of patient samples analyzed, these data are intended only to be hypothesis-generating. A, Kaplan-Meier plot of overall survival vs. the change in Treg percentage post- vs. pre-vaccination. Blue line: change in Treg percentage below median; red line: change in Treg percentage above median (median change was 0.46%). B, Kaplan-Meier plot of overall survival vs. change in frequency of CD4 effector memory (CD4em) cells post- vs. pre-vaccination. Blue line: decrease below median for CD4em frequency; red line: increase above median for CD4em frequency (median change was 8.7%). C, Scatter plot with 75% confidence ellipse showing the % change in Tregs out of all CD4 T cells vs. the change in monocytic monocytic myeloid-derived suppressor cells (mMDSCs); r = 0.82.
Antibody responses (compiled and new data)
When pre- and post-vaccine sera from 349 patients enrolled on 11 studies (2–7, 9, 11, 14, 16, 22, 23) of PSA-based poxviral vaccines were analyzed for evidence of free PSA antibodies, only 2 patients showed evidence of PSA antibody response post-vaccine (Table 3). Since PSA is a secreted protein not found on the cell surface, intact tumor cells could not act as a sink to PSA antibodies. However, it is possible that circulating PSA protein could bind free anti-PSA antibody. Knowing that these circulating antigen/antibody complexes (immune complexes or IC) can be measured, we collaborated with Dr. Gabriel Virella of the Department of Microbiology and Immunology at the Medical University of South Carolina in Charleston to analyze samples from 20 patients treated on one study with PROSTVAC (9) for evidence of PSA-specific circulating IC before and after 3 months of vaccine treatment. Patients selected included all those, whose PSA decreased following vaccine. For the first time we show that of these 20 patients, 14 showed no evidence of IC at any time point, 2 tested borderline positive (one at baseline only; one following vaccine only), 2 had new IC following vaccination (as well as a 2- to 5-fold increase in PSA), and one had pre-existing IC that increased following vaccine (along with a proportional increase in PSA).
Table 3.
Generation of anti-PSA antibodies following vaccination with PSA-based vaccines
| Vaccine | # of positive patients | # of patients tested | Data previously published | Reference |
|---|---|---|---|---|
|
|
||||
| rV-PSA | 1 | 33 | Yes | (4) |
| rV-PSA | 0 | 42 | Yes | (2) |
| rV-PSA | 1 | 6 | Yes | (3) |
| rV-PSA/rF-PSA | 0 | 14 | Yes | (11) |
| rV-PSA/rF-PSA | 0 | 15 | Yes | (14) |
| rV-PSA/rF-PSA | 0 | 9 | Yes | (16) |
| rV-PSA/rF-PSA | 0 | 64 | Yes | (22) |
| PSA-TRICOM | 0 | 82 | Yes | (7) |
| PSA-TRICOM | 0 | 10 | Yes | (6) |
| PSA-TRICOM | 0 | 45 | Yes | (5, 9) |
| PSA-TRICOM | 0 | 29 | No | (23) |
| Total | 2 | 349 (0.57%) | ||
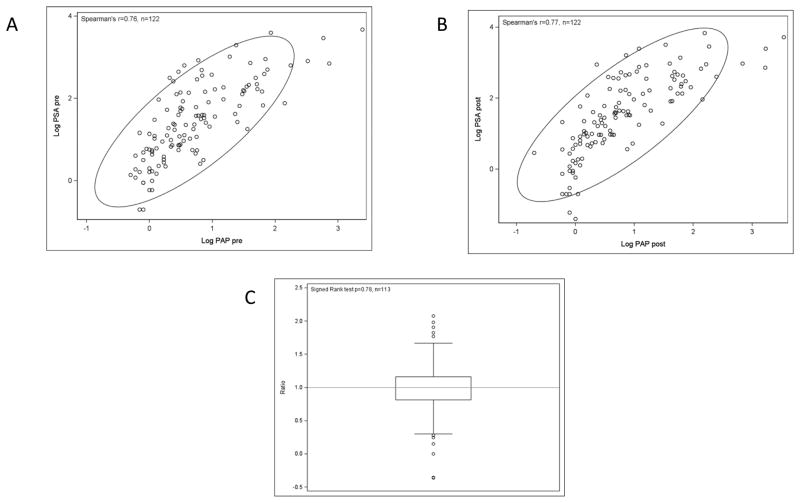
A substantial level of anti-PSA antibodies should result in a decreased level of circulating PSA, as bound antibodies are pulled from the circulation by reticuloendothelial cells, leading to an artificially reduced PSA level. All of our studies have found a moderately strong association between the values for PSA and for PAP, another prostate cancer tumor marker, such that when PSA decreases, so does PAP. For the first time we report here an analysis of 122 patients treated with PROSTVAC (5, 9, 13, 17, 21, 24) which found a moderately strong association between PSA and PAP both before (Fig. 3A, Spearman r = 0.76) and after vaccine (Fig. 3B, Spearman r = 0.77). The median of the ratio (log PSA pre/log PAP pre) / (log PSA post/log PAP post) was exactly 1 (P = 0.78, Wilcoxon signed rank test) (Fig. 3C). Thus, there was no significant difference in PSA/PAP ratios before vs. after treatment.
Figure 3.
Unchanged correlation of PSA to PAP in 122 patients treated with PROSTVAC. A, pre-vaccine scatter plot with 90% confidence ellipse. B, post-vaccine scatter plot with 90% confidence ellipse. C, box-and-whisker plot of the ratio (log PSA pre/log PAP pre)/(log PSA post/log PAP post), n = 113. There were 9 missing observations because of division by zero (5 cases in the pre ratio and 4 cases in the post ratio). If PSA antibodies were pulling PSA out of circulation, the amount of PSA per unit of PAP would decrease.
Vaccine effects on the number of NK cells (new data)
It is possible that a vaccine that induces a Th1 inflammatory response and CD8+ T cells could also alter the quantity or function of NK cells. We performed a small analysis on one study (9) to evaluate whether the vaccine had a significant effect on NK cells. An analysis of 16 paired patient samples available for measurement showed no statistical difference in the number of NK cells pre- vs. post-vaccine. An analysis of the subsets indicated no differences in the number of immature (CD16negCD56bright), mature (CD16posCD56dim), and functional intermediate-stage (CD16posCD56bright) NK cells (data not shown; signed-rank test P > 0.20 for all 3).
Th1 vs. Th2 cytokines (new data)
An analysis of the pre- and post-vaccine serum levels in 29 patients in the phase II trial of PSA-TRICOM found no consistent alteration in most serum Th1 or Th2 cytokines, including IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p70, IL-13, and TNF-α (signed rank test P values: 0.23, 0.84, 0.31, 0.13, 0.0013, 0.78, 0.78, 0.090, 0.89, and 0.14, respectively). Of note, IL-5, a type II cytokine, was significantly decreased following vaccine treatment (Supplemental Figure 2).
Clinical safety (compiled data)
Generation of a strong immune response could theoretically result in adverse events in both on-target and off-target tissue. The safety of PSA-TRICOM was evaluated in 152 patients treated at the NIH Clinical Center and 82 patients on a randomized phase II study. This primary data safety analysis showed that of the 234 patients who received 1,341 vaccinations, grade ≥ 2 injection-site reactions were seen following 21.6% of vaccinations (with only 2 grade 3 and no grade 4 injection-site reactions). Other adverse events possibly related to vaccine (largely limited to fatigue and flu-like symptoms) were seen following < 1.5% (grade 2) and < 0.5% (grade 3 or 4) of the vaccinations. Furthermore, of the 234 patients who received PSA-TRICOM on study, only 2 (< 1%) had to stop treatment due to adverse events. All grade ≥ 2 adverse events believed to be at least possibly related to vaccine are described in Supplemental Table 2.
Discussion
PROSTVAC is known to induce a robust cellular immune response in many patients, with immune responses to PROSTVAC quantitatively similar to the memory responses to influenza. Yet data evaluating immune responses almost certainly underestimate the true therapeutic impact of the vaccine. The ELISPOT assay used to analyze immune response in the trials mentioned above has several inherent limitations. (a) It measures IFN-γ-producing cells circulating in the peripheral blood, but does not take into account those that have trafficked to the site of the tumor. It is possible that the level of tumor-specific T cells measured in the periphery does not reflect the actual number of tumor-specific T cells at the tumor site. Preclinical evidence suggests that TAA-specific CD8+ T cells are enriched at the tumor site. In 2 studies, the level of TAA-specific T cells detected in the periphery was 2.1% to 2.3% in CEA-transgenic (CEA-Tg) mice following vaccination with CEA/TRICOM vaccine (25, 26). In contrast, in 2 studies analyzing tumor-infiltrating T cells, CEA-Tg mice demonstrated 11% to 22% of the infiltrating CD8+ T cells specific for the TAA following vaccination with CEA/TRICOM vaccine (27, 28). Taken together, these data suggest that TAA-specific T cells may be enriched 5- to 10-fold at the site of the tumor. Moreover, much higher levels of tumor-specific T cells at the tumor than in the peripheral blood have previously been reported in patients with cancer (29). A neoadjuvant study in prostate cancer that will analyze intratumoral immune responses following vaccination with PSA-TRICOM is slated to open shortly. (b) In each of the clinical studies of PROSTVAC, immune responses were assayed in PBMCs obtained 28 days after the preceding vaccination. Resultant immune responses likely reflected residual circulating memory cells, since 28 days is well beyond the anticipated peak of an effector-cell response to vaccine. (c) ELISPOT uses only one-nanomer PSA peptide in HLA-A2+ patients, whereas patients may mount immune responses to other PSA epitopes expressed by the viral-vector vaccine, which expresses the entire gene (i.e., 244 amino acids). (d) Unlike the multiple rounds of in vitro stimulation reported in some studies, the ELISPOT assays reported here employed a single overnight incubation of APCs pulsed with antigen and patient samples. (e) The assays used whole PBMC populations, not pure CD8+ populations. It is estimated that CD8+ populations represent only 20% of total PBMCs; thus, a pure CD8+ population used in the ELISPOT assay should give 5-fold higher results. (f) Not all patients were HLA-0201. Therefore, the level of binding of the PSA nanomer in these patients could not be determined, which may have resulted in an underestimation of the proportion of patients responding to the vaccine. (g) Our laboratory has a very conservative definition of what constitutes an ELISPOT, as evidenced by the data for influenza matrix protein-specific T cells (Table 1B).
An additional limitation of the ELISPOT assay is that it does not address 2 fundamental aspects of an immune response: its avidity and breadth of response to a variety of TAAs. T-cell avidity can be defined by the concentration of antigen required to elicit a response, meaning that higher-avidity T cells can be activated to kill tumor cells with much lower concentrations of antigen (30). This is important because only high-avidity T cells can efficiently lyse target cells (31). In preclinical studies, vaccines containing TRICOM produced a 2.4-fold increase in vaccine-specific T cells compared to vaccines without costimulatory molecules. More importantly, however, there was a > 100-fold increase in the avidity of T cells produced by TRICOM-containing vaccines compared to vaccines without costimulatory molecules (Table 4) (26).
Table 4.
Multiple costimulatory molecules encoded within poxviral vectors dramatically increase avidity of tumor-specific T cells in a murine model
| Vaccine | Precursor frequency/105 CD8+ T cells | Δ Precursor | Peptide concentration for CTL (nM) | Δ Avidity* |
|---|---|---|---|---|
| rV-CEA | 321 | 1.0X | 510 | 1X |
| rV-CEA-TRICOM | 769 | 2.4X | 5 | 102X |
Defined as natural log of the peptide concentration that results in 50% maximal target lysis. Similar results were seen with tetramer dissociation. Adapted from (26).
CTL: cytotoxic T lymphocytes.
The most intriguing explanation for the ELISPOT assay’s possible underestimation of a clinically relevant antitumor immune response may be the difficulty of identifying the specific tumor-rejection antigen(s) present in any given prostate cancer patient. However, any initial tumor-specific immune response that leads to immune-mediated tumor killing can also lead to cross-priming of other tumor-specific antigens to the immune system in a process called antigen-spreading or antigen-cascade (32), as demonstrated in multiple preclinical studies (18, 33, 34). One study of intratumoral CEA-TRICOM vaccine demonstrated not only increased numbers of CEA-specific T cells within the tumor compared with the spleen, but also T cells specific to other antigens expressed by the tumor, such as wild-type p53 and an endogenous retroviral epitope of gp70 (18). Moreover, the magnitude of CD8+ T-cell immune responses to gp70 was far greater than responses induced to CEA. In fact, the predominant T-cell population infiltrating the regressing CEA+ tumor after vaccine was specific for gp70. An expanding, cascading immune response may continue over time, eventually broadening into an immune response potentially more clinically relevant than the initial immune response to the vaccine. Clinical trials of PROSTVAC have reported a T-cell antigen-cascade in 68% of the patients tested (Table 2). Furthermore, immune responses to cascade-antigens are often more robust than immune response to the PSA expressed by the vaccine (11, 13, 15). One study with an earlier version of PROSTVAC revealed treatment-associated autoantibody responses in 15/33 (45.5%) patients treated with the combination of vaccine and radiation vs. 1/8 (12.5%) patients treated with radiation alone (35). Others have reported improved clinical outcomes for patients who demonstrated a broadened immune response (36–38).
Multiple studies have demonstrated that the number and/or function of Tregs are increased in patients with prostate cancer compared with healthy volunteers (39–41). PSA-TRICOM may be capable of reversing the immunosuppressive capacity of Tregs by shifting the effector T cell:Treg ratio, or possibly by decreasing the functional capacity of Tregs. Prospective analyses of the correlative trends seen in this analysis, along with other immune endpoints, are planned in the ongoing phase III study.
A poxviral-vector vaccine encoding multiple T-cell costimulatory molecules might be expected to produce an immune response skewed toward Th1, with little or no antibody production. Indeed, some preclinical data support this expectation. An initial safety study of recombinant vaccinia expressing human PSA, performed in rhesus monkeys, found only transient production of IgM antibodies to human PSA and no IgG or IgA antibodies (42). Studies seeking evidence of induction of anti-PSA antibody responses in 349 patients treated with poxviral vaccines encoding PSA found only 2 patients with increased anti-PSA antibodies following vaccination (Table 3) and little direct evidence of circulating PSA/antibody immune complexes. Furthermore, indirect evidence from 122 patients suggests no change in the ratio of PSA to PAP after vaccination. A substantial increase in the post-vaccine ratio would be consistent with an artificial lowering of PSA (more than PAP) due to induction of a PSA antibody. The almost identical correlation coefficients indicate that the vaccine does not alter the ratio of PSA to PAP, suggesting that it is reasonable to use PSA as a marker to analyze the kinetics of a clinical response to PROSTVAC vaccine. Indeed, this lends further credence to a prior analysis and hypothesis on tumor growth rates following vaccine (Supplemental Figure 3) (43, 44).
T-cell response (mostly CD8+) appears predominant, with no evidence of B-cell response. It is interesting to note that IL-5, a cytokine involved in a type II immune response, decreases following vaccine. While this finding would need to be confirmed in subsequent studies, this is consistent with a bias for a cytotoxic T-lymphocyte response over a B-cell response. This T-cell-predominant response is exactly what one would predict to be beneficial, based on preclinical depletion studies. Furthermore, the absence of evidence of antibody/immune complexes to PSA allows for the use of PSA levels to assess disease kinetics in vaccine-treated patients. The ability to generate an apparently clinically significant immune response is associated with only a minority of the patients having transient mild to moderate adverse events from the vaccines. This desirable side-effect profile allows for potential combinations with a variety of different agents and facilitates patient acceptability in earlier stages of the disease.
The ELISPOT assays used to evaluate patients’ immune responses in the trials reported here, and used by many other investigators to evaluate other vaccines, should be considered first-generation biomarkers. The same ELISPOT assay was used in the trials reported here for consistency and comparison of results from one trial to another. Most ELISPOT assays to date, however, have used 9-mer peptides, which represent only one CD8+ epitope, and only the immune responses of patients with one HLA allele (HLA-A2) can be evaluated. Recent studies have begun to employ numerous 15-mer peptides, which span the entire region of the tumor antigen being evaluated. This approach will identify both CD4 and CD8 responses and will not be restricted to evaluating a patient with a particular allele. The use of 15-mer peptides, however, is costly and the concentration of each individual peptide in the assay is limited. In addition to ELISPOT assays, FACS-based assays are being used to evaluate intracellular cytokines and activation markers in response to antigens, as are FACS-based assays using 10 or more colors to evaluate numerous immune-cell subsets pre- and post-therapy. This approach is being used to determine if a given patient has the potential to benefit from a particular vaccine therapy, and to determine early in the vaccine regimen if specific immune cells are being activated. It is unclear at this time, however, whether any immune phenomenon will be a true surrogate for patient benefit. This is due to the complexity of the immune system and the heterogeneous nature of a given patient population in terms of prior and/or current therapy, age, etc. At this time, any one or combination of the above assays can at best define trends in correlation with patient responses. As more clinical studies demonstrate clinical efficacy, better immune correlations with clinical efficacy may become apparent.
These preliminary immune data from over 100 patients treated with PSA-TRICOM suggest clear evidence of immune responses to PSA in the majority of patients post-vaccination. While no surrogates for clinical efficacy have been identified, further detailed analyses of immune endpoints and correlation with clinical endpoints are prospectively designed into the ongoing 1,200-patient, randomized, controlled clinical trial of PSA-TRICOM (8).
Supplementary Material
Acknowledgments
The authors thank Diane J. Poole for technical assistance, and Bonnie L. Casey and Debra Weingarten for editorial assistance in the preparation of this manuscript. We also gratefully acknowledge the collaboration of Dr. Gabriel Virella, Department of Microbiology and Immunology, Medical University of South Carolina, Charleston, SC, on PSA/antibody circulating immune complexes.
Grant Support
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–17. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 3.Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–6. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 4.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–8. [PubMed] [Google Scholar]
- 5.Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–20. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 6.DiPaola RS, Plante M, Kaufman H, Petrylak DP, Israeli R, Lattime E, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A Randomized, Double-blind, Phase 3 Efficacy Trial of PROSTVAC-V/F +/− GM-CSF in Men With Asymptomatic or Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer. 2013 Oct 17; Available from: http://www.cancer.gov/clinicaltrials/search/view?cdrid=698050&version=HealthProfessional&protocolsearchid=11241123.
- 9.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–49. [PubMed] [Google Scholar]
- 11.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 12.Britten CM, Meyer RG, Kreer T, Drexler I, Wolfel T, Herr W. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods. 2002;259:95–110. doi: 10.1016/s0022-1759(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 13.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–46. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 15.Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccine Therapy With PROSTVAC/TRICOM and Flutamide Versus Flutamide Alone to Treat Prostate Cancer. 2013 Oct 17; Available from: http://www.cancer.gov/clinicaltrials/search/view?cdrid=535593&version=HealthProfessional&protocolsearchid=3172647.
- 18.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11:2416–26. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 19.Vergati M, Cereda V, Madan RA, Gulley JL, Huen NY, Rogers CJ, et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother. 2011;60:197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 21.Gulley JL, Heery CR, Madan RA, Walter BA, Merino MJ, Dahut WL, et al. Phase I study of intraprostatic vaccine administration in men with locally recurrent or progressive prostate cancer. Cancer Immunol Immunother. 2013;62:1521–31. doi: 10.1007/s00262-013-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 23.Vaccine Therapy and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) in Treating Patients With Prostate Cancer That Progressed After Surgery and/or Radiation Therapy. 2013 Oct 17; Available from: http://clinicaltrials.gov/ct2/show/NCT00108732?term=NCT00108732&rank=1.
- 24.Heery C, Madan RA, Bilusic M, Kim J, Singh N, Rauckhorst M, et al. A phase II randomized clinical trial of samarium-153 EDTMP (Sm-153) with or without PSA-tricom vaccine in metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. J Clin Oncol. 2013;31(suppl 6):abstr 102. [Google Scholar]
- 25.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother. 2011;60:1227–42. doi: 10.1007/s00262-011-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty M, Gelbard A, Carrasquillo JA, Yu S, Mamede M, Paik CH, et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008;57:1173–83. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 29.Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641–50. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandmaier AG, Leitner WW, Ha SP, Sidney J, Restifo NP, Touloukian CE. High-avidity autoreactive CD4+ T cells induce host CTL, overcome T(regs) and mediate tumor destruction. J Immunother. 2009;32:677–88. doi: 10.1097/CJI.0b013e3181ab1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–92. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulley JL. Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother. 2013;9:219–21. doi: 10.4161/hv.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood LM, Pan ZK, Guirnalda P, Tsai P, Seavey M, Paterson Y. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother. 2011;60:931–42. doi: 10.1007/s00262-011-1002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nesslinger NJ, Ng A, Tsang KY, Ferrara T, Schlom J, Gulley JL, et al. A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res. 2010;16:4046–56. doi: 10.1158/1078-0432.CCR-10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardwick N, Chain B. Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy. 2011;3:731–3. doi: 10.2217/imt.11.62. [DOI] [PubMed] [Google Scholar]
- 38.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 39.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 40.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 41.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodge JW, Schlom J, Donohue SJ, Tomaszewski JE, Wheeler CW, Levine BS, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–7. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 43.Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–75. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.