Abstract
Objective
We sought to determine the accuracy and role of rapid C-reactive protein (CRP) testing in HIV-infected tuberculosis suspects.
Design
We enrolled HIV-infected adults (≥18 years) with a cough for ≥2 weeks and negative sputum smears for acid-fast bacilli (AFB) in KwaZulu-Natal, South Africa. Participants were evaluated for pulmonary tuberculosis by a nurse with rapid CRP, and independently by a physician with a chest radiograph. Rapid CRP test results were compared to laboratory CRP and sputum sent for confirmation of tuberculosis.
Results
Among 93 participants, 55 (59%) were female, mean age was 35 years, and median CD4 count was 177/mm3. Forty-five (54%) participants were diagnosed with pulmonary tuberculosis. Diagnostic sensitivity and specificity were 95% (95% CI 74–99%) and 51% (95% CI 35–66%) for rapid CRP > 8 mg/l, 87% (95% CI 73–96%) and 53% (95% CI 38–68%) for nurse assessment, and 69% (95% CI 52–83%) and 76% (95%CI 61–87%) for physician examination. Combining a positive rapid CRP (> 8 mg/l) to nurse and physician assessments decreased post- test probability of pulmonary tuberculosis from 22% to 6% and 32% to 6%, respectively.
Conclusion
Rapid CRP testing helped exclude pulmonary tuberculosis, and may be a valuable test to assist nurses and physicians in tuberculosis-endemic regions.
Keywords: Tuberculosis, C-reactive protein, HIV/AIDS, point-of-care test, diagnostic testing, South Africa
Introduction
Tuberculosis is a leading cause of AIDS-related deaths worldwide.1 In South Africa, nearly half a million new cases of tuberculosis are diagnosed annually, and over two-thirds of those people are living with HIV.2 The World Health Organization promotes intensified case finding for tuberculosis in adults living with HIV, and suggests evaluating persons with a cough, fever, weight loss, or night sweats for pulmonary tuberculosis.3,4 Conducting microbiological testing for all patients who present with any of those common symptoms may overburden existing infrastructure, and a rapid, clinic-based test to exclude tuberculosis may be valuable.
Existing tests have low diagnostic sensitivity, or a high false negative rate, for detecting pulmonary tuberculosis among HIV-infected adults.5 Sputum smear microscopy for acid-fast bacilli (AFB) has poor sensitivity (9–33%) among HIV-infected adults with culture-confirmed pulmonary tuberculosis.6–8 Mycobacterial culture, which takes weeks to months to obtain results, is not accessible to many health facilities in sub-Saharan Africa.9 The Xpert MTB/RIF assay, which is more sensitive than smear microscopy, has lower diagnostic sensitivity for both sputum- smear negative and HIV-infected adults.10,11 Furthermore, placement in centralized reference laboratories has reduced its potential clinical impact.12 Urine lipoarabinomannan has moderate sensitivity and high specificity for detecting active tuberculosis, so it may be valuable as a ‘rule-in’ test.13,14 A rapid, clinic-based test that could exclude active tuberculosis might improve patient outcomes.5,15
C-reactive protein (CRP), a non-specific acute phase serum protein, is elevated in both HIV-infected and -uninfected people with active tuberculosis,16–19 and has been proposed as a biomarker for active tuberculosis.5 Rapid CRP tests have been developed, but their performance has not been evaluated in HIV-infected adults with suspected pulmonary tuberculosis. We sought to determine the diagnostic accuracy and clinical role of rapid CRP testing in HIV-infected tuberculosis suspects in a tuberculosis-endemic region.
Methods
Study design and participant selection
We conducted a study of HIV-infected tuberculosis suspects referred to Edendale Hospital in KwaZulu-Natal, South Africa from August 2009 to October 2010. Edendale serves a population with a high HIV prevalence and tuberculosis incidence.20,21 Eligible participants were referred from a primary care facility, were ≥18 years old, HIV-seropositive, had a cough for ≥2 weeks, and were either unable to produce a respiratory sputum sample or had ≥2 negative sputum smear microscopy for AFB samples. We excluded people who had taken antituberculosis medications within 3 months. Participants received a 5-day course of amoxicillin/clavulanic acid or doxycycline before enrollment, in accordance with South African guidelines for sputum smear-negative tuberculosis suspects.22
The Research Ethic Committee of the University of KwaZulu-Natal and the KwaZulu-Natal Department of Health approved the study. All participants provided written informed consent prior to enrollment.
Study Procedures
Upon enrollment, a study nurse performed a clinical assessment for pulmonary tuberculosis using a standardized questionnaire for tuberculosis symptoms. A nurse obtained venous blood for laboratory-based CRP and CD4 count testing, and one sputum sample for smear microscopy and mycobacterial culture from all participants (using an ultrasonic nebulizer with 5% hypertonic saline if cough was non-productive). A nurse obtained approximately 5 L of whole blood by finger prick of the index finger and performed rapid CRP testing using the NycoCard CRP test (Axis-Shield; Oslo, Norway), according to the manufacturer’s specifications.23 The finger-prick whole blood drop is placed on a card, which is then read by the machine. The test provided a quantitative CRP result ranging from 8–200 mg/liter (l). The NycoCard kit included a positive control test, and this calibration step was performed each morning before participant CRP testing.
Immediately after the study nurse visit, all participants had a same-day, single-view chest radiograph, symptom review and a targeted physical examination, including chest auscultation and lymph node palpation, by a physician who was blinded to the rapid CRP result. Participants were offered antiretroviral therapy in accordance with South African guidelines.20
Laboratory Testing
Direct microscopy of AFB was performed with both Ziehl-Nielsen and Auramine fluorescent staining. All specimens sent for mycobacterial culture were cultured in liquid culture media (BACTEC™ MGIT™ BD, Sparks, MD, USA) and solid culture media. Positive cultures were identified as M. tuberculosis using a niacin test. Some AFB positive specimens were not cultured due to limited capacity. All laboratory investigations were performed at accredited laboratories by technicians registered with the Health Professions Council of South Africa.
Laboratory-based high-sensitivity CRP testing was performed using a Dimension RXL analyzer from Dade-Behring (Deerfield, IL, USA). The machine had a range of 0.7-400 mg/L with good precision.24
Clinical Diagnosis of Tuberculosis
A study nurse classified participants as having pulmonary tuberculosis with presence of cough, an elevated rapid CRP test result (> 8 mg/L), and at least one of the following tuberculosis-related symptoms: fever, night sweats, or weight loss. Therefore, the diagnostic classification from the study nurse was a designated a priori algorithm that utilized both symptom-based screening and rapid CRP result. The physician, who was blinded to CRP results, reviewed participants clinically and evaluated the chest radiograph.
Statistical Analysis
Culture-confirmed M. tuberculosis was used as the reference-standard diagnostic test for pulmonary tuberculosis. Participants with positive sputum smear microscopy and who did not have a sputum culture result were also classified as having pulmonary tuberculosis. We determined the correlation between the lab-based and rapid CRP results using correlation coefficients and receiver operating curves. We calculated sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for lab-based CRP and rapid CRP testing using thresholds of > 8, ≥25, and ≥50 mg/l. We also calculated sensitivity and specificity for combinations of tuberculosis symptoms, nurse assessment, and physician assessment, both with and without rapid CRP testing. Exact 95% confidence intervals were calculated for test characteristics. We then assessed the change in clinical pre-test probability by multiplying the pre-test odds by the positive or negative likelihood ratio and then converting back to a post-test probability, when using various diagnostic strategies. All reported p-values were two-tailed with α=0.05. We conducted analyses using SAS (version 9.2; SAS Institute, Cary, USA).
Results
Among 93 participants, mean age was 34.7 years [standard deviation (SD) ±8.2], and 55 participants (59.1%) were female (Table 1). The mean duration of cough was 7.5 weeks (SD ±3.6), and most participants reported fevers (94.6%), night sweats (88.2%), or weight loss (92.5%). Mean weight was 56.3 kg (SD ±11.5), and median Karnofsky Performance score was 80 [Interquartile range (IQR) 70–80]. All participants were HIV-positive, and median CD4 count was 177/mm3 (IQR 80–256/mm3). Sixty participants (65.2%) had radiographic findings consistent with consolidation. Forty-five of 84 participants (53.6%) with bacteriology results had pulmonary tuberculosis; 37 were sputum culture positive and 8 were sputum smear positive.
Table 1.
Cohort characteristics of 93 HIV-infected tuberculosis suspects.
| Mean ±SD or N (%) |
|
|---|---|
| Demographic | |
| Age (years) | 34.7 ±8.2 |
| Female | 55 (59.1) |
| Tuberculosis-related Symptoms | |
| Cough | 93 (100) |
| Cough Duration (weeks) | 7.5 ±3.6 |
| Fever | 88 (94.6) |
| Night sweats | 82 (88.2) |
| Weight loss | 86 (92.5) |
| Clinical Assessment | |
| Weight (kg) | 56.3 ±11.5 |
| Karnofsky Performance Status [median, IQR] | 80 [70, 80] |
| ECOG Performance Status [median, IQR] | 1 [1, 2] |
| Laboratory and Radiographic | |
| CD4 count [median, IQR]/mm3 | 177 [80, 256] |
| Chest X-ray with Infiltration or Miliary Pattern | 60 (65.2) |
| Tuberculosis Diagnosis | |
| Sputum Microscopy or Culture Positive* | 45 (53.6) |
ECOG – Eastern Cooperative Oncology Group
N=84
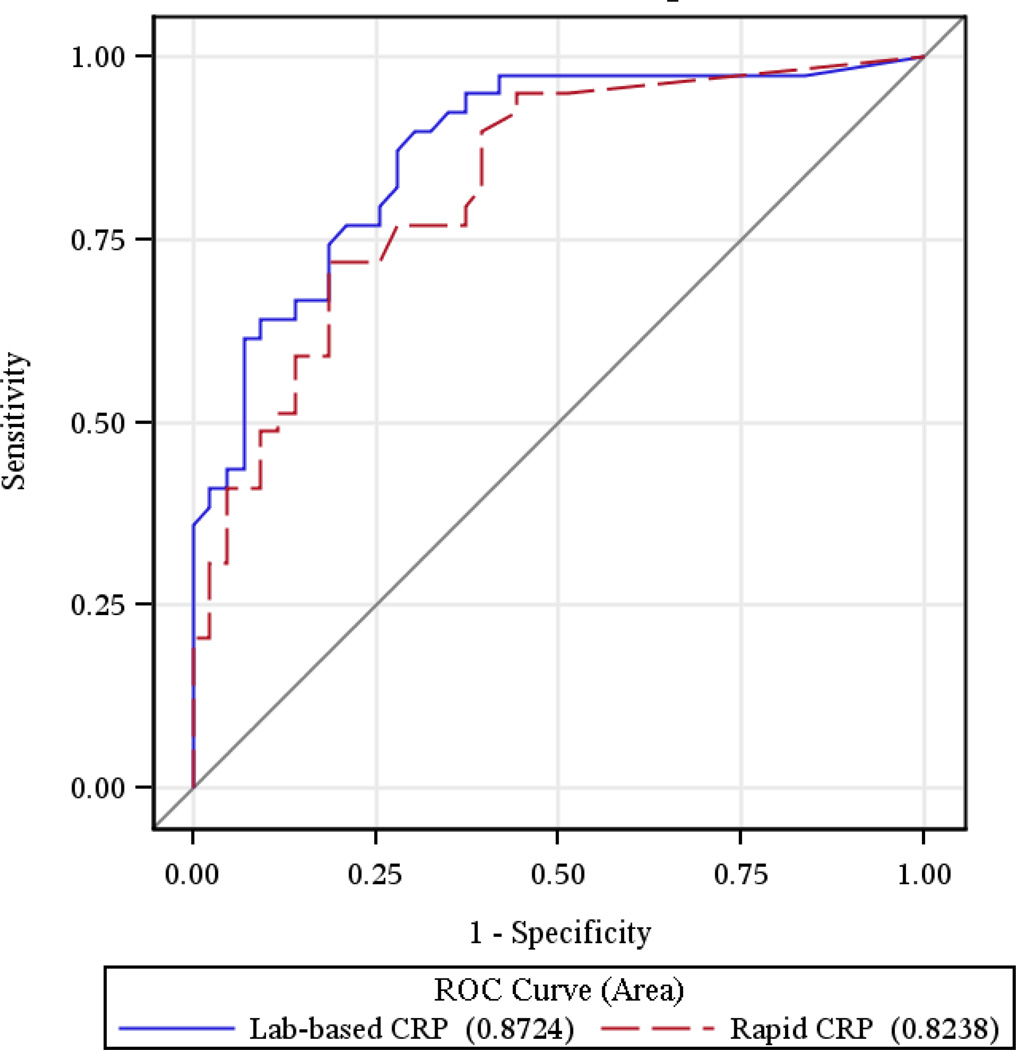
Mean lab-based CRP was 70.7 mg/l (SD ±72.1), and identified 70 participants (77.8%) as having a high value (> 5 mg/l). Mean rapid CRP was 50.7 mg/l (SD ±54.8), and identified 64 participants (71.1%) as having a high value (> 8 mg/l). The correlation coefficient between lab- based CRP and rapid CRP was 0.79 among all observations, and 0.70 when restricting lab-based results to the range of the rapid CRP test (8–200 mg/l). The area under the receiver operating curve for the lab-based and rapid CRP tests were 0.87 (95% CI 0.79–0.95) and 0.82 (95% CI 0.74–0.91), respectively (Figure 1). Study nurses easily performed rapid CRP testing, and results were available within 5 minutes.
Figure 1.
Receiver operating curves (ROC) for lab-based and rapid CRP tests for diagnosing active pulmonary tuberculosis.
Diagnostic Accuracy of CRP Testing
Lab-based CRP > 8 mg/l had a diagnostic sensitivity of 97.4% (95% CI 92.5–99.9%), and specificity of 53.5% (95% CI 37.7–68.8%) (Table 2). Rapid CRP > 8 mg/l had a diagnostic sensitivity of 94.9% (95% CI 82.7–99.4%), and specificity of 51.1% (95% CI 35.8-66.3%). Using a high threshold for CRP positivity decreased sensitivity and increased specificity. Among our cohort, rapid CRP > 8 mg/l had a positive predictive value of 62.7% (95% CI 49.2-75.0), and a negative predictive value of 92.0% (95% CI 74.0-99.0). Positive likelihood ratio (LR+) was highest (5.22) for rapid CRP ≥50 mg/l. Negative likelihood ratio (LR−) was lowest (0.05 and 0.10) for lab-based and rapid CRP > 8 mg/l, respectively.
Table 2.
Diagnostic accuracy of C-reactive protein (CRP) among HIV-infected tuberculosis suspects with bacteriology results (N=84).
| Sensitivity (%) |
Specificity (%) |
Positive PV (%) |
Negative PV (%) |
LR Positive |
LR Negative |
|
|---|---|---|---|---|---|---|
|
Lab-based C-reactive protein CRP >8 mg/l CRP ≥25 mg/l CRP ≥50 mg/l |
97.4 (92.5–99.9) 92.3 (79.1–98.4) 76.9 (60.7–88.9) |
53.5 (37.7–68.8) 62.8 (46.7–77.0) 74.4 (58.8–86.5) |
65.5 (51.9–77.5) 69.2 (54.9–81.3) 73.2 (57.1–85.8) |
95.8 (78.9–99.9) 90.0 (73.5–97.9) 78.1 (62.4–89.4) |
2.18 2.48 3.00 |
0.05 0.12 0.31 |
|
Rapid C-reactive protein CRP >8 mg/l CRP ≥25 mg/l CRP ≥50 mg/l |
94.9 (82.7–99.4) 76.9 (60.7–88.9) 59.0 (42.1–74.4) |
51.1 (35.8–66.3) 73.3 (58.1–85.4) 86.7 (73.2–95.0) |
62.7 (49.2–75.0) 71.4 (55.4–84.3) 79.3 (60.3–92.0) |
92.0 (74.0–99.0) 78.6 (63.2–89.7) 70.9 (57.1–82.4) |
1.94 2.88 5.22 |
0.10 0.32 0.47 |
PV = predictive value; LR = likelihood ratio.
Values in parentheses represent 95% confidence intervals.
Diagnostic Accuracy of CRP Testing with Symptoms and/or Clinical Assessment
Among our cohort, having ≥3 tuberculosis-related symptoms had 94.4% sensitivity (95% CI 86.5-99.9%), but only 4.4% specificity (95% CI 0.5-15.2%) (Table 3). When combined with rapid CRP > 8 mg/l, diagnostic sensitivity decreased slightly to 92.3% (95% CI 79.1-98.4%), but specificity increased to 53.3% (95% CI 37.9-68.3%). Using a higher threshold for rapid CRP decreased sensitivity and increased specificity.
Table 3.
Rapid CRP testing to augment diagnostic accuracy of tuberculosis (TB) symptom screening, nurse assessment, and physician assessment (N=84).
| No Rapid CRP Testing |
Rapid CRP >8 mg/l |
Rapid CRP ≥25 mg/l |
Rapid CRP ≥50 mg/l |
|||||
|---|---|---|---|---|---|---|---|---|
| Sens.(%) | Spec.(%) | Sens.(%) | Spec.(%) | Sens.(%) | Spec.(%) | Sens.(%) | Spec.(%) | |
| Number of TB Symptoms | ||||||||
| ≥2 symptoms | 100(91.0–100) | 2.2(0.1–11.8) | 94.9(82.7–99.4) | 51.1(35.8–66.3) | 76.9(60.7–88.9) | 73.3(58.1–85.4) | 59.0(42.1–74.4) | 86.7(73.2–95.0) |
| ≥3 symptoms | 97.4(86.5–99.9) | 4.4(0.5–15.2) | 92.3(79.1–98.4) | 53.3(37.9–68.3) | 74.4(57.9–87.0) | 73.3(58.1–85.4) | 56.4(39.6–72.2) | 86.7(73.2–95.0) |
| All 4 symptoms | 94.9(82.7–99.4) | 28.9(16.4–44.3) | 89.7(75.8–97.1) | 64.4(48.8–78.1) | 71.8(55.1–85.0) | 82.2(68.0–92.0) | 53.8(37.2–69.9) | 88.9(76.0–96.3) |
| Nurse Assessment | 87.2(72.6–95.7) | 53.3(37.9–68.3) | ||||||
| Nurse AND CRP value | -- | -- | 84.6(69.5–94.1) | 62.2(46.5–76.2) | 71.8(55.1–85.0) | 77.8(62.9–88.8) | 56.4(39.6–72.2) | 86.7(73.2–95.0) |
| Nurse OR CRP value* | -- | -- | 97.4(86.5–99.9) | 42.2(27.7–57.9) | 92.3(79.1–98.4) | 48.9(33.7–64.2) | 92.3(79.1–98.4) | 48.9(33.7–64.2) |
| Physician Assessment | 69.2(52.4–83.0) | 75.6(60.5–87.1) | ||||||
| Physician with CXR AND | -- | -- | 66.7(49.8–80.9) | 80.0(65.4–90.4) | 56.4(39.6–72.2) | 86.7(73.2–95.0) | 43.6(27.8–60.4) | 88.9(76.0–96.3) |
| CRP value | ||||||||
| Physician with CXR OR | -- | -- | 97.4(86.5–99.9) | 46.7(31.7–62.1) | 89.7(75.8–97.1) | 62.2(46.5–76.2) | 84.6(69.5–94.1) | 73.3(58.1–85.4) |
| CRP value* | ||||||||
Values in parentheses represent 95% confidence intervals.
Test considered positive if either clinical assessment or rapid CRP >8 mg/l were positive.
Nursing assessment alone had a sensitivity of 87.2% (95% CI 72.6-95.7%) and specificity of 53.3% (95% CI 37.9-68.3) for diagnosing pulmonary tuberculosis. When using a combined testing strategy of either rapid CRP > 8 mg/l or nurse assessment positive, diagnostic sensitivity increased to 97.4% (95% CI 86.5-99.9%), but specificity decreased to 42.2% (95% CI 27.7-57.9%). Using a higher threshold for rapid CRP caused moderate decreases on diagnostic sensitivity and small increases on specificity. Likelihood ratios positive and negative were 1.87 and 0.24 for the nursing assessment alone, and 1.69 and 0.06 when using a combined strategy with rapid CRP > 8 mg/l.
Physician assessment with a chest radiograph alone had a sensitivity of 69.2% (95% CI 52.4-83.0%) and specificity of 75.6% (95% CI 60.5-87.1) for diagnosing pulmonary tuberculosis. When using a combined testing strategy of either rapid CRP > 8 mg/l or physician assessment positive, diagnostic sensitivity increased to 97.4% (95% CI 86.5-99.9%), but specificity decreased to 46.7% (95% CI 31.7-62.1%). Unlike the nursing assessment, using a higher threshold for rapid CRP appeared to have small decreases on diagnostic sensitivity, but moderate increases on specificity. Likelihood ratios positive and negative were 2.84 and 0.41 for the physician assessment alone, and 1.83 and 0.06 when using a combined strategy with rapid CRP > 8 mg/l.
Value of Rapid CRP on Post-test Probability
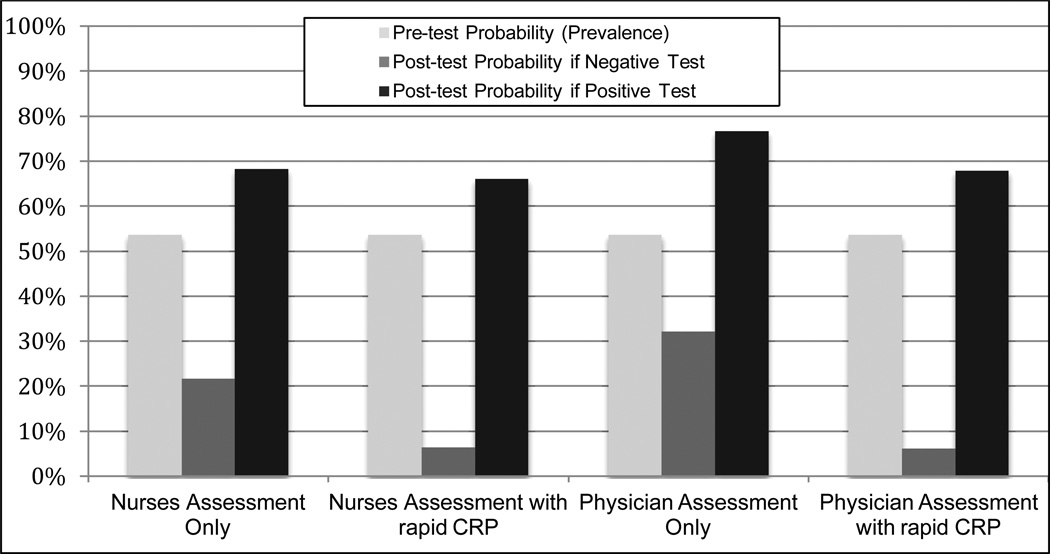
The use of rapid CRP test (> 8 mg/l) substantially improved a nurse or physicians ability to exclude pulmonary tuberculosis (Figure 2). The baseline prevalence (or pre-test probability) of pulmonary tuberculosis was 53.6%. When using nursing assessment alone, the post-test probability of disease was 68.3% for a positive assessment and 21.7% for a negative assessment. The inclusion of rapid CRP decreased the post-test probability of disease to 6.1% for a negative assessment, and had minimal impact (66.1%) on post-test probability for a positive assessment. Similarly, when using physician assessment alone, the post-test probability of disease was 76.6% for a positive assessment and 32.1% for a negative assessment. When either test was positive the post-test probability decreased slightly to 67.9%, but the post-test probability substantially decreased to only 6.1% when using rapid CRP testing.
Figure 2.
Pre- and Post-test probabilities for clinical assessment, with and without rapid CRP testing (≤8 mg/l), among HIV-infected tuberculosis suspects in a tuberculosis-endemic region.
Discussion
Among this cohort of HIV-infected tuberculosis suspects, a positive rapid CRP test (> 8 mg/l) had high overall sensitivity, but low specificity, for diagnosing pulmonary tuberculosis. Combining rapid CRP testing with a nurse or physician assessment provided significant value for excluding or “ruling out” pulmonary tuberculosis. The rapid CRP test, which had a strong correlation with results from the laboratory-based CRP test, was feasible of being operated at the clinical point-of-care by a nurse, was performed on whole blood obtained from a simple finger prick, and returned results within 5 minutes. Rapid, clinic-based CRP testing may an inexpensive and valuable test to assist nurses and physicians in excluding pulmonary tuberculosis among HIV-infected adults in tuberculosis-endemic regions.
Whole blood CRP is known to correlate well with serum CRP. Testing whole blood with the rapid NycoCard CRP test, which was used in this study, has previously showed good correlation with serum in laboratory-based CRP results.25 In a previous study, quantitative discrepancies began to appear at higher CRP concentrations (> 150 mg/l),26 but this was not observed in our study. Our study demonstrates that nurses can accurately perform rapid CRP testing on whole blood in a clinical setting, and obtain results similar to a laboratory-based test.
Serum CRP has a positive association for the presence of pulmonary tuberculosis.16,27 Among a similar cohort of tuberculosis suspects, serum CRP above the upper limit of normal had a sensitivity of 98% and specificity of 59% for diagnosing either smear AFB- or culture-positive pulmonary tuberculosis, which were similar to our findings.28 When used as a screening test among asymptomatic newly-diagnosed HIV-infected adults, serum CRP ≥10 mg/l had a sensitivity of 85% and specificity of 58% for culture-confirmed pulmonary tuberculosis.29 Their findings may be different by using a different CRP machine (Quantikine; R&D Systems Inc., USA) and/or by including asymptomatic individuals. We improve on prior studies by providing the first evaluation of a rapid, clinic-based, whole blood CRP testing as a diagnostic test for pulmonary tuberculosis among HIV-infected adults in a tuberculosis-endemic region.
Our findings suggest that rapid CRP testing may be an efficient and effective method to exclude, or ‘rule out’, pulmonary tuberculosis among HIV-infected tuberculosis suspects, which has been suggested.28–30 In asymptomatic newly-diagnosed HIV-infected adults, CRP ≥10 mg/l had a negative predictive value of 95% and negative likelihood ratio of 0.3, both to which suggest potential use as a “rule out” test.29 Using a similar CRP threshold among tuberculosis suspects, we previously reported a similar negative predictive value (96%), but a negative likelihood ratio of 0.04.28 In our analyses, a negative rapid CRP test decreased the post-test probability of disease of the physician’s assessment by over 5-fold (32% to 6%). Thus, a negative rapid CRP test should give nurses and physicians more confidence for excluding pulmonary tuberculosis.
Several rapid quantitative and semi-quantitative CRP tests are now available. At least three tests, including the one used in this study, are small, portable, battery powered devices that require ≤20 microliters of whole blood and provide a numerical readout (8–160 mg/l) within 4 minutes.31 Two semi-quantitative tests use a test cassette and reagent buffer, without a machine, and provide information on CRP being less than or greater than 10 mg/l.32 Although we evaluated a variety of CRP levels, we used a CRP > 8 mg/l since it is commonly used in rapid tests, has been reported in prior studies,33,34 and maximizes diagnostic sensitivity. In 2012, the average price of 3 rapid CRP machines was Euro (€) 1,837, and €4 per test.35 However, CRP testing may decrease overall costs by reducing expensive and labor-intensive microbiological evaluations of sputum samples. Rapid exclusion of tuberculosis could also assist clinicians to initiate antiretroviral therapy more quickly, which might reduce mortality.36
Our study had several limitations and strengths. We had a limited sample size, conducted the study in a single clinic, and did not assess rapid CRP with extrapulmonary tuberculosis. Sputum culture is an imperfect gold standard test and non-differential misclassification could reduce diagnostic accuracy. We also did not assess CRP as a prognostic indicator, as serum CRP > 10 mg/l and > 50 mg/l predict HIV disease progression and mortality.29,34 Finally, we evaluated rapid CRP as an outpatient diagnostic test among tuberculosis suspects in a tuberculosis-endemic region, and these results apply to HIV-infected sputum smear-negative tuberculosis suspects with cough for more than two weeks and may not be generalizable to other populations or areas with low tuberculosis rates. Rapid CRP testing should be evaluated in other populations, including inpatients, across a wider range of CD4 counts, and include a more diverse range of tuberculosis symptoms. Additional studies may also compare rapid, point-of-care CRP testing to the Xpert MTB/RIF assay conducted in a centralized laboratory for excluding active pulmonary tuberculosis.
In conclusion, rapid whole blood CRP testing can be performed at the clinical point-of- care, and may be an inexpensive and valuable test to assist clinicians in excluding pulmonary tuberculosis among HIV-infected tuberculosis suspects in a tuberculosis-endemic region. If CRP is a marker of tuberculosis bacillary load,29 then rapid CRP testing at the clinical point-of-care, as well as repeated CRP measurement, may be a valuable host biomarker to allow for an earlier evaluation of treatment efficacy. Since rapid CRP testing has several appealing properties, including independence from microbiological laboratories, studies are warranted to determine if rapid whole blood CRP testing predicts disease relapse, treatment failure, or patient outcomes. Rapid CRP testing appears to be a promising tuberculosis test to exclude pulmonary tuberculosis at the clinical point-of-care in certain settings.
Acknowledgements
We would like to acknowledge the support of the KwaZulu-Natal Department of Health. We appreciate the assistance of Andrew Michowicz and Keith Rasmussen. This research was presented in part at the 41st World Union Conference on Lung Health. Finally, we graciously thank all of the men and women who participated in this study.
Financial Support
This study was funded by the Howard Hughes Medical Institute, KwaZulu-Natal Research Initiative for Tuberculosis and HIV (K-RITH). This research was supported by the Harvard Global Health Institute for Global Health (PKD); the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University R24 TW007988 (PKD). Axis- Shield donated the rapid CRP instrument and testing kits.
Footnotes
Conflict of Interest Statement
All authors declare that they have no conflicts of interest.
References
- 1.World Health Organization. Global TB Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.World Health Organization. Joint TB/HIV Interventions. Geneva: World Health Organization; 2009. [Google Scholar]
- 3.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear negative pulmonary tuberculosis in people with HIV infection or AIDS in resource constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization; 2011. [Google Scholar]
- 5.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–1937. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–1880. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- 7.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassim S, Shaw PA, Sangweni P, Malan L, Ntshani E, Mathibedi MJ, et al. Detection of a substantial rate of multidrug-resistant tuberculosis in an HIV-infected population in South Africa by active monitoring of sputum samples. Clin Infect Dis. 2010;50:1053–1059. doi: 10.1086/651119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito S, Howard AA, Reid MJ, Elul B, Scardigli A, Verkuijl S, et al. TB diagnostic capacity in sub-Saharan African health care settings. J Acquir Immune Defic Syndr. 2012;61:216–220. doi: 10.1097/QAI.0b013e3182638ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralized use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicenter implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drain PK, Losina E, Coleman SM, Giddy J, Ross D, Parker G, Katz JN, Freedberg KA, Walensky RP, Bassett IV. A prospective, clinic-based study of a urine lipoarabinomannan (LAM) test for pulmonary or extrapulmonary TB among HIV-infected adults in South Africa. Presentation at the Conference on Retroviruses and Opportunistic Infections on March 5. 2013 [Google Scholar]
- 14.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Wiktor S, Coulibaly D, Ackah AN, Lal RB. Serum C-reactive protein and detection of tuberculosis in persons co-infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2001;95:41–42. doi: 10.1016/s0035-9203(01)90328-1. [DOI] [PubMed] [Google Scholar]
- 17.Schleicher GK, Herbert V, Brink A, Martin S, Maraj R, et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur Respir J. 2005;25:688–692. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 18.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10:31–38. [PubMed] [Google Scholar]
- 19.Choi C-M, Kang C-I, Jeung W-K, Kim D-H, Lee C-H, et al. Role of the C-reactive protein for the diagnosis of TB among military personnel in South Korea. Int J Tuberc Lung Dis. 2007;11:233–236. [PubMed] [Google Scholar]
- 20.Department of Health, Republic of South Africa. Clinical Guidelines for the Management of HIV & AIDS in adults and adolescents. South Africa: National Department of Health; 2010. [Google Scholar]
- 21.The Italian Cooperation in South Africa. [Accessed 19 December 2010];Demographic and Health Indicators KwaZulu-Natal. Available: http://italcoop.co.za/PublicDocuments/Demographic_Health_Statisticaldata_KW_EC_S A%20_EN.pdf.
- 22.Meintjes G, Maartens G, Boulle A, Conradie F, Goemaere E, Hefer E, et al. Guidelines for antiretroviral therapy in adults. S Afr J HIV Med. 2012;13:114–133. [Google Scholar]
- 23.Axis-Shield. NycoCard CRP Test. List website [Google Scholar]
- 24.De BK, Smith LG, Owen WE, Roberts WL. Performance characteristics of an automated high-sensitivity C-reactive protein assay on the Dimension RXL analyzer. Clinica Chemica Acta. 2002;323:151–155. doi: 10.1016/s0009-8981(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 25.Zecca E, Barone G, Corsello M, et al. Reliability of two different bedside assays for C- reactive protein in newborn infants. Clin Chem Lab Med. 2009;47:1081–1084. doi: 10.1515/CCLM.2009.246. [DOI] [PubMed] [Google Scholar]
- 26.Cohen R, Romain O, Levy C, Perreaux F, Decobert M, et al. Impact of CRP rapid test in management of febrile children in paediatric emergency units of Ile-de-France. Arch Pediatr. 2006;13:1566–1571. doi: 10.1016/j.arcped.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Breen RAM, Leonard O, Perrin FMR, Smith CJ, Bhagani S, et al. How good are systemic symptoms and blood inflammatory markers at detecting individuals with tuberculosis? Int J Tuberc Lung Dis. 2008;12:44–49. [PubMed] [Google Scholar]
- 28.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One. 2011;6:e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C- reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2013;17:636–643. doi: 10.5588/ijtld.12.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez GG, Sabri E, Ling D, Cameron DW, Maartens G, Wilson D. A model to rule out smear-negative tuberculosis among symptomatic HIV patients using C-reactive protein. Int J Tuberc Lung Dis. 2012;16:1247–1251. doi: 10.5588/ijtld.11.0743. [DOI] [PubMed] [Google Scholar]
- 31.Diar HA, Nakwa FL, Thomas R, Libhaber EN, Velaphi S. Evaluating the QuikRead C- reactive protein test as a point-of-care test. Paediatr Int Child Health. 2012;32:35–42. doi: 10.1179/1465328111Y.0000000045. [DOI] [PubMed] [Google Scholar]
- 32.Siebenhaar R, Mushholt PB, Forst T, et al. Laboratory evaluation of a new lateral-flow- based point-of-care rapid test for assessment of chronic systemic inflammation. J Diabetes Science Technol. 2010;4:717–722. doi: 10.1177/193229681000400327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grutzmeier S, Sandstrom E. C-reactive protein levels in HIV complicated by opportunistic infections and infections with common bacterial pathogens. Scand J Infect Dis. 1999;31:229–234. doi: 10.1080/00365549950163491. [DOI] [PubMed] [Google Scholar]
- 34.Drain PK, Kupka R, Msamanga GI, Urassa W, Mugusi F, Fawzi WW. C-reactive protein independently predicts HIV disease progression and mortality among women and children in a resource-poor setting. AIDS. 2007 Oct 1;21(15):2067–2075. doi: 10.1097/QAD.0b013e32826fb6c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel MF, Paling FP, Hoepelman AIM, van der Meer V, Oosterheert JJ. Evaluating the evidence for the implementation of C-reactive protein measurement in adult patients with suspected lower respiratory tract infection in primary care: a systematic review. Family Practice. 2012;29:383–393. doi: 10.1093/fampra/cmr119. [DOI] [PubMed] [Google Scholar]
- 36.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]