Abstract
Host antitumor adaptive immune responses are generated as a result of the body’s immunosurveillance mechanisms. How the antitumor immune response is initially primed remains unclear, given that soluble tumor antigens generally are quantitatively insufficient for cross-priming and tumors lack the classical pathogen-associated molecular patterns (PAMPs) to activate costimulation and initiate cross-priming. We explored the interaction of the tumor-derived heat-shock proteins (HSP) with their common receptor (CD91) on antigen presenting cells (APCs) as a mechanism for host-priming of T cell-mediated antitumor immunity. Using targeted genetic disruption of the interaction between HSPs and CD19, we demonstrated that specific ablation of CD91 in APCs prevented the establishment of antitumor immunity. The antitumor immunity was also inhibited when the transfer of tumor-derived HSPs to APCs was prevented using an endogenous inhibitor of CD91. Inhibition was manifested in a reduction of cross-presentation of tumor-derived antigenic peptides in the lymph nodes providing a molecular basis for the observed immunity associated with tumor development. Our findings demonstrate that early in tumor development, the HSP-CD91 pathway is critical for the establishment of antitumor immunity.
Keywords: Tumor immunology, immunosurveillance, antigen transfer, RAP, CD91/LRP-1
INTRODUCTION
In a majority of cancer patients and in murine experimental tumor model systems, recognition of tumor by the host’s immune system occurs resulting in the priming of concomitant immunity and immunoediting (1-3). Conventional mechanisms of priming immune responses to pathogens do not apply to tumors because tumors generally lack sufficient antigens as native proteins for cross-priming (4-5). In addition, being of self origin, tumors in a sterile environment often lack the classical pathogen-associated molecular patterns (PAMPs) for activating innate signals and co-stimulation necessary for priming T cells. Given the ability of endogenous, purified heat-shock proteins (HSPs) to prime for specific immune responses under conditions of limiting antigen (5,6) we tested the role of tumor-derived HSPs in situ and their receptor CD91 on antigen presenting cells (APCs) in the initiation of immune responses to tumors.
We have explored the HSP-CD91 axis as a mechanism for host-priming of antitumor immunity for two reasons; i) antigens in the form of peptides are chaperoned by HSPs and are efficiently cross-presented by APCs (7-11). The increase in efficiency of cross-presentation of HSP-chaperoned peptides versus peptides alone, is several thousand fold and is made possible through the cell surface receptor CD91 on APCs (5,12-16), and ii) we have recently shown that HSPs signal through CD91 and activate APCs for co-stimulatory capacity based on the up-regulated secretion of pro-inflammatory cytokines, including IL-1β, TNF-α, IL-6 and the improved expression of CD40, MHC II and CD86 molecules (17-20). These observations explain the ability of six intracellular HSPs, gp96, hsp90, hsp70, calreticulin, hsp110 and grp170 to prime immune responses, specific for the peptides they chaperone in cells, once they have been purified from various antigen-bearing cells including tumors, pathogen-infected cells, allogeneic cells, and model antigen-expressing cells (6,9,11-14,21-25).
The immunological properties of HSPs make them prime candidates for the initiation of immune responses to tumors. However, HSPs are necessary for the survival of cells, so testing their requirement for priming tumor-specific immune responses in vivo through simultaneous or sequential deletion is not possible. Instead, we test their requirement by targeting and selectively deleting the HSP receptor CD91 in mice. This approach is possible because while structurally unrelated, four of the abundant and immunogenic HSPs, gp96, hsp90, hsp70 and calreticulin, utilize the common receptor CD91 to elicit their immune responses (12-14,17). We show that, unlike wild type mice, mice lacking CD91 expression on dendritic cells fail to elicit tumor-associated immunity. Antitumor immune responses can also be abrogated by the receptor-associated protein (RAP), an endogenous inhibitor of the HSP-CD91 pathway, which prevents exposed HSPs from binding to CD91. We show that endogenously expressed RAP inhibits the localization of HSPs in the draining lymph nodes, the uptake of HSPs by CD91 and the cross-presentation of HSP-chaperoned peptides. Our study demonstrates that the HSP-CD91 pathway is critical for the establishment of tumor-associated immunity.
Materials and Methods
Mice
Female BALB/c, C57BL/6, C.129S7(B6)-Rag1tm1Mom/J (rag1−/− BALB/c), and B6(Cg)-Rag2tm1.1Cgn/J (rag2−/− C57BL/6), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-1), B6.SJL-PtprcaPepb/BoyJ (CD45.1) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in the animal facility at the University of Pittsburgh. All experimental mice were 6–8-weeks old. B6;129S7-Lrp1tm2Her/J (CD91flox/+) mice (Jackson Laboratory, Bar Harbor, ME) were mated to homozygousity. CD91 conditional knockout mice were generated by crossing B6.Cg-Tg(Itgax-cre)1-1Reiz/J (CD11c-Cre) mouse with CD91floxP/flox mouse. Specific depletion of CD91 in CD11c+ cells was confirmed by using immunoblot and flow cytometry. All experiments with mice were approved by the Institutional Animal Care and Use Committee (Pittsburgh, PA) and performed in compliance with its guidelines.
Cells and Reagents
Tumor cell lines: lung carcinoma D122, fibrosarcoma CMS5 and Simian Virus 40 (SV40)-induced SVB6 were obtained from American Type Culture Collection (ATCC) and cultured in complete Dulbecco’s modified Eagle’s medium (DMEM), which includes 1% sodium pyruvate, 1% L-glutamine, 1% non-essential amino acids, 1% penicillin and streptomycin, 0.1% 2-mercaptoethanol and 10% fetal bovine serum (GIBCO). RAP or control vector-transfected tumor cells were cultured in complete DMEM media plus blasticidin (3μg/mL, Invitrogen). Double-transfected tumor cells (gp96EGFP+RAP, or gp96EGFP+control vector) were cultured in complete DMEM media plus blasticidin (3μg/mL, Invitrogen) and geneticin (0.7g/L, GIBCO). All tumor cell lines were free of specific pathogen, tested by IMPACT I PCR Profile (Research Animal Diagnostic Laboratory, RADIL, Columbia, MO). All cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. Ovalbumin, complete Freund's adjuvant, incomplete Freund's adjuvant, LPS, and mitomycin-C were purchased from Sigma (St. Louis, MO). GM-CSF was purchased from Fisher Scientific. Ovalbumin was rendered free of endotoxin by detoxi-Gel columns (Thermo Scientific). Mouse CD8α+ T cell isolation kit, and mouse CD11c microbeads were purchased from Miltenyi Biotec (Auburn, CA). Mouse IL-1β ELISA kit and mouse IL-2 ELISA kit were purchased from eBioscience (San Diego, CA). Pacific Blue anti-mouse CD8α antibody, PerCP-Cy5.5 anti-mouse CD4 antibody, APC anti-mouse CD19 antibody, PE-Cy5 anti-mouse CD3 antibody, PE anti-mouse CD11c antibody, PE-Cy7 anti-mouse CD11b antibody, APC anti-mouse CD45.2, FITC anti-mouse CD40 antibody, PE anti-mouse CD86 antibody, and PE anti-mouse MHC II (I-A/I-E) antibody were purchased from BD Pharmingen. Anti-V5-HRP antibody, 53kDa V5-containing protein (positope), and APC anti-CD45 antibody were purchased from Invitrogen (Carlsbad, CA). Anti-grp94 antibody and anti-hsp90 antibody were purchased from Enzo Life Sciences (Farmingdale, NY). Anti-CD91 β-chain antibody and anti-CD8 depleting antibody were purified from culture supernatant of 11H4 and TIB210 hybridomas (ATCC), respectively. Anti-OVA antibody was purchased from Abcam (Cambridge, MA).
Tumor growth assay and tumor rejection assay
8×105 D122 or 1x106 SVB6 tumor cells were injected intradermally into CD91−/− or control mice, with or without CD8 depletion. Tumor growth was measured on two axes thereafter. CD8 T cells were depleted with anti-CD8 antibody one day before tumor challenge as described (26). To test the effect of RAP on the immunogenicity of tumor, BALB/c (or C57BL/6) mice were challenged with 1x106 RAP- or control vector-transfected CMS5 (or SVB6) cells. In a separate experimental setting, immunocompromised rag1−/− BALB/c or rag2−/− C57BL/6 mice were challenged with 1×106 corresponding RAP- or control vector-transfected tumor cells. Tumor growth was measured on two axes for 2-3 weeks after challenge. The in vitro proliferative rates of those tumors were determined using the Click-iT EdU Assay Kit (Invitrogen, Carlsbad, CA). To test antigen-transfer from RAP-expressing tumor cells to APCs, BALB/c mice were immunized intradermally with titrated dose of mitomycin C-treated, RAP- or control vector-transfected CMS5 cells. Two weeks later, mice were challenged with 1×106 untransfected CMS5 cells, and tumor growth was measured. In the E.G7 tumor system, gp96 was purified from cultured cells as described (6). Mice were immunized intradermally with 1μg gp96 twice one week apart and challenged with 5×105 E.G7 tumor cells in PBS one week later. Tumor growth was measured on two axes and expressed as average tumor diameter.
T cell proliferation assay
Endotoxin-free OVA was introduced into CMS5 cells expressing either RAP or control protein by electroporation at 200 V for 30 ms (Bio-rad). The OVA-loaded cells were then rendered replication-incompetent by treatment with mitomycin-C. CD45.2+ OT-1 cells were harvested from spleens, enriched for CD8+ T cells (Miltenyi Biotec), and labeled with CFSE (Invitrogen). CFSE-labeled OT-1 cells were transferred into CD45.1+ C57BL/6 or CD91−/− mice via the retro-orbital route (1.5×106 cells per mouse). One day later, recipient mice were immunized intradermally with 1×105 or a high dose (1×106) of replication-incompetent OVA-transfected CMS5 cells with or without RAP expression. After 3 days, cells from the draining lymph nodes were harvested and stained with anti-45.2 and anti-CD8 (for the experiment in CD45.1+ C57BL/6 mice, Fig. 5 D and Fig. S2 C) or with anti-CD8 antibody alone (Fig. 5 E) to enrich for the previously transferred OT-1 cells. CFSE dilution of those OT-1 cells was examined. For CD91-independent T cell priming, mice were immunized subcutaneously twice with 20μg OVA 8 peptide emulsified in Freund's adjuvant. One week after the last vaccination, 5×106 splenocytes were harvested and cultured ex vivo for 5 days in the presence of OVA 8 peptide. IL-2 was measured by ELISA. In Fig. 2 F,G, OT-1 cells were harvested from the spleens, enriched for CD8+ T cells (Miltenyi Biotec), and labeled with CFSE (Invitrogen). CFSE-labeled OT-1 cells (2.5×106 cells per mouse) were transferred into CD91−/− or CD91+/+ mice via the retro-orbital route. One day later, recipient mice were immunized intradermally with 20 μg OVA 8 peptide emulsified in Complete Freund's adjuvant. After 3 days, cells from the draining lymph nodes were harvested and stained with anti-CD8 antibody. CFSE dilution of the previously transferred OT-1 cells was examined.
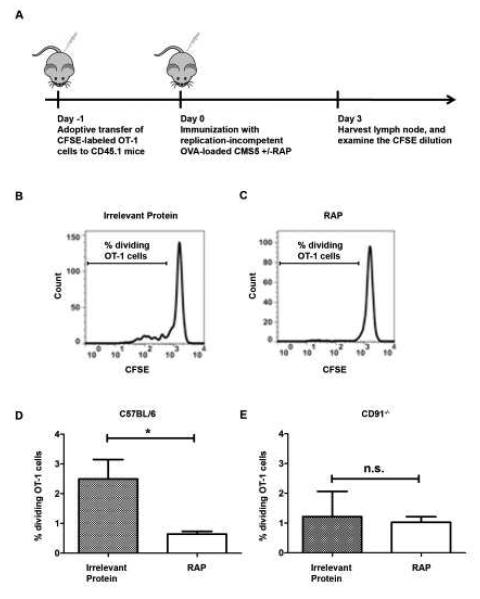
Figure 5. Inhibition of antigen cross-presentation by RAP reduces T cell proliferation in vivo.
The effect of RAP on T cell proliferation in vivo was examined. (A) CD45.1+ C57BL/6 mice were adoptively transferred with CFSE-labeled CD45.2+ OT-1 cells (1.5×106 per mouse), one day before immunization with mitomycin-C treated OVA-loaded CMS5 with or without RAP expression. After 3 days, the draining inguinal and axillary lymph nodes were harvested and stained for CD45.2 and CD8 expression to differentiate adoptively transferred OT-1 cells from endogenous responses. (B and C) Histograms from the flow cytometry analysis of gated CD45.2+CD8+ OT-1 cells from representative mice of each group. (D) The percentages of dividing OT-1 cells were compared in mice immunized with OVA-loaded CMS5 cells, expressing RAP or control protein. (E) CD8+OT-1 proliferation in CD91−/− mice was measured on day 3 following immunization with mitomycin-C treated OVA-loaded CMS5 with or without RAP expression. The percentages of dividing OT-1 cells are shown. * P < 0.05, n.s. not significant. Experiments were independently performed twice with 3-5 mice per group. Error bars indicated s.e.m.
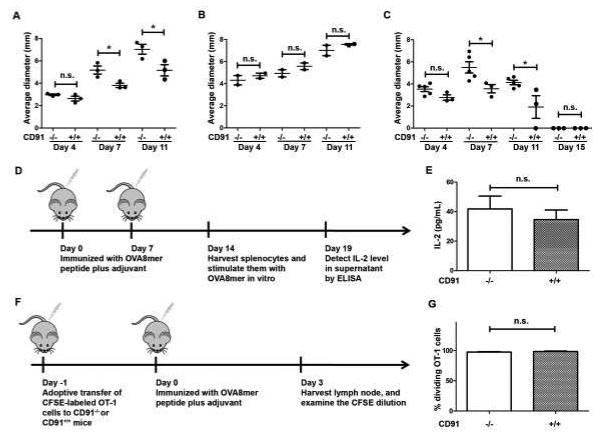
Figure 2. CD91 expression in CD11c+ cells is required for tumor-associated immunity.
(A) CD91−/− or CD91+/+ littermates were challenged intradermally with D122 tumor cells. Tumor growth was measured on two axes. (B) CD8+ cells were depleted from CD91−/− or CD91+/+ mice 24 hours prior to implantation of intradermal D122. CD8 depletion was continuous for >5 days. Tumor growth was monitored as in (A). (C) SVB6 tumor was implanted intradermally into CD91−/− or CD91+/+ littermates. Tumor growth was monitored as in (A). (D) Schema for CD91 independent priming. CD91−/− or CD91+/+ mice were immunized with OVA8 peptide emulsified in CFA (week 1) or IFA (week2). Supernatants from spleen cells cultured ex vivo with OVA8 peptide for 5 days were analyzed for IL-2. (E) IL-2 ELISA was performed on supernatant of cultures. (F) CFSE-labeled OT-1 cells were transferred to mice followed by immunization with OVA8 peptide in CFA. Lymph nodes were harvested on day 3. (G) Percent dividing OT-1 cells in lymph nodes were measured by flow cytometry. n.s., not significant; * P< 0.05, ** P< 0.01. Experiments were independently performed twice with 3-5 (A,C,E,G) or 2 (B) mice per group.
Microscopy
Transfected tumor cells were cultured overnight in 35 mm glass bottom culture dishes (MatTek Corp. Ashland, MA) at 37°C, washed with PBS, fixed in 2% paraformaldehyde, permeabilized in 0.1% Triton X-100, and blocked with 2% BSA. For RAP-transfected cells, mouse anti-V5 antibody and Cy3-conjugated goat anti-mouse IgG antibody were used to stain RAP; rat anti-HSP90 antibody and DyLight488-conjugated goat anti-rat IgM antibody were used to stain HSP90; DAPI was used to stain nuclei. For gp96-EGFP-transfected cells, rhodamine-conjugated Phalloidin (Invitrogen/Life Technologies, Grand Island, NY) was used to stain the cytoskeleton; DAPI was used to stain nuclei, according to a protocol developed by the Center for Biologic Imaging (CBI, University of Pittsburgh). Tumor tissues were incubated with 2% paraformaldehyde for one hour, then with 30% sucrose at 4°C overnight. After freezing, tumor tissues were cut into 8um sections using a cryo-microtome (HM505E Microm). The sections were permeabilized with 0.1% Triton*100 and blocked with 2% BSA. Mouse anti-V5 antibody and Cy3-conjugated goat anti-mouse IgG antibody were used to stain RAP. Rat anti-HSP90 antibody and DyLight488-conjugated goat anti-rat IgM antibody were used to stain HSP90. DAPI was used to stain nuclei. All images were captured using an Olympus FV1000 inverted confocal microscope with 100x objective (Fig. 3 C and D, and 6 B) or 60x objective (Fig. 3 K) and Fluoview v. 2.1 acquisition software (Melville, NY). Imaris v. 7.2.1 (Bitplane, Zürich, Switzerland) and Photoshop v. 7.0 (Adobe, San Jose, CA) were used for analysis and to prepare the images for publication.
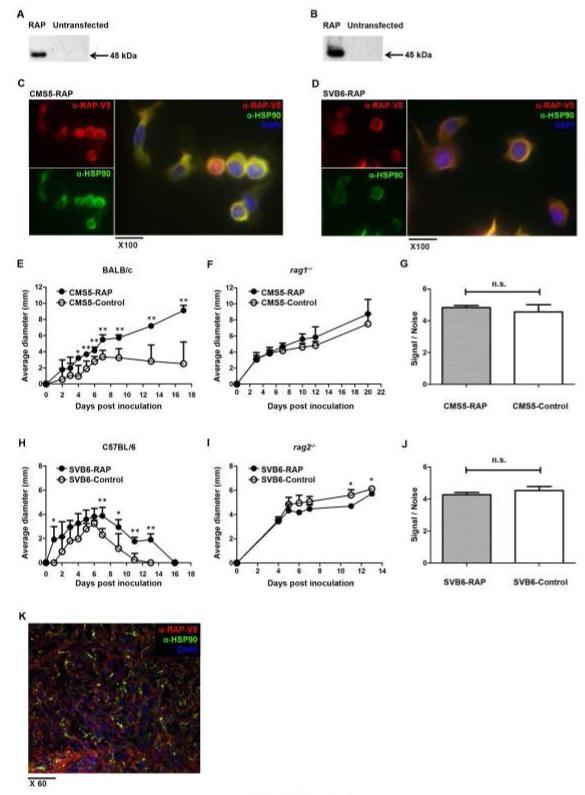
Figure 3. Inhibition of ligand binding to CD91 abrogates priming of tumor immunity.
(A and B) The CD91 inhibitor, RAP, was transfected and stably expressed in tumor cells, as confirmed by immunoblot in CMS5 cells (A), or SVB6 cells (B). (C and D) Cytosolic localization of RAP in CMS5 (C) cells, or SVB6 (D), cells was examined by confocal microscopy comparing the signal of RAP with the cytosolic protein HSP90. The two signals co-localized to give the yellow color. (E and F) CMS5 cells expressing RAP or a control protein were implanted intradermally in BALB/c (E) or rag1−/− (F) mice and tumor growth was monitored. Average tumor diameter was calculated. (G) Proliferation of CMS5 cells with RAP or control protein expression was determined by EdU incorporation into DNA. (H and I) SVB6 cells expressing RAP or control protein were implanted into C57BL/6 (H) or rag2−/− (I) mice and tumor growth was monitored. (J) Proliferation of SVB6 cells with RAP or control protein expression was determined by EdU incorporation into DNA. (K) CMS5 tumor expressing RAP were established intradermally in BALB/c mice for 15 days. Tumor was harvested, sectioned and stained with antibodies to V5-tagged RAP and HSP90. n.s., not significant; * P < 0.05, ** P < 0.01. Experiments were independently performed twice (A-D,G,J,K) or thrice (E,F,H,I) with 3-5 mice per group. Error bars indicated s.e.m.
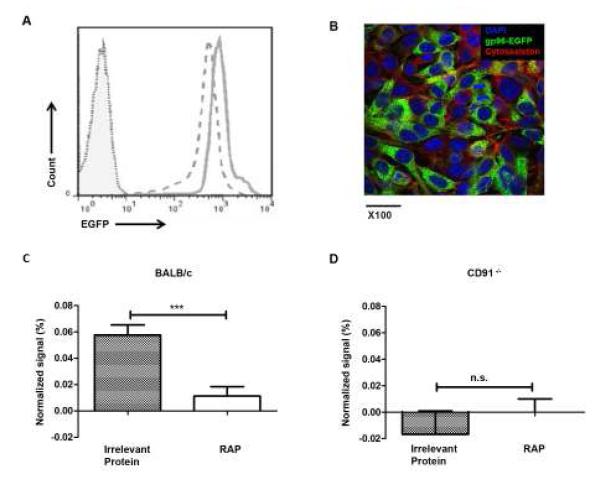
Figure 6. Transfer of HSP-peptide complexes to APCs in the lymph node requires engagement of CD91.
CMS5 cells expressing RAP or control protein were transfected with gp96-EGFP. Robust fluorescence of gp96-EGFP was analyzed by flow cytometry (A) and confocal microscopy (B). The foot pad of BALB/c (C), or CD91−/− (D), mice were implanted with 5×106 of either cell type and the draining or contralateral non-draining lymph nodes were harvested two days later. Cells were stained for the hematopoietic marker CD45 and analyzed by flow cytometry for EGFP. The EGFP signal was normalized against the starting fluorescence in each cell type and the background fluorescence of non-draining lymph nodes. *** P < 0.001, n.s. not significant. Experiments were independently performed twice with 8 mice per group (A), or 3 mice per group (B). Error bars indicated s.e.m.
HSP transfer experiments in vivo
5×106 CMS5 cells co-transfected either with RAP and gp96-EGFP, or with control vector and gp96-EGFP, were injected into one footpad of wild type BALB/c or CD91−/− mice. Two days later, the draining (dLN) and contra lateral (non-draining, ndLN) popliteal lymph nodes were harvested. EGFP signal in CD45+ cells in the lymph nodes were compared. Due to minor variations in auto-fluorescence observed in each mouse and in the fluorescence of the gp96-EGFP-expressing cultured cells over time, the signal was normalized to these two parameters by accounting for background signal in the ndLN and the fluorescence of gp96-EGFP-expressing cells on the day of the experiment, respectively (Supplementary Fig. S3 D-G). The following formula was used;
Statistical analysis
An unpaired two-tailed Student's t-test or a two-way ANOVA test was used for statistical analyses, and a P value of less than 0.05 was considered statistically significant. Error bars were calculated as standard error of the mean (s.e.m).
RESULTS
Selective loss of CD91 in CD11c+ cells renders mice unresponsive to gp96
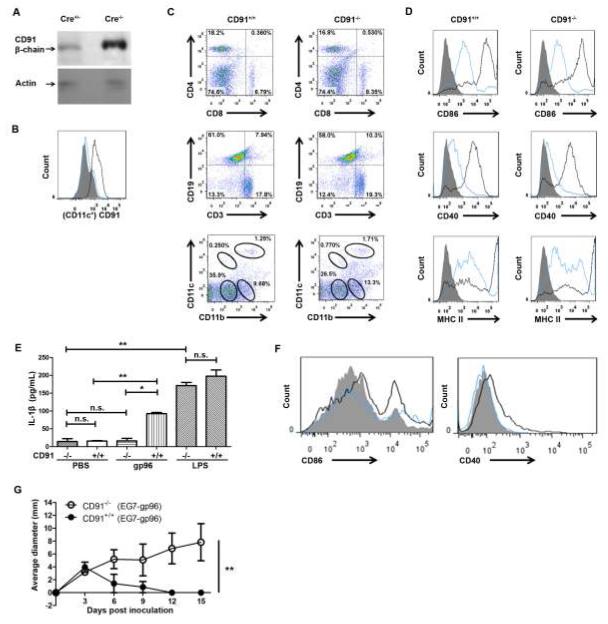
Using multiple approaches in vitro, we and others have demonstrated that CD91 serves as a receptor for immunogenic HSPs including gp96 (12-17). Conventional CD91 knock-out mice are embryonically lethal (27), thus we created mice lacking CD91 expression in CD11c+ cells, to test the role of CD91 in HSP-mediated immunogenicity in vivo. Mice with homozygous floxed CD91/LRP1 (CD91flox/flox) were crossed with mice expressing Cre-recombinase under the CD11c promoter. Lack of CD91 expression was confirmed in the bone marrow dendritic cells (BMDCs) of these mice, generated by culturing bone marrow cells in GM-CSF for 6 days and purified by CD11c expression to 96%. Cells were analyzed by SDS-PAGE and immunoblotting for CD91 protein expression (Fig. 1 A). In addition, CD11c+ cells in the lymph nodes of CD91flox/flox Cre+/− mice were analyzed by flow cytometry. Loss of CD91 expression in CD11c+ cells was confirmed (Fig. 1 B). The CD11c+ cells from the lymph node analyzed in Fig. 1 B included Langerhans cells, CD103+ cells and other subsets of dendritic cells. Loss of CD91 expression in these mice, herein referred to as CD91−/− thus occurred on all these cellular subtypes, regardless of their conceivable role in HSP-dependent immunity. These mice had normal percentages of other cellular immune compartments including CD4 and CD8 T cells, B cells, macrophages and DCs when compared to litter mates, CD91flox/flox Cre−/− mice (CD91+/+) (Fig. 1 C). The ability of BMDCs from CD91−/− mice to mature was tested by pulsing these cells with LPS for 24 hours and monitoring the expression of several maturation markers. CD86, CD40 and MHC II were all up-regulated in response to LPS, and the up-regulation was to similar levels as those in BMDCs from CD91+/+ littermates (Fig. 1 D). BMDCs from CD91−/− mice were also able to respond to LPS by secreting IL-1β, measured by ELISA 24 hours later (Fig. 1 E). Importantly however, CD91−/− BMDCs failed to secrete IL-1β in response to gp96. Control BMDCs from CD91+/+ mice secreted IL-1β after stimulation with LPS or gp96, consistent with our previous observations (17,18). We tested the ability of BMDCs to mature in response to gp96 as previously shown (28). BMDCs from CD91−/− mice failed to upregulate maturation markers CD86 and CD40 in response to incubation with gp96 (Fig. 1F), in contrast to BMDCs from CD91+/+ mice. We next tested the response of CD91−/− mice to gp96 immunization in a prototypical tumor-rejection assay (6,14). CD91−/− or CD91+/+ mice were immunized with E.G7-derived gp96. Mice were then challenged with E.G7 and tumor growth was monitored. While tumors were rejected in gp96-immunized CD91+/+ mice (as observed routinely, 6,14), there was no tumor-rejection in gp96-immunized CD91−/− mice (Fig. 1 G).
Figure 1. Selective loss of CD91 in CD11c+ cells renders mice unresponsive to gp96.
CD91flox/flox mice were mated with CD11c+-Cre recombinase mice and backcrossed. (A) BMDCs were sorted for CD11c to 96% purity and analyzed by immunoblotting for CD91 β-chain or actin as a loading control. (B) Lymph node cells from CD91−/− (blue) or CD91+/+ littermates (black) were stained with anti-CD91 antibody, gated on CD11c+ and analyzed by flow cytometry. Filled grey is secondary antibody alone. (C) Lymph node cells from CD91−/− or CD91+/+ littermates were phenotyped for markers indicated. (D) BMDCs from CD91−/− or CD91+/+ mice were pulsed on day 6 with LPS for 24 hours (black line) or left un-pulsed (blue line). Cells were analyzed for expression of CD86, CD40 and MHC II. Filled grey is secondary antibody alone. (E) BMDCs from CD91−/− or CD91+/+ mice were pulsed with 100μg gp96 or 1EU LPS for 24 hours or with PBS. IL-1β was measured by ELISA. (F) BMDCs from CD91−/− (blue line) or CD91+/+ (black line) mice were pulsed on day 6 with 100μg gp96 for 24 hours or left un-pulsed (filled grey). Cells were analyzed for expression of CD86 and CD40. (G) CD91−/− or CD91+/+ mice were immunized with 1 μg E.G7 tumor-derived gp96 twice, one week apart, followed by E.G7 tumor challenge one week later. Tumor growth was monitored. * P< 0.05, ** P< 0.01, n.s. not significant. Error bars indicate s.e.m. Experiments were independently performed twice with 2 (A-F) or 3-5 (G) mice per group.
Loss of CD91 expression in antigen presenting cells abrogates tumor-associated immunity
As a measure of tumor-associated immunity, we tested the rate of growth of tumors in CD91−/− mice using the moderately immunogenic Lewis Lung Carcinoma D122. When mice were inoculated with 8×105 D122 cells, the tumor growth rate was significantly faster in CD91−/− mice than in CD91+/+ recipient mice (Fig. 2 A). The significant difference in tumor growth rate was observed at early time points, up to day 11, with differences then dissipating. To test the role of adaptive immune response on tumor growth, we depleted CD8+ T cells from CD91−/− or CD91+/+ mice. In the absence of CD8+ cells, D122 tumors grew with identical kinetics in mice from both groups (Fig. 2 B). The rate of tumor growth in both groups was identical to CD91−/− mice in Fig. 2A. Therefore, CD8+ T cell priming was impaired in CD91−/− mice. To test the generality of these observations, similar experiments were performed with the highly immunogenic SV40-transformed regressor tumor SVB6. While SVB6 cells ultimately were rejected in both CD91−/− and CD91+/+ mice, the tumors grew significantly larger and were rejected more slowly in CD91−/− mice (Fig. 2 C). Since CD91−/− mice were deficient in their ability to mount antitumor immune responses, we tested their general competence in mounting immune responses with a regimen that bypasses a requirement for antigen-uptake and intracellular processing. CD91−/− or CD91+/+ mice were immunized with the OVA 8-mer (SIINFEKL) peptide plus Freund’s adjuvant and spleen cells were examined for T cells specific for the peptide. The OVA 8 peptide required no CD91-dependent uptake or further processing by the cell prior to binding MHC I. Following peptide stimulation of spleen cells ex vivo, IL-2 was measured in culture supernatants by ELISA as an indication of OVA 8-specific T cells (Fig. 2D). Equivalent levels of functional responsive OVA 8-specific T cell responses were obtained in both groups of mice (P = 0.5428, Fig. 2 E). A second assay was used to confirm the results in Fig. 2E. CFSE-labeled OT-1 cells were adoptively transferred into CD91−/− or CD91+/+ mice one day prior to immunization with OVA 8 peptide plus adjuvant (Fig. 2 F). Three days later, lymph nodes were harvested and the percent dividing OT-1 cells was quantified. OT-1 cells were observed to divide to similar levels following immunization of CD91−/− and CD91+/+ mice (Fig. 2 G). These results are consistent with a normal immune phenotype observed in Fig. 1 C-E and show that CD91−/− mice are capable of priming T cell responses when immunized with peptide + adjuvant, a regimen that does not require CD91 for uptake.
CD91-HSP interaction is required for priming tumor immunity
CD91 is both an endocytic (12-15) and signaling receptor (17) for immunogenic HSPs. We next tested if CD91 was required for its role in binding extracellular ligands by using the universal CD91-binding antagonist, receptor-associated protein (RAP) (29). RAP interacts with CD91 with high affinity and inhibits the binding of immunogenic HSPs (14). To determine if abrogation of ligand-binding to CD91 was responsible for the decreased immune responses to tumors described in Fig. 2, we expressed and quantified RAP in the moderately immunogenic fibrosarcoma CMS5, or SVB6 (Fig. 3 A-D, Supplementary Fig. S1 A-E). Tumors were determined to express ~10 fg of RAP per cell for both tumors. Tumors expressing RAP or a control (non-CD91 binding) protein were then implanted into BALB/c mice and tumor growth was monitored. In the tumor growth assay, RAP-expressing CMS5 grew with significantly faster kinetics when compared to control CMS5 tumor cells (Fig. 3 E). However, when the same tumor cells were implanted into immunocompromised rag1−/− mice, there was no difference in growth rate between the RAP and non-RAP expressing tumor cells (Fig. 3 F). Since tumor growth rate is determined largely by the balance between tumor cell proliferation and lysis (mediated by immune effectors), we tested if RAP transfection had any effect on cellular proliferation. There was no difference in growth rate between RAP and non-RAP expressing cells when tested in a proliferation assay in vitro that measured incorporation of EdU dye into DNA (Fig. 3 G). These results were confirmed in the SVB6 tumor system in C57BL/6 mice. RAP-expressing SVB6 tumors grew to a significantly bigger size and were rejected more slowly than control SVB6 tumor cells (Fig. 3 H). In immunocompromised rag2−/− mice, however, the growth of tumors was comparable irrespective of RAP expression (Fig. 3 I). Similar to the CMS5 model, SVB6 tumors proliferated comparably whether they expressed RAP or not (Fig. 3 J). The reduction of immunogenicity of RAP-transfected cells depends on the continual expression of RAP. Therefore, RAP expression in tumors was monitored throughout the duration of the experiment and was found to be preserved 15 days after implantation (Fig. 3 K). These results highlight the role of CD91 as a receptor for ligand binding in mounting tumor-associated immunity.
RAP abrogates antigen transfer in vivo and inhibits T cell priming
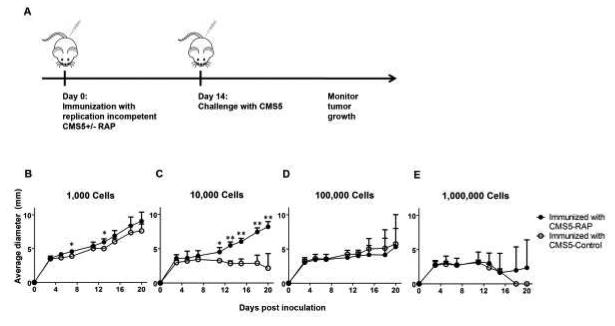
To examine the transfer of antigen from tumor cells to APCs in the presence or absence of RAP in vivo, we rendered the transfected tumor cells replication incompetent and used them as a source of antigen. Mice were immunized with titrated doses of replication-incompetent CMS5 tumor cells expressing RAP or control protein (Fig. 4 A). Two weeks later, mice were challenged with 1×106 wild type CMS5 cells and tumor growth was monitored. There was no protection in either group at the 1,000 immunizing dose (Fig. 4 B). However, when mice were immunized with 10,000 cells, RAP-expressing cells failed to protect mice from a subsequent tumor challenge while the control tumor cells were able to elicit protection (Fig. 4 C). These results are consistent with RAP-expressing cells failing to transfer their antigen to APCs leading to inefficient cross-priming of T cells. At >1×105 immunizing cells, tumor cells expressing either protein were able to protect mice suggesting that at higher antigen doses, CD91-independent mechanisms for antigen transfer to cross-prime APCs may come into play (Fig. 4 D and E).
Figure 4. RAP abrogates antigen transfer in vivo and inhibits T cell priming.
The ability of RAP-expressing CMS5 to transfer antigen and prime immune responses was tested in a tumor prophylaxis assay. (A) Schema showing the experimental design, in which BALB/c mice were immunized with titrated doses of replication incompetent, RAP-expressing cells and later challenged with parental (wild type) CMS5 cells. (B-E) Mice were immunized with 1000 (B), 10,000 (C), 100,000 (D) or 1,000,000 (E), RAP or control protein expressing CMS5 tumor cells rendered replication incompetent. Mice were challenged with 1×106 parental CMS5 cells in all groups and tumor growth was measured. n.s., not significant; * P < 0.05, ** P < 0.01. Experiments were independently performed twice with 3-5 mice per group. Error bars indicated s.e.m.
Inhibition of antigen cross-presentation by RAP reduces T cell proliferation in vivo
We tested the ability of RAP to prevent antigen transfer by measuring levels of antigen presented to T cells in the draining lymph nodes. CFSE-labeled CD45.2+ OT-1 cells were adoptively transferred into naïve mice (CD45.1+) which were immunized a day later with 1×105 ovalbumin-loaded CMS5 tumor cells (Fig. 5 A). This number of cells was estimated to be loaded with a total of approximately 1 ng of ovalbumin (Supplementary Fig. S2 A,B). The ovalbumin-loaded CMS5 tumor cells either expressed RAP or a control protein. The CMS5 tumor is of the H-2d haplotype and cannot directly present the OVA8 peptide to OT-1 cells. Therefore stimulation of the OT-1 cells will be an indication of cross-presentation by resident APCs of the (H-2b, CD45.1+) C57BL/6 mouse. Lymph node cells were harvested after 3 days and the percent of dividing CD8+CD45.2+ cells was determined by flow cytometry. We show CFSE dilution in OT-1 cells from representative mice in Fig. 5 B and C. In multiple mice (Fig. 5 D) we show that the presence of RAP in the tumor cells accounted for a decrease in proliferation of OT-1 cells when compared to cells expressing a control protein. This is despite the fact that both cell types were loaded with exactly the same amount of OVA. In parallel experiments, CFSE-labeled OT-1 cells were transferred into CD91−/− mice one day prior to immunization with replication-incompetent, ovalbumin-loaded CMS5 cells (Fig. 5 E) expressing RAP or an irrelevant protein. Lymph node cells were harvested after 3 days and the percent of all dividing CD8+ cells was determined by flow cytometry. Regardless of RAP expression, no OT-1 proliferation was observed in these CD91−/− mice. When mice were immunized with 10 times more OVA-loaded CMS5 cells (1×106, 10 ng), no difference was observed in dividing OT-1 cells when cells expressed RAP or a control protein (Supplementary Fig. S2 C). This latter result was consistent with results in Fig. 4 D,E.
CD91 is required for uptake of immunogenic HSPs
HSPs transfer antigen from tumor cells to APCs for cross-priming (5). To determine whether CD91 was essential for HSP-peptide cross-presentation in vivo, we developed an assay to monitor HSP transfer to APCs and the localization of the HSP in the lymph node. CMS5 cells were constructed to express gp96-EGFP (Fig. 6A and B). The amount of gp96-EGFP expressed in the CMS5 cells was equivalent to the levels of endogenous gp96 (Supplementary Fig. S3 A, B). RAP or a control protein was simultaneously expressed in the gp96-EGFP expressing cells. The two tumor types were implanted into individual foot pads of BALB/c mice and the corresponding draining lymph nodes were harvested 2 days later. The popliteal lymph node cells were stained and gated on CD45 to identify hematopoietic cells and to exclude tumor cells that potentially drained directly, from the analysis (Supplementary Fig. S3 C). The amount of EGFP fluorescence was measured by flow cytometry (Supplementary Fig. S3 D,E). Due to minor variations in auto-fluorescence observed in each mouse and in fluorescence of the gp96-EGFP-expressing cultured cells (Fig. 6 A), the signal was normalized to these two parameters by accounting for background signal in contralateral (non-draining) popliteal lymph nodes and fluorescence of gp96-EGFP-expressing cells on the day of the experiment, respectively (Supplementary Fig. S3 D-G). Significantly less EGFP was detected in the lymph nodes when tumors expressed RAP compared to control protein (P = 0.0002, Fig. 6 C). We tested the transfer of HSP to APCs in CD91−/− mice using the same system. CMS5 cells expressing gp96-EGFP with or without RAP were implanted in the footpads of CD91−/− mice. Draining lymph node cells were harvested after 2 days and analyzed as in Fig. 6 C. As shown in Fig. 6 D, we did not detect gp96-EGFP signal in the lymph node regardless of whether the cells expressed RAP or not. These results highlighted the importance of CD91 for HSP transfer and trafficking.
DISCUSSION
In this study, we report that mice deficient in the receptor CD91 on antigen presenting cells are unable to mount a substantial CD8+ T cell-mediated immune response to tumors. In addition, tumors expressing molecules known to inhibit binding of ligands to CD91 exhibited the same phenotype of decreased antitumor immunity. We demonstrated that the inhibition was due to the inability of APCs to cross-present antigens chaperoned by heat shock proteins. To our knowledge these findings are the first reports of a direct role for CD91 in antigen cross-priming in vivo. We have utilized a novel system we have developed in which the tumor and the induction of tumor-associated immunity did not require experimental manipulation in vitro.
When antigen is abundant, acquisition of antigens by APCs for cross-presentation and cross-priming can occur through several mechanisms as in most infectious diseases (30). These mechanisms are less reliant on the source of antigen or the type of APC (31). However, most tumor-associated antigens are derived from mutations of self proteins and so are likely to be limited in quantity at the time of the development of tumor-associated immunity (32). As previously demonstrated for transplantable tumors, concomitant immunity is established by day 3 when intradermal tumors are barely visible (1). A careful quantification and titration of the amount of antigens in tumors available for cross-presentation is ~6 orders of magnitude less than is required if the antigen is transferred as an intact protein (5). This necessitates a special mechanism to efficiently transfer antigens from the bearing (tumor) cell to the cross-presenting cell. Here we show that the HSP-peptide complex with its receptor CD91 provides such a mechanism. This study is supported by a large body of work in vitro demonstrating the efficient cross-presentation of HSP-chaperoned peptides by a variety of CD91-expressing APCs (7-11,28,33). In our present study, it is clear that upward titration of tumor cell-associated antigen renders the cross-presentation less dependent on the HSP-CD91 pathway. One possibility is through the utilization of alternative undefined HSP receptors (5). Recently CD91 was also demonstrated to be a signaling receptor for the immunogenic HSPs allowing the initiation of signaling cascades necessary for secretion of various pro-inflammatory cytokines (17). As a single entity, the HSP-peptide complex is able to provide the dual signals of antigen and co-stimulation which are necessary for priming tumor-associated immunity.
Following priming of antitumor immunity, a period called immuno-editing occurs during which the tumor may develop several mechanisms allowing for co-existence with the immune response (3,34,35). These mechanisms include induction of immunosuppressive factors, activation of regulatory cells, loss of antigen-expression, or tumor-induced impairment of antigen presentation. As a mechanism of evasion of the immune response by tumors, preceding immuno-editing, we hypothesize that tumors may over-express RAP, or other as yet identified endogenous CD91 inhibitors, to impair antigen transfer. A previous study showed a correlation between elevated RAP levels and progressive disease in colon cancer patients (36). We expect this mechanism to occur during tumor initiation because antigen burden is very low, and HSP-mediated antigen uptake prevails, and allows the tumor to be firmly established. We note that as tumors grow and tumor antigen becomes more abundant other mechanisms of antigen transfer may be evoked. This is hinted at in Fig. 4 and supported by data in supplementary Fig. S2 C. These other mechanisms of antigen transfer may become dominant in cases of over-expressed self or oncoviral tumor antigens.
We used transplantable tumors in these studies because we can carefully titrate the input of tumors into mice, measure antigen-specific immune responses and stably introduce CD91 inhibitors such as RAP into the tumors. However, the role of CD91 in de novo induction of tumors remains to be tested, as was performed for the dependence of antitumor immunity on IFN-γ (2). Currently we are investigating carcinogen- and UV-induction of tumors in CD91−/− mice. Key steps in spontaneously developed tumors or oncogenesis elicited by carcinogens or UV can be regulated by the immune system. Tumors that are established in CD91−/− mice, and harvested on <6-8 days, are expected to be more immunogenic and rejected faster when transplanted into wild type mice, when compared to tumors established in wild type mice and transplanted into wild type mice. We expect this because those early tumors established in CD91−/− mice are presumed to be less edited, if edited at all, due to a failure in the establishment of antitumor immunity in these mice.
The impact of the loss of expression of CD91 in CD11c+ cells raises two issues; first, CD91 is known to bind to other ligands (29), and of these, only α2M has been shown to have any immunological function (37,38). Thus although not tested here, α2M bound to extracellular tumor antigens may be another mechanism for cross-priming. We also do not know the relative contribution of each HSP known to bind CD91 to the global immune response, since only gp96 was measured here. Second, given the recent observation that CD169+ macrophages are bona fide professional antigen presenting cells in the setting of apoptosis-associated antigen in lymph nodes (39), we expect a more robust phenotype when CD91 expression is eliminated in both macrophages and dendritic cells. Studies are currently underway to create mice with loss of CD91 in both CD11c+ and CD11b+ cells. Our present study is supported by work of Podack and colleagues showing that enforced secretion of gp96 from tumor cells enhances the generation of antitumor CD8 T cell responses. In those reports, CD11c+ cells were identified as the necessary APCs for the immune response (40,41).
Our findings have a bearing on immunotherapy of cancer. A prevalent approach for cancer immunotherapy involves the isolation of tumor-specific T cells from the blood of patients, expanding them in vitro and re-infusing these autologous, in vitro expanded T cells back into the patient (42). Correlations of the absence of T cells and the expression of endogenous CD91 inhibitors by tumors should be firmly established.
Supplementary Material
Acknowledgement
The authors would like to thank Laura Kropp, Megan Beck and Prerana Thapar for maintenance of the mouse colonies and other technical assistance. We would also like to thank Dr. Walter Storkus and Dr. Penny Morel for critically reviewing the manuscript.
This work was supported by NIH Grants AI079057, CA137133 and CA90440 to RJB.
Abbreviations
- HSP
Heat shock protein
- PAMPs
Pathogen associated molecular patterns
- RAP
receptor associated protein
Footnotes
Potential Conflict of Interest: RJB is a named inventor of intellectual property that is being evaluated under his NIH grants, and the technology has been licensed to a company in which RJB has no ownership interest or consulting contract and from which he has no sponsored research agreements. His significant financial interest consists only of a share of license fees and possible future royalties from the commercialization of his invention.
Author Contribution
YJZ, MNM and RJB performed the experiments. RJB conceived the study. YJZ and RJB wrote the manuscript.
References
- 1.North RJ, Kirstein DP. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1997;145:275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, et al. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 5.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. PNAS. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 8.SenGupta D, Norris PJ, Suscovich TJ, Hassan-Zahraee M, Moffett HF, et al. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J Immunol. 2004;173:1987–1993. doi: 10.4049/jimmunol.173.3.1987. [DOI] [PubMed] [Google Scholar]
- 9.Binder RJ, Kelly JB, 3rd, Vatner RE, Srivastava PK. Specific immunogenicity of heat shock protein gp96 derives from chaperoned antigenic peptides and not from contaminating proteins. J Immunol. 2007;179:7254–7261. doi: 10.4049/jimmunol.179.11.7254. [DOI] [PubMed] [Google Scholar]
- 10.Matsutake T, Sawamura T, Srivastava PK. High efficiency CD91- and LOX-1-mediated re-presentation of gp96-chaperoned peptides by MHC II molecules. Cancer Immun. 2010;10:7. [PMC free article] [PubMed] [Google Scholar]
- 11.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 14.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. PNAS. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobian AA, Canaday DH, Boom WH, Harding CV. Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J Immunol. 2004;172:5277–5286. doi: 10.4049/jimmunol.172.9.5277. [DOI] [PubMed] [Google Scholar]
- 16.Tischer S, Basila M, Maecker-Kolhoff B, Immenschuh S, Oelke M, et al. Heat shock protein 70/peptide complexes: potent mediators for the generation of antiviral T cells particularly with regard to low precursor frequencies. J Transl Med. 2011;9:175–189. doi: 10.1186/1479-5876-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- 20.Wang YF, Kelly CG, Singh M, McGowan EG, Carrara AS, et al. Stimulation of Th1-polarizing maturation of dendritic cells, cytokines, C-C chemokines, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 21.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 22.Zügel U, Sponaas AM, Neckermann J, Schoel B, Kaufmann SH. gp96-peptide vaccination of mice against intracellular bacteria. Infect Immun. 2001;69:4164–4167. doi: 10.1128/IAI.69.6.4164-4167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–497. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 24.Navaratnam M, Deshpande MS, Hariharan MJ, Zatechka DS, Jr, Srikumaran S. Heat shock protein-peptide complexes elicit cytotoxic T-lymphocyte and antibody responses specific for bovine herpesvirus 1. Vaccine. 2001;19:1425–1434. doi: 10.1016/s0264-410x(00)00381-9. [DOI] [PubMed] [Google Scholar]
- 25.Mo A, Musselli C, Chen H, Pappas J, Leclair K, et al. A heat shock protein based polyvalent vaccine targeting HSV-2: CD4(+) and CD8(+) cellular immunity and protective efficacy. Vaccine. 2011;29:8530–8541. doi: 10.1016/j.vaccine.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Sayles PC, Johnson LL. Intact immune defenses are required for mice to resist the ts-4 vaccine strain of Toxoplasma gondii. Infect Immun. 1996;64:3088–3092. doi: 10.1128/iai.64.8.3088-3092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 28.Messmer MN, Pasmowitz J, Kropp LE, Watkins SC, Binder RJ. Identification of the cellular sentinels for native immunogenic heat shock proteins in vivo. J Immunol. 2013;191:4456–4465. doi: 10.4049/jimmunol.1300827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 31.Norbury CC, Basta S, Donohue KB, Tscharke DC, Princiotta MF, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–1321. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava N, Srivastava PK. Modeling the repertoire of true tumor-specific MHC I epitopes in a human tumor. PLoS One. 2009;4:e6094. doi: 10.1371/journal.pone.0006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder RJ. Hsp receptors: the cases of identity and mistaken identity. Curr Opin Mol Ther. 2009;11:62–71. [PubMed] [Google Scholar]
- 34.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto K, Bu G, Chen S, Takei Y, Hibi K, et al. Premature ligand-receptor interaction during biosynthesis limits the production of growth factor midkine and its receptor LDL receptor-related protein 1. J Biol Chem. 2011;286:8405–8413. doi: 10.1074/jbc.M110.176479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binder RJ, Karimeddini D, Srivastava PK. Adjuvanticity of alpha 2-macroglobulin, an independent ligand for the heat shock protein receptor CD91. J Immunol. 2001;166:4968–4972. doi: 10.4049/jimmunol.166.8.4968. [DOI] [PubMed] [Google Scholar]
- 38.Chu CT, Pizzo SV. Receptor-mediated antigen delivery into macrophages. Complexing antigen to alpha 2-macroglobulin enhances presentation to T cells. J Immunol. 1993;150:48–58. [PubMed] [Google Scholar]
- 39.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki K, Nguyen T, Podack ER. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999 Nov 15;163(10):5178–82. [PubMed] [Google Scholar]
- 41.Strbo N, Pahwa S, Kolber MA, Gonzalez L, Fisher E, Podack ER. Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal Immunol. 2010;3:182–192. doi: 10.1038/mi.2009.127. [DOI] [PubMed] [Google Scholar]
- 42.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.