Abstract
In this study, we report a Japanese boy with testotoxicosis due to a heterozygous mutation [p. A572V] in the LH/CGR gene, who was the first boy treated with a third-generation aromatase inhibitor (AI) and reached his adult height in Japan. He showed accelerated growth and rapid penile growth from 3 yr of age and was diagnosed as having testotoxicosis. Combined treatment with anastrozole and spironolactone was started when he was 7 yr old and 145.8 cm (+4.45 SD) tall, at which point his bone age (BA) was 13.5 yr. His predicted adult height (PAH) was estimated to be 158.3 cm. The combined treatment was continued until he was 13 yr old and 166.5 cm tall, with his BA being 15.5 yr. He reached his adult height of 166.9 cm at 15 yr of age. Combined treatment with anastrozole and spironolactone successfully decelerated BA advancement, prolonged pubertal period and improved adult height.
Keywords: testotoxicosis, LH/CGR, aromatase inhibitors, anastrozole, spironolactone
Introduction
It is known that estrogen closes epiphysis of bones and stops height growth in both boys and girls. Therefore, the use of aromatase inhibitors (AIs) has now been reported for a variety of disorders, such as peripheral precocious puberty, congenital adrenal hyperplasia (CAH) and idiopathic short stature, in boys to increase their adult height (1, 2). Testotoxicosis, also known as familial male-limited precocious puberty (FMPP), is a form of peripheral precocious puberty characterized by low levels of serum LH (3) and is caused by heterozygous activating mutations of the LH/CGR gene encoding the LH/ choriogonadotropin receptor (LH/CGR) (4, 5).
Here, we report a Japanese boy with testotoxicosis due to a heterozygous mutation [p. A572V] in the LH/CGR gene, who was the first boy treated with a third-generation AI and reached his adult height in Japan.
Materials and Methods
Hormone measurements
Serum LH (prepubertal: 0.02–0.15 mIU/mL) and FSH (prepubertal: 0.38–1.11 mIU/mL) were measured by the immunofluorometric assay method using Delfia kits from Pharmacia Biosystems, Tokyo, Japan. Serum testosterone (prepubertal: <10 ng/dL) was measured by electrochemiluminescence immunoassay (ECLIA) (Elecsys, Roche Diagnostics, Mannheim, Germany).
DNA amplification and sequence analysis of the LH/CGR gene
The genetic study was approved by the Institutional Ethics Review Board at the National Center for Child Health and Development. Written informed consent for the genetic analysis was obtained from the parents.
Genomic DNA was isolated from peripheral blood by proteinase K digestion and phenol/chloroform extraction. The entire exon 11 of the LH/CGR gene was amplified as previously reported (6). The amplified PCR products were isolated and purified on a 1% agarose gel (Bio-Rad Laboratories, Richmond, CA, USA). The purified PCR products were directly sequenced using [α-35S]-dATP (GE Healthcare Bio-Sciences KK, Tokyo, Japan) and a Thermo Sequenase Cycle Sequencing Kit (Affymetrix Japan K.K., Tokyo, Japan).
Bone age (BA) estimation
BA was estimated by one trained doctor using the TW2 RUS method standardized for Japanese children.
Case Report
The patient showed accelerated growth and rapid penile enlargement from 3 yr of age. At his first visit at hospital, his height was 121.4 cm (+4.0 SD) and weight was 24.2 kg (+3.1 SD); he was 4 yr and 10 mo of age at that time. His penile growth was Tanner stage 3, pubic hair was Tanner stage 1 and testicular volumes were 8 ml bilaterally. BA using the TW2 RUS method for Japanese children was 9.2 yr. His serum testosterone level was 289 ng/dL. His serum LH and FSH levels were <2.0 mIU/mL and 1.0 mIU/mL, respectively. After administration of GnRH, his peak LH level was 2.0 mIU/mL (normal range for prepubertal boys: 1.70–3.77 mIU/mL) and peak FSH level was 3.5 mIU/mL (normal range for prepubertal boys: 4.38–9.48 mIU/mL), which suggested a prepubertal response. His serum testosterone level increased from 226 to 611 ng/dL after an HCG stimulation test. His serum concentrations of 17α-hydroxyprogesterone, dehydroepiandrosterone sulfate, hCGβ and hCG were not elevated, and his thyroid function was normal. This patient was diagnosed as having peripheral precocious puberty and referred to our clinic for treatment. The heights of his father and mother were 170 and 160 cm, respectively.
LH/CGR mutation
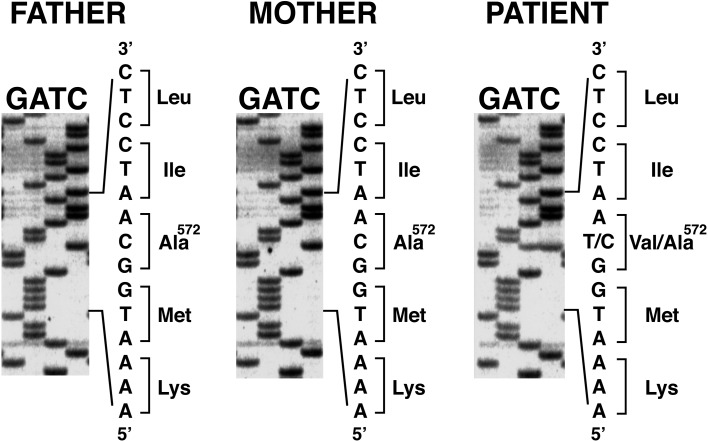
Direct sequencing of PCR products from the patient revealed a heterozygous for a C to T transition at nucleotide c.1715. This mutation changed codon 572 (GCA) encoding alanine to GTA encoding valine (p.A572V mutation). The A572V mutation is known as a causative mutation of testotoxicosis. This mutant exhibited high basal cAMP levels, leading to Leydig cell activation and an increase in testosterone (7). His parents were homozygous for the wild-type allele (Fig. 1). He was diagnosed as having testotoxicosis.
Fig. 1.
Genetic analysis of the LH/CGR gene. The patient is heterozygous for a C to T transition at nucleotide c.1715. This mutation results in substitution of Val for Ala at codon 572 (p.A572V mutation). His parents are homozygous for the wild-type allele.
Treatment course
At 7 yr of age, his height was 142.8 cm (+4.45 SD) and his BA had advanced to 13.5 yr (BA/CA 1.93). His predicted adult height (PAH) was estimated to be 158.3 cm, according to the projected height SD score for his bone age (8). His testicular volume was 10–12 ml, penile growth was Tanner stage 4 and pubic hair was Tanner stage 2. In order to decelerate bone age maturation and pubertal development, anastrozole (1 mg daily) and spironolactone (150 mg daily) were begun to treat testotoxicosis. The dose of anastrozole was cut by half (0.5 mg daily) from 9 to 11 yr of age, as his level of estradiol decreased. At 10 yr of age, his BA was 13.9 yr, advancing by only 0.4 yr compared with his 3-yr increase in chronological age. His increased basal LH and FSH concentrations (2.09 and 5.26 mIU/ml, respectively) suggested that he entered central puberty at 11 yr of age. Metenolone (10 mg daily) was started to promote growth velocity at 12 yr of age. The combination treatment was well tolerated and resulted in deceleration of BA advancement and elongation of his pubertal period. At 13 yr of age, his height increased to 166.5 cm (+1.22 SD) and BA reached 15.5 yr (BA/CA 1.19), and the treatment was discontinued. His bone density measured by a Dichroma Scan (DCS-600EX-III, ALOKA, Japan) indicated 0.78 SD of normal age-matched group. Laboratory data throughout treatment revealed no hepatotoxicity and low estradiol levels. At 15 yr of age, he reached his adult height of 166.9 cm (Table 1, Fig. 2).
Table 1. Clinical and biological data.
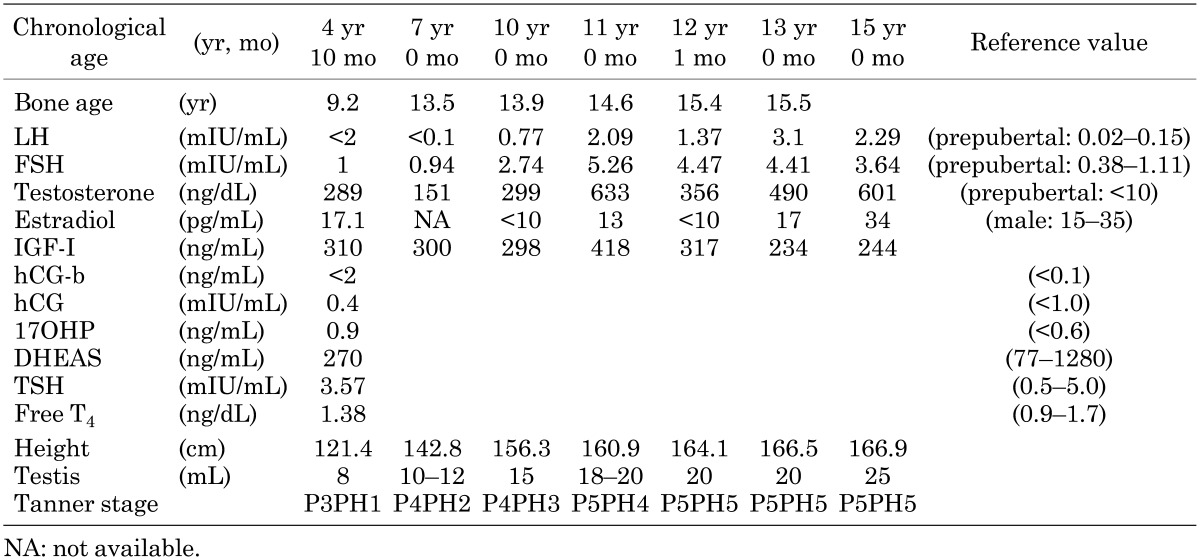
Fig. 2.
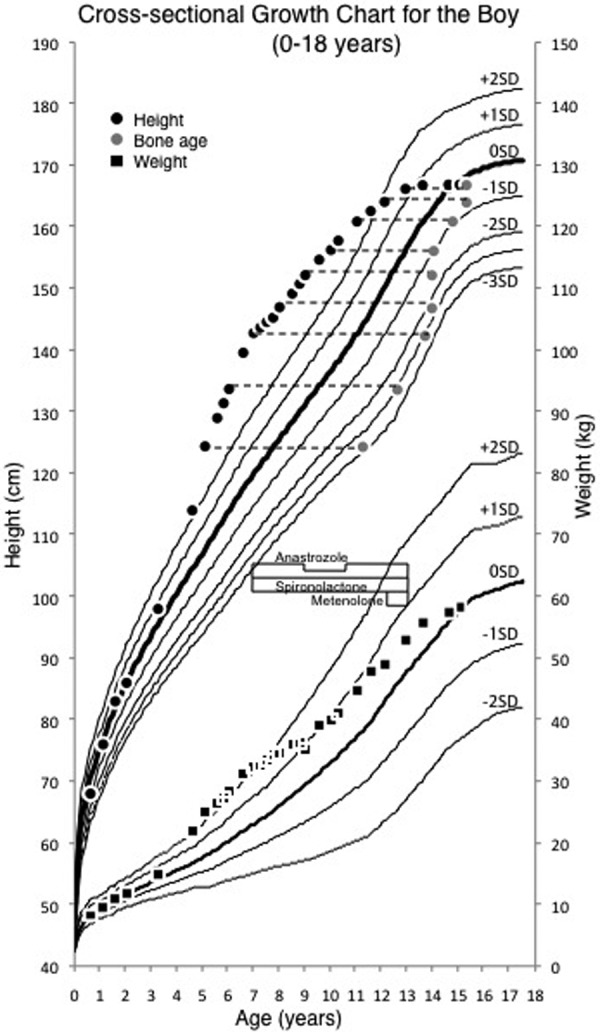
Longitudinal growth record of the patient with testotoxicosis. Gray circles indicate bone age. Administration of anastrozole (1 mg daily) and spironolactone (150 mg daily) was started at 7 yr of age and was continued until 13 yr of age. The dose of anastrozole was cut by half (0.5 mg daily) from 9 to 11 yr of age. Administration of metenolone (10 mg daily) was started at 12 yr of age and was continued until 13 yr of age.
Discussion
Testotoxicosis or FMPP has been previously treated with medroxyprogesterone acetate, spironolactone, cyproterone acetate (a weak antiandrogen agent) and testolactone (first-generation AI) in Japan (6, 7, 9, 10). Furthermore, in cases developing secondary gonadotropin-dependent puberty, patients would be treated with a GnRH analogue, such as buserelin acetate or leuprorelin acetate. Six Japanese patients were reported to have reached adult heights ranging from 146 to 161.8 cm after combination therapy for three to ten yr (6, 7, 9, 10). These previous studies did not result in sufficient deceleration of BA advancement (BA/CA 1.54–2.06). In addition, adult height was not increased compared with the PAH at the start of treatment, although combination therapy with spironolactone, testolactone and GnRH analogue successfully improved prepubertal growth rates and gonadotropin concentrations (11).
In this study, the patient reached his adult height at 166.9 cm, which is taller than those of the other 6 patients reported. This improvement in his adult height was caused by marked deceleration of BA advancement accompanied by prolongation of the pubertal period by the combination therapy with the antiandrogenic agent and third-generation AI. This effect is mainly attributed to the third-generation AI, since the antiandrogenic agent is not reported to be so effective in decelerating BA advancement. Improved adult height might be partly caused by metenolone, an anabolic steroid hormone. Since metenolone is not metabolized to estrogen, it promotes height growth and suppresses gonadotropins through a feedback mechanism without BA advancement. Metenolone administration maintained growth velocity to some extent.
The first-generation AI (testolactone) has demonstrated less than 90% inhibition of cytochrome P450 enzyme (CYP19), whereas a third-generation AI (anastrozole) is more potent (97% inhibition) and has a longer half-life (2 d). The use of a third-generation AI has the advantage of avoiding the use of a GnRH analogue even when the patients enter central puberty. Bicalutamide is a potent nonsteroidal antiandrogen agent used for the treatment of prostate cancer (12). It binds to the androgen receptor and increases androgen-receptor degradation. Since it also has a longer half-life, it can be administered once a day. Bicalutamide was not available in Japan when his treatment was started.
The combination therapy with bicalutamide and anastrozole/letrozole was reported to be effective in decreasing growth rates and BA maturation (13). Lenz et al. (14) reported that BA maturation was decelerated and that the ratios of BA/CA decreased to 1.01 and 1.4, respectively, after longer-term combined treatment (4.5–5 yr) in two boys. PAH in one patient increased from 168.9 cm at start of treatment to 178.2 cm over a total of 5.4 yr of treatment.
The current study is the first report to support the efficacy of a third-generation AI and an antiandrogen agent for the treatment of testotoxicosis in Japan. This combination therapy was well tolerated without adverse events. However, the use of a third-generation AI in children has not been established yet, not only in Japan but also throughout the world, although the first clinical experience in children was reported (2). As bicalutamide is a more potent antiandrogen agent, the combination of a third-generation AI and bicalutamide would be a more favorable treatment. Further experience and controlled clinical trials will be necessary to clarify the efficacy of the combination treatment for testotoxicosis.
Acknowledgements
This work was supported in part by a Grant for Research on Intractable Diseases (11103517) from the Ministry of Health, Labour and Welfare, Japan, and by Grants-in-Aid for Scientific Research (C) (23591523 and 25461517) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure statement: T. Tanaka is a consultant for Japan Chemical Research.
References
- 1.Miller WR, Jackson J. The therapeutic potential of aromatase inhibitors. Expert Opin Investig Drugs 2003;12:337–51. doi: 10.1517/13543784.12.3.337 [DOI] [PubMed] [Google Scholar]
- 2.Dunkel L. Use of aromatase inhibitors to increase final height. Mol Cell Endocrinol 2006;254–255:207–16. doi: 10.1016/j.mce.2006.04.031 [DOI] [PubMed] [Google Scholar]
- 3.Holland FJ. Gonadotropin-independent precocious puberty. Endocrinol Metab Clin North Am 1991;20:191–210 [PubMed] [Google Scholar]
- 4.Shenker A, Laue L, Kosugi S, Merendino JJ, Jr, Minegishi T, Cutler GB., Jr Nature 1993;365:652–4. doi: 10.1038/365652a0 [DOI] [PubMed] [Google Scholar]
- 5.Kremer H, Mariman E, Otten BJ, Moll GW, Jr, Stoelinga GB, Wit JM, et al. Cosegregation of missense mutations of the luteinizing hormone receptor gene with familial male-limited precocious puberty. Hum Mol Genet 1993;2:1779–83. doi: 10.1093/hmg/2.11.1779 [DOI] [PubMed] [Google Scholar]
- 6.Shinagawa T, Katsumata N, Sato N, Horikawa R, Tanae A, Tanaka T. Japanese familial patients with male-limited precocious puberty. Endocr J 2000;47:777–82. doi: 10.1507/endocrj.47.777 [DOI] [PubMed] [Google Scholar]
- 7.Yano K, Saji M, Hidaka A, Moriya N, Okuno A, Kohn LD, et al. A new constitutively activating point mutation in the luteinizing hormone/choriogonadotropin receptor gene in cases of male-limited precocious puberty. J Clin Endocrinol Metab 1995;80:1162–8 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Niimi H, Matsuo N, Fujieda K, Tachibana K, Ohyama K, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on Central Precocious Puberty. J Clin Endocrinol Metab 2005;90:1371–6. doi: 10.1210/jc.2004-1863 [DOI] [PubMed] [Google Scholar]
- 9.Yano K, Hidaka A, Saji M, Polymeropoulos MH, Okuno A, Kohn LD, et al. A sporadic case of male-limited precocious puberty has the same constitutively activating point mutation in luteinizing hormone/choriogonadotropin receptor gene as familial cases. J Clin Endocrinol Metab 1994;79:1818–23 [DOI] [PubMed] [Google Scholar]
- 10.Yano K, Kohn LD, Saji M, Kataoka N, Okuno A, Cutler GB., Jr Biochem Biophys Res Commun 1996;220:1036–42. doi: 10.1006/bbrc.1996.0528 [DOI] [PubMed] [Google Scholar]
- 11.Laue L, Jones J, Barnes KM, Cutler GB., Jr J Clin Endocrinol Metab 1993;76:151–5 [DOI] [PubMed] [Google Scholar]
- 12.Furr BJ. The development of Casodex (bicalutamide): preclinical studies. Eur Urol 1996;29 Suppl 2):83–95 [DOI] [PubMed] [Google Scholar]
- 13.Kreher NC, Pescovitz OH, Delameter P, Tiulpakov A, Hochberg Z. Treatment of familial male-limited precocious puberty with bicalutamide and anastrozole. J Pediatr 2006;149:416–20. doi: 10.1016/j.jpeds.2006.04.027 [DOI] [PubMed] [Google Scholar]
- 14.Lenz AM, Shulman D, Eugster EA, Rahhal S, Fuqua JS, Pescovitz OH, et al. Bicalutamide and third-generation aromatase inhibitors in testotoxicosis. Pediatrics 2010;126:e728–33. doi: 10.1542/peds.2010-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]