Abstract
Migration and anchorage of nuclei within developing and adult tissues rely on Linkers of the Nucleoskeleton to the Cytoskeleton (LINC complexes). These macromolecular assemblies span the nuclear envelope and physically couple chromatin and nuclear lamina to cytoplasmic cytoskeletal networks. LINC complexes assemble within the perinuclear space through direct interactions between the respective evolutionary-conserved SUN and KASH domains of Sun proteins, which reside within the inner nuclear membrane, and Nesprins, which reside within the outer nuclear membrane. Here, we describe and validate a dominant-negative transgenic strategy allowing for the disruption of endogenous SUN/KASH interactions through the inducible expression of a recombinant KASH domain. Our approach, which is based on the Cre/Lox system, allows for the targeted disruption of LINC complexes in a wide array of mouse tissues or specific cell types thereof and bypasses the perinatal lethality and potential cell nonautonomous effects of current mouse models based on germline inactivation of genes encoding Sun proteins and Nesprins. For these reasons, this mouse model provides a useful tool to evaluate the physiological relevance of LINC complexes integrity during development and homeostasis in a wide array of mammalian tissues.
Keywords: Nesprin, Sun proteins, LINC complex, KASH domain, SUN domain
INTRODUCTION
The nuclear envelope (NE) is composed of the inner and outer nuclear membrane (INM and ONM, respectively) that merge at nuclear pores and delineate the perinuclear space (PNS). Linkers of the Nucleoskeleton to the Cytoskeleton (LINC complexes) are large macromolecular scaffolds that span the NE and connect the interior of the nucleus to components of the cytoplasmic cytoskeleton (Burke and Roux 2009, Razafsky and Hodzic 2009, Starr and Fridolfsson 2010). They consist of interactions between Sun (Sad1/Unc84) proteins and Nesprins (Nuclear envelope spectrins). In mammals, Sun1 and Sun2, which are encoded by two distinct genes, are integral transmembrane proteins of the INM that interact directly with nuclear lamins (Hodzic, Yeater et al. 2004, Crisp, Liu et al. 2006, Haque, Lloyd et al. 2006). Within the PNS, the highly conserved C-terminal SUN domain interacts directly with the evolutionary-conserved KASH (Klarsicht/Anc-1, Syne Homology) domain found at the C-terminus of Nesprins (Fig. 1A). Nesprins 1, 2, 3 and 4 are integral transmembrane proteins of the ONM whose cytoplasmic regions can range in size from ~50 kDa to ~1 MDa and interact with a variety of cytoskeletal filaments and molecular motors (Padmakumar, Abraham et al. 2004, Wilhelmsen, Litjens et al. 2005, Meyerzon, Fridolfsson et al. 2009, Roux, Crisp et al. 2009, Fridolfsson and Starr 2010).
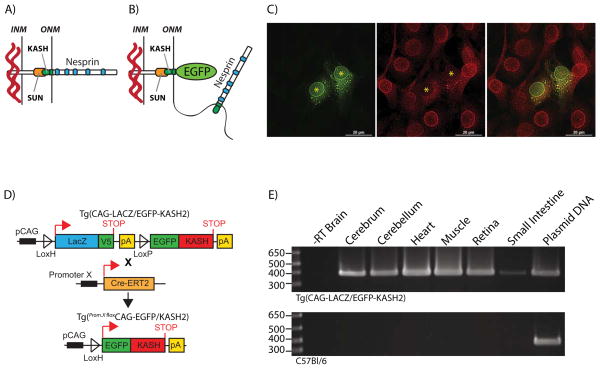
FIGURE 1. EGFP-KASH2 expression disrupts endogenous LINC complexes.
A) LINC complexes consist of interactions between highly conserved SUN and KASH domains, the respective molecular signature of Sun proteins and Nesprins. These macromolecular assemblies connect the interior of the nucleus with cytoskeletal components. B) Depiction of the dominant negative effect of EGFP-KASH2 expression on the integrity of LINC complexes through the displacement of endogenous Nesprins from the NE to the ER. C) Immunostaining of C2C12 myoblasts transfected with EGFP-KASH2 (green) with a Nesprin3 antiserum (red). Note the absence of Nesprin3 rims at the NE of EGFP-KASH2 positive cells. Asterisks denote nuclei centers of EGFP-KASH2+ cells. Scale bars: 20μm. D) Genetic control of EGFP-KASH2 expression in various tissues. EGFP-KASH2 is conditionally expressed upon breeding of the CAG-LacZ/EGFP-KASH2 strain to other strains expressing Cre recombinase under the control of a tissue/cell-specific promoter (Promoter X). Upon developmental activation of promoter X in double transgenic mice, Cre recombinase accesses the nucleoplasm either directly (Cre) or after Tamoxifen injections (CreER-T2) where it excises the LacZ ORF thereby inducing EGFP-KASH2 expression under the control of the CAG promoter. E) RT-PCR amplification of the LacZ transcripts from selected tissues of either Tg(CAG-LacZ/EGFP-KASH2) (upper panel) or C57Bl6/J (lower panel) mice.
Knockout (KO) approaches targeting LINC complex components in mice have established the central role of LINC complexes in nuclear anchorage/migration during the development of embryonic tissues (Zhang, Xu et al. 2007, Meyerzon, Fridolfsson et al. 2009, Yu, Lei et al. 2011). However, the usefulness of these models has been hampered by significant drawbacks. First, Sun proteins and Nesprins are encoded by two and at least four genes, respectively. As a result, germline inactivation of any single Sun or Nesprin gene is most often compensated for by other combinations of Sun and Nesprin proteins (Stewart-Hutchinson, Hale et al. 2008, Wang, Shi et al. 2012). Accordingly, whereas single KO mice of any of these genes develop normally, Sun1/2 and Nesprin1/2 double KO mice display severe CNS development abnormalities (Zhang, Lei et al. 2009). The perinatal death of these mice precludes phenotypical analyses associated with extensive LINC complex disruption in adult tissues (Zhang, Lei et al. 2009). Finally, germline KO approaches inherently complicate the phenotypical interpretation due to possible cell nonautonomous effects.
To overcome these drawbacks, we developed a Cre-Lox based dominant negative strategy that allows for the disruption of LINC complexes in a cell autonomous and developmentally regulated manner. This strategy is based on two main observations: 1) the tethering of Nesprins at the NE relies on the presence of a C-terminal KASH domain that is strictly required for Nesprins’ interaction with available SUN domains (Crisp, Liu et al. 2006, Kracklauer, Banks et al. 2007, Stewart-Hutchinson, Hale et al. 2008) and 2) the KASH domain of any given Nesprin interacts promiscuously with the SUN-domain of either Sun1 or Sun2 (Stewart-Hutchinson, Hale et al. 2008, Wang, Shi et al. 2012). As a consequence, overexpression of the KASH domain of Nesprin1, 2, 3 or 4 fused to EGFP saturates available SUN domains and acts in a dominant-negative manner by displacing endogenous Nesprins from the NE to the ER (Stewart-Hutchinson, Hale et al. 2008, Roux, Crisp et al. 2009), a phenotype we call “LINC complex disruption” (Figure 1B and C and (Stewart-Hutchinson, Hale et al. 2008)). Using these guiding principles, we developed a transgenic mouse model allowing for the targeted and inducible expression of the KASH domain of Nesprin2 fused to EGFP (EGFP-KASH2) in vivo. Here we describe the transgenic expression pattern of EGFP-KASH2 and provide several examples of spatiotemporal disruption of LINC complexes.
RESULTS AND DISCUSSION
To enable disruption of LINC complexes in a cell autonomous and developmentally regulated manner in vivo, we generated transgenic mice harboring the genetic construct depicted in Figure 1D. It consists of an open reading frame (ORF) encoding EGFP-KASH2 cloned downstream of a LoxP-flanked ORF encoding β-galactosidase fused to the V5 epitope. Upon Cre-mediated excision of the LacZ/V5 ORF, EGFP-KASH2 is expressed under the control of the chicken beta-actin promoter coupled with the CMV immediate early enhancer (CAG promoter) (Figure 1D).
To survey the extent of transgenic expression, the LacZ transcript was probed by RT-PCR and In Situ hybridization (ISH). Using primers flanking the LacZ ORF, RT-PCR was performed on total RNA purified from adult tissues dissected from either Tg(CAG-LacZ/EGFP-KASH2) or C57Bl/6 littermates. A single amplicon of the expected size was observed in cerebrum, cerebellum, heart, muscle, retina and small intestine isolated from Tg(CAG-LacZ/EGFP-KASH2) mice (Figure 1E). This amplicon was neither amplified in the absence of reverse transcriptase nor when total RNA from C57Bl/6 littermates was used as template. These results indicated that transgenic expression takes place in a wide variety of adult Tg(CAG-LacZ/EGFP-KASH2) tissues. Developmental transgenic expression pattern was surveyed by ISH with a LacZ probe on cross sections of embryonic and adult tissues from Tg(CAG-LacZ/EGFP-KASH2) mice and control littermates. Embryonic cerebellum, retina and heart were positive for LacZ expression (Figure 2, A to C). In embryonic retina, stronger transgenic expression was observed within post-mitotic cells of the developing ganglion cell layer (GCL) by comparison to the neuroblast layer (NBL) that mostly contains cycling neuronal precursors (Figure 2B). LacZ expression was retained in most adult tissues with variable expression patterns across cell types. For example, Purkinje cells within the cerebellum or retinal cell types belonging to the inner nuclear layer displayed stronger transgenic expression (Figure 2D, E). Transgenic expression was also notably elevated in adult skeletal muscle fibers (Figure 2F).
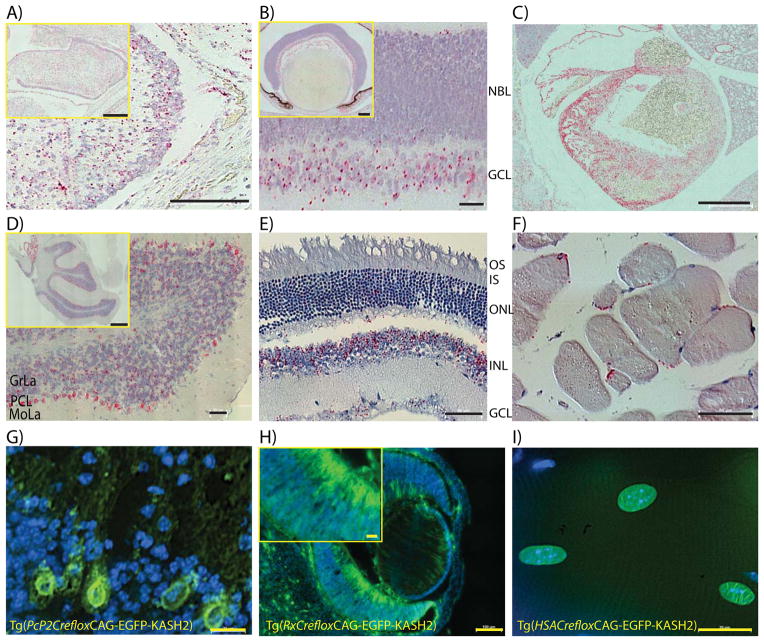
FIGURE 2. LacZ and EGFP-KASH2 transgenic expression in embryonic and adult mouse tissues.
In-situ hybridization performed on E18.5 Cerebellum (A), retina (B) and heart (C) using a LacZ probe. D-F) Same experiment performed on adult cerebellum (D), retina (E) and skeletal muscle fibers (F). G-I) Induction of EGFP-KASH2 expression in vivo within single cell types or tissues. Tg(CAG-LacZ/EGFP-KASH2) mice were bred to Tg(Pcp2-Cre), Tg(Rx-Cre) or Tg(HSA-CreERT2) mice and sections from adult Tg(Pcp2floxCAG-EGFP/KASH2) cerebellum (G), adult Tg(RxfloxCAG-EGFP/KASH2) eyes (H) and adult Tg(HSAfloxCAG-EGFP/KASH2) muscle fibers from mice treated with Tamoxifen (I) were counterstained with DAPI and imaged by direct fluorescence microscopy in the FITC channel. Typical EGFP-KASH2 rims were clearly visible in Purkinje cells (G), retinal precursors and post-mitotic ganglion cells (H) and myonuclei (I). Abbreviations: NBL: neuroblast layer, GCL: ganglion cell layer, GrLa: Granule cell layer, PCL: Purkinje cell layer, MoLa: Molecular layer, OS: outer segment, IS: inner segment, ONL: outer nuclear layer, INL: inner nuclear layer. Scale bars: A) 100μm (inset: 200μm), B) 25μm (inset: 100μm), C) 500μm, D) 50μm (inset: 500μm), E) 50μm, F) 50μm, G) 20μm, H) 100μm (inset: 20μm) and I) 20μm.
To analyze Cre-mediated expression of EGFP-KASH2, Tg(CAG-LacZ/EGFP-KASH2) mice were bred to mouse strains expressing Cre recombinase in specific tissues and/or cell types. To restrict the expression of EGFP-KASH2 within a single cell type, Tg(CAG-LacZ/EGFP-KASH2) mice were bred to Tg(Pcp2-Cre) mice that initiate expression of Cre-recombinase specifically in Purkinje cells (PCs) by ~P6. As expected from ISH results (Figure 2D), EGFP-KASH2+ rims were specifically observed in PCs within cerebellar slices (Figure 2G). Interestingly, we did not observe any abnormal nuclear positioning or morphological defects of PCs in one-month-old Tg(Pcp2floxCAG-EGFP/KASH2) mice (data not shown, manuscript in preparation).
Widespread expression of EGFP-KASH2 was induced in the retina using Tg(Rx-Cre) that initiate Cre recombinase expression in the developing eye field by ~E9.5 (Swindell, Bailey et al. 2006). In Tg(RxCrefloxCAG-EGFP-KASH2), EGFP-KASH2 was detected in direct fluorescence microscopy as rims corresponding to the NE of most cells of embryonic retina (Figure 2H). In agreement with ISH results, the expression level of EGFP-KASH2 was significantly higher in post-mitotic retinal ganglion cells exiting the NBL by comparison to neuroblasts within the NBL (Figure 2H). However, by contrast to the sparse ISH labeling detected in the ONL of adult Tg(CAG-LacZ/EGFP-KASH2) retinas (Figure 2E), over 95% of cone photoreceptors and the majority of rod photoreceptors were found to express EGFP-KASH2 when bred to mouse strains expressing Cre recombinase in post-mitotic cone or rod photoreceptors (data not shown).
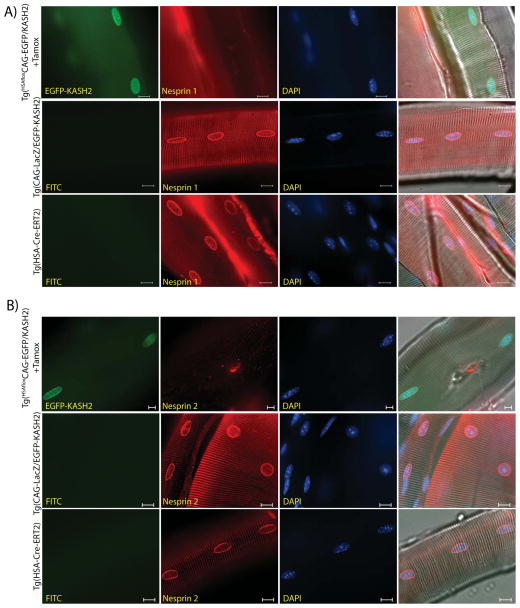
To control the timing of EGFP-KASH2 expression, we used Tg(HSA-Cre-ERT2) mice. In this strain, the expression of Cre-ERT2, which is under the control of the Human α-Skeletal Actin (HSA) promoter, is restricted to skeletal muscle (Schuler, Ali et al. 2005). Despite the constitutive expression of Cre-ERT2 in skeletal muscle, tamoxifen injection is required for translocation of Cre-ERT2 within the nucleus where it excises LoxP-flanked genomic targets (Schuler, Ali et al. 2005). Upon tamoxifen injection, EGFP-KASH2 expression was observed at the NE of more than 90% of adult myonuclei (Figure 2I). To assess the disruption of LINC complexes, skeletal muscle fibers from gastrocnemius of Tg(HSAfloxCAG-EGFP/KASH2) mice treated with tamoxifen were immunostained with Nesprin antisera. We could not detect any Nesprin rims at the NE of EGFP-KASH2+ myonuclei (Figure 3A, B, upper panels). By contrast, the same immunostaining carried out on fibers isolated from control littermates revealed Nesprin1 and 2 rims at the NE of all myonuclei (Figure 3A, B, middle and lower panels). This body of data indicates that, similarly to the displacement of endogenous Nesprins from the NE of cultured cells transfected with EGFP-KASH2 (Figure 1C), transgenic expression of EGFP-KASH2 efficiently displaces endogenous Nesprins from the NE to the ER in vivo. A similar displacement of Nesprins was reliably observed in different tissues/cell types expressing EGFP-KASH2 (data not shown).
FIGURE 3. Inducible transgenic expression of EGFP-KASH2 in skeletal muscle fibers displaces endogenous Nesprins from the nuclear envelope in a Cre-dependent manner. A).
Nesprin1 immunostaining of muscle fibers from adult Tg(HSAfloxCAG-EGFP/KASH2) mice injected with tamoxifen (upper panels), Tg(CAG-LacZ/EGFP-KASH2) and Tg(HAS-Cre-ERT2) control littermates (middle and lower panels). B) Nesprin2 immunostaining of the same samples. Note the absence of endogenous Nesprin1/2 rims at the NE of myonuclei expressing EGFP-KASH2. Scale bars: 10μm.
Taken together, our data indicate that Tg(CAG-LacZ/EGFP-KASH2) mice provide a useful mouse model to induce the Cre-recombinase dependent expression of EGFP-KASH2 that allows for the efficient spatiotemporal disruption of LINC complexes in vivo. By comparison to a similar transgenic strategy that used the CMV promoter to drive transgene expression (Razafsky, Blecher et al. 2012), the Tg(CAG-LacZ/EGFP-KASH2) strain allows for higher expression levels of EGFP-KASH2 both in a significantly higher percentage of a given cell populations and in a wider array of tissues (data not shown). Whereas this model bypasses the drawbacks of existing KO mouse models of Sun proteins and Nesprins (most notably postnatal lethality and cell non-autonomous effects (Ding, Xu et al. 2007, Zhang, Xu et al. 2007, Lei, Zhang et al. 2009, Zhang, Lei et al. 2009, Yu, Lei et al. 2011)), it is not without a main drawback on its own. Indeed, any phenotype observed upon EGFP-KASH2 expression reflects the phenotypical consequences of disruption of endogenous SUN/KASH interactions as a whole. Hence, interpretation of such phenotypes requires the careful analysis of the qualitative and quantitative expression pattern of endogenous Nesprins in biological processes/tissues under investigation. In conclusion, Tg(CAG-LacZ/EGFP-KASH2) mice provide a new and useful genetic tool to analyze the physiological relevance of LINC complexes during development and homeostasis of a wide variety of mammalian tissues.
METHODS
Mice
Animal protocols used in this study adhered to the ethical and sensitive care and use of animals in research and were approved by the Washington University School of Medicine Animal Studies Committee (Animal Welfare Assurance Permit # A-3381-01, protocol # 20130225). A cDNA encoding the KASH domain of mouse Nesprin2 (amino acids 6810 to 6874 from NP_001005510.2) was amplified by RT-PCR from C2C12 myoblasts and cloned into BglII/BamH1 sites of pEGFP-C1 (Razafsky, Blecher et al. 2012). pCAG-flox was obtained by replacing the CMV promoter of pCMV-flox with the chicken beta-actin promoter coupled with the CMV immediate early enhancer (CAG promoter). The EGFP-KASH2 open reading frame was subcloned in HindIII and ApaI sites of the pCAG-flox polylinker. After sequence confirmation, a PacI fragment encompassing the genetic construct described in Figure 1D was purified and microinjected into fertilized eggs that were implanted in pseudopregnant females (Molecular Genetics core, Department of Ophthalmology and Visual Sciences). Founders were identified by genotyping with primers amplifying EGFP-KASH2 (5′ GGAGTTCGTGACCGCCGCCGGGATCACTCT 3′ and 5′ TTTAAACGGGCCCCCTAGGTGGGAGGTGGC 3′). A single F0 male was selected based on efficient germline transmission and widespread transgenic expression for amplification in the C57Bl/6J background. F1 Tg(CAG-LacZ/EGFP-KASH2) mice were indistinguishable from their wild-type littermates indicating that random integration of this transgene does not affect development. These mice will be available to the research community upon acceptance of this manuscript. Tg(HSA-CreERT2) and Tg(Rx-Cre) were kindly provided by Drs. Pierre Chambon and Milam Jamrich, respectively. Tg(Pcp2-Cre) mice were purchased from The Jackson Laboratory (#004146). Mouse colonies were maintained and genotyped at the Mouse Genetics Core (Washington University School of Medicine). Adult Tg(HSAfloxCAG-EGFP/KASH2) and control littermates were injected with one milligram of tamoxifen for five consecutive days as previously described (Schuler, Ali et al. 2005).
Immunofluorescence
Mice were sacrificed via CO2 inhalation and tissues were rinsed in PBS several times before fixing in 4% PFA in PBS. For cerebellar samples, mice were anesthetized with a Ketamine/Xylazine cocktail and transcardially perfused with 30% sucrose in PBS followed by 4% PFA in PBS. Following fixation, tissues were rinsed in PBS several times and incubated in 30% sucrose in PBS overnight at 4°C then embedded in OCT compound. For direct fluorescence microscopy, cryosections (15μm) on Superfrost Plus slides were rinsed in PBS and permeabilized in 0.5% Triton X-100/10% donkey serum in PBS. Samples were washed, counterstained with DAPI and imaged by epifluorescence with an Eclipse Ti inverted microscope (Nikon, Melville, NY, USA) equipped with a CoolSnap HQ2 camera (Photometrics, Tucscon, AZ, USA) and controlled by NIS Elements software. Muscle fibers were dissected, fixed in 4% PFA in PBS overnight at 4°C, permeabilized in 0.5% Triton X-100/10% donkey serum in PBS overnight and further incubated with Nesprin1 or affinity purified Nesprin 2 antisera (Khatau, Bloom et al. 2012, Razafsky, Ward et al. 2013) overnight at 4°C. After several washes, donkey anti-rabbit Alexa-conjugated secondary antibodies (Invitrogen) were incubated overnight at 4°C in permeabilization solution and muscle fibers were counterstained with DAPI. Fibers were separated under a dissecting microscope, mounted and imaged as described above.
RNA extraction and RT-PCR
Mouse tissues were isolated and resuspended in Trizol (Invitrogen, #1559602), grinded with a Bullet Blender (Next Advance, Averill Park, NY, USA) and centrifuged. Supernatants were then used for RNA purification according to the manufacturer’s specifications. One microgram of total RNA was reverse transcribed with Superscript II (Invitrogen, #18064014) following manufacturer recommendations. Ten percent of RT reactions were used with Taq Hi-FI polymerase (Invitrogen, 11304-011). Thirty amplification cycles were performed using LacZ primers p1369 (5′-GTTGCAGTGCACGGCAGATACACTTGCTGA-3′) and p1370 (5′-GCCACTGGTGTGGGCCATAATTCAATTCGC-3′). Ethidium bromide-stained agarose gels were imaged with a G:Box HR16 imaging system (Syngene, Fredrick, ND, USA).
In Situ Hybridization
ISH was performed on sections using the RNAscope 2.0 Red Kit (Advanced Cell Diagnostics, #310036) according to the manufacturer’s instructions. Briefly, sections were deparaffinized in xylene, followed by dehydration in an ethanol series. Sections were then incubated in a boiling citrate buffer, rinsed with water and immediately treated with protease. Hybridization with target probes, preamplifier and amplifier were carried out at 40°C followed by development using the supplied Fast Red reagents. Control hybridizations were carried out in parallel with tissues from non-recombinant littermates. Samples were counterstained with Hematoxylin and tiled images were acquired with an Eclipse Ti inverted microscope fitted with a DS-Fi2 color camera (Nikon).
Acknowledgments
The authors are grateful to Drs. Milan Jamrich and Pierre Chambon for the kind gift of Tg(Rx-Cre) and Tg(HSA-CreERT2) mice, respectively. We thank the staff of the Morphology and Imaging core, of the Molecular Genetics core (Department of Ophthalmology and Visual Sciences) and of the Mouse Genetics Core at Washington University in St. Louis School of Medicine. This work was supported by the National Institute of Health (EY022632 to DH), an NIH Training Grant (T32 EY013360 to DR), the McDonnell Center for Cellular and Molecular Neurobiology at Washington University in St. Louis School of Medicine (to DH) and by awards to the Department of Ophthalmology and Visual Sciences at Washington University in St. Louis from a Research to Prevent Blindness, Inc. Unrestricted grant and the NIH Vision Core Grant (P30 EY002687).
References
- Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell. 2009;17(5):587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172(1):41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12(6):863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Fridolfsson HN, Starr DA. Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. J Cell Biol. 2010;191(1):115–128. doi: 10.1083/jcb.201004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26(10):3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279(24):25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- Khatau SB, Bloom RJ, Bajpai S, Razafsky D, Zang S, Giri A, Wu PH, Marchand J, Celedon A, Hale CM, Sun SX, Hodzic D, Wirtz D. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci Rep. 2012;2:488. doi: 10.1038/srep00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracklauer MP, Banks SM, Xie X, Wu Y, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly (Austin) 2007;1(2):75–85. doi: 10.4161/fly.4254. [DOI] [PubMed] [Google Scholar]
- Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A. 2009;106(25):10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerzon M, Fridolfsson HN, Ly N, McNally FJ, Starr DA. UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development. 2009;136(16):2725–2733. doi: 10.1242/dev.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295(2):330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Razafsky D, Blecher N, Markov A, Stewart-Hutchinson PJ, Hodzic D. LINC complexes mediate the positioning of cone photoreceptor nuclei in mouse retina. PLoS One. 2012;7(10):e47180. doi: 10.1371/journal.pone.0047180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D, Hodzic D. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol. 2009;186(4):461–472. doi: 10.1083/jcb.200906068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky DS, Ward CL, Kolb T, Hodzic D. Developmental regulation of linkers of the nucleoskeleton to the cytoskeleton during mouse postnatal retinogenesis. Nucleus. 2013;4(5):16–15. doi: 10.4161/nucl.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A. 2009;106(7):2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M, Ali F, Metzger E, Chambon P, Metzger D. Temporally controlled targeted somatic mutagenesis in skeletal muscles of the mouse. Genesis. 2005;41(4):165–170. doi: 10.1002/gene.20107. [DOI] [PubMed] [Google Scholar]
- Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008;314(8):1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell EC, Bailey TJ, Loosli F, Liu C, Amaya-Manzanares F, Mahon KA, Wittbrodt J, Jamrich M. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis. 2006;44(8):361–363. doi: 10.1002/dvg.20225. [DOI] [PubMed] [Google Scholar]
- Wang W, Shi Z, Jiao S, Chen C, Wang H, Liu G, Wang Q, Zhao Y, Greene MI, Zhou Z. Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res. 2012;22(10):1440–1452. doi: 10.1038/cr.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171(5):799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20(6):1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64(2):173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007;134(5):901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]