Abstract
The glycemic index (GI) indicates how fast blood glucose is raised after consuming a carbohydrate-containing food. Human metabolic studies indicate that GI is related to patho-physiological responses after meals. Compared with a low-GI meal, a high-GI meal is characterized with hyperglycemia during the early postprandial stage (0~2 h) and a compensatory hyperlipidemia associated with counter-regulatory hormone responses during late postprandial stage (4~6 h). Over the past three decades, several human health disorders have been related to GI. The strongest relationship suggests that consuming low-GI foods prevents diabetic complications. Diabetic retinopathy (DR) is a complication of diabetes. In this aspect, GI appears to be useful as a practical guideline to help diabetic people choose foods. Abundant epidemiological evidence also indicates positive associations between GI and risk for type 2 diabetes, cardiovascular disease, and more recently, age-related macular degeneration (AMD) in people without diabetes. Although data from randomized controlled intervention trials are scanty, these observations are strongly supported by evolving molecular mechanisms which explain the pathogenesis of hyperglycemia. This wide range of evidence implies that dietary hyperglycemia is etiologically related to human aging and diseases, including DR and AMD. In this context, these diseases can be considered metabolic retinal diseases.
Molecular theories that explain hyperglycemic pathogenesis involve a mitochondria-associated pathway and four glycolysis-associated pathways, including advanced glycation end products formation, protein kinase C activation, polyol pathway, and hexosamine pathway. While the four glycolysis-associated pathways appear to be universal for both normoxic and hypoxic conditions, the mitochondria-associated mechanism appears to be most relevant to the hyperglycemic, normoxic pathogenesis. For diseases that affect tissues with highly active metabolism and that frequently face challenge from low oxygen tension, such as retina in which metabolism is determined by both glucose and oxygen homeostases, these theories appear to be insufficient. Several lines of evidence indicate that the retina is particularly vulnerable when hypoxia coincides with hyperglycemia. We propose a novel hyperglycemic, hypoxia-inducible factor (HIF) pathway, to complement the current theories regarding hyperglycemic pathogenesis. HIF is a transcription complex that responds to decreases in oxygen in the cellular environment. In addition to playing a significant role in the regulation of glucose metabolism, under hyperglycemia HIF has been shown to increase the expression of HIF-inducible genes, such as vascular endothelial growth factor (VEGF) leading to angiogenesis. To this extent, we suggest that HIF can also be described as a hyperglycemia-inducible factor.
In summary, while management of dietary GI appears to be an effective intervention for the prevention of metabolic diseases, specifically AMD and DR, more interventional data is needed to evaluate the efficacy of GI management. There is an urgent need to develop reliable biomarkers of exposure, surrogate endpoints, as well as susceptibility for GI. These insights would also be helpful in deciphering the detailed hyperglycemia-related biochemical mechanisms for the development of new therapeutic agents.
1. INTRODUCTION
As the most important energy source for human body, glucose has a broad spectrum of physiological effects and proper regulation of glucose metabolism is required to maintain health and avoid diseases. The blood glucose concentration (glycemia) reflects the combined effects of carbohydrate uptake, delivery to the blood, production and utilization by the body. The concentration of blood glucose is tightly regulated by a homeostatic regulatory system.(Jenkins et al. 2002; Ludwig 2002) Among those determinants for glucose metabolism, diet-induced glycemia results in the greatest daily variation.(Giugliano et al. 2008) Therefore, it is not surprising that glycemic index (GI), which is a physiological measure for classifying carbohydrate-containing foods according to postprandial glycemic potential, has been related to many disorders, such as diabetes and cardiovascular disease (CVD). Importantly, recent evidence shows that GI of the diet also relates to an increased risk for age-related diseases, such as atherosclerosis and age-related macular degeneration (AMD) in non-diabetic population.(Balkau et al. 1998; Chiu et al. 2006a; Chiu et al. 2009a; Chiu et al. 2007a; Chiu et al. 2007b; Chiu et al. 2009b; Kaushik et al. 2008) This accumulating evidence implies that glucose homeostasis and carbohydrate nutrition play an important role in human aging as well as in disease pathogenesis. However, the associations may vary due to subtle patho-physiological mechanistic differences as well as differences in composition, structure, homeostatic systems, micro-environment, and function between metabolically different regions within tissues.(Brownlee 1995; Chiu et al. 2006b; Chiu et al. 2005; Chiu et al. 2010)
Many patho-physiological effects follow postprandial hyperglycemia after eating a high-GI meal (Fig. 1).(Jenkins et al. 2002; Ludwig 2002; Riccardi et al. 2008) Most studies of hyperglycemia have focused on diabetes or diabetic complications but not on age-related disorders, because in diabetes tissue damage develops over a much shorter period and manifests more obvious clinical signs. However, it is proposed that the patho-physiological effects of hyperglycemia that are operative in diabetes also affect non-diabetic people upon aging.(Brownlee 1995) Furthermore, studies also suggest that older people with diabetes are more susceptible to age-related diseases, including AMD, than people without diabetes.(Tumosa 2008)
Fig. 1.

Adverse metabolic events relating high-GI diets to diabetes and cardiovascular disease.
The retina is the most metabolically active tissue in the human body, with dual blood supplies and rapid consumption of glucose and oxygen.(Cohen & Noell 1965) It is not surprising that glucose homeostatsis in the retina plays an important role in retinal health and disease. In people with diabetes, failure to regulate blood glucose leads to biochemical abnormalities in cells and tissues. Diabetic retinopathy (DR) is the most common microvascular complication.(Fong et al. 2004) Although the detailed pathogenesis of DR is not completely understood, large epidemiological trials have established that hyperglycemia is an underlying cause of this disease.(Diabetes Control and Complications Trial Research Group 1993; UK Prospective Diabetes Study Group 1998) However, similar damage also happens to people without diabetes and is manifest in AMD. The range of pathologic lesions in the retina and other vascular beds differ between diabetic and non-diabetic age-related lesions. This may be due to differences in the extent or duration of hyperglycemic exposure and/or in the related biochemical and metabolic abnormalities (Table 1). Interestingly, the GI-related pathogenesis in diabetes and CVD appears to show extensive overlap with etiology for AMD (Fig. 1).
Table 1.
Comparison of characteristics between age-related macular degeneration and diabetic retinopathy.
| Age-related macular degeneration (AMD) | Diabetic retinopathy (DR) | |
|---|---|---|
| Population affected | Elders over 60’s | Type I and II diabetic patients |
| Incidence | The 10-year incidence:
|
0.64/1000 person-year ~ 42.1/1000 person-year in diabetic patients |
| Prevalence | 1.47% in the US population 40 years and older. | 12.7%~75.1% in diabetic patients. |
| Blindness | Over 420,000 cases per year. | Over 10,000 cases per year. |
| Risk factors |
|
|
| Involved blood vessels | Choroidal circulation | Retinal circulation |
| Primary retinal area involved | Macula in the outer retina: RPE, Bruch’s membrane, photoreceptors | Inner retina: Retinal endothelium, pericytes, basement membrane |
| Early stage lesions | Drusen, pigment abnormality | Cotton wool spots |
| Advanced lesions |
|
|
| Prevention | AREDS formulation (high-dose formulation of antioxidants and zinc) delays and possibly prevents intermediate AMD from progression to advanced AMD in people with:
|
Sustained blood glucose control |
| Treatment |
|
|
Although the concept of GI was introduced almost three decades ago, only recently was GI related to retinal health. This review primarily focuses on how dietary hyperglycemia may increase the risk for metabolic retinal diseases. It will begin with the definition and measurement of GI. Then the systemic patho-physiology of GI is described. This is followed by a brief review of the epidemiological evidence for the associations between GI and AMD, diabetes, and CVD. Next, several plausible mechanisms which may link hyperglycemia to retinal pathology are reviewed, with emphasis on AMD and DR. Finally, a summary along with some suggestions for future study and recommendations are proposed.
2. GLYCEMIC INDEX (GI)
In order to confront epidemics of CVD, especially coronary heart disease (CHD), nutritional recommendations in most high-income countries advise a reduced intake of dietary fat. (Hare-Bruun et al. 2008) Since protein intake tends to vary very little in humans, adherence to these recommendations usually leads to an increase in the intake of carbohydrate-rich foods. Americans are eating more. The United States Department of Agriculture (USDA) food consumption survey data indicated that the increased energy intake in Americans was caused primarily by higher carbohydrate intake.(Chanmugam et al. 2003) Data from National Health and Nutrition Examination Surveys (NHANES) for 1971–2000 also indicate similar trends. Specifically, the increase in energy intake is attributable primarily to an increase in carbohydrate intake, with a 62.4-gram increase per day among women and a 67.7-gram increase among men.(Wright et al. 2004) Although a diet with reduced fat and increased carbohydrate could have beneficial effects on lipid metabolism, its high carbohydrate content may reduce its potentially healthful effects in some people, particularly those at risk of CHD. This includes obese people, those with insulin resistance or metabolic syndrome, or those with diabetes.(Kopp 2006; Nordmann et al. 2006; Pala et al. 2006; Riccardi et al. 2008)
Carbohydrate foods represent a heterogeneous category in terms of their metabolic effects, i.e. not all carbohydrate foods have a same blood glucose-raising potential. For example, white bread raises blood glucose much faster than whole-grain bread. Foods with high blood glucose-raising potential also tend to raise plasma insulin, triacylglycerol, etc., which are important risk factors of CHD and diabetic-related macrovascular and microvascular diseases. Management of dietary carbohydrate appears to offer a means to address diabetes-related conditions and disorders.(Garg et al. 1988; Hare-Bruun et al. 2008; Jenkins et al. 2002; Ludwig 2002; Parillo et al. 1996) Unfortunately, despite accumulating evidence relating many important health issues to poor quality of carbohydrate nutrition, the public health value of GI has not received appropriate attention and the GI of the average diet in the United States appears to have risen in recent years.(Hu et al. 2000; Ludwig 2002) However, although GI has not been incorporated into the USDA dietary guidelines, the principle of low-GI diets is applied in the USDA MyPyramid Sample Menus (http://www.mypyramid.gov/downloads/sample_menu.pdf).
2.1. Definition of GI and related measures
The GI, proposed by Dr. David J. Jenkins and colleagues in 1981,(Jenkins et al. 1981) is a measure of the effects of carbohydrate-containing foods on postprandial glycemia. It is defined as the percentage of the area under two hour blood glucose curve (AUC) following the ingestion of a tested food vs. a standard food (Fig. 2). Glucose is usually used as the standard food. White bread can also be used as a reference food, but using white bread as the standard gives a different set of GI values (if white bread = 100, then glucose ≈ 140). The test and standard foods contain a fixed portion of available carbohydrate, usually 50 grams. Foods that break down quickly during digestion and result in higher levels of blood glucose have a high GI. Foods that break down more slowly, releasing glucose more gradually into the bloodstream, have a low GI. Fiber-rich foods generally have a low GI. However, not all foods with a low GI have high fiber content. Neither is it appropriate to assume that all “simple” sugars have a high GI or that “complex” carbohydrates or whole grains have a low GI.(Atkinson et al. 2008; Foster-Powell et al. 2002) For example, pure fructose has a GI of 19, whereas foods like potatoes have GIs around 100. The most updated formal publication of GI values for almost 2,500 food items is available online at http://dx.doi.org/10.2337/dc08-1239.
Fig. 2.

Glycemic responses demonstrate the definition of GI.
GI values have been measured in an ethnically and physiologically wide variety of subjects, including both diabetic and non-diabetic healthy people, and it has been shown that the GI values obtained are roughly similar for the same foods.(Atkinson et al. 2008; Wolever et al. 2008b) Nevertheless, the published GI values for apparently similar foods may vary from study to study. This is because the GI of a food is determined by several factors which affect gastric emptying and rate of intestinal digestion, including amylase, fiber, and moisture content, cooking time, ingredients or processing methods, etc. (Foster-Powell et al. 2002; Riccardi et al. 2008)
Because the test relies on subjects consuming enough of a tested food containing 50 g of available carbohydrate, the GI should be applied only to foods of major dietary carbohydrate sources (carbohydrate-rich foods) or foods with reasonable carbohydrate content. The glycemic response to mixed meals can be predicted with reasonable accuracy from the glycemic index of constituent foods when standard methods are used.(Bornet et al. 1987; Chew et al. 1988; Wolever & Bolognesi 1996; Wolever & Jenkins 1986) The overall GI for a person’s diet (dietary GI) can be calculated as the weighted average of the GI scores for each food item, with the amount of carbohydrate consumed from each food item as the weight [Σ(GIi × Wi)/W],(Wolever et al. 1994) where GIi is the glycemic index of an individual food, W is the weight of total carbohydrate, and Wi is the weight of available carbohydrate of individual food (i.e. the fiber content was subtracted from the carbohydrate content).
Another measure of carbohydrate nutrition, glycemic load (GL), was defined to summarize the combined effects of quantity and quality of carbohydrate foods.(Salmeron et al. 1997b) It is calculated as the product of the GI and the carbohydrate amount (in grams) of the food item divided by 100.(Salmeron et al. 1997b) Because, by definition, GL is an interaction between quantity and quality of dietary carbohydrate, it is likely that high-GL diets have differential physiologic effects from country to country. For example, in the US high-carbohydrate diets are most often dominated by high-GI foods, but in Scandinavian countries high-carbohydrate diets include many low-GI staples. This may result in geographic or ethnic differences in associations between dietary GL and risk for diseases.
2.2. Methodological issues regarding measurement of GI
Recently considerable concerns have been raised about the differences in assigning GI values to food items. The inconsistencies occur in part due to methodologic inconsistencies. Because inconsistencies in GI values may impact the interpretation of data relating GI/GL to diseases and result in inconsistency among studies, it is critical that a standard method is used to obtain GI values. Here we discuss the most relevant methodological considerations and highlight specific issues regarding subjects, test meals, blood sampling procedures, and calculation of area under the glycaemic response curve (AUC). All together, these technical procedures will ensure quality of GI measurement in laboratories, improve the validity of GI-related studies, and help to establish the scientific evidence for using GI in food labeling.(Aziz 2009; Brouns et al. 2005; Granfeldt et al. 2006; van Bakel et al. 2009; Venn & Green 2007; Wolever et al. 2008b)
In theory, the GI is a property of the food, not a property of the subject in whom it is measured. The subjects can be thought of as the analytical instruments used to measure GI. The GI values for foods tested in both normal and diabetic subjects have been shown to be highly correlated (γ=0.94, P<0.001).(Atkinson et al. 2008) The concerns raised by researchers regarding inconsistencies in GI measurement may be mitigated if the same data base, with complete and accurate dietary data, is used to compare subjects within the same cohort, and if the GI test is repeated several times on appropriate groups of 10 or more subjects with normal gastrointestinal function, using standardized conditions, and with an average within-subject coefficient of variation of less than 30% and the results averaged.(Brouns et al. 2005; Mettler et al. 2007) Using such conditions, two inter-laboratory studies involving 28 laboratories around the world showed that the current method for measuring GI is reliable enough to be able to distinguish a low-GI food (GI 55 and below) from a high-GI food (GI 70 and above).(Wolever et al. 2003; Wolever et al. 2008b)
A dose of 50 g available carbohydrate is recommended. Available carbohydrate is defined as total carbohydrate minus dietary fiber and other carbohydrate that does not get absorbed in the intestine. GI values reflect the context and formulation of the food including the amount of a food consumed, size of the food particle swallowed, viscosity, extent of digestion and absorption, addition of other components such as fat, cooking times and temperatures, etc.(Foster-Powell et al. 2002; Miller et al. 2006; Read et al. 1986; Suzuki et al. 2005) Liquid meals (250ml) should be consumed within 5–10 minutes and solids and semisolids should be ingested within 10–15 minutes. Subjects should drink at least 250 ml with each test meal.
Finger-prick capillary blood samples are taken in the morning after an overnight fast (immediately before starting to eat) and at 15, 30, 45, 60, 90 and 120 minutes after starting to eat the test meal (Fig. 2). Glucose in whole blood, serum or plasma (consistent method for all tests) should be measured with an acceptable analytical precision of coefficient of variation <3%.
A recent study showed that over 50% of laboratories did not report correct values for AUC.(Wolever et al. 2008b) The GI value of each test food is the mean of the values of: 100×(AUC elicited by the test food)/(AUC elicited by the reference food) in the same subject. Values which are >2 standard deviations from the mean should be excluded. Final GI values should be expressed on the glucose scale, i.e. the GI of glucose = 100 (Fig. 2).
2.3. GI and systemic patho-physiology
Mainly because of the metabolic demands of the brain, the human body has an obligatory requirement for glucose, approaching 200 g/d.(Cahill 1970) The blood glucose concentration is tightly regulated by homeostatic regulatory systems and maintained between 40 mg/dL (2.2 mmol/L) and 180 mg/dL (10.0 mmol/L). Hypoglycemia below the lower limit may result in coma, seizure, or even death. Hyperglycemia, exceeding the upper limit, is associated with immediate (glycosuria and calorie loss) and long-term (retinopathy, atherosclerosis, renal failure, etc) consequences.(Ludwig 2002; Ludwig 2007) Under normal physiological conditions, hyperglycemia stimulates insulin secretion, promoting uptake of glucose by muscle and adipose tissue. Conversely, hypoglycemia elicits secretion of glucagon, epinephrine, cortisol, growth hormone, and counter-regulatory hormones that antagonize insulin action and restore normal blood glucose levels.(Ludwig 2002)
A low-GI food results in a better postprandial glycemia because it raises blood glucose gradually. Gradual increases in blood glucose reduce the postprandial levels of gut hormones (eg, incretins) and insulin. This will suppress the free fatty acid concentrations(Jenkins et al. 1990; Wolever et al. 1988) and the counter regulatory responses(Jenkins et al. 1990; Ludwig et al. 1999). Under this condition, the respiratory quotient is raised (i.e. sustained tissue insulinization) and glucose is withdrawn from the circulation at a faster rate (i.e. better glucose clearance). Consequently, blood glucose concentrations fluctuate less while they are above baseline and return toward baseline faster despite continued glucose absorption from the small intestine(Jenkins et al. 1990).
Low-GI meals also improve second meal carbohydrate tolerance (i.e., Staub-Traugott effect), which in turn results in lower free fatty acid concentrations of the second standard meal (Granfeldt et al. 2006; Jenkins et al. 1982; Wolever et al. 1988). In diabetic subjects, mimicking the slow digestion of low-GI foods has been shown to reduce glycemic and insulinemic responses over the course of a day compared with the same foods eaten in the same amount in any given 24-h period(Bertelsen et al. 1993; Jenkins et al. 1992). The long-term effects have been related to altered adipose tissue enzyme concentrations(Bray 1972) and reduced fasting blood lipid concentrations(Arnold et al. 1994; Cohn 1964; Fabry & Tepperman 1970; Janannathan et al. 1964; Jenkins et al. 1989; Jones et al. 1993). However, either increasing intake frequency(Tai et al. 1991) or reducing dietary GI(Vega-López & Mayol-Kreiser 2009) does not appear to favor weight reduction.
By contrast, the rapid absorption of glucose following a high-GI meal elicits a sequence of hormonal events that challenge glucose homoeostasis.(Febbraio et al. 2000; Jenkins et al. 1990; Jenkins et al. 1989; Ludwig 2002; Ludwig et al. 1999; Wolever et al. 1995) Compared with a low-GI meal, a high-GI meal induces a significant excursion and fluctuation of blood glucose over the whole postprandial period. This results in a high insulin-to-glucagon ratio during early postprandial stage (0 ~ 2 hours), hypoglycemia and suppressed free fatty acid concentration during middle postprandial stage (2 ~ 4 hours), and counter-regulatory hormone responses and a compensatory increase in free fatty acid concentration during late postprandial stage (4 ~ 6 hours).
In the first hour of the early postprandial period (0 ~ 2 hours) after a high-GI meal (Fig. 2), the rapidly increased blood glucose concentration can be more than twice that which is obtained after consuming a low-GI meal containing identical nutrients and energy. This postprandial hyperglycemia, acting in concert with elevated concentrations of the gut hormones, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide, potently stimulates the pancreas to release insulin from beta cells. Conversely, it also inhibits glucagon release from alpha cells. The dramatically increased insulin/glucagon ratio induces a powerful anabolic stimulus, promoting uptake of nutrients at insulin-responsive tissues, including liver, muscle, and fat (i.e. stimulation of glycogenesis and lipogenesis), and suppressing hepatic glucose output (i.e. suppression of gluconeogenesis and lipolysis). Because of the physiological effects of a high insulin/glucagon ratio, after 60 min of a high-GI meal, blood glucose begins to fall rapidly and the release of free (non-esterified) fatty acids from adipose tissue is suppressed. This can induce abnormal hunger and result in overeating as the body restores the concentration of the metabolic fuels (glucose and free fatty acids) to normal. After 2 h of a high-GI meal, the blood glucose decline often reaches to a level below fasting baseline glucose concentration (hypoglycemia). Between 2 and 4 hours after a high-GI meal (middle postprandial period) (Fig. 2), the hypoglycemia continues because nutrient absorption from the gastrointestinal tract declines but the biological effects of the high insulin and low glucagon levels persist. This postprandial hypoglycemia after consumption of a high-GI meal results in a decrease in glucose oxidation rate.(Ritz et al. 1991)
Approximately 4 to 6 hours after a high-GI meal (late postprandial period) (Fig. 2), the body triggers a counter-regulatory hormone response to restore glycemia by stimulating glycogenolytic and gluconeogenic pathways and to elevate free fatty acid concentration. This combination of elevated counter regulatory hormone and free fatty acid levels resembles a state after many hours without food.(Cahill 1976)
The effects of a high-GI diet on lipid metabolism are prominent. After they were fed high- versus low-GI diets for 18 wk, rats in which diabetes was modeled by partially pancreatectomy, had decreased glucose tolerance, twice the body fat, lower plasma adiponectin and higher plasma triglyceride concentrations.(Pawlak et al. 2004) Mice, fed a high-GI diet for 25 weeks, showed accumulation of fat in liver, adipose tissue, and plasma, and contracted non-alcoholic fatty liver disease.(Scribner et al. 2007) In the same animals, the long-term (40 wk) effects of a low-GI diet include 1) lower respiratory quotient (higher fat oxidation) despite the same energy intake and similar mean body weights; 2) better insulin sensitivity; and 3) higher physical activity despite no differences in energy expenditure throughout the study.(Scribner et al. 2008) Thus, it appears that after a high-GI meal the early postprandial hyperglycemia and hyperinsulinaemia and the late postprandial hypoglycemia and counter-regulatory hormone response could adversely affect body composition, and increase risk for diabetes, CVD, and other age-related diseases. In contrast, the postprandial hypoglycemia and counter-regulatory hormone response do not occur during the postprandial period after a low-GI meal containing identical energy and nutrients. Although the postprandial hypoglycemia following consumption of a high-GI meal may be especially pronounced in obesity and diabetic people,(Ludwig 2002) it is also commonly observed in non-diabetic people, and considered a normal physiological phenomenon.(Brun et al. 1995; Lev-Ran & Anderson 1981; Ludwig et al. 1999)
Diabetic patients are more susceptible to the influence of diet on plasma glucose than non-diabetic persons, because regulation of glucose metabolism is impaired. This is of particular concern during the postprandial period. Therefore, the difference of physiological effects among foods with different GIs is more pronounced in diabetic patients.(Jenkins et al. 1989; Kiens & Richter 1996; Wolever et al. 1985) In people with diabetes insulin resistance appears to be a major etiologic factor for hyperglycemia-related tissue damage.(Ludwig 2002) However, the evidence relating age-related diseases to hyperglycemia in people without diabetes(Balkau et al. 1998; Chiu et al. 2006a; Chiu et al. 2009a; Chiu et al. 2007a; Chiu et al. 2007b; Chiu et al. 2009b) may imply that some insulin resistance-independent mechanisms account for these relationships.
In healthy young men, low-GI diets could have only minor effects on some health indicators in the short term. For example, when consuming a Western diet, switching the carbohydrates from high- to low-GI sources decreases insulin action on whole-body glucose disposal only at a high but not at a physiologic plasma insulin concentration during a 30-day trial study.(Kiens & Richter 1996) However, in another study of healthy men, a low-GI breakfast reduced 24-h urinary C-peptide output,(Jenkins et al. 1987a) low-density lipoprotein (LDL)-cholesterol concentrations and serum C-peptide after 2 wk.
In people with glucose metabolism-impaired disorders, studies suggest beneficial effects and a potential therapeutic utility of low-GI diets despite large variations in the GI-difference between the test and control treatments, the short duration of many studies, and the limited numbers of subjects in others.(Jenkins et al. 2002) For example, in middle-aged, insulin-resistant women a low-GI diet improved insulin sensitivity.(Frost et al. 1998) Patients with hyperlipidemia (i.e. in those with higher triacylglycerol concentrations) consuming a low-GI diet for 1 mo showed reduced LDL-cholesterol and triacylglycerol concentrations without significant change in body weight.(Jenkins et al. 1987b) In studies of persons with type 1 and 2 diabetes, low-GI diets diminished levels of glycated proteins (HbA1c or serum fructosamine) (Brand-Miller et al. 2003; Brand et al. 1991; Collier et al. 1988; Fontvieille et al. 1988; Frost et al. 1994; Giacco et al. 2000; Gilbertson et al. 2001; Järvi et al. 1999; Jenkins et al. 1988; Jenkins et al. 2002; Lafrance et al. 1998; Luscombe et al. 1999; Wolever et al. 1992a; Wolever et al. 1992b) and reduced plasminogen activator inhibitor 1 concentrations(Järvi et al. 1999). Therefore, it is reasonable to postulate that these effects could accrue to a significant health benefit in both diabetic and healthy people from maintaining a low-GI diet for a longer period.
Oxidative stress is thought to be etiologic in hyperglycemia-related disorders and consuming low-GI diets appears to attenuate this burden. Actually, the overproduction of superoxide by the mitochondrial electron-transport chain induced by dietary hyperglycemia has been considered to be the major upstream event of inflammatory responses and endothelial dysfunction in diabetic and age-related macrovacular and microvascular disorders (see 4.2. Hypothesized Mechanisms relating dietary hyperglycemia to AMD and DR).(Brownlee 2001; Dickinson & Brand-Miller 2005) For example, there has been considerable evidence indicating that consuming a high-GI diet is a risk factor of developing CVD, even in individuals without diabetes despite particularly in those with insulin resistance.(Dickinson & Brand-Miller 2005) The pathological sequence may include the generation of reactive oxygen species (ROS),(Hu et al. 2006) tissue damage, and the liberation of proinflammatory factors, such as interleukins (IL), and transcription factors, such as nuclear factor-kappa B (NF-κB), and acute phase proteins such as C-reactive protein,(Dickinson et al. 2008) which is a powerful markers of CHD.(Liu et al. 2002; Ridker et al. 2000) In support of a positive relationship between consuming high-GI diets and oxidative stress, studies have shown that postprandial hyperglycemia depresses serum antioxidants, including lycopene and vitamin E, (Ceriello et al. 1998; Rao & Agarwal 1999) and the higher the glycemia, the greater the postprandial depression of serum antioxidants (Ceriello et al. 1998). These data suggests that a possible beneficial role for low-GI diets may be through reducing oxidative damage.(Botero et al. 2009) In a feeding study evaluating the acute (1 wk) effects of a high-GI diet concluded that oxidative stress, caused by an imbalance between antioxidant capacity and reactive oxygen species, may be an early event in the hyperglycemia-related metabolic cascade and mediate, at least in part, the high-GI effects on health which ultimately increase the risk for CVD and diabetes.(Botero et al. 2009)
3. GI AND HUMAN DISEASES
The quantity (amount) and glycemic quality (GI) of carbohydrate foods have been related to the risk for diabetes, CVD, and AMD in human studies (Table 2). Prior to reviewing the epidemiological or clinical data regarding the GI-disease associations, it is helpful to appreciate the complexity of evaluating diet. Because, for the consideration of energy balance, any assessment of the association between carbohydrate intake and risk for diseases must take each macronutrient into consideration, the complexity of macronutrient replacement often complicates the design and analysis and makes the results difficult to interpret. For example, in order to create low-carbohydrate or low-GI diets without changing calorie intake, fat or protein is often substituted for the carbohydrate. This makes it difficult to differentiate the effect of carbohydrate or GI from effects due to altering fat or protein. In addition to the issue of macronutrient replacement, previous studies relating amount of carbohydrate intake to the risk for diabetes, including epidemiological observations and both long-term and short term interventional trials,(Boden et al. 2005; Brehm et al. 2003; Foster et al. 2003; Gannon & Nuttall 2004; Hales & Randle 1963; Halton et al. 2008; McAuley et al. 2005; Samaha et al. 2003; Stern et al. 2004; Swinburn et al. 1991) are inconsistent (Table 2) and difficult to interpret because of the differences in the degrees of weight loss between the diet groups and in the duration of follow-up. However, overall, GI offers a better measure of carbohydrate foods than quantity for the associations with these diseases.
Table 2.
Current evidence-based evaluation of the impact of quantity (g/d) and glycemic index (GI) of carbohydrate foods on the risk for diabetes, cardiovascular disease, and age-related macular degeneration in human. Overall, GI is a better measure than quantity for the associations between carbohydrate foods and these diseases.
| Overall association | ||
|---|---|---|
| GI | Quantity | |
| Diabetes | ||
| Epidemiological observations | + | ± |
| Intervention studies | ? | ±* |
| Cardiovascular disease | ||
| Epidemiological observations | + | ± |
| Intervention studies | ±* | ±* |
| Age-related macular degeneration | ||
| Epidemiological observations | + | No |
| Intervention studies | ? | ? |
Using intermediate risk factors as the endpoints (surrogate endpoints).
+: Positive association.
±: Uncertain association.
No: No association.
?: Unknown.
3.1. GI and diabetes
With greater availability of energy-rich foods and the rising prevalence of obesity, type 2 diabetes and associated complications are increasing alarmingly. Type 2 diabetes is characterized by insulin resistance and reduced responsiveness of the pancreatic islet cells to glucose, ultimately leading to hyperglycemia and the development of clinical diabetes. In animal models, hyperglycemia contributes to insulin resistance and defects in insulin secretion.(DeFronzo et al. 1992; Leahy et al. 1992) Thus, dietary factors that decrease plasma glucose and insulin responses could plausibly decrease the risk of type 2 diabetes. Therefore, the concept of GI was developed to help diabetic people to choose foods. Over the past three decades, studies have demonstrated that, independent of the effect of fat intake, consuming a low-GI diet may improve long-term blood glucose control and blood lipids in diabetic and, probably, non-diabetic people.(Jenkins et al. 2002) This may help reduce the risk for obesity, insulin resistance, diabetes, cardiovascular disease, etc.(Ludwig 2002) It has been proposed that, in addition to low-fat, high-carbohydrate diets, all alternative dietary approaches for preventing type 2 diabetes share an unifying mechanism: the reduction of postprandial glycemia and insulinemia.(Buyken et al. 2010b) The benefit of lowing GI was also shown to be significant even by substituting whole grains for minor carbohydrate foods in diet, such as white rice in the US.(Sun et al. 2010)
Overall, prospective epidemiological studies support a protective effect of low-GI diets against diabetes (Table 2). Furthermore, use of acarbose reduces diabetes risk.(Chiasson et al. 2002) Acarbose inhibits intestinal glucosidase. In so doing, it delays carbohydrate digestion and thus mimics the effect of a low-GI diet. However, the complex carbohydrates remain in the intestine and can cause gastrointestinal side-effects, such as flatulence and diarrhea.
3.1.1. Epidemiological studies relating GI or GL to diabetes
Positive associations were obtained in seven of the ten prospective epidemiologic studies studies that examined the relation between GI, and risk of type 2 diabetes.(Hodge et al. 2004; Krishnan et al. 2007; Meyer et al. 2000; Sahyoun et al. 2008; Salmeron et al. 1997a; Salmeron et al. 1997b; Schulze et al. 2004; Stevens et al. 2002; Villegas et al. 2007; Zhang et al. 2006) The GL was also positively associated with diabetes(Halton et al. 2008; Krishnan et al. 2007; Patel et al. 2007; Villegas et al. 2007; Zhang et al. 2006) and this finding was confirmed based on 20 years of follow-up.(Halton et al. 2008) Methodological difficluties might explain the three studies with null findings.(Meyer et al. 2000; Sahyoun et al. 2008; Stevens et al. 2002) A possible reason for the lack of association in the Iowa Women’s Study is that the diagnosis of diabetes was made only on self report without confirmation.(Meyer et al. 2000) Stevens et al. used an abbreviated food questionnaire that deliberately focused on dietary fat rather than carbohydrate.(Stevens et al. 2002) Sahyoun et al. assessed only 99 cases of diabetes.(Sahyoun et al. 2008)
In a recent meta-analyses of studies of GI and GL in relation to risk of type 2 diabetes, Barclay calculated 40% and 27% higher summary RRs when comparing the highest with lowest quantiles of GI (95% CI: 1.23, 1.59; P < 0.0001) and for GL (95% CI: 1.12, 1.45; P < 0.0001), respectively.(Barclay et al. 2008) All the studies included in this meta-analysis were adjusted for fiber. Additional data from the Black Women’s Health Study(Krishnan et al. 2007) and the Shanghai Women’s Health Study(Villegas et al. 2007) provide valuable evidence that the adverse effects of GI and GL also apply to non-Caucasian ethnic groups. In summary, although not every study found positive associations between GI and GL and risk of type 2 diabetes, the overall epidemiologic evidence strongly supports a positive relationship.
Although there have been no long-term clinical trials to determine whether low-GI or low-GL diets can prevent diabetes per se,(Radulian et al. 2009) the effect of dietary carbohydrate on comorbidities of diabetes has been investigated. Importantly, those who develop diabetes are unable to compensate for the increased age-related insulin resistance by secreting more insulin.(Festa et al. 2006) In normal subjects and subjects with impaired glucose tolerance(Wolever et al. 2008a; Wolever & Mehling 2002), as well as in subjects with diabetes or CHD, low-GI diets limit reductions in insulin sensitivity(Ebbeling & Ludwig 2001; Liu & Manson 2001; Pereira et al. 2002; Willett et al. 2002; Wolever 1990) and reduced insulin secretion(Frost et al. 1996; Frost et al. 1998; Juntunen et al. 2003; Laaksonen et al. 2005). In contrast, two observational epidemiological studies failed to demonstrate that a low-GI/GL diet improves insulin resistance, which is considered as a metabolic dysfunction predisposing to diabetes in the majority of individuals at risk.(Lau et al. 2005; Liese et al. 2005)
3.1.2. Intervention studies regarding GI and diabetes
No intervention study has evaluated the effect of a low-GI diet on the risk for clinical endpoint of diabetes. However, studies aimed at diabetes management or prevention by life style modifications indicate that an increase in fiber consumption, which is often associated with a low-GI diet, can reduce the risk for diabetes.(Knowler et al. 2002; Tuomilehto et al. 2001)
Both short-term and long-term studies strongly indicate that low-GI diets improve glycaemic control in people with diabetes.(Riccardi et al. 2008; Thomas & Elliott 2009) In a long-term (6 mo) intervention trial in patients with type 1 diabetes, a low-GI diet that is rich in dietary fiber was found to lower mean daily blood glucose concentrations, lower the level of glycated hemoglobin, and caused fewer number of hypoglycemic events.(Giacco et al. 2000) Similar beneficial effects have been observed in patients with type 2 diabetes treated with a low GI diet which was rich in dietary fiber. Additional beneficial effects of this type of diet for type 2 diabetes patients include improved lipid metabolism and insulin sensitivity.(Rizkalla et al. 2004)
Importantly, the beneficial effects of low-GI diets are independent of fiber. Compared with high-GI diets, low-GI diets containing a same fiber content result in lower postprandial plasma glucose concentrations in patients with type 2 diabetes.(Giacco et al. 2001; Parillo et al. 1985) In a study of type 2 diabetes, the effects of 2 diets differing solely in their GI and containing the same amounts of nutrients and dietary fiber were evaluated.(Järvi et al. 1999) The results showed that, after 24 d, subjects consuming the low-GI diet had better blood glucose control, improved insulin sensitivity, and lower LDL cholesterol and plasminogen activator inhibitor-1 activity. Taken together, these data suggest that consuming low-GI diets has therapeutic potential for diabetes.
A meta-analysis of 14 randomized trials of people with diabetes indicated that glycated proteins (HbA1c or fructosamine) were reduced 7.4% (95% CI, 8.8–6.0) more on a low-GI diet compared to a conventional diet with a higher GI after adjusting for baseline differences.(Brand-Miller et al. 2003) A recent trial of type 2 diabetic individuals also showed that a low GI diet, compared with a high fiber control diet, improved HbA1c.(Jenkins et al. 2008) However, neither a low GI diet nor a lower carbohydrate (higher mono-unsaturated fat) diet improved HbA1c in patients with near-normal HbA1c and only the low-GI diet elicited sustained reductions in postprandial glucose and C-reactive protein, a marker for systemic inflammation.(Wolever et al. 2008c) These data suggest that people with diabetes gain more salutary advantage than those without diabetes from low GI diets and low GI diets confer additional advantage compared to high fiber diets.(Hu et al. 2001)
Weight loss has also been related to GI/GL. A 12-week randomized trial of 129 overweight young men and women compared 4 different dietary GLs.(McMillan-Price et al. 2006) Participants on 2 diets with moderately reduced GL (a high-carbohydrate but low-GI diet or a high-protein diet) were twice as likely as those on the conventional high-carbohydrate diet (low-fat/high-GI diet) to achieve a weight loss of 5% or more without inducing differences in plasma lipid profiles. It has been shown that a weight loss of 5% or more of initial body weight can reduce the 4-year cumulative incidence of diabetes by 58% among overweight and obese men and women with impaired glucose tolerance.(Knowler et al. 2002)
In conclusion, while there has not been trial evidence linking GI/GL to clinical endpoint of diabetes, available experimental evidence corroborates the findings from epidemiologic studies that low GI/GL are associated with reduced risk of type 2 diabetes.
3.1.3. GI and glycated hemoglobin A1c (HbA1c)
Currently, measurement of HbA1c concentrations is considered the standard for assessing long-term glycemic control. Control of HbA1c is also considered a key therapeutic target for the prevention of diabetes-related complications.(Giugliano et al. 2008) Despite these uses, HbA1c has its limitations to reflect the GI exposure. It is noteworthy here that the GI-diabetes association does not necessarily imply an association between GI and HbA1c. Neither does the weak association between GI and HbA1c diminish the validity of HbA1c as a biomarker of blood glucose control. As discussed below, the association between GI and HbA1c is largely determined by the molecular properties of HbA1c.
First, the HbA1c concentration only reflects an integrated summary of circadian blood glucose concentrations during the preceding 6–8 wk.(Pecoraro et al. 1982) Unlike GI, which reflects kinetic properties of glucose metabolism,(Jenkins et al. 2002; Ludwig 2002) the HbA1c level does not reveal information on the extent or frequency of blood glucose excursions. Therefore, the HbA1c concentration is not necessarily the best or most clinically useful glycemic indicator of the risk of diseases, particularly at the lower end of elevated HbA1c concentrations.(Giugliano et al. 2008) For example, in diabetic patients frequently consuming high-GI meals whose glucose concentrations fluctuate markedly, the HbA1c concentration may indicate adequate blood glucose control; however, such patients are exposed to the harmful effects of excessive postprandial hyperglycemic excursions and the risks of hypoglycemia (Fig. 2).
Generally the best determinant of HbA1c concentrations in patients with types 1 and 2 diabetes is mean daily glycemia.(Bonora et al. 2001; Rohlfing et al. 2002) However, studies indicate that postprandial hyperglycemia may contributes up to 70% of total daytime hyperglycemia.(Reaven et al. 1988; Riddle 1990) It has been shown that the contribution of postprandial glucose excursions changes with the degree of blood glucose control: the contribution of postprandial glucose in HbA1c concentration predominates in patients with fairly good control, whereas the contribution of fasting hyperglycemia increases as glycemic control worsens.(Monnier et al. 2003) Thus it is not surprising that the association between GI and HbA1c is inconsistent across epidemiological studies.(Hare-Bruun et al. 2008) Therefore, from both systemic and population points of view, HbA1c is not an ideal biomarker for GI exposure. Nor is HbA1c a good indicator for GI exposure from biochemistry and molecular pathogenesis points of view. First, because HbA1c is an Amadori product but not an end product of glycation,(Glenn & Stitt 2009) it can only reflect the internal dose of hyperglycemia but not the biologically effective dose. Second, the formation of advanced glycation end products (AGEs) is only one of the dimensions of hyperglycemic pathology (see 4.2. Hypothesized mechanisms relating dietary hyperglycemia to AMD and DR) and the pathogenic contribution of AGEs may vary from diseases to diseases. These limit the utility of HbA1c as a biomarker of GI or hyperglycemic exposure.
3.2. GI and cardiovascular disease
The effects of the quantity of carbohydrate intake on CVD risk remain controversial (Table 2).(Bravata et al. 2003) Results from a 20-y follow-up study in the Nurses’ Health Study (NHS) suggest that diets lower in carbohydrate and higher in protein and fat are not associated with increased risk of CHD.(Halton et al. 2006) As for the trials, none of them had a sufficient sample size or duration of follow-up to evaluate the effects on the clinical outcomes of CHD. In a meta-analysis of five randomized trials using intermediate biomarkers for CVD as the endpoint, the low-carbohydrate diet, compared with a low-fat diet, was found to have a beneficial effect on high-density lipoprotein (HDL) cholesterol and triglyceride levels but an adverse effect on total cholesterol and LDL cholesterol levels after at least 6-month of intervention.(Nordmann et al. 2006)
3.2.1. Epidemiologic evidence regarding GI or GL and cardiovascular disease
Insulin resistance increases multiple risk factors for cardiovascular disease (CVD), a broad category of circulatory diseases which affect the heart and blood vessels. These risk factors include dyslipidemia, hypertension and hyperglycemia, etc. Many of the same dietary factors that are related to enhanced risk for diabetes also appear to be related to higher risk of CHD.(Liu 2002)
The relation between GI and GL and incidence of CVD has been examined in four prospective studies.(Beulens et al. 2007; Halton et al. 2006; Liu et al. 2000; van Dam et al. 2000) Overall, GL was more closely related to risk of CVD than GI. A high dietary GL was associated with markedly increased risk of CHD, independent of conventional CHD risk factors over 10 years of follow-up(Liu et al. 2000) and a 90% increased risk comparing highest and lowest deciles after 20 years of follow-up(Halton et al. 2006) in a large female American cohort. In a Dutch female cohort, there was a 47% increased risk of CVD for the highest against lowest quartile of GL in women followed up for 9 years.(Beulens et al. 2007) However, results from a much smaller study following 646 elderly Dutch men for 10 years did not corroborate these observations.(van Dam et al. 2000)
In a meta-analysis of CVD, there were 25% higher summary RRs for GI.(Barclay et al. 2008) In a systematic review of prospective cohort studies or randomized trials investigating dietary exposures in relation to CHD, there is strong evidence that dietary GI is a risk factor independent of other dietary factors and patterns, including fiber and Mediterranean dietary pattern.(Mente et al. 2009) Corroborating the data above, GI or GL were also found to be strongly associated with several risk factors for CHD including HDL levels,(Buyken et al. 2001; Ford & Liu 2001; Frost et al. 1999; Hokanson & Austin 1996; McKeown et al. 2009; Shikany et al. 2010) insulin resistance, metabolic syndrome,(McKeown et al. 2004) and C-reactive protein.(Liu et al. 2002)
3.2.2. Intervention studies regarding GI or GL and metabolic risk factors for cardiovascular disease
There have not been randomized trials in humans regarding clinical endpoint of CVD, however, evidence indicates that diets high in carbohydrate can increase plasma levels of triglycerides and reduce HDL cholesterol, both of which are risk factors for CHD.(Mensink et al. 2003) Further, partial replacement of carbohydrate with either protein or unsaturated fat improved CVD risk factors.(Appel et al. 2005; Carey et al. 2005) Three controlled intervention studies show that low-GI diets reduced levels of plasminogen-activator inhibitor-1 (PAI-1), a marker of thrombogenicity, in overweight(Ebbeling et al. 2005) as well as diabetic adults.(Järvi et al. 1999; Rizkalla et al. 2004)
Although the overall evidence is less robust than for diabetes, epidemiological studies tend to support a favorable effect of low-GI diets on CVD risk (Table 2). Furthermore, intervention studies using intermediate risk factors (surrogate endpoints) for CVD demonstrate that lowering GI reduces the risk for CVD.
For over one decade, it has been appreciated that AMD shares some risk factors with CVD. These include age, smoking, hypertension, hypercholesterolemia, diabetes, and dietary intakes of fat and antioxidants, etc.(Snow & Seddon 1999) Evolving data indicate that dietary GI is also a common risk factor for AMD and CVD. Furthermore, diabetic complications can often manifest vascular diseases in the retinal circulation, such as central retinal vein occlusion, central retinal artery occlusion.(Watkinson & Seewoodhary 2008) These associations were noted above and set the stage for our review of the relationship between GI or diabetes and AMD.
3.3. GI and age-related macular degeneration (AMD)
Epidemiologic studies indicate that a low-GI diet is associated with reduced risk for AMD, but no intervention study has been conducted on this topic (Table 2).
3.3.1. Epidemiologic evidence relating GI to AMD
Recent epidemiological studies have consistently found positive relationships between GI and AMD in non-diabetic people, and the associations are independent of fiber intake (Fig. 3).(Chiu et al. 2006a; Chiu et al. 2007a; Chiu et al. 2007b; Kaushik et al. 2008) In the first study published in 2006, women in the third tertile of dietary GI compared with those in the first tertile had ~2.7-fold increased risk for early AMD, mainly pigment abnormality, in a case-control study of the Nutrition and Vision Project (NVP) of the Nurses’ Health Study (NHS).(Chiu et al. 2006a) The findings were replicated in a much larger American cohort, the Age-Related Eye Disease Study (AREDS).(Chiu et al. 2007a) In that case-control study, a diet in the highest quintile of dietary GI compared with a diet in the lowest quintile was associated with an over 40% increased risk for large drusen. When comparing the upper 50% with the lower 50% of the dietary GI, an almost 50% increased risk for advanced AMD was noted. Using this data, the prevalent population attributable fraction of advanced AMD for high dietary GI was estimated to be 20%. In other words, one in five of the existing cases of advanced AMD would have been eliminated if the AREDS participants consumed diets with a dietary GI below the median. The positive relationship between GI and AMD was further strengthened in a prospective study that followed the AREDS subjects for up to 8 years (mean=5.4 years).(Chiu et al. 2007b) Overall the multivariate-adjusted risk of progression was significantly higher (hazard ratio=1.10; 95% CI: 1.00, 1.20; P = 0.047) in the upper 50% of the dietary GI than in the lower 50%. Furthermore, the more advanced the grade of AMD at baseline, the higher the increase of risk for progression during the follow-up period (P for trend < 0.001). We also estimate the incident population attributable fraction for advanced AMD to be 7.8 during the follow-up period, i.e. 7.8% of new advanced AMD cases would be prevented in 5 y if people consumed a low dietary GI diet. This could save over 100,000 cases of AMD-related blindness in the US in 5 years.
Fig. 3.
Studies relating GI to AMD indicate that consuming a low-GI diet is associated with lower risk for both early and advanced AMD.
Importantly, consuming lower GI diets appears to provide ophthalmic benefit in addition to that gained from currently known dietary factors. Analysis of a compound score summarizing dietary intakes of antioxidants (including vitamins C and E, and lutein/zeaxanthin), zinc, omega-3 fatty acids (including docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]), and GI suggested that the associations between the compound score and risk for drusen and advanced AMD are largely driven by dietary GI.(Chiu et al. 2009b) A prospective analysis of the AREDS AMD trial indicated that consuming a low-GI diet augmented the protective effects of the AREDS fomula (antioxidants plus zinc) and of DHA/EPA against progression to advanced AMD.(Chiu et al. 2009a) Even more attractive is that the benefit can be achieved by a minor dietary change, such as by daily substituting as little as 5 slices of whole grain bread (GI=79) for white bread (GI=100).(Chiu et al. 2009a)
The GI-AMD relationship was further confirmed in a 10-year follow-up in the Blue Mountains Eye Study (BMES).(Kaushik et al. 2008) After multivariate adjustment, a higher dietary GI was associated with a 77% increased risk of early AMD comparing the 4th with the 1st quartiles of dietary GI (95% CI: 1.13, 2.78; P for trend = 0.03), and further including cereal fiber in the model did not change the association. Conversely, greater consumption of cereal fiber (in a comparison of quartiles 1 and 4, RR=0.68; 95% CI: 0.44, 1.04; P for trend = 0.05) was associated with a reduced risk of incident early AMD. The relevance of fiber to the association between GI and AMD risk should be considered in future studies. However, the study could not show a relation between GI and late AMD, probably because of insufficient power (late MD developed in only 54 of 1913 persons at risk).
In a prospective study, it is shown that GI played a more important role in individuals with bilateral AMD progression (i.e., those who are more susceptible to AMD progression) than those with unilateral AMD progression, especially in the later stages of AMD.(Chiu et al. 2007b) This finding implies that the interaction between AMD susceptibility and GI affects the risk for AMD progression, and that the interaction plays a more important role in the later stages. The nature of this susceptibility remains to be elucidated. It is possible that genetic susceptibility represents a major component of the underlying relationship between GI and AMD.
3.3.2. Intervention studies regarding GI and AMD
Like diabetes and CVD, an intervention study evaluating the effect of GI on clinical outcomes of AMD would be difficult to execute because feeding people high-GI diets for prolonged periods may be unethical and the study would be very costly. This would be all the more challenging because there are no intermediate metabolic risk factors which can serve as surrogate endpoints for AMD after short-term of intervention.
In conclusion, while interventional data is unavailable, observational epidemiological studies support findings that lowering GI reduces the risk for the progression of both early and late AMD.
3.3.3. Diabetes and AMD
Intuitively, one might expect an epidemiological association between two diseases which share a common risk factor. Specifically, one might expect an association between risk for diabetes and AMD since they both share consuming high-GI diets as a common risk factor. But this expectation is not always reasonable. For example, although alcoholic drinking is a risk factor for both breast cancer in women and prostate cancer in men,(Allen et al. 2009; Middleton Fillmore et al. 2009) an association between the two cancers based on the shared risk factor is not observed. Clearly, the association between two diseases which share a common risk factor depends not only on the similarity of etiologies but also on the susceptible populations between the two diseases.
Epidemiological data regarding the association between diabetes and AMD have been inconsistent. Some studies found a positive association,(Age-Related Eye Disease Study Research Group 2005; Klein et al. 1997; Klein et al. 1992; Leske et al. 2006; Mitchell & Wang 1999; Topouzis et al. 2009) while others not.(Delcourt et al. 2001; Eye Disease Case-Control Study Group 1992; Fraser-Bell et al. 2008; Goldberg et al. 1988; Hyman et al. 2000; Smith et al. 2001; Tomany et al. 2004) But, even in studies that found a positive association, the association with specific types of late AMD (neovascular AMD or geographic atrophy [GA]) was inconsistent, either; Some found an association with neovascular AMD,(Age-Related Eye Disease Study Research Group 2005; Klein et al. 1997; Klein et al. 1992; Topouzis et al. 2009) while others found an association with GA.(Mitchell & Wang 1999; Tomany et al. 2004) None of these studies found an association between diabetes and early AMD.
In the Beaver Dam Eye Study (BDES), diabetes was not associated with early AMD.(Klein et al. 1992) However, in persons older than 75 years, diabetes was found to be associated with neovascular AMD, but not with GA. Further stratification analysis revealed that the association was only in men but not in women; the relative risk (RR) of neovascular AMD was 10.2 (95% CI: 2.4, 43.7) for men, it was 1.1 (95% CI: 0.4, 3.0) for women. The authors could not explain this gender difference, and suggested that it might be due to chance.(Klein et al. 1992) However, in contrast, in the Women’s Health Initiative Sight Exam Ancillary study, a history of diabetes was associated with a 2.5-fold increased risk for neovascular AMD but not with either early AMD or GA in these women.(Klein et al. 1997) Positive associations between diabetes and neovascular AMD have also been identified by the AREDS and the EUREYE study.(Age-Related Eye Disease Study Research Group 2005; Topouzis et al. 2009) In the AREDS, a history of diabetes was associated with increased risk for incident neovascular AMD (odds ratio [OR]=1.88) but not for GA in persons at risk of developing advanced AMD in one eye. In the EUREYE study, subjects with neovascular AMD compared with controls had increased odds for diabetes (OR=1.81; 95% CI: 1.10, 2.98). No significant association of diabetes was found with either early AMD or GA.
In contrast with the data above, the BMES reported that diabetes was significantly associated with the prevalence of GA (OR=4.0; 95% CI: 1.6, 10.3), but no association was found for either neovascular AMD or early AMD.(Mitchell & Wang 1999) In the 5- and 10-year incidence studies in the same cohort, diabetes was also related to increased risk of incident GA (RR=8.3 and 3.9, respectively) but not to neovascular AMD.(Tomany et al. 2004)
In a cohort study of a black population in Barbados, a diabetes history was associated with 2.7-fold increased risk of incident advanced AMD. However, a subtype analysis of advanced AMD was not performed.(Leske et al. 2006) Diabetes history was not associated with early AMD in this study. As with the other studies, diabetes history was not associated with early AMD.
Surprisingly, in the BDES, diabetes at baseline was associated with decreased risk of incident reticular drusen.(Klein et al. 2008) Reticular drusen has been reported to be associated with a high risk of progression to neovascular AMD.(Smith et al. 2006)
4. MECHANISMS RELATING HYPERGLYCEMIA TO DR AND AMD
As reviewed in the previous sections, as a measure of postprandial hyperglycemia, GI has been related to diabetes and AMD. In the following section we will discuss potential underlying mechanisms for the GI-disease associations with emphasis on DR and AMD. We begin with a brief review of the pathologies for DR and AMD with emphasis on the effects of hyperglycemia. This is followed by a discussion of the molecular mechanisms that appear to be etiologic for the pathology. Five well-developed hyperglycemic mechanisms are described, including four glycolysis-associated pathways and one mitochondria-associated pathway. However, these well-established hyperglycemic pathways can only explain the pathogenesis under normoxic conditions. Therefore, we propose a novel hyperglycemic, hypoxic pathway to explain the hyperglycemic pathology under low oxygen tension. Finally, inter-relationships among the six pathways are discussed.
It is useful to mention here that all of the six hyperglycemic pathways could lead to a common intracellular or extracellular insult, oxidative stress. This is consistent with the benefit of antioxidant intake in AMD (Age-Related Eye Disease Study Research Group 2001; Chiu & Taylor 2007) as well as with recent epidemiological data that indicate that a diet high in antioxidants and low in GI brings an additional benefit compared with a diet high in antioxidants or low in GI alone.(Chiu et al. 2009a; Chiu et al. 2009b)
4.1. Hyperglycemic pathology of DR and AMD
Although differences exist between DR and AMD (Table 1), in the following sections we will show that both of them can be considered as metabolic diseases.
4.1.1. Hyperglycemic pathology of DR
Hyperglycemia has long been recognized as the critical factor in the development of DR.(Kohner et al. 1998; Kohner et al. 2001; Madsen-Bouterse & Kowluru 2008; The Diabetes Control and Complications Trial Research Group 1993; The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group 2000; UK Prospective Diabetes Study (UKPDS) Group 1991) The large variations in the prevalence and incidence of DR among different studies are mainly attributable to the difference in the level of blood glucose control across the different study cohorts.(Williams et al. 2004) Prolonged exposure to high glucose causes both acute, reversible changes in cellular metabolism and long-term, irreversible changes in stable macromolecules. In addition, long before disease pathology is detectable, the cells of the retina start to respond to this hyperglycemic environment by altering metabolism.
DR is a retinal microvascular complication of diabetes. It primarily affects the retinal circulation (i.e. inner retina), which receives 20–30% of the blood that flows to the retina through the central retinal artery to nourish the inner neural retina. The retinal capillary wall is surrounded by a connective tissue sheath-the basement membrane. A single layer of the retinal microvascular cells, including pericytes and endothelial cells lies on this membrane.(Lorenzi & Gerhardinger 2001) The pericytes are a type of smooth muscle cell. They directly contact the endothelial cells, providing support to the capillaries, and help regulate the endothelial cells. These functions are critical for the development of a proper retinal network, and, by cooperating with other retinal cells like astrocytes and Müller cells, pericytes appear protective for retinal endothelial cells under hyperglycemic conditions. Importantly, DR is characterized by pericyte loss followed by increased vascular permeability and progressive vascular occlusion.
The inner blood-retinal barrier (iBRB) consists of the basement membrane and the fusion of membranes between retinal endothelial cells forms tight junctional complexes to help stop the outward flow of circulating proteins.(Harhaj & Antonetti 2004) A main pathological feature of very early stage DR is hyperglycemia-associated iBRB breakdown.(Engerman & Kern 1986) The iBRB breakdown begins with the loss of tight junctions between adjacent microvascular endothelial cells. This allows macromolecules to seep out. As barrier breakdown proceeds, the basement membrane of the capillaries thickens and the capillaries become rigid. This could interfere with the ability of the basement membranes to bind various growth factors.(Frank 2004)
Loss of pericytes results in empty, balloon-like spaces on the wall of the retinal capillary. Endothelial cells try to repair the damaged vessel by proliferation on the inner vessel wall. At this stage, the disease remains clinically non-detectable. However, as the pathology progresses, it results in capillary occlusion and appearance of small hemorrhages and yellow deposits (hard exudates), followed by the complete loss of all cellular elements from the retinal microvessels (acellular capillaries), and development of abnormally dilated capillaries around the margins of areas with no capillary blood flow (ischemia). The microaneurysms are the earliest clinically observable lesion of diabetic retinopathy. This leads to non-proliferative diabetic retinopathy and often progresses into diabetic macular edema (DME). If the disease becomes more severe, the non-proliferative retinopathy may progress to pre-proliferative retinopathy. The ischemia and hypoxia of pre-proliferative retinopathy eventually lead to retinal neovascularization (RNV), which is the hallmark of proliferative retinopathy.(D’Amico 1994) These newly formed blood capillaries are fragile and tend to hemorrhage. They can also extend into the vitreous of the eye, and their fibrous proliferation on the retina could scar the vitreous body leading to traction retinal detachment, and ultimately to blindness.(D’Amico 1994; Frank 2004; Madsen-Bouterse & Kowluru 2008; Singh & Stewart 2009)
4.1.2. Hyperglycemia-related pathology in AMD
In contrast with DR, the first indication of AMD is observed in the outer retina, primarily involving the retinal pigment epithelium (RPE) and associated tissues (Table 2).(Glenn & Stitt 2009) The RPE lays on a basal lamina, known as Bruch’s membrane, and together they form the outer blood retinal barrier (oBRB). The oBRB separates the retina from the choroidal plexus. The choroidal circulation receives 65–85% of the blood that flows to the retina through choroidal arteries and is vital for the maintainance of the outer retina (particularly the photoreceptors).(Henkind 1981; Henkind & Walsh 1980) A very high density of mitochondria and lysosomes in RPE also indicates a high metabolic activity. The RPE serves as a headquarters in outer retinal metabolism; it oxygenates and nurtures the outer retina and is also responsible for processing metabolic waste generated from visual cycle. The high energy requirements of the RPE stem from requirements for metabolism including proteolytic burden, because every night each RPE must digest the outer 10% of the photoreceptor discs that are shed by 30 photoreceptors. In fact, the RPE has the highest proteolytic burden in the body.(Young & Bok 1969)
In both the RPE and the photoreceptor inner segments there are large numbers of mitochondria indicative of the involvement of the tricarboxylic acid (TCA) cycle (also known as citric acid cycle or Kreb’s cycle) in energy provision.(Kaur et al. 2008) Glucose is the major fuel for energy metabolism in retina and about 60% of blood glucose entering the retina appears to be supplied to RPE.(Coffe et al. 2006; Foulds 1990) It has been shown that RPE exhibits a high saturation level of glucose transport and high rates of oxygen consumption.(Miceli et al. 1990; To et al. 1998; Vilchis & Salceda 1996) In order to provide an adequate supply of oxygen and glucose to this most energy demanding location in human body-the retinal photoreceptors and the RPE- there is high blood flow in the choroid. Indeed, blood flow in the choroid is the highest of any tissue in the body in terms of blood flow per unit mass of tissue.(Wilson et al. 1973) The RPE also provides a major transport pathway for the exchange of metabolites and ions between the choroidal blood supply and the neural retina to maintain a normal function of the photoreceptor cells.(Pascuzzo et al. 1980; Strauss 2005; Zadunaisky & Degnan 1976) All of these functions indicate that the RPE plays a central role in the health of the outer retina. Therefore, it is not surprising that disorders induced by hyperglycemia in the RPE predispose the retina to the development of AMD.
Tissue aging is associated with a progressive decline in cellular and physiological function, including metabolic capability.(Pawlak et al. 2008) Aging is also associated with diminished capacity to respond to stress and concomitant susceptibility to degenerative disease.(Beckman & Ames 1998; Szweda et al. 2003) Cellular manifestations of aging include increased chemical damage to proteins, accumulation of intracellular and/or extracellular deposits, and decreased efficiency of antioxidant defenses. These processes are pronounced in long-lived post-mitotically differentiated cells, such as RPE.(Boulton et al. 2004; Grune et al. 2004; Louie et al. 2002; Terman et al. 2007; Zhang et al. 2008)
The accumulation of heterogeneous debris within the RPE-Bruch’s membrane-choriocapillaris complex is a major histopathologic hallmark of aging and AMD. A range of age-related macular changes have been described in the RPE and underlying Bruch’s membrane. With the function of RPE compromised during aging, drusen, the early stage of maculopathy, and more advanced lesions can begin to develop. For example, damage to the metabolic waste processing machinery in the RPE, such as lysosomes and microsomal glutathione S transferase 1, have been related to aging retina.(Maeda et al. 2005) This may result in or accelerate the formation of lipofuscin and drusen. Their precursors are generated from RPE phagocytosed photoreceptor outer segments which are not well degraded by the RPE and thus accumulate intracellularly within the RPE and extracelluarly between the RPE and Bruch’s membrane, respectively.(Boulton et al. 1994; Ishibashi et al. 1986a; Ishibashi et al. 1986b; Rakoczy et al. 1996) Clinically, these manifestations are considered the early stage of AMD. They are commonly seen in people over 60’s yrs old without vision loss but a significant proportion of them will progress to the late stage of AMD, including geographic atrophy (GA) and choroidal neovascularization (CNV), which often result in severe vision loss.
In addition to drusen, the accumulation of deposit within the RPE-Bruch’s membrane-choriocapillaris complex includes extracellular basal lamina deposits (BLDs). There are also changes in the chemical composition, physical structure and hydrodynamics of Bruch’s membrane.(Cherepanoff et al. 2009; Moore & Clover 2001; Moore et al. 1995; Sarks et al. 1999; Stitt 2005) Such abnormalities are thought to be important in the development of AMD.(Anderson et al. 2009; Hageman et al. 2001; Johnson et al. 2003)
With aging, the outer segments of photoreceptors become convoluted and lipofuscin accumulates in the inner segment of photoreceptors. RPE cells reduce in number, become pleomorphic, and undergo atrophy, hypertrophy, hyperplasia, and cell migration. Bruch’s membrane becomes thickened, basophilic, and hyaline and the lipid content increases. The RPE is unable to cope with the phagocytosis of outer segments as well as its own high metabolic needs. Drusen formed from the metabolic debris further interfere with the metabolic process of RPE. Ultimately, this leads to cell death.(Farkas et al. 1971) Although the histopathologic characteristics and chemical composition of these deposits are documented, their precise role in the etiology for AMD has only been partly resolved. Carbohydrates have been found to be important components in drusen and BLDs and play an important role in the pathogenesis of AMD.(Hageman et al. 2001) Drusen and, to a lesser extent, BLD have deleterious effects on RPE function and the accumulation of lipofuscin in RPE with age also has a direct influence on outer retinal integrity.(Boulton & Marshall 1986; Johnson et al. 2003; Sarks et al. 1999)
4.2. Hypothesized mechanisms relating dietary hyperglycemia to AMD and DR
In aerobic cellular respiration, glucose is metabolized through three steps: 1) glycolytic pathway, 2) tricarboxylic acid (TCA) cycle (also known as citric acid cycle or Kreb’s cycle), and 3) electron transport chain (ETC). Under normal glucose level (euglycemia, left panel in Fig. 4a), glucose generates energy (i.e. adenosine triphosphate [ATP]) for normal physiological needs without inducing deleterious side reactions. However, under hyperglycemic conditions which exceed the physiological needs (right panel in Fig. 4a), the glycolytic pathway may induce four adverse side pathways to relieve the influx of excess glucose. The four glycolysis-related hyperglycemic pathways include: 1) Intracellular production of AGE precursors, 2) Increased flux through the polyol pathway, 3) PKC activation, and 4) Increased hexosamine pathway activity. Each of these will be discussed below (see sections 4.2.1.~4.2.4.). Under normoxic conditions (normal oxygen tension) the TCA cycle will induce an abnormally high mitochondrial membrane potential. This will further induce the ETC to reduce O2 into superoxide (O2−), which in turn will generate intracellular and even extracellular oxidative stress.(Brownlee 2001; Brownlee 2005)
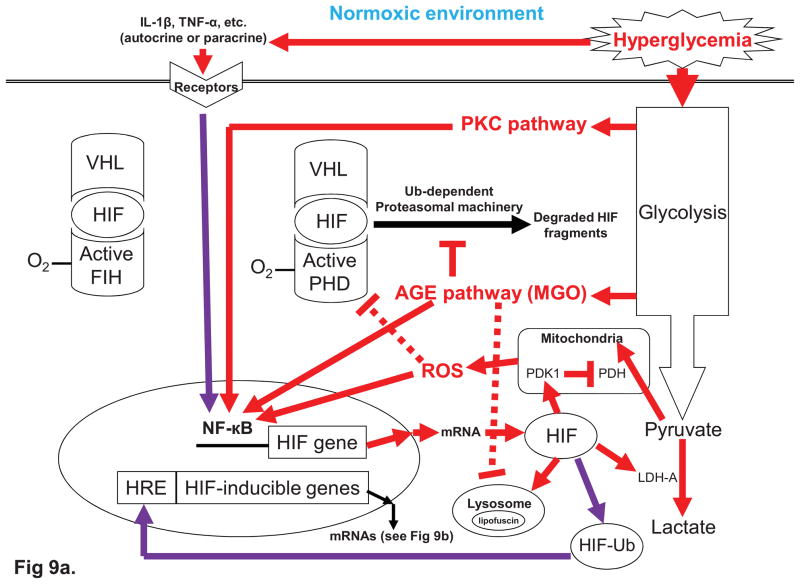
Fig. 4. Cellular responses to euglycemia (normal glycemia) and hyperglycemia under normoxia (4a) and hypoxia (4b).
Fig. 4a. Glucose metabolism in euglycemia vs. hyperglycemia under normoxic conditions. Compared with euglycemia, hyperglycemia induces mitochondria-derived superoxide (O2−) and four glycolysis-related pathways (see Figs 5–8), including polyol, hexosamine, AGE, and PKC pathways, and excess cytosolic HIF. The left panel demonstrates normal aerobic respiration in a euglycemic condition. After glycolysis, the glucose metabolite, pyruvate, is produced. Pyruvate enters the mitochondria to generate ATP and water (H2O). The right panel demonstrates that hyperglycemia drives glycolysis to generate the four adverse side pathways noted above.(also see Fig. 5–8) In the mean time, driven by the hyperglycemia, the ETC is obstructed in coenzyme Q by an abnormally high mitochondrial membrane potential and generates superoxide (O2−), which may activate PARP, a DNA repair enzyme which needs GAPDH as a cofactor and is only found in the nucleus. This gives rise to the decrease of cytosol GAPDH and further exacerbates of the four glycolysis-associated pathways induced by hyperglycemia. The mechanism underlying the movement of GAPDH from the cytosol to the nucleus under high glucose conditions involves the E3 Ub ligase siah-1, which facilitates hyperglycemia-induced GAPDH nuclear translocation via formation of a complex with GAPDH. Furthermore, because hyperglycemic AGEs, PKC, and mitochondrial ROS may give rise to the over expression and decreased degradation of HIF, the excess HIF proteins may switch pyruvate metabolism from transformation through the TCA cycle and oxidative phosphorylation in the ETC to conversion to lactate in the cytoplasm (also see Fig. 9a for more details). Remarkablly, PKC can be also activated through hyperglycemic polyol pathway (Fig. 6) and hyperglycemic hexosamine pathway (Fig. 8). The cell may defend against superoxide using the mitochondrial isoform of superoxide dismutase (Mn-SOD). This enzyme degrades the oxygen free radical to hydrogen peroxide, which is then converted to H2O and O2 by other enzymes.
Fig. 4b. Glucose metabolism in euglycemia vs. hyperglycemia under hypoxic conditions. In euglycemia, HIF pathway is turn on by hypoxia-activated HIF. Under hyperglycemic conditions, the HIF pathway is enhanced by hyperglycemia-induced AGE and PKC pathways. The left panel indicates the HIF is activated and induces two aspects of the cellular responses, including switching glucose metabolism and turning on HIF pathway. Cytosolic HIF switches glucose metabolism from aerobic respiration to fermentation, the end product of which is lactate. The HIF pathway activated by hypoxia-activated HIF may induce a range of deleterious effects. However, when hypoxia coincides with hyperglycemia (right panel), which results in the formation AGEs and activation of PKC during glycolysis, HIF pathway is further enhanced by hyperglycemia. Remarkablly, PKC can be also activated through hyperglycemic polyol pathway (Fig. 6) and hyperglycemic hexosamine pathway (Fig. 8). Furthermore, in adaptation of lower efficiency of ATP generation from fermentation, the activations of some HIF-inducible genes in HIF pathway may increase glucose uptake and up-regulate glycolysis pathway (also see Fig. 9b). Therefore, in hyperglycemic, hypoxic conditions HIF pathway may further deteriorate the four glycolysis-associated pathways.
It has been shown that inhibition of some of these hyperglycemia-related pathways can protect against multiple or specific microvascular complications in diabetic animal models, including retinopathy. For example, studies using the transketolase activator benfotiamine indicate that it can inhibit a common convergent pathway and effectively prevent retinopathy in diabetic animals by inhibiting activation of PKC β II, alterations in hemodynamics, flux through the polyol and hexosamine pathways, and AGE formation.(Hammes et al. 2003) Based on this evidence, a unifying theory proposed by Brownlee indicates that under hyperglycemic states the mitochondria-derived reactive oxygen species (ROS) are the upstream common initiator for the four deleterious pathways derived from glycolysis.(Brownlee 2005) In other words, in addition to inducing oxidative stress, the overproduction of ROS can also, indirectly, accelerate the four glycolysis-related pathways by blocking the downstream flow of glycolysis (Fig. 4a; see 4.2.5. Hyperglycemic mitochondria-derived ROS).(Brownlee 2001; Brownlee 2005; Nishikawa & Araki 2008; Nishikawa et al. 2000)
The relative importance of the four glycolysis-related hyperglycemic pathways may vary by the types of tissue in the body.(Brownlee 2001) In the retina, some suggested that the polyol pathway, which is upstream of glycolysis, is more important,(Diederen et al. 2006; Ola et al. 2006) while others assign priority to the downstream pathways(Ido & Williamson 1997; Nyengaard et al. 2004; Williamson et al. 1993.). The controversy may arise from the different experimental conditions that were used to best reflect the real environment in living human retina during disease progression. It is likely that the relative importance of the four glycolysis-related hyperglycemic pathways may also vary by the stages of disease progression.
Glucose metabolism also depends on the homeostasis of oxygen. Under oxygen-insufficient conditions (low oxygen tension or hypoxia), instead of entering the TCA cycle and ETC in mitochondria, some of the glycolysis metabolite, pyruvate, may proceed to fermentation (Fig. 4b). Although fermentation is less efficient at generating energy from glucose, under certain physiological conditions, such as sprinting, it may be used by a skeletal muscle cell to generate ATP and lactate even before the oxygen levels are depleted. This hypoxia-induced metabolism switch has been shown to be associated with a hypoxia-inducible factor (HIF).(Brahimi-Horn et al. 2007; Kim & Dang 2006) Similarly, despite large numbers of mitochondria in the RPE indicative of the involvement of the TCA cycle in energy provision, fermentation also occurs in the RPE. As a result, concomitant with high oxygen consumption, the RPE also has a high lactate production even under physiological conditions.(Coffe et al. 2006; Kaur et al. 2008; Miceli et al. 1990)
Local occlusive vascular diseases in the eye may also result in retinal hypoxia. Interestingly, in both DR and AMD, hypoxia has been shown to accelerate the progression of NV through a HIF-related pathway.(Arjamaa & Nikinmaa 2006) It has been shown that, in this pathway HIF serves as a transcription factor that controls the expression of many genes (HIF-inducible genes). Some of these genes regulate angiogenesis, such as VEGF.
In this review, we propose a novel hyperglycemic HIF pathway to explain hyperglycemic pathogenesis in the retina under hypoxic conditions (Fig. 4b; also see Fig. 9 in 4.2.6. Hyperglycemic HIF pathway). Like in aerobic respiration (Fig. 4a), under hypoxia conditions the four glycolysis-related pathways and the HIF pathway should be viewed as inter-related and independent mechanisms.
Fig. 9. Hyperglycemic HIF pathway in both normoxic (9a) and hypoxic conditions (9b).
Fig. 9a. Normaxic, hyperglycemic HIF pathway. In normoxia, hyperglycemic PKC activation, AGEs formation, mitochondrial ROS, and proinflammatory cytokines (e.g. IL-1β and TNF-α) decreases the degradation (through impairing proteasomal system) and/or increases the expression of HIF (through activating NF-κB). The elevated cytoplasmic HIF proteins may switch glucose metabolism from aerobic respiration to fermentation giving rise to lactate accumulation (also see Fig. 4a). The hyperglycemia-induced excess cytosolic HIF proteins may also lead to increased autophagy, while the lysosomal proteases are impaired by hyperglycemia. The combination of the two effects may also results in the accumulation of lysosomal lipofuscin. In addition, the excess cytosolic HIF proteins, such as an ubiquitinated form of HIF-1α induced by TNF-α, can also transactivate HIF-inducible genes (also see Fig 9b). However, under hypoxia the hyperglycemia-induced HIF protein is more stable.
Fig. 9b. Hypoxic, hyperglycemic HIF pathway. In hypoxia, the transactivation activity of HIF is turned on because both oxygen sensors, PHD and FIH, become inactive. This eliminates proteasomal degradation of HIF proteins. The HIF proteins are further stabilized by hypoxia-induced HSP90 and bind to the HREs in the promoter or enhancer region of HIF-inducible genes to transactivate the transcriptions of the genes. The HIF pathway consists of many HIF-inducible genes, which encode a wide range of proteins, including RAGE, glycolytic enzymes, GLUTs, Epo, and VEGF. The hyperglycemic, hypoxic HIF pathway may be enhanced by hyperglycemic AGE formation and PKC activation, which can activate RAGE signaling cascades and increase HIF expression. Furthermore, in adaptation of lower efficiency of ATP generation from fermentation, the activation of GLUTs and glycolytic enzymes increase glucose uptake and up-regulate the glycolysis pathway (also see Fig. 4b). Therefore, in hyperglycemic, hypoxic conditions the HIF pathway may further deteriorate the four glycolysis-associated pathways and lactate accumulation in the cytoplasm. Furthermore, the hyperglycemia-induced excess cytosolic HIF proteins may also enhance autophage, while the lysosomal system is impaired by hyperglycemia. Together, they may also result in the accumulation of lysosomal lipofuscin. In addition, hyperglycemia-induced proinflammatory cytokines (e.g. IL-1β and TNF-α) can further enhance the HIF pathway.
Simply speaking, our hypothesized hyperglycemic pathogenesis consists of six pathways, including four glycolysis-related pathways, a mitochondria-derived ROS pathway, and a HIF pathway. The four glycolysis-related pathways are applicable in both normoxic and hypoxic conditions. The mitochondria-derived ROS pathway participates during normoxia and the HIF pathway dominates the hyperglycemic pathogenesis in hypoxia. In the following sections (see sections 4.2.1.~4.2.6.), we will discuss these six molecular mechanisms regarding how dietary hyperglycemia results in cellular dysfunctions which are associated with tissue damage and clinical manifestations of AMD and DR.
4.2.1. Hyperglycemic AGE pathway
One of the major sources leading to the damage to the BRB is caused by non-enzymatic modification of free amino groups of proteins, lipids, and DNA by aldehyde groups on sugars or sugar metabolites (such as dicarbonyls).(Stitt 2005) This reaction, called Maillard reaction, happens naturally during aging in all tissues.(Monnier et al. 1992) The initial unstable Schiff base slowly rearranges into an Amadori adduct, the first stable product formed during glycation of protein(Thorpe & Baynes 2003). These have half lives of several months under physiological conditions.(Lyons et al. 1991) The most well-known Amadori product is glycated hemoglobin A1c (HbA1c), which is used as an indicator for cumulative exposure of hemoglobin to elevated blood glucose.(Glenn & Stitt 2009) Amadori adducts may undergo further oxidation, dehydration reactions, and crosslinking to form AGEs. Interestingly, these reactions are markedly accelerated during aging and even more so in diabetes, but the adducts formed during aging may differ.(Brownlee 2005; Giardino et al. 1994; Glenn & Stitt 2009; Queisser et al. 2010; Shinohara et al. 1998; Tessier et al. 1999) AGEs can come from many sources. A wide range of AGE precursor molecules give rise to a broad array of AGEs. The quantity and types of AGEs which are found at any time depend upon rates of formation and rates of degradation. The most abundant AGE in human body is the Nε-(carboxyl-methyl) lysine (CML).(Ikeda et al. 1996; Reddy et al. 1995)
Amino groups can also react with highly active glucose metabolites, including glyoxal (GO), methylglyoxal (MGO), 3-deoxyglucosone, etc. (Thorpe & Baynes 2003) These dicarbonyls can lead to very rapid AGE formation especially in circumstances of enhanced glycolytic activity (such as in hyperglycemia).(Lal et al. 1995; Thornalley et al. 1999; Thorpe & Baynes 2003) Interestingly, it was recently shown that in human aortic endothelial cells hyperglycemia-induced ROS production increases expression of RAGE and RAGE ligands and this effect is mediated by ROS-induced MGO.(Yao & Brownlee 2010) Furthermore, it is believed that these intracellular glucose-derived dicarbonyls are the major initiating molecules in the formation of both intracellular and extracellular AGEs.(Degenhardt et al. 1998) Therefore, it is reasonable to anticipate that they constitute a more important source of AGEs in a highly energy-demanding tissue, such as retina, than other tissues.
4.2.1.1. Hyperglycemic AGE pathway and DR
Clinical studies have showed that the levels of AGEs in serum,(Dolhofer-Bliesener et al. 1996; Ono et al. 1998; Wagner et al. 2001) skin,(Sell et al. 1992) and cornea(Sato et al. 2001) correlate with the onset or grade of DR. Importantly, AGEs are significantly increased in diabetic pre-pubescent children and adolescents with early or pre-proliferative retinopathy compared to both healthy and diabetic controls who are free from clinical signs of retinopathy.(Chiarelli et al. 1999) While many of the studies measured non-specific AGE moieties, others evaluated the associations between defined adducts, such as CML, pentosidine, or crossline,(Sugiyama et al. 1998; Yamaguchi et al. 1998). There are also studies that reported no correlation between AGE levels and retinopathy in diabetic patients. (Sugiyama et al. 1998; Wagner et al. 2001) The apparent inconsistency with other studies may be due to the variations in patient populations and/or the non-uniform assays for AGE quantification.
AGEs have a wide range of deleterious effects and play a role in initiation and progression of DR. In diabetic patients AGEs and/or late Amadori products have been demonstrated to directly accumulate in retinal pericytes, vessels, neuroglia, etc. Crosslinked AGEs are a significant feature of extracellular matrix dysfunction during diabetes progression.(Gardiner et al. 2003; Hammes et al. 1999a; Hammes et al. 1994; Murata et al. 1997; Schalkwijk et al. 1999; Stitt et al. 1997)
In vivo, retinal pericytes are surrounded by vascular basement membrane and lie outside the inner blood-retina barrier (iBRB) and it is shown that retinal pericytes have a much lower replicative capacity than retinal microvascular endothelium.(Sharma et al. 1985) Toxic AGEs accumulate in retinal pericytes in diabetic animal models.(Chibber et al. 1997; Kalfa et al. 1995; Ruggiero-Lopez et al. 1997; Stitt et al. 1997) In vitro, pericytes grown on a “diabetic-like” AGE-modified basement membrane induces pericyte dysfunction and apoptotic death.(Stitt et al. 2004) Similarly, AGEs accumulation has been reported to have a detrimental influence on the cell function and survival ability of the retinal pericytes. This includes impaired phospholipid hydrolysis and phospholipid enzyme inhibition(Assero et al. 2001) or modification of the antioxidant enzymes catalase and superoxide dismutase.(Paget et al. 1998) Studies also demonstrated that AGEs can induce osteoblastic differentiation, calcification,(Yamagishi et al. 1999) and potent apoptotic death in pericytes.(Yamagishi et al. 2002) These observations are consistent with the pathology of DR. For example, in diabetes retinal endothelial cells and pericytes undergo accelerated apoptosis, and this is related to the development of acellular capillaries and pericyte ghosts in the retinal microvasculature, which are preclinical signs of DR.(Kern et al. 2000; Mizutani et al. 1996) In addition to direct biochemical effects, it appears that AGE-related toxicity in retinal pericytes acts in a receptor mediated fashion.(Chibber et al. 1997) For example, a growing body of evidence indicates that AGEs-RAGE (receptor for AGEs) interaction-mediated oxidative stress generation plays an important role in DR.(Yamagishi et al. 2008)
Exposure to AGEs results in several deleterious effects on the retinal vessels, including increasing vasopermeability, neovascularization, and evoking proinflammatory pathways, etc. For example, in vitro and in vivo studies showed that exposure to AGEs causes significant upregulation of vascular endothelial growth factor (VEGF),(Lu et al. 1998; Stitt et al. 2000; Treins et al. 2001; Yamagishi et al. 2002) which can also be induced by a variety of stimuli, such as PKC (Fig. 7) and HIF (Fig. 9b), to increase vascular permeability and induce DR-related neovascularization. Being a patho-physiological hallmark of DR, the direct damage of excessive vasopermeability to the retinal microvasculature is iBRB dysfunction.(Antonetti et al. 1999) Even, in nondiabetic rats AGEs compromise the retinal capillary unit leading to subtle but significant breakdown of the iBRB with a concomitant increase in intracellular adhesion molecule-1.(Moore et al. 2003; Stitt et al. 2000) The increased levels of adhesion molecules on the surface of retinal microvascular endothelial cells can activate proinflammatory pathways. In conjunction with an enhanced stickiness and reduced deformability of blood-borne leukocytes in the diabetic state, this can lead to a marked leukocyte adhesion to retinal vascular endothelium that precipitates capillary occlusion, vascular cell death and finally diabetic retinopathy.(Kunt et al. 1998; Mamputu & Renier 2004; Miyamoto & Ogura 1999; Moore et al. 2003)
Fig. 7. Hyperglycemic PKC pathway.
The pathogenic consequences of hyperglycemic PKC through activating transcription factors for a wide range of proteins, including cytokines. Many transcription factors, such as NF-κB, are activated through hyperglycemia-induced PKC activation, resulting in oxidatives stress, increased vaso-permeability, angiogenesis, vascular occlusion, capillary occlusion, and abnormal blood flow, etc.
Interestingly, clinical observations indicate that development and progression of hyperglycemia-induced microvascular and macrovascular complications continues for many years after consistent improvement in HbA1c. This is referred to as “hyperglycemic memory.”(Brownlee 1992; Yamagishi et al. 2008) The persistence of accumulated AGEs during periods of normal glucose homeostasis is considered to be the best explanation for this phenomenon.
The importance of AGEs in DR can also be seen from several novel therapeutics of DR. These include peroxisome proliferactor–activated receptor (PPAR) agonist, blockade of the renin-angiotensin system with an angiotensin converting enzyme inhibitor or by using angiotensin II type 1–receptor blockers, and intravitreal anti-VEGF antibody administration,(Simó & Hernández 2009) all of which have been at least indirectly related to AGEs.(Yamagishi et al. 2008)
4.2.1.2. Hyperglycemic AGE pathway and AMD
Among indicators of AMD is elevated levels of immunoreactive AGEs in Bruch’s membrane and drusen.(Farboud et al. 1999; Glenn & Stitt 2009; Hammes et al. 1999b; Handa 1998a; Handa et al. 1999; Hollyfield et al. 2003; Howes et al. 2004; Ishibashi et al. 1998; Schutt et al. 2003; Yamada et al. 2006) In Bruch’s membrane this leads to the progressive thickening and compromised permeability of the membrane.(Moore et al. 1995; Okubo et al. 1999; Okubo et al. 2000) Additional evidence of toxicity of AGEs is the finding that some of the components of drusen, such as lipids, tissue inhibitor of metalloproteinases 3, clusterin, serum albumin, apolipoprotein E, amyloid, and vitronectin,(Hageman et al. 1999; Hollyfield et al. 2003; Mullins et al. 2000) are readily modified by AGEs and/or ALEs during aging.(Hammes et al. 1996; Li & Dickson 1997; Schutt et al. 2003; Tabaton et al. 1997) Because accumulation of AGEs plays an important role in AMD pathogenesis, recently studies have tried to use the fluorescent property of AGE adducts to develop non-invasive predictors for AMD.(Mulder et al. 2010; Pawlak et al. 2008)
AGEs influence the physiological functions of RPE. For example, in vitro AGEs induce RPE to up-regulate the expression of VEGF and platelet-derived growth factor-B (PDGF-B), both of which are important regulators in angiogenesis.(Handa 1998a; Lu et al. 1998; McFarlane et al. 2005) Prolonged exposure of RPE to AGEs or AGE-forming dicarbonyls induces changes in intracellular pH, maintenance of the choriocapillaris, and integrity of the RPE/photoreceptor complex. These dysfunctions may finally lead to apoptotic death in RPE.(Stitt 2005)
As a part of cellular defense systems, some AGEs are transported through a receptor-mediated pathway to the lysosomal compartment for degradation. However, some AGEs escape degradation. AGEs are also substrates for degradation via the ubiquitin (Ub) proteasome pathway. Intracellular accumulation of highly reactive AGE adducts can markedly reduce degradative enzymatic activity,(Kasper et al. 1999; Miyata et al. 1997; Queisser et al. 2010; Sebeková et al. 1998) i.e. the lysosomal and Ub proteasomal degradation systems are also vulnerable to AGEs. This may lead to a reduction of intracellular proteolytic capacity and incomplete proteolysis of phagocytosed photoreceptor outer segments resulting in the accumulation of lipofuscin in the RPE.(Boulton et al. 1989) Significantly, intracellular sequestration of these highly reactive adducts can markedly reduce degradative enzymatic activity in many types of epithelial cells.(Kasper et al. 1999; Miyata et al. 1997; Sebeková et al. 1998; Stitt 2005)
Additional protective mechanisms involve a range of intracellular detoxifying enzymes against reactive dicarbonyls, such as GO and MGO (Fig. 5). These detoxifying enzymes serve to limit advanced adduct formation. For example, a glutathione (GSH)-dependent glyoxalase complex has been found to serve as an effective detoxification system for GO and MGO.(Kuhla et al. 2005) It is interesting to note that this enzyme activity declines with aging and overexpression of this enzyme decreases the accumulation of MGO-derived AGEs in cells and elongate lifespan in Caenorhabditis elegans.(Morcos et al. 2008; Shinohara et al. 1998) It has also been demonstrated that upregulation of glyoxalase-1 can reverse high-glucose mediated AGE formation over a short, 10-day period and prevent AGE-mediated cell abnormalities.(Shinohara et al. 1998) Therefore, alterations in these enzymes during disease may result in AGE accumulation and pathogenic damage in cells and tissues.(Miyata et al. 2001; Thornalley 1993; Thornalley 2003)
Fig. 5. Hyperglycemic AGE pathway.
The hyperglycemia-induced intracellular AGE precursors, such as MGO, induce pathological consequences in four routes, 1) direct intracellular glycation of proteins, including proteins involved in the regulation of gene transcription, such as NF-κB, 2) inhibiting enzymes responsible for protein degradation, such as proteasomal (including ubiquitin) and lysosomal systems, 3) the intracellular AGEs precursors can diffusing out of the cell and modify nearby cells (even the same cell itself), extracellular matrix, such as Bruch’s membrane and choroidal capillary membranes, and 4) the intracellular AGEs precursors diffusing out of the cell to modify circulating proteins in the blood, which in turn activate RAGE on pro-inflammatory cells or CECs, thereby causing the production of inflammatory cytokines and/or growth factors.
In addition to the intracellular detoxifying enzymes, cells have several complex receptor systems that are responsible for removing senescent, glycation-modified molecules and/or degrading existing AGEs cross-links from cells and tissues. Several AGE-binding molecules have been described, such as the receptor for AGEs (RAGE),(Schmidt et al. 1994) AGE-R1,(Li et al. 1996; Stitt et al. 1999) galectin-3,(Pugliese et al. 2001; Stitt et al. 2005) CD36,(Ohgami et al. 2002) and the type I and II scavenger receptor.(Horiuchi et al. 1996) These cell surface receptors interact with AGEs to maintain homeostatic function by clearing/detoxifying extracellular AGE-modified macromolecules from serum and the intercellular matrix. Among them, RAGE is the best characterized. It was first identified in endothelium and now is known to be present in multiple vascular, neural and cardiac tissues (Neeper et al. 1992). The RAGE is a member of the immunoglobulin super-family with a high affinity for several ligands, including AGEs, high mobility group-1 protein, amyloid-β peptide, and S100B/calgranulins, some of which are known components of drusen and Bruch’s membrane deposits.
On the other hand, despite of the protective role of AGE receptors it is thought that many of the adverse effects caused by AGEs are mediated via AGE receptors and that these receptors play a critical role in AGE-related pathbiology associated with diabetes and aging disorders.(Sano et al. 1999; Schmidt et al. 2000; Vlassara 2001) For example, activation of the RAGE evokes downstream pro-inflammatory responses that could play a critical role in aging,(Yan et al. 2007) such as skin ageing (Lohwasser et al. 2006), and age-related diseases (Schmidt et al. 2000; Yan et al. 2007), such as Alzheimer’s disease,(Takeuchi & Yamagishi 2008) atherosclerosis,(Ehlermann et al. 2006), dysfunction of cardiomyocytes (Gao et al. 2008), and retinal diseases(Stitt et al. 2005). The RAGE-ligand signals activate the wide range of patho-physiological responses linked to downstream transcriptional activity of NF-κB (Fig. 5), which induces pro-inflammatory cytokines and oxidative stress.(Bierhaus et al. 2005.; Pawlak et al. 2008)
In the context of the outer retina, studies have showed that RAGE is expressed on RPE and that RAGE levels are significantly increased in AMD (in postmortem tissue), especially on cells adjacent to drusen.(Howes et al. 2004; Pawlak et al. 2008; Yamada et al. 2006) Activation of the RAGE axis in RPE cells up-regulates the expression and secretion of VEGF. (Glenn et al. 2009; Glenn & Stitt 2009; Ma et al. 2007; Yamada et al. 2006) This can elicit or propagate neovascularization. For example, exogenous AGE-albumin and S100B can activate RAGE and modulate pro-angiogenic VEGF expression in RPE,(Justilien et al. 2007.) and, with prolonged exposure, these ligands may lead to apoptosis.(Howes et al. 2004) In vitro, in addition to RAGE,(Ma et al. 2007) an increase of VEGF expression in RPE can also be modulated by another AGE receptor known as galectin-3.(McFarlane et al. 2005) Interestingly, the proteolytic fragment of RAGE, known as soluble RAGE is elevated in serum of elderly kidney disease patients and is associated with decreased glomerular filtration rate (Semba et al. 2009). The AGE-RAGE-NF-κB signal casade in both RPE and photoreceptor cells has also been shown to contribute to the disease progression of early AMD and GA.(Howes et al. 2004) Consistently, suppression of RAGE signaling using peptide analogues or neutralizing antibodies can prevent key pathological events in a range of cells and tissues.(Schmidt et al. 2000; Wautier & Schmidt 2004.)
As summarized in Fig. 5, the intracellular AGEs precursors can damage cells by four routes described below. First, AGE accumulation in RPE can appear as free AGE adducts in the cytoplasm and as AGE-modified proteins in lipofuscin granules.(Schutt et al. 2003) While some cytoplasm AGE-modified transducer proteins can affect the activity of downstream transcriptional factors, others are transported through a receptor-mediated transportation to the lysosomal compartment for degradation. However, because AGEs also compromise proteolytic capacity, the accumulation of lipofuscin in RPE reflects the effects of AGEs on both substrates and enzyme degradation systems.(Stitt 2001) Indeed, incomplete proteolysis of phagocytosed photoreceptor outer segments is linked to the formation of lipofuscin in RPE(Boulton et al. 1989) and it has been shown that AGEs play an important role in the formation of age-related intracellular fluorophores and lipofuscin granules in postmitotic epithelial cells.(Yin 1996)
A special case is that, when the detoxification enzymes per se become vulnerable to their substrates, the situation may be exacerbated. For example, recent studies show that in microvascular endothelial cells of the retina hyperglycemia-induced intracellular MGO reduces levels of the polyubiquitin receptor 19S and decreases the chymotrypsin-like activity of the proteasome and cause polyubiquitinated proteins to accumulate in the cell. This may result in the decline of proteasomal activities over time.(Queisser et al. 2010) In addition, our recent work also showed that consuming high-GI diets causes accumulation of AGEs in RPE and the AGE-modified intracellular proteins become resistant to the degradation by Ub-dependent proteasome system.(Uchiki et al., unpublished) Furthermore, RPE grown on an AGE-modified substrate, show enhanced accumulation of lipofuscin which contributes to suppression of lysosomal enzymatic activity.(Glenn et al. 2009; Glenn & Stitt 2009) The compromised lysosomal enzymatic activity could also account, at least partially, for the age-related RPE dysfunction resulting in the pathological accumulation of AGE cross-links on Bruch’s membrane, described below.
In addition to affecting RPE and Bruch’s membrane, AGEs also occur at comparatively high levels in CNV membranes(Swamy-Mruthinti et al. 2002) where they may play a role in fibrous membrane formation by induction of growth factors, such as transforming growth factor beta (TGF-β ) and PDGF.(Handa 1998a; Rumble et al. 1997) For example, CML has been shown to promote CNV formation in cultured choroidal explants from aged rats via stimulation of growth factors such as vascular endothelial growth factor (VEGF), tumor necrosis factor α (TNF-α) and PDGF-B.(Kobayashi et al. 2007)
The intracellular AGEs precursors can diffuse out of the RPE cell and modify nearby RPE cells (even the same RPE cell itself) and extracellular matrix, such as Bruch’s membrane and choroidal capillary membranes.(Glenn et al. 2009; Glenn & Stitt 2009) In turn, these alterations may compromise cell-matrix signaling (e.g. integrin-laminin between RPE-Bruch’s membrane) and cause physiological dysfunction.(Aisenbrey et al. 2006; Charonis et al. 1990; Fang et al. 2009) Furthermore, dysfunction in the RPE can affect Bruch’s membrane’s function per se, and vice versa, and such disfunction leaves the neural retina vulnerable because all metabolic exchange between the neural retina and choroidal plexus requires passage through the oBRB.
Intracellular AGEs precursors can also diffuse out of the cell to modify circulating proteins in the blood, which can then bind to RAGE on pro-inflammatory cells or choroidal endothelial cells (CEC) to activate them, thereby causing the production of inflammatory cytokines and/or growth factors with associated vascular pathology (Fig. 5).(Abordo & Thornalley 1997; Doi et al. 1992; Kirstein et al. 1992; Li et al. 1996; Neeper et al. 1992; Schmidt et al. 1995; Skolnik et al. 1991; Smedsrod et al. 1997; Vlassara et al. 1988; Vlassara et al. 1995) Furthermore, by analogy with the angiogenesis-promoting abilities of AGEs in diabetic RNV,(Hoffmann et al. 2002; Ishibashi 2000) AGEs have been hypothesized to be involved in the process of CNV formation. Indeed, studies have showed that AGEs accumulate significantly in the choriocapillaris during aging [(Handa 1998b) and are highly expressed in CNV membranes.(Hammes et al. 1999b; Ishibashi et al. 1998) These AGE accumulations can stimulate CEC proliferation, matrix metalloproteinase 2 secretion and VEGF up-regulation and are important promoters of CNV in exudative AMD in vivo.(Hoffmann et al. 2002; Howes et al. 2004; Ma et al. 2007; Yamada et al. 2006) Together, these data indicate that the choriocapillaris is subject to damage from two different sources of AGEs, i.e., one from cell-matrix communication and the other coming from the blood circulation.
4.2.1.3. Advanced lipoxidation end products (ALEs) in retina
Lipids are another important source of chemical modifications of proteins, especially in the lipid-rich and highly oxidative environments in the retina.(Stitt et al. 2005) Lipid peroxidation products also form Maillard products called advanced lipoxidation end products (ALEs).(Onorato et al. 2000) It is not surprising that there is an interesting similarity between fatty acids and glucose (i.e. between ALEs and AGEs) in terms of age-related pathogenesis. Indeed, dyslipidemia, including hyperlipidemia, has been considered as a risk factor for AMD.(Tan et al. 2007) The formation of ALEs through lipid peroxidation reactions may, at least partially, account for the underlying pathogenesis.
The outer retina is rich in polyunsaturated fatty acids (PUFAs), such as DHA.(Bazan 1982.; SanGiovanni & Chew 2005) PUFAs are highly susceptible to lipid peroxidation and this process yields lipid hydroperoxides, which in turn decompose into reactive aldehydes, such as acrolein, 4-hydroxynonenal, or malondialdehyde. Like reactive dicarbonyls derived from glucose, these reactive aldehydes can react with proteins to form stable ALE adducts.(Januszewski et al. 2003.)
ALEs add to the burden of protein modifications in the aging retina. For example, studies have shown that various ALEs can induce pro-angiogenic growth factor expression by RPE in vitro.(Glenn & Stitt 2009; Zhou et al. 2005)
Interestingly, studies indicate that higher intake of DHA and EPA reduces the risk for AMD(SanGiovanni & Chew 2005; SanGiovanni et al. 2008) and that the protective effect may be through modulating postprandial hyperlipidaemia, which is a physiological consequence after consuming a high-GI diet.(Anil 2007) Importantly, recently epidemiological observations also indicate that a diet high in DHA/EPA and low in GI offers a synergistic protection against AMD progression probably because the diet helps to eliminate the additive deleterious effects from AGEs and ALEs.(Chiu et al. 2009a; Chiu et al. 2009b)
4.2.2. Hyperglycemic polyol pathway
Dietary hyperglycemia may manifest age-related or diabetic disorders through increases in the polyol pathway (Fig. 6), which in turn lead to intracellular accumulation of sorbitol and oxidative stress. However, flux through this pathway during hyperglycemia varies from 33% of total glucose use in the rabbit lens to 11% in human erythrocytes. Therefore, the contribution of this pathway to age-related or diabetic disorders may be very much species, site and tissue dependent.(Brownlee 2001) The polyol pathway is primarily controlled by the enzyme aldose reductase (AR). Under euglycemia, AR can reduce toxic aldehydes in the cell to inactive alcohols, but when the glucose concentration in the cell becomes too high, AR also reduces that glucose to sorbitol (a polyol or sugar alcohol), which is later oxidized to fructose. In the process of reducing high intracellular glucose to sorbitol, the AR consumes the cofactor NADPH.(Lee & Chung 1999) However, as shown in Fig. 6, NADPH is also the essential cofactor for regenerating a critical intracellular antioxidant, reduced glutathione (GSH). By competing NADPH with glutathione reductase and hence resulting in reduced amount of GSH, the polyol pathway increases susceptibility to intracellular oxidative stress. Some studies also link this pathway to antioxidant taurine through an inhibitory effect on its Na+-taurine cotransporter (TT).(Hansen 2001; Nakashima et al. 2005; Obrosova et al. 2001.; Pop-Busui et al. 1999.; Stevens et al. 1997; Stevens et al. 1999.; Stevens et al. 1997.)
Fig. 6. Hyperglycemic polyol pathway.
Under hyperglycemia, AR reduces glucose to sorbitol (a polyol or sugar alcohol), which is later oxidized to fructose. In this process, the AR consumes cofactor NADPH. Therefore, the hyperglycemic polyol pathway consumes NADPH and hence results in the depletion of GSH. This increases intracellular oxidative stress.
In vascular smooth muscle cells isolated from rat aorta, AR was also found to affect PKC activation (see 4.2.3. Hyperglycemic PKC pathway for PKC activation).(Ramana et al. 2005) It was shown that inhibition of AR prevents membrane translocation (PKC-β 2 and -δ) and phosphorylation (PKC-β 1 and -ε) of multiple PKC enzymes by inhibiting high glucose-induced generation of diacylglycerol (DAG) from phospholipid hydrolysis.
4.2.2.1. Hyperglycemic polyol pathway and DR
In diabetes, elevated expression of AR may impair antioxidant defense, which may determine tissue susceptibility to chronic diabetic complications. Thus, increased expression of AR has been implicated as the critical link between chronic glucose toxicity and tissue damage.(Burg & Kador 1988.; Dent et al. 1991.; Kasajima 2000.; Nakashima et al. 2005; Shah et al. 1997.; Stevens et al. 1997; Vinores et al. 1988) Although this pathway may play a minor role in retinopathies, there is also evidence linking AR activity with retinal capillary basement membrane thickening in galactosemic rats, suggesting a possible role in DR.(Vinores et al. 1988) For example, electron microscopic immunocytochemical staining suggested that increased AR expression in retinal vascular endothelial cells and perivascular astrocytes is associated with hyperglycemia-related iBRB failure, which may finally lead to DR.(Vinores et al. 1993a; Vinores et al. 1993b)
4.2.2.2. Hyperglycemic polyol pathway and AMD
Human RPE cells contain two NADPH-dependent reductases, AR and aldehyde reductase with AR being the predominant reductase, because the levels of aldehyde reductase are insufficient to generate sugar alcohols (e.g. sorbitol).(Sato et al. 1993) In RPE cells, it is suggested that hyperglycemia up-regulates AR gene expression, protein production and activity,(Henry et al. 2000) and that some hyperglycemia-related ultrastructural changes can be prevented by AR inhibitor, Sorbinil.(Vinores & Campochiaro 1989) Furthermore, hyperglycemia also induces loss of Na+/K(+)-ATPase function in RPE cells, which affects the response to AR inhibitors and results in chronic accumulation of intracellular sorbitol (see Fig. 6 and 4.2.2.2. Hyperglycemic polyol pathway and AMD).(Crider et al. 1997)
The precise patho-physiological mechanism linking polyol pathway to AMD remains uncertain, but depletion of the osmolyte and antioxidant taurine has been invoked(Obrosova et al. 2001.; Pop-Busui et al. 1999.; Stevens et al. 1997; Stevens et al. 1997.) through an inhibitory effect on its Na+-taurine cotransporter (TT).(Nakashima et al. 2005; Stevens et al. 1997; Stevens et al. 1999.; Stevens et al. 1997.) Indeed, it is found that in RPE cells the TT is regulated by oxidative stress and that over-expression of AR and hyperglycemia impair this response (Fig. 6).
4.2.3. Hyperglycemic PKC pathway
Breakdown of the blood-retinal barrier (BRB) occurs in several retinal diseases and is a major cause of visual loss. Hyperglycemia-induced PKC activation may lead to BRB breakdown. For example, it has been shown that in RPE cells hyperglycemia induces protein kinase C (PKC) activation, which increases expression of a variety of genes and further leads to a series of adverse effects. Since RPE forms the oBRB, the breakdown of the oBRB due to the disruption of the RPE tight junctions may lead to the development of AMD.(Erickson et al. 2007) Conversely, inhibition of PKC decreases breakdown of the iBRB and prevents early changes in the diabetic retina.(Bishara et al. 2002; Brownlee 2005; Ishii et al. 1996; Koya et al. 2000; Saishin et al.)
PKC also affects the expression of gap junction proteins, connexins, which are critical for intercellular communication between RPE cells.(Malfait et al. 2001) Many correlations between gap junctional intercellular communication and cellular processes, such as cellular growth control, cell differentiation, regulation of development, tissue homeostasis, etc., have been described.(Goodenough et al. 1996)
As shown schematically in Fig. 7, intracellular hyperglycemia increases the synthesis of DAG, a critical activating cofactor for the isoforms of PKC, -β, -δ, and -α.(DeRubertis & Craven 1994; Koya et al. 1997; Koya & King 1998; Xia et al. 1994) In turn, PKC activation activates transcriptional factors, such as NF-κB, increasing expression of a variety of genes, which in turn give rise to physiological dysfunction. For example, in endothelial cells hyperglycemia induces PKC-β activation, which inhibits the expression of endothelial nitric oxide synthase (eNOS) while increasing the expression of vasoconstrictor endothelin-1 (ET-1).(Ishii et al. 1996) This results in reduced blood flow. PKC-β activation also increases the expression of vascular endothelial growth factor (VEGF) and plasminogen activator inhibitor-1 (PAI-1), which lead to angiogenesis and vascular occlusion, respectively.(Feener et al. 1996; Ishii et al. 1996; Koya et al. 1997; Kuboki et al. 2000; Studer et al. 1993) In the following two sections (4.2.3.1.~ 4.2.3.2.), we will focus on the effects of hyperglycemia-induced PKC activation on VEGF, which is of interest for DR and AMD from therapeutic point of view.(Abdallah & Fawzi 2009; Bressler 2009a; Bressler 2009b; Jardeleza & Miller 2009)
Hyperglycemia-induced PKC activation also increases the expression of transforming growth factor-β (TGF-β ) and contributes to capillary occlusion, probably through inhibition of nitric oxide (NO) production.(Craven et al. 1997) Furthermore, hyperglycemia-induced PKC activation has also been implicated in the activation of membrane-associated NAD(P)H-dependent oxidase, which may increase the generation of ROS.(Brownlee 2001) In addition to diacylglycerol-related pathway, hyperglycemia may also activate the PKC pathway indirectly through ligation of AGE receptors(Portilla et al. 2000) (Fig. 5), increased flux of the polyol pathway(Keogh et al. 1997)(Fig. 6), and increased flux of the hexosamine pathway(Goldberg et al. 2002) (Fig. 8).
Fig. 8. Hyperglycemic hexosamine pathway.
The hyperglycemic hexosamine pathway starting from the glycolytic intermediate, F-6-P, which is converted by GFAT to glucosamine-6-P and eventually to UDPGlcNAc, an O-linked GlcNAc. Intracellular glycosylation by adding GlcNAc moieties to serine and threonine residues of proteins (e.g. transcription factors) is catalysed by OGT. Increased glycosylation of transcription factors, such as Sp1, AP2 and CREB, often at phosphorylation sites, increases the expression of cytokines and enzymes, including TGF- β 1, PAI-1, and glycosyltransferase. In addition, AGEs can exert cellular effects by increasing a-series ganglioside levels to inhibit retinal pericyte cell proliferation. Other cytoplasmic proteins are also subjects to dynamical modification by hyperglycemia-induced O-linked GlcNAc, such as the inhibition of eNOS activity by O-acetylglucosaminylation at the Akt site of the eNOS protein and activations of various PKC isoforms by glucosamine without membrane translocation.
4.2.3.1. Hyperglycemic PKC pathway and DR
VEGF, a potent endothelial cell mitogen and permeability factor, has been implicated as a cause of iBRB breakdown and angiogenesis in DR and other ischemic retinopathies.(Aiello et al. 1994; Aiello et al. 1997) In retinal endothelial cells and pericytes, high glucose causes activation of PKC-β and consequent expression of VEGF contributing to the progression of DR. (Clarke & Dodson 2007; Enaida et al. 1999; Hata et al. 1999) It is also suggested that RPE cells may contribute to the pathogenesis of DR caused by hyperglycemia (Fig. 7) and hypoxia (Fig. 4b) through the PKC-mediated expression of VEGF (see 4.2.6. Hypoxia-inducible factor (HIF) pathway).(Young et al. 2005)
4.2.3.2. Hyperglycemic PKC pathway and AMD
In RPE cells, VEGF is expressed in response to mechanical stretch,(Seko et al. 1999.) hypoxia(Mousa et al. 1999.) and high glucose(Sone et al. 1996.), and may be mediated by PKC activation.(Young et al. 2005) This is corroborated by the observation that inhibition of the PKC pathway using a mixture of ethanol extracts from herbal medicines inhibits high glucose or AGEs-induced VEGF expression in human RPE.(Kim et al. 2007) Interestingly, it is well known that a number of cytokines and VEGF that are synthesized by RPE cells can exert autocrine function in addition to stimulating other cell types. Indeed, VEGF receptors are expressed on the surface of RPE cell itself and increased expression of VEGF in the RPE in maculae is involved in AMD.(Kociok et al. 1998) It is also noted that VEGF expression and secretion by RPE in hyperglycemia and hypoxia are PKC-dependent and the regulation appears to be more complicated than in hyperglycemia alone (see 4.2.6. Hypoxia-inducible factor (HIF) pathway).(Young et al. 2005)
4.2.4. Hyperglycemic hexosamine pathway
As shown schematically in Fig. 8, glucose is metabolized through glycolysis, going first to glucose-6 phosphate (G-6-P), then fructose-6 phosphate (F-6-P), and then on through the rest of the glycolytic pathway. However, when glucose is high inside a cell, some of that F-6-P gets diverted into the hexosamine pathway in which glutamine:fructose-6 phosphate amidotransferase (GFAT) converts the F-6-P to glucosamine-6 phosphate and finally to uridine diphosphate N-acetyl glucosamine (UDPGlcNAc). Studies showed that inhibition of GFAT blocks hyperglycemia-induced increases in the transcription of TGF-α, TGF-β 1 (Kolm-Litty et al. 1998) and PAI-1(Du et al. 2000). It is suggested that, in the hexosamine pathway, the hyperglycemia-induced increases in gene transcription may be through glycosylation of the transcription factor, Sp1, by UDPGlcNAc.(Brownlee 2001) Furthermore, the glycosylated form of Sp1 seems to be more transcriptionally active than the deglycosylated form.(Kadonaga et al. 1988) However, because every RNA polymerase II transcription factor has been found to be O-acetylglucosaminylated,(Hart 1997) it is possible that reciprocal modification by O-acetylglucosaminylation (O-GlcNAcylation) and phosphorylation of transcription factors other than Sp1 may function as a more generalized mechanism for regulating hyperglycemia-induced gene transcriptions in the hexosamine pathway.(Brownlee 2001; Wang et al. 2007) Indeed, it has been shown that, in addition to Sp1, AP2 and CREB operate on the promoters of glycosyltransferases genes, which regulate the ganglioside biosynthetic pathway (see 4.2.4.1. Hexosamine pathway and DR for more details).(Zeng & Yu 2008) Many other nuclear and cytoplasmic proteins are also subject to dynamic modification by O-linked GlcNAc, and may show reciprocal modification by phosphorylation in a manner analogous to Sp1.(Hart 1997) For example, the eNOS activity can be inhibited by hyperglycemia-induced O-acetylglucosaminylation at the Akt site of the eNOS protein(Du et al. 2001). Additionally, various PKC isoforms (-β I and -δ) can be activated by glucosamine without membrane translocation(Goldberg et al. 2002).
4.2.4.1. Hyperglycemic hexosamine pathway and DR
It is known that during the early stages of DR, there is significant death of the retinal microvascular pericytes. For example, it is shown that gangliosides in retinal pericytes are increased in response to the increase flux of the hexosamine pathway and are involved in the anti-proliferative effect of glucosamine.(Masson et al. 2005b) In addition, AGEs can increase a-series ganglioside (GM3, GM2, GM1, GD1a) levels to inhibit bovine retinal pericyte cell proliferation. The possible mechanism could involve an increase in GM3 synthase activity (Fig. 8)(Masson et al. 2005a).
Gangliosides play additional functions, including cellular recognition and adhesion as well as signaling. The expression of gangliosides is not only cell specific and developmentally regulated but also closely related to the differentiation state of the cell.(Yu et al. 2004) In general, ganglioside biosynthesis starts with the common precursor for acidic and nonacidic glycosphingolipids, ceramide (Fig. 8). Ganglioside synthases are glycosyltransferases involved in the biosynthesis of glycoconjugates in the ganglioside biosynthetic pathway. The transcription of glycosyltransferases genes is subject to complex developmental and tissue-specific regulation. The promoters of glycosyltransferases genes are characteristic of house-keeping genes, including TATA-less and lacking a CCAAT box but containing GC-rich boxes. It has been shown that a set of cis-acting elements and transcription factors, including Sp1, AP2, and CREB, operate in the proximal promoters (Fig. 8).(Zeng & Yu 2008) Hyperglycemia-induced increased transcription of glycosyltransferases may result in increased synthesis of gangliosides, which in turn inhibits retinal pericyte cell proliferation and leads to the development of early DR. (Masson et al. 2005a; Masson et al. 2005b)
4.2.4.2. Hyperglycemic hexosamine pathway and AMD
In macula-derivedRPE cells taken from fresh human donor, there are age-related decreases in the activity of N-acetyl-beta-glucosaminidase, an enzyme which is responsible for the degradation of N-acetyl-glucosamine (GlcNAc). Because GlcNAc is the major carbohydrate monomer of the oligosaccaride chains of human rhodopsin, defects in its degradation may lead to the accumulation of undigested residual material in the RPE.(Cingle et al. 1996) Indeed, N-acetyl glucosamine is observed in drusen, RPE, Bruch’s membrane, and photoreceptors in eyes with AMD.(D’Souza et al. 2009) In keeping with Farkas’ observation that drusen are derived from degenerating RPE cells containing abundant photoreceptor remnants, (Farkas et al. 1971) this observation supports the hypothesis that the pathogenesis of drusen is a combined mechanism, involving photoreceptors, Bruch’s membrane, and RPE, and the hexosamine pathway plays a role in AMD pathogenesis.
4.2.5. Hyperglycemic mitochondria-derived ROS
There are four protein complexes in the mitochondrial ETC, including complex I, II, III, and IV. Under euglycemic and normoxic conditions (left panel of Fig. 4a), after pyruvate is metabolized through the TCA cycle, it generates electrons that are passed to coenzyme Q through complexes I and II, and then transferred to complex III, cytochrome-C (Cyt c), complex IV, and finally to molecular oxygen (O2), which is then reduced to water. While electrons are transported from left to right in the left panel of Fig. 4a, some of the energy of those electrons is used to pump protons across the membrane at complexes I, III, and IV to generate a voltage potential across the mitochondrial membrane. The energy from this voltage gradient drives the synthesis of ATP by ATP synthase.(Trumpower 1990; Wallace 1992) Regulation of the rate of ATP generation is achieved in part by uncoupling proteins (UCPs) that can dissipate the voltage gradient to generate heat.
However, under hyperglycemic normoxic conditions (right panel of Fig. 4a), more glucose is oxidized. This pushes more electron donors into the ETC. When the voltage gradient across the mitochondrial membrane increases to a critical threshold, transfer in complex III is blocked,(Korshunov et al. 1997) causing the electrons to accumulate in coenzyme Q. This allows coenzyme Q to donate the electrons one at a time to O2, thereby generating superoxide (O2−). The cell defends itself against this ROS using the mitochondrial isoform of superoxide dismutase (Mn-SOD). This enzyme degrades the oxygen free radical to hydrogen peroxide, which is then converted to H2O and O2 by catalase.
4.2.5.1. Hyperglycemic mitochondria-derived ROS and DR
Brownlee hypothesizes that the hyperglycemia-induced mitochondrial overproduction of ROS (i.e. the O2− described above) may activate poly(ADP-ribose) polymerase (PARP), a DNA repair enzyme which is only found in the nucleus and needs GAPDH as a cofactor.(Sawa et al. 1997; Schmidtz 2001) By competing with glycolysis for GAPDH, the ROS-activated PARP impedes the glycolytic pathway and increases the level of all the glycolytic intermediates that are upstream of GAPDH (Fig. 4a). This exacerbates the deleterious effects of the four glycolysis-associated pathways (see sections 4.2.1.~4.2.4.) that are induced by hyperglycemia.(Brownlee 2005) This unifying hypothesis of hyperglycemia-induced microvascular damage suggests that hyperglycemia-induced mitochondrial overproduction of ROS is the major culprit in the pathogenesis of DR.(Brownlee 2001; Brownlee 2005; Hammes 2005) In support of this theory, it has been shown that hyperglycemia increases superoxide production in mitochondria and therapies that inhibit such superoxide production prevent the development of DR.(Du et al. 2003; Kanwar et al. 2007)
Studies have shown that hyperglycemia-induced GAPDH nuclear translocation and accumulation participates in the development of various degenerative diseases. This is because nuclear translocation of GAPDH induces the formation of oxidative stress and production of pro-inflammatory stimuli, such as nitric oxide (NO) and cytokines.(Chuang et al. 2005; Kanwar & Kowluru 2009) These are associated with increased risk for DR. Translocation of GAPDH from the cytosol to the nucleus is a critical step in the induction of apoptosis in neuronal cells, such as Müller cells.(Kusner et al. 2004) The mechanism underlying the movement of GAPDH from the cytosol to the nucleus under high glucose conditions involves the E3 ubiquitin (Ub) ligase siah-1, which facilitates hyperglycemia-induced GAPDH nuclear translocation via formation of a complex with GAPDH (Fig. 4a).(Yego & Mohr 2010)
Overproduction of ROS may also result in impaired antioxidant defense enzymes, oxidatively modified DNA, and nitrosylated proteins. Furthermore, the mitochondria become dysfunctional because the proapoptotic protein, Bax, translocates from the cytosol into the mitochondria. This results in Cyt c leaking out from the mitochondria. This is accompanied by increased retinal capillary cell apoptosis, and the formation of acellular capillaries and pericyte ghosts, early signs of DR.(Kowluru 2005)
The cellular antioxidant response element is important for the amelioration of oxidative stress. It responds to hyperglycemia and can be used to evaluate the complications of diabetes mellitus. It has been shown that in retinal endothelial cells the induction of mitochondrial oxidative stress is more sensitive to hyperglycemia than the induction of the antioxidant response element. In other words, it appears that endothelial cells are particularly vulnerable to hyperglycemia-induced mitochondrial ROS.(Prow et al. 2008) However, recent studies showed that in human retinal endothelial cells exposure to high glucose did not stimulate endogenous ROS production, activation of NF-κB, interleukin (IL)-1β, or TNF-α production and only slightly affected apoptotic cell death pathways compared with normal glucose. In marked contrast, exposure of human retinal endothelial cells to proinflammatory cytokines IL-1β or TNF-α increased glucose consumption, mitochondrial superoxide production, NF-κB activation, etc. These results suggest that diabetes-related endothelial injury in the retina may be due to glucose-induced cytokine release by other retinal cells (i.e. paracrine mediators), such as RPE and Müller cells, and not a direct effect of high glucose.(Busik et al. 2008) Observations of pharmacological prevention of acellular capillaries without the rescue of pericyte loss corroborate the hypothesis that the retinal endothelium is the primary therapeutic target, at least in experimental DR.(Hammes 2005)
4.2.5.2. Hyperglycemic mitochondria-derived ROS and AMD
Age-related pathology, including AMD has been related to mitochondrial genomic instability. For example, an increased level of the mitochondrial superoxide dismutase (SOD2) (Fig. 4a), has been shown to decrease the disruption of mitochondrial transmembrane potential and the release of Cyt c, and thus to prevent apoptotic cell death in mouse RPE.(Kasahara et al. 2005) Furthermore, it is suggested that in RPE mitochondria are the main target of oxidative injury, that the mitochondrial genome is a weak link in the antioxidant defenses, and that deficits in mitochondrial DNA repair pathways are important contributors to the pathogenesis of AMD.(Cai et al. 2000; Jarrett et al. 2008) These data suggest that oxidative stress-induced mitochondrial genomic instability will result in loss of cell function and greater susceptibility to stress. This mitochondrial overproduction of ROS may play a significant role in AMD pathogenesis.
4.2.6. Hyperglycemic Hypoxia-inducible factor (HIF) pathway
Hypoxia, a condition defined as a decrease in the oxygen tension (oxygen partial pressure) from that observed at sea-level (normoxia), is encountered to different degrees in various tissues and arises as a direct consequence of insufficient blood flow or vascularisation in relation to the energy consumption of a given tissue.(Brahimi-Horn & Pouyssegur 2005) Cells may be rendered hypoxic because more oxygen is consumed in the ETC during hyperglycemia (Fig. 4a). A corollary is that hypoxia can be viewed as a status of reduced availability of oxygen for aerobic oxidation of glucose, particularly during hyperglycemia.
Because cell respiration depends on the balance between glucose homeostasis and oxygen homeostasis, hyperglycemia increases the risk of oxygen depletion and renders cells vulnerable. This is similar to a hypoxic environment. Therefore, hypoxia can be viewed as frequently coincident events with hyperglycemia, even under physiological conditions. In addition, because vascular occlusion diseases, which often develop during the progression of DR or AMD, limit circulation and result in ischemia, they may also induce hypoxia.
It has been proposed that the increased cytosolic ratio of free NADH/NAD+ caused by diabetes-related hyperglycemia mimics the effects of true hypoxia on vascular and neural function and plays an important role in the pathogenesis of diabetic complications, including DR.(Nyengaard et al. 2004; Williamson et al. 1993.) This is referred to as pseudohypoxia because tissue partial pressure oxygen is normal.
The aberrant stabilization of HIF-α proteins (HIF-1α and/or HIF-2α) (see 4.2.6.1. Hypoxia-inducible factor (HIF)) under normoxic conditions is also termed pseudohypoxia. For example, dysfunction of TCA cycle enzymes causes pseudohypoxia, leading to the enhanced neovascularization and glycolysis that support cancer formation.(Gottlieb & Tomlinson 2005) The mechanisms involve inhibition of ETC in the succinate dehydrogenase (SDH) complex due to mutations in the SDHB or SDHD genes, causing a build-up of succinate that inhibits HIF proline hydroxylase (PHD), resulting in stabilization of HIF-1α. Pseudohypoxia can also result from von Hippel-Lindau (VHL) mutations,(Kim & Kaelin 2004) because VHL mutations result in stabilization of HIF-α proteins (see 4.2.6.1. Hypoxia-inducible factor (HIF)). It has been proposed that identifying ways to prevent HIF-α stabilization under pseudohypoxia could lead to treatments for tumors.(MacKenzie et al. 2007)
As discussed later in 4.3. Hyperglycemia induces inflammation and apoptosis, hyperglycemia-induced proinflammatory cytokines, such as IL-1β and TNF-α, can result in stabilization of HIF-1α under normoxic conditions (Fig. 9a). This can be also considered pseudohypoxia.
Although some vertebrate species, such as fish, have adapted to tolerate large variations in ambient oxygen tension during their normal life cycle, most mammals, including man, face serious problems if exposed, even for shorter periods of time, to low oxygen tension.(Stecyk et al. 2004) The human retina is highly sensitive to reduction in oxygen tension,(Arjamaa & Nikinmaa 2006) but, like the effects of hyperglycemia, the effects of hypoxia vary among retinal cell types.(Young et al. 2005)
4.2.6.1. Hypoxia-inducible factor (HIF)
HIF was first characterized from human hepatoma cells in which it influenced the transcription of erythropoietin (Epo) gene.(Semenza & Wang 1992) Roles for HIF in the etiologies of DR and AMD have also been proposed. (Arjamaa & Nikinmaa 2006; Arjamaa et al. 2009) Stabilizing HIF is among the primary responses to low oxygen tension in cells. Actually, because HIF is expressed in a wide range of animal cells and tissues, and plays an indispensable role in cellular reactions, HIF represents a global adaptation of all cells to oxygen homeostasis and can be regarded as a “master switch” of metabolism.(Semenza 2000; Semenza 2003)
Some suggest that the oBRB, including RPE, is highly resistant to hypoxic damage.(Kaur et al. 2008) We posit the novel hypothesis that the resistance to hypoxia is compromised when hypoxia coincides with hyperglycemia. Furthermore, the effects of hypoxia largely depend on the level of hyperglycemia.
With the objective of enhancing understanding of hyperglycemia-related retinal pathology in both normoxia and hypoxia, in the following sections, we will explore relationships between the HIF pathway and the four glycolysis-associated pathways and the mitochondria-derived ROS pathway, which were described above.
HIF is a heterodimeric protein complex that is composed of two subunits, HIF-1α and HIF-1β.(Wang et al. 1995) In contrast to the constitutively expressed HIF-1β subunit, HIF-α is an oxygen labile protein, which in the presence of O2 is hydroxylated and then degraded.(Cockman et al. 2000.; Kamura et al. 2000; Lisztwan et al. 1999; Ohh et al. 2000.; Tanimoto et al. 2000.) HIF-1α becomes stabilized in response to hypoxia, dimerizes with HIF-1β and binds to hypoxia response elements (HRE), thereby activating the expression of numerous target genes (HIF-inducible genes) involved in a wide range of cell patho-physiology.(Park et al. 2006)
Although HIF is expressed under physiological conditions, the expression and degradation of the HIF protein are kept in balance (Fig. 9). Under normoxic conditions (Fig. 9a), there are two mechanisms that cells use to keep HIF inactive.(Iyer & Leung 1998) The first mechanism is through the Ub-dependent proteasomal degradation pathway. In this pathway, the conserved proline residues (402 and 564) of HIF are first hydroxylated by proline hydroxylase (PHD) enzymes. Next, the hydroxylated HIF interacts with a Ub-protein ligase, von Hippel-Lindau (VHL) protein,(Iwai et al. 1999; Lisztwan et al. 1999) becomes ubiquitinated, and is degraded via the proteasomal pathway.(Cockman et al. 2000.; Kamura et al. 2000; Lisztwan et al. 1999; Ohh et al. 2000.; Tanimoto et al. 2000.) The degradation is dependent on the protein motifs found in the carboxyl terminus of HIF proteins, termed hypoxia-responsive domains.(Huang et al. 1998) Thus, HIF is kept at a low level in the cytosol. In the second mechanism, HIF is prevented from binding to the HREs in the promoter or enhancer regions of HIF-inducible genes. Specifically, in normoxia, HIF binding to DNA is inhibited through asparagine hydroxylation by an oxygen-dependent factor inhibiting HIF (FIH) (Fig. 9a).(Lando et al. 2002.; Lisy & Peet 2008.; Mahon et al. 2001.) In this situation, transcription of HIF-inducible genes, such as VEGF, is limited.
Under hypoxia both oxygen sensors, PHD and FIH, become inactive and unable to hydroxylate HIF-1α. The stabilized HIF binds to HIF-inducible genes (Fig. 9b). In addition, hypoxia also induces the expression of heat shock proteins (HSPs). HSPs are molecular chaperones required for the stability and function of a number of conditionally activated and/or expressed protein kinases and transcription factors, such as HIF.(Kaarniranta & Salminen 2009.) For example, the ATP-dependent HSP90 can bind to the Per-Arnt-Sim (PAS) domain of HIF and increase its stability.(Pearl et al. 2008.) The PAS is situated in the amino terminal end of HIF and is involved in the formation and stabilization of HIF heterodimers.(Park et al. 2006; Wang et al. 1995) Therefore, under hypoxia, the transcription activities of HIF-inducible genes are turned on by HIF. As noted earlier, these HIF-inducible genes are responsible for a wide range of hyperglycemic pathogenesis, such as VEGF-induced angiogenesis (Fig. 4b and Fig. 9b).
The HIF system is also affected independently of O2.(Dehne & Brüne 2009) Though oxygen seems to be the major determinant of PHD activity, the enzyme is also sensitive to cellular redox status, iron or metabolite homeostasis, etc.(Kaelin Jr. & Ratcliffe 2008) Among these effectors is nitric oxide (NO). NO is produced by activated macrophages and granulocytes during inflammation.(Thomas et al. 2008) NO is synthesised from L-arginine by nitric oxide synthase (NOS). NOS from neurons (nNOS) and endothelium (eNOS) are constitutively expressed enzymes. Their activities are stimulated by increases in intracellular calcium.(Kaur et al. 2008) Inducible nitric oxide synthase is calcium-independent, and NO generated from this isoform is known to mediate immune functions. Excess production of NO has been reported to increase blood flow and the permeability of the blood brain barrier allowing substances to enter into the brain passively.(Shukla et al. 1996.; Thiel & Audus 2001.) The modulation of NO availability by eNOS seems to be an important determinant in the maintenance of cerebral perfusion in hypoxic conditions. Vasodilatation occurring after hypoxic–ischaemic episodes is mediated by eNOS(Bolanos & Almeida 1999.) leading to increased blood flow. It has also been proposed that eNOS mediates VEGF-induced vascular hyperpermeability.(Fukumura et al. 2001.) NO, exogenously added or endogenously produced, stabilizes HIF protein and causes transactivation of HIF under normoxia.(Brune & Zhou 2007) It is suggested that hypoxia and NO use overlapping signaling pathways to stabilize HIF, because NO attenuates HIF ubiquitination in an in vitro-assay and decreases PHD activity.(Brune & Zhou 2007) FIH activity is also inactivated by NO.(Park et al. 2008) Besides, NO also increased PI3K-dependent HIF protein expression.(Brune & Zhou 2007) Therefore, it is not surprising that NO, similar to hypoxia, can induce HIF-related responses.
In tumor cells, it has been shown that tumor suppressor gene and oncogene activity can also influence HIF activity and subsequent changes in glucose metabolism.(Hammond & Giaccia 2005; Ramanathan et al. 2005; Semenza 2003)
4.2.6.2. Hyperglycemia and HIF
Accumulating evidence implies that there is an undescribed HIF-related mechanism that results in pathology under hyperglycemic, hypoxic conditions (Fig. 9b). Hyperglycemia can enhance the activity of HIF through glycolysis-derived hyperglycemic AGE and PKC pathways (Fig. 9b). First, hypoxia has been shown to result in the activation of NF-kB, which can bind to the HIF promoter in response to hypoxia.(Belaiba et al. 2007.; Bonello et al. 2007.) As described previously, NF-κB can also be activated by hyperglycemia-induced AGE formation (Fig. 5) and PKC activation (Fig. 7). Thus, coincidental hyperglycemia and hypoxia lead to enhanced expression of HIF and HIF-inducible genes (Fig. 9b). Indeed, it has been shown that when hyperglycemia coincides with hypoxia the secretion of VEGF is enhanced. This is partially mediated via activation of PKC.(Young et al. 2005) Remarkablly, PKC can be also activated through hyperglycemic polyol pathway (see 4.2.2. Hyperglycemic polyol pathway and Fig. 6) and hyperglycemic hexosamine pathway (see 4.2.4. Hyperglycemic hexosamine pathway and Fig. 8). Second, the hyperglycemic HIF pathway may amplify the deleterious effects of hyperglycemic AGE formation (Fig. 5) by HIF-inducible RAGE expression (Fig. 9b).(Pichiule et al. 2007).
Another example of synergistic effect of hypoxia and hyperglycemia is on the lysosomal and proteasomal proteolysis systems. Autophagy is one of the cellular mechanisms that are responsible for proteolytic functions. It cooperates with the lysosomal and Ub proteasome pathway in protein clearance in response to cellular stress, such as hypoxia and disturbed energy balance.(Korolchuk et al. 2009; Ryhnen et al. 2009; Salminen & Kaarniranta 2009.) Interestingly, it has been shown that HIF can induce autophagy by preventing ATP depletion and by enhancing elimination of damaged mitochondria.(Bellot et al. 2009.; Zhang et al. 2008.) However, hyperglycemia-induced glycating agents, such as MGO, impair both lysosomal(Kasper et al. 1999; Miyata et al. 1997; Sebeková et al. 1998) and proteasomal functions.(Queisser et al. 2010) Taken together, the higher input (increased autophagocytosis) than output (impaired proteolytic function) in proteolytic machinery may lead to the accumulation of intracellular lipofuscin and the formation of drusen, both of which are hallmarks of early AMD. This mechanistic proposal is corroborated by observations that in human AMD donor samples or in RPE cells, there are increased levels of autophagic markers, decreased lysosomal activity, increased exocytotic activity and release of cytokines. (Wang et al. 2009) Interestingly, the exosomes released by the stressed RPE to remove damaged intracellular proteins are coated with complement and can bind complement factor H (CFH), which has been identified as a major inflammatory factor in AMD pathogenesis.(Edwards et al. 2005; Haines et al. 2005; Klein et al. 2005)
Even under normoxic conditions, hyperglycemia appears to be able to induce some effects of HIF. It has been shown that in RPE cells high concentrations of glucose enhance synthesis and accumulation of HIF.(Xiao et al. 2006) As described above (Fig. 5 and Fig. 7), AGEs and PKC can activate NF-kB. And, as discussed above, PKC can be also activated through hyperglycemic polyol pathway (Fig. 6) and hyperglycemic hexosamine pathway (Fig. 8). This may cause the over expression of HIF (Fig. 9a). The overexpressed HIF may be further stabilized by MGO, which has been shown to impair Ub proteasome function.(Queisser et al. 2010) Elevated mitochondrial ROS can also enhance the expression of HIF through NF-kB signaling(Bonello et al. 2007.; Decanini et al. 2007.; Taylor 2008.; van Uden et al. 2008.; Wang et al. 2010) and stabilize HIF protein by inhibiting PHD(Yuan et al. 2008.) (Fig. 4a and Fig. 9a). Taken together, under hyperglycemic, normoxic conditions, the hyperglycemic AGE and PKC pathways may also result in excess cytosolic HIF proteins (Fig. 9a), which can induce autophagy (Bellot et al. 2009.; Zhang et al. 2008.) and, in conjuction with impaired proteolytic functions, lead to the accumulation of lysosomal lipofuscin.
Actually, under both hypoxic and normoxic conditions (Fig. 9a and Fig. 9b), hyperglycemia can lead to HIF accumulation resulting in the formation of intracellular deposits and expression of HIF-inducible genes (see 4.3. Hyperglycemia induces inflammation and apoptosis). However, studies have shown that under hypoxia the hyperglycemia-induced HIF protein is more stable, and the expression of VEGF is increased.(Xiao et al. 2006; Yao et al. 2003)
The effects of hypoxia can persist for some time after oxygen tension returns to a normoxic level, probably through mitochondrial ROS generation. For example, intermittent hypoxia, followed by reoxygenation, has been shown to potentiate the production of ROS, which may lead to HIF activation and accelerated aging and to the appearance of age-related diseases.(Rapino et al. 2005.; Yuan et al. 2008.) In theory, this phenomenon may occur at the highly active retina during the transition from early to middle postprandial stage after ingesting a high-GI meal (Fig. 3).
It has been known for long that, even under conditions of plentiful oxygen (normoxia), cancer cells switch from aerobic respiration to lactate fermentation.(Warburg 1956) Studies have demonstrated that the phenomenon, including increased glucose uptake, up-regulated glycolytic cascade and reduced aerobic respiration, increased lactate production, and acidosis of the micro-environment, is primarily due to the activation of HIF.(Brahimi-Horn et al. 2007; Kim & Dang 2006) Actually, this phenonmenon also happens in non-tumor cells under physiological conditions.(Brahimi-Horn et al. 2007; Kim & Dang 2006; Kim et al. 2006a; Kim et al. 2006b; Papandreou et al. 2006; Pouyssegur & Mechta-Grigoriou 2006) This may, at least partially, explain the physiological phenomenon of a high lactate production concomitant with high oxygen consumption in the RPE (Fig. 4a and Fig. 9a).(Coffe et al. 2006; Kaur et al. 2008; Miceli et al. 1990) The possibility of using the lactate level in the RPE as a biomarker of hyperglycemic exposure or even a prognostic biomarker as well deserves further study.
The molecular mechanism for the HIF-dependent pyruvate metabolism switching has also been studied (Fig. 9a). The increased metabolism of pyruvate to lactate is mainly a result of activation of two HIF-dependent enzymes, pyruvate dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase A (LDH-A). PDK1 inhibits the activity of pyruvate dehydrogenase (PDH), which is required in the TCA cycle.(Kim et al. 2006a; Kim et al. 2006b; Papandreou et al. 2006; Pouyssegur & Mechta-Grigoriou 2006) LDH-A converts pyruvate into lactate.(Koukourakis et al. 2005)
Since the metabolism of glucose via pyruvate to lactate is less energy efficient, cells must increase glucose uptake and accelerate glycolysis to maintain the ATP level. It is remarkable that these adaptations are also mediated by HIF pathway, which increases the expression of glucose transporters (GLUTs, e.g. GLUT-1) and up-regulates glycolytic enzymes (e.g. LDH and aldolase protein levels) (Fig. 4b and Fig. 9b).(Schofield & Ratcliffe 2004; Semenza 2003) Therefore, in hyperglycemic, hypoxic conditions HIF pathway may further potentiate the four glycolysis-associated pathways. The stabilization of HIF that occurs under conditions of hypoxia and sufficient glucose provides an additional explanation for synergistic effects of hypoxia and hyperglycemia compared with the effect from hypoxia alone.(Vordermark et al. 2005)
4.2.6.3. HIF and VEGF
From a clinical point of view, VEGF is one of the most important HIF-inducible genes (Fig. 9b), because it induces postnatal neovascularization and angiogenesis seen after ischemic events in both DR and AMD patients.(Lee et al. 2000) Although both hyperglycemia and hypoxia can induce VEGF expression, it is remarkable that, while hyperglycemia-induced VEGF expression is mediated by PKC (Fig. 7), hypoxia mediates VEGF expression by increased binding of the active HIF to the HRE of the VEGF promoter and by increasing the stability of the VEGF mRNA transcript through mitogen-activated protein kinase and Akt pathways, respectively (Fig. 9b).(Suzuma et al. 2000) Importantly, it has been shown that exposure to hypoxia as well as AGEs causes additive VEGF expression by RPE cells.(Lu et al. 1998)
Expression of HIF-inducible genes, including VEGF, can also be stimulated through HIF-independent mechanisms, such as the transcriptional coactivator peroxisome-proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α). PGC-1α is a potent metabolic sensor and regulator induced by a lack of nutrients and oxygen. PGC-1α powerfully regulates VEGF expression and angiogenesis in cultured muscle cells and skeletal muscle in vivo.(Arany et al. 2008) Such observations may help explain why people with diabetes can have impaired new blood vessel growth in one tissue compartment (e.g., myocardium) and also exhibit hyperproliferative vascular disease in another (e.g., retina).(Thangarajah et al. 2010b)
Anti-VEGF, such as Lucentis®, Avastin®, and Macugen®, has been used in clinics to treat exudative AMD(Bressler 2009a; Bressler 2009b) and is currently being evaluated for the treatment of proliferative DR and neovascular glaucoma.(Rodriguez-Fontal et al. 2009) Recently, it has been shown that intravitreal ranibizumab (anti-VEGF therapy, trade name Lucentis®) with prompt or deferred laser is more effective through at least 1 year compared with prompt laser alone for the treatment of diabetic macular edema involving the central macula.(The Diabetic Retinopathy Clinical Research Network et al. 2010)
In addition to VEGF, recent studies have also explored the possibility of directly targeting HIF for a new therapeutic option for both DR and AMD, especially the neovascular types.(Arjamaa & Nikinmaa 2006; Arjamaa et al. 2009; Wang et al. 2009; Zhang et al. 2007.)
Some studies suggested that the poor wound healing in diabetic patients is a result of compromised blood vessel formation in response to ischemia and that this impairment in neovascularization results from a MGO-induced defect in transactivation of HIF-1α, leading to the decreased expression of VEGF.(Bento et al. 2010; Thangarajah et al. 2010a; Thangarajah et al. 2009) However, since ischemia is a phenomenon of lacking oxygen, glucose, and serum in the tissues,(Osborne et al. 2004; Wood & Osborne 2001) it should be differentiated from the hypoxic, hyperglycemic conditions discussed here.
4.2.6.4. HIF and erythropoietin
It was shown that erythropoietin (Epo) provides protection against apoptosis of photoreceptor cells in the rodent retina and this protection is through interfering with caspase-1 activation, a downstream event in the intracellular death cascade, but not through inhibiting initial events of the apoptosis cascade such as activator protein-1 activation.(Grimm et al. 2006; Grimm et al. 2002; Junk et al. 2002) It has also been shown that Epo acts as a neuroprotective factor in diabetic neuropathy,(Bianchi et al. 2004; Lipton 2004) However, although Epo overexpression is an early event in the retina of diabetic patients, at this stage it is unrelated to a hypoxic stimulus (i.e. HIF-independent).(Forooghian et al. 2007; García-Ramírez et al. 2008) This is to say that, in the early stage of DR, HIF-independent Epo overexpression actually has beneficial rather than pathogenic actions. It appears that factors, apart from hypoxia, that could be responsible for Epo overexpression include hyperglycemia and inflammation.(García-Ramírez et al. 2008; Sun & Zhang 2001; Watanabe et al. 2005)
However, Epo is also a potent retinal angiogenic factor independent of VEGF and, at least partially, responsible for retinal angiogenesis in proliferative DR, the late stage of the disease. This Epo expression is mainly stimulated by hypoxia (i.e. HIF-dependent) (Fig. 9b).(Mowat et al. 2010; Watanabe et al. 2005)
Therefore, despite its neuroprotective effect,(Bianchi et al. 2004; Lipton 2004) Epo administration may be hazardous for retinal diseases that involve retinal vasoproliferation. Conversely, Epo blockade may be hazardous for retinal diseases that involve apoptosis of retinal photoreceptors.(Becerra & Amaral 2002; Watanabe et al. 2005) The clinical application of Epo needs further study.
4.3. Hyperglycemia induces inflammation and apoptosis
Both DR and AMD have been characterized as chronic inflammatory diseases leading to cell death in the retina.(Anderson et al. 2002; Hageman et al. 2001; Joussen et al. 2004; Mohr 2004) This is consistent with many molecular and epidemiological observations, reviewed above, that hyperglycemia results in increased production of pro-inflammatory cytokines and apoptosis of the cells.(Allen et al. 2005; Buyken et al. 2010a; Node & Inoue 2009)
Caspases, a family of cysteine proteases, are known to be critically involved in both activation of proinflammatory cytokines and the initiation and execution of apoptosis.(Alnemri et al. 1996; Alnemri 1997) Caspase-1 is involved in activation of inflammatory processes.(Mariathasan et al. 2004) Two caspase pathways have been described by which cells undergo apoptosis. The extrinsic (receptor-mediated) pathway is triggered via cell surface receptors which are represented by TNF-α family receptors, leading to activation of caspase-8 and caspase-3 proteolytic enzymes. The intrinsic (mitochondrial) pathway involves the mitochondrial Cyt c release and activation of the caspase-9, with subsequent activation of caspase-3. The intrinsic pathway can be activated by agents that directly target the mitochondria, or indirectly via the extrinsic pathway through caspase-8-mediated cleavage of the inactive cytosolic protein BID. Once activated, BID translocates to the mitochondria where it stimulates Cyt c release.(Fiers et al. 1999; Takahashi et al. 2004)
In Müller cells in the retina of streptozotocin-induced diabetic rats, hyperglycemia caused apoptosis which is associated with the activation of the caspase-3 and mitochondrial caspase-9 pathways.(Xi et al. 2005) Recent studies also showed that inhibiting the activation of caspase-1, or former IL-1β converting enzyme which is responsible for the production of the pro-inflammatory cytokines IL-1β and IL-18, could be a potential new strategy to prevent the development of DR.(Mohr 2004; Mohr et al. 2002; Vincent & Mohr 2007)
In the human RPE, the activation of caspase-8 pathway (i.e. extrinsic pathway), but not the mitochondrial caspase-9 pathway (i.e. intrinsic pathway) was shown to involve in the 7-ketocholesterol (an oxidative stressor)-induced apoptosis.(Luthra et al. 2006) However, in TNF-α-induced apoptosis in the human RPE cells it was shown that the mitochondrial caspase-9 could be used to amplify the death signal mediated by caspase-8.(Yang et al. 2007)
As discussed in the four glycolysis-related pathways above, hyperglycemia can induce proinflammatory cytokines (e.g. Fig. 5), such as IL-1β and TNF-α. Interestingly, IL-1β and TNF-α can prolong the activation of HIF-1α protein under conditions of inflammation via enhancing the translation of HIF-1α mRNA, further leading to increased expression of HIF-inducible genes.(Frede et al. 2007; Frede et al. 2005; Hellwig-Bürgel et al. 1999; Sandau et al. 2001; Zhou et al. 2003) Importantly, this can happen under both normaxic (Fig. 9a) and hypoxic (Fig. 9b) conditions. This gives additional support to the idea that hyperglycemia is able to induce some effects of HIF (also see 4.2.6.2. Hyperglycemia and HIF).
In conclusion, HIF is not only a hypoxia-inducible factor: it can also be described as a “hyperglycemia-inducible factor”. Through this mechanism, the hyperglycemic HIF pathway can affect oxidative stress responses, inflammation, proteolytic mechanisms, etc., all of which are involved in the pathogeneses of DR and AMD. This wide range of HIF-mediated cellular effects may open new treatment indications, e.g., for dry forms of AMD. Nevertheless, caution is advised because suppressing of HIF may be a double-edged sword. By serving as the major regulator in glucose metabolism, HIF is necessary for maintaining physiological homeostasis.
5. SUMMARY
Our health is largely determined by nurture, including the modifiable aspect of diet. Since carbohydrate is our major energy source, it is reasonable that metabolism of sugars plays a significant role in aging and disease. GI reflects the kinetics of blood glucose levels after ingesting a meal in people both with and without diabetes. Recent data from a wide range of epidemiological and molecular evidence offers a strong support for the conclusion that dietary hyperglycemia is associated with risk for major metabolic disorders, including type 2 diabetes, CVD, and retinal diseases such as DR and AMD. Therefore, it remains critical to re-value the management of carbohydrate nutrition as a means to prevent the onset or progression of these diseases. A low-GI diet should be recommended to those at high risk. In terms of identifying high risk populations, development of susceptibility biomarkers, exposure biomarkers, and surrogate endpoints for a disease will be valuable. Future studies should focus on the relationship between management and efficacy of low-GI diets on disease risk. Deciphering the biochemical mechanisms which link consuming high-GI diets to increased disease risk and salutary effects of consuming low-GI diets will further our understanding of the underlying pathogenesis and enhance therapeutic options.
Acknowledgments
Financial support for this project has been provided by the U.S. Department of Agriculture under agreements, 1950-5100-060-01A (CJC, AT) and R01-13250 and R03-EY014183-01A2 from the National Institutes of Health (AT), and to CJC from the Ross Aging Initiative.
ABBREVIATIONS
- AGEs
advanced glycation end products
- ALEs
advanced lipoxidation end products
- AMD
age-related macular degeneration
- AR
aldose reductase
- AREDS
Age-Related Eye Disease Study
- ATP
adenosine triphosphate
- AUC
area under blood glucose curve and above the baseline blood glucose level
- BDES
Beaver Dam Eye Study
- BLDs
basal lamina deposits
- BMES
Blue Mountains Eye Study
- BRB
blood-retinal barrier
- CEC
choroidal endothelial cells
- CHD
coronary heart disease
- CI
confidence interval
- CML
Nε-(carboxyl-methyl) lysine
- CNV
choroidal neovascularization
- CVD
cardiovascular disease
- Cyt c
cytochrome-C
- DAG
diacylglycerol
- DHA
docosahexaenoic acid
- DME
diabetic macular edema
- DR
diabetic retinopathy
- eNOS
endothelial nitric oxide synthase
- EPA
eicosapentaenoic acid
- Epo
erythropoietin
- ET-1
vasoconstrictor endothelin-1
- ETC
electron transport chain
- F-6-P
fructose-6-phosphate
- FIH
factor inhibiting HIF
- G-6-P
glucose-6-phosphate
- GA
geographic atrophy
- GA-3-P
glyceraldehyde-3-phosphate
- GAPDH
glyceraldehyde-3 phosphate dehydrogenase
- GFAT, glutamine
fructose-6 phosphate amidotransferase
- GI
glycemic index
- GL
glycemic load
- Glucosamine-6-P
glucosamine-6-phosphate
- GLUTs
glucose transporters
- GO
glyoxal
- GSH
glutathione
- HbA1c
hemoglobins A1c
- HDL
high-density lipoprotein
- HIF
hypoxia-inducible factor
- HREs
hypoxia-responsive elements
- HSP90
heat shock proteins 90
- HSPs
heat shock proteins
- iBRB
inner blood-retinal barrier
- LDH-A
lactate dehydrogenase A
- LDL
low-density lipoprotein
- IL
interleukin
- MGO
methylglyoxal
- Mn-SOD
mitochondrial isoform of superoxide dismutase
- NF-κB
nuclear factor kappa B
- NHANES
National Health and Nutrition Examination Surveys
- NHS
Nurses Health Study
- nNOS
neurons nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NVP
Nutrition and Vision Project
- oBRB
outer blood retinal barrier
- O-GlcNAcylation
O-acetylglucosaminylation
- OGT
O-GlcNAc transferase
- OR
odds ratio
- PAI-1
plasminogen activator inhibitor-1
- PARP
poly(ADP-ribose) polymerase
- PAS
Per-Arnt-Sim
- PDGF-B
platelet-derived growth factor-B
- PDH
pyruvate dehydrogenase
- PDK1
pyruvate dehydrogenase kinase 1
- PGC-1α
peroxisome-proliferator-activated receptor-gamma coactivator-1alpha
- PHD
praline hydroxylase
- PKC
protein kinase C
- PPAR
peroxisome proliferactor–activated receptor
- PUFAs
polyunsaturated fatty acids
- RAGE
receptors for AGEs
- RNV
retinal neovascularization
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- RR
relative risk
- SDH
succinate dehydrogenase
- SOD2
mitochondrial superoxide dismutase
- TCA
tricarboxylic acid
- TGF-β1
transforming growth factor-β1
- TNF-α
tumor necrosis factor α
- TT
Na+-taurine cotransporter
- Ub
ubiquitin
- UCPs
uncoupling proteins
- UDPGlcNAc
uridine diphosphate N-acetyl glucosamine
- USDA
the United States Department of Agriculture
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau
Footnotes
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
The funding sources had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah W, Fawzi AA. Anti-VEGF therapy in proliferative diabetic retinopathy. Int Ophthalmol Clin. 2009;49:95–107. doi: 10.1097/IIO.0b013e31819fd84a. [DOI] [PubMed] [Google Scholar]
- Abordo EA, Thornalley PJ. Synthesis and secretion of tumour necrosis factor-alpha by human monocytic THP-1 cells and chemotaxis induced by human serum albumin derivatives modified with methylglyoxal and glucose-derived advanced glycation endproducts. Immunol Lett. 1997;58:139–147. doi: 10.1016/s0165-2478(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research Group. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no.19. Ophthalmology. 2005;112:533–9. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, LES, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- Aisenbrey S, Zhang M, Bacher D, Yee J, Brunken WJ, Hunter DD. Retinal pigment epithelial cells synthesize laminins, including laminin 5, and adhere to them through alpha3- and alpha6-containing integrins. Invest Ophthalmol Vis Sci. 2006;47:5537–44. doi: 10.1167/iovs.05-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem. 2005;16:705–13. doi: 10.1016/j.jnutbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J Million Women Study Collaborators. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- Alnemri E, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature (Letter) Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. doi: 10.1002/(sici)1097-4644(199701)64:1<33::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Bowes Rickman C, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog Retin Eye Res. 2009 doi: 10.1016/j.preteyeres.2009.11.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil E. The impact of EPA and DHA on blood lipids and lipoprotein metabolism: influence of apoE genotype. Proc Nutr Soc. 2007;66:60–8. doi: 10.1017/S0029665107005307. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–7. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ERr, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473–83. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K. Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD) Ageing Res Rev. 2009;8:349–58. doi: 10.1016/j.arr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Arnold L, Ball M, Mann J. Metabolic effects of alterations in meal frequency in hypercholesterolaemic individuals. Atherosclerosis. 1994;108:167–74. doi: 10.1016/0021-9150(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Assero G, Lupo G, Anfuso CD, Ragusa N, Alberghina M. High glucose and advanced glycation end products induce phospholipid hydrolysis and phospholipid enzyme inhibition in bovine retinal pericytes. Biochim Biophys Acta. 2001;1533:128–40. doi: 10.1016/s1388-1981(01)00151-2. [DOI] [PubMed] [Google Scholar]
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–3. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz A. The glycemic index: methodological aspects related to the interpretation of health effects and to regulatory labeling. J AOAC Int. 2009;92:879–87. [PubMed] [Google Scholar]
- Balkau B, Shipley M, Jarrett RJ, Pyörälä K, Pyörälä M, Forhan A, Eschwège E. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360–7. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- Barclay AW, Ptocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Millar JC. Glycemic index, glycemic load, and chronic disease risk – a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–37. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Metabolism of phospholipids in the retina. Vision Res. 1982;22:1539–1548. doi: 10.1016/0042-6989(82)90221-8. [DOI] [PubMed] [Google Scholar]
- Becerra SP, Amaral J. Erythropoietin--an endogenous retinal survival factor. N Engl J Med. 2002;347:1968–70. doi: 10.1056/NEJMcibr022629. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Belaiba RS, Bonello S, Zhringer C, Schmidt S, Hess J, Kietzmann T, Grlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssgur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento CF, Fernandes R, Matafome P, Sena C, Seiça R, Pereira P. Methylglyoxal-induced imbalance in the ratio VEGF/Ang-2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Exp Physiol. 2010;95:955–70. doi: 10.1113/expphysiol.2010.053561. [DOI] [PubMed] [Google Scholar]
- Bertelsen J, Christiansen C, Thomsen C, Poulsen PL, Vestergaard S, Steinov A, Rasmussen LH, Rasmussen O, Hermansen K. Effect of meal frequency on blood glucose, insulin, and free fatty acids in NIDDM subjects. Diabetes Care. 1993;16:4–7. doi: 10.2337/diacare.16.1.4. [DOI] [PubMed] [Google Scholar]
- Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50:14–21. doi: 10.1016/j.jacc.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tataroglu C, Cerami A, Ghezzi P. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101:823–8. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Stern DM, Arnold B, Nawroth PP. Advanced glycation end product receptor-mediated cellular dysfunction. Ann NY Acad Sci. 2005;1043:676–680. doi: 10.1196/annals.1333.077. [DOI] [PubMed] [Google Scholar]
- Bishara NB, Dunlop ME, Murphy TV, Darby IA, Sharmini Rajanayagam MA, Hill MA. Matrix protein glycation impairs agonist-induced intracellular Ca2+ signaling in endothelial cells. J Cell Physiol. 2002;193:80–92. doi: 10.1002/jcp.10153. [DOI] [PubMed] [Google Scholar]
- Boden G, Sargrad K, Homko C, Mozzoli M, Stein T. Effect of a low-carbohydrate diet on appetite, blood glucose levels and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta. 1999;1411:415–436. doi: 10.1016/s0005-2728(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Bonello S, Zhringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Grlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Bonora E, Calcaterra F, Lombardi S, Bonfante N, Formentini G, Bonadonna RC, Muggeo M. Plasma glucose levels throughout the day and HbA1c interrelationship in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care. 2001;24:2023–9. doi: 10.2337/diacare.24.12.2023. [DOI] [PubMed] [Google Scholar]
- Bornet FR, Costagliola D, Rizkalla SW, Blayo A, Fontvieille AM, Haardt MJ, Letanoux M, Tchobroutsky G, Slama G. Insulinemic and glycemic indexes of six starch-rich foods taken alone and in a mixed meal by type 2 diabetics. Am J Clin Nutr. 1987;45:588–95. doi: 10.1093/ajcn/45.3.588. [DOI] [PubMed] [Google Scholar]
- Botero D, Ebbeling CB, Blumberg JB, Ribaya-Mercado JD, Creager MA, Swain JF, Feldman HA, Ludwig DS. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring) 2009;17:1664–70. doi: 10.1038/oby.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Marshall J. Effects of increasing numbers of phagocytic inclusions on human retinal pigment epithelial cells in culture: a model for aging. Br J Ophthalmol. 1986;70:808–15. doi: 10.1136/bjo.70.11.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The formation of autofluorescent granules in cultured human RPE. Invest Ophthalmol Vis Sci. 1989;30:82–9. [PubMed] [Google Scholar]
- Boulton M, Moriarty P, Jarvis-Evans J, Marcyniuk B. Regional variation and age-related changes of lysosomal enzymes in the human retinal pigment epithelium. Br J Ophthalmol. 1994;78:125–9. doi: 10.1136/bjo.78.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Rozanowska M, Rozanowski B, Wess T. The photoreactivity of ocular lipofuscin. Photochem Photobiol Sci. 2004;3:759–64. doi: 10.1039/b400108g. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–9. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Pouyssegur J. The hypoxia-inducible factor and tumor progression along the angiogenic pathway. Int Rev Cytol. 2005;242:157–213. doi: 10.1016/S0074-7696(04)42004-X. [DOI] [PubMed] [Google Scholar]
- Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–7. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- Brand JC, Colagiuri S, Crossman S, Allen A, Roberts DC, Truswell AS. Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care. 1991;14:95–101. doi: 10.2337/diacare.14.2.95. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD, Bravata DM. Efficacy and safety of low-carbohydrate diets: a systematic review. JAMA. 2003;289:1837–50. doi: 10.1001/jama.289.14.1837. [DOI] [PubMed] [Google Scholar]
- Bray GA. Lipogenesis in human adipose tissue: some effects of nibbling and gorging. J Clin Invest. 1972;51:537–48. doi: 10.1172/JCI106842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology. 2009a;116(10 Suppl):S15–23. doi: 10.1016/j.ophtha.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Bressler SB. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009b;116(10 Suppl):S1–7. doi: 10.1016/j.ophtha.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TMS. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835–1843. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Brun JF, Fedou C, Bouix O, Raynaud E, Orsetti A. Evaluation of a standardized hyperglucidic breakfast test in postprandial reactive hypoglycaemia. Diabetologia. 1995;38:494–501. doi: 10.1007/BF00410289. [DOI] [PubMed] [Google Scholar]
- Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–282. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Burg MB, Kador PF. Sorbitol, osmoregulation, and the complications of diabetes. J Clin Invest. 1988;81:635–640. doi: 10.1172/JCI113366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–65. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyken AE, Flood V, Empson M, Rochtchina E, Barclay AW, Brand-Miller J, Mitchell P. Carbohydrate nutrition and inflammatory disease mortality in older adults. Am J Clin Nutr. 2010a;92:634–43. doi: 10.3945/ajcn.2010.29390. [DOI] [PubMed] [Google Scholar]
- Buyken AE, Mitchell P, Ceriello A, Brand-Miller J. Optimal dietary approaches for prevention of type 2 diabetes: a life-course perspective. Diabetologia. 2010b;53:406–18. doi: 10.1007/s00125-009-1629-8. [DOI] [PubMed] [Google Scholar]
- Buyken AE, Toeller M, Heitkamp G, Karamanos B, Rottiers R, Muggeo M, Fuller JH EURODIAB IDDM Complications Study Group. Glycemic index in the diet of European outpatients with type 1 diabetes: relations to glycated hemoglobin and serum lipids. Am J Clin Nutr. 2001;73:574–81. doi: 10.1093/ajcn/73.3.574. [DOI] [PubMed] [Google Scholar]
- Cahill GFJ. Starvation in man. N Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cahill GFJ. Starvation in man. Clin Endocrinol Metab. 1976;5:397–415. doi: 10.1016/s0300-595x(76)80028-x. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg PJ, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–21. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Carey VJ, Bishop L, Charleston J, Conlin P, Erlinger T, Laranjo N, McCarron P, Miller E, Rosner B, Swain J, Sacks FM, Appel LJ. Rationale and design of the Optimal Macro-Nutrient Intake Heart Trial to Prevent Heart Disease (OMNI-Heart) Clin Trials. 2005;2:529–37. doi: 10.1191/1740774505cn123oa. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Bortolotti N, Motz E. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care. 1998;21:1529–1533. doi: 10.2337/diacare.21.9.1529. [DOI] [PubMed] [Google Scholar]
- Chanmugam P, Guthrie JF, Cecilio S, Morton JF, Basiotis PP, Anand R. Did fat intake in the United States really decline between 1989–1991 and 1994–1996? J Am Diet Assoc. 2003;103:867–72. doi: 10.1016/s0002-8223(03)00381-x. [DOI] [PubMed] [Google Scholar]
- Charonis AS, Reger LA, Dege JE, Kouzi-Koliakos K, Furcht LT, Wohlhueter RM, Tsilibary EC. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990;39:807–814. doi: 10.2337/diab.39.7.807. [DOI] [PubMed] [Google Scholar]
- Cherepanoff S, McMenamin PG, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2009 Dec 3; doi: 10.1136/bjo.2009.165563. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chew I, Brand JC, Thorburn AW, Truswell AS. Application of glycemic index to mixed meals. Am J Clin Nutr. 1988;47:53–6. doi: 10.1093/ajcn/47.1.53. [DOI] [PubMed] [Google Scholar]
- Chiarelli F, de Martino M, Mezzetti A, Catino M, Morgese G, Cuccurullo F, Verrotti A. Advanced glycation end products in children and adolescents with diabetes: relation to glycemic control and early microvascular complications. J Pediatr. 1999;134:486–91. doi: 10.1016/s0022-3476(99)70208-8. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002 Jun 15;359(9323):2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Chibber R, Molinatti PA, Rosatto N, Lambourne B, Kohner EM. Toxic action of advanced glycation end products on cultured retinal capillary pericytes and endothelial cells: relevance to diabetic retinopathy. Diabetologia. 1997;40:156–64. doi: 10.1007/s001250050657. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack JLT, Hankinson SE, Willett WC, Taylor A. Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am J Clin Nutr. 2006a;83:880–6. doi: 10.1093/ajcn/83.4.880. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A. Does eating particular diets alter risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009a;93:1241–1246. doi: 10.1136/bjo.2008.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Gensler G, Taylor A. Dietary carbohydrate and glycemic index in relation to cortical and nuclear lens opacities in the Age-Related Eye Disease Study. Am J Clin Nutr. 2006b;83:1177–84. doi: 10.1093/ajcn/83.5.1177. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007a;86:180–8. doi: 10.1093/ajcn/86.1.180. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A. Dietary carbohydrate and progression of age-related macular degeneration, a prospective study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2007b;86:1210–1218. doi: 10.1093/ajcn/86.4.1210. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A. Dietary compound score and risk of age-related macular degeneration in the Age-Related Eye Disease Study. Ophthalmology. 2009b;116:939–46. doi: 10.1016/j.ophtha.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Morris MS, Rogers G, Jacques PF, Chylack LTJ, Tung W, Hankinson SE, Willett WC, Taylor A. Carbohydrate intake and glycemic index in relation to the odds of early cortical and nuclear lens opacities. Am J Clin Nutr. 2005;81:1411–1416. doi: 10.1093/ajcn/81.6.1411. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Robman L, McCarty CA, Mukesh BN, Hodge A, Taylor HR, Taylor A. Dietary carbohydrate in relation to cortical and nuclear lens opacities in the Melbourne Visual Impairment Project. Invest Ophthalmol Vis Sci. 2010;51:2897–905. doi: 10.1167/iovs.08-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007;84:229–45. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Hough C, Senatorov V. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2005;45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- Cingle KA, Kalski RS, Bruner WE, O’Brien CM, Erhard P, Wyszynski RE. Age-related changes of glycosidases in human retinal pigment epithelium. Curr Eye Res. 1996;15:433–8. doi: 10.3109/02713689608995834. [DOI] [PubMed] [Google Scholar]
- Clarke M, Dodson PM. PKC inhibition and diabetic microvascular complications. Best Pract Res Clin Endocrinol Metab. 2007;21:573–86. doi: 10.1016/j.beem.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factoralpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Coffe V, Carbajal RC, Salceda R. Glucose metabolism in rat retinal pigment epithelium. Neurochem Res. 2006;31:103–8. doi: 10.1007/s11064-005-9236-7. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Noell WK. Biochemistry of the Retina. New York: Academic Press; 1965. Relationships between visual function and metabolism. [Google Scholar]
- Cohn C. Feeding patterns and some aspects of cholesterol metabolism. Fed Proc. 1964;23:76–81. [PubMed] [Google Scholar]
- Collier G, Giudici S, Kalmusky J. Low glycaemic index starchy foods improve glucose control and lower serum cholesterol in diabetic children. Diabetes Nutr Metab. 1988;1:11–9. [Google Scholar]
- Craven PA, Studer RK, Felder J, Phillips S, DeRubertis FR. Nitric oxide inhibition of transforming growth factor-beta and collagen synthesis in mesangial cells. Diabetes. 1997;46:671–681. doi: 10.2337/diab.46.4.671. [DOI] [PubMed] [Google Scholar]
- Crider JY, Yorio T, Sharif NA, Griffin BW. The effects of elevated glucose on Na+/K(+)-ATPase of cultured bovine retinal pigment epithelial cells measured by a new nonradioactive rubidium uptake assay. J Ocul Pharmacol Ther. 1997;13:337–52. doi: 10.1089/jop.1997.13.337. [DOI] [PubMed] [Google Scholar]
- D’Souza YB, Jones CJ, Bonshek RE. Comparison of lectin binding of drusen, RPE, Bruch’s membrane, and photoreceptors. Mol Vis. 2009;15:906–11. [PMC free article] [PubMed] [Google Scholar]
- D’Amico DJ. Diseases of the retina. N Engl J Med. 1994;331:95–106. doi: 10.1056/NEJM199407143310207. [DOI] [PubMed] [Google Scholar]
- Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2007;143:607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Degenhardt TP, Thorpe SR, Baynes JW. Chemical modification of proteins by methylglyoxal. Cell Mol Biol. 1998;44:1139–45. [PubMed] [Google Scholar]
- Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009;315:1791–7. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Delcourt C, Michel F, Colvez A, Lacroux A, Delage M, Vernet MH POLA Study Group. Association of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237–49. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- Dent MT, Tebbs SE, Gonzalez AM, Ward JD, Wilson RM. Neutrophil aldose reductase activity and its association with established diabetic microvasulcar complications. Diabet Med. 1991;8:439–442. doi: 10.1111/j.1464-5491.1991.tb01628.x. [DOI] [PubMed] [Google Scholar]
- DeRubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes: mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1993;46:271–286. [PubMed] [Google Scholar]
- Dickinson S, Brand-Miller J. Glycemic index, postprandial glycemia and cardiovascular disease. Curr Opin Lipidol. 2005;16:69–75. doi: 10.1097/00041433-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Dickinson S, Hancock DP, Petocz P, Ceriello A, Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am J Clin Nutr. 2008;87:1188–93. doi: 10.1093/ajcn/87.5.1188. [DOI] [PubMed] [Google Scholar]
- Diederen RM, Starnes CA, Berkowitz BA, Winkler BS. Reexamining the hyperglycemic pseudohypoxia hypothesis of diabetic oculopathy. Invest Ophthalmol Vis Sci. 2006;47:2726–31. doi: 10.1167/iovs.06-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Vlassara H, Kirstein M, Yamada Y, Striker GE, Striker LJ. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci USA. 1992;89:2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhofer-Bliesener R, Lechner B, Gerbitz KD. Possible significance of advanced glycation end products in serum in end-stage renal disease and in late complications of diabetes. Eur J Clin Chem Clin Biochem. 1996;34:355–61. doi: 10.1515/cclm.1996.34.4.355. [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35:1491–9. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Leidig MM, Sinclair KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am J Clin Nutr. 2005;81:976–82. doi: 10.1093/ajcn/81.5.976. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Ludwig DS. Treating obesity in youth: should dietary glycemic load be a consideration? Adv Pediatr. 2001;48:179–212. [PubMed] [Google Scholar]
- Edwards AO, Ritter Rr, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enaida H, Kabuyama Y, Oshima Y, Sakamoto T, Kato K, Kochi H, Homma Y. VEGF-dependent signaling in retinal microvascular endothelial cells. Fukushima J Med Sci. 1999;45:77–91. [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Hyperglycemia as a cause of diabetic retinopathy. Metabolism. 1986;35:20–3. doi: 10.1016/0026-0495(86)90182-4. [DOI] [PubMed] [Google Scholar]
- Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- Eye Disease Case-Control Study Group. Risk factors for neovascular age-related macular degeneration. Arch Ophthalmol. 1992;110:1701–1708. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- Fabry P, Tepperman J. Meal frequency—a possible factor in humanpathology. Am J Clin Nutr. 1970;23:1059–68. doi: 10.1093/ajcn/23.8.1059. [DOI] [PubMed] [Google Scholar]
- Fang IM, Yang CH, Yang CM, Chen MS. Overexpression of integrin alpha6 and beta4 enhances adhesion and proliferation of human retinal pigment epithelial cells on layers of porcine Bruch’s membrane. Exp Eye Res. 2009;88:12–21. doi: 10.1016/j.exer.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT. Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch’s membrane of the aging eye. Mol Vis. 1999;5:11. [PubMed] [Google Scholar]
- Farkas TG, Sylvester V, Archer D. The ultrastructure of drusen. Am J Ophthalmol. 1971;71:1196–205. doi: 10.1016/0002-9394(71)90963-9. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Keenan J, Angus DJ, Campbell SE, Garnham AP. Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: effect of the glycemic index. J Appl Physiol. 2000;89:1845–1851. doi: 10.1152/jappl.2000.89.5.1845. [DOI] [PubMed] [Google Scholar]
- Feener EP, Xia P, Inoguchi T, Shiba T, Kunisaki M, King GL. Role of protein kinase C in glucose- and angiotensin II-induced plasminogen activator inhibitor expression. Contrib Nephrol. 1996;118:180–187. doi: 10.1159/000425092. [DOI] [PubMed] [Google Scholar]
- Festa A, Williams K, D’Agostino RJ, Wagenknecht LE, Haffner SM. The Natural Course of [beta]-Cell Function in Nondiabetic and Diabetic Individuals: The Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–20. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- Fong DS, Aiello LP, Ferris FLr, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540–53. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- Fontvieille AM, Acosta M, Rizkalla SW. A moderate switch from high to low glycaemic-index foods for 3 weeks improves the metabolic control of type 1 (IDDM) diabetic subjects. Diabetes Nutr Metab. 1988;1:139–43. [Google Scholar]
- Ford ES, Liu S. Glycemic index and serum high-density lipoprotein cholesterol concentration among us adults. Arch Intern Med. 2001;161:572–6. doi: 10.1001/archinte.161.4.572. [DOI] [PubMed] [Google Scholar]
- Forooghian F, Razavi R, Timms L. Hypoxia-inducible factor expression in human RPE cells. Br J Ophthalmol. 2007;91:1406–10. doi: 10.1136/bjo.2007.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Foulds WS. The choroid circulation and retinal metabolism. Part 2: An overview. Eye. 1990;4:243–248. doi: 10.1038/eye.1990.35. [DOI] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Fraser-Bell S, Wu J, Klein R, Azen SP, Hooper C, Foong AW, Varma R. Cardiovascular risk factors and age-related macular degeneration: The Los Angeles Latino Eye study. Am J Ophthalmol. 2008;145:308–16. doi: 10.1016/j.ajo.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–19. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. doi: 10.1158/0008-5472.CAN-04-3877. [DOI] [PubMed] [Google Scholar]
- Frost G, Keogh B, Smith D, Akinsanya K, Leeds A. The effect of low-glycemic carbohydrate on insulin and glucose response in vivo and in vitro in patients with coronary heart disease. Metabolism. 1996;45:669–72. doi: 10.1016/s0026-0495(96)90129-8. [DOI] [PubMed] [Google Scholar]
- Frost G, Leeds A, Trew G, Margara R, Dornhorst A. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic index diet. Metabolism. 1998;47:1245–51. doi: 10.1016/s0026-0495(98)90331-6. [DOI] [PubMed] [Google Scholar]
- Frost G, Leeds AA, Dore CJ, Madeiros S, Brading S, Dornhorst A. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet. 1999;353:1045–8. doi: 10.1016/s0140-6736(98)07164-5. [DOI] [PubMed] [Google Scholar]
- Frost G, Wilding J, Beecham J. Dietary advice based on the glycaemic index improves dietary profile and metabolic control in type 2 diabetic patients. Diabet Med. 1994;11:397–401. doi: 10.1111/j.1464-5491.1994.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- Gannon M, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53:2375–82. doi: 10.2337/diabetes.53.9.2375. [DOI] [PubMed] [Google Scholar]
- Gao ZQ, Yang C, Wang YY, Wang P, Chen HL, Zhang XD, Liu R, Li WL, Qin XJ, Liang X, Hai CX. RAGE upregulation and nuclear factor-kappaB activation associated with ageing rat cardiomyocyte dysfunction. Gen Physiol Biophys. 2008;27:152–8. [PubMed] [Google Scholar]
- García-Ramírez M, Hernández C, Simó R. Expression of erythropoietin and its receptor in the human retina: a comparative study of diabetic and nondiabetic subjects. Diabetes Care. 2008;31:1189–94. doi: 10.2337/dc07-2075. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Anderson HR, Stitt AW. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J Pathol. 2003;201:328–33. doi: 10.1002/path.1429. [DOI] [PubMed] [Google Scholar]
- Garg A, Bonanome A, Grundy SM, Zhang ZJ, Unger RH. Comparison of a high-carbohydrate diet with a high-monounsaturated-fat diet in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;319:829–34. doi: 10.1056/NEJM198809293191304. [DOI] [PubMed] [Google Scholar]
- Giacco R, Brighenti F, Parillo M, Capuano M, Ciardullo AV, Rivieccio A, Rivellese AA, Riccardi G. Characteristics of some wheat-based foods of the Italian diet in relation to their influence on postprandial glucose metabolism in patients with type 2 diabetes. Br J Nutr. 2001;85:33–40. doi: 10.1079/bjn2000218. [DOI] [PubMed] [Google Scholar]
- Giacco R, Parillo M, Rivellese AA, Lasorella G, Giacco A, D’Episcopo L, Riccardi G. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care. 2000;23:1461–6. doi: 10.2337/diacare.23.10.1461. [DOI] [PubMed] [Google Scholar]
- Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity: a model for intracellular glycosylation in diabetes. J Clin Invest. 1994;94:110–117. doi: 10.1172/JCI117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson HR, Brand-Miller JC, Thorburn AW, Evans S, Chondros P, Werther GA. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care. 2001;24:1137–43. doi: 10.2337/diacare.24.7.1137. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Esposito K. Glucose metabolism and hyperglycemia. Am J Clin Nutr. 2008;87:217S–222S. doi: 10.1093/ajcn/87.1.217S. [DOI] [PubMed] [Google Scholar]
- Glenn JV, Mahaffy H, Wu K, Smith G, Nagai R, Simpson DA, Boulton ME, Stitt AW. Advanced glycation end product (AGE) accumulation on Bruch’s membrane: links to age-related RPE dysfunction. Investig Ophthalmol Vis Sci. 2009;50:441–451. doi: 10.1167/iovs.08-1724. [DOI] [PubMed] [Google Scholar]
- Glenn JV, Stitt AW. The role of advanced glycation end products in retinal ageing and disease. Biochim Biophys Acta. 2009;1790:1109–16. doi: 10.1016/j.bbagen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Goldberg HJ, Whiteside CI, Fantus IG. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-beta I and -delta. J Biol Chem. 2002;277:33833–41. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MOM. Factors associated with age-related macular degeneration. Am J Epidemiol. 1988;128:700–710. doi: 10.1093/oxfordjournals.aje.a115023. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- Granfeldt Y, Wu X, Björck I. Determination of glycaemic index; some methodological aspects related to the analysis of carbohydrate load and characteristics of the previous evening meal. Eur J Clin Nutr. 2006;60:104–12. doi: 10.1038/sj.ejcn.1602273. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Acar N, Keller S, Seeliger M, Gassmann M. Hypoxic preconditioning and erythropoietin protect retinal neurons from degeneration. Adv Exp Med Biol. 2006;588:119–31. doi: 10.1007/978-0-387-34817-9_11. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Remé CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–24. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–30. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hales CN, Randle PJ. Effects of low carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose tolerance tests. Lancet. 1963;1:790–4. doi: 10.1016/s0140-6736(63)91501-0. [DOI] [PubMed] [Google Scholar]
- Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr. 2008;87:339–46. doi: 10.1093/ajcn/87.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 2005;37(Suppl 1):39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Alt A, Niwa T, Clausen JT, Bretzel RG, Brownlee M, Schleicher ED. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia. 1999a;42:728–36. doi: 10.1007/s001250051221. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Brownlee M, Edelstein D, Saleck M, Martin S, Federlin K. Aminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive rat. Diabetologia. 1994;37:32–5. doi: 10.1007/BF00428774. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–9. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, Laqua H. N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999b;40:1855–9. [PubMed] [Google Scholar]
- Hammes HP, Weiss A, Hess S, Araki N, Horiuchi S, Brownlee M, Preissner KT. Modification of vitronectin by advanced glycation alters functional properties in vitro and in the diabetic retina. Lab Invest. 1996;75:325–338. [PubMed] [Google Scholar]
- Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun. 2005;331:718–725. doi: 10.1016/j.bbrc.2005.03.154. [DOI] [PubMed] [Google Scholar]
- Handa JT. The advanced glycation endproduct pentosidine induces the expression of PDGF-B in human retinal pigment epithelial cells. Exp Eye Res. 1998a;66:411–419. doi: 10.1006/exer.1997.0442. [DOI] [PubMed] [Google Scholar]
- Handa JT. Immunohistochemical evidence for deposition of advanced glycation endproducts (AGEs) in Bruch’s membrane and choroid with age. Invest Ophthalmol Vis Sci. 1998b;39:1725–B1606. [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci. 1999;40:775–9. [PubMed] [Google Scholar]
- Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- Hare-Bruun H, Nielsen BM, Grau K, Oxlund AL, Heitmann BL. Should glycemic index and glycemic load be considered in dietary recommendations? Nutr Rev. 2008;66:569–90. doi: 10.1111/j.1753-4887.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–37. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- Hata Y, Rook SL, Aiello LP. Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes. 1999;48:1145–1155. doi: 10.2337/diabetes.48.5.1145. [DOI] [PubMed] [Google Scholar]
- Hellwig-Bürgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- Henkind P. Retinal blood vessels. Neovascularisation, collaterals, and shunts. Trans Ophthalmol Soc N Z. 1981;33:46–50. [PubMed] [Google Scholar]
- Henkind P, Walsh JB. Retinal vascular anomalies. Pathogenesis, appearance, and history. Trans Ophthalmol Soc U K. 1980;100:425–33. [PubMed] [Google Scholar]
- Henry DN, Frank RN, Hootman SR, Rood SE, Heilig CW, Busik JV. Glucose-specific regulation of aldose reductase in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 2000;41:1554–60. [PubMed] [Google Scholar]
- Hodge AM, English DR, O’Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–6. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Friedrichs U, Eichler W, Rosenthal A, Wiedemann P. Advanced glycation end products induce choroidal endothelial cell proliferation, matrix metalloproteinase-2 and VEGF upregulation in vitro. Graefes Arch Clin Exp Ophthalmol. 2002;240:996–1002. doi: 10.1007/s00417-002-0568-6. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–9. [PubMed] [Google Scholar]
- Hollyfield JG, Salomon RG, Crabb JW. Proteomic approaches to understanding age-related macular degeneration. Adv Exp Med Biol. 2003;533:83–89. doi: 10.1007/978-1-4615-0067-4_11. [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Higashi T, Ikeda K, Saishoji T, Jinnouchi Y, Sano H, Shibayama R, Sakamoto T, Araki N. Advanced glycation end products and their recognition by macrophage and macrophage-derived cells. Diabetes. 1996;45(Suppl 3):S73–6. doi: 10.2337/diab.45.3.s73. [DOI] [PubMed] [Google Scholar]
- Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:3713–20. doi: 10.1167/iovs.04-0404. [DOI] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Manson JE, Grodstein F, Colditz GA, Speizer FE, Willett WC. Trends in the incidence of coronary heart disease and changes in diet and lifestyle in women. N Engl J Med. 2000:530–7. doi: 10.1056/NEJM200008243430802. [DOI] [PubMed] [Google Scholar]
- Hu FB, van Dam RM, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44:805–17. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr. 2006;84:70–6. doi: 10.1093/ajcn/84.1.70. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–92. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–8. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- Ido Y, Williamson JR. Hyperglycemic cytosolic reductive stress ‘pseudohypoxia’: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:1467–70. [PubMed] [Google Scholar]
- Ikeda K, Higashi T, Sano H, Jinnouchi Y, Yoshida M, Araki T, Ueda S, Horiuchi S. N (epsilon)-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996;35:8075–83. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- Ishibashi T. Cell biology of intraocular vascular diseases. Jpn J Ophthalmol. 2000;44:323–324. doi: 10.1016/s0021-5155(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, Ryan SJ. Advanced glycation end products in age related macular degeneration. Arch Ophthalmol. 1998;116:1629–1632. doi: 10.1001/archopht.116.12.1629. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ. Formation of drusen in the human eye. Am J Ophthalmol. 1986a;101:342–53. doi: 10.1016/0002-9394(86)90830-5. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Sorgente N, Patterson R, Ryan SJ. Pathogenesis of drusen in the primate. Invest Ophthalmol Vis Sci. 1986b;27:184–93. [PubMed] [Google Scholar]
- Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, Klausner RD, Pause A. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Nat Acad Sci USA. 1999;96:12436–12441. doi: 10.1073/pnas.96.22.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Leung SW. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. doi: 10.1006/geno.1998.5416. [DOI] [PubMed] [Google Scholar]
- Janannathan SN, Connel WF, Bevridge JM. Effect of gormandizing and semicontinuous eating of equicaloric amounts of formula-type high-fat diets on plasma cholesterol and triglyceride levels in humanvolunteer subjects. Am J Clin Nutr. 1964;15:90–3. doi: 10.1093/ajcn/15.2.90. [DOI] [PubMed] [Google Scholar]
- Januszewski AS, Alderson NL, Metz TO, Thorpe SR, Baynes JW. Role of lipids in chemical modification of proteins and development of complications in diabetes. Biochem Soc Trans. 2003;31:1413–1416. doi: 10.1042/bst0311413. [DOI] [PubMed] [Google Scholar]
- Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008;27:596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Järvi AE, Karlström BE, Granfeldt YE, Björck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22:10–8. doi: 10.2337/diacare.22.1.10. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, Corrigan S, Banach MS, Ares S, Mitchell S, Emam A, Augustin LS, Parker TL, Leiter LA. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–53. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Ocana A, Jenkins AL, Wolever TM, Vuksan V, Katzman L, Hollands M, Greenberg G, Corey P, Patten R, et al. Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. Am J Clin Nutr. 1992;55:461–7. doi: 10.1093/ajcn/55.2.461. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Buckley G, Lam KY, Giudici S, Kalmusky J, Jenkins AL, Patten RL, Bird J, Wong GS, et al. Low-glycemic-index starchy foods in the diabetic diet. Am J Clin Nutr. 1988;48:248–54. doi: 10.1093/ajcn/48.2.248. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, Buckley G, Lam Y, Mayer A, Thompson LU. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr. 1987a;46:968–75. doi: 10.1093/ajcn/46.6.968. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Kalmusky J, Guidici S, Giordano C, Patten R, Wong GS, Bird JN, Hall M, Buckley G, et al. Low-glycemic index diet in hyperlipidemia: use of traditional starchy foods. Am J Clin Nutr. 1987b;46:66–71. doi: 10.1093/ajcn/46.1.66. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Ocana AM, Vuksan V, Cunnane SC, Jenkins M, Wong GS, Singer W, Bloom SR, Blendis LM, et al. Metabolic effects of reducing rate of glucose ingestion by single bolus versus continuous sipping. Diabetes. 1990;39:775–81. doi: 10.2337/diab.39.7.775. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Taylor RH. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, Lawrie JA, Bennett CM, Goff DV, Sarson DL, Bloom SR. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr. 1982;35:1339–46. doi: 10.1093/ajcn/35.6.1339. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Cunnane SC, Rao AV, Jenkins AL, Buckley G, Patten R, Singer W, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–34. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, Augustin LSA, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76 (suppl):266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Lewis GP, Talaga KC, Brown MN, Kappel PJ, Fisher SK, Anderson DH, Johnson LV. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44:4481–8. doi: 10.1167/iovs.03-0436. [DOI] [PubMed] [Google Scholar]
- Jones PJ, Leitch CA, Pederson RA. Meal-frequency effects on plasma hormone concentrations and cholesterol synthesisin humans. Am J Clin Nutr. 1993;57:868–74. doi: 10.1093/ajcn/57.6.868. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2002;99:10659–64. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntunen KS, Laaksonen DE, Poutanen KS, Niskanen LK, Mykkanen HM. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am J Clin Nutr. 2003;77:385–91. doi: 10.1093/ajcn/77.2.385. [DOI] [PubMed] [Google Scholar]
- Justilien V, Pang JJ, Renganathan K, Zhan X, Crabb JW, Kim SR, Sparrow JR, Hauswirth WW, Lewin AS. SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci. 2007;48:4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K, Salminen A. Age-relatedmacular degeneration: activation of innate immunity system via pattern recognition receptors. J Mol Med. 2009;87:117–123. doi: 10.1007/s00109-008-0418-z. [DOI] [PubMed] [Google Scholar]
- Kadonaga JT, Courey AJ, Ladika J, Tjian R. Distinct regions of Sp1 modulate DNA binding and transcriptional activation. Science. 1988;242:1566–1570. doi: 10.1126/science.3059495. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kalfa TA, Gerritsen ME, Carlson EC, Binstock AJ, Tsilibary EC. Altered proliferation of retinal microvascular cells on glycated matrix. Invest Ophthalmol Vis Sci. 1995;36:2358–67. [PubMed] [Google Scholar]
- Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–5. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–11. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- Kanwar M, Kowluru RA. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58:227–34. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara E, Lin LR, Ho YS, Reddy VN. SOD2 protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46:3426–34. doi: 10.1167/iovs.05-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasajima H. Enhanced in situ expression of aldose reductase in peripheral nerve and renal glomeruli in diabetic patients. Virchows Arch. 2000;439:46–54. doi: 10.1007/s004280100444. [DOI] [PubMed] [Google Scholar]
- Kasper M, Schinzel R, Niwa T, Münch G, Witt M, Fehrenbach H, Wilsch-Bräuninger M, Pehlke K, Hofer A, Funk RH. Experimental induction of AGEs in fetal L132 lung cells changes the level of intracellular cathepsin D. Biochem Biophys Res Commun. 1999;261:175–182. doi: 10.1006/bbrc.1999.0902. [DOI] [PubMed] [Google Scholar]
- Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–47. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P. Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr. 2008;88:1104–10. doi: 10.1093/ajcn/88.4.1104. [DOI] [PubMed] [Google Scholar]
- Keogh RJ, Dunlop ME, GLR Effect of inhibition of aldose reductase on glucose flux, diacylglycerol formation, protein kinase C, and phospholipase A2 activation. Metabolism. 1997;46:41–47. doi: 10.1016/s0026-0495(97)90165-7. [DOI] [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–8. [PubMed] [Google Scholar]
- Kiens B, Richter EA. Types of carbohydrate in an ordinary diet affect insulin action and muscle substrates in humans. Am J Clin Nutr. 1996;63:47–53. doi: 10.1093/ajcn/63.1.47. [DOI] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–30. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006a;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WGJ. Failure to prolyl hydroxylate hypoxia-inducible factor a phenocopies VHL inactivation in vivo. EMBO J. 2006b;25:4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Jung DH, Kim NH, Lee YM, Jang DS, Song GY, Kim JS. KIOM-79 inhibits high glucose or AGEs-induced VEGF expression in human retinal pigment epithelial cells. J Ethnopharmacol. 2007;112:166–72. doi: 10.1016/j.jep.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Aston C, Hintz R, Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992;90:439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Jeensen SC. The relationship of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy. The Beaver Dam Eye study. Ophthalmology. 1997;104:1804–12. doi: 10.1016/s0161-6420(97)30023-2. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE. Diabetes, hyperglycemia, and age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1527–34. doi: 10.1016/s0161-6420(92)31770-1. [DOI] [PubMed] [Google Scholar]
- Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145:317–26. doi: 10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Nomura M, Nishioka T, Kikuchi M, Ishihara A, Nagai R, Hagino N. Overproduction of N(epsilon)-(carboxymethyl)lysine-induced neovascularization in cultured choroidal explant of aged rat. Biol Pharm Bull. 2007;30:133–138. doi: 10.1248/bpb.30.133. [DOI] [PubMed] [Google Scholar]
- Kociok N, Heppekausen H, Schraermeyer U, Esser P, Thumann G, Gristanti G, Heimann K. The mRNA expression of cytokines and their receptors in cultured iris pigment epithelial cells: a comparison with retinal pigment epithelial cells. Exp Eye Res. 1998;67:237–250. doi: 10.1006/exer.1998.0517. [DOI] [PubMed] [Google Scholar]
- Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, Turner RC. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- Kohner EM, Stratton IM, Aldington SJ, Holman RR, Matthews DR UK Prospective Diabetes Study (UKPDS) Group. Relationship between the severity of retinopathy and progression to photocoagulation in patients with Type 2 diabetes mellitus in the UKPDS (UKPDS 52) Diabet Med. 2001;18:178–84. doi: 10.1046/j.1464-5491.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp W. The atherogenic potential of dietary carbohydrate. Prev Med. 2006;42:336–42. doi: 10.1016/j.ypmed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of crosstalk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.12.047. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–87. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115–126. doi: 10.1172/JCI119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Rosenberg L, Singer M, Hu FB, Djoussé L, Cupples LA, Palmer JR. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med. 2007;167:2304–9. doi: 10.1001/archinte.167.21.2304. [DOI] [PubMed] [Google Scholar]
- Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- Kuhla B, Lüth HJ, Haferburg D, Boeck K, Arendt T, Münch G. Methylglyoxal, glyoxal, and their detoxification in Alzheimer’s disease. Ann N Y Acad Sci. 2005;1043:211–6. doi: 10.1196/annals.1333.026. [DOI] [PubMed] [Google Scholar]
- Kunt T, Forst T, Harzer O, Buchert G, Pfützner A, Löbig M, Zschäbitz A, Stofft E, Engelbach MJB. The influence of advanced glycation endproducts (AGE) on the expression of human endothelial adhesion molecules. Exp Clin Endocrinol Diabetes. 1998;106:183–8. doi: 10.1055/s-0029-1211974. [DOI] [PubMed] [Google Scholar]
- Kusner LL, Sarthy VP, Mohr S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Müller cells. Invest Ophthalmol Vis Sci. 2004;45:1553–61. [PubMed] [Google Scholar]
- Laaksonen DE, Toppinen LK, Juntunen KS, Autio K, Liukkonen KH, Poutanen KS, Niskanen L, Mykkanen HM. Dietary carbohydrate modification enhanced insulin secretion in persons with the metabolic syndrome. Am J Clin Nutr. 2005;82:1218–27. doi: 10.1093/ajcn/82.6.1218. [DOI] [PubMed] [Google Scholar]
- Lafrance L, Rabasa-Lhoret R, Poisson D, Ducros F, Chiasson JL. Effects of different glycaemic index foods and dietary fibre intake on glycaemic control in type 1 diabetic patients on intensive insulin therapy. Diabet Med. 1998;15:972–8. doi: 10.1002/(SICI)1096-9136(1998110)15:11<972::AID-DIA704>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lal S, Szwergold BS, Taylor AH, Randall WC, Kappler F, Wells-Knecht K, Baynes JW, Brown TR. Metabolism of fructose-3-phosphate in the diabetic rat lens. Arch Biochem Biophys. 1995;318:191–199. doi: 10.1006/abbi.1995.1220. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Lau C, Faerch K, Glümer C, Tetens I, Pedersen O, Carstensen B, Jørgensen T, Borch-Johnsen K Inter99 study. Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance: the Inter99 study. Diabetes Care. 2005;28:1397–403. doi: 10.2337/diacare.28.6.1397. Erratum in: Diabetes Care. 2005 Sep;28(9):2340–1. [DOI] [PubMed] [Google Scholar]
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992;15:442–55. doi: 10.2337/diacare.15.3.442. [DOI] [PubMed] [Google Scholar]
- Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–33. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Hennis A, Nemesure B, Yang L, Hyman L, Schachat AP Barbados Eye Studies Group. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology. 2006;113:29–35. doi: 10.1016/j.ophtha.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycemia. Diabetes. 1981;30:996–999. doi: 10.2337/diab.30.12.996. [DOI] [PubMed] [Google Scholar]
- Li YM, Dickson DW. Enhanced binding of advanced glycation endproducts (AGE) by the ApoE4 isoform links the mechanism of plaque deposition in Alzheimer’s disease. Neurosci Lett. 1997;226:155–158. doi: 10.1016/s0304-3940(97)00266-8. [DOI] [PubMed] [Google Scholar]
- Li YM, Mitsuhashi T, Wojciechowicz D, Shimizu N, Li J, Stitt A, He C, Banerjee D, Vlassara H. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996;93:11047–52. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese AD, Schulz M, Fang F, Wolever TM, D’Agostino RBJ, Sparks KC, Mayer-Davis EJ. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2832–8. doi: 10.2337/diacare.28.12.2832. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Erythropoietin for neurologic protection and diabetic neuropathy. N Engl J Med. 2004;350:2516–7. doi: 10.1056/NEJMcibr041121. [DOI] [PubMed] [Google Scholar]
- Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008;15:642–649. doi: 10.1038/sj.cdd.4402315. [DOI] [PubMed] [Google Scholar]
- Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–33. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease. J Am Coll Nutr. 2002;21:298–306. doi: 10.1080/07315724.2002.10719227. [DOI] [PubMed] [Google Scholar]
- Liu S, Manson JE. Dietary carbohydrates, physical inactivity, obesity, and the ‘metabolic syndrome’ as predictors of coronary heart disease. Curr Opin Lipidol. 2001;12:395–404. doi: 10.1097/00041433-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol. 2006;126:291–299. doi: 10.1038/sj.jid.5700070. [DOI] [PubMed] [Google Scholar]
- Lorenzi M, Gerhardinger C. Early cellular and microvascular changes induced by diabetes in the retina. Diabetologia. 2001;44:791–804. doi: 10.1007/s001250100544. [DOI] [PubMed] [Google Scholar]
- Louie JL, Kapphahn RJ, Ferrington DA. Proteasome function and protein oxidation in the aged retina. Exp Eye Res. 2002;75:271–84. [PubMed] [Google Scholar]
- Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998;101:1219–24. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. Clinical update: the low-glycaemic-index diet. Lancet. 2007;369:890–2. doi: 10.1016/S0140-6736(07)60427-9. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:e26. doi: 10.1542/peds.103.3.e26. serial online. [DOI] [PubMed] [Google Scholar]
- Luscombe ND, Noakes M, Clifton PM. Diets high and low in glycemic index versus high monounsaturated fat diets: effects on glucose and lipid metabolism in NIDDM. Eur J Clin Nutr. 1999;53:473–8. doi: 10.1038/sj.ejcn.1600779. [DOI] [PubMed] [Google Scholar]
- Luthra S, Fardin B, Dong J, Hertzog D, Kamjoo S, Gebremariam S, Butani V, Narayanan R, Mungcal JK, Kuppermann BD, Kenney MC. Activation of caspase-8 and caspase-12 pathways by 7-ketocholesterol in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5569–75. doi: 10.1167/iovs.06-0333. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Silvestri G, Dunn JA, Dyer DG, Baynes JW. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes. 1991;40:1010–5. doi: 10.2337/diab.40.8.1010. [DOI] [PubMed] [Google Scholar]
- Ma W, Lee SE, Guo J, Qu W, Hudson BI, Schmidt AM, Barile GR. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007;48:1355–61. doi: 10.1167/iovs.06-0738. [DOI] [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–9. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–27. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- Maeda A, Crabb JW, Palczewski K. Microsomal glutathione S-transferase 1 in the retinal pigment epithelium: protection against oxidative stress and a potential role in aging. Biochemistry. 2005;44:480–9. doi: 10.1021/bi048016f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1, a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait M, Gomez P, van Veen TA, Parys JB, De Smedt H, Vereecke J, Himpens B. Effects of hyperglycemia and protein kinase C on connexin43 expression in cultured rat retinal pigment epithelial cells. J Membr Biol. 2001;181:31–40. doi: 10.1007/s0023200100082. [DOI] [PubMed] [Google Scholar]
- Mamputu JC, Renier G. Advanced glycation end-products increase monocyte adhesion to retinal endothelial cells through vascular endothelial growth factor-induced ICAM-1 expression: inhibitory effect of antioxidants. J Leukoc Biol. 2004;75:1062–9. doi: 10.1189/jlb.0603265. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack D, Vucic D, French D, Lee W, Roose-Girma M, Erickson S, Dixit V. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Masson E, Troncy L, Ruggiero D, Wiernsperger N, Lagarde M, El Bawab S. a-Series gangliosides mediate the effects of advanced glycation end products on pericyte and mesangial cell proliferation: a common mediator for retinal and renal microangiopathy? Diabetes. 2005a;54:220–7. doi: 10.2337/diabetes.54.1.220. [DOI] [PubMed] [Google Scholar]
- Masson E, Wiernsperger N, Lagarde M, El Bawab S. Involvement of gangliosides in glucosamine-induced proliferation decrease of retinal pericytes. Glycobiology. 2005b;15:585–91. doi: 10.1093/glycob/cwi039. [DOI] [PubMed] [Google Scholar]
- McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW, Mann JI. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Glenn JV, MLA Characterisation of the advanced glycation endproduct receptor complex in the retinal pigment epithelium. Br J Ophthalmol. 2005;89:107–12. doi: 10.1136/bjo.2004.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, Jacques PF. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr. 2009;28:150–8. doi: 10.1080/07315724.2009.10719766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–46. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- McMillan-Price J, Petocz P, Atkinson F, O’neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166:1466–75. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–69. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- Mettler S, Lamprecht-Rusca F, Stoffel-Kurt N, Wenk C, Colombani PC. The influence of the subjects’ training state on the glycemic index. Eur J Clin Nutr. 2007;61:19–24. doi: 10.1038/sj.ejcn.1602480. [DOI] [PubMed] [Google Scholar]
- Meyer KA, Kushi LH, Jacobs DRJ, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921–30. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- Miceli MV, Newsome DA, Schriver GW. Glucose uptake, hexose monophosphate shunt activity, and oxygen consumption in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1990;31:277–83. [PubMed] [Google Scholar]
- Middleton Fillmore K, Chikritzhs T, Stockwell T, Bostrom A, Pascal R. Alcohol use and prostate cancer: a meta-analysis. Mol Nutr Food Res. 2009;53:240–55. doi: 10.1002/mnfr.200800122. [DOI] [PubMed] [Google Scholar]
- Miller CK, Gabbay RA, Dillon J, Apgar J, Miller D. The effect of three snack bars on glycemic response in healthy adults. J Am Diet Assoc. 2006;106:745–8. doi: 10.1016/j.jada.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Wang JJ. Diabetes, fasting blood glucose and age-related maculopathy: The Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1999;27:197–9. doi: 10.1046/j.1440-1606.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin Ophthalmol. 1999;14:233–9. doi: 10.3109/08820539909069542. [DOI] [PubMed] [Google Scholar]
- Miyata S, Liu BF, Shoda H, Ohara T, Yamada H, Suzuki K, Kasuga M. Accumulation of pyrraline-modified albumin in phagocytes due to reduced degradation by lysosomal enzymes. J Biol Chem. 1997;272:4037–4042. doi: 10.1074/jbc.272.7.4037. [DOI] [PubMed] [Google Scholar]
- Miyata T, van Ypersele de Strihou C, Imasawa T, Yoshino A, Ueda Y, Ogura H, Kominami K, Onogi H, Inagi R, Nangaku M, Kurokawa K. Glyoxalase I deficiency is associated with an unusual level of advanced glycation end products in a hemodialysis patient. Kidney Int. 2001;60:2351–9. doi: 10.1046/j.1523-1755.2001.00051.x. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–90. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. Potential new strategies to prevent the development of diabetic retinopathy. Expert Opin Investig Drugs. 2004;13:189–98. doi: 10.1517/13543784.13.3.189. [DOI] [PubMed] [Google Scholar]
- Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–9. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients. Diabetes Care. 2003;26:881–5. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Sell DR, Nagaraj RH, Miyata S, Grandhee S, Odetti P, Ibrahim SA. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41(Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Clover GM. The effect of age on the macromolecular permeability of human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:2970–5. [PubMed] [Google Scholar]
- Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch’s membrane. Invest Ophthalmol Vis Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, Campbell IL, Stitt AW, Gardiner TA, Archer DB, Adamis AP. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci. 2003;44:4457–64. doi: 10.1167/iovs.02-1063. [DOI] [PubMed] [Google Scholar]
- Morcos M, Du X, Pfisterer F, Hutter H, Sayed AA, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, Schlotterer A, Bozorgmehr F, El Baki RA, Stern D, Moehrlen F, Ibrahim Y, Oikonomou D, Hamann A, Becker C, Zeier M, Schwenger V, Miftari N, Humpert P, Hammes HP, Buechler M, Bierhaus A, Brownlee M, Nawroth PP. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–9. doi: 10.1111/j.1474-9726.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Lorelli W, Campochiaro PA. Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem. 1999;74:135–143. [PubMed] [Google Scholar]
- Mowat FM, Luhmann UF, Smith AJ, Lange C, Duran Y, Harten S, Shukla D, Maxwell PH, Ali RR, Bainbridge JW. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS One. 2010;5:e11103. doi: 10.1371/journal.pone.0011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder DJ, Bieze M, Graaff R, Smit A, Hooymans A. Skin Autofluorescence is Elevated in Neovascular Age-Related Macular Degeneration. Br J Ophthalmol. 2010;94:622–5. doi: 10.1136/bjo.2009.162990. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. Faseb J. 2000;14:835–46. [PubMed] [Google Scholar]
- Murata T, Nagai R, Ishibashi T, Inomuta H, Ikeda K, Horiuchi S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia. 1997;40:764–9. doi: 10.1007/s001250050747. [DOI] [PubMed] [Google Scholar]
- Nakashima E, Pop-Busui R, Towns R, Thomas TP, Hosaka Y, Nakamura J, Greene DA, Killen PD, Schroeder J, Larkin DD, Ho YL, Stevens MJ. Regulation of the human taurine transporter by oxidative stress in retinal pigment epithelial cells stably transformed to overexpress aldose reductase. Antioxid Redox Signal. 2005;7:1530–42. doi: 10.1089/ars.2005.7.1530. [DOI] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–5004. [PubMed] [Google Scholar]
- Nishikawa T, Araki E. Investigation of a novel mechanism of diabetic complications: impacts of mitochondrial reactive oxygen species. Rinsho Byori. 2008;56:712–9. [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Node K, Inoue T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol. 2009;8:23. doi: 10.1186/1475-2840-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WSJ, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes. 2004;53:2931–2938. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Fathallah L, Stevens MJ. Taurine counteracts oxidative stress and nerve growth factor deficit in early experimental diabetic neuropathy. Exp Neurol. 2001;172:211–219. doi: 10.1006/exnr.2001.7789. [DOI] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Ikemoto M, Arai H, Miyazaki A, Hakamata H, Horiuchi S, Nakayama H. CD36: serves as a receptor for advanced glycation endproducts (AGE) J Diabetes Complications. 2002;16:56–9. doi: 10.1016/s1056-8727(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nature Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Okubo A, Rosa RHJ, Bunce CV, Alexander RA, Fan JT, Bird AC, Luthert PJ. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Invest Ophthalmol Vis Sci. 1999;40:443–449. [PubMed] [Google Scholar]
- Okubo A, Sameshima M, Unoki K, Uehara F, Bird AC. Ultrastructural changes associated with accumulation of inclusion bodies in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2000;41:4305–4312. [PubMed] [Google Scholar]
- Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF. Analysis of glucose metabolism in diabetic rat retinas. Am J Physiol Endocrinol Metab. 2006;290:E1057–67. doi: 10.1152/ajpendo.00323.2005. [DOI] [PubMed] [Google Scholar]
- Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract. 1998;41:131–7. doi: 10.1016/s0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J Biol Chem. 2000;275:21177–84. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Paget C, Lecomte M, Ruggiero D, Wiernsperger N, Lagarde M. Modification of enzymatic antioxidants in retinal microvascular cells by glucose or advanced glycation end products. Free Radic Biol Med. 1998;25:121–9. doi: 10.1016/s0891-5849(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Pala V, Sieri S, Masala G, Palli D, Panico S, Vineis P, Sacerdote C, Mattiello A, Galasso R, Salvini S, Ceroti M, Berrino F, Fusconi E, Tumino R, Frasca G, Riboli E, Trichopoulou A, Baibas N, Krogh V. Associations between dietary pattern and lifestyle, anthropometry and other health indicators in the elderly participants of the EPIC-Italy cohort. Nutr Metab Cardiovasc Dis. 2006;16:186–201. doi: 10.1016/j.numecd.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Parillo M, Giacco R, Ciardullo AV, Rivellese AA, Riccardi G. Does a high-carbohydrate diet have different effects in NIDDM patients treated with diet alone or hypoglycemic drugs? Diabetes Care. 1996;19:498–500. doi: 10.2337/diacare.19.5.498. [DOI] [PubMed] [Google Scholar]
- Parillo M, Giacco R, Riccardi G, Pacioni D, Rivellese A. Different glycaemic responses to pasta, bread, and potatoes in diabetic patients. Diabetic Med. 1985;2:374–7. doi: 10.1111/j.1464-5491.1985.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Park EJ, Kong D, Fisher R, Cardellina J, Shoemaker RH, Melillo G. Targeting the PAS-A domain of HIF-1alpha for development of small molecule inhibitors of HIF-1. Cell Cycle. 2006;5:1847–53. doi: 10.4161/cc.5.16.3019. [DOI] [PubMed] [Google Scholar]
- Park YK, Ahn DR, Oh M, Lee T, Yang EG, Son M, Park H. Nitric oxide donor, (+/−)-S-nitroso-N-acetylpenicillamine, stabilizes transactive hypoxia-inducible factor-1alpha by inhibiting von Hippel–Lindau recruitment and asparagine hydroxylation. Mol Pharmacol. 2008;74:236–245. doi: 10.1124/mol.108.045278. [DOI] [PubMed] [Google Scholar]
- Pascuzzo GJ, Johnson JE, Pautler EL. Glucose transport in isolated mammalian pigment epithelium. Exp Eye Res. 1980;30:53–58. doi: 10.1016/0014-4835(80)90123-2. [DOI] [PubMed] [Google Scholar]
- Patel AV, McCullough ML, Pavluck AL, Jacobs EJ, Thun MJ, Calle EE. Glycemic load, glycemic index, and carbohydrate intake in relation to pancreatic cancer risk in a large US cohort. Cancer Causes Control. 2007;18:287–94. doi: 10.1007/s10552-006-0081-z. [DOI] [PubMed] [Google Scholar]
- Pawlak AM, Glenn JV, Beattie JR, McGarvey JJ, Stitt AW. Advanced glycation as a basis for understanding retinal aging and noninvasive risk prediction. Ann N Y Acad Sci. 2008;1126:59–65. doi: 10.1196/annals.1433.066. [DOI] [PubMed] [Google Scholar]
- Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364:778–85. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- Pecoraro RE, Chen MS, Porte DJ. Glycosylated hemoglobin and fasting plasma glucose in the assessment of outpatient glycemic control in NIDDM. Diabetes Care. 1982;5:592–9. doi: 10.2337/diacare.5.6.592. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Jacobs DRJ, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–55. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J Biol Chem. 2007;282:36330–40. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R, Van Huysen C, Beyer L, Stevens MJ. Attenuation of nerve vascular and functional deficits by taurine in the streptozotocin-diabetic rat. Diabetes. 1999;47:A229. [Google Scholar]
- Portilla D, Dai G, Peters JM, Gonzalez FJ, Crew MD, Proia AD. Etomoxir -induced PPARalpha-modulated enzymes protect during acute renal failure. Am J Physiol Renal Physiol. 2000;278:F667–F675. doi: 10.1152/ajprenal.2000.278.4.F667. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem. 2006;387:1337–1346. doi: 10.1515/BC.2006.167. [DOI] [PubMed] [Google Scholar]
- Prow TW, Bhutto I, Grebe R, Uno K, Merges C, McLeod DS, Lutty GA. Nanoparticle-delivered biosensor for reactive oxygen species in diabetes. Vision Res. 2008;48:478–85. doi: 10.1016/j.visres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese G, Pricci F, Iacobini C, Leto G, Amadio L, Barsotti P, Frigeri L, Hsu DK, Vlassara H, Liu FT, Di Mario U. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J. 2001;15:2471–9. doi: 10.1096/fj.01-0006com. [DOI] [PubMed] [Google Scholar]
- Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, Brownlee M, Preissner KT. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes. 2010;59:670–8. doi: 10.2337/db08-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulian G, Rusu E, Dragomir A, Posea M. Metabolic effects of low glycaemic index diets. Nutr J. 2009;8:5. doi: 10.1186/1475-2891-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoczy PE, Baines M, Kennedy CJ, Constable IJ. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp Eye Res. 1996;63:159–167. doi: 10.1006/exer.1996.0104. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–29. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci USA. 2005;102:5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr Res. 1999;19:305–323. [Google Scholar]
- Rapino C, Bianchi G, Di Giulio C, Centurione L, Cacchio M, Antonucci A, Cataldi A. HIF-1alpha cytoplasmic accumulation is associated with cell death in old rat cerebral cortex exposed to intermittent hypoxia. Aging Cell. 2005;4:177–185. doi: 10.1111/j.1474-9726.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- Read NW, Welch IM, Austen CJ, Barnish C, Bartlett CE, Baxter AJ, Brown G, Compton ME, Hume KE, Storie I, Worlding J. Swallowing food without chewing; a simple way to reduce postprandial glycaemia. Br J Nutr. 1986;55:43–7. doi: 10.1079/bjn19860008. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–4. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87:269S–274S. doi: 10.1093/ajcn/87.1.269S. [DOI] [PubMed] [Google Scholar]
- Riddle MC. Evening insulin strategy. Diabetes Care. 1990;13:676–86. doi: 10.2337/diacare.13.6.676. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ritz P, Krempf M, Cloarec D, Champ M, Charbonnel B. Comparative continuous-indirect-calorimetry study of two carbohydrates with different glycemic indices. Am J Clin Nutr. 1991;54:855–859. doi: 10.1093/ajcn/54.5.855. [DOI] [PubMed] [Google Scholar]
- Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27:1866–72. doi: 10.2337/diacare.27.8.1866. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fontal M, Alfaro V, Kerrison JB, Jablon EP. Ranibizumab for diabetic retinopathy. Curr Diabetes Rev. 2009;5:47–51. doi: 10.2174/157339909787314239. [DOI] [PubMed] [Google Scholar]
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–8. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- Ruggiero-Lopez D, Rellier N, Lecomte M, Lagarde M, Wiernsperger N. Growth modulation of retinal microvascular cells by early and advanced glycation products. Diabetes Res Clin Pract. 1997;34:135–42. doi: 10.1016/s0168-8227(96)01352-6. [DOI] [PubMed] [Google Scholar]
- Rumble JR, Cooper ME, Soulis T, Cox A, Wu L, Youssef S, Jasik M, Jerums G, Gilbert RE. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J Clin Invest. 1997;99:1016–1027. doi: 10.1172/JCI119229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryhnen T, Hyttinen JM, Kopitz J, Rilla K, Kuusisto E, Mannermaa E, Viiri J, Holmberg CI, Immonen I, Meri S, Parkkinen J, Eskelinen EL, Uusitalo H, Salminen A, Kaarniranta K. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J Cell Mol Med. 2009;13:3616–31. doi: 10.1111/j.1582-4934.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun NR, Anderson AL, Tylavsky FA, Lee JS, Sellmeyer DE, Harris TB, Health A Body Composition Study. Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am J Clin Nutr. 2008;87:126–31. doi: 10.1093/ajcn/87.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Melia M, Vinores SA, Campochiaro PA. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:210–9. doi: 10.1002/jcp.10238. [DOI] [PubMed] [Google Scholar]
- Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997a;20:545–50. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997b;277:472–7. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–24. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- Sandau KB, Zhou J, Kietzmann T, Brune B. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001;276:39805–39811. doi: 10.1074/jbc.M107689200. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Agrón E, Clemons TE, Ferris FLr, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R, Sperduto RD Age-Related Eye Disease Study Research Group. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–9. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Nagai R, Matsumoto K, Horiuchi S. Receptors for proteins modified by advanced glycation endproducts (AGE)--their functional role in atherosclerosis. Mech Ageing Dev. 1999;107:333–46. doi: 10.1016/s0047-6374(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83:358–68. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Mori F, Igarashi S, Abiko T, Takeda M, Ishiko S, Yoshida A. Corneal advanced glycation end products increase in patients with proliferative diabetic retinopathy. Diabetes Care. 2001;24:479–82. doi: 10.2337/diacare.24.3.479. [DOI] [PubMed] [Google Scholar]
- Sato S, Lin LR, Reddy VN, Kador PF. Aldose reductase in human retinal pigment epithelial cells. Exp Eye Res. 1993;57:235–41. doi: 10.1006/exer.1993.1119. [DOI] [PubMed] [Google Scholar]
- Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci USA. 1997;94:11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk CG, Ligtvoet N, Twaalfhoven H, Jager A, Blaauwgeers HG, Schlingemann RO, Tarnow L, Parving HH, Stehouwer CD, van Hinsbergh VW. Amadori albumin in type 1 diabetic patients: correlation with markers of endothelial function, association with diabetic nephropathy, and localization in retinal capillaries. Diabetes. 1999;48:2446–53. doi: 10.2337/diabetes.48.12.2446. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–8. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Schmidtz HD. Reversible nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase upon serum depletion. Eur J Cell Biol. 2001;80:419–427. doi: 10.1078/0171-9335-00174. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–56. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663–8. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- Scribner KB, Pawlak DB, Aubin CM, Majzoub JA, Ludwig DS. Long-term effects of dietary glycemic index on adiposity, energy metabolism, and physical activity in mice. Am J Physiol Endocrinol Metab. 2008;295:E1126–31. doi: 10.1152/ajpendo.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner KB, Pawlak DB, Ludwig DS. Hepatic steatosis and increased adiposity in mice consuming rapidly vs. slowly absorbed carbohydrate. Obesity (Silver Spring) 2007;15:2190–9. doi: 10.1038/oby.2007.260. [DOI] [PubMed] [Google Scholar]
- Sebeková K, Schinzel R, Ling H, Simm A, Xiang G, Gekle M, Münch G, Vamvakas S, Heidland A. Advanced glycated albumin impairs protein degradation in the kidney proximal tubules cell line LLC-PK1. Cell Mol Biol. 1998;44:1051–1060. [PubMed] [Google Scholar]
- Seko Y, Seko Y, Fujikura H, Pang J, Tokoro T, Shimokawa H. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest Ophthalmol Vis Sci. 1999;40:3287–3291. [PubMed] [Google Scholar]
- Sell DR, Lapolla A, Odetti P, Fogarty J, Monnier VM. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes. 1992;41:1286–92. doi: 10.2337/diab.41.10.1286. [DOI] [PubMed] [Google Scholar]
- Semba RD, Ferrucci L, Fink JC, Sun K, Beck J, Dalal M, Guralnik JM, Fried LP. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis. 2009;53:51–8. doi: 10.1053/j.ajkd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah VO, Dorin RI, Sun Y, Braun M, Zager PG. Aldose reductase gene expression is increased in diabetic nephropathy. J Clin Endocrinol Metab. 1997;82:2294–2298. doi: 10.1210/jcem.82.7.4082. [DOI] [PubMed] [Google Scholar]
- Sharma NK, Gardiner TA, Archer DB. A morphologic and autoradiographic study of cell death and regeneration in the retinal microvasculature of normal and diabetic rats. Am J Ophthalmol. 1985;100:51–60. doi: 10.1016/s0002-9394(14)74982-7. [DOI] [PubMed] [Google Scholar]
- Shikany JM, Tinker LF, Neuhouser ML, Ma Y, Patterson RE, Phillips LS, Liu S, Redden DT. Association of glycemic load with cardiovascular disease risk factors: The Women’s Health Initiative Observational Study. Nutrition. 2010;26:641–7. doi: 10.1016/j.nut.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142–7. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Dikshit M, Srimal RC. Nitric oxide-dependent blood–brain barrier permeability alteration in the rat brain. Experientia. 1996;52:136–140. doi: 10.1007/BF01923358. [DOI] [PubMed] [Google Scholar]
- Simó R, Hernández C. Advances in the Medical Treatment of Diabetic Retinopathy. Diabetes Care. 2009;32:1556–1562. doi: 10.2337/dc09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Stewart JM. Pathophysiology of diabetic macular edema. Int Ophthalmol Clin. 2009;49:1–11. doi: 10.1097/IIO.0b013e31819fd164. [DOI] [PubMed] [Google Scholar]
- Skolnik EY, Yang Z, Makita Z, Radoff S, Kirstein M, Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991;174:931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrod B, Melkko J, Araki N, Sano H, Horiuchi S. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J. 1997;322:567–573. doi: 10.1042/bj3220567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:5495–504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–43. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- Sone H, Kawakami Y, Okuda Y, Kondo S, Hanatani M, Suzuki H, Yamashita K. Vascular endothelial growth factor is induced by long-term high glucose concentration and upregulated by acute glucose deprivation in cultured bovine retinal pigmented epithelial cells. Biochem Biophys Res Commun. 1996;221:193–198. doi: 10.1006/bbrc.1996.0568. [DOI] [PubMed] [Google Scholar]
- Stecyk JA, Stensløkken KO, Farrell AP, Nilsson GE. Maintained cardiac pumping in anoxic Crucian carp. Science. 2004;306:77. doi: 10.1126/science.1100763. [DOI] [PubMed] [Google Scholar]
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- Stevens J, Ahn K, Juhaeri Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care. 2002;25:1715–21. doi: 10.2337/diacare.25.10.1715. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Feldman EL, Thomas TP, Greene DA. The pathogenesis of diabetic neuropathy. In: Veves A, Conn PMC, editors. Clinical Management of Diabetic Neuropathy. Totowa, NJ: Humana Press; 1997. pp. 13–47. [Google Scholar]
- Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Physiol. 1999;277:E760–E771. doi: 10.1152/ajpendo.1999.277.4.E760. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Larkin D, Hosaka Y, Porcellati C, Thomas TP, Masterson JM, Killen PD, Greene DA. Suppression of endogenous osmoregulatory genes in human retinal pigment epithelial cells transfected to overpress aldose reductase. Diabetologia. 1997;40:A491. [Google Scholar]
- Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–53. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW. The Maillard Reaction in Eye Diseases. Ann NY Acad Sci. 2005;1043:582–597. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Bhaduri T, McMullen CB, Gardiner TA, Archer DB. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell Biol Res Commun. 2000;3:380–8. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- Stitt AW, He C, Vlassara H. Characterization of the advanced glycation end-product receptor complex in human vascular endothelial cells. Biochem Biophys Res Commun. 1999;256:549–56. doi: 10.1006/bbrc.1999.0291. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Hughes SJ, Canning P, Lynch O, Cox O, Frizzell N, Thorpe SR, Cotter TG, Curtis TM, Gardiner TA. Substrates modified by advanced glycation end-products cause dysfunction and death in retinal pericytes by reducing survival signals mediated by platelet-derived growth factor. Diabetologia. 2004;47:1735–46. doi: 10.1007/s00125-004-1523-3. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150:523–31. [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, McGoldrick C, Rice-McCaldin A, McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR, Gardiner TA. Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–94. doi: 10.2337/diabetes.54.3.785. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Studer RK, Craven PA, Derubertis FR. Role for protein kinase C in the mediation of increased fibronectin accumulation by mesangial cells grown in high-glucose medium. Diabetes. 1993;42:118–126. doi: 10.2337/diab.42.1.118. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Miyata T, Ueda Y, Tanaka H, Maeda K, Kawashima S, Van Ypersele de Strihou C, Kurokawa K. Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol. 1998;9:1681–8. doi: 10.1681/ASN.V991681. [DOI] [PubMed] [Google Scholar]
- Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–9. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XM, Zhang YX. Effects of glucose on growth, metabolism and EPO expression in recombinant CHO cell cultures. Sheng Wu Gong Cheng Xue Bao. 2001;17:698–702. [PubMed] [Google Scholar]
- Suzuki H, Fukushima M, Okamoto S, Takahashi O, Shimbo T, Kurose T, Yamada Y, Inagaki N, Seino Y, Fukui T. Effects of thorough mastication on postprandial plasma glucose concentrations in nonobese Japanese subjects. Metabolism. 2005;54:1593–9. doi: 10.1016/j.metabol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, King GL. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem. 2000;275:40725–40731. doi: 10.1074/jbc.M006509200. [DOI] [PubMed] [Google Scholar]
- Swamy-Mruthinti S, Miriam KC, Kumar SK, Biswas J, Ramakrishnan S, Nagaraj RH, Sulochana KN. Immunolocalization and quantification of advanced glycation end products in retinal neovascular membranes and serum: a possible role in ocular neovascularization. Curr Eye Res. 2002;25:139–145. doi: 10.1076/ceyr.25.3.139.13480. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Boyce VL, Bergman RN, Howard BV, Bogardus C. Deterioration in carbohydrate metabolism and lipoprotein changes induced by modern, high fat diet in Pima Indians and Caucasians. J Clin Endocrin Metab. 1991;73:156–65. doi: 10.1210/jcem-73-1-156. [DOI] [PubMed] [Google Scholar]
- Szweda PA, Camouse M, Lundberg KC, Oberley TD, Szweda LI. Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Ageing Res Rev. 2003;2:383–405. doi: 10.1016/s1568-1637(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Tabaton M, Perry G, Smith M, Vitek M, Angelini G, Dapino D, Garibaldi S, Zaccheo D, Odetti P. Is amyloid beta-protein glycated in Alzheimer’s disease? Neuroreport. 1997;8:907–909. doi: 10.1097/00001756-199703030-00018. [DOI] [PubMed] [Google Scholar]
- Tai MM, Castillo P, Pi-Sunyer FX. Meal size and frequency: effect on the thermic effect of food. Am J Clin Nutr. 1991;54:783–7. doi: 10.1093/ajcn/54.5.783. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Masuda A, Sun M, Centonze VE, Herman B. Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm) Brain Res Bull. 2004;62:497–504. doi: 10.1016/j.brainresbull.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Yamagishi S. Possible involvement of advanced glycation endproducts (AGEs) in the pathogenesis of Alzheimer’s disease. Curr Pharm Des. 2008;14:973–978. doi: 10.2174/138161208784139693. [DOI] [PubMed] [Google Scholar]
- Tan JS, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2007;114:1143–50. doi: 10.1016/j.ophtha.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor 1alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Gustafsson B, Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–43. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J Biol Chem. 1999;274:20796–804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP, Wong VW, Brownlee M, Gurtner GC. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010a;9:75–9. doi: 10.4161/cc.9.1.10371. [DOI] [PubMed] [Google Scholar]
- Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP, Wong VW, Brownlee M, Gurtner GC. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010b;9:75–9. doi: 10.4161/cc.9.1.10371. [DOI] [PubMed] [Google Scholar]
- Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106:13505–10. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, III, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK The Diabetic Retinopathy Clinical Research Network. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel VE, Audus KL. Nitric oxide and blood–brain barrier integrity. Antioxid Redox Signal. 2001;3:273–278. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;1:CD006296. doi: 10.1002/14651858.CD006296.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Protecting the genome: defence against nucleotide glycation and emerging role of glyoxalase I overexpression in multidrug resistance in cancer chemotherapy. Biochem Soc Trans. 2003;31(Pt 6):1372–7. doi: 10.1042/bst0311372. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(Pt 1):109–16. [PMC free article] [PubMed] [Google Scholar]
- Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins:New products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- To CH, Cheung KK, Chiu SH, Lai HM, Lung KS. The saturation characteristics of glucose transport in bovine retinal pigment epithelium. Yan Ke Xue Bao. 1998;14:126–129. [PubMed] [Google Scholar]
- Tomany SC, Wang JJ, van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, de Jong PT. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–7. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Topouzis F, Anastasopoulos E, Augood C, Bentham GC, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Vingerling JR, Vioque J, Young IS, Fletcher AE. Association of diabetes with age-related macular degeneration in the EUREYE study. Br J Ophthalmol. 2009;93:1037–41. doi: 10.1136/bjo.2008.146316. [DOI] [PubMed] [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem. 2001;276:43836–41. doi: 10.1074/jbc.M106534200. [DOI] [PubMed] [Google Scholar]
- Trumpower BL. The protonmotive Q cycle: energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- Tumosa N. Eye disease and the older diabetic. Clin Geriatr Med. 2008;24:515–27. vii. doi: 10.1016/j.cger.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinnen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991;34:877–90. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- van Bakel MM, Slimani N, Feskens EJ, Du H, Beulens JW, van der Schouw YT, Brighenti F, Halkjaer J, Cust AE, Ferrari P, Brand-Miller J, Bueno-de-Mesquita HB, Peeters P, Ardanaz E, Dorronsoro M, Crowe FL, Bingham S, Rohrmann S, Boeing H, Johansson I, Manjer J, Tjonneland A, Overvad K, Lund E, Skeie G, Mattiello A, Salvini S, Clavel-Chapelon F, Kaaks R. Methodological challenges in the application of the glycemic index in epidemiological studies using data from the European Prospective Investigation into Cancer and Nutrition. J Nutr. 2009;139:568–75. doi: 10.3945/jn.108.097121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam RM, Visscher AW, Feskens EJ, Verhoef P, Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr. 2000;54:726–31. doi: 10.1038/sj.ejcn.1601086. [DOI] [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-López S, Mayol-Kreiser SN. Use of the glycemic index for weight loss and glycemic control: a review of recent evidence. Curr Diab Rep. 2009;9:379–88. doi: 10.1007/s11892-009-0059-9. [DOI] [PubMed] [Google Scholar]
- Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr. 2007;61(Suppl 1):S122–31. doi: 10.1038/sj.ejcn.1602942. [DOI] [PubMed] [Google Scholar]
- Vilchis C, Salceda R. Characterization of [3H] deoxy-D-glucose uptake in retina and retinal pigment epithelium of normal and diabetic rats. Neurochem Int. 1996;28:213–219. doi: 10.1016/0197-0186(95)00068-2. [DOI] [PubMed] [Google Scholar]
- Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, Shu XO. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007;167:2310–6. doi: 10.1001/archinte.167.21.2310. [DOI] [PubMed] [Google Scholar]
- Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–30. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Campochiaro PA. Prevention or moderation of some ultrastructural changes in the RPE and retina of galactosemic rats by aldose reductase inhibition. Exp Eye Res. 1989;49:495–510. doi: 10.1016/0014-4835(89)90057-2. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Campochiaro PA, Williams EH, May EE, Green WR, Sorenson RL. Aldose reductase expression in human diabetic retina and retinal pigment epithelium. Diabetes. 1988;37:1658–64. doi: 10.2337/diab.37.12.1658. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical demonstration of blood-retinal barrier breakdown in human diabetics and its association with aldose reductase in retinal vascular endothelium and retinal pigment epithelium. Histochem J. 1993a;25:648–63. doi: 10.1007/BF00157879. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical evidence for the mechanism of blood-retinal barrier breakdown in galactosemic rats and its association with aldose reductase expression and inhibition. Exp Eye Res. 1993b;57:723–35. doi: 10.1006/exer.1993.1180. [DOI] [PubMed] [Google Scholar]
- Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436–43. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Li YM, Imani F, Wojciechowicz D, Yang Z, Liu FT, Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1:634–646. [PMC free article] [PubMed] [Google Scholar]
- Vordermark D, Kraft P, Katzer A, Bolling T, Willner J, Flentje M. Glucose requirement for hypoxic accumulation of hypoxia-inducible factor-1a (HIF-1a) Cancer Lett. 2005;230:122–133. doi: 10.1016/j.canlet.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Wagner Z, Wittmann I, Mazák I, Schinzel R, Heidland A, Kientsch-Engel R, Nagy J. N(epsilon)-(carboxymethyl)lysine levels in patients with type 2 diabetes: role of renal function. Am J Kidney Dis. 2001;38:785–91. doi: 10.1053/ajkd.2001.27695. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Diseases of the mitochondrial DNA (Review) Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;24:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J Immunol. 2010;184:582–90. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–9. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pandey A, Hart GW. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics. 2007;6:1365–79. doi: 10.1074/mcp.M600453-MCP200. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- Watkinson S, Seewoodhary R. Ocular complications associated with diabetes mellitus. Nurs Stand. 2008;22:51–7. doi: 10.7748/ns2008.03.22.27.51.c6429. quiz 58, 60. [DOI] [PubMed] [Google Scholar]
- Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- Willett WC, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76:274S–80S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye. 2004;18:963–983. doi: 10.1038/sj.eye.6701476. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- Wilson TM, Strang R, Wallace J, Horton PW, Johnson NF. The measurement of the choroidal blood flow in the rabbit using Krypton-85. Exp Eye Res. 1973;16:421–425. doi: 10.1016/0014-4835(73)90099-7. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Bentum-Williams A, Jenkins DJ. Physiological modulation of plasma free fatty acid concentrations by diet: metabolic implications in nondiabetic subjects. Diabetes Care. 1995;18:962–970. doi: 10.2337/diacare.18.7.962. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996;126:2807–12. doi: 10.1093/jn/126.11.2807. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–72. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemicresponse. Am J Clin Nutr. 1988;48:1041–7. doi: 10.1093/ajcn/48.4.1041. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Jenkins DJ, Vuksan V, Jenkins AL, Buckley GC, Wong GS, Josse RG. Beneficial effect of a low glycaemic index diet in type 2 diabetes. Diabet Med. 1992a;9:451–8. doi: 10.1111/j.1464-5491.1992.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Jenkins DJ, Vuksan V, Jenkins AL, Wong GS, Josse RG. Beneficial effect of low-glycemic index diet in overweight NIDDM subjects. Diabetes Care. 1992b;15:562–4. doi: 10.2337/diacare.15.4.562. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Mehling C, Chiasson JL, Josse RG, Leiter LA, Maheux P, Rabasa-Lhoret R, Rodger NW, Ryan EA. Low glycaemic index diet and disposition index in type 2 diabetes (the Canadian trial of Carbohydrates in Diabetes): a randomised controlled trial. Diabetologia. 2008a;51:1607–15. doi: 10.1007/s00125-008-1093-x. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Nguyen PM, Chiasson JL, Hunt JA, Josse RG, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH. Determinants of diet glycemic index calculated retrospectively from diet records of 342 individuals with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994;59:1265–9. doi: 10.1093/ajcn/59.6.1265. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Nuttall FQ, Lee R, Wong GS, Josse RG, Csima A, Jenkins DJ. Prediction of the relative blood glucose response of mixed meals using the white bread glycemic index. Diabetes Care. 1985;8:418–28. doi: 10.2337/diacare.8.5.418. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Vorster HH, Bjorck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL, Venter C, Xiaomei W. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57:475–82. doi: 10.1038/sj.ejcn.1601551. [DOI] [PubMed] [Google Scholar]
- Wolever TMS. The glycemic index. World Rev Nutr Diet. 1990;62:120–185. [PubMed] [Google Scholar]
- Wolever TMS, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, Björck I, Brighenti F, Brown R, Brynes A, Casiraghi MC, Cazaubiel M, Dahlqvist L, Delport E, Denyer GS, Erba D, Frost G, Granfeldt Y, Hampton S, Hart VA, Hätänen KA, Henry CJ, Hertzler S, Hull S, Jerling J, Johnston KL, Lightowler H, Mann N, Morgan L, Panlasigui LN, Pelkman C, Perry TFH, Pfeiffer AFH, Pieters M, Ramdath DD, Ramsingh RT, Robert SD, Robinson C, Sarkkinen E, Scazzina F, Sison DCD, Sloth B, Staniforth J, Tapola N, Valsta LM, Verkooijen I, Weickert MO, Weseler AR, Wilkie P, Zhang J. Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr. 2008b;87(suppl):S247–S57. doi: 10.1093/ajcn/87.1.247S. [DOI] [PubMed] [Google Scholar]
- Wolever TMS, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG, Leiter LA, Maheux P, Rabasa-Lhoret R, Rodger NW, Ryan EA. The Canadian trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008c;87:114–25. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- Wolever TMS, Mehling C. High-carbohydrate/low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Brit J Nutr. 2002;87:477–87. doi: 10.1079/BJNBJN2002568. [DOI] [PubMed] [Google Scholar]
- Wood JP, Osborne NN. The influence of zinc on caspase-3 and DNA breakdown in cultured human retinal pigment epithelial cells. Arch Ophthalmol. 2001;119:81–8. [PubMed] [Google Scholar]
- Wright JD, Kennedy-Stephenson J, Wang CY, McDowell MA, Johnson CL National Center for Health Statistics C. Trends in Intake of Energy and Macronutrients - United States, 1971–2000. MMWR CDC. 2004;53:80–82. [Google Scholar]
- Xi X, Gao L, Hatala DA, Smith DG, Codispoti MC, Gong B, Kern TS, Zhang JZ. Chronically elevated glucose-induced apoptosis is mediated by inactivation of Akt in cultured Müller cells. Biochem Biophys Res Commun. 2005;326:548–53. doi: 10.1016/j.bbrc.2004.11.064. [DOI] [PubMed] [Google Scholar]
- Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–1129. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zeng S, Ling S, Lv M. Up-regulation of HIF-1alpha and VEGF expression by elevated glucose concentration and hypoxia in cultured human retinal pigment epithelial cells. J Huazhong Univ Sci Technolog Med Sci. 2006;26:463–5. doi: 10.1007/s11596-006-0422-x. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ishibashi K, Ishibashi K, Bhutto IA, Tian J, Lutty GA, Handa JT. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840–8. doi: 10.1016/j.exer.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Amano S, Inagaki Y, Okamoto T, Koga K, Sasaki N, Yamamoto H, Takeuchi M, Makita Z. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun. 2002;290:973–8. doi: 10.1006/bbrc.2001.6312. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Fujimori H, Yonekura H, Tanaka N, Yamamoto H. Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochem Biophys Res Commun. 1999;258:353–7. doi: 10.1006/bbrc.1999.0625. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des. 2008;14:962–8. doi: 10.2174/138161208784139729. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Nakamura N, Nakano K, Kitagawa Y, Shigeta H, Hasegawa G, Ienaga K, Nakamura K, Nakazawa Y, Fukui I, Obayashi H, Kondo M. Immunochemical quantification of crossline as a fluorescent advanced glycation endproduct in erythrocyte membrane proteins from diabetic patients with or without retinopathy. Diabet Med. 1998;15:458–62. doi: 10.1002/(SICI)1096-9136(199806)15:6<458::AID-DIA601>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Yan SF, D’Agati V, Schmidt AM, Ramasamy R. Receptor for advanced glycation endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging. Curr Mol Med. 2007;7:699–710. [PubMed] [Google Scholar]
- Yang P, Peairs JJ, Tano R, Zhang N, Tyrell J, Jaffe GJ. Caspase-8-mediated apoptosis in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48:3341–9. doi: 10.1167/iovs.06-1340. [DOI] [PubMed] [Google Scholar]
- Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–55. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Guan M, Zhao XQ, Huang YF. Downregulation of the pigment epithelium derived factor by hypoxia and elevated glucose concentration in cultured human retinal pigment epithelial cells [Article in Chinese] Zhonghua Yi Xue Za Zhi. 2003;83:1989–92. [PubMed] [Google Scholar]
- Yego EC, Mohr S. siah-1 Protein is necessary for high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation and cell death in Muller cells. J Biol Chem. 2010;285:3181–90. doi: 10.1074/jbc.M109.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic Biol Med. 1996;21:871–88. doi: 10.1016/0891-5849(96)00175-x. [DOI] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TA, Wang H, Munk S, Hammoudi DS, Young DS, Mandelcorn MS, Whiteside CI. Vascular endothelial growth factor expression and secretion by retinal pigment epithelial cells in high glucose and hypoxia is protein kinase C-dependent. Exp Eye Res. 2005;80:651–62. doi: 10.1016/j.exer.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Yu RK, Bieberich E, Xia T, Zeng G. Regulation of ganglioside biosynthesis in the nervous system. J Lipid Res. 2004;45:783–93. doi: 10.1194/jlr.R300020-JLR200. [DOI] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadunaisky JA, Degnan KJ. Passage of sugars and urea across the isolated retina pigment epithelium of the frog. Exp Eye Res. 1976;23:191–196. doi: 10.1016/0014-4835(76)90202-5. [DOI] [PubMed] [Google Scholar]
- Zeng G, Yu RK. Cloning and transcriptional regulation of genes responsible for synthesis of gangliosides. Curr Drug Targets. 2008;9:317–24. doi: 10.2174/138945008783954925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29:2223–30. doi: 10.2337/dc06-0266. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou J, Du H. Inhibition of VEGF expression by targeting HIF-1 alpha with small interference RNA in human RPE cells. Ophthalmologica. 2007;221:411–417. doi: 10.1159/000107502. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, Shang F. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:3622–30. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cai B, Jang YP, Pachydaki S, Schmidt AM, Sparrow JR. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp Eye Res. 2005;80:567–80. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou J, Schmid T, Brune B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol Biol Cell. 2003;14:2216–2225. doi: 10.1091/mbc.E02-09-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]