Abstract
T-type calcium channels are expressed in many diverse tissues, including neuronal, cardiovascular, and endocrine. T-type calcium channels are known to play roles in the development, maintenance, and repair of these tissues but have also been implicated in disease when not properly regulated. Calcium channel blockers have been developed to treat various diseases and their use clinically is widespread due to both their efficacy as well as their safety. Aside from their established clinical applications, recent studies have suggested neuroprotective effects of T-type calcium channels blockers. Many of the current T-type calcium channel blockers could act on other molecular targets besides T-type calcium channels making it uncertain whether their neuroprotective effects are solely due to blocking of T-type calcium channels. In this review, we discuss these drugs as well as newly developed chemical compounds that are designed to be more selective for T-type calcium channels. We review in vitro and in vivo evidence of neuroprotective effects by these T-type calcium channel blockers. We conclude by discussing possible molecular mechanisms underlying neuroprotective effects by T-type calcium channel blockers.
Keywords: Calcium channels, CaV3.1, CaV3.2, CaV3.3, Neurologic disease, Hearing loss
Introduction
Calcium signaling plays a vital role in the survival of neurons. After acute injury or with increasing age, calcium homeostasis can be disrupted, leading to neuronal dysfunction. Theoretically, pharmacologic interventions modulating calcium may yield neuroprotective effects. Recent studies have successfully shown the use of T-type calcium channel blockers (CCBs) in treating a number of neurological diseases in animal models.
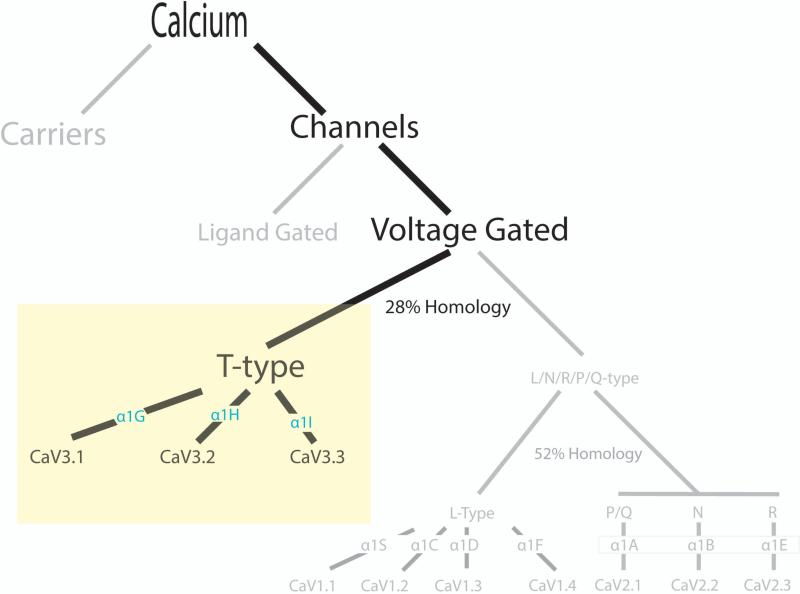
Calcium carriers and calcium channels located on the plasma membrane of many cell types are responsible for calcium homeostasis within the cell. Calcium channels can be further divided into ligand bound or voltage gated calcium channels (VGCC). VGCCs can be divided into two groups: high-voltage activated calcium channels (L, N, P/Q, and R-types) and low-voltage activated calcium channels (T-types) (Figure 1). The VGCCs are defined by their alpha (α) subunits sub-categorized as the L-types CaV1.1 (α1S), 1.2 (α1C), 1.3 (α1D), 1.4 (α1F), the P/Q-type CaV2.1 (α1A), the N-type CaV2.2 (α1B), the R-type (α1E) and the T-types as CaV3.1 (α1G), 3.2 (α1H), 3.3 (α1I) [83,85,28,31]. L-type calcium channels possess at least two additional subunits that may help differentiate them from the T-type calcium channel [3,31]. T-type calcium channels are predominantly found in neurons but have been found in other cells including cardiac myocytes, pacemaker cells, glial cells, fibroblasts, osteoblasts, retinal cells, and adrenocortical cells [16,40]. At the systemic level, inhibition of T-type calcium channels may result in long-term organ protection due to improvement of local microcirculation and reduction of adverse hormonal effects [67].
Figure 1. Calcium Homeostasis through T-type Calcium Channels.
Calcium can enter a cell through either calcium carriers or calcium channels. Calcium channels can be further subdivided into ligand gated or voltage gated. Voltage gated calcium channels can be characterized by high voltage (CaV1 L-type and CaV2 N, P/Q, and R-types) or low voltage (T-type). L, N, P/Q, R-types and T-type calcium channels are further characterized by their alpha subunits into CaV1.1 (α1S), 1.2 (α1C), 1.3 (α1D), 1.4 (α1F), CaV 2.1 (α1A), 2.2 (α1B), 2.3 (α1E), and CaV3.1 (α1G), 3.2 (α1H), 3.3 (α1I). The degrees of homology between classes are noted.
At the cellular level, T-type (“T” is for transient) calcium channels open at approximately −70mV whereas L-type (“L” is for large or long-lasting) open at a more depolarized potential of approximately −20mV. Specific T-type calcium channel properties include having a low-open channel conductance, selective regulation by GTPases, and playing roles in pacemaking [65,18,74,78]. It is important to note that there are differences of biophysical properties, regional expression, functionality, pharmacological sensitivity, potential for activation, kinetics of inactivation and deactivation, and permeability among the various T-type calcium channels [30,66,23,46,47]; however, given that current drugs are not yet channel subtype specific, we will not discuss this further. When the membrane potential is between −80mV and −40mV, T-type calcium channels can cycle from open to closed and back to open, such that at all times, some T-type calcium channels are open producing a “window” current. The calcium influx from this “window” current is counter-balanced by an energy-consuming pumping mechanism. A disruption of this balance during aging or after injury could contribute to neuronal malfunction. As we will now discuss, blockers for T-type calcium channels have been developed to treat various diseases. These blockers show neuroprotective effects both in vitro and in vivo [59,80,42,5]. However, molecular mechanisms underlying their neuroprotective effects are still unclear.
Overview of T-type Calcium Channel Blockers
One major class of CCBs is a family of antiepileptic drugs which includes ethosuximide, trimethadione, and zonisamide (Table 1). Ethosuximide has a succinimide structure whereas trimethadione is an oxazolidinedione. Both ethosuximide and trimethadione are used for the treatment of absence seizures. Absence seizures have a generalized, non-convulsive pattern with a characteristic 3-Hz spike and wave electrical pattern on electroencephalography that is due to the T-type calcium channels providing the neurons with an oscillatory capacity [26,7,12,13]. Of the three T-type calcium channel subtypes, CaV3.1 is expressed in the thalamocortical relay nucleus. CaV3.1 knockout mice provide protection from absence seizures [35,63]. It is believed that ethosuximide and trimethadione effectively block this channel. While absence seizures involve a generalized cortical involvement, partial seizures affect only a small region of the brain. Zonisamide is chemically classified as a sulfonamide and is unrelated to other antiepileptic agents. Zonisamide is effective in the treatment partial seizures [11,24], childhood epilepsy [17], West syndrome (infantile spasms), and juvenile myoclonic epilepsy. General side effects of these three antiepileptic medications include nausea, vomiting, headache, mental status changes, neuropathy, and change in weight. All three of these antiepileptic drugs reach therapeutic concentration to effectively block the T-type calcium channels and show neuroprotective functions (Table 1).
Table 1.
Clinically Used Drugs with Possible Neuroprotective Effects
| Drug | Pathways Inhibited | MW (g/mole) | Clinical Dose/mM | IC50 (mM)-T channel | Neuroprotective? |
|---|---|---|---|---|---|
| Anti-Epileptics | |||||
| Ethosuximide | T-Type (CaV3.1, CaV3.2); Na | 141.17 | 250-750mg PO BID/3.5-10 | 0.3-1.0 | Y |
| Trimethadione | T-Type (CaV3.2) | 143.14 | 300-600mg TID-QID/6-16.7 | ? | Y |
| Zonisamide | T-Type; Oxidative; Carbonic Anhydrase Inhibitor | 212.23 | 400-600mg qD/1.89-2.83 | 0.05-0.5 | Y |
| Anti-Hypertensives/Angina | |||||
| Amlodipine | T-Type (CaV3.2>CaV3.1 or CaV3.3) and L-Type; Na; K | 408.88 | 5-10mg qD/0.12-0.24 | 0.031 | Y |
| Aranidipine*J | T-Type and L-Type | 388.37 | 5-20mg qD/0.012-0.051 | 0.03-0.04 | ? |
| Azelnidipine*CJ | T-Type and L-Type | 582.65 | 8-16mg qD/0.014-0.028 | 0.04-0.07 | Y |
| Barnidipine*SJO | T-Type and L-Type | 528 | 10-15mg qD/0.019-0.028 | 0.005-0.02 | ? |
| Benidipine*IJCO | T-Type and L-Type | 542.02 | 2-4mg qD/0.0037-0.0074 | 0.003-0.2 | ? |
| Efonidipine*J | T-Type and L-Type | 631.66 | 40mg qD/0.63 | 0.0029 | ? |
| Mibefradil*O | T-Type and L-Type; Na; K | 568.55 | 400mg qD/0.70 | 0.00017-0.00029 | Y |
| Nicardipine | T-Type and L-Type | 515.99 | 20mg TID-120mg qD/0.11-0.23 | 0.0028 | Y |
| Nimodipine | T-Type and L-Type | 418.44 | 60mg q4h/0.86 | 0.0056 | Y |
| Other | |||||
| Lomerizine*J | T-Type and L-Type | 541.46 | 5-20mg qD/0.0009-0.0036 | 0.00000046 | Y |
| Pimozide | T-Type; Dopamine | 461.55 | 2-10mg BID/0.0089-0.043 | 0.000036-0.000054 | Y |
Not currently FDA approved; J= Japan, C= China, I= India, S= Spain, O= Other; MW = Molecular Weight
Traditionally, many antihypertensive/antianginal CCBs were thought to function through their blockade of L-type calcium channels. However, many common CCBs are now known to block both L-type and T-type calcium channels with similar potencies [19,20,52]. Among clinically relevant CCBs to treat hypertension, three separate classes exist: dihydropyridines (amlodipine), phenylakylamines (verapamil), and benzothiazepines (diltiazem). Among the dihydropyridine class, one subclass blocks only L-type calcium channels and the other subclass blocks both L-type and T-type calcium channels. The latter subclass includes amlodipine, aranidipine, azelnidipine, barnidipine, benidipine, efonidipine, nicardipine, and nimodipine [2,21,69,67,6,19,20,45,52]. Of these listed, amlodipine, nicardipine, and nimodipine are FDA approved for use in the United States (Table 1). The other drugs are used elsewhere or are undergoing clinical trials to establish their safety profiles. Verapamil, a phenylakylamine, blocks both L-type and T-type channels with higher affinity for depolarized channels than for resting channels [8]. Mibefradil, another phenylalkylamine, was withdrawn from the market due to its interactions with other drugs metabolized through the cytochrome P450 pathway although it is highly effective to block T-type calcium channels. In general, side effects of these drugs include headache, fatigue, flushing, chest pain, shortness of breath, limb edema, and in rare cases, can cause arrhythmias, syncope, erectile dysfunction, and depression. Many of these CCBs have demonstrated neuroprotective effects (Table 1).
There are several other drugs able to block T-type calcium channels. T-type calcium channels are potently blocked by a subset of neuroleptic drugs such as pimozide and penfluridol from the diphenylbutylpiperidine family [4,57]. The diphenyldiperazine flunarizine can also block T-type calcium channels, preferentially for α1G and α1I. Fluoxetine and trazodone, primarily for treating depression, block T-type calcium channels [72,73,38]. Lomerizine is an antimigraine medication used in Japan and has been shown to have neuroprotective effects [32]. Many anesthetic agents such as isoflurane, propofol, and nitrous oxide can effectively block T-type calcium channels [70,69,50], although they also block many other ion channels as well.
Understanding through which mechanisms these drugs work is important to providing safe and efficacious interventions to neurological diseases. This requires the exploration of specific T-type CCBs with the understanding that each T-type calcium channel is unique. Unlike L-type CCBs, there are no specific blockers for T-type calcium channels on the market, providing an active field to develop specific T-type CCBs (Table 2). Table 2 presents a partial list of current drug candidates that have been designed to be more specific for T-type calcium channels with some of them already having demonstrated neuroprotective effects. A number of T-type specific CCBs have been engineered and are at various phases of testing such as neuroactive steroids [71], tetramethylcyclopropanecarboxamide derivatives [60], and pyridyl amides [53]. In addition, structure-based approaches have been applied to explore new blockers [51], and T-type calcium channels can also be blocked by activation of other molecular pathways including GTPases [68,27,29]. Some specific examples of more channel selective blockers include TTA-A2 and KYS05047 which have been shown to have effects on neuronal circuits [75,37,55]. A1048400 blocks both N-type and T-type calcium channels and has been shown to reduce tactile pain [9]. 202-W92 is a weak T-type calcium channel antagonist and is a similar compound to lamotrigine and sipatrigine. T-type calcium channels can be inhibited by sipatrigine but not lamotrigine [1,39]. 202-W92 inhibits neuronal calcium channels in a dose-dependent manner. In a stroke model, 202-W92 reduced infarct volume by 75-80% (Table 2). Given the novelty of these chemicals, not much is known about their mechanisms of neuroprotection or their compatibility with human use.
Table 2.
Drug Candidates with Possible Neuroprotective Effects
| Drug Candidate | Pathways Inhibited | IC50 -T channel | Neuroprotection |
|---|---|---|---|
| A1048400 | T-Type and N-Type | 1.2-4.6μM | ? |
| KYS05044 | T-Type (CaV3.1, CaV3.2) | 1μM | ? |
| ML218 | T-Type (CaV3.1-CaV3.3) | 150-310nM | Y |
| NNC 55-0396 | T-Type | 7μM | Y |
| RQ-00311610 | T-Type | 110-170nM | ? |
| TTA-A2 | T-Type (CaV3.1-3.3) | 50-100nM | Y |
| TTA-P2 | T-Type | 22nM | ? |
| VH04 | T-Type (CaV3.1) | 100nM | ? |
| Z941/944 | T-Type | 50-160nM | Y |
Neuroprotection evidence from in vitro and in vivo models
Ischemia Model: Oxygen Glucose Deprivation (OGD)
The early evidence for neuroprotection of T-type CCBs was demonstrated in vitro [44,43,54,48,56]. In an OGD model commonly used to mimic ischemic insult in vitro, significant neuroprotection was observed when extracellular calcium levels were artificially lowered. In the same OGD culture with normal calcium level but with the addition of CCBs, there was nearly identical neuroprotection as in the calcium depleted culture, suggesting that the modification of calcium levels can protect neurons [48]. Why is modification of calcium neuroprotective in the OGD model? One theory is that T-type calcium channels remain open at membrane potentials near threshold providing a small “window” current. This “window” current is necessary for normal development but may play a role in calcium toxicity. Small increases (provided by the “window” current) in intracellular calcium trigger the mitochondrial calcium uniporter to intake calcium and activates the TCA cycle resulting in physiologically normal increases of ATP. However, excess calcium triggers increased pore formation and the release of multiple pro-apoptotic enzymes into the cell, resulting in activation of the cell death cascade. During OGD, ATP stores are rapidly depleted as ATP is used by plasma ion channels in an attempt to restore homeostasis. In cells where either the mitochondrial calcium uniporter was effectively blocked (regardless of intracellular calcium levels), the extracellular calcium was removed, or the calcium channels were blocked, there was a significant delay in the depletion of ATP during OGD resulting in prolonged neuronal protection [22]. Also of note, neuroprotection in ischemia models has been noted for other CCBs including L and N-type channels [10].
Neuronal Cell Line Model
Overload of intracellular calcium due to the use of local anesthetics such as bupivicaine is neurotoxic. Cell viability is dose dependent with bupivicaine. In the SH-SY5Y cells treated with bupivicaine alone, intracellular calcium was dramatically increased leading to cell death; however, when pretreated with dihydrochloride (a T-type CCB), intracellular calcium was reduced leading to neuroprotection [79].
Peripheral Neuropathy Model
T-type calcium channels are upregulated in patients with peripheral neuropathy. A week after sciatic nerve injury, rats had hyperalgesia. This hyperalgesia was significantly reduced with the use of mibefrabil and ethosuximide [33]. In mice with an L5-L6 spinal nerve injury, the density of T-type calcium channels in the small dorsal root ganglion neurons in the injured set increased whereas the density did not change in the medium and large injured neurons. Small neurons convey pain and temperature whereas neurons with a large diameter convey touch and mechanoreceptive information. An increase in density of calcium channels in the small nerve fibers decreases the resting potential of the pain fibers making them hyperexcitable resulting in hyperalgesia. Mibefradil and ethosuximide alleviate the decrease in resting potential that was induced by the nerve injury, thus reducing the pain response [82].
Hearing Loss Model
Cisplatin is a widely used chemotherapeutic drug that results in irreversible hearing loss by damaging outer hair cells in the cochlea. L-type CCBs diltiazem, nicardipine, nifedipine, and dantrolene do not protect hair cells from cisplatin damage while T-type CCBs flunarizine and pimozide protect hair cells after exposure [62]. However, this protection is not completely through T-type calcium channels [61]. Flunarazine acts through calcium independent mechanisms to activate the antioxidant response element to drive Nrf2 into the nucleus to drive the transcription of phase II antioxidants by binding to the promoter regions. Among the phase II elements, heme oxidase1 forms both carbon monoxide and bilirubin, both of which are known to protect against cisplatin. Flunarizine protects hair cells against cisplatin by inhibiting the translocation of NF-κB (which can be pro-apoptotic) to the nucleus (which subsequently degrades intracellularly) and by increasing the levels of Nrf2 [61].
Noise-induced hearing loss is the second most common form of sensorineural hearing loss. Previously, only the L-type CCBs appeared to reduce the auditory loss [76]. Our recent studies found that trimethadione and ethosuximide were protective of auditory hair cells and spiral ganglion neurons (SGNs) [59,5]. However, similar to in vitro studies, it was unclear whether this neuroprotection in vivo was due to blocking of T-type calcium channels or regulating other signaling pathways. It is possible that these blockers act on oxidative, neurotrophic, or both pathways to protect auditory neurons against acoustic trauma [14].
Recently, we provided compelling data that blockers for T-type calcium channels protect against age-related hearing loss of SGNs via the α1H subunit of CaV3.2 T-type calcium channels [42]. Age-related hearing loss or presbycusis is the most common age-related neurodegenerative disease. α1H (CaV3.2) is strongly expressed in SGNs and increases in expression with age [42], suggesting that with increasing age, more calcium can be in the SGNs. α1H is not expressed in inner ear hair cells whereas α1G and α1I are expressed weakly in hair cells [59]. C57BL/6 mice null for α1H had preserved hearing with aging compared to control mice consistent with preserved number of SGNs. This indicates that T-type calcium channels may play a role in the progressive hearing loss in this strain. Interestingly, the administration of trimethadione or ethosuximide to α1H null mice provided no additional hearing protection, indicating that the T-type CCBs protect hearing function through CaV3.2 channel [42] in the C57 mouse.
Neuroprotective Mechanisms of T-type Calcium Channels
We have illustrated a number of examples highlighting the neuroprotective effects of T-type CCBs. Except in one case [42], where we showed that the neuroprotective effect was due to direct inhibition of the T-type calcium channel alone, the neuronal protection in the examples did not provide direct evidence that the current blockers act on T-type calcium channels for their neuroprotective effects due to the fact that T-type CCBs do not specifically act solely on T-type calcium channels. We can only conclude that the neuroprotective effects of the class of drugs specified as T-type CCBs provide neuronal protection by blocking T-type calcium channels and/or other signaling cascades.
Because no current blockers for T-type calcium channels are highly specific, every blocker may act through a unique but partially overlapping mechanism for its neuroprotective effect. With that in mind, we focus on one T-type CCB, zonisamide, and discuss its possible neuroprotective mechanisms. Zonisamide is an FDA-approved antiepileptic drug developed in the 1970's that has recently gained interest in treating neuropathic pain, headaches, eating disorders, and Parkinson's disease. Zonisamide blocks T-type calcium channels, blocks other channels, and binds to other proteins common for this type of blocker.
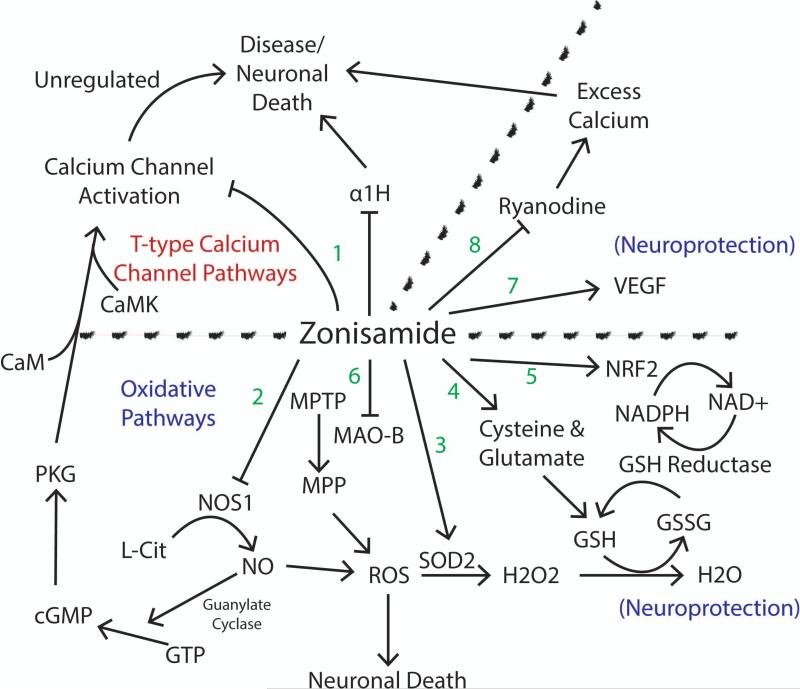
Besides zonisamide's blocking T-type calcium channels such as CaV3.2 (α1H) to control the excess calcium influx seen in disease states (Figure 2 Pathway 1), it may be neuroprotective due to scavenging oxygen free radicals. Zonisamide has been shown to reduce brain ischemia through inhibition of nitric oxide synthase. Zonisamide blocks the induction of post-ischemic long-term potentiation (iLTP). While zonisamide reduces iLTP, the protective effects can be antagonized by a cGMP analog. Nitric oxide activates soluble guanylate cyclase which increases cGMP. Zaprinast, an inhibitor of phosphodiesterases, will elevate cGMP levels and even in the presence of zonisamide, will restore the damage from iLTP. Therefore, nitric oxide and cGMP are important in the pathogenesis of iLTP and are modulated in part by zonisamide [15] (Figure 2 Pathway 2). Zonisamide can also increase neuronal viability through upregulation of superoxide dismutase (SOD2). Treatment with zonisamide reduces the number of pro-apoptotic molecules such as caspase 3, caspase 9, p-JNK [34] which are important molecules in the oxidative cascade (Figure 2 Pathway 3). Zonisamide increases glutathione (an antioxidant) by increasing the cysteine/glutamate exchange transporter (Figure 2 Pathway 4). Cisplatin causes damage by generation of reactive oxygen species. Ebselen is a glutathione peroxide mimic with antioxidant capacity has been found to be protective against cisplatin toxicity. It acts by increasing the levels of Nrf2 in the nucleus which activates phase II antioxidant genes (heme oxygenase, NADPH, quinine oidoreductase, and gamma glutamylcysteine synthetase). In Nrf2 knockout mice, hearing rapidly deteriorates, corresponding to loss of hair cells and SGNs [25] (Figure 2 Pathway 5). Zonisamide preserves neurons by reducing free radical stresses [36,41].
Figure 2. Possible Pathways of T-type Calcium Channel Blocker Zonisamide.
Zonisamide functions through multiple mechanisms. Due to its multifactorial mechanisms of action, it is not clear by which set of pathways provides its neuroprotective effects. 1) Zonisamide functions as a T-type calcium channel blocker. 2) Zonisamide inhibits NOS1. 3) Zonisamide upregulates SOD2. 4) Zonisamide increases GSH. 5) Zonisamide increases NRF2. 6) Zonisamide inhibits MAO-B. 7) Zonisamide increases VEGF. 8) Zonisamide inhibits ryanodine receptors resulting in a decrease of excess calcium. Arrows indicate increase or activation. Ended lines indicate inhibition. References in text.
Zonisamide also ameliorated the dopamine depletion seen in mice treated with MPTP, whose metabolite MPP is a toxin that affects the nigrostriatal neurons. Parkinson's disease appears to be a result of mitochondrial dysfunction with a significant reduction in mitochondrial respiratory chain complex I activity. MPP inhibits complex I activity and is often used as a model for Parkinson's disease. In the MPP model, levels of intracellular calcium were increased, suggesting a possible role of toxicity to the neurons. Zonisamide's inhibition of MPTP metabolism is by inhibiting MAO-B, an activator of MPTP. In zonisamide treated groups, levels of calcium were significantly lower. Likewise, in the MPP group, levels of caspase-3, a marker for apoptosis were dramatically increased compared to the levels in the zonisamide group [84]. Other drugs that inhibit MAO-B may be neuroprotective as well [64] (Figure 2 Pathway 6).
Zonisamide can act on other pathways to protect neurons. It can upregulate mRNA for astrocyte-derived neurotrophic factor VEGF providing neuroprotection [14] (Figure 2 Pathway 7). During a hyperexcitable state, zonisamide inhibits both GABA and glutamate and also prevents calcium overload by suppressing ryanodine [81] (Figure 2 Pathway 8). The calcium induced calcium release system is comprised of the ryanodine receptor contributing to an elevation of intraneuronal calcium. Zonisamide prevents the overload response of induced ryanodine activation.
Conclusion
Drug development is time consuming and costly. It is estimated that it takes ten years and $1.8 billion dollars to take a drug from discovery to market approval [77]. Not only is this system burdened with cost and time, there are many hurdles that suppress innovative designs [49,58]. This makes the novel applications of already proven safe and efficacious medications within tested dosage ranges for various therapeutic applications more appealing.
The therapeutic role of CCBs is an exciting frontier that exists at the intersection of personalized medicine and pharmacogenomics. Numerous CCBs with T-type calcium channel activity are being used by millions of people in a safe and efficacious manner. Combining the broad array of epidemiologic data and translational science, we can assess which commonly used drugs can be repurposed for neuroprotection. Which drugs are useful? Are certain populations better protected? Is there a genetic basis to this protection? The importance of such identifications of currently available drugs cannot be understated. As the disease prevalence of many neurological diseases continues to increase, the immediate availability and protocolled use of ameliorating agents is required. The data we have shown illustrates the immense potential that T-type CCBs have in a number of neurological diseases. Our next steps are to continue to unveil currently approved agents and to prove their efficacy, safety, and at the same time, to characterize underlying molecular mechanisms as they provide neuronal protection.
Acknowledgements
The project was supported by grants to J.B. from the National Institute of Health (DC010489 and DC011793) and the National Organization for Hearing Research Foundation.
Footnotes
Ethics Standards
No animal or human subjects were used for this study.
Conflicts of Interest
BK and RL declare that they have no conflicts of interest. Dr. Bao is a co-inventor on U.S. Patent Application 60806344, which is assigned to Washington University in St. Louis. Dr. Bao is aslo a co-founder of Gateway Biotechnology Inc. and has disclosed this relationship to the Conflict of Interest Board at Washington University.
References
- 1.AH H, NC M, A P, T S, AD R. Actions of Sipatrigine, 202W92 and Lamotrigine on R-type and T-type Ca+2 channel currents. European Journal of Pharmacology. 2003;467:77–80. doi: 10.1016/s0014-2999(03)01625-x. [DOI] [PubMed] [Google Scholar]
- 2.Arnoult C, Villaz M, Florman HM. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Molecular pharmacology. 1998;53(6):1104–1111. [PubMed] [Google Scholar]
- 3.Bae J, Suh EJ, Lee C. Interaction of T-type calcium channel Ca(V)3.3 with the beta-subunit. Molecules and cells. 2010;30(3):185–191. doi: 10.1007/s10059-010-0106-z. doi:10.1007/s10059-010-0106-z. [DOI] [PubMed] [Google Scholar]
- 4.Bancila M, Copin JC, Daali Y, Schatlo B, Gasche Y, Bijlenga P. Two structurally different T-type Ca 2+ channel inhibitors, mibefradil and pimozide, protect CA1 neurons from delayed death after global ischemia in rats. Fundamental & clinical pharmacology. 2011;25(4):469–478. doi: 10.1111/j.1472-8206.2010.00879.x. doi:10.1111/j.1472-8206.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- 5.Bao J, Hungerford M, Luxmore R, Ding D, Qiu Z, Lei D, Yang A, Liang R, Ohlemiller KK. Prophylactic and therapeutic functions of drug combinations against noise-induced hearing loss. Hearing research. 2013;304:33–40. doi: 10.1016/j.heares.2013.06.004. doi:10.1016/j.heares.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton ME, Eberle EL, Shannon HE. The antihyperalgesic effects of the T-type calcium channel blockers ethosuximide, trimethadione, and mibefradil. European journal of pharmacology. 2005;521(1-3):79–85. doi: 10.1016/j.ejphar.2005.08.017. doi:10.1016/j.ejphar.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62(5):612–632. doi: 10.1016/j.neuron.2009.05.015. doi:10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergson P, Lipkind G, Lee SP, Duban ME, Hanck DA. Verapamil block of T-type calcium channels. Molecular pharmacology. 2011;79(3):411–419. doi: 10.1124/mol.110.069492. doi:10.1124/mol.110.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bladen C, Zamponi GW. Common mechanisms of drug interactions with sodium and T-type calcium channels. Molecular pharmacology. 2012;82(3):481–487. doi: 10.1124/mol.112.079715. doi:10.1124/mol.112.079715. [DOI] [PubMed] [Google Scholar]
- 10.Burns LH, Jin Z, Bowersox SS. The neuroprotective effects of intrathecal administration of the selective N-type calcium channel blocker ziconotide in a rat model of spinal ischemia. Journal of vascular surgery. 1999;30(2):334–343. doi: 10.1016/s0741-5214(99)70145-x. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick DW, Marson AG. Zonisamide add-on for drug-resistant partial epilepsy. The Cochrane database of systematic reviews. 2005;4:CD001416. doi: 10.1002/14651858.CD001416.pub2. doi:10.1002/14651858.CD001416.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Cheong E, Kim C, Choi BJ, Sun M, Shin HS. Thalamic ryanodine receptors are involved in controlling the tonic firing of thalamocortical neurons and inflammatory pain signal processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(4):1213–1218. doi: 10.1523/JNEUROSCI.3203-10.2011. doi:10.1523/JNEUROSCI.3203-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheong E, Shin HS. T-type Ca2+ channels in normal and abnormal brain functions. Physiological reviews. 2013;93(3):961–992. doi: 10.1152/physrev.00010.2012. doi:10.1152/physrev.00010.2012. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury ME, Sugimoto K, Kubo M, Iwaki H, Tsujii T, Kyaw WT, Nishikawa N, Nagai M, Tanaka J, Nomoto M. Zonisamide up-regulated the mRNAs encoding astrocytic anti-oxidative and neurotrophic factors. European journal of pharmacology. 2012;689(1-3):72–80. doi: 10.1016/j.ejphar.2012.05.012. doi:10.1016/j.ejphar.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Costa C, Tozzi A, Siliquini S, Galletti F, Cardaioli G, Tantucci M, Pisani F, Calabresi P. A critical role of NO/cGMP/PKG dependent pathway in hippocampal post-ischemic LTP: modulation by zonisamide. Neurobiology of disease. 2011;44(2):185–191. doi: 10.1016/j.nbd.2011.06.015. doi:10.1016/j.nbd.2011.06.011 10.1016/j.nbd.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Errington AC, Stohr T, Lees G. Voltage gated ion channels: targets for anticonvulsant drugs. Current topics in medicinal chemistry. 2005;5(1):15–30. doi: 10.2174/1568026053386872. [DOI] [PubMed] [Google Scholar]
- 17.Eun SH, Kim HD, Eun BL, Lee IK, Chung HJ, Kim JS, Kang HC, Lee YM, Suh ES, Kim DW, Eom S, Lee JS, Moon HK. Comparative trial of low- and high-dose zonisamide as monotherapy for childhood epilepsy. Seizure : the journal of the British Epilepsy Association. 2011;20(7):558–563. doi: 10.1016/j.seizure.2011.04.005. doi:10.1016/j.seizure.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14469–14474. doi: 10.1073/pnas.2437756100. doi:10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa T, Nukada T, Namiki Y, Miyashita Y, Hatsuno K, Ueno Y, Yamakawa T, Isshiki T. Five different profiles of dihydropyridines in blocking T-type Ca(2+) channel subtypes (Ca(v)3.1 (alpha(1G)), Ca(v)3.2 (alpha(1H)), and Ca(v)3.3 (alpha(1I))) expressed in Xenopus oocytes. European journal of pharmacology. 2009;613(1-3):100–107. doi: 10.1016/j.ejphar.2009.04.036. doi:10.1016/j.ejphar.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Ge W, Ren J. Combined L-/T-type calcium channel blockers: ready for prime time. Hypertension. 2009;53(4):592–594. doi: 10.1161/HYPERTENSIONAHA.108.127548. doi:10.1161/HYPERTENSIONAHA.108.127548. [DOI] [PubMed] [Google Scholar]
- 21.Gomora JC, Daud AN, Weiergraber M, Perez-Reyes E. Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Molecular pharmacology. 2001;60(5):1121–1132. [PubMed] [Google Scholar]
- 22.Gouriou Y, Bijlenga P, Demaurex N. Mitochondrial Ca2+ uptake from plasma membrane Cav3.2 protein channels contributes to ischemic toxicity in PC12 cells. The Journal of biological chemistry. 2013;288(18):12459–12468. doi: 10.1074/jbc.M112.428128. doi:10.1074/jbc.M112.428128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrand ME, David LS, Hamid J, Mulatz K, Garcia E, Zamponi GW, Snutch TP. Selective inhibition of Cav3.3 T-type calcium channels by G alpha(q/11)-coupled muscarinic acetylcholine receptors. Journal of Biological Chemistry. 2007;282(29):21043–21055. doi: 10.1074/jbc.M611809200. doi:DOI 10.1074/jbc.M611809200. [DOI] [PubMed] [Google Scholar]
- 24.Holder JL, Jr., Wilfong AA. Zonisamide in the treatment of epilepsy. Expert opinion on pharmacotherapy. 2011;12(16):2573–2581. doi: 10.1517/14656566.2011.622268. doi:10.1517/14656566.2011.622268. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino T, Tabuchi K, Nishimura B, Tanaka S, Nakayama M, Ishii T, Warabi E, Yanagawa T, Shimizu R, Yamamoto M, Hara A. Protective role of Nrf2 in age-related hearing loss and gentamicin ototoxicity. Biochemical and biophysical research communications. 2011;415(1):94–98. doi: 10.1016/j.bbrc.2011.10.019. doi:10.1016/j.bbrc.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends in neurosciences. 2007;30(7):350–356. doi: 10.1016/j.tins.2007.05.007. doi:10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, Turner RW, Zamponi GW. Regulation of T-type calcium channels by Rho-associated kinase. Nature neuroscience. 2007;10(7):854–860. doi: 10.1038/nn1921. doi:Doi 10.1038/Nn1921. [DOI] [PubMed] [Google Scholar]
- 28.Iftinca MC. Neuronal T-type calcium channels: what's new? Iftinca: T-type channel regulation. Journal of medicine and life. 2011;4(2):126–138. [PMC free article] [PubMed] [Google Scholar]
- 29.Iftinca MC, Hamid J, Turner RW, Zamponi GW. ROCK-mediated inhibition of T-type calcium channels. Biophysical journal. 2007:601a–601a. [Google Scholar]
- 30.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends in pharmacological sciences. 2009;30(1):32–40. doi: 10.1016/j.tips.2008.10.004. doi:DOI 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends in pharmacological sciences. 2009;30(1):32–40. doi: 10.1016/j.tips.2008.10.004. doi:10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Ishii M, Iizuka R, Kiuchi Y, Mori Y, Shimizu S. Neuroprotection by lomerizine, a prophylactic drug for migraine, against hydrogen peroxide-induced hippocampal neurotoxicity. Molecular and cellular biochemistry. 2011;358(1-2):1–11. doi: 10.1007/s11010-011-0913-3. doi:10.1007/s11010-011-0913-3. [DOI] [PubMed] [Google Scholar]
- 33.Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. Journal of neurophysiology. 2008;99(6):3151–3156. doi: 10.1152/jn.01031.2007. doi:10.1152/jn.01031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawajiri S, Machida Y, Saiki S, Sato S, Hattori N. Zonisamide reduces cell death in SH-SY5Y cells via an anti-apoptotic effect and by upregulating MnSOD. Neuroscience letters. 2010;481(2):88–91. doi: 10.1016/j.neulet.2010.06.058. doi:10.1016/j.neulet.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31(1):35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, Park C, Han AL, Youn MJ, Lee JH, Kim Y, Kim ES, Kim HJ, Kim JK, Lee HK, Chung SY, So H, Park R. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hearing research. 2009;251(1-2):70–82. doi: 10.1016/j.heares.2009.03.003. doi:10.1016/j.heares.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, Doran SM, Barrow JC, Yang ZQ, Reger TS, Koblan KS, Renger JJ. In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. The Journal of pharmacology and experimental therapeutics. 2010;335(2):409–417. doi: 10.1124/jpet.110.171058. doi:10.1124/jpet.110.171058. [DOI] [PubMed] [Google Scholar]
- 38.Kraus RL, Li Y, Jovanovska A, Renger JJ. Trazodone inhibits T-type calcium channels. Neuropharmacology. 2007;53(2):308–317. doi: 10.1016/j.neuropharm.2007.05.011. doi:10.1016/j.neuropharm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 39.L C, AH H, F L, MJ L, NC M, NB M, AD R, F S, JH S, A S. Neuroprotective actions in vivo and electrophysiological action in vitro of 202W92. Brain research. 2001;919(2):259–261. doi: 10.1016/s0006-8993(01)03029-3. [DOI] [PubMed] [Google Scholar]
- 40.Lacinova L. Voltage-dependent calcium channels. General physiology and biophysics. 2005;24(Suppl 1):1–78. [PubMed] [Google Scholar]
- 41.Lee YJ, Kang HC, Seo JH, Lee JS, Kim HD. Efficacy and tolerability of adjunctive therapy with zonisamide in childhood intractable epilepsy. Brain & development. 2010;32(3):208–212. doi: 10.1016/j.braindev.2009.02.003. doi:10.1016/j.braindev.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Lei D, Gao X, Perez P, Ohlemiller KK, Chen CC, Campbell KP, Hood AY, Bao J. Anti-epileptic drugs delay age-related loss of spiral ganglion neurons via T-type calcium channel. Hearing research. 2011;278(1-2):106–112. doi: 10.1016/j.heares.2011.05.010. doi:10.1016/j.heares.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loikkanen J, Naarala J, Vahakangas KH, Savolainen KM. Glutamate increases toxicity of inorganic lead in GT1-7 neurons: partial protection induced by flunarizine. Archives of toxicology. 2003;77(12):663–671. doi: 10.1007/s00204-003-0498-z. doi:10.1007/s00204-003-0498-z. [DOI] [PubMed] [Google Scholar]
- 44.Manev H, Favaron M, De Erausquin G, Guidotti A, Brooker G, Costa E. Destabilization of ionized Ca2+ homeostasis in excitatory amino acid neurotoxicity: antagonism by glycosphingolipids. Cell biology international reports. 1990;14(1):3–14. doi: 10.1016/0309-1651(90)90066-8. [DOI] [PubMed] [Google Scholar]
- 45.Matar N, Jin W, Wrubel H, Hescheler J, Schneider T, Weiergraber M. Zonisamide block of cloned human T-type voltage-gated calcium channels. Epilepsy research. 2009;83(2-3):224–234. doi: 10.1016/j.eplepsyres.2008.11.010. doi:10.1016/j.eplepsyres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 46.McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. European Journal of Neuroscience. 2006;24(9):2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. doi:DOI 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 47.Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5555–5560. doi: 10.1073/pnas.0601261103. doi:DOI 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikonenko I, Bancila M, Bloc A, Muller D, Bijlenga P. Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. Molecular pharmacology. 2005;68(1):84–89. doi: 10.1124/mol.104.010066. doi:10.1124/mol.104.010066. [DOI] [PubMed] [Google Scholar]
- 49.Nutt D, Goodwin G. ECNP Summit on the future of CNS drug research in Europe 2011: report prepared for ECNP by David Nutt and Guy Goodwin. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2011;21(7):495–499. doi: 10.1016/j.euroneuro.2011.05.004. doi:10.1016/j.euroneuro.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Orestes P, Bojadzic D, Chow RM, Todorovic SM. Mechanisms and functional significance of inhibition of neuronal T-type calcium channels by isoflurane. Molecular pharmacology. 2009;75(3):542–554. doi: 10.1124/mol.108.051664. doi:10.1124/mol.108.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JH, Choi JK, Lee E, Lee JK, Rhim H, Seo SH, Kim Y, Doddareddy MR, Pae AN, Kang J, Roh EJ. Lead discovery and optimization of T-type calcium channel blockers. Bioorganic & medicinal chemistry. 2007;15(3):1409–1419. doi: 10.1016/j.bmc.2006.11.004. doi:10.1016/j.bmc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Reyes E, Van Deusen AL, Vitko I. Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. The Journal of pharmacology and experimental therapeutics. 2009;328(2):621–627. doi: 10.1124/jpet.108.145672. doi:10.1124/jpet.108.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reger TS, Yang ZQ, Schlegel KA, Shu Y, Mattern C, Cube R, Rittle KE, McGaughey GB, Hartman GD, Tang C, Ballard J, Kuo Y, Prueksaritanont T, Nuss CE, Doran SM, Fox SV, Garson SL, Li Y, Kraus RL, Uebele VN, Renger JJ, Barrow JC. Pyridyl amides as potent inhibitors of T-type calcium channels. Bioorganic & medicinal chemistry letters. 2011;21(6):1692–1696. doi: 10.1016/j.bmcl.2011.01.089. doi:10.1016/j.bmcl.2011.01.089. [DOI] [PubMed] [Google Scholar]
- 54.Rekling JC. Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neuroscience letters. 2003;335(3):167–170. doi: 10.1016/s0304-3940(02)01193-x. [DOI] [PubMed] [Google Scholar]
- 55.Rim HK, Lee HW, Choi IS, Park JY, Choi HW, Choi JH, Cho YW, Lee JY, Lee KT. T-type Ca2+ channel blocker, KYS05047 induces G1 phase cell cycle arrest by decreasing intracellular Ca2+ levels in human lung adenocarcinoma A549 cells. Bioorganic & medicinal chemistry letters. 2012;22(23):7123–7126. doi: 10.1016/j.bmcl.2012.09.076. doi:10.1016/j.bmcl.2012.09.076. [DOI] [PubMed] [Google Scholar]
- 56.Salthun-Lassalle B, Hirsch EC, Wolfart J, Ruberg M, Michel PP. Rescue of mesencephalic dopaminergic neurons in culture by low-level stimulation of voltage-gated sodium channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(26):5922–5930. doi: 10.1523/JNEUROSCI.5668-03.2004. doi:10.1523/JNEUROSCI.5668-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santi CM, Cayabyab FS, Sutton KG, McRory JE, Mezeyova J, Hamming KS, Parker D, Stea A, Snutch TP. Differential inhibition of T-type calcium channels by neuroleptics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(2):396–403. doi: 10.1523/JNEUROSCI.22-02-00396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schellekens H, Moors E, Leufkens HG. Drug regulatory systems must foster innovation. Science. 2011;332(6026):174–175. doi: 10.1126/science.332.6026.174. doi:10.1126/science.332.6026.174. [DOI] [PubMed] [Google Scholar]
- 59.Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao X, Wang Q, Ohlemiller KK, Piccirillo J, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hearing research. 2007;226(1-2):52–60. doi: 10.1016/j.heares.2006.12.011. doi:10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimshoni JA, Bialer M, Yagen B. Synthesis and anticonvulsant activity of aromatic tetramethylcyclopropanecarboxamide derivatives. Bioorganic & medicinal chemistry. 2008;16(11):6297–6305. doi: 10.1016/j.bmc.2008.03.051. doi:10.1016/j.bmc.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 61.So H, Kim H, Kim Y, Kim E, Pae HO, Chung HT, Kim HJ, Kwon KB, Lee KM, Lee HY, Moon SK, Park R. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. Journal of the Association for Research in Otolaryngology : JARO. 2008;9(3):290–306. doi: 10.1007/s10162-008-0126-y. doi:10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.So HS, Park C, Kim HJ, Lee JH, Park SY, Lee JH, Lee ZW, Kim HM, Kalinec F, Lim DJ, Park R. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hearing research. 2005;204(1-2):127–139. doi: 10.1016/j.heares.2005.01.011. doi:10.1016/j.heares.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Song I, Kim D, Choi S, Sun M, Kim Y, Shin HS. Role of the alpha1G T-type calcium channel in spontaneous absence seizures in mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(22):5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. doi:10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonsalla PK, Wong LY, Winnik B, Buckley B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: clinical relevance. Experimental neurology. 2010;221(2):329–334. doi: 10.1016/j.expneurol.2009.11.018. doi:10.1016/j.expneurol.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stotz SC, Zamponi GW. Structural determinants of fast inactivation of high voltage-activated Ca(2+) channels. Trends in neurosciences. 2001;24(3):176–181. doi: 10.1016/s0166-2236(00)01738-0. [DOI] [PubMed] [Google Scholar]
- 66.Sun H, Varela D, Chartier D, Ruben PC, Nattel S, Zamponi GW, Leblanc N. Differential interactions of Na+ channel toxins with T-type Ca2+ channels. Journal of General Physiology. 2008;132(1):101–113. doi: 10.1085/jgp.200709883. doi:DOI 10.1085/jgp.200709883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka H, Shigenobu K. Efonidipine hydrochloride: a dual blocker of L- and T-type ca(2+) channels. Cardiovascular drug reviews. 2002;20(1):81–92. doi: 10.1111/j.1527-3466.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 68.Tao J, Hildebrand ME, Liao P, Liang MC, Tan G, Li S, Snutch TP, Soong TW. Activation of corticotropin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Molecular pharmacology. 2008;73(6):1596–1609. doi: 10.1124/mol.107.043612. doi:10.1124/mol.107.043612. [DOI] [PubMed] [Google Scholar]
- 69.Todorovic SM, Jevtovic-Todorovic V, Mennerick S, Perez-Reyes E, Zorumski CF. Ca(v)3.2 channel is a molecular substrate for inhibition of T-type calcium currents in rat sensory neurons by nitrous oxide. Molecular pharmacology. 2001;60(3):603–610. [PubMed] [Google Scholar]
- 70.Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. Journal of neurophysiology. 1998;79(1):240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- 71.Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, Nilsson KR, Zorumski CF, Covey DF, Jevtovic-Todorovic V. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Molecular pharmacology. 2004;66(5):1223–1235. doi: 10.1124/mol.104.002402. doi:10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- 72.Traboulsie A, Chemin J, Chevalier M, Quignard JF, Nargeot J, Lory P. Subunit-specific modulation of T-type calcium channels by zinc. The Journal of physiology. 2007;578(Pt 1):159–171. doi: 10.1113/jphysiol.2006.114496. doi:10.1113/jphysiol.2006.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Traboulsie A, Chemin J, Kupfer E, Nargeot J, Lory P. T-type calcium channels are inhibited by fluoxetine and its metabolite norfluoxetine. Molecular pharmacology. 2006;69(6):1963–1968. doi: 10.1124/mol.105.020842. doi:10.1124/mol.105.020842. [DOI] [PubMed] [Google Scholar]
- 74.Turner RW, Anderson D, Zamponi GW. Signaling complexes of voltage-gated calcium channels. Channels. 2011;5(5):440–448. doi: 10.4161/chan.5.5.16473. doi:10.4161/chan.5.5.16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uebele VN, Nuss CE, Santarelli VP, Garson SL, Kraus RL, Barrow JC, Stauffer SR, Koblan KS, Renger JJ, Aton S, Seibt J, Dumoulin M, Jha SK, Coleman T, Frank MG. T-type calcium channels regulate cortical plasticity in-vivo. [corrected]. Neuroreport. 2009;20(3):257–262. doi: 10.1097/WNR.0b013e3283200111. doi:10.1097/WNR.0b013e3283200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uemaetomari I, Tabuchi K, Nakamagoe M, Tanaka S, Murashita H, Hara A. L-type voltage-gated calcium channel is involved in the pathogenesis of acoustic injury in the cochlea. The Tohoku journal of experimental medicine. 2009;218(1):41–47. doi: 10.1620/tjem.218.41. [DOI] [PubMed] [Google Scholar]
- 77.Van Den Ham HA, Klungel OH, Leufkens HG, Van Staa TP. The patterns of anticoagulation control and the risk of stroke, bleeding and mortality in patients with non-valvular atrial fibrillation. Journal of thrombosis and haemostasis : JTH. 2013;11(1):107–115. doi: 10.1111/jth.12041. doi:10.1111/jth.12041. [DOI] [PubMed] [Google Scholar]
- 78.Varela DL, Hermosilla T, Zamponi GW. Making the T-type even tinier: corticotropin-releasing factor-mediated inhibition of low-voltage-activated calcium channel activity. Molecular pharmacology. 2008;73(6):1589–1591. doi: 10.1124/mol.108.046961. doi:10.1124/mol.108.046961. [DOI] [PubMed] [Google Scholar]
- 79.Wen X, Xu S, Liu H, Zhang Q, Liang H, Yang C, Wang H. Neurotoxicity induced by bupivacaine via T-type calcium channels in SH-SY5Y cells. PloS one. 2013;8(5):e62942. doi: 10.1371/journal.pone.0062942. doi:10.1371/journal.pone.0062942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Wildburger NC, Lin-Ye A, Baird MA, Lei D, Bao J. Neuroprotective effects of blockers for T-type calcium channels. Molecular neurodegeneration. 2009;4:44. doi: 10.1186/1750-1326-4-44. doi:10.1186/1750-1326-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida M, Ishikawa M, Izumi H, De Santis R, Morisawa M. Store-operated calcium channel regulates the chemotactic behavior of ascidian sperm. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):149–154. doi: 10.1073/pnas.0135565100. doi:10.1073/pnas.0135565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue J, Liu L, Liu Z, Shu B, Zhang Y. Upregulation of T-type Ca2+ channels in primary sensory neurons in spinal nerve injury. Spine. 2013;38(6):463–470. doi: 10.1097/BRS.0b013e318272fbf8. doi:10.1097/BRS.0b013e318272fbf8. [DOI] [PubMed] [Google Scholar]
- 83.Yunker AM, McEnery MW. Low-voltage-activated (“T-Type”) calcium channels in review. Journal of bioenergetics and biomembranes. 2003;35(6):533–575. doi: 10.1023/b:jobb.0000008024.77488.48. [DOI] [PubMed] [Google Scholar]
- 84.Yurekli VA, Gurler S, Naziroglu M, Uguz AC, Koyuncuoglu HR. Zonisamide attenuates MPP+-induced oxidative toxicity through modulation of Ca2+ signaling and caspase-3 activity in neuronal PC12 cells. Cellular and molecular neurobiology. 2013;33(2):205–212. doi: 10.1007/s10571-012-9886-3. doi:10.1007/s10571-012-9886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Jiang X, Snutch TP, Tao J. Modulation of low-voltage-activated T-type Ca(2)(+) channels. Biochimica et biophysica acta. 2013;1828(7):1550–1559. doi: 10.1016/j.bbamem.2012.08.032. doi:10.1016/j.bbamem.2012.08.032. [DOI] [PubMed] [Google Scholar]