Preface
Symbiotic microorganisms that reside in the human intestine are adept at foraging glycans and polysaccharides, including those in dietary plants (starch, hemicellulose, pectin), animal-derived cartilage and tissue (glycosaminoglycans and N-linked glycans), and endogenous glycans from host mucus (O-linked glycans). Fluctuations in the abundance of dietary and endogenous glycans, combined with the immense chemical variation among these molecules, create a dynamic and heterogeneous environment in which gut microorganisms proliferate. In this review, we describe how glycans shape the composition of the gut microbiota over various lengths of time, the mechanisms by which individual microorganisms degrade these glycans, and potential opportunities to intentionally influence this ecosystem for better health and nutrition.
Introduction
The dense microbial community (microbiota) that is established in the human intestine shortly after birth has a profound effect on health and physiology, providing benefits such as modulation of immune development1, digestion of recalcitrant dietary nutrients2, and inhibition of pathogen colonization3. However, abnormalities in microbiota composition (dysbiosis) have been implicated in several disease states, including inflammatory bowel disease (IBD)4, colon cancer5, antibiotic-associated colitis6and obesity7. Dysbiosis is postulated to result when a typically healthy microbial community becomes unbalanced, due to either increased abundance of potentially harmful microorganisms8–10or increased flux through harmful metabolic pathways. The normal composition of the gut microbiota, both at single time points and over longer periods of human life, has only been deeply probed within the last several years11–15. Current investigations seek to define the dominant forces shaping the microbiota in order to better understand the causes of dysbiosis and develop strategies to restore a healthy community.
One major factor shaping the composition and physiology of the microbiota is the influx of glycans into the intestine, mostly from diet and host mucosal secretions. Humans consume dozens of different plant and animal-derived dietary glycans, most of which cannot be degraded by enzymes encoded in the human genome. Microbial fermentation transforms these indigestible glycans into short chain fatty acids (SCFA), which serve as nutrients for colonocytes and other gut epithelial cells. Gut microorganisms therefore play a pivotal symbiotic role in helping humans access calories from otherwise indigestible nutrients16. Individual microorganisms prefer different glycans. Thus, selective consumption of these nutrients can influence which microbial groups proliferate and persist in the gastrointestinal tract, pointing to dietary glycans as a non-invasive strategy with which humans can directly influence the balance of species in the gut.
In addition to dietary glycans, which fluctuate in composition and abundance, some members of the microbiota are able to degrade glycans found in host mucus secretions or shed epithelial cells. These endogenous glycans provide consistent sources of nutrients to the microbiota, despite potentially drastic changes in diet. Endogenous host glycans are presented to bacteria in the intestinal lumen as O-linked glycans attached to secreted or cell-associated mucin glycoproteins (the major component of mucus), or as N-linked glycans present in shed epithelial cells. Some proportion of endogenous glycans are likely to be concentrated directly adjacent to host tissue in the protective mucus layer. The ability of certain microorganisms to penetrate and degrade mucus as a nutrient source positions them in close proximity to host cells. As a consequence, species that are adept at utilizing these endogenous glycans may exert a disproportionate effect on colonic health, especially during states of dysbiosis.
This review explores the role of glycans in shaping the microbiota by first considering its assembly from birth to adulthood and how this process is catalyzed by changes in glycan availability. We then consider the glycan acquisition mechanisms that have been evolved by some of the most abundant (and therefore successful) members of the human gut microbiota. Finally, we consider how the spatial abundance and diversity of glycans in different gut regions (i.e., lumen versus mucosa, proximal versus distal) may select for regional sub-populations, some of which may be of particular interest in pathologies resulting from dysbiosis.
Glycans shape the gut microbiota throughout life and from meal-to-meal
The glycan landscape in the human gut
The biochemistry of the various host and dietary glycans that enter the gut is exceptionally diverse. Many different glycosidic linkages may be incorporated into a single polymer, which correspondingly require several linkage-specific degradative enzymes. The human genome is capable of fully degrading a very small subset of glycans, namely starch, lactose and sucrose, each of which contains only one or two different linkages. In contrast, some microorganisms in the intestinal tract target dozens of glycans and possess the corresponding enzymatic tools for depolymerizing each of these molecules into their component sugars. Gut microorganisms vary widely in the number of different glycans that they are capable of targeting17,18. For example, the human gut symbiont Bacteroides thetaiotaomicron can degrade over a dozen different types of glycans17,19, while some species are restricted to one or a few18. From an ecological perspective, species with broad glycan-degrading abilities may be thought of as “generalists” that shift their metabolism from meal-to-meal, while species with narrower glycan degrading potential may be considered “specialists” that focus on one or a few glycans. Specialists run the risk of becoming extinct in a host if their preferred nutrients wane for too long, thus it most likely that such microorganisms would only evolve to degrade ubiquitously abundant dietary glycans or host derived mucins.
The task of degrading glycans in the gut is further complicated by the fact that many of these substrates are sequestered within larger structures like the plant cell wall, or regional microhabitats like the mucus layer, which may be difficult for some species to access (Fig. 1). Plant cell wall glycans (cellulose, hemicellulose and pectin) are intertwined in a polysaccharide matrix in many foods. In addition, hemicelluloses and pectins vary substantially in their fine-level structure between plant sources20,21. Thus, the dietary glycans available in whole-wheat bran differ from those available in a potato skin or in an apple. Intracellular plant glycans such as starch may be contained in either insoluble granules or as chemical forms that are resistant to degradative enzymes (Focus Box 1). Cooking, milling and other food preparation processes can all influence the abundance of these “resistant starch” forms and the availability of other plant glycans to intestinal microbes. Finally, the chemical diversity of endogenous O- and N-linked glycans (hundreds of different structures may be attached to a single mucin glycoprotein22) requires that mucosal bacteria produce many different degradative enzymes, a substantial metabolic investment, to effectively utilize these heterogeneous polymers. Indeed, one reason why such glycan diversity exists in secreted mucus could be to deter microbial species from evolving to be too efficient at harvesting these structures, thus protecting the integrity of this important barrier.
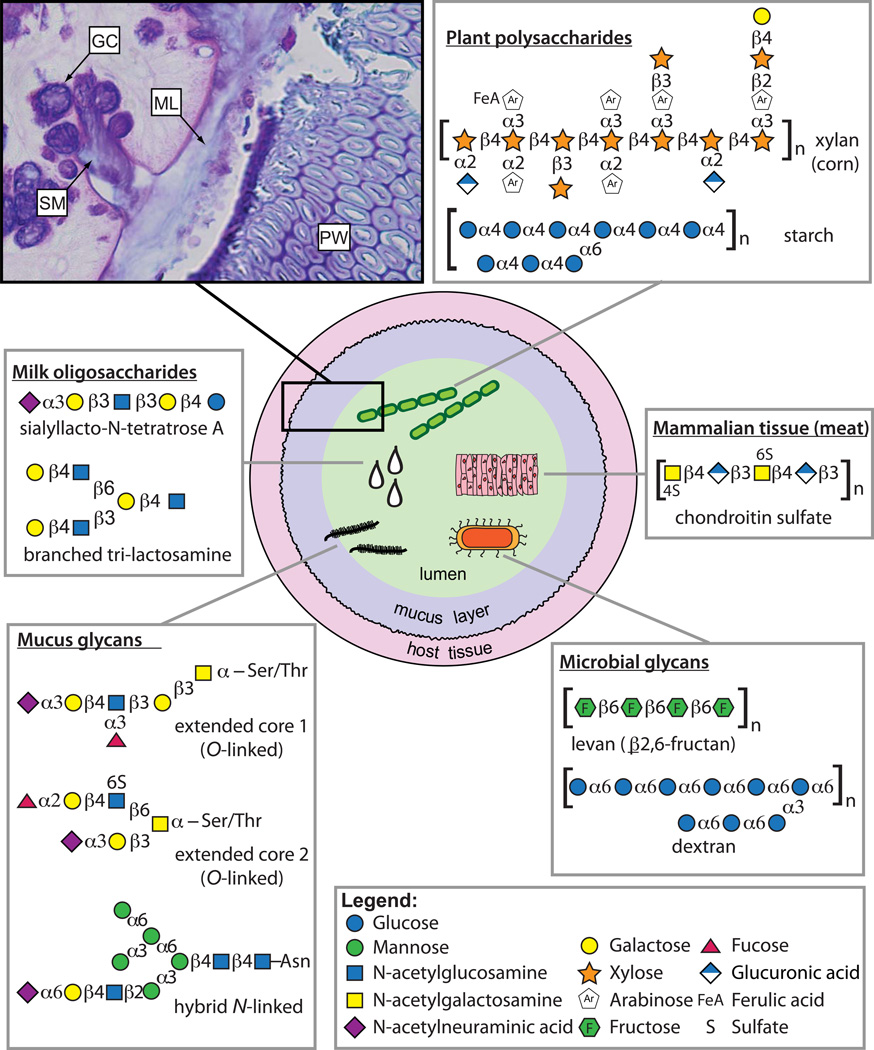
Fig. 1. Sources and chemical variation of glycans in the gut.
The center illustration shows a cross-sectional view of the intestine depicting five different sources of glycans: dietary plants, dietary animal tissue, endogenous microorganisms (e.g., capsules), mucus and breast milk. Some representative glycan structures are shown for each source. However, the complexity of all possible glycans in each category is much more expansive than shown. Monosaccharides are schematized according to the legend and interconnecting linkages are also indicated120. Brackets at the end of horizontal glycan chains indicate that they may extend further with a similar linkage pattern. The inset in the upper left shows a section of germfree mouse colon stained with periodic acid-Schiff base and Alcian blue stains for various carbohydrates. The section is oriented similarly as the corresponding box in the gut illustration in the center and highlights the locations of host mucus-secreting goblet cells (GC), secreted mucus (SM), the mucus layer (ML) and a fragment of plant cell wall (PW) located immediately adjacent to the mucus layer.
Focus Box 1: Starch in the human diet.
Starch is a plant storage glycan that consists of glucose monomers joined via α1,4-glycosidic linkages with possible α1,6-branching. Two common sub-forms of starch are delineated based on their molecular structure: amylose is an unbranched α1,4-linked polymer that tends to form helices that further aggregate into crystalline structures, and amylopectin is a less dense form containing variable amounts of α1,6-linked branches. Mammals secrete several amylase and glucoamylase enzymes in the upper gastrointestinal tract that degrade some parts of ingested starch and the resulting glucose is directly absorbed in the small intestine. Dietary starches are often sub-divided into rapidly-digesting, slowly-digesting and resistant forms109,110. Resistant starch (RS) is the portion of dietary starch that cannot be accessed by human intestinal enzymes and therefore transits to the colon undigested. The properties of RS can be altered by cooking and subsequent food handling steps: gelatinization occurs when starch-containing foods are heated in the presence of water forcing the hydration of starch granules and subsequent leaking of solubilized starch chains; retrogradation occurs when a gelatinized product is cooled for sufficient time allowing amylose regions to re-crystallize. Four types of RS are recognized: RS1 is physically inaccessible starch present in seeds or partly milled whole grains, RS2 is naturally granular starch like that contained in uncooked potato, RS3 is retrograded starch, composed largely of re-crystallized amylose regions, and RS4 is starch that has been chemically or enzymatically modified to avoid digestion. Many studies suggest that diet supplementation with RS improves colonic health and stimulates SCFA production, particularly butyrate, which has been linked with a range of colonic health benefits. In addition, creating foods from resistant starches eliminates the amount of glucose that is directly liberated during digestion, changing both the impact on human physiology and making it more acceptable in a diabetic diet.
Regardless of the particular glycan substrate degraded by gut microorganisms, the colonic epithelium benefits from the end result of this microbial metabolism by absorbing short chain fatty acids, such as butyrate, propionate and acetate. Butyrate that is produced in the colon exerts local effects on the colonic epithelium because it is a preferred energy source of colonocytes and has also been associated with suppressed growth of colonic tumors23. Acetate and propionate are absorbed into the bloodstream and travel to the liver where they are incorporated into lipid and glucose metabolism, respectively24. In addition to being absorbed by the host, the presence of acetate is also manifest in the colonic environment, where it may augment butyrate production by some species25,26and prevent colonization of some enteric pathogens27.
Glycan catalyzed changes in the infant gut microbiota
The human gut microbiota is established in the first few days of life and is initially seeded from microorganisms encountered during passage through the birth canal and incidental environmental exposures. After initial colonization, the gut experiences a series of progressive changes (ecological successions) in the richness and diversity of its inhabitants28,29. Early in life, fewer types of glycans transit the gut as diet is restricted to mother’s milk or formula. Many more glycans become available to the microbiota post-weaning as a diet rich in plant and animal matter is introduced. Despite potential fluctuations in dietary carbohydrates, endogenous host glycans represent a stable source of nutrients for the microbiota over our lifespan. However, at both points (pre- vs post-weaning), the carbohydrate composition of the gut is one important factor that guides the establishment of the microbial community.
Immediately after birth, infants consume a steady diet of breast milk or infant formula. Several hundred different glycan structures have been identified in human breast milk30,31with the primary components being lactose, glucose, galactose, N-acetylglucosamine, fucose, sialic acid, and a mixture of complex human milk oligosaccharides (HMOs). This latter class of highly diverse glycans, which seems to be uniquely abundant in human breast milk but not that of other mammals32, is composed of repeating and variably branched lactose or N-acetyllactosamine units that are often decorated with sialic acid and fucose monosaccharides30,31(Fig. 1). HMOs share structural similarities with human blood group antigens and the O-linked structures present in mucus. In contrast to the simpler lactose, most HMOs are not digested by human enzymes, suggesting that they have evolved as natural prebiotics to guide the development of the infant gut microbiota by selectively feeding certain species33–35.
Studies using culture independent techniques to sample the infant microbiota report substantial temporal and inter-individual variation compared to adults28,29,36and the nascent microbiota typically exhibits an abundance of bacteria from four phyla: Bacteroidetes, Proteobacteria, Firmicutes and Actinobacteria28. Although members of these same groups also dominate the adult gut, their proportions are different and more variable in the infant intestine, often varying in genus or species level taxa28. At the genus level, higher proportions of Lactobacillus and Bifidobacterium are observed in infants that are exclusively fed breast milk, suggesting that they may have co-evolved to occupy this niche, outcompeting other colonizers for available HMOs. Consistent with this, some species of Bifidobacterium (B. infantis and B. bifidum) directly metabolize HMOs19,37–41. Conversely, Lactobacilli seem to prefer the monosaccharide components of HMOs, which may suggest a synergistic effect of Lactobacilli and Bifidobacteria digestion of human milk in the infant gut42–44.
In contrast to breast-fed infants, formula-fed infants display lower abundance of Lactobacilli and Bifidobacteria and show increased abundance of Clostridium, Bacteroides, and members of the Enterobacteriaceae36,45–50. These observations reveal that cow’s milk-based formula, which lacks the amount and diversity of oligosaccharides present in human milk32, selects for different microorganisms during infancy. The potential long-term effects of these differences remain to be fully evaluated. However, one recent study found that adult mice exhibited variable susceptibility to chemically induced colitis based on the structure of milk sialyl-lactose oligosaccharides that they consumed during infancy32. This study, which found measurable differences in the bacteria that were present in adult mice several weeks after weaning, suggests that some members of the mammalian microbiota with the capacity to impact host health can be selected based on early nutritional conditions and persist in the gut after these conditions have been removed.
The ability of some members of the microbiota to access glycans attached to mucus may also have a role in early colonization by providing some bacteria with a source of endogenous nutrients during a period when dietary glycans are still absent. Due to their chemical similarity (Fig. 1) bacterial strategies for degrading HMOs and O-linked mucin glycans are likely to overlap. It was recently reported that outer membrane enzyme systems in the Gram-negative human gut symbiont B. thetaiotaomicron, which are used to degrade host mucus O-glycans51–53, are also deployed during metabolism of HMOs19. An additional study using germfree mice colonized with a B. thetaiotaomicron mutant lacking expression of 5 different gene clusters implicated in host glycan foraging in the adult gut was outcompeted by >200-fold relative to wild-type bacteria in a model of natural inter-generational transmission53. In this study, pre-weaned pups were exposed to similar amounts of these bacteria from their mother’s fecal microbiota, but selectively retained the mucin-degrading strain. Thus, the ability to forage host glycans in the neonatal gut prior to introduction of a more complex diet may be one key parameter that helps species establish colonization.
Post-weaning and adulthood
The carbohydrate composition of the human diet undergoes a somewhat abrupt change at ~6 months, when complex foods such as cereals, fruits and vegetables are introduced. When such complex plant glycans enter the gut, the composition of the microbiota shifts and microorganisms that prefer these glycans, such as the Gram-negative Bacteroidetes and new species of Firmicutes, become more prevalent17,28,29. Recent culture independent metagenomic studies characterizing the functionality of microbial genes present at various times points in the developing human microbiota have noted the presence of genes for plant carbohydrate degradation prior to the introduction of solid food29,49. These genes may be harbored in the genomes of glycan generalists like B. thetaiotaomicron, which degrade milk oligosaccharides or host mucin glycans prior to weaning and shift their metabolism to dietary glycans as they are introduced. The presence of glycan-adaptable species pre-weaning suggests that the gut microbiota is primed for the post-weaning dietary change perhaps because the cyclical, fecal-oral transmission of microorganisms from parent to child selects for species that can target glycans present in both the infant and adult gut.
As a fully omnivorous diet is achieved post-weaning, the composition of the microbiota as measured by abundance of broad taxonomic groups stabilizes and experiences fewer temporal changes54–56. Studies using culture-independent techniques to enumerate the human gut microbiota have found that two bacterial phyla, the Firmicutes and Bacteroidetes, are numerically dominant in the adult microbiota11,13,57; although, it has been documented that a third phylum (Actinobacteria) is frequently underestimated using molecular approaches and “universal” primers for the 16S rRNA gene and is therefore also likely to be more abundant than reported58. The abundance of Firmicutes is usually greatest. However, the ratios of Bacteroidetes and Firmicutes can change over time and be influenced by different diets, especially those that promote changes in host adiposity7,57, although a mechanistic explanation for these changes remains to elucidated. Another human study examined the differences between the gut microbiota of African children consuming a predominantly vegetarian, fibre-rich diet and European children consuming a lower-fibre diet that is more typical of Western societies. This study found a higher prevalence of Bacteroidetes/Actinobacteria compared to Firmicutes/Proteobacteria in the African children, and the opposite trend in European children, suggesting that the higher-fibre African diet was conducive to growth of specific fibre-degrading species59. Interestingly, the Bacteroidetes genera were quite different between groups: the microbiota of African children contained members of Prevotella and Xylanibacter, the latter being a genus that is very rarely, if ever, detected in Western samples7,11,60. By contrast, European children harbored Bacteroides and Alistipes as the dominant Bacteroidetes genera. In light of these clear genus differences, an interesting question for future work will be to measure the glycan degrading abilities of these different Bacteroidetes to determine if they have evolved to specialize on the different glycans contained in each diet.
Beyond the influence of certain types of diets in shaping the composition of the microbiota, supplementing the diet with particular glycans can impact species abundance. Not all species that possess the potential to degrade a given glycan will do so successfully in vivo. For example, inulin and smaller fructo-oligosaccharides (FOS), selectively increase the abundance of Bifidobacteria61, although many Bacteroides species are also able to use these glycans62. More recently, attention has focused on the ability of resistant starch (RS) to direct changes in the composition of the microbiota (Focus Box 1). Based on human and animal feeding studies, some microbial species may be more adept than others at degrading various forms of RS and are responsive to diets augmented with this nutrient. Consumption of some RS forms by humans preferentially results in increases in the short chain fatty acid butyrate which has been reported to exert anti-inflammatory23,63–66as well as anti-tumorigenic effects23,67–70, and has been suggested as a possible therapeutic for inflammatory bowel disease71. Butyrate is produced by members of the Firmicutes but is rarely associated with the SCFA profiles of Bacteroidetes72,73. Human volunteers consuming RS2 experienced increases in the Firmicutes Ruminococcus spp. and Eubacterium rectale74; likewise, overweight individuals consuming a diet high in RS3 exhibited increases in Eubacterium rectale, Roseburia spp. and Ruminococcus bromii75. These findings are consistent with in vitro observations that these species bind directly to insoluble starch particles and may be primary components of bacterial food-chains that target starch76,77.
Responses to rapid diet changes
In contrast to long-term changes between infancy and adulthood, our diets can also elicit rapid changes in microbiota composition as dietary glycans and other nutrients fluctuate from meal-to-meal62,78–80. Studies using germfree mice colonized with a transplanted human microbiota demonstrated that a rapid shift from a high fat diet to a high carbohydrate diet resulted in community changes that were observable after just one day, but took several days to stabilize78. In addition, a recent study of 10 human subjects who were fed either high-fat/low-fiber or low-fat/high-fiber diets in a controlled setting demonstrated that detectable changes in the microbiota are observable within 24 hours of a dietary shift81. Observations like these underscore the relationship between the microbiota and diet, suggesting that some proportion of our gut microorganisms is constantly fluctuating in abundance as a result of meal-to-meal variations. In contrast to protein and fat, which are more readily targeted by human absorptive systems, the low digestibility of non-starch dietary glycans suggests that changes in their abundance may exert a major impact on the microbiota. With this in mind, the effects on the microbiota of the high-fat diets mentioned above could serve to enrich for species that are capable of digesting host mucosal glycans by reducing dietary fiber. Indeed, in one study involving germfree mice colonized with a simplified microbiota, consisting of just E. rectale and B. thetaiotaomicron, it was found that the latter microorganism increased expression of host glycan-degrading genes when colonized animals were switched to a high-fat/low-fibre diet82. Much work is still needed to determine the precise relationships governing these diet-microbiota interactions, the locations along the length and width of the gut that are influenced by different dietary glycans, and the microbial populations that should be targeted for enrichment or depletion during certain states of dysbiosis.
Microbial strategies for harvesting glycans
Bacteroidetes Sus-like systems
Given the broad diversity of glycans that enter the gut, microorganisms must possess efficient strategies for competing for these nutrients. The most studied strategy for glycan acquisition by human gut bacteria is one that is employed by members of the Gram-negative Bacteroidetes. Work performed by Salyers and colleagues83,84provided the first mechanistic insight into how the prototypic symbiont, B. thetaiotaomicron, approaches starch metabolism and revealed a glycan-acquisition paradigm that is universal among the Bacteroidetes85,86. To metabolize starch, B. thetaiotaomicron requires a single eight-gene locus encoding the starch utilization system (Sus)87. The products of this system are located in the outer membrane and the periplasm of the bacterium and work to sequentially bind starch to the cell surface, degrade it into oligosaccharides and transport these pieces into the periplasmic space where they are degraded to even simpler sugars like glucose and imported into the cell (Fig. 2).
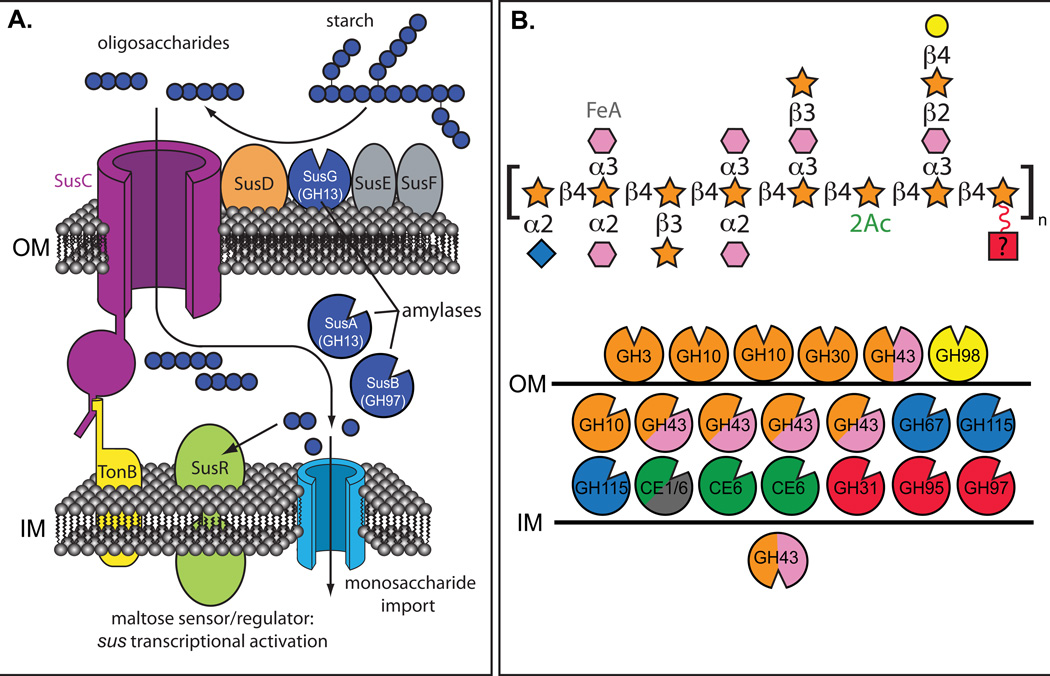
Fig. 2. Variations in functional complexity among Sus-like systems.
Two different representations of Sus-like systems in human gut Bacteroides. A. A model of the B. thetaiotaomicron starch utilization system (Sus). The TonB-dependent transporter SusC works in concert with the starch-binding lipoproteins SusD, SusE, SusF and SusG, which is a glycoside hydrolase family 13 (GH13) α-amylase. Starch binding is initiated by SusD/E/F, followed by initial degradation by SusG, and oligosaccharides are transported into the periplasm via SusC. In the periplasm, maltooligo-saccharides are further degraded to glucose by another GH13 enzyme (SusA, neopullulanse) and a GH97 enzyme (SusB, α-glucosidase). Homologs of the proteins SusC (TonB-dependent porin) and SusD (starch-binding protein) are a hallmark of every Sus-like system, but carbohydrate-binding proteins akin to SusE and SusF as well as glycoside hydrolases, vary substantially between Sus-like loci. B. Depiction of the enzymes encoded in two polysaccharide-utilization loci (PULs) from Bacteroides ovatus that targets the hemicellulose arabinoxylan, a heteropolymer with multiple monosaccharides and glycosidic linkages21. Unlike panel A, only the glycoside hydrolases in this locus are depicted, along with their predicted cellular locations: above the outer membrane (OM) are extracellular lipoproteins, between the OM and inner membrane (IM) are periplasmic enzymes and below the IM are cytoplasmic enzymes. A representation of maize arabinoxylan21is presented using the same monosaccharide scheme as presented in Fig. 1. The various B. ovatus glycan-degrading enzymes are color coded based on the linkages in maize arabinoxylan that they are predicted to degrade. Glycoside hydrolases are color-coded to represent the monosaccharide linkages hydrolyzed by each enzyme; labeling with two colors means that the enzyme family listed includes members capable of degrading two linkages present in arabinoxylan.
Subsequent sequencing of the B. thetaiotaomicron genome revealed the presence of as many as 88 related gene clusters, which contain homologs of at least two of the genes (susC and susD) present in the sus locus53,88. These loci are termed polysaccharide utilization loci (PULs) and their products, which collectively encompass ~18% of all genes in the B. thetaiotaomicron type strain VPI-5482, have been termed “Sus-like systems” because they function by a similar mechanism as Sus but harbor enzymes predicted to target glycans other than starch. These Sus-like systems are widespread among the human gut Bacteroidetes and are unique to this phylum, with many species dedicating similar proportions of their genomes as B. thetaiotaomicron to encoding these pathways52,85,89. A combination of whole-genome transcriptional profiling, gene disruption experiments and analysis of purified enzymes have been used to determine the cognate glycan substrates for the non-starch Sus-like PULs, and establish how glycan recognition and specificity are achieved53,62,89. Investigations of just a few human gut Bacteroidetes species have identified Sus-like systems for virtually all glycans, so far with the exception of cellulose, that are common in plant and animal tissue and that would be expected to enter the human gut (Table 1)53,62,89,90. In addition, many Sus-like systems appear to be devoted towards the breakdown of O-linked glycans found in host mucosal secretions. Additional Sus-like systems with substrate specificities for chitin, cellular N-linked glycans, and algal polysaccharides have been identified in non-human gut Bacteroidetes that inhabit the bovine rumen, canine mouth, soil and ocean, indicating that this glycan acquisition strategy is also common among non-human gut Bacteroidetes91–95.
Table 1.
Relationship between glycan complexity and the enzyme content in Sus-like systems
| Glycan | Unique linkages* |
Degrading species | Number of PULs in the species |
Number of enzymes in the system |
|---|---|---|---|---|
| Pectic galactan (β1,4-galactan)20 | 1 | Bacteroides thetaiotaomicron | 1 | 2 |
| Levan (β2,6-fructan)68 | 1 | B. thetaiotaomicron | 1 | 3 |
| Inulin (β2,1-fructan)68 | 1 | Bacteroides ovatus and Bacteroides caccae | 1 | 4 |
| Starch89 | 2 | B. thetaiotaomicron | 1 | 3 |
| Barley β-glucan20 | 2 | B. ovatus | 1 | 3 |
| Galactomannan and glucomannan20 | 3 | B. ovatus | 1 | 4 |
| Homogalacturonan20 | 4 | B. thetaiotaomicron | 1 | 7 |
| Arabinan20 | 4 | B. thetaiotaomicron | 2 | 6 |
| Xyloglucan20 | 4 | B. ovatus | 1 | 8 |
| Arabinogalactan20 | 4 | B. thetaiotaomicron | 2 | 8 |
| Yeast α-mannan60 | 4 | B. thetaiotaomicron | 3 | 12 |
| Heparin60 | 5 | B. thetaiotaomicron | 1 | 5 |
| Hyaluronan, dermatan and chondroitin sulphates60 | 7 | B. thetaiotaomicron | 1 | 5 |
| Xylan20 | 11 | B.ovatus | 2 | 21 |
| Mucin O-linked glycans60 | 12 | B. thetaiotaomicron | 15 | 17 |
| Rhamnogalacturonan I20 | 13‡ | B. thetaiotaomicron | 1 | 20 |
| Rhamnogalacturonan II20 | 22 | B. thetaiotaomicron | 1 | 32 |
PULs, polysaccharide utilization loci.
Includes all potential glycosidic linkages, plus methyl, acetyl, sulphate or ferulic acid linkages.
Linkages for rhamnogalacturonan I include side chains that are typically attached to a backbone chain25.
Examination of individual Sus-like systems provides a portrait of how broadly they have evolved. The number of enzymes in a given system is directly correlated with the complexity of the target glycan (Table 1). The prototypic Sus is among the simplest of systems with only 3 enzymes, while a pair of Bacteroides ovatus systems for processing branched arabinoxylans together contain 21 annotated enzymes (Fig. 2). Most of these enzymes are predicted to target glycosidic linkages or chemical substituents present in arabinoxylans from various cereal plants, though some enzymes do not target known linkages (illustrated in red in Fig. 2), suggesting that they may target variations in glycan structures not yet discovered.
Given the range of complexity among individual Sus-like systems, an important question is: how do the individual components of each system function together? Structural biology and protein biochemistry studies have provided substantial insight into the molecular mechanism of two of the Sus outer membrane components, each of which makes direct binding interactions with starch96,97. In addition, cross-linking and protein stability experiments with the B. thetaiotaomicron Sus and Sus-like systems in other species suggest that some of the Sus components interact directly with each other, but the stoichiometry and specific interactions within these potential complexes remain unclear84,95. Given the abundance of these systems in the human gut microbiome it is essential to understand how the individual components of the more simple systems work together both before and after an encounter with an extracellular glycan. This knowledge could be extrapolated to investigate more complex systems like those involved in degrading plant cell wall glycans.
Gram-positive glycan acquisition strategies
Bacteroidetes only compose a fraction of the human gut microbiota. Glycan acquisition strategies by the Firmicutes, which are typically more prominent in Western microbiota samples, have been less well defined. On average, sequenced Firmicutes encode fewer carbohydrate degrading enzymes than the Bacteroidetes but possess increased numbers of carbohydrate ABC transporters82. ABC transporters are frequently located adjacent to genes encoding glycoside hydrolases and these two groups of functions may also be co-regulated, suggesting that they function together98. Similar ABC transport systems are also employed by members of the Actinobacteria such as Bifidobacterium infantis whose genome encodes many Family 1 solute binding proteins (extracellular adaptors that work with ABC transporters) that bind HMOs and mucin glycans41. The discovery of glycan-specific ABC transport systems in many human gut Firmicutes and Actinobacteria suggests that they represent a second general paradigm for carbohydrate degradation and uptake by human gut bacteria that is worthy of attention in future studies.
Another well-studied Gram-positive glycan acquisition paradigm, the cellulosome, has been conspicuously absent to date from studies of the human gut microbiome. Cellulosomes are present in carbohydrate degrading microorganisms from the bovine rumen and soil, and, as their name implies, are especially important in cellulose degradation99,100. At least one metagenomic study of the human gut microbiota revealed the presence of protein components (dockerins and cohesins) that are signatures of cellulosomes; these functions were binned with Firmicute genomes, suggesting Faecalibacterium, Eubacterium and Ruminococcus groups as their possible genomic sources12. This exciting finding implies that at least some human gut Firmicutes have evolved to employ the cellulosome strategy in the human gut.
Glycan gradients in the gut
Glycan microhabitats
No gut microorganism characterized to date is capable of tackling all of the glycan structures that enter the intestine. Rather, individual species and strains typically display a subset of glycan degrading phenotypes that equip them to target just part of the overall glycan repertoire present at certain times or locations18,41,101. Since dietary glycans vary in solubility and accessibility in digesting food particles, and mucus glycans are enriched in a layer overlying the epithelium, particular glycans are likely to be concentrated in regional microhabitats based on their location or the variable rates at which they are depleted (Fig. 3).
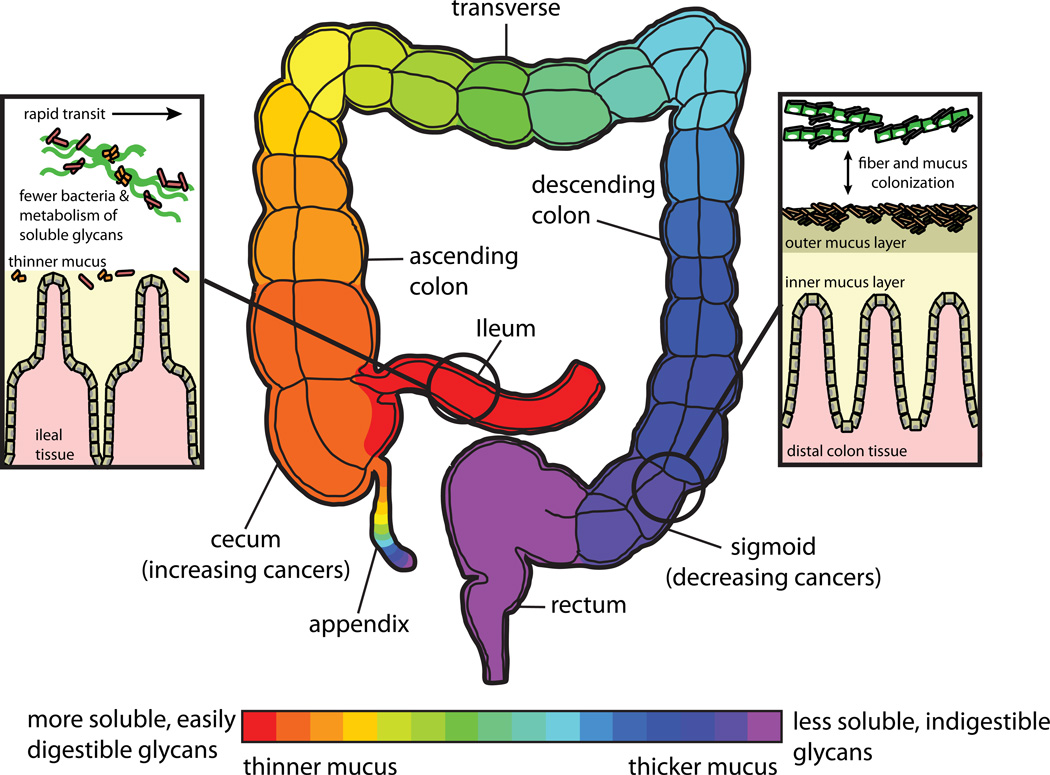
Fig. 3. Glycan utilization along the length of the gut and its potential health effects.
A schematic of the human ileum and colon that is color-coded to reflect potential glycan gradients (schematized according to the color bar at the bottom). The solubility and digestibility of dietary glycans that transit the lumen are variable and therefore each glycan is likely digested at a different rate. The thickness of intestinal mucus also follows a longitudinal gradient along the gut, but may be reciprocal to that of glycan digestibility, with greatest thickness present in the sigmoid colon and rectum where mostly insoluble/indigestible glycans are likely to be present121. The insets on the left and the right show schematics of the luminal and mucosal niches in the ileum and distal (sigmoid) colon. In the ileum, the mucus layer is relatively thin, transit time of contents is more rapid and bacteria are likely to target more soluble and rapidly digestible glycans, such as inulin and different oligosaccharide side-chains, such as α-arabinans and β-galactans, that are commonly attached to pectin (rhamnogalacturonan) backbones. In contrast, the distal colon has a much thicker mucus layer, transit time is slower and the residual glycans that fuel bacterial growth are likely to be less soluble and therefore take longer to degrade. Note the presence of inner and outer mucus layers, with bacterial colonization largely present in just the outer layer122. A possible reason for the increased mucus thickness in the distal gut may be to shield the epithelium from the more prolonged exposure to larger numbers of bacteria, which have more time to proliferate given the slower transit rate. It is widely accepted that increased dietary fibre intake is beneficial for colon health. In light of this idea, it is interesting that the incidence of colon cancers in several developed countries in North America, Europe and Asia are showing decreased abundance in the distal colon, and are increasing in more proximal regions over the last several decades123–126. One explanation offered for this phenomenon involves changing dietary habits in these societies, specifically reduced fiber intake and increased consumption of fat and animal protein. This trend could alter the microbiota or its metabolism in more distal regions, leading to carcinogenesis by several possible mechanisms (reduced transit time, increased production of toxic metabolites, or decreased production of protective metabolites like butyrate).
Regional variations in microbial colonization along the gut have been investigated in few studies using human and animal samples. One seminal culture-independent study of three healthy adult humans found that members of the phylum Bacteroidetes were enriched in colonic mucosal biopsies relative to fecal samples11. This study also noted “patchy and heterogeneous” abundance of mucosal species among different biopsy sites along the colon, suggesting variation in community structure along the length of the gut. Studies in mice using fluorescence in situ hybridization also revealed an enrichment of Bacteroidetes in the mucus layer102, although in neither case were the taxonomic identities of mucus-specific bacteria defined to finer levels. A recent study using germfree mice colonized with just two intestinal symbionts, E. coli and Bacteroides fragilis103, revealed that only the latter species, which is known to degrade mucin glycoproteins104, penetrates the mucus layer in the mouse colon. Thus, the ability to metabolize O-linked oligosaccharides and possibly the underlying peptide backbone that compose mucus are likely to be key factors in determining which microorganisms physically associate with this layer. Future work will be required to determine how widespread mucosal glycan degradation is among bacteria. Given the link between the microbiota and some inflammatory bowel disease (IBD) states4,10, the mucosal sub-population may be of particular interest given its close proximity to host tissue.
In addition to the lumen-mucus gradient, the longitudinal length of the gastrointestinal tract represents a much longer distance (~8 metres in humans from stomach to anus) along which microbial composition varies. In the proximal small intestine both microbial diversity and density are low, with only a few species predominating. Colonization density and diversity increase in the distal small intestine (ileum) and colon, the site of most dietary glycan fermentation, yet the effect of glycan in shaping local microbial composition along this length has not been extensively explored. A recent study using germfree mice colonized with a human fecal microbiota found that members of the Bacteroidetes increased from ~14% to ~42% between the small intestine and the cecum/colon78, suggesting that the slower rate of transit after the ileo-cecal transition provides a more optimal habitat for these glycophilic species. The wide variation in solubility and accessibility of glycans that transit the gut likely affects species composition along the intestinal tract. For example, highly soluble glycans such as inulin could be metabolized more rapidly by bacteria and may be processed in proximal regions of the gut, while insoluble or complicated glycans may take longer to degrade and thus reach more distal regions (Fig. 3). Further understanding the relationships between dietary glycans and the regional or temporal composition of the microbiota may lead to more precise prebiotic strategies for manipulating the region-specific microbiota to improve intestinal health.
Microbial food chains
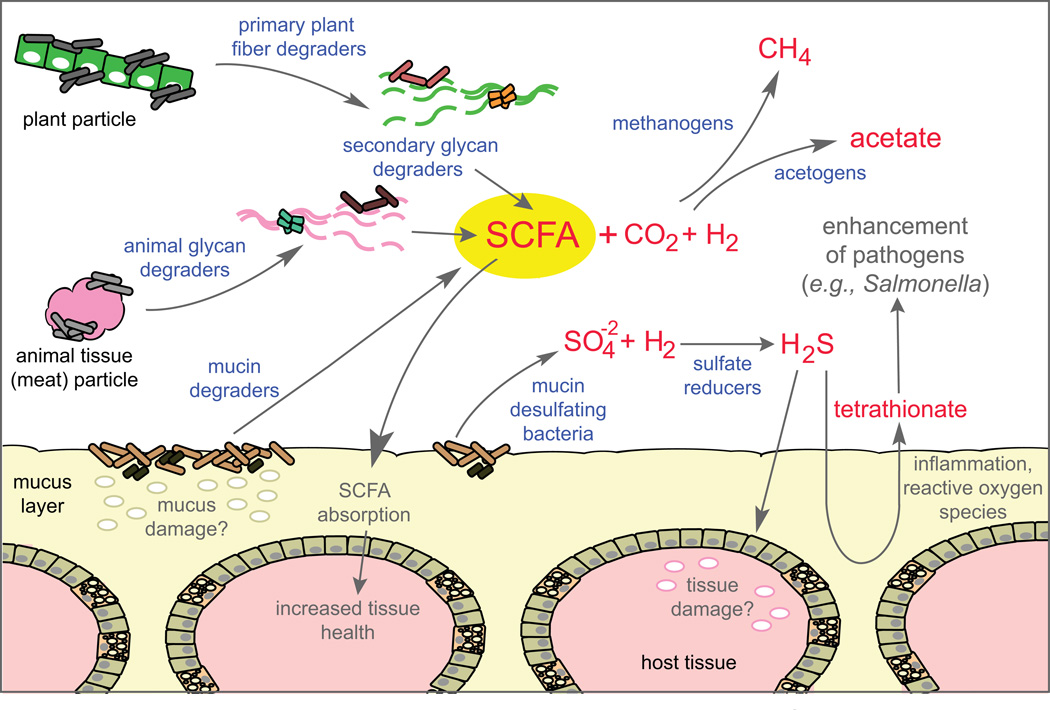
Microbial species interact in vivo to form more complicated food chains and some of these relationships center on glycan metabolism (Fig. 4). Species in food chains may be dependent on others for partial glycan degradation or for the production of certain metabolites (SCFA, vitamins, gases). One example of a glycan-driven bacterial food chain, suggested based on in vitro fecal batch fermentation, is the initial processing of insoluble plant cell wall fibres by certain members of the Firmicutes and Actinobacteria76. These organisms adhere most tightly to dietary fibres, a trait that may allow them to perform the initial glycan digestion steps to liberate shorter, more soluble polysaccharides for other bacteria76. Additional interactions in the gut are demonstrated by co-colonization of germfree mice with B. thetaiotaomicron and Eubacterium rectale82, or B. thetaiotaomicron and Bifidobacterium longum105, which leads to different bacterial glycan degradation profiles compared with monoassociation experiments. These latter studies suggest that bacteria adjust their metabolism based not only on the glycans present but also who else is there, perhaps in an attempt to either synergize or avoid competition. Metabolic cross-feeding (syntrophy) between two or more species also serves to augment the efficiency of glycan metabolism. Several bacterial sugar fermentation pathways generate hydrogen gas (H2) as a mechanism to recover NAD+106. Accumulated H2inhibits fermentation efficiency, but can be removed by methanogenesis, acetogenesis, or sulfate reduction catalyzed by different groups of microorganisms (Fig. 4). The presence of H2-scavenging microorganisms in the same microhabitat or vicinity as sugar-fermenting bacteria benefits both types of organisms and increases the overall metabolic efficiency.
Fig. 4. Glycan microhabitats and food chains in the gut.
An illustration of the ways in which different gut microorganisms are thought to interact during processing of various glycan substrates. Digesta derived from plant cell wall or meat particles will be rich in source-specific glycans, such as cellulose, hemicellulose and pectin (plant) or glycosaminoglycans and cellular glycoproteins (meat). These types of nutrients are likely to enter the distal gut as particulate forms that will be attacked by primary glycan degraders (e.g., Roseburia, Eubacterium, Clostridium, Ruminococcus and Bifidobacterium species) that are capable of directly binding to these insoluble particles and digesting their glycan components76. After initial degradation of glycan-containing particles, more soluble glycan fragments can be digested by other bacteria, which contribute to the liberated pool of SCFA fermentation products derived from both primary and secondary glycan degraders. A similar food chain of primary and secondary degraders has been proposed to occur in the mucus layer127; whereby some primary species are capable of directly degrading high molecular weight mucin glycoproteins and others are optimized to target the resulting oligosaccharide products. Bacterial glycan fermentation is enhanced by removal of downstream H2consumers, which convert this gas to methane, acetate or hydrogen sulfide depending on the types of microorganisms present. The latter pathway also requires free sulfate, which can be derived from many food products, but also from the degradation of animal proteins, sulfated glycans abundant in animal tissue (e.g., chondroitin sulfate) or in mucus. The resulting H2S is toxic to host cells, but is readily metabolized and detoxified by colonic tissue to form thiosulfate128. In the context of mucosal inflammation, thiosulfate can be converted to tetrathionate via reactive oxygen species, an event that has been recently tied to metabolic enhancement of the intestinal pathogen Salmonella enterica subspecies typhimurium129.
In food chains like those described above, the presence or absence of certain keystone members could influence the abundance of other dependent species. Interestingly, a recent metagenomic study of the microbiomes of 33 individual humans revealed the presence of just three different human “enterotypes”14. An enterotype is defined as a microbial community structure that is similar to communities from other individuals based on the presence and relative abundance of detectable species. Each enterotype contains a subset of all possible gut microbiota species in a signature combination that may have evolved to function optimally as a community. If the latter idea is true, then each enterotype should hold clues about functional food chains between human gut microorganisms, including those related to glycan metabolism. For example, in one enterotype the abundance of bacteria from the genus Bacteroides was positively correlated with bacteria from two other groups (Parabacteroides and Clostridiales) and negatively correlated with several others. By contrast, a second enterotype was defined by an abundance of the Bacteroidetes genus Prevotella, which exhibited positive and negative correlations with different groups relative to the first enterotype. These correlations may provide insight into which species are able to thrive in a microbial food chain together and which are potentially competing against each other for nutrients. An additional question is how do different dietary components, including various glycans, interact with the microbiota and do these contribute to enterotype formation? This question was addressed in a recent study involving 98 human subjects that examined the connections between long- and short-term dietary habits and microbiota enterotypes81. This study found that long-term consumption of a high-fiber diet was associated with an enterotype enriched for Prevotella species. In contrast, long-term consumption of diets rich in protein and animal fat were correlated with an enterotype containing increased Bacteroides and Ruminococcus species. As mentioned above, the absence of dietary fiber in high-fat or high-protein diets could select for species that are capable of resorting to mucus glycan degradation as an alternative nutrient base.
Glycans microhabitats and bacterial genome evolution
Microbial species in the gut evolve based on selective pressures that shape each species’ behavior (phenotype) and underlying genome architecture (genotype). When the first genomes of broadly saccharolytic human gut microorganisms were completed, it was observed that some species encode a diverse armament of glycan degrading enzymes. For example, the B. thetaiotaomicron VPI-5482 genome predicts the presence of 296 different glycan degrading enzymes as currently defined in the carbohydrate active enzymes (CAZy) database107. Many of these enzymes are not represented in the human genome, highlighting the complementarity between human and microbial carbohydrate degrading enzymes. Ongoing Human Microbiome Projects and other independent projects have collectively provided several hundred sequenced reference genomes of cultured human gut species108. The availability of genomic blueprints for so many gut microorganisms provides an unprecedented opportunity to compare the evolution of a large number of species that have evolved together in a similar environment. A recent study found that two closely related human symbionts B. thetaiotaomicron and B. ovatus, which share 96.5% identity in their 16S rRNA gene sequences, differ widely in their respective glycan utilization phenotypes and corresponding genomic structures89. Each of these species dedicates ~20% of its genes to encoding Sus-like systems, yet less than 1/3 are homologous between the two species. More strikingly, the Sus-like systems that are unique to each species are scattered throughout the two species’ respective genomes and correlate with a glycan utilization “theme”: mucin O-glycan degradation for B. thetaiotaomicron and plant cell wall hemicellulose degradation for B. ovatus. Thus, these two species seem to have acquired or retained niche-specific Sus-like systems that could direct them to distinct glycan microhabitats: the mucus layer and cell walls of digesting plant matter. Future work will be needed to determine if this theme extrapolates to other sequenced human gut bacteria and how the resulting phenotypic diversity among species and strains directs regional variation in microbial colonization.
Summary and prospectus.
The variations in microbiota composition that result from differing abilities of gut microorganisms to metabolize glycans could have profound implications for understanding both how the microbiota assembles over a human lifetime and how short-term community variations influence health. Due to their proximity to host tissue, mucus-associated microorganisms may have a disproportionate impact on host responses and may therefore be a sub-population of particular interest in investigating diseases thought to result from dysbiosis. Towards this point, a recent study using a mouse model of IBD successfully fulfilled Koch’s Postulates, finding that some individual Bacteroides species including B. thetaiotaomicron were sufficient to elicit disease in the context of a natural microbiota10. A future goal is to develop strategies to manipulate microbiota function using prebiotic, probiotic or pharmacological strategies. In order for this to be achieved, it is imperative to gain deeper insight into the fine structure of microbiota composition and the metabolism of its constituents in different gut regions. Glycan availability is likely to be a major factor in determining these relationships.
Focus Box 2: “Prescription” diets: Prebiotics for treating disease.
Because the microbiota responds to dietary changes, this community can be manipulated to promote host health through the addition of specific dietary components (prebiotics) to one’s existing diet. The long-chain β-fructan inulin, as well as smaller fructo-oligosaccharides, are well-studied prebiotic fibers, and their ability to promote the growth of Bifidobacteria is well-documented61, although many other beneficial human gut species such as the Roseburia spp.111and Faecalibacterium prausnitzii112also increase in abundance in response to dietary inulin. Dietary supplementation with inulin and frucotoligosaccharide has been shown to curb the metabolic effects of a high-fat diet, including improved glucose-tolerance, reduction in colonic inflammation and endotoxemia, and improved colonic barrier function113,114. Inulin may also be useful as a prebiotic preventative for colon cancer; in rats with chemically-induced colon cancer, tumor incidence decreases up to 70% when the diet is supplemented with a combination of inulin and soybean meal115. In this therapy, fructan stimulates microbial metabolism, which is thought to enhance the bioavailability of soybean isoflavones. In another study, microbial metabolites from inulin fermentation stimulated increased apoptosis in human colon cancer cell lines, especially in early adenoma cells116. Prebiotics involving resistant starch (see Focus Box 1and main text) and butyrylated starch70may also have anti-cancer and anti-inflammatory properties in the colon as they stimulate the production of the short chain fatty acid butyrate by some microbiota members. More recently, prebiotic formulations involving arabinoxylans, and chitin-glucan have shown promise for their potential to restore microbial community balance and colonic function (i.e., the Bacteroidetes: Firmicutes ratio) even within a high-fat diet117,118. Systemically, these prebiotics may also help lower cholesterol, as they stimulate the production of propionate by the microbiota, a short chain fatty acid that is absorbed into the bloodstream and travels to the liver where it can inhibit cholesterol synthesis119.
Glossary
- glycan
A polymer of multiple simple sugars connected by covalent linkages. Glycans may be attached to other molecules such as lipids (glycolipids) and proteins (glycoproteins). Like nucleic acids, glycans have polarity, with a linear molecule having one reducing end and one non-reducing end. In this review, the term glycan is used synonymously with polysaccharide.
- short chain fatty acids (SCFA)
Also referred to as volatile fatty acids (VFA), these molecules include linear and branched end products of bacterial fermentation that contain 6 or fewer carbon atoms and are produced in addition to lactic and formic acids. Examples include acetic, propionic and butyric acids.
- mucus
A viscous mixture consisting predominantly of mucin glycoproteins, which may be either attached to cell membranes or secreted in soluble form from the cell. Mucus frequently contains other secreted host compounds, such as secretory IgA and antimicrobial peptides.
- glycosidic linkage
A chemical connection between one of the numbered carbon atoms in a sugar monomer to another carbon atom in a second sugar that is mediated by a shared oxygen atom. These bonds can be in α or β conformation and multiple linkages may be connected in linear or branched chains to construct more complex glycan structures.
- hemicellulose
A heterogeneous class of glycans that are found in the matrix of plant cell walls associated with cellulose. Unlike highly insoluble cellulose, hemicelluloses have more amorphous and flexible structures that help to bind cellulose to pectin fibrils. The type and amount of hemicellulose in the plant cell wall is dependent upon the botanical origin, and includes molecules such as xylan, xyloglucan, galactomannan, and glucomannan.
- pectin
A diverse class of polysaccharides composed of either a homopolymer of α1,4-linked galacturonic acid or a heteropolymer containing galacturonic acid and rhamnose (rhamnogalacturon I). Each of these core pectin backbones can be extensively substituted with a variety of modifications and glycan branches, including methyl and acetyl groups, monosaccharides like xylose, and longer chains such β-galactans and α-arabinans20.
- prebiotic
A functional food component that selectively enhances the abundance or physiology of a subset of microbiota bacteria with the goal of increasing beneficial effects. Plant fibres resistant to human digestion are among the most common prebiotic therapies.
- germfree mice
Mice that are raised in the complete absence of microbial colonization, usually following aseptic delivery by caesarian section and maintenance in sterile isolators that exclude access by environmental microorganisms. Other animal species such as rats, pigs and chickens have also been reared under germfree conditions.
- food chains
An arrangement of multiple species in space and time that allows some members to feed either directly on others or from their by products. Keystone members, which act first in a food chain, are particular important because their absence also influences the status of dependent species that exist downstream in the food chain.
- cellulosome
Extracellular multi-enzyme complexes formed by some Gram-positive bacteria and fungi. Cellulosomes bind and degrade plant cell wall polysaccharides that are resistant to degradation, including cellulose. Scaffoldin, the major non-enzymatic structural component, connects the enzymes via interactions between a dockerin, a binding module of scaffoldin, and cohesion modules present on the enzymes.
- ABC transporter
ATP Binding Cassette (ABC) transporters are a protein superfamily that are found in almost every form of life from bacteria to man. These systems are typically composed of three main components: a solute-binding protein that binds ligand and dictates specificity, a membrane transporter through which the ligand passes, and a ATPase that provides energy to drive ligand transport. Bacteria use ABC importers to take up nutrients like iron, peptides or sugars and ABC efflux transporters to pump toxic compounds out of the cell.
- inflammatory bowel disease (IBD)
This term refers to a group of pathologies, most notably Crohn’s disease and ulcerative colitis, characterized by inflammation in the distal small intestine or colon, respectively. These diseases are thought to stem from a congruence of host susceptibility factors, such as genetic predisposition to uncontrolled inflammatory responses or reduced mucosal immunity, and stimulation by environmental or microbiological (bacteria and virus) triggers4,8
- Human Microbiome Projects
Any of several ongoing efforts to sequence the microbial communities associated with various human body sites including the gut. A major component of these projects is to sequence cultured “reference” organisms. However, because many human-associated microorganisms have not yet been isolated in laboratory culture, a second approach is to directly sequence DNA extracted from microbial community samples (metagenomics).
- Koch’s Postulates
A strategy for establishing causality between a potential microbial pathogen and a disease, published by Robert Koch in 1890. The postulates state that a microorganism that causes a disease should be: 1) abundant in animals suffering from that disease, 2) isolated from diseased specimens, 3) able to be eradicated from diseased animals, resulting in disease cure, and 4) able to be introduced into healthy animals to cause disease.
References
- 1.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 3.Wardwell LH, Huttenhower C, Garrett WS. Current concepts of the intestinal microbiota and the pathogenesis of infection. Curr Infect Dis Rep. 2011;13:28–34. doi: 10.1007/s11908-010-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Current opinion in infectious diseases. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Keefe SJ, et al. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044–2048. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Current opinion in gastroenterology. 2011;27:321–327. doi: 10.1097/MOG.0b013e32834661b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett WS, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom SM, et al. Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, et al. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc Natl Acad Sci U S A. 2010;107:7503–7508. doi: 10.1073/pnas.1002355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011 doi: 10.1038/nature09944. Metagenomic-based analysis of a total of 272 new and published human fecal microbiomes, revealing that presence of just 3 dominant enterotypes.
- 15. Caporaso JG, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12:R50. doi: 10.1186/gb-2011-12-5-r50. The first long term study of human fecal microbiome dynamics with daily time point analysis.
- 16.McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- 17.Salyers AA, Vercellotti JR, West SE, Wilkins TD. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977;33:319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salyers AA, West SE, Vercellotti JR, Wilkins TD. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977;34:529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcobal A, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucusutilization pathways. Cell host & microbe. 2011;10:507–514. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohnen D. Pectin structure and biosynthesis. Current opinion in plant biology. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Saulnier L, Marot C, Chanliaud E, Thibault J-F. Cell wall polysaccharide interactions in maize bran. Carbohydrate Polymers. 1995;26:279–287. [Google Scholar]
- 22.Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 23.Hamer HM, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 24.Rombeau JL, Kripke SA. Metabolic and intestinal effects of short-chain fatty acids. JPEN. Journal of parenteral and enteral nutrition. 1990;14:181S–185S. doi: 10.1177/014860719001400507. [DOI] [PubMed] [Google Scholar]
- 25.Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncan SH, et al. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91:915–923. doi: 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 28.Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koenig JE, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2010;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. A 16S and metagenomic-based study of microbiota formation in a single human child over the first three years of life, correlating changes with life events such as diet shifts, illness and antibiotics.
- 30.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 31.Ninonuevo MR, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–7480. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- 32.Fuhrer A, et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–2854. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. Adv Exp Med Biol. 2001;501:315–323. doi: 10.1007/978-1-4615-1371-1_39. [DOI] [PubMed] [Google Scholar]
- 34.German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:205–218. doi: 10.1159/000146322. discussion 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–3020. doi: 10.1093/jn/130.12.3014. [DOI] [PubMed] [Google Scholar]
- 36.Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sela DA. Bifidobacterial utilization of human milk oligosaccharides. Int J Food Microbiol. 2011;149:58–64. doi: 10.1016/j.ijfoodmicro.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Miwa M, et al. Cooperation of beta-galactosidase and beta-N-acetylhexosaminidase from Bifidobacteria in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology. 2010;20:1402–1409. doi: 10.1093/glycob/cwq101. [DOI] [PubMed] [Google Scholar]
- 39.LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol. 2010;76:7373–7381. doi: 10.1128/AEM.00675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turroni F, et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci U S A. 2010;107:19514–19519. doi: 10.1073/pnas.1011100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS One. 2011;6:e17315. doi: 10.1371/journal.pone.0017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez RC, et al. In vitro evaluation of gastrointestinal survival of Lactobacillus amylovorus DSM 16698 alone and combined with galactooligosaccharides, milk and/or Bifidobacterium animalis subsp. lactis Bb-12. Int J Food Microbiol. 2011;149:152–158. doi: 10.1016/j.ijfoodmicro.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Diaz J, Monedero V, Yebra MJ. Utilization of natural fucosylated oligosaccharides by three novel alpha-L-fucosidases from a probiotic Lactobacillus casei strain. Appl Environ Microbiol. 2011;77:703–705. doi: 10.1128/AEM.01906-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab C, Ganzle M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol Lett. 2011;315:141–148. doi: 10.1111/j.1574-6968.2010.02185.x. [DOI] [PubMed] [Google Scholar]
- 45.Coppa GV, et al. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88:89–94. doi: 10.1111/j.1651-2227.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 46.Favier CF, de Vos WM, Akkermans AD. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe. 2003;9:219–229. doi: 10.1016/j.anaerobe.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Fallani M, et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology. 2011;157:1385–1392. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 48.Harmsen HJ, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Kurokawa K, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariat D, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sonnenburg JL, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. The first in vivo transcriptomic-based study of a human gut symbiont (B. thetaiotaomicron) in the intestines of gnotobiotic mice consuming diets with varying glycan content.
- 52.Bjursell MK, Martens EC, Gordon JI. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. The Journal of biological chemistry. 2006;281:36269–36279. doi: 10.1074/jbc.M606509200. [DOI] [PubMed] [Google Scholar]
- 53. Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–457. doi: 10.1016/j.chom.2008.09.007. Identifies B. thetaiotaomicron genes involved in host glycan degradation by transcriptional profiling of in vitro grown cultures and demonstrates a link between host glycan foraging and inter-generational transmission.
- 54.Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franks AH, et al. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ley RE, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill JE, et al. Improvement of the representation of bifidobacteria in fecal microbiota metagenomic libraries by application of the cpn60 universal primer cocktail. Applied and environmental microbiology. 2010;76:4550–4552. doi: 10.1128/AEM.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. Analyzed the microbiota composition of two different populations of children in Africa and Europe that consume very diverse diets.
- 60.Tap J, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 61.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr. 2009;63:1277–1289. doi: 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- 62. Sonnenburg ED, et al. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. A mechanistic study of fructan utilization by multiple species of human Bacteroides, revealing that a single gene cluster can be evolutionarily altered between species to switch glycan substrate specificity.
- 63.Segain JP, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luhrs H, et al. Cytokine-activated degradation of inhibitory kappaB protein alpha is inhibited by the short-chain fatty acid butyrate. Int J Colorectal Dis. 2001;16:195–201. doi: 10.1007/s003840100295. [DOI] [PubMed] [Google Scholar]
- 65.Hamer HM, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28:88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 67.Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res. 2000;12:83–95. doi: 10.3727/096504001108747558. [DOI] [PubMed] [Google Scholar]
- 68.McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34:386–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dronamraju SS, Coxhead JM, Kelly SB, Burn J, Mathers JC. Cell kinetics and gene expression changes in colorectal cancer patients given resistant starch: a randomised controlled trial. Gut. 2009;58:413–420. doi: 10.1136/gut.2008.162933. [DOI] [PubMed] [Google Scholar]
- 70.Clarke JM, Topping DL, Bird AR, Young GP, Cobiac L. Effects of high-amylose maize starch and butyrylated high-amylose maize starch on azoxymethane-induced intestinal cancer in rats. Carcinogenesis. 2008;29:2190–2194. doi: 10.1093/carcin/bgn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Immerseel F, et al. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J Med Microbiol. 2010;59:141–143. doi: 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- 72.Pan N, Imlay JA. How does oxygen inhibit central metabolism in the obligate anaerobe Bacteroides thetaiotaomicron. Molecular microbiology. 2001;39:1562–1571. doi: 10.1046/j.1365-2958.2001.02343.x. [DOI] [PubMed] [Google Scholar]
- 73.Sakamoto M, et al. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family 'Porphyromonadaceae' isolated from rat faeces. International journal of systematic and evolutionary microbiology. 2009;59:1748–1753. doi: 10.1099/ijs.0.007674-0. [DOI] [PubMed] [Google Scholar]
- 74.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Walker AW, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. Isme J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. Human volunteer study tracking the changes in the microbiota upon shifting to a low-carbohydrate diet, then to a resistant starch diet, and finally to a non-starch polysaccharide rich diet.
- 76.McWilliam Leitch EC, Walker AW, Duncan SH, Holtrop G, Flint HJ. Selective colonization of insoluble substrates by human faecal bacteria. Environ Microbiol. 2007;9:667–679. doi: 10.1111/j.1462-2920.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 77.Macfarlane S, Macfarlane GT. Composition and metabolic activities of bacterial biofilms colonizing food residues in the human gut. Appl Environ Microbiol. 2006;72:6204–6211. doi: 10.1128/AEM.00754-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Turnbaugh PJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. Used culture independent methods to monitor alterations in microbiota of humanized mice in response to rapid diet shift.
- 79.Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science. 2011 doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goodman A, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahowald MA, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D'Elia JN, Salyers AA. Contribution of a neopullulanase, a pullulanase, and an alpha-glucosidase to growth of Bacteroides thetaiotaomicron on starch. J Bacteriol. 1996;178:7173–7179. doi: 10.1128/jb.178.24.7173-7179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho KH, Salyers AA. Biochemical analysis of interactions between outer membrane proteins that contribute to starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 2001;183:7224–7230. doi: 10.1128/JB.183.24.7224-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu J, et al. Evolution of Symbiotic Bacteria in the Distal Human Intestine. PLoS Biol. 2007;5:e156. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. The Journal of biological chemistry. 2009;284:24673–24677. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tancula E, Feldhaus MJ, Bedzyk LA, Salyers AA. Location and characterization of genes involved in binding of starch to the surface of Bacteroides thetaiotaomicron. J Bacteriol. 1992;174:5609–5616. doi: 10.1128/jb.174.17.5609-5616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu J, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 89. Martens EC, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. The first transcriptomic and genetic study linking specific genes in human gut Bacteroides with degradation of all major plant cell wall polysaccharides except cellulose.
- 90. Hehemann JH, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. Identified an enzyme system involved in degrading a polysaccharide present in seaweed, which was transferred from the marine metagenome to the human microbiome as a function of dietary seaweed consumption.
- 91.Dodd D, Moon YH, Swaminathan K, Mackie RI, Cann IK. Transcriptomic analyses of xylan degradation by Prevotella bryantii and insights into energy acquisition by xylanolytic bacteroidetes. The Journal of biological chemistry. 2010;285:30261–30273. doi: 10.1074/jbc.M110.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyazaki K, Hirase T, Kojima Y, Flint HJ. Medium- to large-sized xylo-oligosaccharides are responsible for xylanase induction in Prevotella bryantii B14. Microbiology. 2005;151:4121–4125. doi: 10.1099/mic.0.28270-0. [DOI] [PubMed] [Google Scholar]
- 93.Bauer M, et al. Whole genome analysis of the marine Bacteroidetes 'Gramella forsetii' reveals adaptations to degradation of polymeric organic matter. Environ Microbiol. 2006;8:2201–2213. doi: 10.1111/j.1462-2920.2006.01152.x. [DOI] [PubMed] [Google Scholar]
- 94.McBride MJ, et al. Novel features of the polysaccharide-digesting gliding bacterium Flavobacterium johnsoniae revealed by genome sequence analysis. Appl Environ Microbiol. 2009;75:6864–6875. doi: 10.1128/AEM.01495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Renzi F, et al. The N-glycan Glycoprotein Deglycosylation Complex (Gpd) from Capnocytophaga canimorsus Deglycosylates Human IgG. PLoS pathogens. 2011;7:e1002118. doi: 10.1371/journal.ppat.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koropatkin NM, Smith TJ. SusG: A Unique Cell-Membrane-Associated alpha-Amylase from a Prominent Human Gut Symbiont Targets Complex Starch Molecules. Structure. 2010;18:200–215. doi: 10.1016/j.str.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 97. Koropatkin NM, Martens EC, Gordon JI, Smith TJ. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure. 2008;16:1105–1115. doi: 10.1016/j.str.2008.03.017. Structure of the B. thetaiotaomicron starch-binding protein SusD, revealing a novel fold and site of glycan interaction in this broadly expanded family of Bacteroidetes proteins.
- 98.Rey FE, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. The Journal of biological chemistry. 2010;285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fontes CM, Gilbert HJ. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu Rev Biochem. 2010;79:655–681. doi: 10.1146/annurev-biochem-091208-085603. [DOI] [PubMed] [Google Scholar]
- 100.Bayer EA, Lamed R, White BA, Flint HJ. From cellulosomes to cellulosomics. Chem Rec. 2008;8:364–377. doi: 10.1002/tcr.20160. [DOI] [PubMed] [Google Scholar]
- 101.Pokusaeva K, Fitzgerald GF, van Sinderen D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol. 2005;11:1131–1140. doi: 10.3748/wjg.v11.i8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang JY, Lee SM, Mazmanian SK. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe. 2011;17:137–141. doi: 10.1016/j.anaerobe.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macfarlane GT, Gibson GR. Formation of glycoprotein degrading enzymes by Bacteroides fragilis. FEMS Microbiol Lett. 1991;61:289–293. doi: 10.1016/0378-1097(91)90567-t. [DOI] [PubMed] [Google Scholar]
- 105.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gibson GR, et al. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990;31:679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cantarel BL, et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson KE, et al. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang G, Hamaker BR. Slowly digestible starch: concept, mechanism, and proposed extended glycemic index. Crit Rev Food Sci Nutr. 2009;49:852–867. doi: 10.1080/10408390903372466. [DOI] [PubMed] [Google Scholar]
- 110.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. European journal of clinical nutrition. 1992;46(Suppl 2):S33–S50. [PubMed] [Google Scholar]
- 111.Scott KP, et al. Substrate-driven gene expression in Roseburia inulinivorans: importance of inducible enzymes in the utilization of inulin and starch. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4672–4679. doi: 10.1073/pnas.1000091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramirez-Farias C, et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 113.Cani PD, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 114.Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr. 2007;137:2547S–2551S. doi: 10.1093/jn/137.11.2547S. [DOI] [PubMed] [Google Scholar]
- 115.Gourineni VP, Verghese M, Boateng J, Shackelford L, Bhat KN. Chemopreventive potential of synergy1 and soybean in reducing azoxymethane-induced aberrant crypt foci in fisher 344 male rats. Journal of nutrition and metabolism. 2011;2011:983038. doi: 10.1155/2011/983038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munjal U, Glei M, Pool-Zobel BL, Scharlau D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br J Nutr. 2009;102:663–671. doi: 10.1017/S0007114509274770. [DOI] [PubMed] [Google Scholar]
- 117.Neyrinck AM, et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One. 2011;6:e20944. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neyrinck AM, et al. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. The Journal of nutritional biochemistry. 2011 doi: 10.1016/j.jnutbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Van den Abbeele P, et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ Microbiol. 2011;13:2667–2680. doi: 10.1111/j.1462-2920.2011.02533.x. [DOI] [PubMed] [Google Scholar]
- 120.Varki A, et al. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 121.Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997;40:782–789. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park SY, et al. Proximal shift in the distribution of adenomatous polyps in Korea over the past ten years. Hepato-gastroenterology. 2009;56:677–681. [PubMed] [Google Scholar]
- 124.Larsen IK, Bray F. Trends in colorectal cancer incidence in Norway 1962–2006: an interpretation of the temporal patterns by anatomic subsite. International journal of cancer. Journal international du cancer. 2010;126:721–732. doi: 10.1002/ijc.24839. [DOI] [PubMed] [Google Scholar]
- 125.Toyoda Y, Nakayama T, Ito Y, Ioka A, Tsukuma H. Trends in colorectal cancer incidence by subsite in Osaka, Japan. Japanese journal of clinical oncology. 2009;39:189–191. doi: 10.1093/jjco/hyn144. [DOI] [PubMed] [Google Scholar]
- 126.Singh H, Demers AA, Xue L, Turner D, Bernstein CN. Time trends in colon cancer incidence and distribution and lower gastrointestinal endoscopy utilization in Manitoba. The American journal of gastroenterology. 2008;103:1249–1256. doi: 10.1111/j.1572-0241.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 127.Png CW, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. The American journal of gastroenterology. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 128.Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanediol to thiosulate by rat tissues: a specialized function of the colonic mucosa. Biochemical Pharmacology. 2001;62:255–259. doi: 10.1016/s0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 129. Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. Demonstrates that tetrathionate, a combined metabolic by-product of the microbiota and human tissue, serves as an electron acceptor to enhance the physiology of a gut pathogen.