Introduction
Von Hippel-Lindau (VHL) disease is an autosomal dominantly inherited multisystem disorder characterized by the development of a variety of benign and malignant tumors. Ninety per cent of patients with VHL syndrome will manifest symptoms by age 65, but most already have clinically significant issues in their teens or twenties. Retinal vascularized lesions (hemangioblastomas) remain the most common manifestation of VHL disease, occurring in the majority of VHL patients, often as the first sign of the disease1. Patients affected by retinal hemangioblastomas present with loss of vision and may develop a painful secondary glaucoma with advanced lesions2. Since the risk of blindness is high in eyes with symptomatic retinal hemangioblastomas, diagnosis and treatment before symptoms develop is beneficial. Despite the considerable morbidity associated with these tumors, retinal and CNS hemangioblastomas are classified as benign tumors due to their generally limited local invasion and lack of metastatic spread3. Here we report an unusual case of a 56 year-old woman with known VHL disease and a history of bilateral retinal hemangioblastomas. This normally benign vascular tumor filled the vitreous cavity in one eye, and penetrated the scleral with extraocular extension, ultimately requiring enucleation.
Case Description
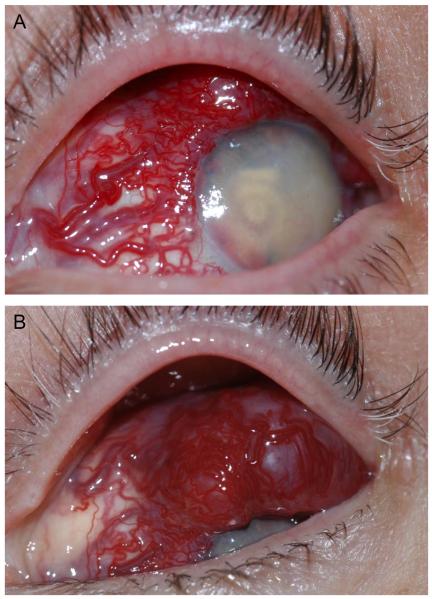
A 56 year-old Caucasian woman with a known history of VHL disease and bilateral retinal hemangioblastomas unresponsive to laser photocoagulation or cryotherapy was referred to an oculoplastics specialist for a vascular lesion evident on the upper surface of the left eye over the previous several months. On clinical examination, the vision without correction was no light perception in each eye. She had phthisis of both eyes. Examination of the left eye revealed a large, poorly circumscribed vascularized epibulbar mass extending from the limbus posteriorly (Figure 1A and B).
Figure 1.
External photos of the highly vascularized lesion. A. Dilated tortuous vessels involving the nasal, superior, and temporal conjunctiva feed a highly vascularized subconjunctival lesion. The cornea is translucent; there is florid neovascularization with a layered hyphema in the anterior chamber. B. Views of the superior conjunctiva reveal that the lesion overrides the corneal limbus superiorly.
High resolution (20 MHz using a fluid standoff) echography revealed a large, irregularly shaped, irregularly structured lesion involving the external surface of the eye (Figure 2A). Multiple large vessels were noted within the mass. The anterior sclera could be followed adjacent to the lesion but not beneath the lesion (Figure 2B), supporting a diagnosis of extraocular extension of an intraocular lesion. Contact B-scan (10MHz) of the vitreous revealed a small globe filled with dense opacities and membranes (Figure 2C). There was marked calcification of the globe wall with an area of discontinuity noted superiorly.
Figure 2.
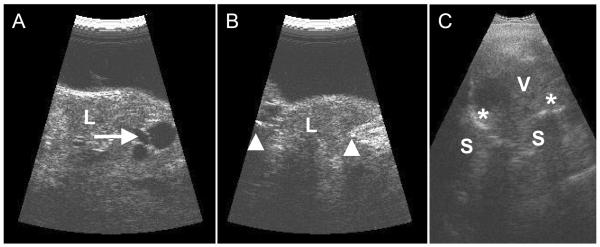
Ultrasound exam of the vascularized lesion. A. and B. Transverse (cross-section; A) and longitudinal (radial section; B) images using a 20 MHz probe (using a fluid standoff) of the anterior segment reveals that the lesion (L) on the outer surface of the globe is highly reflective with low reflective areas consistent with vessels and/or cystic spaces (arrow); the lesion protrudes through the sclera (arrow heads). C. Contact B-scan (10 MHz) showing the disorganized intraocular structures (V) and the marked calcification of the globe wall (asterick) with the associated acoustic shadowing (S).
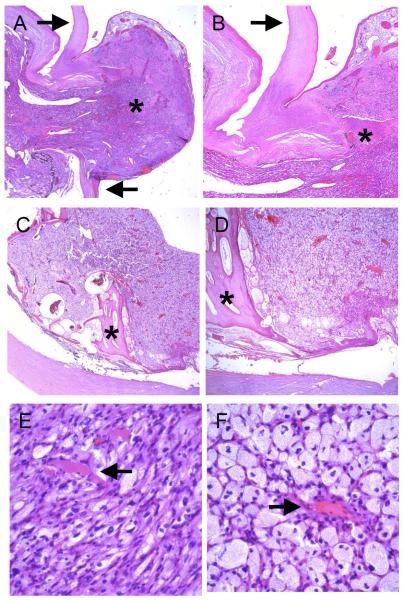
The echographic findings were most suggestive of extrascleral extension of an intraocular hemangioblastoma secondary to Von Hipple-Lindau disease. Enucleation of the left eye was recommended to provide the patient with a better chance of long-term comfort and for definitive diagnosis for the lesion. Microscopic examination disclosed an eye with an intraocular vascularized lesion covered by conjunctival epithelium that extended through a 2 mm defect in the superior limbus (Figure 3A and B). The majority of retina, choroid and vitreous were replaced by the lesion, which was comprised of bland vacuolated stromal cells and numerous interspersed thin-walled blood vessels (Figure 3 C – F). No mitotic figures were identified within the hemangioblastoma. A few larger arteries and veins were also present around the tumor. Bony retinal pigment epithelial metaplasia was present posteriorly at the periphery of the lesion (Figure 3 C, D asterisks).
Figure 3.
Microscopic examination. A. and B. The highly vascularized intraocular lesion extended through a defect in the superior limbus (arrows) and formed an extraocular mass (asterisk, original magnifications 20X and 40X). The lesion was covered by conjunctival epithelium. C. and D. The tumor largely replaced the retina and choroid, and bony RPE metaplasia was noted in the posterior portion of the globe (asterisks, original magnifications 20X and 40X). E and F. The hemangioblastoma was composed of thin-walled, capillary-like blood vessels (arrows) and numerous lipidized stromal (tumor) cells (original magnification 400X).
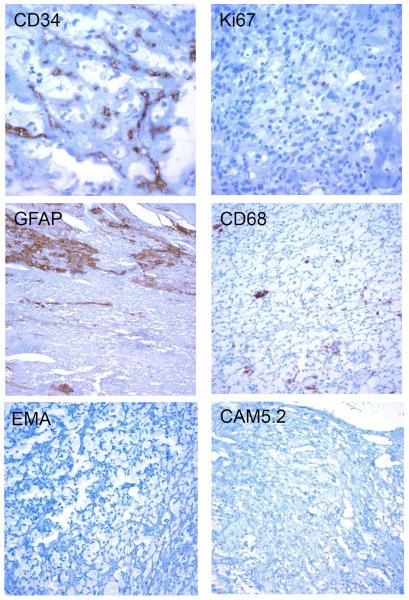
Immunohistochemical analysis confirmed the presence of numerous CD34 positive capillaries surrounding lipidized stromal (tumor) cells with a very low Ki67 proliferation index (Figure 4). There were rare CD68 immunoreactive macrophages and GFAP expressing entrapped glial cells within the tumor (Figure 4). The vacuolated tumor cells were negative for GFAP, Inhibin, CD68, S100, EMA, and CAM5.2, an immunoprofile consistent with a hemangioblastoma rather than a metastatic carcinoma or other malignancy.
Figure 4.
Immunohistochemical analysis. The lesion contained numerous small vessels that stained positive for the endothelial marker CD34. The lipidized stromal (tumor) cells had a low proliferation index, as demonstrated by only rare Ki67 positive cells. There were entrapped glial elements (GFAP) and rare macrophages (CD68). The tumor cells were immunonegative for EMA and CAM5.2. (Original magnifications 200X or 400X).
Discussion
The spectrum of ocular vascular lesions in VHL patients is broad. In addition to the typical retinal hemangioblastomas seen in VHL, Wong et al. have reported cases of retinal neovascularization that may mimic diabetic retinal neovascular disease in 8.3% of their large patient cohort4. In their report, they suggested that these cases “most likely represent one end of the spectrum of hemangioblastoma formation.” Although highly aggressive hemangioblastomas, with extrascleral extension, were not reported, we suggest that the case reported here may represent the other end of the spectrum of these enigmatic vascularized tumors. Review of the literature suggests that our case may be the first reported case in which a retinal hemangioblastoma infiltrated the eye wall. This may be in part due to the limited number of patients with VHL disease, combined with the efficacy of laser photocoagulation or cryotherapy for limiting the growth of most established tumors5,6. Typical of larger hemangioblastomas, our patient's lesions proved difficult to treat and required repeated treatments with both laser photocoagulation and cryotherapy over the course of decades. It is possible that the repeated cryotherapy may have led to scleral thinning that allowed extrascleral extension of this aggressively growing hemangioblastoma.
Retinal hemangioblastomas are indolent vascularized tumors that are believed to be a congenital, benign, neoplastic process1. Accumulated evidence suggests that the vacuolated, foamy, “stromal” cells – also present in cerebellar hemangioblastomas and renal cell carcinoma in patients with VHL disease – are the true tumor cells, with the benign vascular proliferation a response to the secretion of angiogenic growth factors (e.g. VEGF) from these tumor cells.7,8 Reasonable disagreement exists as to the origin of the stromal tumor cell9; while ultrastructural and immunohistochemical studies have suggested that the stromal tumor cells may represent lipidized fibrous astrocytes or glial cells,10,11 immunohistochemical analysis of these lesions support an origin from vascular stem cells or perhaps developmentally arrested hemangioblasts12–14. Although the case reported here does not point to one specific cell of origin, it does demonstrate the aggressive potential of this tumor.
Collectively, our observations demonstrate that even with early aggressive management, VHL-associated retinal hemangioblastomas may continue to grow and scleral infiltration and extraocular extension is possible. This further supports the need for continued investigation into the early and aggressive treatment of these enigmatic vascularized tumors.
Acknowledgments
This work was supported by National Eye Institute, National Institutes of Health Grant, K08-EY021189 (AS), a research grant from the VHL Family Alliance, and an Unrestricted Grant from Research to Prevent Blindness. Dr. Sodhi gratefully acknowledges the support he receives as a Career Development Award recipient from the Research to Prevent Blindness Foundation and as the Stephen J. Ryan, Jr., M.D. Scholar at the Wilmer Eye Institute. We would like to thank Cathy Dibernardo for interpretation of the echography images.
References
- 1.Wong WT, Chew EY. Ocular von Hippel-Lindau disease: clinical update and emerging treatments. Curr Opin Ophthalmol. 2008;19:213–217. doi: 10.1097/ICU.0b013e3282fb7c04. doi:10.1097/ICU.0b013e3282fb7c04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- 3.Wizigmann-Voos S, Plate KH. Pathology, genetics and cell biology of hemangioblastomas. Histol Histopathol. 1996;11:1049–1061. [PubMed] [Google Scholar]
- 4.Wong WT, et al. Retinal vascular proliferation as an ocular manifestation of von Hippel-Lindau disease. Arch Ophthalmol. 2008;126:637–643. doi: 10.1001/archopht.126.5.637. doi:10.1001/archopht.126.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109:1799–1806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 6.Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46:117–142. doi: 10.1016/s0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 7.Morii K, Tanaka R, Washiyama K, Kumanishi T, Kuwano R. Expression of vascular endothelial growth factor in capillary hemangioblastoma. Biochem Biophys Res Commun. 1993;194:749–755. doi: 10.1006/bbrc.1993.1885. doi:10.1006/bbrc.1993.1885. [DOI] [PubMed] [Google Scholar]
- 8.Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358–1364. [PubMed] [Google Scholar]
- 9.Kamitani H, Masuzawa H, Sato J, Kanazawa I. Capillary hemangioblastoma: histogenesis of stromal cells. Acta Neuropathol. 1987;73:370–378. doi: 10.1007/BF00688262. [DOI] [PubMed] [Google Scholar]
- 10.Jurco S, 3rd, et al. Hemangioblastomas: histogenesis of the stromal cell studied by immunocytochemistry. Hum Pathol. 1982;13:13–18. doi: 10.1016/s0046-8177(82)80133-0. [DOI] [PubMed] [Google Scholar]
- 11.Jakobiec FA, Font RL, Johnson FB. Angiomatosis retinae. An ultrastructural study and lipid analysis. Cancer. 1976;38:2042–2056. doi: 10.1002/1097-0142(197611)38:5<2042::aid-cncr2820380529>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Chan CC, Chew EY, Shen D, Hackett J, Zhuang Z. Expression of stem cells markers in ocular hemangioblastoma associated with von Hippel-Lindau (VHL) disease. Mol Vis. 2005;11:697–704. [PMC free article] [PubMed] [Google Scholar]
- 13.Glasker S, et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 2006;66:4167–4172. doi: 10.1158/0008-5472.CAN-05-3505. doi:10.1158/0008-5472.CAN-05-3505. [DOI] [PubMed] [Google Scholar]
- 14.Park DM, et al. von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4:e60. doi: 10.1371/journal.pmed.0040060. doi:10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]