Abstract
Neurocysticercosis has been recognized as a major cause of secondary epilepsy worldwide. So far, most of the knowledge about the disease comes from Latin America and the Indian subcontinent. Unfortunately, in sub-Saharan Africa the condition was neglected for a long time, mainly owing to the lack of appropriate diagnostic tools. This review therefore focuses on the prevalence of neurocysticercosis in sub-Saharan Africa, the clinical picture with emphasis on epilepsy, as well as the diagnosis and treatment of neurocysticercosis and its related epilepsy/epileptic seizures in African resource-poor settings.
Keywords: Cysticercosis, Epilepsy, Sub-Saharan Africa
Neurocysticercosis (NCC) represents the commonest helminthic infection of the central nervous system and one of the most important causes of secondary epilepsy worldwide.1,2 The disease is reported to cause between 20 and 50% of all late-onset epilepsy cases globally1,3–7 and is also assumed to be a common cause of juvenile epilepsy in certain parts of the world, in particular southern Africa.8–12 NCC is not only the major cause of acquired epilepsy/epileptic seizures in many developing countries, but is also of increasing concern in northern/western countries due to globalisation and migration of infected people.13–17
Life Cycle of Taenia solium cysticercus and Its Development in the Brain
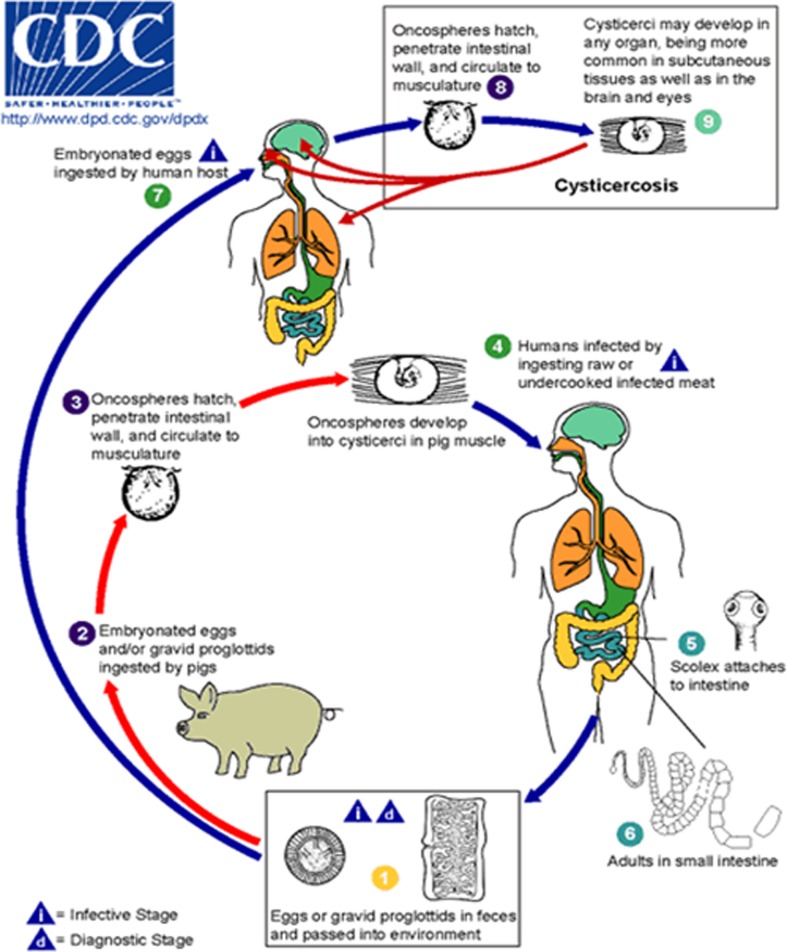
Cysticercosis, a zoonotic disease, is caused by the larval stage (cysticercus) of the porcine tapeworm Taenia solium. The parasite’s life cycle is shown in Fig. 1. In humans, cysticerci are mainly found in the central nervous system (brain and spine), and in subcutaneous tissue, skeletal muscle, and the eye, whereas in pigs cysticerci mainly lodge in skeletal muscle.18 In the brain, immature cysticerci appear within some weeks after ingestion of T. solium eggs (stage 1). Stage 2 (some months after egg ingestion) is characterized by mature cysticerci with virtually no inflammatory response which may persist for many years. Eventually, after some years, asymptomatic stage 2 cysticerci develop into symptomatic stage 3 degenerating cysticerci that no longer prevent the host’s immune response with resulting intense inflammation which may lead to clinical signs and symptoms. In stage 4, the cysticercus calcifies or resolves without scarring.19
Figure 1.
Life cycle of Taenia solium cysticerci. Humans become infected with the adult worm by eating undercooked pork containing cysticerci  and develop taeniosis (tapeworm infection)
and develop taeniosis (tapeworm infection)  ,
,  . Tapeworm eggs or gravid proglottids are excreted from an infected human host into the environment
. Tapeworm eggs or gravid proglottids are excreted from an infected human host into the environment  and can be taken up by freely roaming pigs
and can be taken up by freely roaming pigs  that develop porcine cysticercosis with cysticerci that mainly form in their muscles
that develop porcine cysticercosis with cysticerci that mainly form in their muscles  . The porcine cysticercosis/taeniosis cycle is complete once undercooked infected pork meat is again consumed by a human host
. The porcine cysticercosis/taeniosis cycle is complete once undercooked infected pork meat is again consumed by a human host  . Taenia solium eggs are not only infectious to pigs (paratenic or intermediate hosts) but also to humans
. Taenia solium eggs are not only infectious to pigs (paratenic or intermediate hosts) but also to humans  ,
,  . They can be ingested following direct or indirect (via faecal matter) contact with tapeworm carriers
. They can be ingested following direct or indirect (via faecal matter) contact with tapeworm carriers  ,
,  , which represents the most common route of infection, as well as through the consumption of water or food contaminated with tapeworm eggs
, which represents the most common route of infection, as well as through the consumption of water or food contaminated with tapeworm eggs  . However, the latter is of much less relevance. When humans ingest Taenia solium eggs
. However, the latter is of much less relevance. When humans ingest Taenia solium eggs  through faecal–oral transmission or possible autoinfection, they become accidental hosts of the larval stage of the parasite and develop human cysticercosis
through faecal–oral transmission or possible autoinfection, they become accidental hosts of the larval stage of the parasite and develop human cysticercosis  . Centers for Disease Control and Prevention’s website for parasite identification: http://www.dpd.cdc.gov/dpdx/HTML/Taeniasis.htm.
. Centers for Disease Control and Prevention’s website for parasite identification: http://www.dpd.cdc.gov/dpdx/HTML/Taeniasis.htm.
Prevalence of NCCin Sub-Saharan Africa
Suggested calculation of the prevalence rates of NCC
In sub-Saharan Africa, the presence of porcine cysticercosis is well established,11,20,21 but so far only few studies on human cysticercosis/NCC have been conducted.22 Studies in rural populations of Uganda, Zambia, and Burkina Faso and in an urban population of Tanzania, that are combining serology and neuroimaging data, are underway. A recent meta-analysis on the prevalence of NCC in people with epilepsy, including 12 studies mainly from Latin America, India and sub-Saharan Africa, found that NCC was the cause of epilepsy in almost 30% of people with epilepsy.23
If extrapolating the above result to the entire population of sub-Saharan Africa (approximately 850 million people)24 and assuming a prevalence of epilepsy of 4–13/1000,25,26 3.40–11.05 million people would suffer from epilepsy. In 2010, 631 776 908 people lived in the T. solium taeniosis/cysticercosis endemic areas of sub-Saharan Africa (endemic countries: WHO 2010;27 populations in these endemic countries: World Atlas 201028), yielding an epilepsy population of 2.53–8.21 million. Thirty per cent of epilepsy in endemic regions is due to NCC,23 amounting to 0.76–2.46 million people with epilepsy due to NCC in sub-Saharan Africa. Epilepsy makes up for 80% of symptomatic NCC29 and therefore 0.95–3.08 million people would suffer from symptomatic NCC, including all cases with any (not just epilepsy) neurological symptom/sign due to NCC. We also know that symptomatic NCC is only the tip of the iceberg and that the majority of people with NCC are asymptomatic. Data regarding asymptomatic NCC cases vary, but autopsy studies and community-based neuroimaging studies indicate that between approximately 50 and 80% of all people affected with NCC may be asymptomatic.30,31 Using the conservative estimate of 50% another 0.95–3.08 million people would have latent NCC. Therefore, the total of all people suffering from NCC (symptomatic and asymptomatic) in sub-Saharan endemic countries would be somewhere between 1.90 and 6.16 million. These figures, however, represent only very crude estimates, but this is the closest one can get to reality. Prevalence of porcine cysticercosis varies from country to country, region to region, village to village and even household to household. Theoretically, one could take the above numbers and subtract all areas with predominantly Muslim and/or urban populations assuming that NCC may not occur in these populations. However, in urban populations pigs reared in rural communities are sold and eaten and Muslim people mix with pork eating neighbours. Contamination of the environment with T. solium eggs therefore is also possible in non-pig rearing communities. Teasing out all these variables is virtually impossible but calls for more country-based prevalence data on NCC in order to get a clearer picture of the focal distribution of NCC in sub-Saharan Africa.
Asymptomatic NCC and mass drug administration
Although latent NCC does not contribute to disease burden, people with living cysticerci can become symptomatic at any time based on the natural course of the disease (see above). In addition, there is also a potential risk that treatment with drugs targeting soil-transmitted helminths, lymphatic filariasis, and schistosomiasis may precipitate the conversion of latent NCC to symptomatic disease through mass drug administration. At therapeutic doses (see below) praziquantel and albendazole are both known to be able to convert latent/asymptomatic cysticerci to symptomatic cysticerci by destroying the parasite and potentially provoking brain oedema. This is the reason for which co-administration with steroids is recommended (see below). However, reports of sudden onset of serious neurological signs and/or death after mass drug administration during which antihelminthic drugs are used at lower doses [praziquantel 40 mg/kg (single dose; frequency depending on endemicity level: maximum once a year) for schistosomiasis and 5 mg/kg/year (single dose) for taeniosis; albendazole as a 400 mg single dose for both soil-transmitted helminths and lymphatic filariasis (frequency depending on endemicity level: maximum every 6 months)] have also been noted.32–35 Neurological side effects associated with imaging-confirmed exacerbation of NCC have been noted in a 12-year-old girl after a one–off intake of praziquantel at a dose as low as 5 mg/kg during mass treatment against human taeniosis for the control of cysticercosis34,36 and in a young woman after self-medication for taeniosis with a single dose of 400 mg albendazole.35
In 2010, 82 800 490 people received albendazole for lymphatic filariasis, 92 300 833 for soil-transmitted helminths and 27 811 183 people were given praziquantel for schistosomiasis.37 Approximately 20 million people received praziquantel in areas co-endemic for taeniosis/cysticercosis (countries endemic for taeniosis/cysticercosis;27 praziquantel distribution in these endemic countries: WHO 2011: ‘Informal Consultation Meeting on Schistosomiasis and Soil-Transmitted Helminthes’). Assuming that 1–2% of the population in a taeniosis/cysticercosis endemic area harbour asymptomatic cysticerci in their brains,30,38 200 000–400 000 people alone would be at potential risks of developing neurological side effects after praziquantel distribution. This does not take into account the distribution of albendazole which is not only given for lymphatic filariasis to communities in many countries of eastern and western Africa, but also to school children for soil-transmitted helminths in most African countries and in addition most times is co-administered with praziquantel. According to WHO, in 2010 albendazole was given to 57.5 million people in areas co-endemic for lymphatic filariasis and taeniois/cysticercosis (countries endemic for taeniosis/cysticercosis;27 albendazole distribution in these endemic countries39). In view of the large-scale distribution of these drugs reports of precipitation of neurological symptoms/signs are of concern. In national schistosomiasis programmes no neurological signs to date have been routinely reported (personal communication Professor Alan Fenwick and Dr Wendy Harrison, Schistosomiasis Control Initiative, Imperial College, London), although no large-scale study on the side effect of mass drug administration in carriers of latent cysticerci have as yet been performed. Such studies are desperately needed to evaluate the risk of symptomatic NCC in asymptomatic carriers of cerebral cysticerci in sub-Saharan Africa.
Symptomatic NCC in people with epilepsy
Data on sub-Saharan prevalence rates of NCC in people with epilepsy/epileptic seizures come from few countries only with results of over 40% (Cameroon) depending on the serological tests used.11,20,40 Details on countries excluding South Africa are summarized in Winkler et al.22 A recent meta-analysis that only included African studies showed a significant association between epilepsy and cysticercosis with an odds ratio of 3.4.41 More details on the prevalence of NCC (serology and imaging) are available from South African studies.11 The highest imaging-based prevalence rates were found in two South African studies, showing 37% of people with epilepsy attending an outpatient clinic and 51% of newly diagnosed people with epilepsy referred to hospital to harbour NCC lesions on neuroimaging.3,7 The only neuroimaging study in sub-Saharan Africa outside South Africa was performed from our own group. Definite NCC lesions on cerebral computed tomography (cCT) were demonstrated in 2.4% (5/212), lesions highly suggestive of NCC were present in 11.3% (24/212), and lesions compatible with NCC were found in 4.2% (9/212) of people with epilepsy attending an epilepsy clinic in northern Tanzania. NCC lesions were significantly more frequent in people with epilepsy compared to those without epilepsy who underwent cCT for other reasons.42
Clinical Characteristics of NCC
Overview on pathological and clinical characteristics of NCC
NCC can cause a variety of symptoms and signs depending on the number, size, stage, and location of the pathological changes, as well as the inflammatory host response, or it can also be clinically asymptomatic.4,29,43,44 There may be single or multiple cysticerci in the brain (intraparenchymal NCC, approximately 80%) and, in extreme cases, encephalitis may ensue. Cysticerci can also occur in the ventricular system and/or the subarachnoid space (extraparenchymal NCC, approximately 20%). Ventricular disease may cause ependymitis and/or increased intracranial pressure. Arachnoiditis, especially in the basal cisterns, which can lead to communicating hydrocephalus, vasculitis and/or compression of cerebral vessels, can result from subarachnoid disease. Intramedullary cysticerci can be found in the spinal cord, causing focal neurological symptoms/signs, and extramedullary cysticerci can cause radicular symptoms and/or signs in the forefront.44,45 A recent publication from Peru reports the association of subarachnoid NCC and spinal cord disease with involvement of the spinal subarachnoid space in 60% of patients. The authors conclude that more rigorous performance of magnetic resonance imaging of the spine in people with subarachnoid NCC is needed.46
Classification of epilepsy/epileptic seizures in resource-poor settings with reference to NCC
If cerebral cysticerci or calcifications are intraparenchymal, epileptic seizures and/or epilepsy may ensue. It is mainly during the stage of cysticerci degeneration that new-onset acute symptomatic epileptic seizures occur that usually resolve after the inflammation has died down. In the case of remaining calcifications, recurrent ‘unprovoked’ epileptic seizures not related to an acute intracerebral disease ( = epilepsy) can develop, although fortunately most patients remain asymptomatic.47 A systematic review on the clinical manifestations in people with NCC showed that the majority of symptomatic cases (78.8%) had epileptic seizures. This was followed by headaches in 37.9% of people.29,43
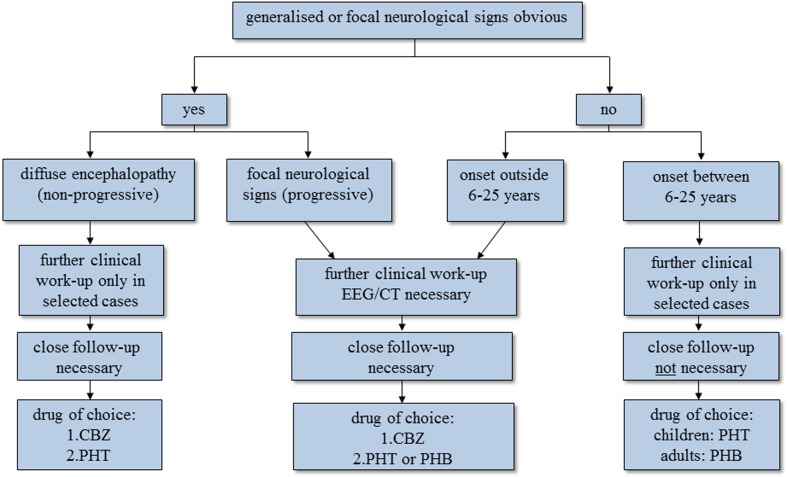
Classifying epileptic seizures in resource-poor countries is a challenge. Whether seizures reported in rural Africa are primarily generalised or focal is still controversial. Some research groups in different African countries found that primary generalised seizures were more prevalent, although others report more focal seizures.1 While members of the International League Against Epilepsy (ILAE) talk about the best possible way of classifying epileptic seizures mainly referring to the western world, appropriate classification systems for epilepsy in developing countries are missing. To date the ILAE classification of epileptic seizures48 and epilepsy syndromes49 has been used too loosely in the context of studies conducted in sub-Saharan Africa. Not only seizure semiology, the description of which due to sociocultural barriers may be incomplete, but important seizure-related issues such as aetiology and concomitant diseases may be relevant for the classification of epilepsy/epileptic seizures in these countries.50 We therefore suggest an adapted more appropriate version of classification of epilepsy/epileptic seizures for sub-Saharan Africa with special emphasis on ensuing therapeutic and prognostic aspects (Fig. 2).51,52
Figure 2.
Algorithm on how to classify people with epilepsy/epileptic seizures in resource-poor countries with diagnostic and therapeutic implications. The first question one has to ask is whether there is any evidence of focal neurological signs or even more widespread encephalopathy. If there is, the approach in the two groups is different. In most people, mainly children, with epilepsy with diffuse encephalopathy, diagnostic tests are rarely needed as the extent of the cerebral sequelae is known and the condition is not progressive. In contrast, in people with epilepsy who show focal signs without diffuse encephalopathy further diagnostic steps are essential in order to identify the underlying, potentially treatable cause. If neurological examination is unremarkable, the so-called primary generalised seizures are split according to the age of onset (see text); the two groups have a different diagnostic and therapeutic approach. Patients with primary generalised seizures that start outside 6–25 years are more likely to have cerebral lesions compared to those with seizures that start within the range of 6–25 years, where genetically determined epilepsy prevails. The former therefore need further investigation. In all four groups, treatment depends mainly on the age of the patient, seizure activity, seizure frequency, the presence of mental handicap and the presence of focal neurological signs, bearing in mind that the choice of antiepileptic medication is limited. People with epilepsy/epileptic seizures due to NCC can potentially be found in all four groups, but are mainly seen in patients with generalised late-onset seizures or in patients with generalised seizures and focal neurological signs without major encephalopathy. For further explanations refer to the main text. CBZ = carbamazepine, PHT = phenytoin, PHB = phenobarbitone.
The classification we propose is based on description of epileptic seizures by the patients and at least one witness, history of seizures with special emphasis on age of onset, associated illness, family history and birth history, as well as a complete neurological examination. The diagnosis is based on clinical symptomatology alone as in most African hospitals electroencephalogram and neuroimaging are not available. The term ‘primary generalised seizure’ as opposed to ‘secondary generalised seizure’ is used to indicate that there is no focal start of the seizure in a patient who otherwise is healthy.
-
Generalised types of epileptic seizures/epilepsy:
Generalised epileptic seizures within a specific age range: primary generalised seizures that start within a specific age group (mainly between 6 and 25 years, which is the age group in which most of the genetically determined epilepsies start). There is no obvious cause for the seizures, encephalopathy ( = diffuse brain damage) is absent. There may however be a positive family history, suggesting a possible genetic background. Seizures of this group can also be termed idiopathic generalised epilepsies.
Generalised epileptic seizures outside a specific age range: primary generalised seizures that start outside the specific age range of most of the idiopathic generalised epilepsies, but have no focal start and no clinical signs of encephalopathy. There may be a cause which cannot be diagnosed with the currently available ancillary means, thus these seizures may be termed ‘cryptogenic’.
-
Partial (secondary generalised) types of epileptic seizures/epilepsy:
Generalised epileptic seizures with encephalopathy: secondary generalised seizures that start in a generalised way, however, serious encephalopathy is obvious, which is the major difference when compared to the group below. Causes are static and may be due to perinatal adverse events or post-traumatic sequelae, among others. All age groups can be affected, but there tends to be a shift to the younger ages.
Generalised epileptic seizures with focal signs: secondary generalised seizures with a focal start or clear unilateral seizures but without major encephalopathy. There may be developmental delay, subtle signs of a cerebral disorder and/or focal neurological signs on examination. Underlying causes are often progressive. All age groups can be affected.
Complex partial seizures: as defined by the ILAE.48
Simple partial seizures: as defined by the ILAE.48
In summary, we suggest a classification of epilepsy/epileptic seizures based on that of the ILAE but adapted to local circumstances of resource-poor countries. The classification has two major categories: (1) generalised epileptic seizures divided by age of onset without obvious underlying brain disorders and (2) partial epileptic seizures with underlying brain disorders divided by whether the underlying causes are static or progressive. People with epileptic seizures/epilepsy due to NCC may be found in all four groups, but are mainly seen in the group with generalized epilepsies without focal neurological signs that start outside the age range of the genetically determined epilepsies (implying that there may be structural brain lesions (group 1b) and in the group with generalized epilepsies with clear focal signs (group 2b).53 For more details see Fig. 2.
Variance of NCC phenotypes and cysticercosis genotypes across regions
Although the above described pathological and clinical presentations of NCC are common, they are far from uniform. A different phenotype of NCC, termed single enhancing lesion, which presents as an enhancing round shaped lesion (in most cases there is ring enhancement)54 usually below 2 cm in diameter and without gross perifocal oedema or subsequent growth is mainly found in India and represents an important differential diagnosis for tuberculoma in this region of the world.54–57 These people often show new-onset seizures of the partial type and most cases present with single seizures or acute symptomatic seizures; chronic epilepsy seems to be rare.58 Interestingly, this form of NCC also seems to be common in travellers returning from taeniosis/cysticercosis endemic countries, but only represents the minority of cases in people with NCC from other Asian countries such as China, countries of Latin America and the African continent.59–61
In patients with incident (first seizure within the previous year) or prevalent epilepsy/epileptic seizures from countries other than India one usually sees multiple intracerebral lesions consisting of cysticerci in various stages including calcifications (Latin America,6,30,38,62 sub-Saharan Africa,3,7,42,63,64 Asia65–67). Often calcifications are the only pathology and most of the patients seem to be asymptomatic with it.6,38 The onset of seizure (whether incident or prevalent) certainly plays a role when it comes to the prevailing lesion and it can be assumed that cysticerci stage 2 and 3 are more likely to be seen in patients with recent-onset epileptic seizures, whereas calcifications may be the only pathology in chronic epilepsy simply because of the time factor.7,47
These different presentations of intracerebral NCC lesions and associated epileptic seizures not only seem to vary between countries but also between individuals. The presentation of single enhancing lesions may be a result of mild infection (single enhancing lesions are clustering in travellers and young people from India with relatively little exposure to the parasite) associated with the potential of the host to overcome the infection. A genetic predisposition may play a role in this process.60 Also, it is not well understood why most people with NCC lesions are asymptomatic,38 but evidence emerges that the individual reaction of the immune system may play a role and that there is a genetic predisposition of who will acquire symptomatic disease.68
The presentation of cysticercosis in sub-Saharan Africa clinically seems to be similar to that of Latin America, not only with regards to the appearance of the intracerebral lesions, but also with regards to its extraneural features. Subcutaneous cysticerci in patients with NCC are frequent in Asia, but rarely found in Latin America and unequally distributed in Africa.65,69 Subcutaneous cysticerci were reported in people who suffered from onchocerciasis but otherwise were healthy in the Northwest of Uganda70 and in people suffering from epilepsy in Togo,71 whereas thorough examination of almost 1400 people with epilepsy from highly endemic T. solium taeniosis/cysticercosis areas of northern Uganda revealed absence of subcutaneous nodules (unpublished data). In a population of people with epilepsy and confirmed NCC from northern Tanzania, a few people showed calcified lesions in muscular tissues of unknown origin (incidental findings on X-ray), but none had palpable subcutaneous nodules (unpublished data). These differences in extraneural presentation of cysticercosis correlate well with the two main genotypes of T. solium that were found to exist worldwide: a pure Asian and a Latin American/African mixed genotype.69,72,73 This genetic variation not only seems to contribute to the overall different clinical phenotypes of cysticercosis of the various continents, but may also impact on serological diagnoses of T. solium cysticercosis. Antigenic variations of T. solium cysticerci belonging to different genotypes can be postulated and was corroborated by findings of differences in immunoblot banding patterns when using cyst fluid from Asia compared to that from Latin America/Africa.69 Variation in genotypes may therefore impact on serodiagnosis and has to be considered when testing serum from people living in T. solium taeniosis/cysticercosis endemic areas with T. solium cysticerci antigen from a different region as is the case with commercially available immunoblots. Whether this is at all relevant for routine diagnosis needs to be established and currently is evaluated in studies conducted in Zambia, Tanzania, and Uganda.
Diagnosis of NCC in Sub-Saharan Africa
Diagnosis of NCC has been well established and is mainly based on neuroimaging and immunodiagnosis.45,74 Diagnostic testing should be initiated if NCC is suspected on clinical grounds, e.g. epileptic seizures in people coming from areas endemic for cysticercosis. In sub-Saharan Africa, due to the lack of diagnostic facilities, this is only possible in a minority of cases.
The analysis of cerebrospinal fluid of patients with NCC may indicate parasitic disease or show mononuclear pleocytosis and eosinophilia, depending on disease activity and the location of lesions. Cell counts rarely exceed 100 cells/μl. Protein levels can be increased to within the range of 50–300 mg/dl, but glucose levels are usually normal.75 In addition to or instead of a standard analysis of cerebrospinal fluid, the diagnostic value of which is debatable and unremarkable in most cases, serological tests for T. solium cysticercosis should be performed in suspected cases. Antigen/antibody enzyme-linked immunosorbent assay (ELISA) and immunoblots in serum and/or cerebrospinal fluid are available, whereby a positive antigen ELISA indicates active disease with viable cysticerci and a positive antibody ELISA and/or immunoblot demonstrates exposure to the parasite, but not necessarily active disease.18,22,74,76–79 Also, a positive test only indicates cysticercosis but not necessarily NCC, and the sensitivity and specificity can vary considerably according to the test used.74,76 In addition, most of these tests are unavailable in sub-Saharan Africa, although the capacity has been transferred to some African centres.22 Furthermore, a DNA-based method of diagnosis of T. solium cysticercosis is under development. Preliminary results in cerebrospinal fluid indicate variable levels of sensitivity but 100% specificity.80,81 Again, these techniques, with high-cost equipment, might not be available in African countries.
Although positive serological and/or DNA-based tests may give the first indication, the gold standard of NCC diagnosis is neuroimaging, including cCT and/or cerebral magnetic resonance imaging, both of which are generally not available in sub-Saharan Africa. Serology may help in areas without CT scanners or may indicate who should go for cCT examination and thus save resources. A recent study from Tanzania indicated a sensitivity of 100% and a specificity of 84% for diagnosis of active NCC using T. solium cysticercosis antigen ELISA, whereas a study from South Africa demonstrated much lower sensitivity and specificity using the same T. solium cysticercosis antigen ELISA in a different study population.7,82 Neuroimaging not only is essential for confirmation of diagnosis, but also represents the only method that differentiates between active and inactive disease. Active NCC is defined as the presence of at least one NCC-like cystic lesion (a round shaped hypodensity on cCT with or without a hyperdense dot representing the head of the cysticercus ( = scolex) usually no bigger than 1 cm in diameter and without perifocal inflammatory reaction ( = oedema)) or at least one ring- or fully enhancing lesion ( = granuloma). Only a lesion showing the scolex is classified as a definite NCC lesion and considered pathognomonic.18,76–78 In contrast, parenchymal calcifications are classified as inactive.47,83 Cystic brain lesions, granulomas and calcifications not only appear in the context of NCC, but include many parasitological and non-parasitological diagnoses, and therefore always have to be combined with immunodiagnostic tests. Results gained from neuroimaging and T. solium cysticercosis serology, but also from clinical and pathological examination, X-ray investigation and treatment with antihelminthic drugs together with epidemiological criteria are graded as absolute, major and minor criteria according to their significance and summarized in the diagnostic criteria for NCC (definitive, probable and possible) suggested by Del Brutto et al.77,78,84
Treatment of NCC in Sub-Saharan Africa
Summary of current state of the art treatment
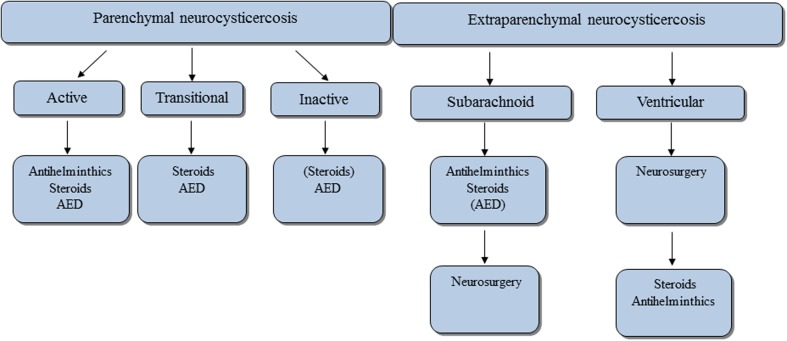
Nash and Garcia45 and Carpio85 in their reviews have summarized the latest treatment aspects which are also outlined in Fig. 3. Briefly, treatment approach depends on whether the disease is intraparenchymal or extraparenchymal. Neuroimaging is mandatory to visualize the location of the NCC lesion(s). Therefore, the algorithm of Fig. 3 cannot be transferred one to one to resource-poor settings. In that algorithm, only active intraparenchymal and symptomatic (e.g. epileptic seizures) disease requires treatment with triple therapy, i.e. antihelminthic drugs (praziquantel 50 mg/kg per day for 15–30 days or albendazole 15 mg/kg per day for 8–15 days), steroids and antiepileptic medication. Steroids should be initiated together with antihelminthic drugs to prevent perifocal oedema in intraparenchymal disease and ideally should be administered for as long as the patient is symptomatic (e.g. severe progressive headache, acute symptomatic epileptic seizures), but at least for the first days antihelminthic treatment is given. So far, steroid doses in the treatment of NCC have not been standardized.86 Once the parasite has gone into the transitional stage ( = stage 3 degenerating cysticercus; see above), antihelminthics may no longer be needed as the parasite is already attacked and destroyed by the host. Current advice is that the symptomatic patient with epileptic seizures should be maintained on symptomatic treatment only, i.e. steroids and antiepileptic medication. There is, however, some controversy over the topic of treatment of the transitional stage and new results are expected shortly (personal communication with Professor Hugo Garcia, Cysticercosis Working Group in Peru). In inactive symptomatic disease the patient should receive antiepileptic mediation only if seizures are present. If perilesional oedema is obvious, steroids may be beneficial, although there are only anecdotal reports.86 Treatment of extraparenchymal disease is much more tedious and in most cases requires lengthy treatment regimens with antihelminthic medication and steroids.45 For summary refer to Fig. 3.
Figure 3.
Overview on treatment of NCC according to current guidelines.45,85 Treatment approach in people with NCC is divided into those with intraparenchymal and those with extraparenchymal disease. According to disease stage and localisation of lesions different approaches are advocated. Antihelminthic drugs are only administered in active disease. If there are signs of cerebral oedema steroids are used with and without antihelminthic medication. Steroids are also used prophylactically together with antihelminthic medication in active disease without cerebral oedema, as oedema may develop over the course of treatment. In multicystic disease with obvious oedema ( = encephalitis) antihelminthic medication must not be used and the treatment of choice are steroids alone or in combination with antiepileptic medication. The latter is used in all stages whenever epileptic seizures are present. In subarachnoid disease antihelminthic medication together with steroids may have to be given in higher doses and over longer time than in patients with intraparenchymal disease. The same is true for ventricular disease, although the first line treatment is surgery with removal of the ventricular cysts.45 AED = antiepileptic medication.
Difficulties of treating NCC in sub-Saharan Africa
Treatment in the presence of neuroimaging
In sub-Saharan Africa, the treatment algorithm of Fig. 3 should be adhered to, if possible. In urban centres, CT scanners may be available and in these areas NCC-suspected cases should undergo cCT. However, in the majority of cases, especially in rural areas, CT scanners are not available or the financial means to get a CT scan are not at hand. In both scenarios, with and without CT scan, the attending doctor faces various ‘black boxes’. If a cCT scan shows active NCC then treatment according to guidelines (antihelminthic medication, i.e. praziquantel that is mainly available in sub-Saharan Africa, and steroids; Fig. 3) should be started (in encephalitis antihelminthic medication must not be administered and patients should be given steroids only).
Also, in resource-poor settings, patients may not be particularly compliant with treatment due to various reasons, or they may take one tablet and not the other, which may seriously jeopardize their health. There is no easy way out. Obviously, the patient has to be informed about the condition and the potential side effects of the medication. The best solution may be to keep the patient in hospital for the time of treatment, but due to financial expenses the patient may not agree. To minimise costs an elegant solution may be a high-dose short-term treatment with 100 mg/kg praziquantel in three divided doses every 2 h followed by corticosteroids. This treatment regimen has been demonstrated to be successful in single-cysticercus disease, but unfortunately efficacy could not be demonstrated in patients with multiple cysticerci which represent the major phenotype in sub-Saharan Africa.87,88
Follow-up of patients and monitoring of treatment success represents another challenge. The CT scanner may not be functional anymore or the financial constraints may be too high. An alternative could be the performance of T. solium cysticercosis antigen-ELISA which has been shown to indicate active disease82 and the course of antigen levels has been suggested as a successful tool to monitor treatment response.89 In the eastern and southern African region, the T. solium cysticercosis antigen-ELISA has been used for detecting the infection in both pigs and humans. These tests have been conducted in Zambia at the Cysticercosis Working Group of Eastern and Southern Africa Regional Reference Centre for Immunodiagnosis of T. solium Infections at the University of Zambia. Member countries of the Cysticercosis Working Group of Eastern and Southern Africa usually send samples for antigen-ELISA testing to this reference laboratory. Recently, antigen-ELISA facilities have also been established in Mozambique, Madagascar, South Africa, Burkina Faso, and Cameroon.
Treatment in the absence of neuroimaging
The majority of patients in resource-poor settings have neither access to neuroimaging nor to T. solium cysticercosis antigen ELISA. Therefore, patients should be treated symptomatically only. If NCC becomes symptomatic, this is mainly with epileptic seizures, either as acute symptomatic seizures caused by perifocal oedema or as chronic epilepsy caused by calcifications. Apart from epilepsy the patient may suffer from severe progressive headache alone or in combination with epileptic seizures. Other neurological/neuropsychiatric symptoms/signs are less frequent.29 Neurological examination in most cases is unremarkable and there is often no clue as to the origin of the epileptic seizures other than the patient coming from a T. solium cysticercosis endemic area which does not really help differentiate NCC-related seizures from other types of seizures.
In case of acute symptomatic seizures other potential causes such as cerebral malaria, bacterial and Tb-meningitis, as well as encephalitis, etc. have to be excluded and often lumbar puncture is necessary. In the majority of cases, especially in patients with intraparenchymal NCC lesions cerebrospinal fluid may be normal. However, analysis of cerebrospinal fluid is still important to exclude potential differential diagnoses which require fast and specific treatment such as bacterial or Tb-meningitis. Once the most important differential diagnoses have been excluded and household contact with free-roaming pigs and/or consumption of pork has been established and the patient deteriorates with signs of increased intracranial pressure, hospitalisation is advisable and a trial of steroids should be given together with antiepileptic medication on the assumption that the epileptic seizures are caused by acutely symptomatic NCC.
One may argue that by treating symptomatic patients with steroids only (without antihelminthic medication) the long-term outcome of the patients both in terms of cysticercus clearance and severity of epileptic seizures may be jeopardized. However, whether antihelminthic drugs increase clearance of the parasite and ultimately control epileptic seizures remains controversial. A well-designed study showed that treatment with albendazole in addition to antiepileptic medication significantly cleared the parasite and reduced seizure frequency, especially those with generalisation, when compared to the control group that received antiepileptic medication only.90 However, another study reported that albendazole and antiepileptic treatment did not show greater benefit than therapy with antiepileptic drugs alone. The combination treatment led to increased hospital admission, increased seizure frequency, more cases of encephalopathy and deaths. A greater proportion of lesions calcified compared to the group that was treated with antiepileptic drugs alone, in which more lesions resolved completely.91 As sub-Saharan Africa is lacking imaging facilities and ‘blind treatment’ with antihelminthic medication may cause severe side effects in terms of cerebral oedema, most African physicians do not have a choice but to treat symptomatically, i.e. with steroids and/or antiepileptic medication alone.
Treatment with antiepileptic medication and withdrawal in the context of NCC
Antiepileptic treatment should be initiated if seizure activity is recurrent, irrespective of whether epileptic seizures occur within the setting of symptomatic cysticerci or calcifications. According to the latest guidelines of the ILAE92 treatment should already be initiated after the first epileptic seizure if an underlying lesion is present, or in the circumstances of a resource-poor setting, is suspected. Unfortunately, the choice of antiepileptic treatment is limited in sub-Saharan Africa with mainly phenobarbitone, phenytoin and carbamazepine available. Valproate is found rarely and often antiepileptic drugs are out of stock. Dosing and potential side effects are given in Table 1. Carbamazepine would be the drug of choice in generalised epilepsies with an obvious or assumed focal start as is the case with epileptic seizures related to NCC. Phenobarbitone and phenytoin can also be used for treatment of generalised epilepsy with a focal start, but have substantial side effects, especially the former, which are better avoided.
Table 1. Antiepileptic medication available in sub-Saharan Africa: drug loading, titration, and maintenance doses as well as side effects (in the order of their availability).
| Antiepileptic medication | Starting dose | Titration (usual adult maintenance dose) | Side effects (list non-exhaustive) | Route of administration |
| Phenobarbitone | 1. 30 mg p.o. | 30–60 mg p.o. every 3 days (long half-life) (∼60–180 mg/day) | Fatigue, pronounced cognitive decline, headaches, ataxia, nystagmus, depression, agitation, aggression, hyperkinesia (children), megaloblastic anaemia,teratogenicity, vitamin K deficiency in new-borns; rapid titration in status: respiratory depression | Oral, i.v., s.c., i.m. |
| 2. (rapid) 50 mg i.v., s.c. or i.m. every 6 hours diluted 1∶10 with inj. water | ||||
| 3. (status) 20 mg/kg i.v. diluted 1∶10 with inj. water (start with 200–400 mg) (maximum rate: 100 mg/minute) | ||||
| Carbamazepine | 200 mg p.o. | 200 mg p.o. every 3 days (∼800–2000 mg/day) | Vertigo, double vision, nystagmus, ataxia, movement disorders, hyponatraemia, leucopenia, rash, nausea, fatigue, liver failure, teratogenicity | Oral |
| Phenytoin | 1. 300 mg p.o. | 25–50 mg p.o. per day (∼200–500 mg/day) | Vertigo, double vision, nystagmus, tremor, movement disorders, rash, megaloblastic anaemia, leuco-, thrombopenia, fatigue, acne, osteopathy, gingival hyperplasia, liver failure, irreversible cerebellar atrophy, teratogenicity; rapid titration in status: cardiac dysrhythmias, hypotonia | Oral, i.v. (beware of phlebitis) |
| 2. (rapid) 600 mg p.o. for 3 days | ||||
| 3. (status) 1.5 g i.v. diluted 1∶10 with inj. water (first 250 mg as bolus, next 500 mg in 0.5 to 6 hours, next 750 mg in 1–24 hours according to clinic) (maximum rate: 20 mg/minute) |
Therefore, results on T. solium cysticercosis serology and/or neuroimaging may guide the choice towards the right antiepileptic drugs for people with epilepsy in areas endemic for T. solium taeniosis/cysticercosis and should be sought for, if possible. As the majority of patients in sub-Saharan Africa do not have access to these diagnostics, antiepileptic treatment has to be started empirically and the response has to be monitored. If seizures still recur on sufficient doses, switching to another antiepileptic drug may be necessary. If possible the patient should be maintained on monotherapy. A recent study has shown that most patients with NCC-related epilepsy are well controlled on monotherapy compared to those with epilepsy due to other reasons.53 It is self-evident that patients have to be informed about side effects of antiepileptic medication, especially the potentially ensuing tiredness, in order to increase compliance. Short follow-up visits at the epilepsy clinic are necessary.
Prolonged usage of antiepileptic medication may have short- and long-term side effects. Hence, when a patient with epilepsy is in remission (free of seizures for some time) discontinuation of medication can be justified. Controlled withdrawal of antiepileptic treatment in sub-Saharan Africa is important as the majority of patients may stop medication at once when the seizures have died down, which may lead to severe withdrawal seizures. There are no guidelines as to withdrawal of antiepileptic mediation in the context of NCC and therefore this has to be decided on a case-by-case basis. In neurological practice, discontinuation of antiepileptic treatment usually is considered in people with epilepsy who have been seizure-free for two years or longer. In the context of sub-Saharan Africa one year may be justified.25 The decision to withdraw antiepileptic mediation must weigh the risk of seizure recurrence against the benefits in terms of medical, emotional and social implication of treatment. The factors associated with a higher-than-average risk of seizure relapse include partial seizures, the presence of an underlying neurological condition or brain lesions and abnormalities on electroencephalogram at the time of withdrawal, among others.93 Predictors for remaining seizure-free after withdrawal of antiepileptic medication over one year were normal neurological examination and use of carbamazepine prior to withdrawal.94 In the context of NCC, this means that patients with normal neurological examination, those with clearance of NCC lesions and those that were treated with carbamazepine stand a good chance of remaining seizure-free after withdrawal of antiepileptic medication. However, well planned longitudinal studies are necessary to confirm the above assumptions.
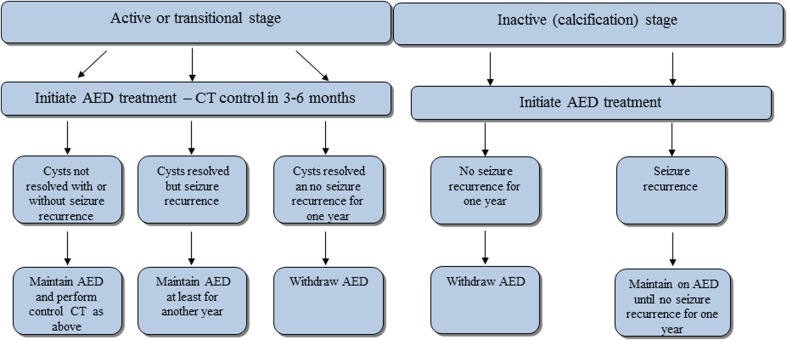
For withdrawal of antiepileptic medication in patients with NCC that have access to CT scanners various possible scenarios are laid out in Fig. 4 that may guide the clinician as to when withdrawal of antiepileptic medication in patients with epilepsy due to NCC seems justified. In the absence of CT the clinician may attempt to get serial T. solium cysticercosis antigen levels89 or electroencephalogram recordings and if this is not available, he may start slow tapering of antiepileptic medication under close supervision after the patient was seizure-free for at least one year.
Figure 4.
Withdrawal algorithm of antiepileptic medication in people with epilepsy due to NCC. If CT is at hand, we suggest following the indicated algorithm which refers to people with NCC of various disease stages who previously have been put on antiepileptic medication. In a nutshell, withdrawal is guided by the presence or absence of intracerebral lesions and by seizure recurrence. We have defined seizure recurrence as at least one seizure during the last year as this seems to be the accepted time frame for initiation of antiepileptic treatment in resource-poor settings.25 For more details refer to the main text. AED = antiepileptic medication, CT = computed tomography. Adapted from Carpio 2012: http://emedicine.medscape.com/article/1168784-overview#a0199.
Factors influencing access to antiepileptic treatment in sub-Saharan Africa
Biomedical treatment of people with epilepsy in sub-Saharan Africa may be affected by important sociocultural factors such as traditional beliefs and/or stigma95 and knowledge of these factors is crucial to make treatment successful. In this context the term ‘epilepsy treatment gap’ has been coined which represents the number of people with active epilepsy who have not accessed biomedical services or who are not on treatment or are on inadequate treatment.96 This treatment gap has two important components and includes access to health care and adherence to treatment.97
The ‘access gap’ is caused by factors such as inconsistent access to health facilities with antiepileptic medication, lack of knowledge of affected people and their families, medical personnel and entire communities on the topic of epilepsy, traditional concepts as to the origin of epilepsy and resulting stigma towards people with epilepsy, among other factors.96–99 This stigma was clearly demonstrated in a recent study from Zambia which showed that people with epilepsy are disadvantaged regarding social and economic matters compared to people with other chronic diseases without an attached stigma, such as asthma, diabetes mellitus, hypertension, and rheumatic heart disease.100
The ‘adherence gap’ which describes the failure of compliance with antiepileptic medication often is due to lack of information and education of the patients and their families as well as lack of time and lack of knowledge on treatment of health personnel. Adherence to antiepileptic medication not only is influenced by health service related factors but again by stigma towards people with epilepsy and by people’s belief systems which may view epilepsy as caused by demoniac possession or punishment for sins, among others.96–99 Those and other factors that may be responsible for the ‘epilepsy treatment gap’ have to be taken into consideration when dealing with people with epilepsy/epileptic seizures in sub-Saharan Africa, irrespective of the origin of the epileptic seizures.
Prevention of NCC
In sub-Saharan Africa most likely more than in other parts of the world preventative and educational aspects of T. solium cysticercosis play an important role. T. solium taeniosis/cysticercosis is a disease of the poor and is rampant in communities with a low standard of sanitation and hygiene. Further risk factors include free-range pig farming, close contact of humans and pigs and inadequate meat inspection.101,102 As free access of pigs to human faeces plays a crucial role in the maintenance of the life cycle of T. solium cysticerci, education on proper community-based sanitation, building and usage of latrines that are inaccessible to pigs and education on community-friendly pig rearing (restraining pigs, vaccination programmes) are indispensable. Furthermore, meat inspection procedures as well as controlled slaughter have to be in place and farmers have to be educated about how to recognize infected pork. Hygienic measures such as hand washing after toilet use and before preparing food, among others, has to be advocated as it can prevent human cysticercosis which develops through ingestion of eggs from a tapeworm carrier through the faecal–oral route (contact with a tapeworm carrier, contaminated water or food).22
In addition to prevention of human taeniosis/cysticercosis and porcine cysticercosis both diseases should be treated adequately. In theory, eradication of NCC through eradication of human taeniosis (destroying the source of infection and preventing the spread of cysticercosis, both in humans and in pigs)103 and porcine cysticercosis (prevention of new cases of taeniosis)103 seems possible. Although the term eradication has been replaced by ‘control and reduction of NCC’ in T. solium taeniosis/cysticercosis endemic areas,101,104,105 the aim is certainly still a valid one. How could this be achieved?
For the control of human taeniosis mass drug administration of chemotherapeutics directed against T. solium (the adult pork tapeworm) to communities endemic for T. solium taeniosis/cysticercosis has been discussed at length, but so far no firm decision as to its instalment in sub-Saharan Africa has been reached.103 The drug of choice for treatment of human taeniosis would be niclosamide (2 g) in a single dose. It is not absorbed and therefore has virtually no side effects, but unfortunately it is not available in most countries of sub-Saharan Africa.104 Praziquantel, which is available, seems to be effective as well (dosage 5–10 mg/kg).104,106 However, when given by its own without steroids perifocal oedema around cysticerci in the brain or spinal cord may develop due to release of parasite antigen from viable cysticerci and may lead to neurological symptoms/signs in the treated individual (epileptic seizures, severe progressive headache; see above). So far, only case reports on the development of neurological symptoms/signs after administration of praziquantel have been published,32–34 but a large community-based study on neurological side effects after mass drug administration of praziquantel is underway. Another approach may be a more focally directed treatment as clustering of human cysticercosis around tapeworm carriers has been shown to be present.103,107,108 However, the uncertainty of the occurrence of undesirable neurological side effects still remains, although their detection seems more favourable in a focal setting rather than in whole communities. Considering transmission dynamics, chemotherapy of human taeniosis seems a crucial step towards control of NCC and definitely deserves further evaluation, whereas treatment of porcine cysticercosis (e.g. oxfendazole)109 seems more like an add-on procedure stabilizing the results of human chemotherapy.107 However, the combination of the above described measures, i.e. prevention/education and treatment of humans and pigs will have to be tailored to affected communities and strongly depends on local policies, financial means and available expertise, among others, and, in an ideal setting, should include all of the above components.
In summary, symptomatic NCC may affect between one and three million people throughout T. solium taeniosis/cysticercosis endemic areas of sub-Saharan Africa. Asymptomatic cases are potentially at risk of developing neurological symptoms/signs through mass drug administration directed against schistosomiasis, lymphatic filariasis and soil-transmitted helminths. The clinical presentation of cysticercosis/NCC not only is determined by the prevailing genotype of T. solium cysticerci, which seems closely related to that of Latin America, but may also vary individually based on genetic, immunological and environmental factors, among others. Diagnosis and treatment of NCC and its most common clinical presentation, i.e. epilepsy/epileptic seizures, is rendered difficult by the scarcity of neuroimaging facilities and the lack of an adapted epilepsy classification system together with appropriate antiepileptic treatment, respectively. Thus, the focus has to be on symptomatic treatment of epileptic seizures with locally available antiepileptic medication and steroids in selected cases. Besides treating people with NCC, prevention strategies as well as education have to be considered.
Acknowledgments
I am extremely grateful to the patients and their relatives of the Epilepsy Clinics of Haydom Lutheran Hospital (northern Tanzania) and Mahenge Hospital (southern Tanzania) for helping me gain experience with epilepsy and/or NCC within the African setting. I am especially indebted to Dr Wendy Harrison, Schistosomiasis Control Initiative, Imperial College, London, and Professor Hugo Garcia, Cysticercosis Working Group in Peru for their advice and critical review of parts of the manuscript. I am also very thankful to the German Research Foundation (DFG) and the Bill and Melinda Gates foundation for their support of further work into the subject of epilepsy and NCC.
References
- 1.Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 2.Del Brutto OH.Helminthic infections of the central nervous system Noseworthy J H, ed, editor. Neurological therapeutic principles and practice. 2nd edAbingdon, Oxon: Informa Health Care; 2006. p. 1133–52. [Google Scholar]
- 3.Campbell CD, Farrell VJR. Brain scans, epilepsy and cerebral cysticercosis. S Afr Med J. 1987;72:885–6. [PubMed] [Google Scholar]
- 4.Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis. 1999;29:1203–9. doi: 10.1086/313470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59:1730–4. doi: 10.1212/01.wnl.0000036320.69823.ea. [DOI] [PubMed] [Google Scholar]
- 6.Del Brutto OH, Santibáñez R, Hidrovo L, Rodríguez S, Dìaz-Calderón E, Navas C, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–7. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 7.Foyaca-Sibat H, Cowan LD, Carabin H, Targonska I, Anwary MA, Serrano-Ocaña G, et al. Accuracy of serological testing for the diagnosis of prevalent neurocysticercosis in outpatients with epilepsy, Eastern Cape Province, South Africa. PLoS Negl Trop Dis. 2009;3:e562. doi: 10.1371/journal.pntd.0000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shasha W, Pammenter MD. Sero-epidemiological studies of cysticercosis in school children from two rural areas of Transkei, South Africa. Ann Trop Med Parasitol. 1991;85:349–35. doi: 10.1080/00034983.1991.11812573. [DOI] [PubMed] [Google Scholar]
- 9.Thomson AJG. Neurocysticercosis — experience at the teaching hospitals of the University of Cape Town. S Afr Med J. 1993;83:332–4. [PubMed] [Google Scholar]
- 10.Grill J, Rakotomalala W, Andriantsimahavandy A, Boisier P, Guyon P, Roux J, et al. High prevalence of serological markers of cysticercosis among epileptic Malagasy children. Ann Trop Paediatr. 1996;16:185–91. doi: 10.1080/02724936.1996.11747824. [DOI] [PubMed] [Google Scholar]
- 11.Mafojane NA, Appleton CC, Krecek RC, Michael LM, Willingham AL., 3rd The current status of neurocysticercosis in eastern and southern Africa. Acta Trop. 2003;87:25–33. doi: 10.1016/s0001-706x(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 12.Gaffo AL, Guillen-Pinto D, Campos-Olazabal P, Burneo JG. Cysticercosis as the main cause of partial seizures in children in Peru. Rev Neurol. 2004;39:924–6. [PubMed] [Google Scholar]
- 13.Wallin M, Kurtzke J. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004;63:1559–64. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 14.Willingham AL III, Harrison LJ, Fèvre EM, Parkhouse ME. Inaugural meeting of the Cysticercosis Working Group in Europe. Emerg Infect Dis. 2008;14:e2. doi: 10.3201/1412.080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Brutto OH. A review of cases of human cysticercosis in Canada. Can J Neurol Sci. 2012;39:319–22. doi: 10.1017/s0317167100013445. [DOI] [PubMed] [Google Scholar]
- 16.Del Brutto OH. Neurocysticercosis among international travelers. J Travel Med. 2012;19:112–7. doi: 10.1111/j.1708-8305.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- 17.Del Brutto OH. Neurocysticercosis in Western Europe: a re-emerging disease? Acta Neurol Belg. Epub 2012 Apr 18. [DOI] [PubMed] [Google Scholar]
- 18.Serpa JA, Yancey LS, White AC., Jr Advances in the diagnosis and management of neurocysticercosis. Expert Rev Anti Infect Ther. 2006;4:1051–61. doi: 10.1586/14787210.4.6.1051. [DOI] [PubMed] [Google Scholar]
- 19.Davis LE.Neurocysticercosis Power C, Johnson R T, editors. Emerging neurological infectionsBoca Raton: Taylor & Francis; 2005. p. 261–87. [Google Scholar]
- 20.Zoli A, Shey-Njila O, Assana E, Nguekam JP, Dorny P, Brandt J, et al. Regional status, epidemiology and impact of Taenia solium cysticercosis in western and central Africa. Acta Trop. 2003;87:35–42. doi: 10.1016/s0001-706x(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 21.Sikasunge CS, Phiri IK, Phiri AM, Siziya S, Dorny P, Willingham AL., III Prevalence of Taenia solium porcine cysticercosis in the Eastern, Southern and Western provinces of Zambia. Vet J. 2008;176:240–4. doi: 10.1016/j.tvjl.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Winkler AS, Willingham AL, Sikasunge CS, Schmutzhard E. Epilepsy and neurocysticercosis in sub-Saharan Africa. Wien Klin Wochenschr. 2009;121(Suppl 3):3–12. doi: 10.1007/s00508-009-1242-3. [DOI] [PubMed] [Google Scholar]
- 23.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Bank 2011 [cited 2012 Aug] Available from: http://data.worldbank.org/sites/default/files/adi_2011-web.pdf. [Google Scholar]
- 25.Edwards T, Scott AG, Munyoki G, Odera V, Chengo E, Bauni E, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7:50–6. doi: 10.1016/S1474-4422(07)70292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler AS, Kerschbaumsteiner K, Stelzhammer B, Meindl M, Kaaya J, Schmutzhard E. Prevalence, incidence and clinical characteristics of epilepsy — a community-based door-to-door study in northern Tanzania. Epilepsia. 2009;50:2310–3. doi: 10.1111/j.1528-1167.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- 27.WHO. 2010. Working to overcome the global impact of neglected tropical diseases. First WHO report on neglected tropical diseases [cited 2012 Aug]. Available from: http://whqlibdoc.who.int/publications/2010/9789241564090_eng.pdf. [Google Scholar]
- 28.World Atlas 2010 [cited 2012 Aug] Available from: http://www.worldatlas.com/aatlas/populations/ctypopls.htm. [Google Scholar]
- 29.Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5:e1152. doi: 10.1371/journal.pntd.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez AL, Lindback J, Schantz PM, Sone M, Sakai H, Medina MT, et al. A population based, case-control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93:247–58. [PubMed] [Google Scholar]
- 31.De Almeida SM, Torres LFB. Neurocysticercosis — retrospective study of autopsy reports, a 17-year experience. J Community Health. 2011;36:698–702. doi: 10.1007/s10900-011-9389-z. [DOI] [PubMed] [Google Scholar]
- 32.Johnson RB. Potential hazard of mass praziquantel use. Am J Med. 1986;80:A88. [PubMed] [Google Scholar]
- 33.Torres JR, Noya O, de Noya BA, Mondolfi A. Seizures and praziquantel. A case report. Rev Inst Med Trop Sao Paulo. 1988;30:433–6. doi: 10.1590/s0036-46651988000600008. [DOI] [PubMed] [Google Scholar]
- 34.Flisser A, Madrazo I, Plancarte A, Schantz P, Allan J, Craig P, et al. Neurological symptoms in occult neurocysticercosis after single taeniacidal dose of praziquantel. The Lancet. 1993;342:748. doi: 10.1016/0140-6736(93)91743-6. [DOI] [PubMed] [Google Scholar]
- 35.Garcia HH, Gonzalez I, Mija L. Neurocysticercosis uncovered by single-dose albendazole. N Engl J Med. 2007;356:1277–8. doi: 10.1056/NEJMc062891. [DOI] [PubMed] [Google Scholar]
- 36.Sarti E, Schantz PM, Avila G, Ambrosio J, Medina-Santillán R, Flisser A. Mass treatment against human taeniasis for the control of cysticercosis: a population-based intervention study. Trans R Soc Trop Med Hyg. 2000;94:85–9. doi: 10.1016/s0035-9203(00)90451-6. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Weekly epidemiological record — Relevé épidémiologique hebdomadaire No. 2, 2012, 87, 17–28 [cited 2012 Aug]. Available from: http://www.who.int/wer. [Google Scholar]
- 38.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Weekly epidemiological record - Relevé épidémiologique hebdomadaire No. 35, 2011, 86, 377–8 [cited 2012 Aug]. Available from: http://www.who.int/wer. [Google Scholar]
- 40.Zoli AP, Nguekam JP, Shey-Njila O, Nsame Nforninwe D, Speybroeck N, Ito A, et al. Neurocysticercosis and epilepsy in Cameroon. Trans R Soc Trop Med Hyg. 2003;97:683–6. doi: 10.1016/s0035-9203(03)80103-7. [DOI] [PubMed] [Google Scholar]
- 41.Quet F, Guerchet M, Pion SDS, Ngoungou EB, Nicoletti A, Preux PM. Meta-analysis of the association between cysticercosis and epilepsy in Africa. Epilepsia. 2010;51:830–7. doi: 10.1111/j.1528-1167.2009.02401.x. [DOI] [PubMed] [Google Scholar]
- 42.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, Schmutzhard E. Epilepsy and neurocysticercosis in rural Tanzania — an imaging study. Epilepsia. 2009;50:987–93. doi: 10.1111/j.1528-1167.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 43.Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42:389–92. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 44.Takayanagui OM, Odashima NS. Clinical aspects of neurocysticercosis. Parasitol Int. 2006;55:S111–5. doi: 10.1016/j.parint.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol. 2011;7:584–94. doi: 10.1038/nrneurol.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE. Cysticercosis Working Group in Peru. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology. 2012;78:1394–400. doi: 10.1212/WNL.0b013e318253d657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.International League Against Epilepsy (ILAE) Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 49.International League Against Epilepsy (ILAE) Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 50.Birbeck GL. Revising and refining the epilepsy classification system: Priorities from a developing world perspective. Epilepsia. 2012;53(Suppl 2):18–21. doi: 10.1111/j.1528-1167.2012.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkler AS, Schaffert M, Schmutzhard E. Epilepsy in resource poor countries – suggestion of an adjusted classification. Epilepsia. 2007;48:1029–30. doi: 10.1111/j.1528-1167.2007.01009_1.x. [DOI] [PubMed] [Google Scholar]
- 52.Winkler AS, Schaffert M, Schmutzhard E. The pattern of epilepsy in a rural African hospital – an approach adapted to local circumstances. Trop Doct. 2009;39:44–7. doi: 10.1258/td.2008.080057. [DOI] [PubMed] [Google Scholar]
- 53.Blocher J, Schmutzhard E, Wilkins PP, Gupton P, Schaffert M, Auer H, et al. A cross-sectional study of people with epilepsy and neurocysticercosis: clinical characteristics and diagnostic approaches. PLoS Negl Trop Dis. 2011;5:e1185. doi: 10.1371/journal.pntd.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh MK, Garg RK, Nath G, Verma DN, Misra S. Single small enhancing computed tomographic (CT) lesions in Indian patients with new-onset seizures: a prospective follow-up in 75 patients. Seizure. 2001;10:573–8. doi: 10.1053/seiz.2001.0558. [DOI] [PubMed] [Google Scholar]
- 55.Chandy MJ, Rajshekhar V, Ghosh S, Prakash S, Joseph T, Abraham J, et al. Single small enhancing CT lesions in Indian patients with epilepsy: clinical, radiological and pathological considerations. J Neurol Neurosurg Psychiatry. 1991;54:702–5. doi: 10.1136/jnnp.54.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajshekhar V, Joshi DD, Doanh NQ, van De N, Xiaonong Z. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop. 2003;87:53–60. doi: 10.1016/s0001-706x(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 57.Singh G, Rajshekhar V, Murthy JMK, Prabhakar S.S, Modi M, Khandelwal N, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology. 2010;75:2236–45. doi: 10.1212/WNL.0b013e31820202dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh G, Singh P, Singh I, Rani A, Kaushal S, Avasthi G. Epidemiologic classification of seizures associated with neurocysticercosis: observations from a sample of seizure disorders in neurologic care in India. Acta Neurol Scand. 2006;13:233–40. doi: 10.1111/j.1600-0404.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 59.Rosenfeld EA, Byrd SE, Shulman ST. Neurocysticercosis among children in Chicago. Clin Infect Dis. 1996;23:262–8. doi: 10.1093/clinids/23.2.262. [DOI] [PubMed] [Google Scholar]
- 60.García HH, Gonzalez AE, Rodriguez S, Tsang VC, Pretell EJ, Gonzales I, et al. Neurocysticercosis: unraveling the nature of the single cysticercal granuloma. Neurology. 2010;75:654–8. doi: 10.1212/WNL.0b013e3181ed9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del Brutto OH, Nash TE. Garcia HH. Cysticerci-related single parenchymal brain enhancing lesions in non-endemic countries. J Neurol Sci. 2012;319:32–6. doi: 10.1016/j.jns.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruz ME, Schantz PM, Cruz I, Espinosa P, Preux PM, Cruz A, et al. Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 63.van As AD, Joubert J. Neurocysticercosis in 578 black epileptic patients. S Afr Med J. 1991;80:327–8. [PubMed] [Google Scholar]
- 64.Mason P, Houston S, Gwanzura L. Neurocysticercosis: experience with diagnosis by ELISA serology and computerised tomography in Zimbabwe. Cent Afr J Med. 1992;38:149–54. [PubMed] [Google Scholar]
- 65.Ky H, van Chap N. Radioclinical aspects of cerebral and muscular cysticercosis: 20 cases. J Neuroradiol. 2000;27:264–6. French. [PubMed] [Google Scholar]
- 66.Huang B, Li G, Jia F, Liu F, Ge L, Li W, et al. Determination of specific IgG4 for diagnosis and therapeutic evaluation of cerebral cysticercosis. Chin Med J. (Engl) 2002;115:580–3. [PubMed] [Google Scholar]
- 67.Yuan Z, Ren HJ, Ding YZ, Zhang JS, Wang WP, Wu XL, et al. Clinical study on the treatment of severe neurocysticercosis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2004;22:213–7. Chinese. [PubMed] [Google Scholar]
- 68.Verma A, Prasad KN, Gupta RK, Singh AK, Nyati KK, Rizwan A, et al. Toll-Like receptor 4 polymorphism and its association with symptomatic neurocysticercosis. J Infect Dis. 2010;202:1219–25. doi: 10.1086/656395. [DOI] [PubMed] [Google Scholar]
- 69.Ito A, Yamasaki H, Nakao M, Sako Y, Okamoto M, Sato MO, et al. Multiple genotypes of Taenia solium — ramifications for diagnosis, treatment and control. Acta Trop. 2003;87:95–101. doi: 10.1016/s0001-706x(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 70.Katabarwa M, Lakwo T, Habumogisha P, Richards F, Eberhard M. Could neurocysticercosis be the cause of ‘onchocerciasis-associated’ epileptic seizures? Am J Trop Med Hyg. 2008;78:400–1. [PubMed] [Google Scholar]
- 71.Grunitzky E, Balogou AK, M’Bella M, Belo M, Sadzo A, Bouteille B, et al. Cysticercosis in patients with neurologic diseases in hospital milieu in Lomé, Togo. Ann Med Interne (Paris) 1995;146:419–22. French. [PubMed] [Google Scholar]
- 72.Nakao M, Okamoto M, Sako Y, Yamasaki H, Nakaya K, Ito A. A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology. 2002;124:657–62. doi: 10.1017/s0031182002001725. [DOI] [PubMed] [Google Scholar]
- 73.Campbell G, Garcia HH, Nakao M, Ito A, Craig PS. Genetic variation in Taenia solium. Parasitol Int. 2006;55:S121–6. doi: 10.1016/j.parint.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 74.Dorny P, Brandt J, Zoli A, Geerts S. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 2003;87:79–86. doi: 10.1016/s0001-706x(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 75.Sotelo J, Del Brutto OH. Review of neurocysticercosis. Neurosurg Focus. 2002;12:e1. doi: 10.3171/foc.2002.12.6.2. [DOI] [PubMed] [Google Scholar]
- 76.Pal DK, Carpio A, Sander JWAS. Neurocysticercosis and epilepsy in developing countries. J Neurol Neurosurg Psychiatry. 2000;68:137–43. doi: 10.1136/jnnp.68.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Brutto OH. Neurocysticercosis. Semin in Neurol. 2005;25:243–51. doi: 10.1055/s-2005-917661. [DOI] [PubMed] [Google Scholar]
- 79.Abraham R, Livramento JA, Leite CDA, Pardini AX, Vaz AJ, Machado Lidos R. Neurocysticercosis: relationship between Taenia antigen levels in CSF and MRI. Arq Neuropsiquiatr. 2010;68:7–11. doi: 10.1590/s0004-282x2010000100003. [DOI] [PubMed] [Google Scholar]
- 80.Almeida CR, Ojopi EP, Nunes CM, Machado LR, Takayanagui OM, Livramento JA, et al. Taenia solium DNA is present in cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur Arch Psychiatr Clin Neurosci. 2006;56:307–10. doi: 10.1007/s00406-006-0612-3. [DOI] [PubMed] [Google Scholar]
- 81.Hernández M, Gonzalez LM, Fleury A, Saenz B, Parkhouse RM, Harrison LJ, et al. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol. 2008;102:317–23. doi: 10.1179/136485908X278856. [DOI] [PubMed] [Google Scholar]
- 82.Gabriël S, Blocher J, Dorny P, Abatih E, Schmutzhard E, Ombay M, et al. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis. 2012 doi: 10.1371/journal.pntd.0001851. (in print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nash TE, Singh G, White AC, Rajshekhar V, Loeb JA, Proaño JV, et al. Treatment of neurocysticercosis: current status and future research needs. Neurology. 2006;67:1120–7. doi: 10.1212/01.wnl.0000238514.51747.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Del Brutto OH, Wadia NH, Dumas M, Cruz M, Tsang VCW, Schantz PM. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci. 1996;142:1–6. doi: 10.1016/0022-510x(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 85.Carpio A. Neuroimaging in Neurocysticercosis [cited 2012 Aug]. Available from: http://emedicine.medscape.com/article/1168784-differential. [Google Scholar]
- 86.Nash TE, Mahanty S, Garcia HH. Cysticercosis Group in Peru. Corticosteroid use in neurocysticercosis. Expert Rev Neurother. 2011;11:1175–83. doi: 10.1586/ern.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corona T, Lugo R, Medina R, Sotelo J. Single-day praziquantel therapy for neurocysticercosis. N Engl J Med. 1996;334:125. doi: 10.1056/NEJM199601113340216. [DOI] [PubMed] [Google Scholar]
- 88.Pretell EJ, Garcia HH, Gilman RH, Saavedra H, Martinez M. The Cysticercosis Working Group in Peru. Failure of one-day praziquantel treatment in patients with multiple neurocysticercosis lesions. Clin Neurol Neurosurg. 2001;103:175–7. doi: 10.1016/s0303-8467(01)00137-8. [DOI] [PubMed] [Google Scholar]
- 89.Nguekam JP, Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite. 2003;10:65–8. doi: 10.1051/parasite/2003101p65. [DOI] [PubMed] [Google Scholar]
- 90.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350:249–58. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 91.Das K, Mondal GP, Banerjee M, Mukherjee BB, Singh OP. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8-year randomized study. J Clin Neurosci. 2007;14:1172–7. doi: 10.1016/j.jocn.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 93.Specchio LM, Beghi E. Should antiepileptic drugs be withdrawn in seizure-free patients? Epilepsia. 2008;49:455–63. doi: 10.2165/00023210-200418040-00001. [DOI] [PubMed] [Google Scholar]
- 94.Lossius MI, Hessen E, Mowinckel P, Stavem K, Erikssen J, Gulbrandsen P, et al. Consequences of antiepileptic drug withdrawal: a randomized, double-blind study (Akershus Study). CNS Drugs. 2004;18:201–12. doi: 10.1111/j.1528-1167.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 95.Birbeck GL. Barriers to care for patients with neurologic disease in rural Zambia. Arch Neurol. 2000;57:414–17. doi: 10.1001/archneur.57.3.414. [DOI] [PubMed] [Google Scholar]
- 96.Mbuba CK, Ngugi AK, Fegan G, Ibinda F, Muchohi SN, Nyundo C, et al. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol. 2012;11:688–96. doi: 10.1016/S1474-4422(12)70155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winkler AS. Measuring the epilepsy treatment gap in sub Saharan Africa. Lancet Neurol. 2012;11:655–7. doi: 10.1016/S1474-4422(12)70160-6. [DOI] [PubMed] [Google Scholar]
- 98.Winkler AS, Mayer M, Schnaitmann S, Ombay M, Mathias B, Schmutzhard E, et al. Belief systems of epilepsy and attitudes towards people living with epilepsy in a rural community of northern Tanzania. Epilepsy Behav. 2010;19:596–601. doi: 10.1016/j.yebeh.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 99.Winkler AS, Mayer M, Ombay M, Mathias B, Schmutzhard E, Jilek-Aall L. Attitudes towards African traditional medicine and Christian spiritual healing regarding treatment of epilepsy in a rural community of northern Tanzania. Afr J Trad CAM. 2010;7:162–70. doi: 10.4314/ajtcam.v7i2.50877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birbeck G, Chomba E, Atadzhanov M, Mbewe W, Haworth A. The social and economic impact of epilepsy in Zambia: a cross-sectional study. Lancet Neurol. 2007;6:39–44. doi: 10.1016/S1474-4422(06)70629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Willingham AL, 3rd, Engels D. Control of Taenia solium cysticercosis/taeniosis. Adv Parasitol. 2006;61:509–6. doi: 10.1016/S0065-308X(05)61012-3. [DOI] [PubMed] [Google Scholar]
- 102.Pondja A, Neves L, Mlangwa J, Afonso S, Fafetine J, Willingham AL, 3rd, et al. Prevalence and risk factors of porcine cysticercosis in Angonia District, Mozambique. PLoS Negl Trop Dis. 2010;4:e594. doi: 10.1371/journal.pntd.0000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pawlowski ZS. Control of neurocysticercosis by routine medical and veterinary services. Trans R Soc Trop Med Hyg. 2008;102:228–32. doi: 10.1016/j.trstmh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Pawlowski ZS, Allan JC, Meinardi H.Control measures for taeniosis and cysticercosis Murrell K D, ed, editor. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/CysticercosisParis: OIE; 2005. p. 73–99. [Google Scholar]
- 105.Pawlowski ZS, Allan J, Sarti E. Control of Taenia solium taeniasis/cysticercosis; from research towards implementation. Int J Parasitol. 2005;35:1221–32. doi: 10.1016/j.ijpara.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 106.Pawlowski ZS. Perspectives on the control of Taenia solium. Parasitol Today. 1990;6:371–3. doi: 10.1016/0169-4758(90)90142-q. [DOI] [PubMed] [Google Scholar]
- 107.Pawlowski ZS. Role of chemotherapy of taeniasis in prevention of neurocysticercosis. Parasitol Int. 2006;55:S105–S109. doi: 10.1016/j.parint.2005.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Rodriguez S, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS Negl Trop Dis. 2009;3:e371. doi: 10.1371/journal.pntd.0000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gonzales AE, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Bernal T, et al. Effective, single-dose treatment of porcine cysticercosis with oxfendazole. Am J Trop Med Hyg. 1996;54:391–4. doi: 10.4269/ajtmh.1996.54.391. [DOI] [PubMed] [Google Scholar]