Abstract
Calcified granulomas are the most common radiological finding in neurocysticercosis (10–20% of endemic populations). A small proportion serves as foci of seizure activity, which results in large numbers of persons with epilepsy. Calcified granulomas are not all the same. Some demonstrate blood–brain barrier dysfunction (magnetic resonance imaging enhancement) most likely due to the presence of inflammation, visualizable scolices, and/or gliosis. About half the patients with a recent history of seizures, positive serology, and only calcified lesions develop perilesional edema at the time of a seizure recurrence. The natural history, treatment, and pathophysiology of this phenomenon are not well studied. Episodes are usually associated with seizures or other neurological manifestations, resolve by 4–6 weeks, sometimes occur repeatedly, and usually involve a subset of the same calcifications. Treatment is supportive. Histopathological examination of one calcification associated with multiple perilesional edema episodes revealed significant inflammation and supports the concept that perilesional edema is inflammatory in nature. This most likely is due to host responses to released or newly recognized parasite antigen and/or upregulation of the host immune response. Immunosuppressive and anti-inflammatory agents may be useful in prevention and/or treatment of this phenomenon.
Keywords: Neurocysticercosis, Taenia solium, Calcifications, Perilesional edema
Degenerating Taenia solium cysts in human brain incite an inflammatory reaction that resolves into calcified or non-calcified granulomas.1,2 In the absence of calcification, resolved granulomas are not visualized by magnetic resonance imaging (MRI) or computed tomography (CT) imaging unless they enhance. On the other hand, the calcium deposits in calcified granulomas are easily detected by CT imaging and less efficiently by gradient echo-based MRI protocols.3 These are stable in the brain and are a cumulative measure of prior healed parenchymal cysts.4 Although there is considerable variability in size and shape, calcifications are typically small and round. The number of calcified cysts varies considerably in any individual: 1–2 is usual,5–7 but there may be hundreds. Calcified granulomas are frequently referred to as ‘inactive’ cysticercosis to differentiate this stage from ‘active’ neurocysticercosis that refers to viable or degenerating cystic lesions.8,9 The clinical implication is that viable cysts more commonly result in symptoms and seizures whereas calcified lesions are inert and clinically silent. Contrary to prior thinking, recent studies implicate calcified granulomas as a common cause of seizures4 and other neurological symptoms as well as foci of perilesional edema.10 Degenerating viable cysts have a greater propensity to cause seizures than calcified granulomas. But because calcified lesions are so common in endemic populations, the small minority associated with seizures far outnumber those due to degenerating cysts. Therefore, a large majority of seizures in most regions are due to calcified granulomas rather than degenerating cysts.4,6,11–14
Punctate round calcifications that are common in CT imaging in endemic regions are mostly due to neurocysticercosis. The CT appearance of calcifications is almost always non-diagnostic. However, the presence of a positive serology for cysticercosis gives credence that the calcification(s) is due to neurocysticercosis. Unfortunately, cysticercosis serology is negative in a substantial minority of patients with calcifications.15,16 Although there is the distinct impression that typical round punctate lesions are very common in endemic regions and unusual in non-endemic regions, this observation has not been verified by any formal study. Older literature mostly describes the gross description of calcified granulomas as hard, chalky nodules.1 Histopathological analyses of brain calcifications are sparse in the recent literature but support the T. solium origin of cerebral calcifications.1,2,17,18 Uncommonly, parasite remnants are recognizable but usually membrane-like structures, amorphous calcifications and calcareous corpuscles are present. The latter are diagnostic of cestodes and therefore in the usual clinical and epidemiological setting are almost always indicative of degenerated T. solium cysts.19 Also supporting are serial observations of antihelminthic-treated T. solium-infected patients.20–24 Degenerating viable cysts develop into typical calcifications and otherwise indistinguishable calcifications sometimes show diagnostic scolices in a subset of typical calcified lesions using gradient echo MRI as well.25 Additionally, typical calcified lesions are frequently found in the presence of cystic lesions in individuals with a certain diagnosis of neurocysticercosis. Although other central nervous system infections can result in calcifications, they usually do not result in typical calcification or can be distinguished by other criteria.4 Therefore, the data strongly suggest that typical calcifications in patients with reasonable exposure are mostly due to degenerated T. solium cysts.
Brain calcifications are a relatively common finding in endemic regions, and the frequency ranges roughly from 10 to 20%.5–7,26,27 In selected populations with a history of seizures/epilepsy, the frequency of calcifications is generally greater, ranging from 13 to 83% compared to 5 to 15% without the history, 19 to 34% for persons with a positive serology, and 8 to 25% in the absence of a positive serology.4 Routine CT imaging to evaluate mild head trauma in Brazil revealed that about 7.6% had one or more calcifications.28
Information from four types of studies implicates calcifications as foci of seizures. In unselected persons presenting with seizures residing in endemic countries or in non-endemic regions with high endemic immigrant populations, calcifications are frequently the only abnormality on CT scans and are usually more common than degenerating cysts.29 As noted above, patients with a history of seizures from endemic regions have an increased prevalence of calcifications compared to those without a history. In patients with calcifications and seizures, they are implicated as seizure foci in about 50–73% of those studied.30–32 Certain calcifications are more likely to cause seizure activity such as those with gliosis.24,33 Lastly, the presence of perilesional edema around calcifications at the time of seizures implicates specific calcified lesions as foci of seizure activity (Figure 1).
Figure 1.
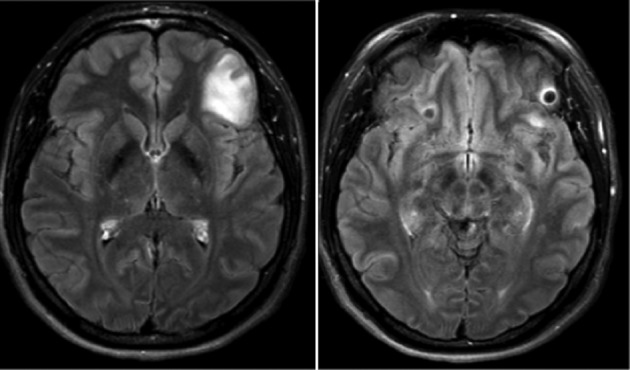
A joining fast attenuated fluid inversion post-gadolinium injection images of a patient who presented with seizures associated with perilesional edema around a calcification in the left frontal lobe. Repeated episodes involving the same and other calcifications have been documented. The right image demonstrates an enhancing calcification with some edema, which is better visualized in the left image.
Perilesional edema associated with calcifications was first recognized by Del Brutto et al.34 in a group of patients who had seizure relapse and calcifications. Of the 16 of 20 persons who had seizures after discontinuation of anti-seizures medication, five had perilesional edema around calcifications. He was the first to suggest antigen release from dead parasites as a possible cause of the edema. White35 and a year later Garg et al.36 briefly mentioned the presence of perilesional edema in a series of patients.
The first full descriptions were published by Sheth et al.37 who described a complicated case and Nash and Patronas38 who published a detailed history of three patients, one with recurrent episodes over 10 years. Subsequently, a number of cases or series describing perilesional edema episodes were reported from different geographic locations,10,25,39–44 indicating that the phenomenon is a relatively common general finding in neurocysticercosis.
Episodes of perilesional edema have been reported from many endemic regions worldwide but the natural history and characteristics of those severely affected have not been well studied (Table 1). Most patients are evaluated close to the onset of clinical symptoms, which are usually seizures. However, other clinical manifestations also occur such as severe headaches, aphasia, confusion, or flashing/moving lights due to occipital lobe calcifications.38,41 These complaints may be overlooked and go unstudied. We have observed asymptomatic perilesional edema episodes in patients who undergo periodic MRI examinations in prospective studies (Nash and Mahanty, unpublished observations). Although edema around a single calcification appears to be most common, two or more calcifications can be affected at the same time.10,38,41 Repeated episodes in the same patient are not uncommon and usually involve a subset of calcified lesions.38–42 Most episodes are self-limiting with edema usually resolved in 4–6 weeks38,40 (Nash and Garcia, Nash and Mahanty, unpublished observations).
Table 1. Features of perilesional edema around T. solium-calcified granulomas.
| 1. Common |
| 2. Intermittent |
| 3. Usually, but not always, associated with symptoms |
| 4. Symptoms mostly seizures can be focal neurological abnormalities or severe headaches |
| 5. Involvement of a subset of calcifications |
| 6. Can be recurrent |
| 7. Edema frequently resolves in 4–6 weeks |
| 8. Predictors include presence of increasing number of enhancing calcified cysts and a visualizable scolex seen on gradient echo MRI sequences |
| 9. Pathophysiology, natural history, and long-term sequelae largely unstudied |
| 10. No proven treatment other than antiepileptic medication |
| 11. Abrupt withdrawal of corticosteroids may lead to symptomatic episodes |
The relative ease of observing perilesional edema episodes suggested that the phenomenon is frequent. Nash et al. prospectively followed 110 patients with only calcifications, a history of seizures and/or severe headaches and a positive serology.10 At the time of an event, which was almost always a seizure, MRIs were obtained in the patient and a person drawn from the cohort without symptoms who was matched by age, sex, and number of calcifications. Twenty-nine persons experienced seizures and 24 of them underwent an MRI exam within 5 days of the event. Fifty percent of those with symptoms and 9% of controls demonstrated perilesional edema. The study concluded that perilesional episodes are usually, but not always associated with symptoms and that recurrent seizures are frequent in this cohort, estimated at 36% in 5 years. Although the studied cohort differs in some ways from rural endemic populations with a history of recent seizures, the results highly suggest that perilesional edema is a common cause of seizures and morbidity in persons living in endemic regions.
What variables predict perilesional episodes? As noted above, most patients with calcifications are asymptomatic but a small subset develops seizures and/or epilepsy and half of these demonstrate perilesional edema. Individuals with a greater proportion of calcified lesion showing enhancement had a significant increase in perilesional edema episodes41 while another report associated perilesional edema episodes with the identification of a scolex in the calcification.25 Surprisingly, neither the number of calcifications, size of the calcifications, or locations predicted perilesional edema recurrences.
The pathophysiology of perilesional edema is unknown. One of the most frequent hypotheses is that edema represents inflammation brought about by host’s reaction to released or sequestered parasite antigen as is known to occur to non-calcified degenerating cysts22,23 and another is that perilesional edema occurs as a consequence of seizure activity.4,36,38 Cellular edema caused by seizures is well documented in the literature. However, there are differences between edema associated with seizures and those associated with perilesional edema around calcifications.38,45–47 The edema associated with seizures is mostly described in patients with status epilepticus, which is uncommon in most patients with calcified lesions and seizures. Electroencephalograms, obtained shortly after onset, fail to show epileptiform activity. Perilesional edema in calcific neurocysticercosis is frequently detected but is uncommonly found after seizure activity caused by other etiologies. The edema associated with seizure activity is diffuse, usually lacks a defined maximal area of activity and presumably is caused by loss of fluid by damaged cells. On the other hand, perilesional edema associated with calcifications is maximal around the lesion and almost always accompanied by a large amount of enhancement, which is more consistent with a vasogenic origin of the edema. In fact, perilesional edema strongly resembles inflammatory responses to non-calcified degenerating cysts. Since the cause of most disease including immune responses to degenerating parenchymal cysts is inflammation and perilesional edema episodes around calcifications are similar, it is reasonable to believe that perilesional edema is a continuation of inflammatory responses to the parasite seen at earlier stages of degeneration.
There are several lines of evidence that support an inflammatory hypothesis. First, calcified cysts are likely not all in the same state; some are quiescent without discernible enhancement or edema while others demonstrate varying amounts of enhancement.41,48 It is likely that residual inflammation results in brain barrier disruption allowing dye, normally confined in the vasculature, to leak into the contiguous brain tissues.48 A role of inflammation is supported by analysis of calcified granuloma due to T. solium that was excised because of recurring episodes of perilesional edema.43 Histopathology showed a large amount of mononuclear inflammation and perivascular cuffing in the adjacent brain tissue. This finding is in contrast to the few images and descriptions of calcified lesions in the literature, which are for the most part associated with amorphous calcifications and little or no residual inflammation.1,2,17,49
Does the calcium in the calcification take part in the pathogenesis of perilesional edema or is it just an easily visualized marker of degenerated cysts? Calcification of granulomas is a risk factor for seizure recurrence;22,23,50 however, there is little direct evidence to implicate calcium deposits directly. On the other hand, calcification may just be an easily detectable feature of granulomas and play little or no role. It is of interest that Del Brutto23 in his early description of perilesional edema mentions a number of instances where transient edema occurred at sites of prior degenerating cysts that failed to calcify and therefore could not be visualized by CT. The author has noted 1–2 similar episodes.
An immunologically based pathophysiology is additionally supported by a small number of case reports revealing induction or worsening of perilesional edema following administration and then cessation of corticosteroids. Sheth et al.’s 37report described a patient with known calcified granulomas that developed after anthleminthic treatment, who was prescribed corticosteroids for erythema marginatum and subsequently developed perilesional edema around lesions known to be uninvolved earlier. Another report by Park et al.39 described a child with multiple calcifications who spontaneously developed symptoms associated with enhancement around a calcification in the pons prompting corticosteroid administration. Over the next 7 months, repeated courses of albendazole and corticosteroids were given accompanied by fluctuating enhancement and edema of the initial lesion as well as involvement of previously quiescent calcifications. Nash et al.41 reported a patient with fluctuating perilesional edema around multiple previously uninvolved calcified lesions following administration of corticosteroids. Poeschl et al.42 described a patient treated for viable parenchymal cysts with albendazole and corticosteroids who became symptomatic after cessation of corticosteroids and albendazole. MRI showed that 25 small lesions had developed enhancement and/or surrounding edema including eight previously quiescent calcifications. This author is aware of two other similar unreported cases. Abrupt cessation of corticosteroids may induce enhancement and/or edema around uninvolved calcifications or exacerbate already existing responses.51 This satisfies the clinical definition of rebound, a common clinically defined complication of a number of diseases associated with too rapid cessation and/or taper of corticosteroids.
There is no proven specific treatment for perilesional edema episodes other than symptomatic therapy with anti-seizure medications. Treatment of brain edema with corticosteroids due to practically any cause is an almost universal, generally accepted practice. It may be helpful in the treatment of perilesional edema as well but the benefit is unclear. Current information suggests that perilesional edema episodes usually resolve spontaneously38,40 without corticosteroid treatment and furthermore corticosteroid treatment may induce or worsen perilesional edema and/or symptoms due to rebound upon cessation.51
If the process of perilesional edema around calcifications is immunologically based and caused by intermittent acute inflammatory responses, then it may be useful to suppress the immune response in order to prevent and/or treat this phenomenon. Two variables could be important. One is the state of the lesion since not all calcifications are associated with perilesional edema episodes and second is the state of the systemic immune response. The former explains why a subset of calcifications are involved and the latter accounts for why multiple lesions can be involved at the same time. Seizure control in this population may benefit from anti-inflammatory measures. This was apparently seen in a patient administered methotrexate41,52 for repeated episodes of perilesional edema who showed a dramatic decrease in the number of subsequent seizures over the subsequent decade beginning after start of the drug.
Perilesional edema around calcifications is common in neurocysticercosis and very likely responsible for a substantial amount of seizure activity in endemic populations. If immune mechanisms are involved, then anti-inflammatory/immunosuppressive treatments in addition to anti-seizure medication may be a novel approach to control seizure activity.10
Acknowledgments
This work was supported by the intramural NIAID, research program, NIH.
References
- 1.Hennenberg R.Die tierischen Parasiten des Zentralnervensystems Lewandowsky M, ed, editor. Handbuch der neurologie Berlin: Springer; 1912Vol. 3, pp. 642–83. [Google Scholar]
- 2.Escobar A.Pathology of the nervous system Palacios E, Rodriguez-Carbajal J, Taveras J M, editors. Cysticercosis of the central nervous system Springfield, IL: Charles Thomas; 1983. pp. 27–54. [Google Scholar]
- 3.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol. 2011;7:584–94. doi: 10.1038/nrneurol.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–45. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 6.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez AL, Lindback J, Schantz PM, Sone M, Sakai H, Medina MT, et al. A population-based, case–control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93:247–58. [PubMed] [Google Scholar]
- 8.Carpio A, Placencia M, Santillan F, Escobar A. A proposal for classification of neurocysticercosis. Can J Neurol Sci. 1994;21:43–7. doi: 10.1017/s0317167100048757. [DOI] [PubMed] [Google Scholar]
- 9.Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms. A study of 753 cases. Arch Intern Med. 1985;145:442–5. [PubMed] [Google Scholar]
- 10.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case–control study. Lancet Neurol. 2008;7:1099–105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Noval J, Allan JC, Fletes C, Moreno E, DeMata F, Torres-Alvarez R, et al. Epidemiology of Taenia solium taeniasis and cysticercosis in two rural Guatemalan communities. Am J Trop Med Hyg. 1996;55:282–9. doi: 10.4269/ajtmh.1996.55.282. [DOI] [PubMed] [Google Scholar]
- 12.Palacio LG, Jimenez I, Garcia HH, Jiménez ME, Sánchez JL, Noh J, et al. Neurocysticercosis in persons with epilepsy in Medellin, Colombia. The Neuroepidemiological Research Group of Antioquia. Epilepsia. 1998;39:1334–9. doi: 10.1111/j.1528-1157.1998.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 13.Cruz ME, Preux PM, Debrock C, Cruz I, Schantz PM, Tsang VC, et al. Epidemiology of cerebral cysticercosis in an Andean community in Ecuador. Bull Soc Pathol Exot. 1999;92:38–41. [PubMed] [Google Scholar]
- 14.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–9. doi: 10.1212/01.wnl.0000249113.11824.64. [DOI] [PubMed] [Google Scholar]
- 15.Garcia HH, Herrera G, Gilman RH, Tsang VC, Pilcher JB, Diaz JF, et al. Discrepancies between cerebral computed tomography and western blot in the diagnosis of neurocysticercosis. The Cysticercosis Working Group in Peru (Clinical Studies Coordination Board). Am J Trop Med Hyg. 1994;50:152–7. doi: 10.4269/ajtmh.1994.50.152. [DOI] [PubMed] [Google Scholar]
- 16.Rawlings D, Ferriero DM, Messing RO. Early CT reevaluation after empiric praziquantel therapy in neurocysticercosis [see comments]. Neurology. 1989;39:739–41. doi: 10.1212/wnl.39.5.739. [DOI] [PubMed] [Google Scholar]
- 17.Marquez-Monter H.Cysticercosis Marcial-Rojas R A, ed, editor. Pathology of protozoal and helminthic diseases Baltimore, MD: The Williams and Wilkens Company; 1971. pp. 592–617. [Google Scholar]
- 18.Raliela-Cervantes MTR, Rivas-Hernandez A, Rodriguez-Ibarra J, Castillo-Medina S, Cancino FdM.Anatomopathological aspects of human brain cysticercosis Flisser A, Willms K, Laclette J P, Larralde C, Ridaura C, Beltran F, editors. Cysticercosis: present state of knowledge and perspectives New York: Academic Press; 1982. pp. 179–200. [Google Scholar]
- 19.Chacko G, Rajshekhar V, Chandy MJ, Chandi SM. The calcified intracorporeal vacuole: an aid to the pathological diagnosis of solitary cerebral cysticercus granulomas. J Neurol Neurosurg Psychiatry. 2000;69:525–7. doi: 10.1136/jnnp.69.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robles C, Sedano AM, Vargastentori N, Galindovirgen S. Long-term results of praziquantel therapy in neurocysticercosis. J Neurosurg. 1987;66:359–63. doi: 10.3171/jns.1987.66.3.0359. [DOI] [PubMed] [Google Scholar]
- 21.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350:249–58. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 22.Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology. 2004;62:2236–40. doi: 10.1212/01.wnl.0000130471.19171.d8. [DOI] [PubMed] [Google Scholar]
- 23.Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology. 1994;44:1706–9. doi: 10.1212/wnl.44.9.1706. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan S, Kathuria MK, Gupta RK. Perilesional gliosis and seizure outcome: a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Ann Neurol. 2000;48:181–7. [PubMed] [Google Scholar]
- 25.Gupta RK, Kumar R, Chawla S, Pradhan S. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia. 2002;43:1502–8. doi: 10.1046/j.1528-1157.2002.21302.x. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Noval J, Moreno E, de Mata F, Soto de Alfaro H, Fletes C, Craig PS, et al. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol. 2001;95:167–75. doi: 10.1080/00034980120050260. [DOI] [PubMed] [Google Scholar]
- 27.Cruz ME, Schantz PM, Cruz I, Espinosa P, Preux PM, Cruz A, et al. Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 28.Bordignon KC, Arruda WO. CT scan findings in mild head trauma: a series of 2,000 patients. Arq Neuropsiquiatr. 2002;60:204–10. doi: 10.1590/s0004-282x2002000200004. [DOI] [PubMed] [Google Scholar]
- 29.Monteiro L, Nunes B, Mendonca D, Lopes J. Spectrum of epilepsy in neurocysticercosis — a long-term follow-up of 143 patients. Acta Neurol Scand. 1995;92:33–40. doi: 10.1111/j.1600-0404.1995.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 30.Murthy JM, Reddy VS. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure. 1998;7:153–7. doi: 10.1016/s1059-1311(98)80072-1. [DOI] [PubMed] [Google Scholar]
- 31.Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino Junior R. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr. 1994;52:289–94. doi: 10.1590/s0004-282x1994000300001. [DOI] [PubMed] [Google Scholar]
- 32.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41:718–26. doi: 10.1111/j.1528-1157.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Raghav S, Husain M, Kumar R, Gupta RK. Epilepsy with focal cerebral calcification: role of magnetization transfer MR imaging. Neurol India. 2004;52:197–9. [PubMed] [Google Scholar]
- 34.Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42:389–92. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 35.White AC., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–13. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]
- 36.Garg RK, Karak B, Mohan Kar A. 1998. Neuroimaging abnormalities in Indian patients with uncontrolled partial seizures; pp. 497–500. Seizure. [DOI] [PubMed] [Google Scholar]
- 37.Sheth TN, Lee C, Kucharczyk W, Keystone J. Reactivation of neurocysticercosis: case report. Am J Trop Med Hyg. 1999;60:664–7. doi: 10.4269/ajtmh.1999.60.664. [DOI] [PubMed] [Google Scholar]
- 38.Nash TE, Patronas NJ. Edema associated with calcified lesions in neurocysticercosis. Neurology. 1999;53:777–81. doi: 10.1212/wnl.53.4.777. [DOI] [PubMed] [Google Scholar]
- 39.Park SY, Barkovich AJ, Weintraub PS. Clinical implications of calcified lesions of neurocysticercosis. Pediatr Infect Dis J. 2000;19:581–3. doi: 10.1097/00006454-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Antoniuk SA, Bruck I, Dos Santos LH, Pintarelli VL, Navolar FB, Brackmann PC, Jr, et al. Seizures associated with calcifications and edema in neurocysticercosis. Pediatr Neurol. 2001;25:309–11. doi: 10.1016/s0887-8994(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 41.Nash TE, Pretell J, Garcia HH. Calcified cysticerci provoke perilesional edema and seizures. Clin Infect Dis. 2001;33:1649–53. doi: 10.1086/323670. [DOI] [PubMed] [Google Scholar]
- 42.Poeschl P, Janzen A, Schuierer G, Winkler J, Bogdahn U, Steinbrecher A. Calcified neurocysticercosis lesions trigger symptomatic inflammation during antiparasitic therapy. Am J Neuroradiol. 2006;27:653–5. [PMC free article] [PubMed] [Google Scholar]
- 43.Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. Short report: a calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg. 2011;85:460–3. doi: 10.4269/ajtmh.2011.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina MT, Dubon-Murcia SA, Aquilar-Estrada RL, Chaves-Sell F, Bu J. Neurocysticercosis and epilepsy. Epilepsies. 2010;22:126–33. [Google Scholar]
- 45.Milligan TA, Zamani A, Bromfield E. Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure. 2009;18:104–8. doi: 10.1016/j.seizure.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Raghavendra S, Ashalatha R, Krishnamoorthy T, Kesavadas C, Thomas SV, Radhakrishnan K. Reversible periictal MRI abnormalities: clinical correlates and long-term outcome in 12 patients. Epilepsy Res. 2007;73:129–36. doi: 10.1016/j.eplepsyres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Cole AJ. Status epilepticus and periictal imaging. Epilepsia. 2004;45 Suppl 4:72–7. doi: 10.1111/j.0013-9580.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 48.Sheth TN, Pillon L, Keystone J, Kucharczyk W. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR Am J Neuroradiol. 1998;19:79–82. [PMC free article] [PubMed] [Google Scholar]
- 49.Rabiela MT, Rivas A, Flisser A. Morphological types of Taenia solium cysticerci. Parasitol Today. 1989;5:357–9. doi: 10.1016/0169-4758(89)90111-7. [DOI] [PubMed] [Google Scholar]
- 50.Singhi PD, Dinakaran J, Khandelwal N, Singhi SC. One vs. two years of anti-epileptic therapy in children with single small enhancing CT lesions. J Trop Pediatr. 2003;49:274–8. doi: 10.1093/tropej/49.5.274. [DOI] [PubMed] [Google Scholar]
- 51.Nash TE, Mahanty S, Garcia HH Cysticercosis Group in Peru. Corticosteroid use in neurocysticercosis. Expert Rev Neurother. 2011;11:1175–1183. doi: 10.1586/ern.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44:549–53. doi: 10.1086/511040. [DOI] [PubMed] [Google Scholar]


