Abstract
Cysticercosis, the infection with the larval stage of Taenia solium, is a cause of neurological symptoms including seizures, affecting the quality of life of patients and their families. Diagnosis focuses on brain imaging and serological tests are mostly used as confirmatory tools. Most cases, however, occur in poor endemic areas, where both kinds of diagnostic tools are poorly available. Development of point of care diagnostic tests is one of the most important priorities for cysticercosis researches today. The ideal point of care test would require detection of viable cysticercosis and hopefully identify cases with severe or progressive forms of neurocysticercosis, leading to referral of the patient for specialized medical attention. This manuscript describes the evolution of the serological diagnosis of cysticercosis over time, and the characteristics of the most common currently available tools, their advantages and disadvantages, and their potential use in future diagnostic tests.
Keywords: Cysticercosis, Neurocysticercosis, Immunodiagnosis, Antibody, Antigen, Serology, Taenia solium, Western blot
Introduction
The varied clinical presentations of neurocysticercosis (NCC) result from a series of factors, which include the number, stage, size, and location of parasites in the nervous system of the human host, factors which also influence case management and prognosis. In this context, the utility of immunodiagnosis as a tool on which to base clinical decisions by itself is quite limited. Diagnosis and characterization of human NCC should be based on a brain imaging examination to observe the characteristics of the lesions, accompanied by a serological test result to confirm the etiology. In the best possible scenario, the immunological test should not only be highly sensitive and highly specific for etiological confirmation but also be able to discriminate infections with living parasites from inactive infections, and correlate the characteristics of the infection with parasite load, for patient management and follow-up. A century of serological assay development for Taenia solium cysticercosis has provided some tests which fulfill several of the above requirements, albeit the ideal assay has yet to be developed.
This review will discuss most of the available immunodiagnostic tests for cysticercosis, focusing on the lentil lectin glycoprotein enzyme-linked immunoelectrotransfer blot (LLGP-EITB) assay as the test of choice, the advantages and disadvantages of antigen and antibody detection, as well as the importance of the selection of the type of sample to be processed. We will also stress the importance of evaluating new diagnostic assays with well-defined sets of sera reflecting the entire spectrum of NCC infection (active/inactive, intra/extraparenchymal, diverse numbers of lesions including viable or degenerating cysts for single-lesion cases, cases from diverse geographic areas).
Antibody Detection Tests for Cysticercosis
Early in the twentieth century, the laboratory diagnosis of tissue parasites was limited to non-specific findings of increased white cell counts, strongly valuing the presence of increased eosinophil numbers.1,2 The first serological assays for parasitic infections were complement precipitation and fixation techniques. In 1909, Weinberg used complement fixation with cystic fluid from cysticerci to demonstrate specific antibodies in the sera of a group of cysticercotic pigs.3 This test became known as ‘Weinberg’s reaction’ and was used until a few decades ago.4–6 In 1911, Arthur Moses reported the use of an aqueous cysticercal extract to demonstrate the presence of antibodies in the serum of three patients with subcutaneous cysticercosis and in the cerebrospinal fluid (CSF) of a patient with cysticercosis encephalitis, thus demonstrating for the first time, the presence of anti-cysticercal antibodies in CSF.1,7
In the following decades, many attempts to develop better diagnostic tests focused on indirect, antibody detection assays. Antibody detection does not distinguish active from inactive infections, and is not useful to monitor changes over short periods;8 however, its diagnostic efficacy is much higher than that of antigen detection assays.9,10 These indirect assays include indirect hemagglutination, immunoelectrophoresis, double immunediffusion, precipitation, indirect immunofluorescence, and skin reaction, among others,11–17 and are comprehensively described in Flisser et al.18 By 1971, Engvall and Perlmann described the enzyme-linked immunosorbent assay (ELISA) technique.19 The ELISA is a quite simple technique, is sensitive, quantitative, and can process many samples at the same time, thus it soon became the most frequently used antibody detection assay (ELISA-Ab). It was initially applied in 1978 for the diagnosis of NCC by Arambulo et al. in cases with high suspicion for NCC, reporting better sensitivity than the indirect hemagglutination, the test in use at that time.20 Coker-Vann et al. then applied the ELISA technique to detect T. solium antibodies in epidemiological studies.21 Many other laboratories adopted the ELISA-Ab with varied sensitivities according to the antigen and serum panels used. It was evident, however, that the ELISA performed better than the previous techniques. Initial assays used crude metacestode antigens.21–24 Better results were obtained using cystic fluid as the antigens, but not with membrane or scolex antigens.25
Unfortunately, a series of factors affected the diagnostic capacity of these early techniques: the sensitivity and specificity of each technique, the difficulties in defining appropriate reference sera sets, and the use of crude or minimally purified antigens, leading to non-specific reactions mainly with echinococcosis, schistosomiasis, angiostrongyliasis, sparganosis, and fasciolasis.22,26–29 Case definitions and reference serum batteries were greatly improved with the advent of computed tomography (CT) in 1977 and magnetic resonance imaging in 1986. Cases of NCC could then be differentiated in terms of number of lesions, stage, and location (intraparenchymal or extraparenchymal NCC), variables, which strongly influence the host’s humoral immune response.30
Antigen characterization became then the objective of researchers looking for improved serological tests. One of the more studied antigens was antigen B, described by Flisser et al. in 1980 as the antigen more frequently recognized by sera from NCC-infected patients,31 producing a strong antibody response.32–34 Use of antigen B in an antibody detecting ELISA in serum35 as well as in CSF, did not demonstrate much advantage over other antigen sources.36,37 Grogl et al. in 1985 characterized a series of 37 antigenic proteins from the total metacestode extract as suitable candidates for immunodiagnosis,38 using for the first time the EITB technique, originally used for immunodiagnosis of schistosomiasis.38,39 A series of other antigens were then purified using chromatographic techniques and were reported to perform with high sensitivity in cysticercosis immunodiagnosis.27,28,40–43
In 1989, the EITB (also known as western blot or immunoblot) using the LLGP fraction was developed and quickly became the assay of choice for serodiagnosis. The LLGP-EITB combines the specificity of using antigens previously purified by chromatography plus the resolution capacity of polyacrylamide gel electrophoresis with sodium dodecyl sulfate coupled with the sensitivity of enzyme-based immunodetection. Seven antigenic glycoproteins (GP) were isolated from a total metacestode homogenate and then purified using lentil-lectin chromatography, namely GP50, GP42-39, GP24, GP21, GP18, GP14, and GP13, where the numbers referred to their molecular mass in kilodaltons (Fig. 1). The presence of any one of the seven antibody bands defines a positive test, with an initial sensitivity and specificity reported to be 98 and 100%, respectively. No cross-reactions were found in 376 sera from 18 heterologous infections.44 Further comparative testing demonstrated the superiority of the LLGP-EITB over ELISA for the diagnosis of human10,45–48 and porcine cysticercosis.49
Figure 1.
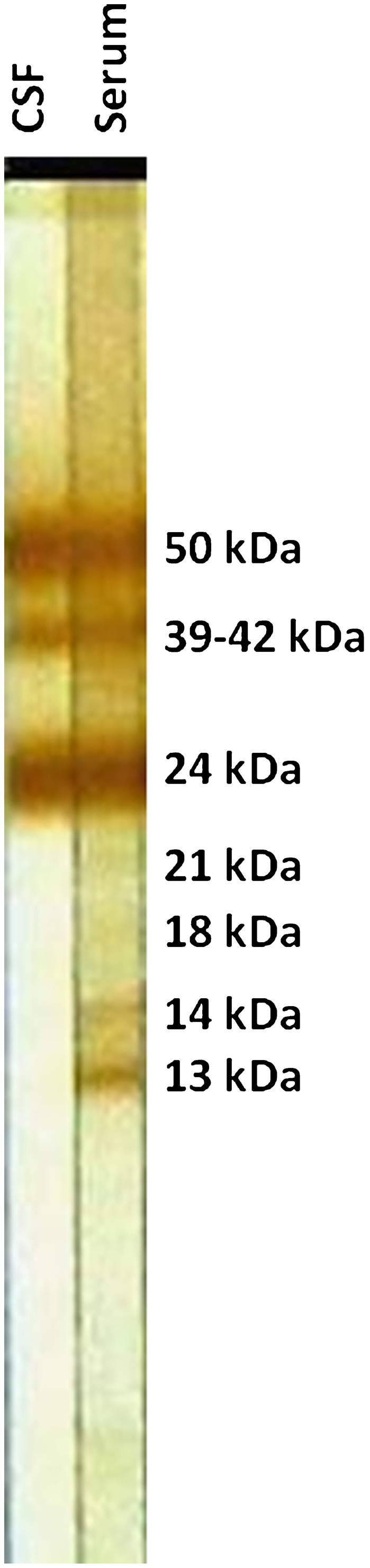
Lentil lectin glycoprotein enzyme-linked immunoelectrotransfer blot (LLGP-EITB) showing the molecular weights of the seven diagnostic bands, and the difference between antibody levels in paired cerebrospinal fluid (CSF) and serum samples from the same individual.
Antibody-detecting techniques in general do not have the capacity to distinguish between exposure, inactive infection and active infection, have a low positive predictive value in cases with viable cysticercosis (due to positive antibody reactions in individuals with calcified cysticerci and a high background of seroprevalence in the general population in endemic areas), and have low sensitivity in cases with a single brain lesion.50,51 Even though the LLGP-EITB is currently considered the test of choice for serodiagnosis of cysticercosis, it has its own drawbacks, which include the source of antigen (the method requires fresh cysts from infected pigs), and is also a complicated procedure. Thus, more recent research efforts have focused on the characterization and synthesis or production of recombinant forms of the seven LLGP diagnostic antigens to produce simpler and more reproducible assays.
Molecular studies showed that the seven LLGP diagnostic antigens comprise three protein families: GP50, T24/T42, and the 8 kDa family.52–54 GP50 is the largest of the LLGP antigens.53 Although no defined cross-reactions have been reported to any of the LLGPs, a ‘bogus’ band can appear slightly above GP50 and generates a problem of interpretation when reading the strips,44 even in patients without evidence of exposure to T. solium, thus its presence as a single reactive band should be taken with caution.55,56 GP50 and GP39–42 are the more immunodominant antigens, inducing vigorous IgG-response,57 both are membrane proteins. Studies have demonstrated that GP24 is a monomeric form and GP42–39 is a homodimeric form of the same protein.53,58 Both have already been produced as recombinants (rGP50 and rT24H) in an eukaryotic expression system, with good diagnostic performances in EITB and in ELISA,53,54,59 as well as in a novel proprietary technique, the QuickELISA.60 In general, rT24H performs slightly better than rGP50, but neither antigen alone reaches the sensitivity and specificity of the combined native LLGP set.
The remaining LLGPs, of lower molecular weights, correspond to a complex group of 8 kDa peptides, which can be found alone or in oligomeric structures, which have molecular masses as large as 42 kDa.61 Similar antigens have been reported in other taeniid cestodes, such as T. hydatigena, T. multiceps, and Echinococcus granulosus.62–65 These small peptides have been described as excretory/secretory (E/S) products and have been associated with immune evasion functions.61,66 In some cases, the immunogenic activity of synthetic and native forms correlate well and thus do not seem to depend on secondary structure resulting from post-translational modifications such as a glycosylation;67,68 in some cases, there seems to be a component of the immune response that depends on conformational epitopes.69 The presence of low molecular bands in the LLGP-EITB is rarely seen in absence of reactivity to the higher LLGP antigens and seems to be associated with more severe infections. The use of these smaller 8 kDa LLGP peptides as serodiagnostic tools has been proposed because of their capacity to discriminate between active and inactive infections,70,71 and their availability as synthetic peptide. TsRS1 and Ts18var1 are two peptides in this family, with reasonable sensitivity and specificity in ELISA, which greatly improve when used in EITB format. They however, show lower diagnostic utility when compared to the native forms to detect single lesion cases.68 Other diagnostic candidates in the 8 kDa family have been expressed and produced as recombinant proteins including Ts8B1, Ts8B2, and Ts8B3. Among these, the Ts8B2 antigen was better able to discriminate between cases of active and inactive NCC,72 although some cross-reactions with echinococcosis and schistosomiasis were observed.73 Splitting the Ts8B2 in smaller synthetic peptides greatly affected the antigen performance.69 Yang et al. described a 10 kDa antigen from cyst fluid, also belonging to the 8 kDa family, reacting mainly to IgG4 and IgG1.74 This 10 kDa antigen was also produced in recombinant form and showed a good performance to differentiate active from inactive NCC.71
Other native antigens under research include parasite proteases. In 2005, Baig et al. described a protease from the T. solium metacestode with L-cathepsin activity and able to degrade IgG (suggesting a role in immune evasion).75 A second similar protease was also identified by a different group soon after. This protease, produced in recombinant form, had antigenic activity recognized by sera from patients with NCC.76 Two other protease fractions highly abundant in cystic fluid have been isolated and evaluated in ELISA and EITB with promising results, and in dot blot form, with lower sensitivity, albeit higher specificity.77,78
Synthetic peptide production is appealing for its ease of production and inherent reproducibility. However, to date no synthetic peptide has performed at the level of native antigens for diagnostic purposes.61,67,79–81 A possible alternative is to use more than one synthetic peptide in the same assay,82 as in the multiantigen print immunoassay, in which several recombinant or synthetic antigens are printed at different positions along a single strip and thus obviating the need for electrophoretic separation.83 Recombinant proteins perform better than synthetic peptides, most likely because part of the response is directed towards conformational epitopes.69 Recombinants to several of the seven LLGP described by Tsang et al., have shown better results than related synthetic peptides in EITB as well as in ELISA.53,54,60,84 A recently reported recombinant protein, Tsolp27, promisingly detected all cases, but specimens from only 13 cases were tested (Table 1).85
Table 1. Antigens used to diagnose NCC by antibody capture.
| Test | Type of antigen | Sensitivity (1 lesion) | Sensitivity (>2 lesions) | Specificity | Cross-reactions | CSF better than serum | References |
| LLGP-EITB | Native semi-purified from whole cyst | ∼50% | 98% | 100% | No | No | [10,44,48,50,51,109,179] |
| rGP50-EITB | Recombinant from 50 kDa | N/E | 90%* | 100% | N/E | N/E | [53] |
| rGP50-ELISA | Recombinant from 50 kDa | N/E | 95%* | 94%* | No | Yes | [59] |
| rGP50-QuickELISA | Recombinant from 50 kDa | 42%* | 94%* | 99%* | No | N/E | [60] |
| rGP50-MAPIA | Recombinant from 50 kDa | 53% | 93% | 100% | No | N/E | [83] |
| rT24-EITB | Recombinant from 24 kDa | 63%* | 94%* | 98% | No | N/E | [54] |
| rT24-QuickELISA | Recombinant from 24 kDa | 47%* | 96%* | 99%* | No | N/E | [60] |
| rT24-MAPIA | Recombinant from 24 kDa | 60% | 97% | 99% | S. mansoni | N/E | [83] |
| rT24-MICT | Recombinant from 24 kDa | N/E | 94% | 99% | No | N/E | [180] |
| TsRS1-EITB | Synthetic from 8 kDa | 85%* | 96%* | 98% | N/E | N/E | [68] |
| TsRS1-ELISA | Synthetic from 8 kDa | 50%* | 77%* | 93% | N/E | N/E | [68] |
| TsRS1-FASTELISA | Synthetic from 8 kDa | N/E | 100%* | 100% | N/E | N/E | [61] |
| Ts18var1-EITB | Synthetic from 8 kDa | 35%* | 97%* | 100% | N/E | N/E | [68] |
| Ts18var1-ELISA | Synthetic from 8 kDa | 44%* | 95%* | 85% | N/E | N/E | [68] |
| Ts18var1-ELISA | Synthetic from 8 kDa | N/E | 90%* | 90%* | No | Yes | [59] |
| Ts18var1-FASTELISA | Synthetic from 8 kDa | N/E | 97%* | 100% | N/E | N/E | [61] |
| Ts18var1-QuickELISA | Synthetic from 8 kDa | 16%* | 90%* | 96%* | No | N/E | [60] |
| Ts8B2-ELISA | Recombinant from 8 kDa | N/E | 97% | 93% | Echinoccocosis Schistosomiasis | No | [72,73] |
| 10 kDa-EITB | Native 10 kDa from fluid cyst | N/E | 85%* | N/E | Echinoccocosis | N/E | [74] |
| 10 kDa-EITB | Recombinant from 10 kDa | N/E | 97%* | 98% | Echinoccocosis | N/E | [71] |
| 53/25 kDa EITB | Cathepsine L-like 53/25 kDa | 78%* | 96%* | 98%* | Echinococcosis | N/E | [77] |
| 53/25 kDa ELISA | Cathepsine L-like 53/25 kDa | 84%* | 98%* | 93%* | Echinococcosis T. saginata | N/E | [77] |
| 53/25 kDa dotELISA | Cathepsine L-like 53/25 kDa | 29.4%* | 84.8%* | 100%* | Echinococcosis | N/E | [78] |
Notes: NCC, neurocysticercosis; LLGP-EITB, lentil lectin glycoprotein enzyme-linked immunoelectrotransfer blot; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; MAPIA, multiantigen print immunoassay; MICT, Magnetic Immuno-Chromatographic Test; N/E, not evaluated.
*Calculated using sera with previously positive serology.
Other attempts to develop immunodiagnostic tests include the lymphocyte transformation test (LTT) described by Prasad et al., in 2008, who found 94% of sensitivity and 96% of specificity. This assay requires lymphocyte separation, long incubation time, and a radioactive developer. The authors proposed their use in patients with a single brain lesion but further evaluations are still missing.86 LTT seems to offer a good alternative to evaluate a host’s exposure to a given antigen but will likely not differentiate active from inactive cases because the assay is based on the presence of memory T cells.87
Phage display peptide selection was reported as early as in 1999.88 Almost 10 years later, Hell et al. produced a synthetic peptide against a scolex antigen with this technology.89 Initial promising results have been reported for two other peptides produced with this technique.90,91 As proposed by Esquivel-Velazquez et al., in 2011, new tools like phage display peptide selection, production of synthetic, and recombinant antigens, could permit us to shorten the path to identify specific antigens capable to distinguish not only the stages of the parasite, but also exposure from viable and non-viable infection.92 In this way, a good alternative to distinguish exposure from infection could be the use of oncospheral antigens, which to date have mostly been used as vaccine candidates. The 8 kDa antigens seem to be promising candidates to distinguish viable from non-viable NCC; however, the sensitivity of these assays needs improving.70,71,73,93
Advantages and disadvantages of antibody detection in cysticercosis
Sound use of serological assays goes beyond the choice of a test and greatly depends on appropriate interpretation of results in the context of a given patient or a given population. Antibody detection with a sensitive and specific assay is the best alternative to diagnose whether somebody has been infected with cysticerci.9,10 Antibody detection however does not discriminate between active and inactive infections and thus its clinical utility is restricted to etiological confirmation (although strong antibody reactions suggest severe infections94–96 and, unlike total IgG,8 IgG4 detection can be associated with active infection97,98 as well as provide a good monitoring marker99,100 for cure).
In field conditions, antibody seroprevalence overestimates the actual prevalence of infection because persons with antibodies from exposure and from past infections are also detected. Even more, there is evidence that almost 40% of the positive results in an endemic area are produced by transient antibodies, which become undetectable within 1 year.101 Detection of parasite specific antibodies in asymptomatic individuals has limited clinical use. Antibody prevalence, however, can provide valuable information on exposure to the parasite, transmission dynamics, risk factors, and incidence calculations, thus it should still be considered a tool for control programs.47,102–107
In summary, a positive antibody test associated with a suggestive brain image strongly supports the diagnosis of NCC, while in endemic regions where no CT or magnetic resonance imaging is available, a positive antibody test should be mainly used to refer patients with neurological symptoms to a more equipped center for imaging diagnosis and etiological case management.
Appropriate samples for antibody detection
Selection of a particular type of sample depends on the available test and antigen. In a study of Sahu et al., serum performed better than CSF when using E/S antigens but there were no differences when using somatic antigens.108 In general, the evidence suggests that with properly specific assays, serum performs better than CSF for antibody detection.10,46,48,50,109,110 Some authors note that the simultaneous use of both samples can provide important information on the infection status of the patient.111
Tests with less specificity and/or less purified antigens give better results with CSF due to the lower frequency of cross-reacting antibodies in CSF. However, a lumbar puncture to obtain CSF is, however, painful and invasive, and poorly accepted by patients. It is important to note that CSF taken from the ventricles (during surgery or through a shunt) or cisterns (cisternal puncture) can differ from samples collected by lumbar puncture in terms of protein concentration. A gradient along the neuroaxis has been described.112,113 Also, there may be intrathecally produced antigens in the CSF, particularly during antiparasitic treatment.114–119
Venipuncture is the preferred collection method for clinical laboratory studies; however, blood can be also collected by finger prick on filter paper. This procedure is quite advantageous for sample storage and transport and is quite well accepted by the population and can be of great use in field studies or whenever venipuncture is not possible. The LLGP-EITB works well in blood samples eluted from filter paper,120,121 with a high agreement with paired serum samples,122,123 although some antibody activity is lost along the process. Placing the piece of filter paper in a liquid buffer preserves the amount of recovered antibodies.124
Detection of antibodies in other biological fluids has not been extensively explored. Two reports showed low sensitivity in urine.125,126 Saliva gave promising results45,127 for diagnosis or even for IgG4 monitoring,100 but no further experiments have been reported. Tears have also been used for diagnosis of ophthalmic cysticercosis (IgA response) with 100% sensitivity and 92% specificity, although this was tested on only a few cases.128
Antigen Detection Tests for Cysticercosis
Direct immunodiagnosis (detection of products of the infective agent in the host) has the advantage of demonstrating active infection and in most cases, the antigen levels are associated with the infective burden and thus the severity of the infection, so this type of test can be used to determine therapeutic decisions and guide the prognosis of the patients. Cure is frequently associated with negative antigen results, and on the other hand, relapses, reinfections, or complications result in increases in circulating antigen levels. Unfortunately, in most cases, the sensitivity of antigen detection assays is inferior to that of indirect, antibody-detecting assays.
The initial reports on finding T. solium antigens in the CSF of patients with NCC used ELISA assays with rabbit polyclonal antisera raised against crude cysticercal extracts. Their results were promising, particularly in terms of specificity (likely resulting from the use of CSF instead of serum, as detailed above).129,130 As expected, circulating antigen cannot be demonstrated in the CSF of all NCC patients. Also, only a fraction of all antigens present in the cyst fluid can be detected in the patient’s CSF.131,132 Circulating antigen can also be detected in serum, as initially demonstrated for T. saginata cysticercosis in cattle133 and later in human samples.134
Monoclonal antibody (MoAb)-based antigen detection greatly improved the performance of these assays. The initial tests for T. solium antigen detection originated from assays developed against T. saginata and performed well thanks to an unexpected interspecies cross-reaction. In 1989, Harrison et al. developed a MoAb against a repetitive epitope from excretory/secretory glycoprotein products of the T. saginata metacestode, HP10. In an ELISA format, HP10 detected circulating antigen in cattle with 200 or more live cysts, with levels detectable in cattle serum as early as 4–5 weeks post-infection. No cross-reactions other than the above described with T. solium were reported.133 The sensitivity of the HP10 ELISA in CSF of confirmed NCC cases was 72%.36 A similar method was pursued by Brandt et al. in 1992. They found eight MoAbs of IgM isotype, which when used in combination, had a lower detection limit of 88 live cysts in infected cattle, and also were able to detect antigens as late as 5 weeks post-infection.134 These MoAbs were also directed against repetitive glycoprotein epitopes.135 Further studies generated IgG MoAbs, which improved the antigen assay performance, reaching 92% sensitivity and 98.7% specificity in sera from cysticercosis-infected cattle. They also showed that the target antigen was thermostable. Heat treatment of samples prior to testing gave better results, in particular fewer non-specific reactions (Fig. 2).136
Figure 2.
Antigen detection enzyme-linked immunosorbent assay (ELISA) using MoAbs B158 and B60 showing serial samples of different cases.
MoAbs against T. solium were first described in 1991.137 The initial report concludes that the antigen detection test performed well for diagnosis of T. solium cysticercosis, but was not 100% sensitive; the test worked better on CSF than on serum, and antigen levels dropped to undetectable levels after successful treatment.137 Another anti-T. solium MoAb targeted cyst fluid (1F11, IgG1 isotype) and had a diagnostic sensitivity of 82%, mainly missing cases with fewer lesions or only calcifications.138 This same group also developed MoAb 4F8. A 4F8-based ELISA was used by them to demonstrate that patients with subcutaneous nodules had higher levels of circulating antigen, likely because subcutaneous cysticercosis is found in patients with higher parasite burdens.139
Interesting and very promising tools are nanobodies (Nbs), single domain antibodies that are produced in immunized camelids. These molecules are, highly stable and soluble, devoid of light chains, and capable of binding to antigens with high affinity and specificity. Their small size (12–15 kDa) allows detection of hidden epitopes and expression in various microorganisms.140 Nbs directed against an 8 kDa antigen of the metacestode have been developed and proven specific for T. solium, without cross-reactions with T. hydatigena, T. saginata, T. crassiceps, and Trichinella spiralis (Table 2).141 Further work is needed to determine the utility of these reagents for antigen detection in T. solium cysticercosis.
Table 2. Antibodies used to diagnose NCC by antigen capture.
| Test | Type of antibody | Sensitivity (1 lesion) | Sensitivity (>2 lesions) | Specificity | Cross-reactions | CSF better than serum | References |
| HP10-ELISA | MoAbs against T. saginata larval antigens from fluid and tegument of cyst | N/E | 75%** | 100%** | T. solium | No | [36,133] |
| T. hydatigena | |||||||
| B158/B60-ELISA | MoAbs against T. saginata excretory/secretory larval antigens | N/E | N/E | N/E | T. solium | Yes | [10,134,136,181] |
| T. hydatigena |
Notes: NCC, neurocysticercosis; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; N/E, not evaluated.
**Calculated using only CSF.
Advantages and disadvantages of antigen detection in cysticercosis
Currently available antigen capture assays do not reach the same sensitivity and specificity as antibody-detecting assays, so they are not the best option for initial diagnosis of clinical cases. They, however, provide an exceptional tool for clinical management and follow-up of confirmed cases. As described above, the levels of circulating antigen correlate with the severity and type of NCC infection. In intraparenchymal brain cysticercosis, the higher the number of viable cysts, the higher the antigen level, both in humans142,143 and in pigs.144 In cases of extraparenchymal NCC, which carry a poorer prognosis, the levels of antigen are much higher, particularly in patients with basal subarachnoid NCC10,145–147 and also correlate with the volume of the lesions except when hydrocephalus is present. (unpublished data, the Cysticercosis Working Group in Peru 2012) Very high antigen levels should thus raise the suspicion of extraparenchymal NCC or massive intraparenchymal NCC. Since the levels of antigen drop quickly in cured NCC patients, serum antigen monitoring is of great help in the follow-up of clinical cases.142,145–149 Detectable levels of circulating antigen do not always correlate with neurological symptoms. A study in India followed 42 antigen-positive individuals for up to 5 years, and none of them developed neurological symptoms associated with NCC.150 It follows that the isolated finding of circulating antigen without neurological symptoms or compatible neuroimage should not be interpreted as an indication for therapeutic intervention.
The usefulness of population-based antigen detection surveys is yet unclear. From the available data, the expected proportion of asymptomatic individuals with viable brain cysticerci and thus positive antigen levels should be quite small. Its use would likely be more productive if focused in patients with compatible symptoms such as seizures or intracranial hypertension.151,152 In field studies, one would expect to find a higher prevalence of antibody-positive individuals than antigen-positive individuals, since antibody responses occur in non-viable infections and individuals with exposure only, and for an unknown time after resolution. However, population-based studies show discordant results. In a community in Mexico, 1% of all people were positive to circulating antigen versus 4.8% seropositive to antibodies.151 In Ecuador, 2.25% of 800 participants in a rural community were positive for antigen, while the antibody seroprevalence measured in a subset of 100 randomly selected samples was 40%.153 On the other hand, a study in South Africa found more antigen-positive than antibody-positive cases,154 and another study in Burundi found higher prevalence of antibodies in patients with epilepsy than in controls but no differences in the frequencies of antigen-positive cases between these groups.155
Appropriate samples for antigen detection
Compared to serum, CSF had better sensitivity (78% versus 68%) and specificity (73% versus 60%) for antigen detection, when MoAbs are used as capture antibodies.10,137 Assays using polyclonal rabbit antisera against specific purified antigens did not show differences in serum versus CSF.156 Also no advantage was demonstrated when using saliva.157
Urine samples are easy to collect, non-invasive and thus are easily provided by patients or villagers. Immunodiagnostic tests in urine have been developed for a variety of infectious diseases,158–161 including cysticercosis. The initial study found detectable antigen levels in five out of eight confirmed NCC cases, in an agglutination assay using rabbit antisera to a T. solium total metacestode extract.162 We have found 92% sensitivity in patients with two or more viable cysts, and 62.5% in single lesion cases using the MoAbs described by Van Kerckhoven et al.,136 as well as a strong correlation between serum and urine levels of antigen.163 Mwape et al.164 tested paired urine and serum samples collected in Zambia and Ecuador. A very high agreement (90%) was found between samples of the same individual, but lower specificity when urine samples were tested. The Zambian samples had more non-specific reactions. Urine can also be concentrated to take advantage of the large sample volumes, which can be obtained. Lyophilization avoids the need for further refrigeration or freezing and also results in a slight increase in sensitivity. It, however, also decreases specificity by 2–4%. Other concentration methods were less efficient.163
Single-Lesional NCC
A single brain-enhancing lesion is a very frequent presentation of NCC in the Indian subcontinent, where it is also a major contributor to seizures in younger patients.165–167 In Latin America, the proportion of NCC patients with a single-enhancing lesion varies from 3.5 to 34%,168 likely reflecting different definitions of a single-enhancing lesion. More importantly, other infections, tumors, vascular lesions, and other etiologies need to be considered in the differential diagnosis of single brain lesions,168–170 and thus serology could be of great help in saving unnecessary invasive procedures or treatments.171
In general, all immunodiagnostic tests show low sensitivity for the diagnosis of single-lesional NCC, even worse when the lesion has entered in an involutive, degenerative process of resolution following antiparasitic treatment or by natural evolution (‘single-enhancing lesion’ or single cysticercal granuloma’). A proportion of these cases could also correspond to early lesions, caused by cysticerci, that resolved soon after infection, before full establishment and thus not provoking a strong immune response.172 Most seronegative NCC cases in ELISA111 or in the LLGP-EITB48,50,51 correspond to cases with a single lesion, although the estimates of sensitivity of the assays in this type of NCC vary greatly. Prabhakaran et al. reported an increase in sensitivity following the use of urea to expose the tertiary structure of the antigenic glycoproteins, detecting 46% of previously negative cases in EITB.173
DNA-based Technology
The greatest contribution of DNA-based technology has been in the genotyping of the genus Taenia, which has served to determine the phylogeny and taxonomy of its species and to understand the level of genetic diversity in the genus.174 Another important contribution of molecular biology is the identification and production of antigenic molecules used as vaccines candidates or as candidates for serological tests.
Direct use of molecular techniques for NCC diagnosis was first reported in 2006 and demonstrated T. solium DNA in the CSF of 29 of 30 consecutive patients, using a PCR with primers against pTsol9, specific for T. solium.175 Another study, using primers against HDP2, based on a non-coding sequence of T. saginata, which cross-reacts with T. solium, also found parasite DNA in human CSF, as well as reported higher sensitivity by type of NCC (10/14 extraparenchymal NCC cases compared to 4/24 of intraparenchymal, degenerating NCC).176 A recent study compared antigen and antibody capture techniques with a pTsol9 PCR. In 150 CSFs of patients with different types of NCC, PCR had the best sensitivity, although its specificity was only 80% using negative controls from Mexico. Unexpectedly, 28/31 patients with only calcified NCC were PCR-positive (compared to 19 antibody-positive and seven antigen-positive by EITB and ELISA-HP10, respectively).177 Only one study using PCR in porcine cysticercosis has been published, with poor performance: sensitivities of 23% and 32% in heavily infected animals, which improved to 64% using a nested PCR. On the other hand, these PCR assays were 100% specific.178
Conclusion
A variety of laboratory methods are available to support the diagnosis of NCC. The LLGP-EITB remains the optimal assay for clinical diagnosis, while antigen detection is useful to monitor patients after anthelminthic treatment (Table 3). Despite initial reports, molecular methods have not yet proven useful to diagnose NCC in clinical settings. More research is necessary to evaluate their real potential.
Table 3. Challenges for new serological tests for NCC diagnosis.
| Test | Challenges |
| Antibody test | Identify antigens or epitopes to maintain high sensitivity and specificity compared to native antigens. |
| Increase the sensitivity for single brain lesion. | |
| Identify antigens capable to differentiate exposure from infection. | |
| Identify antigens capable to differentiate viable and non-viable cysticercosis. | |
| Produce these antigens or epitopes in recombinant or synthetic way to have an easier and reproducible source of antigen. | |
| Use these antigens or epitopes to develop a point of care test, to have a primary tool in field settings. | |
| Antigen test | Produce MoAbs or nanobodies against T. solium metacestodes in order to increase sensitivity and specificity. |
| Standardize a qualitative assay with a better reproducibility and repeatability. | |
| Produce a test capable of differentiating viable and non-viable cysticercosis, with a high PPV for extraparenchymal NCC. | |
| Assay needs to perform well in urine samples to avoid invasive and risky sampling methods. | |
| Develop a point of care test, to have a primary tool in field settings. |
Note: NCC, neurocysticercosis.
References
- 1.Moses A. Dos metodos biolojicos de diagnostico nas cisticercozes. Mem Inst Oswaldo Cruz. 1911;3:322–6. [Google Scholar]
- 2.Waterhouse R. Cysticercus cellulosae in the central nervous system: with an account of two cases. Q J Med. 1913;6:469–85. [Google Scholar]
- 3.Weinberg M. Recherche des anticorps specifiques dans la distomatose et la cysticercose. C R Soc Biol Paris. 1909;66:219–21. [Google Scholar]
- 4.Takayanagui OM, Jardim E. [Clinical aspects of neurocysticercosis: analysis of 500 cases]. Arq Neuro-psiquiatr. 1983;41:50–63. doi: 10.1590/s0004-282x1983000100004. [DOI] [PubMed] [Google Scholar]
- 5.Salinas P, Sandoval L, Rugiero E, Contreras MC. [Diagnosis of human neurocysticercosis by ELISA-IgG using a purified antigen]. Bol Chil Parasitol. 1996;51:85–90. [PubMed] [Google Scholar]
- 6.Bueno EC, Snege M, Vaz AJ, Leser PG. Serodiagnosis of human cysticercosis by using antigens from vesicular fluid of Taenia crassiceps cysticerci. Clin Diagn Lab Immunol. 2001;8:1140–4. doi: 10.1128/CDLI.8.6.1140-1144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teive HA, de Almeida SM, Werneck LC. The Brazilian contribution to the study of neurocysticercosis: Moses and Lange’s role in cerebrospinal fluid diagnosis. Arq Neuro-psiquiatr. 2006;64:534–7. doi: 10.1590/s0004-282x2006000300036. [DOI] [PubMed] [Google Scholar]
- 8.Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VC. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. Cysticercosis Working Group in Peru. J Infect Dis. 1997;175:486–9. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito A, Craig PS. Immunodiagnostic and molecular approaches for the detection of taeniid cestode infections. Trends Parasitol. 2003;19:377–81. doi: 10.1016/s1471-4922(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez S, Dorny P, Tsang VC, Pretell EJ, Brandt J, Lescano AG, et al. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. 2009;199:1345–52. doi: 10.1086/597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biagi F, Navarrete F, Pina A, Santiago AM, Tapia L. [Study of 3 serological reactions in the diagnosis of cysticercosis]. Rev Med Hosp Gen (Mex). 1961;24:501–8. [PubMed] [Google Scholar]
- 12.Biagi F, Tay J. A precipitation reaction for the diagnosis of cysticercosis. Am J Trop Med Hyg. 1958;7:63–5. doi: 10.4269/ajtmh.1958.7.63. [DOI] [PubMed] [Google Scholar]
- 13.Flisser A, Tarrab R, Willms K, Larralde C. [Immunoelectrophoresis and double immunodiffusion in the diagnosis of human cerebral cysticercosis]. Arch Invest Med. 1975;6:1–12. [PubMed] [Google Scholar]
- 14.Flisser A, Bulnes I, Diaz ML, Luna R, Woodhouse E, Beltran F, et al. [Seroepidemiologic study of human cysticerosis in the predominantly indigenous rural Indian population of the State of Chiapas]. Arch Invest Med. 1976;7:107–13. [PubMed] [Google Scholar]
- 15.Schoop G, Lamina J. [Immunological evidence of cysticercosis in the living animal. I. Allergic intracutaneous test]. Deutsch Tierarztl Wochenschr. 1970;77:156–61. [PubMed] [Google Scholar]
- 16.Schenone H, Aranda R, Concha L, Knierim F, Rojas A, Cofre H. [Survey on undiagnosed hydatidosis and cysticercosis by means of immunobiological reactions]. Bol Chil Parasitol. 1971;26:121–3. [PubMed] [Google Scholar]
- 17.Rydzewski AK, Chisholm ES, Kagan IG. Comparison of serologic tests for human cysticercosis by indirect hemagglutination, indirect immunofluorescent antibody, and agar gel precipitin tests. J Parasitol. 1975;61:154–5. [PubMed] [Google Scholar]
- 18.Flisser A, Perez-Montfort R, Larralde C. The immunology of human and animal cysticercosis: a review. Bull World Health Organ. 1979;57:839–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–4. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 20.Arambulo PV, 3rd, Walls KW, Bullock S, Kagan IG. Serodiagnosis of human cysticercosis by microplate enzyme-linked immunospecific assay (ELISA). Acta Trop. 1978;35:63–7. [PubMed] [Google Scholar]
- 21.Coker-Vann MR, Subianto DB, Brown P, Diwan AR, Desowitz R, Garruto RM, et al. ELISA antibodies to cysticerci of Taenia solium in human populations in New Guinea, Oceania, and Southeast Asia. Southeast Asian J Trop Med Public Health. 1981;12:499–505. [PubMed] [Google Scholar]
- 22.Diwan AR, Coker-Vann M, Brown P, Subianto DB, Yolken R, Desowitz R, et al. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibody to cysticerci of Taenia solium. Am J Trop Med Hyg. 1982;31364–9. [DOI] [PubMed] [Google Scholar]
- 23.Costa JM, Ferreira AW, Makino MM, Camargo ME. Spinal fluid immunoenzymatic assay (ELISA) for neurocysticercosis. Rev Inst Med Trop Sao Paulo. 1982;24:337–41. [PubMed] [Google Scholar]
- 24.Mohammad IN, Heiner DC, Miller BL, Goldberg MA, Kagan IG. Enzyme-linked immunosorbent assay for the diagnosis of cerebral cysticercosis. J Clin Microbiol. 1984;20:775–9. doi: 10.1128/jcm.20.4.775-779.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa JM. [Immunoenzymatic test (ELISA) in the diagnosis of neurocysticercosis: study of various antigenic extracts in the detection of IgG antibodies in serum and cerebrospinal fluid samples]. Arq Neuro-psiquiatr. 1986;44:15–31. doi: 10.1590/s0004-282x1986000100002. [DOI] [PubMed] [Google Scholar]
- 26.Schantz PM, Shanks D, Wilson M. Serologic cross-reactions with sera from patients with echinococcosis and cysticercosis. Am J Trop Med Hyg. 1980;29:609–12. doi: 10.4269/ajtmh.1980.29.609. [DOI] [PubMed] [Google Scholar]
- 27.Coker-Vann M, Brown P, Gajdusek DC. Serodiagnosis of human cysticercosis using a chromatofocused antigenic preparation of Taenia solium cysticerci in an enzyme-linked immunosorbent assay (ELISA). Trans R Soc Trop Med Hyg. 1984;78:492–6. doi: 10.1016/0035-9203(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 28.Gottstein B, Tsang VC, Schantz PM. Demonstration of species-specific and cross-reactive components of Taenia solium metacestode antigens. Am J Trop Med Hyg. 1986;35:308–13. doi: 10.4269/ajtmh.1986.35.308. [DOI] [PubMed] [Google Scholar]
- 29.Mendoza J, Molina JM, Rojas A, Leiva J, Sanchez JC, de la Rosa M. [Evaluation of 2 serological techniques in the diagnosis of neurocysticercosis: complement fixation reaction and Western blot]. Enferm Infecc Microbiol Clin. 1991;9:537–42. [PubMed] [Google Scholar]
- 30.Chavarria A, Fleury A, Garcia E, Marquez C, Fragoso G, Sciutto E. Relationship between the clinical heterogeneity of neurocysticercosis and the immune-inflammatory profiles. Clin Immunol. 2005;116:271–8. doi: 10.1016/j.clim.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Flisser A, Woodhouse E, Larralde C. Human cysticercosis: antigens, antibodies and non-responders. Clin Exp Immunol. 1980;39:27–37. [PMC free article] [PubMed] [Google Scholar]
- 32.Plancarte A, Flisser A, Larralde C. Fibronectin-like properties in antigen B from the cysticercus of Taenia solium. Cytobios. 1983;36:83–93. [PubMed] [Google Scholar]
- 33.Laclette JP, Merchant MT, Willms K. Histological and ultrastructural localization of antigen B in the metacestode of Taenia solium. J Parasitol. 1987;73:121–9. [PubMed] [Google Scholar]
- 34.Laclette JP, Landa A, Arcos L, Willms K, Davis AE, Shoemaker CB. Paramyosin is the Schistosoma mansoni (Trematoda) homologue of antigen B from Taenia solium (Cestoda). Mol Biochem Parasitol. 1991;44:287–95. doi: 10.1016/0166-6851(91)90015-x. [DOI] [PubMed] [Google Scholar]
- 35.Espinoza B, Ruiz-Palacios G, Tovar A, Sandoval MA, Plancarte A, Flisser A. Characterization by enzyme-linked immunosorbent assay of the humoral immune response in patients with neurocysticercosis and its application in immunodiagnosis. J Clin Microbiol. 1986;24:536–41. doi: 10.1128/jcm.24.4.536-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correa D, Sandoval MA, Harrison LJ, Parkhouse RM, Plancarte A, Meza-Lucas A, et al. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg. 1989;83:814–6. doi: 10.1016/0035-9203(89)90340-4. [DOI] [PubMed] [Google Scholar]
- 37.Das S, Mahajan RC, Ganguly NK, Sawhney IM, Dhawan V, Malla N. Detection of antigen B of Cysticercus cellulosae in cerebrospinal fluid for the diagnosis of human neurocysticercosis. Trop Med Int Health. 2002;7:53–8. doi: 10.1046/j.1365-3156.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- 38.Grogl M, Estrada JJ, MacDonald G, Kuhn RE. Antigen-antibody analyses in neurocysticercosis. J Parasitol. 1985;71:433–42. [PubMed] [Google Scholar]
- 39.Tsang VC, Peralta JM, Simons AR. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–91. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- 40.Kim SI, Kang SY, Cho SY, Hwang ES, Cha CY. Purification of cystic fluid antigen of Taenia solium metacestodes by affinity chromatography using monoclonal antibody and its antigenic characterization. Kisaengch'unghak Chapchi. 1986;24:145–8. doi: 10.3347/kjp.1986.24.2.145. [DOI] [PubMed] [Google Scholar]
- 41.Kumar D, Gaur SN. Serodiagnosis of porcine cysticercosis by enzyme-linked immunosorbent assay (ELISA) using fractionated antigens. Vet Parasitol. 1987;24:195–202. doi: 10.1016/0304-4017(87)90040-9. [DOI] [PubMed] [Google Scholar]
- 42.Rossi CL. [Evaluation of antigenic fractions of Cysticercus cellulosae for the immunodiagnosis of neurocysticercosis using antibody-lectin conjugates]. Arq Neuro-psiquiatr. 1989;47:287–90. doi: 10.1590/s0004-282x1989000300006. [DOI] [PubMed] [Google Scholar]
- 43.da Silva AD, Quagliato EM, Rossi CL. A quantitative enzyme-linked immunosorbent assay (ELISA) for the immunodiagnosis of neurocysticercosis using a purified fraction from Taenia solium cysticerci. Diagn Microbiol Infect Dis. 2000;37:87–92. doi: 10.1016/s0732-8893(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 44.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. 1989;159:50–9. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 45.Feldman M, Plancarte A, Sandoval M, Wilson M, Flisser A. Comparison of two assays (EIA and EITB) and two samples (saliva and serum) for the diagnosis of neurocysticercosis. Trans R Soc Trop Med Hyg. 1990;84:559–62. doi: 10.1016/0035-9203(90)90040-l. [DOI] [PubMed] [Google Scholar]
- 46.Diaz JF, Verastegui M, Gilman RH, Tsang VC, Pilcher JB, Gallo C, et al. Immunodiagnosis of human cysticercosis (Taenia solium): a field comparison of an antibody-enzyme-linked immunosorbent assay (ELISA), an antigen-ELISA, and an enzyme-linked immunoelectrotransfer blot (EITB) assay in Peru. The Cysticercosis Working Group in Peru (CWG). Am J Trop Med Hyg. 1992;46:610–5. doi: 10.4269/ajtmh.1992.46.610. [DOI] [PubMed] [Google Scholar]
- 47.Schantz PM, Sarti E, Plancarte A, Wilson M, Criales JL, Roberts J, et al. Community-based epidemiological investigations of cysticercosis due to Taenia solium: comparison of serological screening tests and clinical findings in two populations in Mexico. Clin Infect Dis. 1994;18:879–85. doi: 10.1093/clinids/18.6.879. [DOI] [PubMed] [Google Scholar]
- 48.Proano-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, Garcia-Jeronimo RC, Correa D. Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol. 2002;40:2115–8. doi: 10.1128/JCM.40.6.2115-2118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez AE, Cama V, Gilman RH, Tsang VC, Pilcher JB, Chavera A, et al. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg. 1990;43:194–9. doi: 10.4269/ajtmh.1990.43.194. [DOI] [PubMed] [Google Scholar]
- 50.Wilson M, Bryan RT, Fried JA, Ware DA, Schantz PM, Pilcher JB, et al. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164:1007–9. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]
- 51.Singh G, Kaushal V, Ram S, Kaushal RK, Dhanuka AK, Khurana S. Cysticercus immunoblot assay in patients with single, small enhancing lesions and multilesional neurocysticercosis. J Assoc Phys India. 1999;47:476–9. [PubMed] [Google Scholar]
- 52.Greene RM, Wilkins PP, Tsang VC. Diagnostic glycoproteins of Taenia solium cysts share homologous 14- and 18-kDa subunits. Mol Biochem Parasitol. 1999;99:257–61. doi: 10.1016/s0166-6851(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 53.Hancock K, Pattabhi S, Greene RM, Yushak ML, Williams F, Khan A, et al. Characterization and cloning of GP50, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol. 2004;133:115–24. doi: 10.1016/j.molbiopara.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, et al. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol. 2006;147:109–17. doi: 10.1016/j.molbiopara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Kojic EM, White AC., Jr A positive enzyme-linked immunoelectrotransfer blot assay result for a patient without evidence of cysticercosis. Clin Infect Dis. 2003;36:e7–9. doi: 10.1086/344445. [DOI] [PubMed] [Google Scholar]
- 56.Furrows SJ, McCroddan J, Bligh WJ, Chiodini P. Lack of specificity of a single positive 50-kDa band in the electroimmunotransfer blot (EITB) assay for cysticercosis. Clin Microbiol Infect. 2006;12:459–62. doi: 10.1111/j.1469-0691.2006.01381.x. [DOI] [PubMed] [Google Scholar]
- 57.Tsang VC, Pilcher JA, Zhou W, Boyer AE, Kamango-Sollo EI, Rhoads ML, et al. Efficacy of the immunoblot assay for cysticercosis in pigs and modulated expression of distinct IgM/IgG activities to Taenia solium antigens in experimental infections. Vet Immunol Immunopathol. 1991;29:69–78. doi: 10.1016/0165-2427(91)90053-f. [DOI] [PubMed] [Google Scholar]
- 58.Plancarte A, Hirota C, Martinez-Ocana J, Mendoza-Hernandez G, Zenteno E, Flisser A. Characterization of GP39-42 and GP24 antigens from Taenia solium cysticerci and of their antigenic GP10 subunit. Parasitol Res. 1999;85:680–4. doi: 10.1007/s004360050615. [DOI] [PubMed] [Google Scholar]
- 59.Bueno EC, Scheel CM, Vaz AJ, Machado LR, Livramento JA, Takayanagui OM, et al. Application of synthetic 8-kD and recombinant GP50 antigens in the diagnosis of neurocysticercosis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2005;72:278–83. [PubMed] [Google Scholar]
- 60.Lee YM, Handali S, Hancock K, Pattabhi S, Kovalenko VA, Levin A, et al. Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in QuickELISA. Am J Trop Med Hyg. 2011;84:587–93. doi: 10.4269/ajtmh.2011.10-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hancock K, Khan A, Williams FB, Yushak ML, Pattabhi S, Noh J, et al. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J Clin Microbiol. 2003;41:2577–86. doi: 10.1128/JCM.41.6.2577-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maddison SE, Slemenda SB, Schantz PM, Fried JA, Wilson M, Tsang VC. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDA. Am J Trop Med Hyg. 1989;40:377–83. doi: 10.4269/ajtmh.1989.40.377. [DOI] [PubMed] [Google Scholar]
- 63.Poretti D, Felleisen E, Grimm F, Pfister M, Teuscher F, Zuercher C, et al. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am J Trop Med Hyg. 1999;60:193–8. doi: 10.4269/ajtmh.1999.60.193. [DOI] [PubMed] [Google Scholar]
- 64.Monteiro KM, Scapin SM, Navarro MV, Zanchin NI, Cardoso MB, da Silveira NP, et al. Self-assembly and structural characterization of Echinococcus granulosus antigen B recombinant subunit oligomers. Biochim Biophys Acta. 2007;1774:278–85. doi: 10.1016/j.bbapap.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Jia WZ, Yan HB, Lou ZZ, Ni XW, Liu HX, Li HM, et al. Genetic variation of the 8-kDa glycoprotein family from Echinococcus granulosus, Taenia multiceps and Taenia hydatigena. Chin Med J. 2011;124:2849–56. [PubMed] [Google Scholar]
- 66.Rigano R, Profumo E, Bruschi F, Carulli G, Azzara A, Ioppolo S, et al. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect Immun. 2001;69:288–96. doi: 10.1128/IAI.69.1.288-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greene RM, Hancock K, Wilkins PP, Tsang VC. Taenia solium: molecular cloning and serologic evaluation of 14- and 18-kDa related, diagnostic antigens. J Parasitol. 2000;86:1001–7. doi: 10.1645/0022-3395(2000)086[1001:TSMCAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 68.Scheel CM, Khan A, Hancock K, Garcia HH, Gonzalez AE, Gilman RH, et al. Serodiagnosis of neurocysticercosis using synthetic 8-kD proteins: comparison of assay formats. Am J Trop Med Hyg. 2005;73:771–6. [PubMed] [Google Scholar]
- 69.Ferrer E, Sanchez J, Milano A, Alvarez S, La Rosa R, Lares M, et al. Diagnostic epitope variability within Taenia solium 8kDa antigen family: implications for cysticercosis immunodetection. Exp Parasitol. 2012;130:78–85. doi: 10.1016/j.exppara.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Simac C, Michel P, Andriantsimahavandy A, Esterre P, Michault A. [Value of immunodiagnosis by ELISA and Western blotting for the diagnostic and therapeutic management of neurocysticercosis]. Arch Inst Pasteur Madagascar. 1994;61:21–7. [PubMed] [Google Scholar]
- 71.Chung JY, Bahk YY, Huh S, Kang SY, Kong Y, Cho SY. A recombinant 10-kDa protein of Taenia solium metacestodes specific to active neurocysticercosis. J Infect Dise. 1999;180:1307–15. doi: 10.1086/315020. [DOI] [PubMed] [Google Scholar]
- 72.Ferrer E, Bonay P, Foster-Cuevas M, Gonzalez LM, Davila I, Cortez MM, et al. Molecular cloning and characterisation of Ts8B1, Ts8B2 and Ts8B3, three new members of the Taenia solium metacestode 8 kDa diagnostic antigen family. Mol Biochem Parasitol. 2007;152:90–100. doi: 10.1016/j.molbiopara.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Ferrer E, Martinez-Escribano JA, Barderas ME, Gonzalez LM, Cortez MM, Davila I, et al. Peptide epitopes of the Taenia solium antigen Ts8B2 are immunodominant in human and porcine cysticercosis. Mol Biochem Parasitol. 2009;168:168–71. doi: 10.1016/j.molbiopara.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Yang HJ, Chung JY, Yun D, Kong Y, Ito A, Ma L, et al. Immunoblot analysis of a 10 kDa antigen in cyst fluid of Taenia solium metacestodes. Parasite Immunol. 1998;20:483–8. doi: 10.1046/j.1365-3024.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- 75.Baig S, Damian RT, Molinari JL, Tato P, Morales-Montor J, Welch M, et al. Purification and characterization of a metacestode cysteine proteinase from Taenia solium involved in the breakdown of human IgG. Parasitology. 2005;131(Pt 3):411–6. doi: 10.1017/s0031182005007821. [DOI] [PubMed] [Google Scholar]
- 76.Li AH, Moon SU, Park YK, Na BK, Hwang MG, Oh CM, et al. Identification and characterization of a cathepsin L-like cysteine protease from Taenia solium metacestode. Vet Parasitol. 2006;141:251–9. doi: 10.1016/j.vetpar.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Zimic M, Pajuelo M, Rueda D, Lopez C, Arana Y, Castillo Y, et al. Utility of a protein fraction with cathepsin L-like activity purified from cysticercus fluid of Taenia solium in the diagnosis of human cysticercosis. Am J Trop Med Hyg. 2009;80:964–70. [PMC free article] [PubMed] [Google Scholar]
- 78.Pina R, Gutierrez AH, Gilman RH, Rueda D, Sifuentes C, Flores M, et al. A dot-ELISA using a partially purified cathepsin-L-like protein fraction from Taenia solium cysticerci, for the diagnosis of human neurocysticercosis. Ann Trop Med Parasitol. 2011;105:311–8. doi: 10.1179/136485911X12987676649782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hernandez M, Beltran C, Garcia E, Fragoso G, Gevorkian G, Fleury A, et al. Cysticercosis: towards the design of a diagnostic kit based on synthetic peptides. Immunol Lett. 2000;71:13–7. doi: 10.1016/s0165-2478(99)00166-2. [DOI] [PubMed] [Google Scholar]
- 80.Fleury A, Beltran C, Ferrer E, Garate T, Harrison LJ, Parkhouse RM, et al. Application of synthetic peptides to the diagnosis of neurocysticercosis. Trop Med Int Health. 2003;8:1124–30. doi: 10.1046/j.1360-2276.2003.01132.x. [DOI] [PubMed] [Google Scholar]
- 81.Ferrer E, Cortez MM, Cabrera Z, Rojas G, Davila I, Alarcon de Noya B, et al. Oncospheral peptide-based ELISAs as potential seroepidemiological tools for Taenia soliumcysticercosis/neurocysticercosis in Venezuela. Trans R Soc Trop Med Hyg. 2005;99:568–76. doi: 10.1016/j.trstmh.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Noya O, Patarroyo ME, Guzman F, Alarcon de Noya B. Immunodiagnosis of parasitic diseases with synthetic peptides. Curr Protein Peptide Sci. 2003;4:299–308. doi: 10.2174/1389203033487153. [DOI] [PubMed] [Google Scholar]
- 83.Handali S, Klarman M, Gaspard AN, Noh J, Lee YM, Rodriguez S, et al. Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin Vaccine Immunol. 2010;17:68–72. doi: 10.1128/CVI.00339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Handali S, Pattabhi S, Lee YM, Silva-Ibanez M, Kovalenko VA, Levin AE, et al. Development and evaluation of porcine cysticercosis QuickELISA in Triturus EIA analyzer. J Immunoassay Immunochem. 2010;31:60–70. doi: 10.1080/15321810903405068. [DOI] [PubMed] [Google Scholar]
- 85.Salazar-Anton F, Lindh J. Taenia solium: a two-dimensional Western blotting method combined with the use of an EST-library for the identification of immunogenic proteins recognized by sera from neurocysticercosis patients. Exp Parasitol. 2011;128:371–6. doi: 10.1016/j.exppara.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Prasad A, Prasad KN, Yadav A, Gupta RK, Pradhan S, Jha S, et al. Lymphocyte transformation test: a new method for diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis. 2008;61:198–202. doi: 10.1016/j.diagmicrobio.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 87.Amit P, Prasad KN, Kumar GR, Shweta T, Sanjeev J, Kumar PV, et al. Immune response to different fractions of Taenia solium cyst fluid antigens in patients with neurocysticercosis. Exp Parasitol. 2011;127:687–92. doi: 10.1016/j.exppara.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Manoutcharian K, Sotelo J, Garcia E, Cano A, Gevorkian G. Characterization of cerebrospinal fluid antibody specificities in neurocysticercosis using phage display peptide library. Clin Immunol. 1999;91:117–21. doi: 10.1006/clim.1998.4669. [DOI] [PubMed] [Google Scholar]
- 89.Hell RC, Amim P, de Andrade HM, de Avila RA, Felicori L, Oliveira AG, et al. Immunodiagnosis of human neurocysticercosis using a synthetic peptide selected by phage-display. Clin Immunol. 2009;131:129–38. doi: 10.1016/j.clim.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 90.da Silva Ribeiro V, Manhani MN, Cardoso R, Vieira CU, Goulart LR, Costa-Cruz JM. Selection of high affinity peptide ligands for detection of circulating antibodies in neurocysticercosis. Immunol Lett. 2010;129:94–9. doi: 10.1016/j.imlet.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Manhani MN, Ribeiro VS, Cardoso R, Ueira-Vieira C, Goulart LR, Costa-Cruz JM. Specific phage-displayed peptides discriminate different forms of neurocysticercosis by antibody detection in the serum samples. Parasite Immunol. 2011;33:322–9. doi: 10.1111/j.1365-3024.2011.01283.x. [DOI] [PubMed] [Google Scholar]
- 92.Esquivel-Velazquez M, Ostoa-Saloma P, Morales-Montor J, Hernandez-Bello R, Larralde C. Immunodiagnosis of neurocysticercosis: ways to focus on the challenge. J Biomed Biotechnol. 2011;2011:516042. doi: 10.1155/2011/516042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Handali S, Gonzalez AE, Hancock K, Garcia HH, Roberts JM, Gilman RH, et al. Porcine antibody responses to Taenia solium antigens rGp50 and sTs18var1. Am J Trop Med Hyg. 2004;71:322–6. [PubMed] [Google Scholar]
- 94.Corona T, Pascoe D, Gonzalez-Barranco D, Abad P, Landa L, Estanol B. Anticysticercous antibodies in serum and cerebrospinal fluid in patients with cerebral cysticercosis. J Neurol Neurosurg Psychiatry. 1986;49:1044–9. doi: 10.1136/jnnp.49.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michault A, Leroy D, Coubes P, Laporte JP, Bertil G, Mignard C. [Immunologic diagnosis of the cerebrospinal fluid and serum in developing brain cysticercosis]. Pathol Biol (Paris). 1989;37:249–53. [PubMed] [Google Scholar]
- 96.Zini D, Farrell VJ, Wadee AA. The relationship of antibody levels to the clinical spectrum of human neurocysticercosis. J Neurol Neurosurg Psychiatry. 1990;53:656–61. doi: 10.1136/jnnp.53.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Short JA, Heiner DC, Hsiao RL, Andersen FL. Immunoglobulin E and G4 antibodies in cysticercosis. J Clin Microbiol. 1990;28:1635–9. doi: 10.1128/jcm.28.7.1635-1639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Intapan PM, Khotsri P, Kanpittaya J, Chotmongkol V, Maleewong W, Morakote N. Evaluation of IgG4 and total IgG antibodies against cysticerci and peptide antigens for the diagnosis of human neurocysticercosis by ELISA. Asian Pac J Allergy Immunol. 2008;26:237–44. [PubMed] [Google Scholar]
- 99.Huang B, Li G, Jia F, Liu F, Ge L, Li W, et al. Determination of specific IgG4 for diagnosis and therapeutic evaluation of cerebral cysticercosis. Chin Med J. 2002;115:580–3. [PubMed] [Google Scholar]
- 100.Malla N, Kaur R, Ganguly NK, Sawhney IM, Mahajan RC. Utility of specific IgG4 response in saliva and serum samples for the diagnosis and follow up of human neurocysticercosis. Nepal Med Coll J. 2005;7:1–9. [PubMed] [Google Scholar]
- 101.Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, et al. Short report: transient antibody response in Taenia solium infection in field conditions-a major contributor to high seroprevalence. Am J Trop Med Hyg. 2001;65:31–2. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 102.Sarti-Gutierrez EJ, Schantz PM, Lara-Aguilera R, Gomez Dandoy H, Flisser A. Taenia solium taeniasis and cysticercosis in a Mexican village. Trop Med Parasitol. 1988;39:194–8. [PubMed] [Google Scholar]
- 103.Schantz PM, Sarti-Gutierrez E. Diagnostic methods and epidemiologic surveillance of Taenia solium infection. Acta Leiden. 1989;57:153–63. [PubMed] [Google Scholar]
- 104.Diaz Camacho SP, Candil Ruiz A, Suate Peraza V, Zazueta Ramos ML, Felix Medina M, Lozano R, et al. Epidemiologic study and control of Taenia solium infections with praziquantel in a rural village of Mexico. Am J Trop Med Hyg. 1991;45:522–31. doi: 10.4269/ajtmh.1991.45.522. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez AE, Gilman R, Garcia HH, McDonald J, Kacena K, Tsang VC, et al. Use of sentinel pigs to monitor environmental Taenia solium contamination. The Cysticercosis Working Group in Peru (CWG). Am J Trop Med Hyg. 1994;51:847–50. doi: 10.4269/ajtmh.1994.51.847. [DOI] [PubMed] [Google Scholar]
- 106.McManus DP. Improved diagnosis as an aid to better surveillance of Taenia solium cysticercosis, a potential public health threat to Papua New Guinea. Papua New Guinea Med J. 1995;38:287–94. [PubMed] [Google Scholar]
- 107.Rodriguez-Canul R, Allan JC, Dominguez JL, Villegas S, Cob L, Rodriguez RI, et al. Application of an immunoassay to determine risk factors associated with porcine cysticercosis in rural areas of Yucatan, Mexico. Vet Parasitol. 1998;79:165–80. doi: 10.1016/s0304-4017(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 108.Sahu PS, Parija SC, Narayan SK, Kumar D. Evaluation of an IgG-ELISA strategy using Taenia solium metacestode somatic and excretory-secretory antigens for diagnosis of neurocysticercosis revealing biological stage of the larvae. Acta Trop. 2009;110:38–45. doi: 10.1016/j.actatropica.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 109.Aguilar-Rebolledo F, Meza-Lucas A, Torres J, Cedillo-Rivera R, Enciso A, Garcia RC, et al. Evaluation of the enzyme-linked immunoelectrotransfer blot assay for diagnosis of neurocysticercosis in children. J Child Neurol. 2002;17:416–20. doi: 10.1177/088307380201700604. [DOI] [PubMed] [Google Scholar]
- 110.Barcelos IS, Ferreira MS, Moura LP, Biondi GF, Costa-Cruz JM. Use of the paired samples (cerebrospinal fluid and serum) in immunodiagnostic of active and inactive human neurocysticercosis. Mem Inst Oswaldo Cruz. 2005;100:427–9. doi: 10.1590/s0074-02762005000400014. [DOI] [PubMed] [Google Scholar]
- 111.Cho SY, Kim SI, Kang SY, Choi DY, Suk JS, Choi KS, et al. Evaluation of enzyme-linked immunosorbent assay in serological diagnosis of human neurocysticercosis using paired samples of serum and cerebrospinal fluid. Kisaengch'unghak Chapchi. 1986;24:25–41. doi: 10.3347/kjp.1986.24.1.25. [DOI] [PubMed] [Google Scholar]
- 112.Braga FM, dos Reis-Filho JB, de Camargo-Lima JG. [Ventriculo-lumbar gradient of concentration of total cerebrospinal fluid proteins: 1 - mechanisms of origin]. Arq Neuro-psiquiatr. 1983;41:254–65. doi: 10.1590/s0004-282x1983000300006. [DOI] [PubMed] [Google Scholar]
- 113.Torres-Corzo JG, Tapia-Perez JH, Sanchez-Aguilar M, Della Vecchia RR, Chalita Williams JC, Cerda-Gutierrez R. Comparison of cerebrospinal fluid obtained by ventricular endoscopy and by lumbar puncture in patients with hydrocephalus secondary to neurocysticercosis. Surg Neurol. 2009;71:376–9. doi: 10.1016/j.surneu.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Miller B, Goldberg MA, Heiner D, Myers A, Goldberg A. A new immunologic test for CNS cysticercosis. Neurology. 1984;34:695–7. doi: 10.1212/wnl.34.5.695. [DOI] [PubMed] [Google Scholar]
- 115.Miller BL, Staugaitis SM, Tourtellotte WW, Shapshak P, Goldberg M, Heiner D, et al. Intra-blood-brain barrier IgG synthesis in cerebral cysticercosis. Arch Neurol. 1985;42:782–4. doi: 10.1001/archneur.1985.04210090046013. [DOI] [PubMed] [Google Scholar]
- 116.Livramento JA. [A cerebrospinal fluid syndrome in neurocysticercosis]. Arq Neuro-psiquiatr. 1987;45:261–75. doi: 10.1590/s0004-282x1987000300006. [DOI] [PubMed] [Google Scholar]
- 117.Estanol B, Juarez H, Irigoyen Mdel C, Gonzalez-Barranco D, Corona T. Humoral immune response in patients with cerebral parenchymal cysticercosis treated with praziquantel. J Neurol Neurosurg Psychiatry. 1989;52:254–7. doi: 10.1136/jnnp.52.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Estanol Vidal B, Diaz Granados J, Corona Vazquez T. [Integrity of the blood-brain barrier and intrathecal synthesis of IgG in parenchymatous and subarachnoid cerebral cysticercosis]. Rev Invest Clin. 1989;41:327–30. [PubMed] [Google Scholar]
- 119.Machado LR, Livramento JA, Vaz AJ, Bueno EC, Mielli SR, Bastouly V, et al. IgG intrathecal synthesis and specific antibody index in patients with neurocysticercosis. Arq Neuro-psiquiatr. 2002;60:395–9. doi: 10.1590/s0004-282x2002000300011. [DOI] [PubMed] [Google Scholar]
- 120.Jafri HS, Torrico F, Noh JC, Bryan RT, Balderrama F, Pilcher JB, et al. Application of the enzyme-linked immunoelectrotransfer blot to filter paper blood spots to estimate seroprevalence of cysticercosis in Bolivia. Am J Trop Med Hyg. 1998;58:313–5. doi: 10.4269/ajtmh.1998.58.313. [DOI] [PubMed] [Google Scholar]
- 121.Fleury A, Bouteille B, Garcia E, Marquez C, Preux PM, Escobedo F, et al. Neurocysticercosis: validity of ELISA after storage of whole blood and cerebrospinal fluid on paper. Trop Med Int Health. 2001;6:688–93. doi: 10.1046/j.1365-3156.2001.00767.x. [DOI] [PubMed] [Google Scholar]
- 122.Wang LN, Ge LY, Miao F, Yu ZH, Liu YB, Zhen TM, et al. [Application of EITB in immunodiagnosis of cysticercosis]. Chin J Parasitol Parasitic Dis. 2004;22:98–100. [PubMed] [Google Scholar]
- 123.Ishida MM, Almeida MS, Espindola NM, Iha A, Pereira DA, Souza JG, et al. Seroepidemiological study of human cysticercosis with blood samples collected on filter paper, in Lages, State of Santa Catarina, Brazil, 2004–2005. Rev Soc Brasil Med Trop. 2011;44:339–43. doi: 10.1590/s0037-86822011005000040. [DOI] [PubMed] [Google Scholar]
- 124.Handali S, Rodriguez S, Noh J, Gonzalez AE, Garcia HH, Gilman RH, et al. A simple method for collecting measured whole blood with quantitative recovery of antibody activities for serological surveys. J Immunol Methods. 2007;320:164–71. doi: 10.1016/j.jim.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 125.Atluri SR, Singhi P, Khandelwal N, Malla N. Neurocysticercosis immunodiagnosis using Taenia solium cysticerci crude soluble extract, excretory secretory and lower molecular mass antigens in serum and urine samples of Indian children. Acta Trop. 2009;110:22–7. doi: 10.1016/j.actatropica.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 126.Atluri SR, Singhi P, Khandelwal N, Malla N. Evaluation of excretory secretory and 10–30 kDa antigens of Taenia solium Cysticerci by EITB assay for the diagnosis of neurocysticercosis. Parasite Immunol. 2009;31:151–5. doi: 10.1111/j.1365-3024.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 127.Acosta E. Antibodies to the metacestode of Taenia solium in the saliva from patients with neurocysticercosis. J Clin Lab Anal. 1990;4:90–4. doi: 10.1002/jcla.1860040204. [DOI] [PubMed] [Google Scholar]
- 128.Sahu PS, Parija SC, Sahu PK. Tear IgA-ELISA: a novel and sensitive method for diagnosis of ophthalmic cysticercosis. Acta Trop. 2008;106:168–74. doi: 10.1016/j.actatropica.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 129.Estrada JJ, Kuhn RE. Immunochemical detection of antigens of larval Taenia solium and anti-larval antibodies in the cerebrospinal fluid of patients with neurocysticercosis. J Neurol Sci. 1985;71:39–48. doi: 10.1016/0022-510x(85)90035-8. [DOI] [PubMed] [Google Scholar]
- 130.Tellez-Giron E, Ramos MC, Dufour L, Alvarez P, Montante M. Detection of Cysticercus cellulosae antigens in cerebrospinal fluid by dot enzyme-linked immunosorbent assay (Dot-ELISA) and standard ELISA. Am J Trop Med Hyg. 1987;37:169–73. doi: 10.4269/ajtmh.1987.37.169. [DOI] [PubMed] [Google Scholar]
- 131.Estrada JJ, Estrada JA, Kuhn RE. Identification of Taenia solium antigens in cerebrospinal fluid and larval antigens from patients with neurocysticercosis. Am J Trop Med Hyg. 1989;41:50–5. [PubMed] [Google Scholar]
- 132.Choromanski L, Estrada JJ, Kuhn RE. Detection of antigens of larval Taenia solium in the cerebrospinal fluid of patients with the use of HPLC and ELISA. J Parasitol. 1990;76:69–73. [PubMed] [Google Scholar]
- 133.Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989;11:351–70. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 134.Brandt JR, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–7. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 135.Draelants E, Brandt JR, Kumar V, Geerts S. Characterization of epitopes on excretory-secretory antigens of Taenia saginata metacestodes recognized by monoclonal antibodies with immunodiagnostic potential. Parasite Immunol. 1995;17:119–26. doi: 10.1111/j.1365-3024.1995.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 136.Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, Brandt J. Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol. 1998;76:269–74. doi: 10.1016/s0304-4017(97)00226-4. [DOI] [PubMed] [Google Scholar]
- 137.Chen JP, Zhang XY, Tan W, Liu MF, Liu GL, Hu YX. [Determination of circulating antigen in cysticercosis patients using McAb-based ELISA]. Chin J Parasitol Parasitic Dis. 1991;9:122–5. [PubMed] [Google Scholar]
- 138.Wang CY, Zhang HH, Ge LY. A MAb-based ELISA for detecting circulating antigen in CSF of patients with neurocysticercosis. Hybridoma. 1992;11:825–7. doi: 10.1089/hyb.1992.11.825. [DOI] [PubMed] [Google Scholar]
- 139.Wang CY, Li QS, Zhang HH, Su JJ, Zhang JJ, Li H, et al. [Detection of CAg in CSF of 231 cerebral cysticercosis patients]. Chin J Parasitol Parasitic Dis. 1993;11:276–8. [PubMed] [Google Scholar]
- 140.Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deckers N, Saerens D, Kanobana K, Conrath K, Victor B, Wernery U, et al. Nanobodies, a promising tool for species-specific diagnosis of Taenia solium cysticercosis. Int J Parasitol. 2009;39:625–33. doi: 10.1016/j.ijpara.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 142.Garcia HH, Parkhouse RM, Gilman RH, Montenegro T, Bernal T, Martinez SM, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2000;94:673–6. doi: 10.1016/s0035-9203(00)90228-1. [DOI] [PubMed] [Google Scholar]
- 143.Fleury A, Hernandez M, Fragoso G, Parkhouse RM, Harrison LJ, Sciutto E. Detection of secreted cysticercal antigen: a useful tool in the diagnosis of inflammatory neurocysticercosis. Trans R Soc Trop Med Hyg. 2003;97:542–6. doi: 10.1016/s0035-9203(03)80019-6. [DOI] [PubMed] [Google Scholar]
- 144.Deckers N, Kanobana K, Silva M, Gonzalez AE, Garcia HH, Gilman RH, et al. Serological responses in porcine cysticercosis: a link with the parasitological outcome of infection. Int J Parasitol. 2008;38:1191–8. doi: 10.1016/j.ijpara.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 145.Bobes RJ, Hernandez M, Marquez C, Fragoso G, Garcia E, Parkhouse RM, et al. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med Int Health. 2006;11:943–50. doi: 10.1111/j.1365-3156.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- 146.Fleury A, Hernandez M, Avila M, Cardenas G, Bobes RJ, Huerta M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78:970–4. doi: 10.1136/jnnp.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Garcia HH, Dorny P, Castillo Y, Pretell EJ, Rodriguez S, Mija L, et al. Circulating antigen levels follow post-treatment evolution of subarachnoid neurocysticercosis. J Neuroparasitol. 2010;1:1–3. [Google Scholar]
- 148.Garcia HH, Harrison LJ, Parkhouse RM, Montenegro T, Martinez SM, Tsang VC, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The Cysticercosis Working Group in Peru. Trans R Soc Trop Med Hyg. 1998;92:411–4. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nguekam, Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite (Paris, France). 2003;10:65–8. doi: 10.1051/parasite/2003101p65. [DOI] [PubMed] [Google Scholar]
- 150.Alexander AM, Prabhakaran V, Rajshekhar V, Muliyil J, Dorny P. Long-term clinical evaluation of asymptomatic subjects positive for circulating Taenia solium antigens. Trans R Soc Trop Med Hyg. 2010;104:809–10. doi: 10.1016/j.trstmh.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 151.Aranda-Alvarez JG, Tapia-Romero R, Alcantara-Anguiano I, Meza-Lucas A, Mata-Ruiz O, Celis-Quintal G, et al. Human cysticercosis: risk factors associated with circulating serum antigens in an open community of San Luis Potosi, Mexico. Ann Trop Med Parasitol. 1995;89:689–92. doi: 10.1080/00034983.1995.11813004. [DOI] [PubMed] [Google Scholar]
- 152.Correa D, Sarti E, Tapia-Romero R, Rico R, Alcantara-Anguiano I, Salgado A, et al. Antigens and antibodies in sera from human cases of epilepsy or taeniasis from an area of Mexico where Taenia solium cysticercosis is endemic. Ann Trop Med Parasitol. 1999;93:69–74. doi: 10.1080/00034989958816. [DOI] [PubMed] [Google Scholar]
- 153.Rodriguez-Hidalgo R, Benitez-Ortiz W, Praet N, Saa LR, Vercruysse J, Brandt J, et al. Taeniasis-cysticercosis in Southern Ecuador: assessment of infection status using multiple laboratory diagnostic tools. Mem Inst Oswaldo Cruz. 2006;101:779–82. doi: 10.1590/s0074-02762006000700012. [DOI] [PubMed] [Google Scholar]
- 154.Krecek RC, Michael LM, Schantz PM, Ntanjana L, Smith MF, Dorny P, et al. Prevalence of Taenia solium cysticercosis in swine from a community-based study in 21 villages of the Eastern Cape Province, South Africa. Vet Parasitol. 2008;154:38–47. doi: 10.1016/j.vetpar.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 155.Newell E, Vyungimana F, Geerts S, Van Kerckhoven I, Tsang VC, Engels D. Prevalence of cysticercosis in epileptics and members of their families in Burundi. Trans R Soc Trop Med Hyg. 1997;91:389–91. doi: 10.1016/s0035-9203(97)90251-0. [DOI] [PubMed] [Google Scholar]
- 156.Lin X, Li G, Huo H, Xu F, Li Q, Zhao Z. [Detection of circulating antigen in serum and cerebrospinal fluid for diagnosis of cerebral cysticercosis]. Chin J Parasitol Parasitic Dis. 1999;17:146–8. [PubMed] [Google Scholar]
- 157.Flisser A, Plancarte A, Correa D, Rodriguez-Del-Rosal E, Feldman M, Sandoval M, et al. New approaches in the diagnosis of Taenia solium cysticercosis and taeniasis. Ann Parasitol Hum Comp. 1990;65(Suppl 1):95–8. doi: 10.1051/parasite/1990651095. [DOI] [PubMed] [Google Scholar]
- 158.Freilij HL, Corral RS, Katzin AM, Grinstein S. Antigenuria in infants with acute and congenital Chagas’ disease. J Clin Microbiol. 1987;25:133–7. doi: 10.1128/jcm.25.1.133-137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zheng HJ, Tao ZH, Reddy MV, Harinath BC, Piessens WF. Parasite antigens in sera and urine of patients with bancroftian and brugian filariasis detected by sandwich ELISA with monoclonal antibodies. Am J Trop Med Hyg. 1987;36:554–60. doi: 10.4269/ajtmh.1987.36.554. [DOI] [PubMed] [Google Scholar]
- 160.Kremsner PG, de Jonge N, Simarro PP, Muhlschlegel F, Mir M, Sima FO, et al. Quantitative determination of circulating anodic and cathodic antigens in serum and urine of individuals infected with Schistosoma intercalatum. Trans R Soc Trop Med Hyg. 1993;87:167–9. doi: 10.1016/0035-9203(93)90474-5. [DOI] [PubMed] [Google Scholar]
- 161.Parija SC, Ravinder PT, Rao KS. Detection of hydatid antigen in urine by countercurrent immunoelectrophoresis. J Clin Microbiol. 1997;35:1571–4. doi: 10.1128/jcm.35.6.1571-1574.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Parija M, Biswas R, Harish BN, Parija SC. Detection of specific cysticercus antigen in the urine for diagnosis of neurocysticercosis. Acta Trop. 2004;92:253–60. doi: 10.1016/j.actatropica.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 163.Castillo Y, Rodriguez S, Garcia HH, Brandt J, Van Hul A, Silva M, et al. Urine antigen detection for the diagnosis of human neurocysticercosis. Am J Trop Med Hyg. 2009;80:379–83. [PubMed] [Google Scholar]
- 164.Mwape KE, Praet N, Benitez-Ortiz W, Muma JB, Zulu G, Celi-Erazo M, et al. Field evaluation of urine antigen detection for diagnosis of Taenia solium cysticercosis. Trans R Soc Trop Med Hyg. 2011;105:574–8. doi: 10.1016/j.trstmh.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 165.Rajshekhar V. Etiology and management of single small CT lesions in patients with seizures: understanding a controversy. Acta Neurol Scand. 1991;84:465–70. doi: 10.1111/j.1600-0404.1991.tb04996.x. [DOI] [PubMed] [Google Scholar]
- 166.Sethi PP, Wadia RS, Kiyawat DP, Ichaporia NR, Kothari SS, Sangle SA, et al. Ring or disc enhancing lesions in epilepsy in India. J Trop Med Hyg. 1994;97:347–53. [PubMed] [Google Scholar]
- 167.Kumar Garg R, Kumar Singh M, Misra S. Single-enhancing CT lesions in Indian patients with seizures: a review. Epil Res. 2000;38:91–104. doi: 10.1016/s0920-1211(99)00092-3. [DOI] [PubMed] [Google Scholar]
- 168.Del Brutto OH.Solitary cysticercus granuloma in Latin America Rajshekhar V, Chandy M J, editors. Solitary cysticercus granuloma: the disappearing lesionChennai: Orient Longman; 2000. p. 153–63. [Google Scholar]
- 169.Garg RK. Single enhancing computerized tomography-detected lesion in immunocompetent patients. Neurosurg Focus. 2002;12:e4. doi: 10.3171/foc.2002.12.6.5. [DOI] [PubMed] [Google Scholar]
- 170.Carod-Artal FJ. [Tropical causes of epilepsy]. Rev Neurol. 2009;49:475–82. [PubMed] [Google Scholar]
- 171.Rajshekhar V, Oommen A. Utility of the cysticercus immunoblot in a patient with an atypical solitary cerebral cysticercus granuloma. Neurol India. 2001;49:75–7. [PubMed] [Google Scholar]
- 172.Garcia HH, Gonzalez AE, Rodriguez S, Tsang VC, Pretell EJ, Gonzales I, et al. Neurocysticercosis: unraveling the nature of the single cysticercal granuloma. Neurology. 2010;75:654–8. doi: 10.1212/WNL.0b013e3181ed9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prabhakaran V, Rajshekhar V, Murrell KD, Oommen A. Conformation-sensitive immunoassays improve the serodiagnosis of solitary cysticercus granuloma in Indian patients. Trans R Soc Trop Med Hyg. 2007;101:570–7. doi: 10.1016/j.trstmh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 174.McManus DP.Molecular discrimination of taeniid cestodes. Parasitol Int. 200655Suppl:S31–7. [DOI] [PubMed] [Google Scholar]
- 175.Almeida CR, Ojopi EP, Nunes CM, Machado LR, Takayanagui OM, Livramento JA, et al. Taenia solium DNA is present in the cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur Arch Psychiatry Clin Neurosci. 2006;256:307–10. doi: 10.1007/s00406-006-0612-3. [DOI] [PubMed] [Google Scholar]
- 176.Hernandez M, Gonzalez LM, Fleury A, Saenz B, Parkhouse RM, Harrison LJ, et al. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol. 2008;102:317–23. doi: 10.1179/136485908X278856. [DOI] [PubMed] [Google Scholar]
- 177.Michelet L, Fleury A, Sciutto E, Kendjo E, Fragoso G, Paris L, et al. Human neurocysticercosis: comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol. 2011;49:195–200. doi: 10.1128/JCM.01554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramahefarisoa RM, Rakotondrazaka M, Jambou R, Carod JF. Comparison of ELISA and PCR assays for the diagnosis of porcine cysticercosis. Vet Parasitol. 2010;173:336–9. doi: 10.1016/j.vetpar.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 179.Praet N, Rodriguez-Hidalgo R, Speybroeck N, Ahounou S, Benitez-Ortiz W, Berkvens D, et al. Infection with versus exposure to Taenia solium: what do serological test results tell us? Am J Trop Med Hyg. 2010;83:413–5. doi: 10.4269/ajtmh.2010.10-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Handali S, Klarman M, Gaspard AN, Dong XF, Laborde R, Noh J, et al. Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin Vaccine Immunol. 2010;17:631–7. doi: 10.1128/CVI.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sato MO, Yamasaki H, Sako Y, Nakao M, Nakaya K, Plancarte A, et al. Evaluation of tongue inspection and serology for diagnosis of Taenia solium cysticercosis in swine: usefulness of ELISA using purified glycoproteins and recombinant antigen. Vet Parasitol. 2003;111:309–22. doi: 10.1016/s0304-4017(02)00383-7. [DOI] [PubMed] [Google Scholar]