Abstract
Diagnosis of neurocysticercosis (NCC) can be a challenge. Clinical manifestations are non-specific, most neuroimaging findings are non-pathognomonic, and some serologic tests have low sensitivity or specificity. A set of diagnostic criteria was proposed in 2001 to avoid the over diagnosis of NCC that occurs in epidemiologic surveys, and to help clinicians evaluating patients with suspected NCC. The set included four stratified categories of criteria, including: (1) absolute: histological demonstration of cysticerci, cystic lesions showing the scolex on neuroimaging studies, and direct visualization of subretinal parasites by fundoscopic examination; (2) major: lesions highly suggestive of NCC on neuroimaging studies, positive serum enzyme-linked immunoelectrotransfer blot (EITB) for the detection of anticysticercal antibodies, resolution of intracranial cystic lesions after cysticidal drug therapy, and spontaneous resolution of single enhancing lesions; (3) minor: lesions compatible with NCC on neuroimaging studies, suggestive clinical manifestations, positive cerebrospinal fluid (CSF) ELISA for detection of anticysticercal antibodies or cysticercal antigens, and cysticercosis outside the nervous system; and (4) epidemiological: evidence of a household contact with Taenia solium infection, individuals coming from or living in cysticercosis endemic areas, and history of travel to disease-endemic areas. Interpretation of these criteria permits two degrees of diagnostic certainty: (1) definitive diagnosis, in patients who have one absolute criterion or in those who have two major plus one minor and one epidemiological criteria; and (2) probable diagnosis, in patients who have one major plus two minor criteria, in those who have one major plus one minor and one epidemiological criteria, and in those who have three minor plus one epidemiological criteria. After 10 years of usage, this set has been proved useful in both, field studies, and hospital settings. Recent advances in neuroimaging and immune diagnostic methods have enhanced its accuracy for the diagnosis of NCC.
Keywords: Cysticercosis, Neurocysticercosis, Diagnosis
Introduction
Neurocysticercosis (NCC), a parasitic infection of the nervous system caused by the encysted larval stage of the tapeworm Taenia solium, is a major public health problem in the developing world, where it represents a major cause of acquired epilepsy.1 In these regions, prevalence rates of active epilepsy are twice of that reported from developed nations.2–5 In addition, recent evidence also indicate an increasing prevalence of NCC in developed countries, including the USA, Australia, Canada, and some Western European countries.6–11 While most NCC cases in the developing world occur in immigrants from disease-endemic areas, a sizable percentage of autochthonous cases has also been reported from these regions, creating some worry on the risks of further disease spread. The increasing number of NCC patients in non-endemic countries is of concern as physicians working these regions may not be familiar with the different aspects of NCC, which in turn, may cause diagnostic pitfalls.8,11
While NCC has a well-defined causal agent, its diagnosis could be a challenge, as histological demonstration of the parasite is not possible in most cases. Moreover, clinical manifestations are non-specific, neuroimaging findings are most often not pathognomonic, and immune diagnostic tests are faced with problems related to poor sensitivity or specificity. In 1996, it was published the first attempt to settle a set of diagnostic criteria for human cysticercosis, based on the objective evaluation of clinical, radiological, immunological, and epidemiological data of patients.12 After some years of experience, the same panel of investigators considered that set to be somewhat complex, as it was developed for both the diagnosis of patients with NCC as well as for those with systemic cysticercosis. With few exceptions, cysticercosis outside the central nervous system is not clinically relevant. Therefore, it was considered that a set of criteria exclusively devoted to the diagnosis of NCC would be more practical yet more comprehensible than the initial ones. The panel agreed upon the elaboration of more accurate and stringent revised criteria for the diagnosis of NCC.13 As in the 1996 publication,12 the revised criteria included four categories of diagnosis — absolute, major, minor, and epidemiological — stratified on the basis of their individual strength, where absolute criteria allowed unequivocal diagnosis of NCC, major criteria strongly suggested the diagnosis but could not be used alone to confirm the disease, minor criteria were frequent but non-specific manifestations of the disease, and epidemiological criteria referred to circumstantial evidence favoring the diagnosis. Interpretation of these criteria allowed two degrees of diagnostic certainty, definitive and probable, according to the likelihood that NCC is present in a given patient (Table 1). This set of diagnostic criteria was promptly adopted by the medical community, and is currently considered by many as the gold standard for the diagnosis of NCC.14 Here, the 2001 set of diagnostic criteria for NCC13 will be reviewed, with some notes on recent advances in neuroimaging and immune diagnostic techniques that can be incorporated to improve its diagnostic accuracy in selected cases.
Table 1. Diagnostic criteria for neurocysticercosis13.
| Diagnostic criteria |
| Absolute |
| • Histological demonstration of the parasite from biopsy of a brain or spinal cord lesion |
| • Evidence of cystic lesions showing the scolex on neuroimaging studies |
| • Direct visualization of subretinal parasites by fundoscopic examination |
| Major |
| • Evidence of lesions highly suggestive of neurocysticercosis on neuroimaging studies |
| • Positive serum immunoblot for the detection of anticysticercal antibodies |
| • Resolution of intracranial cystic lesions after therapy with albendazole or praziquantel |
| • Spontaneous resolution of small single enhancing lesions |
| Minor |
| • Evidence of lesions compatible with neurocysticercosis on neuroimaging studies |
| • Presence of clinical manifestations suggestive of neurocysticercosis |
| • Positive CSF ELISA for detection of anticysticercal antibodies or cysticercal antigens |
| • Evidence of cysticercosis outside the central nervous system |
| Epidemiological |
| • Individuals coming from or living in an area where cysticercosis is endemic |
| • History of travel to disease-endemic areas |
| • Evidence of a household contact with T. solium infection |
| Degrees of diagnostic certainty |
| Definitive |
| • Presence of one absolute criterion |
| • Presence of two major plus one minor and one epidemiological criteria |
| Probable |
| • Presence of one major plus two minor criteria |
| • Presence of one major plus one minor and one epidemiological criteria |
| • Presence of three minor plus one epidemiological criteria |
Diagnostic Criteria
Absolute diagnostic criteria
There are three criteria that were originally selected as ‘absolute’ since they represent the only way to make the diagnosis of NCC with 100% confidentially using only one criterion:
Histological demonstration of the parasite from biopsy of a brain or spinal cord lesion. Microscopic visualization of the scolex with its characteristics four suckers and a double crown of hooks, or the presence of parasitic membranes, confirm the diagnosis of NCC.15 However, biopsy of many granular and almost all calcified cysticerci may not confirm the diagnosis since the scolex and the membranes are not present in most of these lesions, and the only biopsy finding might be the so-called ‘calcareous corpuscles’ which are seen in a number of parasites.
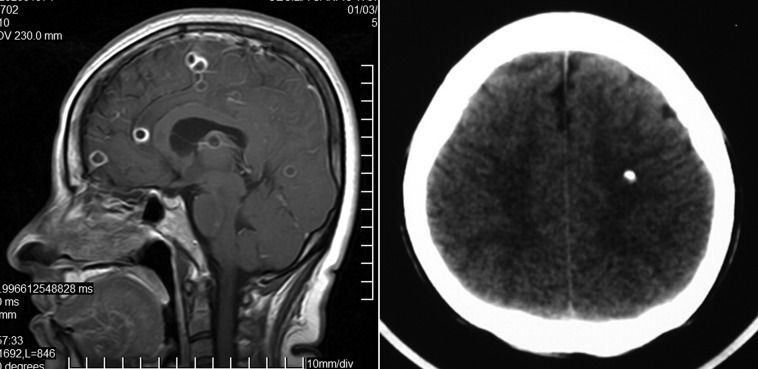
Cystic lesions showing the scolex on CT or MRI. From all the neuroimaging forms of presentation of cysticerci, only the presence of cystic lesions demonstrating the scolex can be considered pathognomonic of NCC.16 In these cases, scolices are often visualized on T1-weighted images as eccentric bright nodules within the cysts. This produces the so-called ‘hole-with-dot’ imaging that is seen in some viable (vesicular) cysts located in the brain parenchyma, the subarachnoid space, or the ventricular system (Fig. 1). New MRI protocols, such as diffusion-weighted imaging and ADC maps, may allow the visualization of the scolex in some coloidal cysts, where T1-weighted, FLAIR, and T2-weigthed sequences fail to visualize it.17
Direct visualization of subretinal parasites by fundoscopic examination. Since the retina is considered part of the central nervous system, patients with subretinal cysticerci are considered to have NCC. These cysts usually have a yellowish color with a central dark spot representing the scolex.18 Despite being an absolute criterion, the occurrence of subretinal cysticercus is rare, even in endemic areas.
Figure 1.
Contrast-enhanced CT scan showing vesicular parenchymal brain cysticerci showing the pathognomonic ‘hole-with-dot’ imaging (arrow).
Major diagnostic criteria
As noted, ‘major’ criteria strongly suggest the diagnosis of NCC, but must always be evaluated on the light of other criteria to confirm or discard NCC. They include some neuroimaging findings, immune diagnostic tests and evolutive aspects of the disease. The set included four major diagnostic criteria.13 MRI techniques developed from that time should be added to enhance the diagnostic accuracy of neuroimaging for the so-called ‘highly-suggestive’ lesions of NCC.19,20
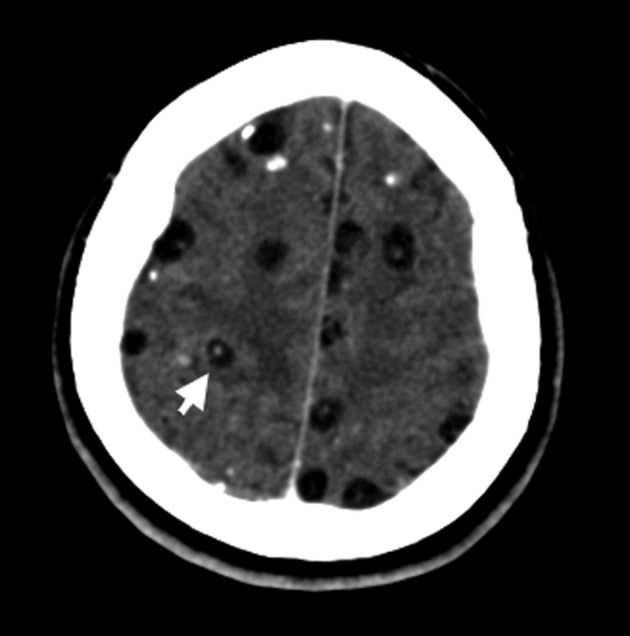
Lesions highly suggestive of NCC on neuroimaging studies. These include: cystic lesions without showing the scolex, single or multiple ring or nodular enhancing lesions, and small parenchymal round calcifications (Fig. 2). Such findings may also be observed in other diseases of the central nervous system and must be interpreted with caution to avoid over diagnosis of NCC. Main differential diagnoses include primary or metastatic brain tumors, toxoplasmosis and tuberculosis. It has recently been shown that the use of diffusion-weighted imaging and MRI spectroscopy may help to differentiate NCC from neurotuberculosis in selected cases.19,20 A common finding in NCC is the presence of intracranial lesions in different evolutive stages. As these findings provides further support for the diagnosis of NCC, the presence of two different highly suggestive lesions on neuroimaging should be considered as two major diagnostic criteria.
Positive serum enzyme-linked immunoelectrotransfer blot (EITB) assay for the detection of antibodies to T. solium glycoprotein antigens. The most reliable test for the detection of antibodies specific for T. solium antigens in serum, is the EITB using purified antigenic extracts.21 The assay has a reported specificity approaching 100% and a sensitivity of up to 98% for patients with two or more parasites in the nervous system.22 While initially considered that reaction to any of seven specific bands should be regarded as diagnostic for cysticercosis, further studies have shown a low specificity of a single positive 50 kDa in this setting.23 Another major problem with the EITB is its low sensitivity in patients with single intracranial cysticerci and in those with only calcified parasites, where up to 50% of cases may test false negative.24,25 A recent preliminary study suggested that the use of conformation-sensitive immunoassay may detect almost 50% of patients with a single cysticercal granuloma who were negative with the EITB, thus improving the sensibility of the test.26 Moreover, as antibody detection in serum reflects cysticercus infection in any tissue, not only patients with NCC, but also those with muscular or subcutaneous cysticercosis, may test positive. Consequently, results of the EITB must be evaluated with caution since extraneural cysticercosis or even exposure without development of infection may result in a positive result.21
Spontaneously resolving small single enhancing lesions. A single parenchymal brain enhancing lesion is a diagnostic challenge. While it is a common finding in NCC, many other infectious and neoplasic diseases of the central nervous system may present with this neuroimaging finding as the sole evidence of the disease. However, it has been demonstrated that when those lesions fulfill a rigid set of clinical and radiological criteria, the diagnosis of NCC can be done with a sensitivity of 99.5% and a specificity of 98.9%.27 Cysticerci-related enhancing lesions are smaller than 20 mm in diameter, are most often located supratentorially, and rarely cause displacement of the midline or focal neurological deficits.27,28 When these lesions resolve spontaneously, either disappearing or transforming into a calcified nodule, the diagnosis of NCC is almost certain. Solitary cysticercus granuloma account for most cases of NCC reported from India, and are also prevalent in other regions of the world where this disease is endemic.27–30 Resolution of a ring-enhancing intracranial lesion with the use of steroids should not be considered as a major criterion for NCC, because other diseases that present with similar findings (i.e. intracranial lymphomas) may also vanish after steroid therapy.
Resolution of intracranial cystic lesions after cysticidal drug therapy. A therapeutic trial with either albendazole or praziquantel is an useful diagnostic tool in cases where the diagnosis of NCC is suspected on the first visit, as disappearance of intracranial cystic or ring-enhancing lesions or their transformation into calcified nodules after therapy is a strong argument favoring the diagnosis of NCC, and then should be considered a ‘major’ diagnostic criteria.31
Figure 2.
Left: T1-weighted MRI showing multiple parenchymal brain cysticerci in the acute encephalitic phase, appearing as small ring-enhancing lesions surrounded by edema; right: plain CT scan showing a small parenchymal brain calcification.
Minor diagnostic criteria
Minor criteria are frequent but non-specific manifestations of the NCC that do not have enough diagnostic strength as to be considered major diagnostic criteria. In the 2001 set, four minor diagnostic criteria were recognized, including:
Lesions compatible with NCC on neuroimaging studies. Hydrocephalus and abnormal enhancement of the leptomeninges are common but non-specific neuroimaging findings in patients with NCC (Fig. 3). Many other conditions — tuberculous and fungal meningitis, meningeal carcinomatosis — may produce similar changes on neuroimaging studies. In these cases, cerebrospinal fluid (CSF) analysis provides useful diagnostic clues that must be interpreted on the light of the clinical and radiological manifestations of the patient.
Clinical manifestations suggestive of NCC. Up to 70% of symptomatic patients with NCC develop recurrent seizures as the primary or sole manifestation of the disease.32 It has been demonstrated that NCC is a common cause of acquired epilepsy in developing countries, and the presence of new onset seizures in an otherwise healthy middle-age individual coming from endemic areas should be considered as suggestive of NCC.5 Other clinical manifestations of the disease include focal neurological deficits, signs, and symptoms of increased intracranial pressure, and cognitive decline. Fever is not a common manifestation of NCC and its presence should suggest other diagnoses.
-
Positive CSF ELISA for detection of anticysticercal antibodies or cysticercal antigens. The detection of anticysticercal antibodies by ELISA using CSF has been shown to be 87% sensitive and 95% specific for the diagnosis of NCC, and remains a relatively useful diagnostic tool in areas with limited access to the EITB assay.33 The ELISA has proven to be false-negative in patients with parenchymal brain cysticercosis or in those with inactive disease, and false-positive in patients with other helminthic infections of the central nervous system. A recently developed QuickELISATM using recombinant and synthetic antigens from purified proteins of T. solium cysticerci has proven, in a preliminary study, to be more than 90% sensitive and specific for detecting anticysticercal antibodies in serum.34
It has also been suggested that a specific antigen-detection ELISA using a monoclonal antibody is useful for the demonstration of excretory-secretory cysticercal antigens, with a sensitivity ranging from 72% to 86%, and false-negative cases restricted to patients with a single intracranial cysticercus and inactive disease.35 The sensitivity of antigen detection tests has been, in general terms, better when performed in CSF than in serum.36–38 Inasmuch as the specificity of this assay has not been assessed in patients with other diseases, it should only be considered as minor diagnostic criteria.
Cysticercosis outside the central nervous system. In endemic regions, a patient may have systemic cysticercosis and neurological manifestations due to an unrelated cause. Moreover, in cysticercosis-endemic areas, some patients may present with subcutaneous nodules related to other infections, i.e. onchocerciasis. To qualify as minor diagnostic criteria for NCC, the presence of extraneural cysticercosis requires one of the following: (1) histological demonstration of the parasite from biopsy of a subcutaneous nodule; (2) plain X-ray films or CT scans showing multiple ‘cigar-shaped’ calcifications in thigh and calf muscles;39 or (3) direct visualization of a cysticercus in the anterior chamber of the eye.18
Figure 3.
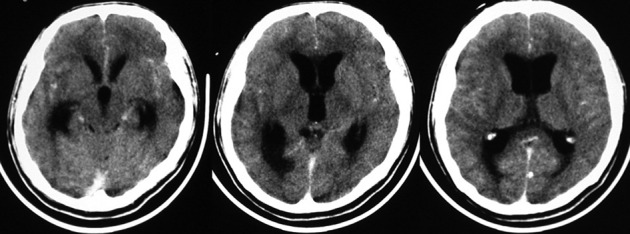
Contrast-enhanced CT scan showing obstructive hydrocephalus associated with mild abnormal enhancement of leptomeninges.
Epidemiological diagnostic criteria
Data including the place of birth and residence, and travel history, provide important information when evaluating patients with suspected NCC. However, such information should only be considered as circumstantial evidence favoring the diagnosis of NCC.
As previously noted, the disease is endemic in Latin America, the sub-Saharan Africa, the Indian subcontinent, and vast regions of southeast Asia.1 While NCC has been considered rare in most Western European countries, in North America, in Oceania, and in Muslim countries of Asia and Africa, massive migratory movements and increased overseas traveling have recently increased the number of NCC cases in these areas.6–11 NCC in non-endemic countries may be locally acquired, or may occur in immigrants from — or travelers to — disease-endemic areas. Locally-acquired NCC most often occurs in persons who are in close contact with a taenia carrier living in the non-endemic country. Human cysticercosis is mostly transmitted from person to person, and the role of infected swine is to perpetuate the infection cycle only. Therefore, to acquire NCC, travelers to disease-endemic areas must be in contact with a taenia carrier, who will most often infect them through non-hygienic handling of food.11
Degrees of Diagnostic Certainty
Interpretation of the above described diagnostic criteria allowed two degrees of diagnostic certainty for NCC: (1) definitive diagnosis, in patients who had one absolute criterion or in those who had two major plus one minor and one epidemiological criteria; and (2) probable diagnosis, in patients who had one major plus two minor criteria, in those who had one major plus one minor and one epidemiological criteria, and in those who had three minor plus one epidemiological criteria. Some authors have manifested concern on the reliability of these degrees of diagnostic certainty because of the fact that one or more of the proposed diagnostic criteria are uncommon in a given region, i.e. subretinal cysticerci are rare in Indian patients,40 or by the lack of availability of neuroimaging equipments in very poor countries.41 However, the set of diagnostic criteria was proposed to be used worldwide, with some minor adaptations probably needed according to the most common patterns of disease expression in a given region.
Comment
Before the introduction of the above described set of diagnostic criteria, it was common to diagnose NCC in patients presenting with seizures and a positive test for the detection of anticysticercal antibodies in serum. This probably resulted in the inclusion of many patients who had cryptogenic epilepsy and false-positive results on immunological testing. On the other hand, some infected persons may had escaped detection just because immune diagnostic tests were negative. While the set of diagnostic criteria may be considered too complex to be applied in field studies, it seems to be the only way to avoid the overdiagnosis of NCC that occurred in epidemiological surveys. This set of diagnostic criteria is also of value for hospitalized patients with suspected NCC. In areas where this disease is endemic, this parasitic disease could be overdiagnosed if the diagnosis rests only on non-specific neuroimaging findings. In contrast, NCC tends to be overlooked in other regions of the world simply because it is rare. Such diagnostic pitfalls often lead to the practice of unnecessary invasive procedures.8,11
Ten years after its publication, and after being quoted in more than 400 papers published in peer-reviewed medical journals and major textbooks, the set of diagnostic criteria for NCC is widely accepted (http://scholar.google.com/scholar?q = diagnostic+criteria+for+neurocysticercosis&hl = en&btnG = Search&as_sdt = 1%2C5&as_sdtp = on; accessed on 5 January 2012). It has become a widely used and highly useful tool for the diagnosis of NCC in patients living in endemic as well as in non-endemic countries, avoiding over- and under-diagnosis of NCC in both, the field and the hospital settings.
Recent advances in neuroimaging have enhanced the diagnostic accuracy of some of these criteria but have not moved them from one category to another (from minor to major or absolute), since the original conception of the set was not directed to a particular imaging technique, but to its diagnostic ability to characterize imaging findings as pathognomonic, highly suggestive, or common. In the case of immune diagnostic tests, it is possible that not-yet widely used highly specific and sensitive tests join the EITB as a major diagnostic criteria in the future, but they would never be considered as an absolute criterion since immune tests only measure the occurrence of an antigen-antibody reaction in the blood or body fluids, and are not able to objectively visualize the presence of cysticerci within the central nervous system.21 In an ideal scenario, immune diagnostic tests must be highly sensitive and specific, and should be able to differentiate between active and inactive infections, to better orientate patient management and follow up.
References
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–61. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Santibáñez R, Idrovo L, Rodriguez S, Díaz-Calderon E, Navas C, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–7. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 3.Medina MT, Durón RM, Martínez L, Osorio JR, Estrada AJ, Zúñiga C, et al. Prevalence, incidence and etiology of epilepsies in rural Honduras: the Salamá study. Epilepsia. 2005;46:124–31. doi: 10.1111/j.0013-9580.2005.11704.x. [DOI] [PubMed] [Google Scholar]
- 4.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis. Association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–34. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 5.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States. Review of an important emerging infection. Neurology. 2004;63:1559–64. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 7.Croker C, Reporter R, Mascola L. Use of statewide hospital discharge data to evaluate the economic burden of neurocysticercosis in Los Angeles County (1991–2008). Am J Trop Med Hyg. 2010;83:106–10. doi: 10.4269/ajtmh.2010.09-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Brutto OH. 2012. Neurocysticercosis in Western Europe. A re-emerging disease? Acta Neurol Belg. Epub 2012 Apr 18. [Google Scholar]
- 9.Del Brutto OH. Neurocysticercosis in Australia: still free of autochthonous cases? Med J Aust. 2012;196:385. doi: 10.5694/mja11.11443. [DOI] [PubMed] [Google Scholar]
- 10.Del Brutto OH. A review of cases of human cysticercosis in Canada. Can J Neurol Sci. 2012;39:319–22. doi: 10.1017/s0317167100013445. [DOI] [PubMed] [Google Scholar]
- 11.Del Brutto OH. Neurocysticercosis among international travelers to disease-endemic areas. J Travel Med. 2012;19:112–7. doi: 10.1111/j.1708-8305.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- 12.Del Brutto OH, Wadia NH, Dumas M, Cruz M, Tsang VC, Schantz PM. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci. 1996;142:1–6. doi: 10.1016/0022-510x(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 13.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White ACJr, Weller PF.Cestodes Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. 17thed.New York: McGraw-Hill; 2008. p.1336–41. [Google Scholar]
- 15.Pittella JE. Neurocysticercosis. Brain Pathol. 1997;7:681–93. doi: 10.1111/j.1750-3639.1997.tb01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Trop. 2003;87:71–8. doi: 10.1016/s0001-706x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 17.Faria do Amaral LL, Ferreira RM, da Rocha AJ, Fortes Ferreira NP. Neurocysticercosis. Evaluation with advanced magnetic resonance techniques and atypical forms. Top Mag Reson Imaging. 2005;16:127–44. doi: 10.1097/01.rmr.0000189106.78146.98. [DOI] [PubMed] [Google Scholar]
- 18.Lozano-Elizondo D.Ophthalmic cysticercosis In: Palacios E, Rodriguez-Carbajal J, Taveras JM, editors. Cysticercosis of the central nervous systemSpringfield, IL: Charles C. Thomas Publisher; 1983. p.84–100. [Google Scholar]
- 19.Gupta RK, Prakash M, Mishra AM, Husain M, Prasad KN, Husain N. Role of diffusion weighted imaging in differentiation of intracranial tuberculoma and tuberculous abscess from cysticercus granulomas — a report of more than 100 lesions. Eur J Radiol. 2005;55:384–392. doi: 10.1016/j.ejrad.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Pretell EJ, Martinot C, Jr, Garcia HH, Alvarado M, Bustos JA, Martinot C. Differential diagnosis between cerebral tuberculosis and neurocysticercosis by magnetic resonance spectroscopy. J Comput Assist Tomogr. 2005;29:112–4. doi: 10.1097/01.rct.0000149959.63294.8f. [DOI] [PubMed] [Google Scholar]
- 21.Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 2010;26:137–44. doi: 10.1016/j.pt.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. 1989;159:50–9. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Furrows SJ, McCroddan J, Bligh WJ, Chiodini P. Lack of specificity of a single positive 50-kDa band in the electroimmunotransfer blot (EITB) assay for cysticercosis. Clin Microbiol Infect. 2006;12:459–62. doi: 10.1111/j.1469-0691.2006.01381.x. [DOI] [PubMed] [Google Scholar]
- 24.Rajshekhar V, Oommen A. Serological studies using ELISA and EITB in patients with solitary cysticercus granuloma and seizures. Neurol Infect Epidemiol. 1997;2:177–80. [Google Scholar]
- 25.Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VC. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. J Infect Dis. 1997;175:486–9. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prabhakaran V, Rajshekhar V, Murrell KD, Oommen A. Conformation-sensitive immunoassays improve the serodiagnosis of solitary cysticercus granuloma in Indian patients. Trans R Soc Trop Med Hyg. 2007;101:570–7. doi: 10.1016/j.trstmh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Rajshekhar V, Chandy MJ. Validation of diagnostic criteria for solitary cerebral cysticercus granuloma in patients with seizures. Acta Neurol Scand. 1997;96:76–81. doi: 10.1111/j.1600-0404.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 28.Singh G, Rajshekhar V, Murthy JM, Prabhakar S, Modi M, Khandelwal N, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology. 2010;75:2236–45. doi: 10.1212/WNL.0b013e31820202dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Brutto OH. Single parenchymal brain cysticercus in the acute encephalitic phase: definition of a bening form of neurocysticercosis with a benign prognosis. J Neurol Neurosurg Psychiatry. 1995;58:247–9. doi: 10.1136/jnnp.58.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yodnopaklow P, Mahuntussanapong A. Single small enhancing CT lesion in Thai patients with acute symptomatic seizures: a clinico-radiological study. Trop Med Int Health. 2000;5:250–5. [PubMed] [Google Scholar]
- 31.Del Brutto OH, Roos KL, Coffey CS, Garcia HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med. 2006;145:43–51. doi: 10.7326/0003-4819-145-1-200607040-00009. [DOI] [PubMed] [Google Scholar]
- 32.Del Brutto OH, Santibañez R, Noboa CA, Aguirre R, Diaz-Calderón E, Alarcón TA. Epilepsy due to neurocysticercosis. An analysis of 203 patients. Neurology. 1992;42:389–92. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 33.Rosas N, Sotelo J, Nieto D. ELISA in the diagnosis of neurocysticercosis. Arch Neurol. 1986;43:353–6. doi: 10.1001/archneur.1986.00520040039016. [DOI] [PubMed] [Google Scholar]
- 34.Lee YM, Handali S, Hancock K, Pattabhi S, Kovalenko VA, Levin A, et al. Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in QuickELISATM. Am J trop Med Hyg. 2011;84:587–93. doi: 10.4269/ajtmh.2011.10-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García HH, Harrison LJ, Parkhouse RM, Montenegro T, Martinez SM, Tsang VC, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. Trans R Soc Trop Med Hyg. 1998;92:411–4. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleury A, Hernandez M, Avila M, Cardenas G, Bobes RJ, Huerta M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78:970–4. doi: 10.1136/jnnp.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez S, Dorny P, Tsang VC, Pretell EJ, Brandt J, Lescano AG, et al. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. 2009;199:1345–52. doi: 10.1086/597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michelet L, Fleury A, Sciutto E, Kendjo E, Fragoso G, Paris L, et al. Human neurocysticercosis: comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol. 2011;49:195–200. doi: 10.1128/JCM.01554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustos JA, Garcia HH, Dorregaray R, Naranjo M, Pretell J, Gonzalez AE, et al. Detection of muscle calcifications by thigh CT scan in neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2005;99:775–9. doi: 10.1016/j.trstmh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Garg RK. Diagnostic criteria for neurocysticercosis: some modifications are needed for Indian patients. Neurol India. 2004;52:171–7. [PubMed] [Google Scholar]
- 41.Gabriel S, Blocher J, Dorny P, Abatih E, Schmutzhard E, Ombay M, et al. Re-visiting the ‘Del Brutto diagnostic criteria’ for the diagnosis of neurocysticercosis [document on the Internet]. [cited 2012 Apr 9]. Barcelona, ECTMIH, 2011. Available from: http://www.ectmihbarcelona2011.org/docs/Gabriel.pdf. [Google Scholar]