Abstract
Alogliptin (Nesina) for adults with type-2 diabetes
INTRODUCTION
Type-2 diabetes, which accounts for approximately 90% to 95% of all diagnosed incidence of diabetes, is a chronic disease characterized by insulin resistance and abnormal pancreatic beta-cell function.1 Diabetes is the leading cause of a multitude of complications, including kidney failure, lower limb amputations, and blindness, and is the seventh leading cause of death in the United States.2
There are numerous antihyperglycemic agents on the market to treat type-2 diabetes, including biguanides, sulfonylureas, meglitinides, thiazolidinediones, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and insulin. Bile acid sequestrants, amylin mimetics, and dopamine 2-agonists are also available but less frequently recommended.1 Canagliflozin (Invokana, Janssen), the first sodium-glucose co-transporter-2 inhibitor, was approved by the Food and Drug Administration (FDA) in March 2013.3
The preferred and most widely utilized first-line agent in the treatment of type-2 diabetes is metformin (Glucophage, Bristol-Myers Squibb).1,4 However, for those who cannot tolerate metformin or whose diabetes is inadequately controlled with this single agent, another agent is required. A profile of agents recommended after metformin is provided in Table 1. The selection of the antihyperglycemic agent is based on patient characteristics and goals and the pharmacological profile of medication.1
Table 1.
Profile of Agents Recommended After Metformin
| Efficacy | Hypoglycemia | Weight | Major Side Effects | Cost | |
|---|---|---|---|---|---|
| Sulfonylurea | High | Moderate risk | Gain | Hypoglycemia | Low |
| Thiazolidinedione | High | Low risk | Gain | Edema, heart failure, bone fractures | High |
| DPP-4 Inhibitor | Intermediate | Low risk | Neutral | Rare | High |
| GLP-1 Receptor | High | Low risk | Loss | Gastrointestinal | High |
| Agonist | |||||
| Insulin | Highest | High risk | Gain | Hypoglycemia | Varies |
From Inzucchi SE, et al. Diabetes Care 2012;35:1364–1379.1
DPP-4 inhibitors are among the agents recommended after metformin.1 DPP-4 inhibitors have demonstrated their capacity to reduce blood glucose levels in type-2 diabetes when utilized alone or in combination with agents such as metformin, sulfonylureas, or meglitinides.5 Four DPP-4 inhibitors are currently available in the United States. Sitagliptin (Januvia, Merck) was approved in October 2006; saxagliptin (Onglyza, Bristol-Myers Squibb) was approved in July 2009; and linagliptin (Tradjenta, Boehringer Ingelheim) was approved in May 2011. The newest DPP-4 inhibitor, alogliptin, was approved in January 2013. Alogliptin is available as a single ingredient agent (Nesina, Takeda) as well as in combination with pioglitazone (Oseni, Takeda) and metformin (Kazano, Takeda).6 This review will focus on alogliptin.
PHARMACOLOGY
Alogliptin is a potent, highly selective, noncovalent inhibitor of DPP-4.7 It is prepared as a benzoate salt with the chemical name 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl) benzonitrile monobenzoate. The chemical structure is shown in Figure 1 (C18H21N5O2C7H6O2) and the molecular weight is 461.51 daltons.8
Figure 1.
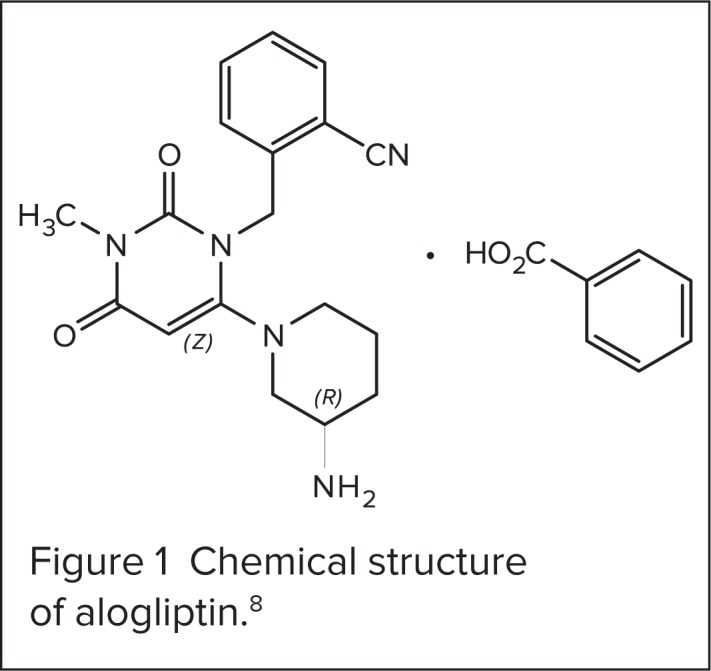
Chemical structure of alogliptin.8
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are incretin hormones that potentiate the release of insulin from pancreatic beta cells in a glucose-dependent manner; they are released in response to a meal.7,8 GLP-1 also decreases pancreatic glucagon secretion, resulting in less hepatic gluconeogenesis. DPP-4 enzymes inactivate GLP-1 and GIP within minutes. Thus, DPP-4 inhibition results in increased concentrations of these incretin hormones, ultimately reducing both fasting and postprandial glucose concentrations.8 The selectivity of alogliptin for the DPP-4 enzyme is more than 10,000-fold greater than that of other DPP isoenzymes, DPP-2, 8, and 9.9
PHARMACODYNAMICS
When administered at therapeutic doses, alogliptin diminishes plasma DPP-4 activity by 80% or more, providing a twofold to threefold increase in GLP-1 levels.7,10 In a randomized, double-blind, placebo-controlled, parallel-group trial, 56 patients with type-2 diabetes were randomized to receive alogliptin 25 mg, 100 mg, 400 mg, or placebo once daily for 14 days. Following a single oral dose of alogliptin, the mean maximal inhibition of DPP-4 in all alogliptin groups ranged from 94.7% to 98.8% compared with 25.3% in the placebo group. Time to achieve maximal inhibition was 1 to 1.4 hours. Twenty-four hours after a single dose, the mean DPP-4 inhibition in patients receiving alogliptin ranged from 78.3% to 95.7%. Following 14 days of dosing, the mean maximal inhibition of DPP-4 for those receiving alogliptin ranged from 93.8% to 98.9% and mean inhibition after 24 hours ranged from 81.8% to 96.7%. The concentration of alogliptin required to produce 50% inhibition of DPP-4 was 6.6 ng/mL (19.4 nmol/L).11
PHARMACOKINETICS
Alogliptin is rapidly absorbed with an approximate bioavailability of 100% and achieves peak plasma concentration (Tmax) one to two hours after administration.8,9 Absorption occurs primarily in the small intestine and steady state is achieved by day 7.10,12,13 There appears to be minimal accumulation; after 14 days of once-daily alogliptin dosing (25 mg to 400 mg daily) in patients with type-2 diabetes, the area under the curve (AUC) from time 0 to 24 hours increased by 34% (90% CI, 28%–40%) and the maximum concentration by 9% (90% CI, −1 to 21) compared with day 1.11 In healthy subjects who received alogliptin 100 mg under fed and fasted conditions, food had no effect on the AUC and only a minimal, clinically insignificant effect on its Cmax.14,15 Consequently, alogliptin can be taken without regard to meals.8
Alogliptin distributes well into tissues; the volume of distribution following a 12.5-mg intravenous infusion of alogliptin was 417L. Alogliptin is predominantly free in the plasma, with only 20% bound to plasma proteins.8 In patients with type-2 diabetes, the terminal half-life (t½) of alogliptin ranged from 12.5 to 21.1 hours, with an average 21 hours for those receiving the maximum recommended dose of 25 mg.8,11
Alogliptin is primarily excreted unchanged in the urine (60% to 71%).8 The renal clearance of alogliptin 25 mg, 100 mg, and 400 mg once daily in patients with type-2 diabetes ranged from 165 to 254 mL/min.11 The prescribing information gives the value of 9.6 L/hour as the renal clearance of alogliptin. Alogliptin undergoes limited metabolism, and in vitro data suggests that the hepatic enzymes CYP2D6 and CYP3A4 are involved. The two minor metabolites that have been detected are M-I and M-II. Alogliptin undergoes N-demethylation to the active metabolite M-I and N-acetylation to the inactive metabolite M-II. M-I accounts for less than 2% of alogliptin concentrations in the urine, while M-II accounts for less than 6%.8,14
CLINICAL TRIALS
The safety and efficacy of alogliptin as monotherapy and combination therapy in patients with type-2 diabetes have been evaluated in numerous clinical trials. Key clinical trials leading to the approval of alogliptin by the FDA are summarized below and in Table 2. Adverse events data from clinical trials are further discussed within the Safety and Tolerability section.
Table 2.
Summary of Clinical Trials
|
Reference Study Duration |
Treatment (Number of Subjects) | Mean Baseline A1c (%) | Least–Squares Mean Change in A1c (%) |
|---|---|---|---|
| In Patients With No Prior Antihyperglycemic Therapy | |||
| Defronzo et al. 200816 26 weeks |
Placebo (n = 64) | 7.9 | −0.02 |
| Alogliptin 12.5 mg (n = 133) | −0.56 (P < 0.001, vs. placebo) | ||
| Alogliptin 25 mg (n = 131) | −0.59 (P < 0.001, vs. placebo) | ||
| Rosenstock et al. 201017 26 weeks |
Alogliptin 25 mg (n = 164) | 8.80 | −0.96 |
| Pioglitazone 30 mg (n = 163) | 8.76 | −1.15 | |
| Alogliptin 12.5 mg + pioglitazone 30 mg (n = 164) | 8.85 | −1.56 (P < 0.05, vs. pioglitazone alone) | |
| Alogliptin 25 mg + pioglitazone 30 mg (n = 164) | 8.80 | −1.71 (P < 0.05, vs. pioglitazone alone, vs. alogliptin alone) | |
| Pratley et al. 20128,18 26 weeks |
Placebo (n = 102) | 8.5 | 0.1 |
| Alogliptin 12.5 mg b.i.d. (n = 104) | 8.4 | −0.6 | |
| Metformin 500 mg b.i.d. (n = 103) | 8.5 | −0.7 | |
| Metformin 1,000 mg b.i.d. (n = 108) | 8.4 | −1.1 | |
| Alogliptin 12.5 mg + metformin 500 mg b.i.d. (n = 102) | 8.5 | −1.2 (P < 0.001, vs alogliptin 12.5 mg b.i.d., vs. metformin 500 mg b.i.d.) | |
| Alogliptin 12.5 mg + metformin 1,000 mg b.i.d. (n = 111) | 8.4 | −1.6 (P < 0.001, vs alogliptin 12.5 mg b.i.d., vs. metformin 1,000 mg b.i.d.) | |
| In Patients Receiving Metformin | |||
| Nauck et al. 200819 26 weeks |
Placebo + metformin MTD (n = 104) | 8.0 | −0.1 |
| Alogliptin 12.5 mg + metformin MTD (n = 213) | 7.9 | −0.6 (P < 0.001, vs. placebo) | |
| Alogliptin 25 mg + metformin MTD (n = 210) | 7.9 | −0.6 (P < 0.001, vs. placebo) | |
| Defronzo et al. 20128,20 26 weeks |
Placebo + metformin* (n = 126) | 8.5 | −0.1 |
| Alogliptin 25 mg + metformin (n = 123) | 8.6 | −0.9 | |
| Pioglitazone 15 mg + metformin (n = 127) | 8.5 | −0.8 | |
| Pioglitazone 30 mg + metformin (n = 123) | 8.5 | −0.9 | |
| Pioglitazone 45 mg + metformin (n = 126) | 8.5 | −1.0 | |
| Pioglitazone 15 mg + alogliptin 25 mg + metformin (n = 127) | 8.5 | −1.3 (P < 0.01, vs. pioglitazone 15 mg, vs. alogliptin 25 mg) | |
| Pioglitazone 30 mg + alogliptin 25 mg + metformin (n = 124) | 8.5 | −1.4 (P < 0.01, vs. pioglitazone 30 mg, vs. alogliptin 25 mg) | |
| Pioglitazone 45 mg + alogliptin 25 mg + metformin (n = 126) | 8.6 | −1.6 (P < 0.01, vs. pioglitazone 45 mg, vs. alogliptin 25 mg) | |
| In Patients Receiving Thiazolidinedione | |||
| Pratley et al. 200921 26 weeks |
Placebo + pioglitazone 30 or 45 mg (n = 97) | 8.0 | −0.19 |
| Alogliptin 12.5 mg + pioglitazone 30 or 45 mg (n = 197) | 8.1 | −0.66 (P < 0.001, vs. placebo) | |
| Alogliptin 25 mg + pioglitazone 30 or 45 mg (n = 199) | 8.0 | −0.80 (P < 0.001, vs. placebo) | |
| In Patients Receiving Pioglitazone and Metformin | |||
| Bosi et al. 201122 52 weeks |
Metformin (≥ 1,500 mg or MTD) + pioglitazone 30 mg + Alogliptin 25 mg (n = 404) | 8.3 | −0.70 (P < 0.001) |
| Metformin (≥ 1,500 mg or MTD) + pioglitazone 30 mg + pioglitazone 15 mg (n = 399) | 8.1 | −0.29 | |
| In Patients Receiving Sulfonylurea | |||
| Pratley et al. 200923 26 weeks |
Glyburide + placebo (n = 99) | 8.1 | +0.01 |
| Glyburide + alogliptin 12.5 mg (n = 203) | 8.1 | −0.39 (P < 0.001, vs. placebo) | |
| Glyburide + alogliptin 25 mg (n = 198) | 8.1 | −0.53 (P < 0.001, vs. placebo) | |
| In Patients Receiving Insulin | |||
| Rosenstock et al. 200924 26 weeks |
Insulin + placebo ± metformin (n = 130) | 9.3 | −0.13 |
| Insulin + alogliptin 12.5 mg ± metformin (n = 131) | 9.3 | −0.63 (P < 0.001, vs. placebo) | |
| Insulin + alogliptin 25 mg ± metformin (n = 129) | 9.3 | −0.71 (P < 0.001, vs. placebo) | |
b.i.d. = twice daily
MTD = maximum tolerated dose
Metformin was titrated to stable dose
In Drug-Naïve Patients
Monotherapy
Defronzo et al. (2008) conducted a 26-week, double-blind, placebo-controlled study to assess the efficacy and safety of alogliptin in drug-naïve patients with inadequately controlled type-2 diabetes.16 A total of 329 patients with a mean age of 53.4 years were randomized to receive once-daily dosing of alogliptin 12.5 mg, alogliptin 25 mg, or placebo. At week 26, the least-squares mean change in glycosylated hemoglobin (HbA1c) was significantly lower in the alogliptin 12.5-mg group (−0.56%; P < 0.001) and 25-mg group (−0.59%; P < 0.001) compared with the placebo group (−0.02%). Statistically significant HbA1c reductions were noted as early as week 4. Fasting plasma glucose (FPG) also decreased significantly with both doses of alogliptin (−10.3 mg/dL for alogliptin 12.5 mg; −16.4 mg/dL for alogliptin 25 mg) compared with the 11.3 mg/dL increase observed with placebo (P < 0.001).
The occurrence of adverse effects (67.4% to 70.3%) was similar among the treatment groups; most effects were of mild to moderate intensity. Although the skin-related adverse events remained low, the incidence of dermatologic effects was greater in the alogliptin groups (12.8% to 15.2%) compared with the placebo group (6.3%). The authors concluded that the efficacy and safety of alogliptin when administered as monotherapy were analogous to that of other DPP-4 inhibitors.16
With Pioglitazone
In a 26-week, randomized, double-blind, parallel-group clinical trial, Rosen-stock et al. (2010) evaluated the efficacy and tolerability of alogliptin plus pioglitazone in 655 patients with type-2 diabetes who had not previously received antihyperglycemic therapy.17 Patients had a mean age of 53 years, mean BMI of 31 kg/m2, and an average three years of diabetes duration. Patients were randomized into four arms: alogliptin 25 mg once daily, pioglitazone 30 mg once daily, alogliptin/pioglitazone 12.5 mg/30 mg once daily, and alogliptin/pioglitazone 25 mg/30 mg once daily.
Combination therapy of alogliptin/pioglitazone 25 mg/30 mg resulted in greater reduction of HbA1c compared with the other four arms of the trial at week 26. Results are shown in Table 2. A greater reduction of FPG was also observed in the combination groups compared with the monotherapy groups; the mean FPG reduction from baseline was −2.7 mmol/L in the alogliptin/pioglitazone 12.5 mg/30 mg arm (P < 0.05 vs. pioglitazone alone) and −2.8 mmol/L in the alogliptin/pioglitazone 25 mg/30 mg arm (P < 0.05, vs. pioglitazone alone, vs. alogliptin alone). The authors concluded that initial combination therapy of alogliptin plus pioglitazone was generally well tolerated and effective for the treatment of type-2 diabetes in drug-naïve patients with inadequate glycemic control.17
With Metformin
The results of a 26-week, double-blind, placebo-controlled trial comparing the efficacy and safety of alogliptin plus metformin and each agent alone were presented by Pratley et al. at the 48th Annual Meeting of the European Association for the Study of Diabetes.18 The data are also represented in the prescribing information for Nesina.8 A total of 784 subjects diagnosed with type-2 diabetes inadequately controlled with diet and exercise were randomized to one of seven treatment groups: placebo, alogliptin 25 mg once daily, alogliptin 12.5 mg twice daily, metformin 500 mg twice daily, metformin 1,000 mg twice daily, alogliptin/metformin 12.5 mg/500 mg twice daily, or alogliptin/metformin 12.5 mg/1,000 mg twice daily. The HbA1c reduction in the combination groups was significantly greater than the corresponding monotherapy groups (P < 0.001). Results are shown in Table 2. (Data for the alogliptin 25-mg once daily group were not provided.) Weight decrease was observed in those receiving metformin (alone or in combination), while the group receiving alogliptin remained weight-neutral. Both monotherapy and combination therapy were very well tolerated, with similar safety profiles and few hypoglycemic events.
In Patients Receiving Metformin
With Metformin
In a multicenter, randomized, double-blind, placebo-controlled study, Nauck et al. (2009) evaluated the safety and efficacy of adding alogliptin to metformin in patients with type-2 diabetes with inadequate glycemic control.19 Patients (N = 527) with a historical diagnosis of type-2 diabetes and HbA1c between 7% and 10% while on metformin monotherapy were randomized by a 2:2:1 ratio to receive one of the following treatments in addition to metformin: placebo, alogliptin 12.5 mg once daily, or alogliptin 25 mg once daily. Prior to the addition of the study medication, metformin was titrated to the maximum tolerated dose (MTD). Treatment was continued for 26 weeks. Patients had a mean age of 55 years with a mean six-year duration of diabetes and a mean metformin daily dose of 1,847 mg.
The addition of alogliptin produced a significantly greater decrease in HbA1c at both doses when compared with placebo (P < 0.001). Results are shown in Table 2. The significant changes were observed starting with week 4 and persisted throughout the trial until completion at week 26. A rapid and significant FPG reduction from baseline was also perceived as early as week 1 and continued through the length of trial to week 26 for both alogliptin treatment groups versus placebo.19
With Metformin and Pioglitazone
Defronzo et al. (2012) conducted a 26-week, randomized, double-blind, double-dummy, placebo-controlled, parallel-arm clinical trial to evaluate the efficacy and tolerability of alogliptin and pioglitazone in patients with type-2 diabetes inadequately controlled with metformin monotherapy.20 A total of 1,554 patients were randomized to receive one of the following 12 treatments in addition to their stabilized dose of metformin: placebo, alogliptin 12.5 mg once daily, alogliptin 25 mg once daily, pioglitazone 15 mg once daily, pioglitazone 30 mg once daily, pioglitazone 45 mg once daily, alogliptin/pioglitazone 12.5 mg/15 mg once daily, alogliptin/pioglitazone 12.5 mg/30 mg once daily, alogliptin/pioglitazone 12.5 mg/45 mg once daily, alogliptin/pioglitazone 25 mg/15 mg once daily, alogliptin/pioglitazone 25 mg/30 mg once daily, and alogliptin/pioglitazone 25 mg/45 mg once daily. The mean duration of diabetes was 6.2 years and the mean metformin daily dose was 1,887 mg.
The combination of alogliptin and pioglitazone added to metformin resulted in greater reductions in HbA1c and FPG than either agent alone with metformin. Results are shown in Table 2 (data for the groups receiving alogliptin 12.5 mg are not shown). Body weight decreased in the placebo and alogliptin 12.5-mg and 25-mg treatment groups (−0.7 kg, −0.02 kg, and −0.7 kg, respectively), while weight increased in groups receiving pioglitazone therapy. The combination therapy of alogliptin with pioglitazone was generally safe and well tolerated. The authors concluded that the addition of alogliptin with pioglitazone was well tolerated and produced significant reductions in HbA1c as well as FPG. When used alone, alogliptin 25 mg showed greater efficacy than alogliptin 12.5 mg; however, in combination with pioglitazone, no dose-response relationship was observed.20
In Patients Receiving Thiazolidinediones
With Pioglitazone
A double-blind, placebo-controlled study (Pratley, 2009) was conducted in 493 patients with type-2 diabetes inadequately controlled with a thiazolidinedione (pioglitazone or rosiglitazone) to evaluate the efficacy and safety of add-on alogliptin therapy.21 Patients had a mean age of 55 years and had been diagnosed with diabetes for an average 7.4 to 7.8 years prior to the study. In addition to the thiazolidinedione, 56.2% of patients were receiving metformin (mean dose 1,688 mg/day) and 21.1% were receiving a sulfonylurea at baseline. Patients were randomized to one of three groups: placebo (n = 97), alogliptin 12.5 mg once daily (n = 197), or alogliptin 25 mg once daily (n = 199). All patients also received pioglitazone 30 mg or 45 mg and continued previous metformin or sulfonylurea therapy.
Patients taking alogliptin in conjunction with pioglitazone experienced a greater reduction of HbA1c at week 26 than patients receiving placebo (Table 2). Mean reductions of FPG were also greater in the alogliptin groups compared with the placebo group; mean FPG decrease was −19.7 mg/dL for alogliptin 12.5 mg, −19.9 mg/dL for alogliptin 25 mg, and −5.7 mg/dL for placebo (P = 0.003). Incidences of drug-related adverse events were similar across all groups. The incidence of severe adverse events was 9% in the alogliptin 25-mg group, 5.6% in the alogliptin 12.5-mg group, and 6.2% in the placebo group. Cardiac adverse events occurred more frequently in the alogliptin 25-mg group (6.5%) compared with the alogliptin 12.5-mg group (3.0%) and the placebo group (1.0%), with all severe cardiac adverse events occurring in the alogliptin groups.21 Cardiovascular adverse events are further discussed in the Safety and Tolerability section.
In Patients Receiving Metformin and Pioglitazone
With Metformin and Pioglitazone
Bosi et al. (2011) conducted a 52-week, randomized, double-blind, active-controlled, parallel-group study to assess the efficacy and safety of alogliptin 25 mg once daily added to dual therapy with metformin and pioglitazone.22 Patients inadequately controlled on metformin (1,500 mg or more or MTD) and pioglitazone 30 mg were randomized to receive either alogliptin 25 mg (n = 404) or pioglitazone 15 mg (n = 399). Metformin and pioglitazone 30 mg were open-label, while pioglitazone 15 mg and alogliptin 25 mg were double-blinded. Patients had a mean age of 55.1 years, a mean BMI of 31.6 kg/m2, and a mean duration of diabetes of 7.2 years. The median baseline metformin dose was 1,700 mg.
The changes from baseline in HbA1c at weeks 26 and 52 were significantly greater (P < 0.001) in the group taking at least 1,500 mg or the MTD of metformin plus pioglitazone 30 mg plus alogliptin 25 mg (triple therapy) compared with the group taking at least 1,500 mg or the MTD of metformin plus pioglitazone 30 mg plus pioglitazone 15 mg (dual therapy). Results at week 52 are shown in Table 2. Changes from baseline in FPG were also greater in the group receiving alogliptin (−0.8 mmol/L at week 52) compared with the group receiving the additional pioglitazone (−0.2 mmol/L at week 52, P < 0.05). Proinsulin/insulin ratio and HOMA beta-cell function at week 52 were improved in the alogliptin group compared with the pioglitazone group (P < 0.001). More patients in the triple therapy group experienced drug-related dermatologic and subcutaneous adverse events (5.2%) compared with the dual therapy group (2.3%), with pruritus and rash being the most common events. Hypoglycemia was reported more frequently in the triple therapy group (4.5%) compared with the dual therapy group (1.5%). (Skin-related adverse events and incidence of hypoglycemia are further discussed in the Safety and Tolerability section.) The authors concluded that the addition of alogliptin to therapy with metformin and pioglitazone was largely well tolerated, with no clinically meaningful safety distinctions between the two treatment groups.22
In Patients Receiving Sulfonylureas
With Glyburide
Pratley et al. (2009) conducted a 26-week, double-blind, placebo-controlled study to evaluate the efficacy and safety of alogliptin in combination with glyburide in 500 patients with type-2 diabetes inadequately controlled on sulfonylurea monotherapy.23 Patients on sulfonylureas other than glyburide were switched to equivalent doses of glyburide and randomized to one of the following treatments: placebo plus glyburide (n = 99), alogliptin 12.5 mg plus glyburide (n = 203), or alogliptin 25 mg plus glyburide (n = 198). Patients had a mean age of 57 years and a mean BMI of 30 kg/m2. There were greater decreases in HbA1c at week 26 in both the alogliptin 12.5-mg (−0.39%) and 25-mg (−0.53%) groups compared with the glyburide monotherapy group (+0.01%, P <0.001). Reductions in HbA1c were observed as early as four weeks into the study. However, there was no statistically significant difference in FPG reduction at week 26 among groups.23
In Patients Receiving Insulin
With Insulin
A 26-week, double-blind, randomized, placebo-controlled trial (Rosenstock, 2009) was conducted in 390 patients with type-2 diabetes inadequately controlled with insulin alone or combined with metformin.24 Patients were randomized to receive placebo (n = 130), alogliptin 12.5 mg once daily (n = 131), or alogliptin 25 mg once daily (n= 129) in addition to their current regimen of insulin with or without metformin. Patients had a mean age of 55 to 56 years and a mean BMI of 32 kg/m2. The majority of patients were receiving both insulin and metformin therapy. Insulin use included premixed, combination, long-acting, and short-acting formulations.
A decrease of HbA1c was observed as early as week 4 and at week 26 was significant in the alogliptin groups compared with the placebo group. Results are shown in Table 2. There was an increase in the mean FPG in the placebo group (0.3 mmol/L) and a statistically significant decrease in mean FPG in the alogliptin 25-mg group (−0.6 mmol/L, P = 0.03). The change in mean FPG in the alogliptin 12.5-mg group was not significantly different from placebo. Hyperglycemic rescues were lower in the alogliptin groups (20% to 21%) compared with placebo (40%), with a statistically significant P value of 0.001. The authors concluded that the add-on therapy of alogliptin to existing insulin therapy with or without metformin may significantly improve glycemic control without adverse weight effects or increased hypoglycemia.24
In Elderly Patients
A 52-week, randomized, double-blind, active-controlled, noninferiority study was conducted to compare the efficacy and safety of alogliptin and glipizide in elderly patients (ages 65 to 90 years) with type-2 diabetes.25 A total of 441 patients who had inadequate glycemic control with diet and exercise or monotherapy were randomized to receive alogliptin 25 mg once daily (n = 222) or glipizide 5 mg once daily (n = 219). Glipizide was titrated to 10 mg once daily if needed. Patients had a mean age of 69.9 years and a mean baseline HbA1c of 7.5%.
The percentage of patients who completed the study was 59.9% for the alogliptin group and 57.1% for the glipizide group. Reasons for discontinuation included adverse events, hyperglycemic rescue, voluntary withdrawal, and loss to follow-up. The least-squares mean change from baseline in HbA1c in patients who completed the study was −0.42% with alogliptin (n = 125) and −0.33% with glipizide (n = 117). The requirements for noninferiority were met, but superiority of alogliptin was not achieved. Adverse events were reported in 73.4% of patients receiving alogliptin and 68.9% of patients receiving glipizide, with urinary tract infection, dizziness, and headache being the most commonly reported events. The incidence of hypoglycemia was 5.4% in the alogliptin group and 26% in the glipizide group. A modest decrease in body weight was observed in the alogliptin group (−0.62 kg), while patients receiving glipizide had mild weight gain (0.60 kg) by week 52 (P < 0.001). The authors concluded that alogliptin was noninferior to glipizide in elderly patients and resulted in fewer hypoglycemic episodes.25
SAFETY AND TOLERABILITY
Warnings and Precautions
The warnings and precautions noted in the Nesina prescribing information include acute pancreatitis, hypersensitivity, hepatic effects, and hypoglycemia when used with insulin or insulin secretagogues (e.g., sulfonylureas).8
Pancreatitis
In March 2013, the FDA released a safety communication indicating the possible link between use of incretin mimetics and increased risk of pancreatitis and precancerous cellular changes. Incretin mimetics include exenatide, liraglutide, sitagliptin, saxagliptin, alogliptin, and linagliptin. The FDA has not reached a conclusion in regard to the risk of pancreatitis with incretin mimetics use and advises patients to continue therapy as directed by their health care professional.26
During clinical trials, pancreatitis was reported in 0.2% of patients (11 of 5,902) receiving alogliptin 25 mg compared with less than 0.1% of patients (5 of 5,183) receiving comparators. Acute pancreatitis was also reported during postmarketing surveillance.8 A recent meta-analysis of clinical trials with DPP-4 inhibitors found no significant increase in pancreatitis in patients receiving DPP-4 inhibitors (sitagliptin, vildagliptin, saxagliptin, alogliptin, and linagliptin) versus comparators.27 However, caution is still advisable and alogliptin should be discontinued quickly in anyone with symptoms consistent with pancreatitis.
Hypersensitivity
In clinical trials, the overall incidence of hypersensitivity reactions in pooled analysis was similar among those taking alogliptin 25 mg (0.6%) and comparators (0.8%). However, there have been post-marketing reports of severe hypersensitivity reactions including anaphylaxis, angioedema, Stevens-Johnson syndrome, and other severe cutaneous reactions in patients receiving alogliptin. Alogliptin should be discontinued immediately in patients with suspected hypersensitivity reactions. For those with a history of hypersensitivity reactions to other DPP-4 inhibitors, alogliptin should be initiated with caution, since the incidence of cross-sensitivity is unknown.8
Hepatic Effects
While hepatic abnormalities were not significantly different in alogliptin-and comparator-treated patients in clinical trials, there have been postmarketing reports of fatal and nonfatal hepatic failure in patients taking alogliptin. Although some of the cases did not contain enough information to determine causality, others were deemed possibly to probably related to alogliptin therapy. The incidence is very low, but since the risk may exist for hepatic abnormalities, patients should have liver test panels conducted prior to initiating alogliptin and periodically while using it. Alogliptin should be used with caution in patients with abnormal liver tests and should be discontinued in patients with suspected hepatic toxicity.28
Hypoglycemia
In general, the incidence of hypoglycemia with alogliptin is low. Patients treated with alogliptin had similar or lower incidence of hypoglycemia compared with patients treated with placebo or active comparators in clinical trials. In the clinical trial evaluating monotherapy with alogliptin, the incidence of hypoglycemia was 1.5% in patients treated with alogliptin and 1.6% in patients treated with placebo.8 In a pooled analysis of phase 2 and phase 3 clinical trials, hypoglycemia occurred in 8% or less of patients receiving alogliptin and in 7% to 11% of patients receiving placebo.29 Caution is advisable when using alogliptin with agents known to cause hypoglycemia, such as insulin or insulin secretagogues. The dose of insulin or the insulin secretagogue may need to be decreased to avoid hypoglycemic episodes.8
ADVERSE REACTIONS
Common Adverse Events and Overall Incidence
An estimated 8,500 patients have been treated with alogliptin in 14 randomized, double-blind clinical trials with an average exposure of 40 weeks. The incidence of adverse events in a pooled analysis of all the clinical trials was 66% in patients receiving alogliptin 25 mg, 62% in patients receiving placebo, and 70% in patients receiving an active comparator. The discontinuation rates due to adverse events were similar among those receiving alogliptin 25 mg (4.7%) and placebo (4.5%). The discontinuation rate was 6.2% in those receiving an active comparator. The most commonly reported adverse events occurring more frequently in patients receiving alogliptin compared with placebo were nasopharyngitis (4.4%), headache (4.2%), and upper respiratory tract infection (4.2%). Alogliptin did not result in significant weight gain in clinical trials.8
Skin-Related Adverse Events
Skin and subcutaneous adverse events were specifically monitored during clinical trials with alogliptin due to concerns raised with other DPP-4 inhibitors.9,29 In clinical trials, there appears to be a marginal increase in skin-related adverse events in the alogliptin group compared with placebo, with the main finding being pruritus.9 However, very few patients in clinical trials discontinued therapy due to skin-related adverse events. In the monotherapy clinical trial, only one patient discontinued therapy due to the adverse event; in the combination therapy trials, less than 0.1% of patients discontinued therapy due to the adverse events.29
Cardiovascular Events
As patients with diabetes are at increased risk for cardiovascular (CV) events, the FDA has requested that data from clinical trials demonstrate that new antidiabetic therapy does not increase CV risk to an unacceptable extent.30 For alogliptin, clinical trial data do not suggest an increased CV risk. A meta-analysis reviewing pooled data found no additional CV risk with alogliptin compared with placebo and other antihyperglycemic agents in patients with type-2 diabetes.31 Eleven phase 2 and 3 clinical trials including 4,168 patients exposed to alogliptin (12.5 mg and 25 mg), 691 patients exposed to placebo, and 1,169 patients exposed to active comparators were analyzed to evaluate the incidence of the major adverse cardiovascular event endpoints of CV death, nonfatal myocardial infarction, and nonfatal stroke. No significant difference in major CV events was observed between patients treated with alogliptin and those treated with placebo or active comparators.
To further evaluate the CV safety of alogliptin, the EXAMINE study (Examination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type-2 diabetes mellitus and acute coronary syndrome) was conducted.32 A total of 5,380 patients with previously diagnosed type-2 diabetes and recent acute coronary syndrome were randomized to receive alogliptin or placebo in addition to standard of care treatment for type-2 diabetes and CV risk factors. Results demonstrated that after a median duration of alogliptin exposure of 533 days (interquartile range: 280 to 751 days), patients receiving alogliptin had an occurrence of major CV events similar to those receiving placebo. The incidence of the primary endpoint (composite of CV-associated death, nonfatal myocardial infarction, or nonfatal stroke) was 11.3% and 11.8% in the alogliptin and placebo groups, respectively, with a hazard ratio of 0.96 (95% CI ≤ 1.16; P = 0.32). Alogliptin did not increase CV risk in patients with recent acute coronary syndrome.33
DRUG INTERACTIONS
Alogliptin is a renally excreted drug that has minimal cytochrome P450 (CYP) metabolism. No clinically significant pharmacokinetic changes or alterations were discovered when alogliptin coadministration was studied with: p-glycoprotein inhibitors and substrates (cyclosporine, digoxin), CYP inhibitors (atorvastatin, fluconazole, ketoconazole, and gemfibrozil), CYP substrates (pioglitazone, glyburide, warfarin, ethinyl estradiol, norethindrone), and renally excreted drugs such as metformin and cimetidine. No drug interactions or dose adjustments are warranted with concomitant use of CYP inhibitors, CYP substrates, and renally excreted drugs.8
DOSAGE AND ADMINISTRATION
The recommended dosage of alogliptin is 25 mg once daily. The tablets can be taken without regard to meals. Dosage adjustments are required for patients with renal impairment. For patients with a creatinine clearance rate (CrCl) of at least 30 but less than 60 mL/min, the recommended dose is 12.5 mg once daily. In patients with severe renal impairment or end-stage renal disease (CrCl of at least 15 but less than 30 mL/min and CrCl less than 15 mL/min or on hemodialysis), the dose is 6.25 mg once daily. Patients on hemodialysis may be given alogliptin without regard to timing of the hemodialysis, although it has not been studied in patients on peritoneal dialysis.8 A trial conducted by Fujii et al. on the safety and efficacy of alogliptin in patients with end-stage renal disease on hemodialysis found that as monotherapy or in combination, alogliptin safely improved glycemic control significantly (P < 0.0001) in all patients who were previously inadequately controlled.34 Patients with mild to moderate hepatic insufficiency do not need dosage adjustments. However, alogliptin was not studied in patients with severe impairment. Alogliptin should be used with caution in patients with liver disease.8
COST
The average wholesale price (AWP) for a 30-day supply of alogliptin 6.25 mg, 12.5 mg, and 25 mg is $340.64. As of February 2014, the AWPs for linagliptin, saxagliptin, and sitagliptin were similar to alogliptin.35 AWPs for DPP-4 inhibitors available in the United States are shown in Table 3.
Table 3.
Key Features of DPP-4 Inhibitors
| Product | Alogliptin (Nesina) | Linagliptin (Tradjenta) | Saxagliptin (Onglyza) | Sitagliptin (Januvia) |
|---|---|---|---|---|
| Cost (30 count) | 6.25-, 12.5-, 25-mg tablets: $340.64 | 5-mg tablets: $340.62 | 2.5-, 5-mg tablets: $334.68 | 25-, 50-, 100-mg tablets: $340.61 |
| Usual daily dose | 25 mg once daily without regard to meals | 5 mg once daily without regard to meals | 2.5 to 5 mg once daily with-out regard to meals | 100 mg once daily without regard to meals |
| Pregnancy category | B | B | B | B |
| Renal impairment | Dose adjustment required for CrCl < 60 mL/min | No dose adjustment required | Dose adjustment required for CrCl ≤ 50 mL/min | Dose adjustment required for CrCl < 50 mL/min |
| Hepatic impairment | No dosage adjustment required for mild to moderate hepatic impairment; not studied in severe impairment (Child-Pugh Grade C) Use with caution | No dose adjustment required | No dose adjustment required | No dosage adjustment required for mild to moderate hepatic impairment; not studied in severe impairment (Child-Pugh score > 9) |
| Warnings/precautions |
|
|
|
|
| Common adverse events |
|
|
|
|
| Drug interactions | No significant clinical interactions noted | P-glycoprotein and CYP3A4 inducers may decrease the efficacy of linagliptin | Strong CYP3A4/5 inhibitors increase saxagliptin exposure; limit saxagliptin to 2.5 mg | No significant clinical interactions noted |
| Combination products |
|
Jentadueto: with metformin | Kombiglyze: with metformin |
|
P&T COMMITTEE CONSIDERATIONS
Alogliptin is the latest DPP-4 inhibitor to be approved by the FDA for use in adults with type-2 diabetes as an adjunct to diet and exercise. Clinical trials have evaluated alogliptin as monotherapy as well as in combination with metformin, pioglitazone, glyburide, and insulin. Patients newly diagnosed with diabetes as well as those with inadequate glycemic control on multiple drug therapy have experienced reduction of HbA1c with alogliptin therapy. Mean baseline HbA1c values of patients receiving alogliptin in clinical trials have ranged from 7.9% to 9.3% and mean HbA1c reductions with alogliptin therapy have ranged from 0.4% to 1%. In drug-naïve patients with type-2 diabetes, monotherapy with alogliptin resulted in a mean 0.6% reduction in HbA1c.8
Alogliptin has greater selectivity for DPP-4 over DPP-8/DPP-9 than sitagliptin, saxagliptin, and linagliptin.7 However, the clinical significance of this theoretical advantage is unknown and has not been noted in practice. While there are no direct comparative clinical trials with alogliptin and other DPP-4 inhibitors, a review of clinical trials suggests that all have comparable efficacy and tolerability.36,37
There does not appear to be a therapeutic advantage of using one DPP-4 inhibitor over another. However, select features of the different DPP-4 inhibitors may make one preferable in specific situations. Alogliptin, like sitagliptin, does not have significant drug-drug interactions and so may be preferred in patients on multiple medications. However, dosage adjustment is required with renal impairment and caution is advised in hepatic impairment. Linagliptin is the only DPP-4 inhibitor that does not require dosage adjustment in both renal and hepatic impairment. Key features of the available DPP-4 inhibitors are summarized in Table 3.8,38,39,40,41
CONCLUSION
Diabetes affects more than 25 million people in the United States and is associated with a multitude of complications and increased risk of morbidity and mortality.2 Alogliptin, a selective DPP-4 inhibitor, is approved for use in adults with type-2 diabetes as an adjunct to diet and exercise.8 Similar to the other DPP-4 inhibitors already on the market, alogliptin is dosed once daily without regard to meals, is weight-neutral, and has a low incidence of hypoglycemia. Alogliptin is predominately eliminated renally and is not associated with significant drug interactions.
Various clinical trials have demonstrated the efficacy and safety of alogliptin as monotherapy and combination therapy in drug-naïve and previously treated patients with type-2 diabetes. Alogliptin lowers HbA1c approximately 0.6% and is generally well tolerated. Its side-effect profile is similar to that of other DPP-4 inhibitors. The cost of alogliptin is comparable to other DPP-4 inhibitors but higher than metformin or sulfonylureas. Alogliptin provides another oral option for diabetes patients inadequately controlled on or unable to take metformin. Data from long-term, clinical-outcomes-based studies should further clarify the safety of DPP-4 inhibitors in patients with type-2 diabetes.
Footnotes
Disclosure: The authors report that they have no commercial or financial relationships in regard to this article.
REFERENCES
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention 2011 National Diabetes Fact Sheet. Available at: www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed June 10, 2013.
- 3.Food and Drug Administration FDA approves Invokana to treat type-2 diabetes: first in a new class of diabetes drugs. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htm. Updated March 29, 2013. Accessed August 20, 2013.
- 4.American Diabetes Association Standards of medical care in diabetes—2013: position statement. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247–1258. doi: 10.1016/j.clinthera.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration FDA approved drug products. Available at: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed May 29, 2013.
- 7.Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors. Drugs. 2011;71(11):1441–1467. doi: 10.2165/11591400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Nesina (alogliptin), prescribing information. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; Jun, 2013. [Google Scholar]
- 9.Andukuri R, Drincic A, Rendell M. Alogliptin: a new addition to the class of DPP-4 inhibitors. Diabetes Metab Syndr Obes. 2009;2:117–126. [PMC free article] [PubMed] [Google Scholar]
- 10.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012;51(8):501–514. doi: 10.1007/BF03261927. [DOI] [PubMed] [Google Scholar]
- 11.Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type-2 diabetes. Clin Ther. 2008;30(3):499–512. doi: 10.1016/j.clinthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Karim A, Laurent A, Munsaka M, et al. Coadministration of pioglitazone or glyburide and alogliptin: pharmacokinetic drug interaction assessment in healthy participants. J Clin Pharmacol. 2009;49:1210–1219. doi: 10.1177/0091270009338938. [DOI] [PubMed] [Google Scholar]
- 13.Scott LJ. Alogliptin: a review of its use in the management of type-2 diabetes mellitus. Drugs. 2010;70:2051–2072. doi: 10.2165/11205080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Scheen AJ. Pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2010;12:648–658. doi: 10.1111/j.1463-1326.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 15.Karim A, Covington P, Christopher R, et al. Pharmacokinetics of alogliptin when administered with food, metformin, or cimetidine: a two-phase, crossover study in healthy subjects. Int J Clin Pharmacol Ther. 2010;48:46–58. doi: 10.5414/cpp48046. [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type-2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–2317. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenstock J, Inzucchi SE, Seufert J, et al. Initial combination therapy with alogliptin and pioglitazone in drug-naïve patients with type-2 diabetes. Diabetes Care. 2010;33:2406–2408. doi: 10.2337/dc10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratley R, Wilson C, Fleck P. Alogliptin plus metformin combination therapy vs. alogliptin or metformin monotherapy for type 2 DM (abstract). Presented at 48th Annual Meeting of the European Association for the Study of Diabetes; Berlin, Germany. October 2012; Available at: www.abstractsonline.com/plan/ViewAbstract.aspx?mID=2978&sKey=590037db-1ab0-4abd-a68f-e98c3351797f&cKey=78e391c0-8d4a-4924-b6e8-f7d9fd2275dd&mKey=%7B2DBFCAF7-1539-42D5-8DDA-0A94ABB089E8%7D#. Accessed July 10, 2013. [Google Scholar]
- 19.Nauck MA, Ellis GC, Fleck PR, et al. for the Alogliptin Study 008 Group Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type-2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomized, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46–55. doi: 10.1111/j.1742-1241.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 20.Defronzo RA, Burant CF, Fleck P, et al. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type-2 diabetes. J Clin Endocrinol Metab. 2012;97:1615–1622. doi: 10.1210/jc.2011-2243. [DOI] [PubMed] [Google Scholar]
- 21.Pratley RE, Reusch JEB, Fleck PR, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type-2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009;25:2361–2371. doi: 10.1185/03007990903156111. [DOI] [PubMed] [Google Scholar]
- 22.Bosi E, Ellis GC, Wilson CA, Fleck PR. Alogliptin as a third oral antidiabetic drug in patients with type-2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes Metab. 2011;13:1088–1096. doi: 10.1111/j.1463-1326.2011.01463.x. [DOI] [PubMed] [Google Scholar]
- 23.Pratley R, Kipnes M, Fleck P, et al. Alogliptin Study 007 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type-2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167–176. doi: 10.1111/j.1463-1326.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosenstock J, Rendell MS, Gross JL, et al. Alogliptin added to insulin therapy in patients with type-2 diabetes reduces HbA1c without causing weight gain or increased hypoglycemia. Diabetes Obes Metab. 2009;11:1145–1152. doi: 10.1111/j.1463-1326.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstock J, Wilson C, Fleck P. Alogliptin versus glipizide monotherapy in elderly type-2 diabetes mellitus patients with mild hyperglycemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab. 2013 doi: 10.1111/dom.12102. [DOI] [PubMed] [Google Scholar]
- 26.Food and Drug Administration FDA drug safety communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type-2 diabetes. Available at: www.fda.gov/Drugs/DrugSafety/ucm343187.htm. Updated March 18, 2013. Accessed February 9, 2014.
- 27.Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013 doi: 10.1111/dom.12176. [DOI] [PubMed] [Google Scholar]
- 28.Food and Drug Administration, Center for Drug Evaluation and Research Application number: 022271Orig1s000: Summary Review. Available at: www.accessdata.fda.gov/drugsatfda_docs/nda/2013/022271Orig1s000SumR.pdf. Accessed February 9, 2014.
- 29.Scott LJ. Alogliptin: a review of its use in the management of type-2 diabetes mellitus. Drugs. 2010;70:2051–2072. doi: 10.2165/11205080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration, Center for Drug Evaluation and Research Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type-2 diabetes. 2008 Dec; Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071627.pdf. Accessed February 9, 2014. [Google Scholar]
- 31.White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type-2 diabetes mellitus. Diabetes Obes Metab. 2013;15:668–673. doi: 10.1111/dom.12093. [DOI] [PubMed] [Google Scholar]
- 32.White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type-2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type-2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620–626. doi: 10.1016/j.ahj.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 33.White WB, Canon CP, Heller SR. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 34.Fuji Y, Abe M, Higuchi T, et al. The dipeptidyl peptidase-4 inhibitor alogliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Expert Opin Pharmacother. 2013;14:259–267. doi: 10.1517/14656566.2013.761690. [DOI] [PubMed] [Google Scholar]
- 35. Red Book Online via Micromedex. Available at: www.micromedexsolutions.com. Accessed February 2, 2014.
- 36.Rendell M, Drincic A, Andukuri R. Alogliptin benzoate for the treatment of type-2 diabetes. Expert Opin Pharmacother. 2012;13:553–563. doi: 10.1517/14656566.2012.656088. [DOI] [PubMed] [Google Scholar]
- 37.Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type-2 diabetes mellitus. Pharmacotherapy. 2010;30:463–484. doi: 10.1592/phco.30.5.463. [DOI] [PubMed] [Google Scholar]
- 38.Tradjenta (linagliptin), prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; Jun, 2013. [Google Scholar]
- 39.Onglyza (saxagliptin), prescribing information. Princeton, NJ: Bristol-Myers Squibb Company; Accessed February 2, 2014. [Google Scholar]
- 40.Januvia (sitagliptin), prescribing information. Whitehouse Station, NJ: Merck & Co, Inc; Accessed February 2, 2014. [Google Scholar]
- 41. Micromedex Healthcare Series Website. Alogliptin. Available at: www.thomsonhc.com.ezproxy.samford.edu/home/dispatch. Accessed July 27, 2013.


