Abstract
Background:
The WHO recommendation for parasitological diagnosis of malaria wherever possible is challenged by evidence of poor-quality microscopy in African hospitals but the reasons are not clear.
Methods:
All 12 of the busier district hospital laboratories from three regions of Tanzania were assessed for quality of the working environment and slide readers read 10 reference slides under exam conditions. Slides that had been routinely read were removed for expert reading.
Results:
Of 44 slide readers in the study, 39 (88.6%) correctly read >90% of the reference slides. Of 206 slides that had been routinely read, 33 (16%) were judged to be unreadable, 104 (51%) were readable with difficulty, and 69 (34%) were easily readable. Compared to expert reading of the same slide, the sensitivity of routine slide results of easily readable slides was 85.7% (95% confidence interval: 77.4–94.0), falling to 44.4% (95% confidence interval: 34.5–54.4) for slides that were ‘readable with difficulty’.
Conclusions:
The commonest cause of inaccurate results was the quality of the slide itself, correction of which is likely to be achievable within existing resources. A minority of slide readers were unable to read slides even under ideal conditions, suggesting the need for a ‘slide reading licence’ scheme.
Keywords: Plasmodium falciparum, Malaria, Microscopy, Quality
Introduction
Malaria is commonly overdiagnosed in health facilities in malaria-endemic areas of Africa and this is largely the result of the overlap of symptoms with those of other common illnesses and application of the WHO policy of presumptive treatment for malaria in young children.1,2 While this policy has been effective in high transmission areas, there has been growing awareness over the last decade of the negative effects of overdiagnosis of malaria that include the relatively high cost of artemisinin combination drugs, high selection pressure for drug resistance, inaccurate surveillance data, and neglect of alternative diagnoses.3–7 As a result, the most recent (2010) WHO guideline for malaria diagnosis and treatment recommends that, wherever possible, malaria treatment should depend on a positive parasitological test, either using microscopy or a rapid immunochromatographic diagnostic test.8
This policy presents a substantial challenge to diagnostic services in many African countries. While there are few systematic studies of the quality of malaria microscopy in Africa, the limited evidence that is available suggests that quality assurance (QA) systems are rarely operating and the results of malaria microscopy are often highly discordant with expert reading of the same slide or a concurrent slide taken from the same patient.5,9,10 In spite of this, malaria microscopy remains the gold standard of malaria diagnosis.
The large-scale roll-out of rapid immunochromatographic diagnostic tests and the need to build confidence among prescribers in parasitological test results both demand a critical review of the quality of slide reading, especially in district hospital laboratories that provide the most accurate test result for the whole district. We therefore conducted this study to assess the accuracy of slide reading and the reasons for any deficiencies that were identified in district hospital laboratories in three regions of Tanzania.
Methods
Study area and hospitals
Two administrative regions in the northeast of Tanzania and one in the mid-west were chosen for their accessibility to investigators. Within these regions, 12 district hospital laboratories had read more than 3000 slides in 2009 and were included in the study; an additional four hospital laboratories had read <3000 slides in 2009 and were excluded. In 2007, the national malaria prevalence study reported mean parasite prevalence in children under the age of 10 years in the three regions to be 1, 9.7 and 13.9%, respectively.11
Retrospective and observational data
Hospitals were visited for study sensitisation and laboratory staff gave verbal consent to participate. The chief laboratory technician in each laboratory provided information on the number of laboratory staff who regularly read slides (excluding trainees) and their qualifications. Staff were classified as follows: ‘laboratory technician’ with 3 years of full-time training at a regional laboratory, ‘laboratory assistant’ with 2 years full-time training at a regional laboratory, and ‘laboratory attendant’ with 6 months training at a district laboratory and whose duties include cleaning and sample preparation. In Tanzania, laboratory assistants commonly read blood slides under supervision although they are not officially qualified to do so. Laboratory slide readers who were available on the day of the study visit were briefly interviewed for their opinion on constraints to good slide results.
Laboratories were inspected by an experienced laboratory technician for the presence of electricity and clean water, adequacy of the workspace and staining technique and the quality of microscopes. The total slides read and the slide positivity rate in the year 2009 were recorded for each hospital from routine data or extracted from the laboratory register.
Accuracy of slide results
A standard set of 10 test slides was selected from a large archive of slides that had been read for clinical trials. The slides were selected according to the following criteria: slide results had been independently agreed by two expert slide readers with concordance of slide density ±10%. Slides were selected to include three slides that were negative for Plasmodium parasites, three that were positive for P. falciparum asexual parasites to a density of 5–8 parasites per 200 white cells, and three that were positive to a density of 50–80/200 white cells. Initially 20 slides meeting these criteria were selected from which the best quality 10 slides were selected by an expert laboratory technician. Slides were ‘thick films’ only, stained with Giemsa according to a standard laboratory procedure and mounted under cover slips.
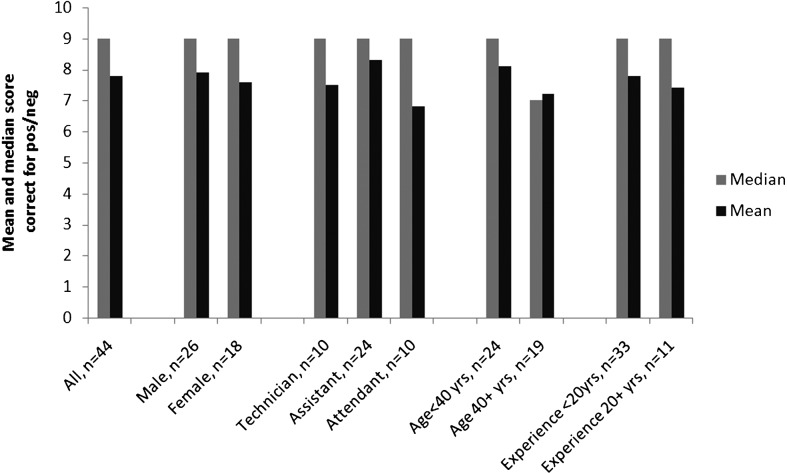
Readers who were available in each site on the day of the study visit were asked to examine these 10 slides using a good-quality microscope in quiet and clean surroundings and where no time pressure was imposed. Readers were asked to record results as negative or positive for P. falciparum and readers were informed that no other Plasmodium species or other pathogen would be present (Figure 1). If a slide was reported as positive, readers were asked to record the density of asexual parasites per 200 white blood cells (the standard counting system in all laboratories).
Figure 1.
Median and mean scores for the correct identification of the presence or absence of P. falciparum asexual parasites by 44 slide readers who each read 10 high-quality test slides.
After completion of the slide reading test, staff were asked to rank in order of importance, the factors that in their opinion constrained their ability to produce good-quality slide results.
As is common practice in Tanzania, all of the laboratories in this study routinely washed and re-used slides; 20 slides from each hospital were randomly selected from among blood slides that had been read and were waiting to be washed. These slides were numbered and the local laboratory result of each slide was recorded from the laboratory register. The quality of these slides was independently assessed by two experienced microscopists, with any discordance resolved by discussion between the readers, according to whether or not a result could be given with reasonable confidence (‘good quality’), or with difficulty (‘poor quality’) or a result could not be obtained (‘unreadable’). Slides that were judged to be readable were double read for presence or absence of P. falciparum with any discordance resolved by discussion and review of the slide by the two readers.
Data analysis and ethical approval
Data were double entered into MS Access (Microsoft Corp., Redmond, VA, USA) and analyzed using Stata-11 (Statacorp, College Station, TX, USA).
Ethical clearance, including a waiver for the need for written consent, was obtained from the Ethics Committee of the Kilimanjaro Christian Medical Center in Tanzania.
Results
Laboratory staff
Across the 12 hospitals, 49 laboratory staff regularly read slides. Of these, 11 (22.5%) were laboratory technicians, 25 (51%) were laboratory assistants, and 13 (26.5%) were laboratory attendants. The average age was 38 years (range 23–59 years) and 21 (42.9%) were female; the average length of experience in slide reading was 12 years (range 1–41 years). The average number of slides read per hospital ranged from 21 to 50 slides/slide reader/working day (Table 1).
Table 1. Staff levels and slides read in the 12 study laboratories.
| Hospital | Lab staff | Technician* | Assistant† | Attendant‡ | Slide readers§ | Total slides read, 2009 | % positive |
| 1 | 11 | 1 | 7 | 3 | 9 | 16 997 | 68% |
| 2 | 5 | 1 | 2 | 2 | 5 | 7597 | 33% |
| 3 | 10 | 1 | 5 | 4 | 10 | 35 571 | 62% |
| 4 | 16 | 3 | 5 | 8 | 5 | 13 995 | 50% |
| 5 | 4 | 0 | 2 | 2 | 2 | 10 355 | 2% |
| 6 | 7 | 2 | 4 | 1 | 6 | 6031 | 23% |
| 7 | 10 | 1 | 5 | 4 | 6 | 13 241 | 20% |
| 8 | 5 | 2 | 1 | 2 | 4 | 6127 | 13% |
| 9 | 3 | 1 | 1 | 2 | 6978 | 13% | |
| 10 | 6 | 2 | 1 | 3 | 6 | 6165 | 0% |
| 11 | 5 | 2 | 2 | 1 | 4 | 3800 | 9% |
| 12 | 6 | 1 | 2 | 3 | 6 | 3240 | 10% |
Notes: *Laboratory technicians are senior staff with 3 years of training.
†Laboratory assistants are middle-grade staff with 2 years of training.
‡Laboratory attendants have 6 months of training and are not qualified for slide reading.
§Slide reader defined as staff who were reported to read slides on at least 3 days per working week.
Standard slide reading test
The majority of the hospital slide readers were able to accurately read the 10 test slides, and parasite density did not influence this result (P = 0.43) (Table 2).
Table 2. Quality of slides that had been read in study hospitals according to re-examination by expert slide readers.
| Hospital no. | Good | Poor | Unreadable |
| 1 | 4 (18.2) | 13 (59.1) | 5 (22.7) |
| 2 | 2 (10.0) | 14 (70.0) | 4 (20.0) |
| 3 | 2 (10.5) | 7 (36.8) | 10 (52.6) |
| 4 | 2 (11.7) | 15 (88.2) | 0 |
| 5 | 2 (10.0) | 14 (70.0) | 4 (20.0) |
| 6 | 13 (86.7) | 0 | 2 (13.3) |
| 7 | 6 (30.0) | 12 (60.0) | 2 (10.0) |
| 8 | 5 (62.5) | 3 (37.5) | 0 |
| 9 | 1 (16.7) | 4 (66.7) | 1 (16.7) |
| 10 | 2 (10.0) | 13 (65.0) | 5 (25.0) |
| 11 | 13 (65.0) | 7 (35.0) | 0 |
| 12 | 17 (89.5) | 2 (10.5) | 0 |
| Total | 69 (33.5) | 104 (50.5) | 33 (16.0) |
Quality of routine hospital slides
Overall, the quality of routine hospital slides was poor. Only 33% (69/208) of slides examined were judged to be good quality, the rest were judged to be poor in quality or unreadable. However, there was wide variation between laboratories in the proportion of slides that were judged to be unreadable (0–62%).
The level of agreement between the routine hospital slide results and an expert result (used as gold standard) was reasonable for good-quality slides but low for poor-quality slides (Table 3).
Table 3. Sensitivity and specificity of 173 routinely read slides that were judged to be readable compared to double-reading by expert microscopists.
| Good slides only | Poor but readable slides | |
| Sensitivity | 85.7% (95% CI: 77.4–94.0) | 44.4% (95% CI: 34.5–54.4) |
| Specificity | 91.8% (95% CI: 85.3–98.3) | 88.4% (95% CI: 82.0–94.8) |
| Positive predictive value | 54.6% (95% CI: 42.7–66.4) | 60.0% (95% CI: 50.2–69.8) |
| Negative predictive value | 98.3% (95% CI: 95.1–101.4) | 80.3% (95% CI: 72.3–88.2) |
Note: CI, confidence interval.
When the slide quality was found to be poor or unreadable, expert slide readers recorded the specific defect(s) of the slide and the large majority of these defects were due to some combination of dirt, poor staining, precipitates, or poor smearing technique (Table 4).
Table 4. Reasons for poor quality of 173 routine slides examined by expert slide readers.
| Reason for poor quality | Number (%) |
| Dirt or scratch on slide | 17 (13.4) |
| Smear too thick or thin | 10 (7.9) |
| Over- or under-stained | 14 (11) |
| Precipitates present | 18 (14.2) |
| Poor smear and staining | 18 (14.2) |
| Poor staining and precipitates present | 19 (15) |
| Dirt and precipitates present | 22 (17.3) |
| Poor smear and dirt on slide | 5 (3.9) |
| Poor staining and dirty slide | 2 (1.6) |
| Other | 2 (1.6) |
Laboratory inspection
The expert assessor judged that space was often inadequate to read slides (9/12; 75%) and some laboratory staff also mentioned this. By contrast, laboratory staff complained of lack of office space and rest areas while the expert assessor did not consider these to be a problem. Laboratory equipment and stains were generally judged to be adequate. Most laboratory staff reported that microscopes were shared between 2–3 readers although this was not thought to be a problem in producing good-quality results. Field stain was more commonly used than Giemsa stain (75% versus 25%) but this was not considered to impair the quality of slide results.
Staff interviews
Forty-four laboratory staff were asked to rank the relative importance in constraining their ability to produce correct slide results; ‘low salary’ (16; 36%) and ‘lack of training’ (11; 25%) were mostly commonly ranked as most important, followed by ‘physical space’ (7; 16%) and ‘high workload’ (6; 14%). Only 5/44(11%) slide readers ranked ‘poor slides’ as the most important factor followed by ‘lack of QA’ (2; 5%) and ‘poor microscope’ (1; 2%).
Only 22/44 (50%) laboratory staff defined ‘QA’ with a definition that included the examination of reference slides. Two of the senior laboratory technicians reported that there was no QA scheme operating in their laboratory. While 10 of the 12 laboratories were reported to be part of a QA scheme, eight of these reported that there was no documentation or specific corrective action taken when problems were identified.
Discussion
The findings of this study are consistent with earlier studies and suggest that currently many district hospital laboratories do not produce malaria blood slide results that meet minimum standards needed to guide clinical decisions.12 This inevitably results in a number of adverse consequences including failure to treat patients who actually have malaria, waste of scarce resources, and loss of confidence among prescribers and their patients in the validity of laboratory results. In addition, poor-quality slide results can result in misleading surveillance data. These problems will inevitably limit the success of current initiatives to move from presumptive to parasitological diagnosis of malaria.
Accurate slide results rely on a chain of events including availability of a clean slide, good smearing technique and drying, proper stain preparation and staining procedures, availability of a functioning microscope, and a trained slide reader who has sufficient time to provide an accurate result. This is achievable with modest resources as evidenced by the large number of research and other facilities that meet WHO minimum standards of 95% sensitivity and 90% specificity when compared to expert slide reading.12 In addition, high levels of training are not essential in determining the presence or absence of asexual Plasmodium parasites; in Malawi, school leavers were able to produce good results in determining the presence or absence of P. falciparum asexual parasitemia and good-quality slide results were reported in Zambia following expansion of services for blood slide microscopy.13,14 There is also good evidence from Uganda that relatively brief additional training of laboratory staff can result in marked improvements in the quality of slide preparation and reading.15
In this study, we found that poor results were primarily due to two factors. Firstly, a minority of slide readers appeared unable to read even high-quality thick films. Secondly, almost half of the routine slides were judged to be poor quality or unreadable, and routine slide results among good slides reached almost 90% sensitivity but declined sharply for poor slides, a finding consistent with at least one other study.16 We did not find major defects in microscopes although this has been previously found to be a problem and this may reflect the investment in hospitals and laboratories that has occurred progressively across Africa in the last 10 years.17
These findings suggest that relatively simple and achievable remedial action could result in a marked improvement. Firstly, slide readers should be evaluated under controlled conditions to ascertain their ability to read high-quality reference slides. Our findings suggest that staff qualifications (including laboratory attendants who are not actually qualified to read slides) were only weakly correlated with competence in slide reading, a finding that suggests that personal characteristics such as good eyesight and coordination or a careful and methodical approach may be more important than formal qualifications.
Among slide readers who demonstrated basic competence, the quality of results was determined by a number of common faults in slide preparation, and these can be improved with relatively basic training of ward and laboratory staff. The wide range in the quality of slides between sites suggests that this is achievable within existing resources.
In contrast to the study findings, few of the laboratory staff rated the quality of slides as an important constraint but more than half rated high workload or low pay as the most important causes of poor slide results. Clearly, staff motivation and time to read a blood slide are important and our study was unable to evaluate this in detail but the lack of recognition of slide quality as a key problem suggests that staff have become accustomed or resigned to having to read poor-quality slides. Only one laboratory worker mentioned a functioning QA scheme to be important while this would be considered by international standards to be essential for any hospital laboratory.9
Limitations
Although covering a large area (and approximately 10% of all district hospital laboratories in Tanzania), our sample was not random across the country or region, and we cannot assume that the study findings are nationally representative. Unfortunately we were unable to assess the impact of speed in reading slides as a determinant of quality and this needs to be addressed in future studies. We were unable to identify which individual slide readers had read the routinely collected blood slides. Staff interviews were brief and predominantly consisted of closed questions while the opinions, concerns, and level of motivation of laboratory staff must be important elements in any quality review.
Conclusion
Our results suggest the need for a validation procedure for blood slide readers; staff qualifications might not be a good guide to competence and direct testing of slide reader’s ability to read reference slides is necessary. Correction of common problems relating to slide smearing, dirty slides, and poor staining is likely to result in a large and relatively low-cost improvement in the quality of blood slide results for malaria. A functioning QA scheme could address these problems and is an essential step in malaria diagnosis and surveillance.
References
- 1.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364(9448):1896–8. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Guidelines for the diagnosis and treatment of malaria in the African region., Geneva: WHO; 2003. AFR/MAL/03.01. [Google Scholar]
- 3.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329(7476):1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeung S, Van Damme W, Socheat D, White NJ, Mills A. Cost of increasing access to artemisinin combination therapy: the Cambodian experience. Malar J. 2008;7:84. doi: 10.1186/1475-2875-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zurovac D, Midia B, Ochola SA, English M, Snow RW. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006;11(4):432–40. doi: 10.1111/j.1365-3156.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 6.Zurovac D, Rowe AK. Quality of treatment for febrile illness among children at outpatient facilities in sub-Saharan Africa. Ann Trop Med Parasitol. 2006;100(4):283–96. doi: 10.1179/136485906X105633. [DOI] [PubMed] [Google Scholar]
- 7.Rowe AK, Rowe SY, Snow RW, Korenromp EL, Schellenberg JR, Stein C, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35(3):691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Guidelines for malaria diagnosis and treatment. Geneva: WHO; 2010. [Google Scholar]
- 9.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42(3):377–82. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 10.Reyburn H, Ruanda J, Mwerinde O, Drakeley C. The contribution of microscopy to targeting antimalarial treatment in a low transmission area of Tanzania. Malar J. 2006;5:4. doi: 10.1186/1475-2875-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanzania Commission for AIDS. National AIDS and malaria indicator survey. Dar es Salaam: United Republic of Tanzania; 2008. [Google Scholar]
- 12.WHO. The role of laboratory diagnosis to support malaria disease managment: report of a WHO technical consultation. Geneva: WHO; 2004. [Google Scholar]
- 13.Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60(6):1024–30. doi: 10.4269/ajtmh.1999.60.1024. [DOI] [PubMed] [Google Scholar]
- 14.Jonkman A, Chibwe RA, Khoromana CO, Liabunya UL, Chaponda ME, Kandiero GE, et al. Cost-saving through microscopy-based versus presumptive diagnosis of malaria in adult outpatients in Malawi. Bull World Health Organ. 1995;73(2):223–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Kiggundu M, Nsobya SL, Kamya MR, Filler S, Nasr S, Dorsey G, et al. Evaluation of a comprehensive refresher training program in malaria microscopy covering four districts of Uganda. Am J Trop Med Hyg. 2011;84(5):820–4. doi: 10.4269/ajtmh.2011.10-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishengoma DR, Derua YA, Rwegoshora RT, Tenu F, Massaga JJ, Mboera LE, et al. The performance of health laboratories and the quality of malaria diagnosis in six districts of Tanzania. Ann Trop Med Parasitol. 2010;104(2):123–35. doi: 10.1179/136485910X12607012373993. [DOI] [PubMed] [Google Scholar]
- 17.Mundy CJ, Bates I, Nkhoma W, Floyd K, Kadewele G, Ngwira M, et al. The operation, quality and costs of a district hospital laboratory service in Malawi. Trans R Soc Trop Med Hyg. 2003;97(4):403–8. doi: 10.1016/s0035-9203(03)90070-8. [DOI] [PubMed] [Google Scholar]