Abstract
The authors have described Chinius samarensis n. sp. (Diptera; Psychodidae) from the Philippines (Island of Samar). This is the fourth known species belonging to the Asiatic genus Chinius Leng, 1987. Like the other Chinius, C. samarensis n. sp. is a cavernicolous species. The genital ducts of the male are four times shorter than the spermathecal ducts. However, the authors think that male and female belong to the same species due to their similar cytochrome b mt DNA sequences. A differential diagnosis with the other species belonging to the genus Chinius is provided.
Keywords: Phlebotomine sandflies, Chinius, New species, Description, Caves, Philippines, Cytochrome b mt DNA
Introduction
The genus Chinius was created by Leng for a cavernicolous sandfly, Chinius junlianensis, from Sichuan and Guizhou, China.1 The female has been re-described by Depaquit et al.2 Later on, two other species of the same genus: C. barbazani Depaquit, Léger & Beales, 2006 and C. eunicegalatiae Léger, Depaquit & Gay, 2010 have been described in the same type of habitat (caves) respectively in Thailand and Laos.3,4
In this paper, we have described a fourth cavernicolous species belonging to the genus Chinius: C. samarensis from Samar Island (Philippines).
Material and Methods
Captures have been performed on 1 June and 2 June 2011 with CDC miniature incandescent white light traps without CO2 in and at the entrance of a cave near Borongan (Basiad) (11°15′33″N, 125°09′47″E; alt. 0–12 m above sea level).
Collected sandflies (42 males and 53 females) were kept in 96% ethanol and mounted in toto for morphological study according to Abonnenc’s5 method: 4–8 hours in 10% KOH solution followed by eight baths (20 minutes each) of water, then at least 1 hour in Marc–André solution. Females were directly mounted in this liquid with their spermathecae dissected, then re-mounted in chloral gum when possible, or in Canada balsam as described below. Some males and a few females were dehydrated in ethanol of growing concentrations (from 70% to absolute ethanol), then in beech creosote, and finally mounted in Canada balsam.
Moreover, two specimens (one male and one female) were prepared for both morphological and molecular studies. The head and genitalia of each sandfly were cut off in a drop of ethanol, cleared in boiling Marc–André solution, and mounted under a cover slip in chloral gum for identification. Genomic DNA was extracted from thorax and abdomen with the QIAmp DNA Mini Kit (Qiagen, Germany) as explained by Depaquit et al. (2004).6 Cytochrome b polymerase chain reactions were performed according to the primers N1N-PDR and C3B-PDR and protocols used by Esseghir et al. (1997).7 Direct sequencing of both DNA strands was performed using the primers used for DNA amplification.
Results
Description of the male of Chinius samarensis n. sp. (Figs. 1 and 2)
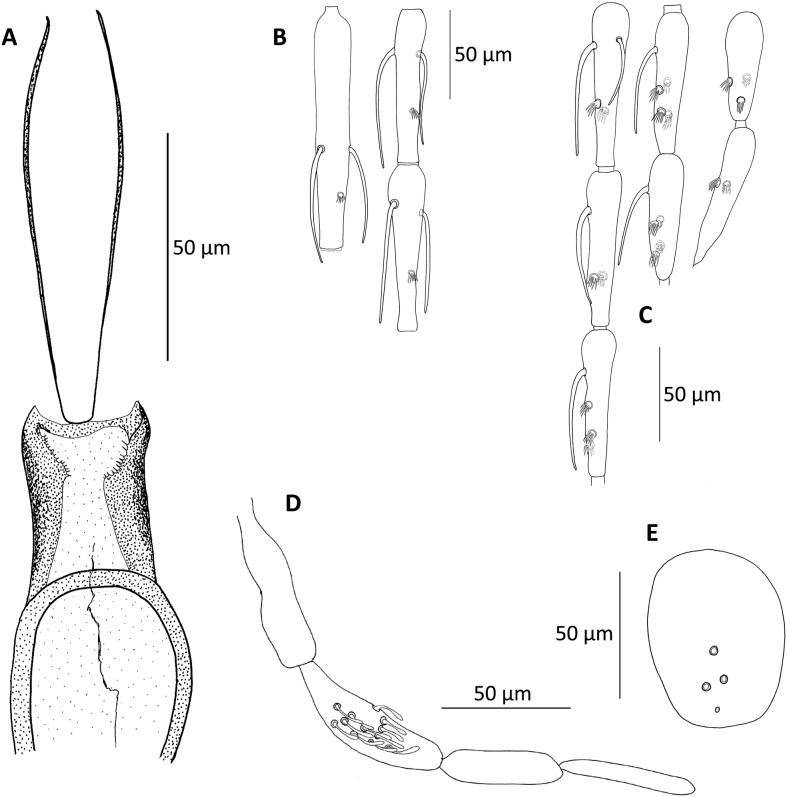
Figure 1.
Chinius samarensis n. sp. male. (A) Pharynx and cibarium; (B) antennal segments III–V; (C) antennal segments X–XVI; (D) palp; (E) clypeus.
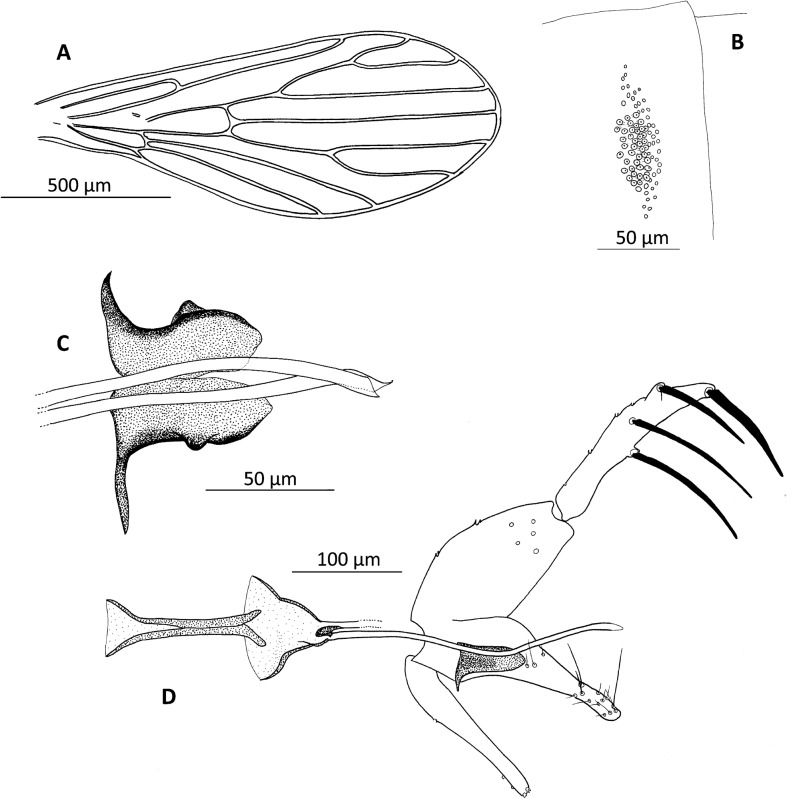
Figure 2.
Chinius samarensis n. sp. male. (A) Wing; (B) ‘trumpet glands’ of the third abdominal; (C) aedeagus; (D) genitalia.
This species is small and very fragile (very few specimens had antennae). The head is difficult to mount dorso-ventrally. Consequently, we used several specimens for the description.
Head
Eye: with generally 18 facets.
AIII = 137 μm; AIV = 91 μm; AV = 93 μm (measured on one specimen).
Ascoid formula (on one specimen): 2/III–X; 1/XI–XIV. Ascoids do not reach the next articulation. One papilla on antennal segments III, IV, V, two papillae on segments X, XII, XVI, three papillae on segments XII, XV, and four papillae on segments XII and XIV.
Palpal formula: 1–(2–4)–5–3. About 10 club-like Newstead’s scales on the third segment.
Labrum (L) = 90–100 μm
Clypeus: length = 55–70 μm; 3–4 setae on the dorsal side.
Cibarium: margins ornamented with tiny teeth. Absence of central teeth.
Pharynx: free of ornamentation, narrowed at its posterior part.
Thorax
Pleural setae absent
Wings rounded with a R2 (alpha) short = 150–170 μm. Length = 1.400–1.500 μm; width = 500–520 μm; alpha: 140–180 μm; beta: 240–260 μm; gamma: 150–200 μm; delta: 28–48 μm; pi: 60–80 μm.
Abdomen and genitalia
Antero-lateral groups of about 50 trumpet-like structures on tergites 4 and 5, similar to those of other species of the genus Chinius.1,3
Coxite measures about 145–170 μm in length and 70–90 μm in breadth, with the inner side covered with about 20 stout hairs.
Style measures 155–175 μm in length and about 30 μm in breadth with four nearly equidistant spines.
Paramere measures 150–170 μm in length, becoming gradually narrowed towards the apex, slightly curved, with a round extremity.
Surstyle is 100–130 μm long.
Aedeagus 57–70 μm in length, strongly chitinized, large, with a special form (Fig. 2).
The lengths of the genital filaments (250–320 μm) and genital pump (185–215 μm) give a genital filament/pump ratio of about 1.4.
Description of the presumed female of Chinensis samarensis n. sp. (Figs. 3 and 4)
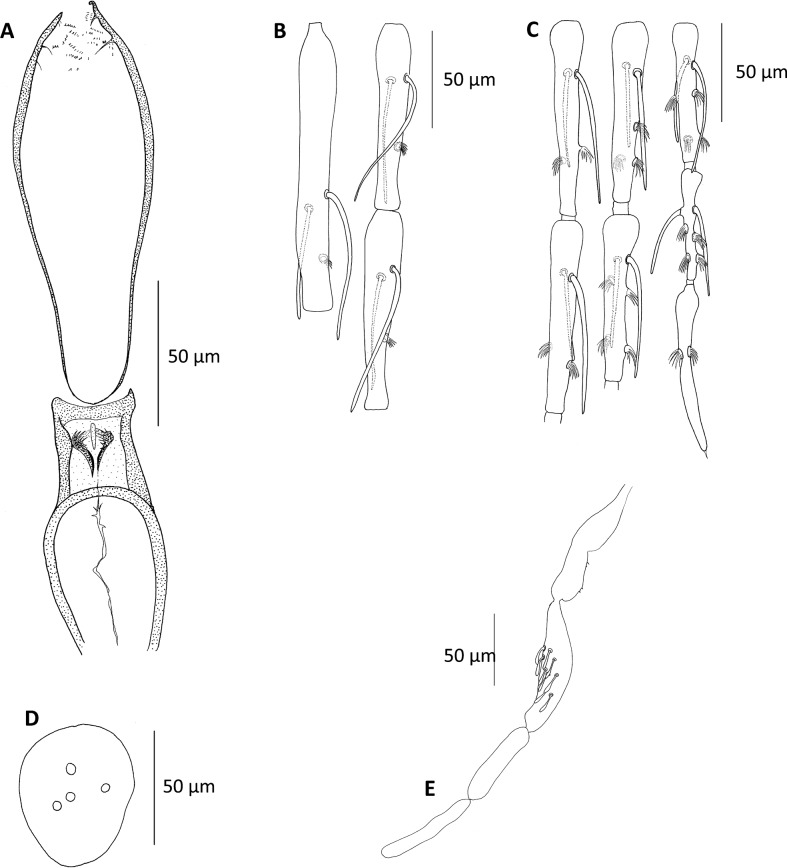
Figure 3.
Chinius samarensis n. sp. female. (A) Pharynx and cibarium; (B) antennal segments III–V; (C) antennal segments X–XVI; (D) clypeus; (E) palp.
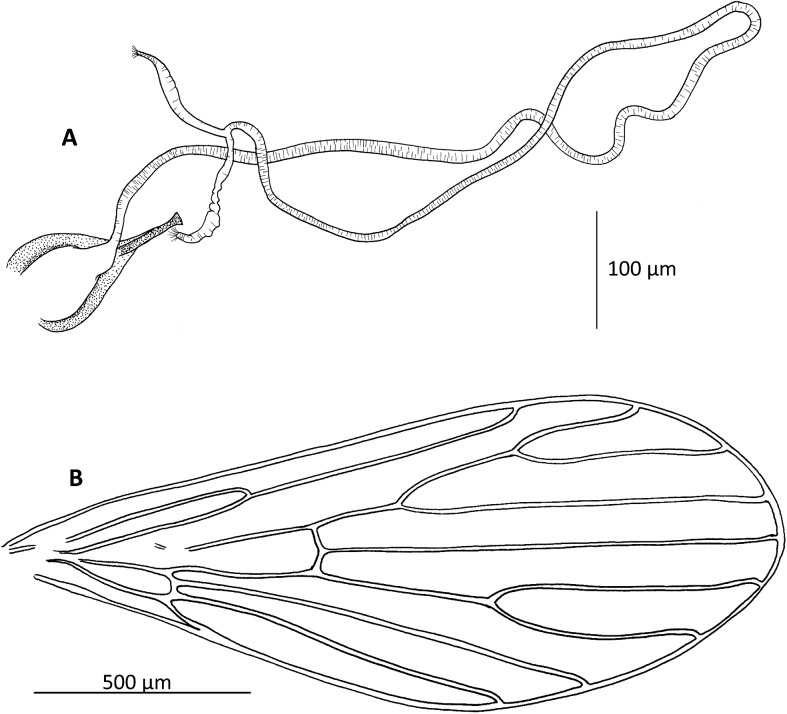
Figure 4.
Chinius samarensis n. sp. female. (A) Furca and spermathecae; (B) wing.
Head
Eye with 18–20 facets.
AIII = 125–160 μm; AIV = 85–110 μm; AV = 90–120 μm.
Ascoid formula (on two preparations): 2/III–XV but some specimens showed only one ascoid on segments XIII–XIV. Ascoids slender, as long as the segment. Number of papillae similar to that observed in males, except one more (four) on the antennal segment XV.
Palpal formula: 1–2–3–5–4. About 10 club-shaped Newstead’s scale on the third segment.
Labrum = 120–130 μm.
Clypeus measures 80–100 μm in length with three to six setae on the dorsal side.
Cibarium: thin teeth arranged along two diagonally oriented lines located in the center of the cibarial chamber.
Pharynx: wide, narrowed at its posterior part which is covered with a few large crossing folds and with a few fringes of tiny scales.
Thorax
Pleural setae absent.
Wings rounded with an R2 (alpha) not very short = 230–270 μm. Length = 1.750–1.950 μm; width = 650–780 μm; alpha: 220–280 μm; beta: 250–320 μm; gamma: 200–240 μm; delta: 20–50 μm; pi: 120–160 μm.
Genitalia
Spermathecae are very long, tubular, and narrow with short individual ducts with no clear limit between each spermatheca and its individual duct.
The combined length of the individual duct and spermatheca is 100–130 μm.
Common duct measures 850–1300 μm in length. It is thin and slightly pleated.
Furca thick, morphologically typical of the genus Chinius.
Molecular data
Sequences analyzed of partial cytochrome b obtained from a male and from a female (436 bp each) have been deposited in GenBank under accession numbers JX485975 and JX485976. They are exactly similar (100% homology).
Derivatio nominis
From the name of the Island of Samar where all our samples were obtained.
Depositories of Type Material
Museum National d’Histoire Nature (Paris): holotype male, seven paratypes males, and four paratypes females, including an allotype mounted in choral gum (five slides);
Natural History Museum (London): six paratype males and five paratype females (two slides);
Bernice Pauahi Bishop Museum (Honolulu): three paratype males (one slide).
Discussion
We classified C. samarensis n. sp. in the genus Chinius because it possesses the morphological characters of the genus Chinius, as revised by Depaquit et al.:3
absence of pleural setae;
presence in the males of trumpet-like organs on abdominal tergites 4 and 5;
interocular suture complete, very few setae on vertex and clypeus;
short labrum;
cibarium with several rows of fine teeth;
broad and rounded wings with reduced or lacking R2;
Moreover, males have
trumpet-like organs on abdominal tergites 4 and 5;
style with 4 spines.
And females have
smooth spermathecae with a common duct;
typical thick furca.
R2 is present as in the type species (absent in C. barbazani and C. eunicegalatiae) but longer in the female than in the male (same unequality observed in C. junlianensis). This state of character corroborates our opinion on the terminal synapomorphism of the character ‘absence of R2’ in Phlebotominae and more generally in Diptera.3
A question must be raised: the short genital filaments of the males are about four times shorter than the long spermathecal ducts of the female. For phlebotomine sandflies, the length of these genital organs is usually more or less similar. However, when they differ, the male genital filaments are usually longer than female spermathecal ducts, except that of Sergentomyia clydei.8 Consequently, we decided to describe the female as ‘the presumed female of C. samarensis n. sp.’ as we did previously for that of C. eunicegalatiae. However, the link between females and males was based on the following items: (1) they were captured together, the same day, in the same trap; (2) both belong to the same genus, and we suppose, to the same species; and (3) the cytochrome b sequences obtained from a male and from a female are exactly similar.
As other Chinius, C. samarensis is a cave dweller associated with bats and reptiles. The states of characters ‘small eyes with few facets’ and ‘short labrum’ are probably adaptive ones.
The Chinius geographical repartition corresponds to the classical oriento-Australasian track used by many groups of insects. Note that the fossil species: Palaeomyia burmitis Poinar, 2004 from Burma, has a rounded wing showing a very short R2, looking like that of C. junlianensis.9
Differential Diagnosis of the Species Belonging to the Genus Chinius
A — males
No. R2 vein on the wing:
Insertion of the basal spine in the middle of the style: C. barbazani;
Insertion of the basal spine at the proximal third of the style: C. eunicegalatiae.
a short R2 vein on the wing:
genital filaments long (>1000 μm): C. junlianensis;
genital filaments short (<400 μm): C. samarensis.
B — females:
No. R2 vein on the wing:
Individual ducts/common duct = about 1: C. barbazani;
Individual ducts/common duct = about 0.35: C. eunicegalatiae.
a short R2 vein on the wing:
pharynx: enlarged posterior part with thick walls and armed surface: C. junlianensis;
pharynx: not enlarged, without ornaments: C. samarensis.
References
- 1.Leng YJ. A preliminary survey of phlebotomine sandflies in limestone caves of Sichuan and Guizhou Provinces, south-west China, with description and discussion of a primitive new genus Chinius. Ann Trop Med Parasitol. 1987;81:311–7. doi: 10.1080/00034983.1987.11812125. [DOI] [PubMed] [Google Scholar]
- 2.Depaquit J, Léger N, Zhang LM, Leng YJ. Chinius junlianensis Leng, 1987 (Diptera: Psychodidae): new morphological data. Ann Trop Med Parasitol. 2007;101:181–4. doi: 10.1179/136485907X154915. [DOI] [PubMed] [Google Scholar]
- 3.Depaquit J, Léger N, Beales P. Chinius barbazani n. sp. de Thailande (Diptera: Psychodidae). Parasite. 2006;13:151–8. doi: 10.1051/parasite/2006132151. [DOI] [PubMed] [Google Scholar]
- 4.Léger N, Depaquit J, Gay F. Chinius eunicegalatiae n. sp. (Diptera; PSychodidae), a cavernicolous sandfly from Laos. Ann Trop Med Parasitol. 2010;104:595–600. doi: 10.1179/136485910X12786389891524. [DOI] [PubMed] [Google Scholar]
- 5.Abonnenc E. Les phlébotomes de la région éthiopienne (Diptera, Psychodidae). Entomol Méd Parasitol. 1972;55:1–239. [Google Scholar]
- 6.Depaquit J. Léger N., Ferté H. & Robert V. Les Phlébotomes de Madagascar (Diptera – Psychodidae). II - Description de la femelle de Phlebotomus (Anaphlebotomus) fertei Depaquit, Léger & Robert, 2002 ; description du mâle et redescription de la femelle de Phlebotomus (Anaphlebotomus) berentiensis (Léger & Rodhain, 1978) comb. nov. Parasite, 2004a, 11, 201-209. [DOI] [PubMed] [Google Scholar]
- 7.Esseghir S. Ready P., Killick-Kendrick R. & Ben-Ismail R. Mitochondrial haplotypes and phylogeography of Phlebotomus vectors of Leishmania major. Insect Molecular Biology, 1997, 6, 211-225. [DOI] [PubMed] [Google Scholar]
- 8.Ilango K, Lane RP. Coadaptation of male aedeagal filaments and female spermathecal ducts of the old world phlebotomine sand flies (Diptera: Psychodidae). J Med Entomol. 2000;37:653–59. doi: 10.1603/0022-2585-37.5.653. [DOI] [PubMed] [Google Scholar]
- 9.Poinar G., Jr Paleomyia burmitis (Diptera: Phlebotomidae) a new genus and species of Cretaceous sand flies with evidence of blood-sucking habits. Proc Entomol Soc Wash. 2004;106:598–605. [Google Scholar]