Abstract
BACKGROUND:
There is a wide geographic and temporal variability of bacterial resistance among microbial causes of ventilator-associated pneumonia (VAP). The contribution of multi-drug resistant (MDR) pathogens to the VAP etiology in Saudi Arabia was never studied. We sought to examine the extent of multiple-drug resistance among common microbial causes of VAP.
MATERIALS AND METHODS:
We conducted a retrospective susceptibility study in the adult intensive care unit (ICU) of King Abdulaziz Medical City, Riyadh, Saudi Arabia. Susceptibility results of isolates from patients diagnosed with VAP between October 2004 and June 2009 were examined. The US National Healthcare Safety Network definition of MDR was adopted.
RESULTS:
A total of 248 isolates including 9 different pathogens were included. Acinetobacter spp. was highly (60-89%) resistant to all tested antimicrobials, including carbapenems (three- and four-class MDR prevalence were 86% and 69%, respectively). Pseudomonas aeruginosa was moderately (13-31%) resistant to all tested antimicrobials, including antipseudomonal penicillins (three- and four-class MDR prevalence were 13% and 10%, respectively). With an exception of ampicillin (fully resistant), Klebsiella spp. had low (0-13%) resistance to other tested antimicrobials with no detected MDR. Staphylococcus aureus was fully susceptible to vancomycin with 42% resistance to oxacillin. There were significant increasing trends of MDR Acinetobacter spp. however not P. aeruginosa during the study. Resistant pathogens were associated with worse profile of ICU patients but not patients’ outcomes.
CONCLUSION:
Acinetobacter in the current study was an increasingly resistant VAP-associated pathogen more than seen in many parts of the world. The current finding may impact local choice of initial empiric antibiotics.
Keywords: Acinetobacter, antimicrobial resistance, microbiology, Saudi Arabia, ventilator-associated pneumonia
Ventilator-associated pneumonia (VAP) is a leading health care-associated infection (HAI) among critically ill-patients accounting for 25% of all types of an intensive care unit (ICU)-acquired infections.[1] In addition to its huge impact on morbidity and health care costs, VAP is considered the most deadly HAI with a VAP-associated mortality rates range from 24 to 50% respectively.[2,3] Increasing drug resistance rates among Gram-negative pathogens that frequently cause VAP may compromise treatment and result in prolongation of hospital stays, inflation of inpatient health care costs and further increase in hospital mortality.[4,5] The infecting pathogens can be resistant to the chosen antibiotic at the start of its administration or acquire resistance during therapy, particularly with a single-agent treatment.[5] The appropriateness for initial empiric antimicrobial therapy for VAP is greatly reliant on the type of causative pathogen and its resistance pattern.[5]
There is a wide geographic and temporal variability of bacterial resistance among microbial causes of VAP and other HAIs.[6,7,8] The resistance against commonly used antimicrobials for VAP-associated pathogens, such as Pseudomonas aeruginosa and Acinetobacter baumannii, has been shown to be higher in the ICUs of developing countries than ICUs in the US.[2,9] Moreover, this variability was observed between different types of HAI.[10] These findings emphasize the importance of local VAP surveillance and antibiogram programs to correctly guide empirical therapy. Although a number of local studies examined antimicrobial resistance in clinical isolates from adult ICU patients, none of them was specific to VAP.[8,11,12] Moreover, the data evaluating the contribution of multi-drug resistant (MDR) pathogens to the VAP etiology are lacking both locally and globally. The objective of the current study was to examine single- and multiple-drug resistance among common microbial causes of VAP at an adult ICU at a tertiary care center in Saudi Arabia.
Materials and Methods
Setting
The current study was conducted at adult ICU of King Abdulaziz Medical City (KAMC) in Riyadh, Saudi Arabia. KAMC is an approximately 900-bed tertiary care facility that provides health care services to about 600,000 Saudi National Guard soldiers, employees and their families. The care provided ranges from primary and preventive care to tertiary care. The adult ICU at KAMC is a 21-bed closed medical-surgical-trauma unit covered by onsite board-certified intensivists 24 h/day, 7 days a week and admits approximately 900 patients/year. The nurse-patient ratio is 1:1.
VAP surveillance
The details of VAP surveillance were described elsewhere.[13] Briefly, a prospective surveillance program was established in 2003 as a joint project between the intensive care department and infection prevention and control department to provide regular reports about VAP rate to guide quality improvement projects. The same VAP definition was used throughout the study period, which was based on that of the US Centers for Disease Control.[14] Accordingly, VAP was defined as a pneumonia occurring more than 48 h after endotracheal intubation according to the following diagnostic criteria: new or progressive infiltrates, consolidation or cavitation on chest X-ray with one of the following: (a) New onset purulent bronchial secretions with leukopenia (white blood cell <1500/mm3) or leukocytosis (≥12,000/mm3), or core temperature ≥38.5 or ≤36°C without other cause, (b) significant positive culture from blood, or (c) bronchoalveolar lavage (BAL) or endotracheal aspirate or culture from another relevant site of infection. Both primary (first) and recurrent (second or third) episodes of VAP during the same hospitalization were included in the analysis.
Microbiological examination
Since the susceptibility data were not collected prospectively as we did with VAP episodes, we retrospectively collected the susceptibility data for all isolates obtained from patients diagnosed with VAP between October 2004 and June 2009. Isolates examined included blood, BAL, tracheal aspirate and pleural fluid. Up to two isolates were allowed per episode. Only the first positive culture was included. Multiple cultures with the same results from one episode of VAP were recorded just once. Gram-positive and negative bacteria were identified to the species level using MicroScan Walkaway (Siemens, Frimley, Camberley, UK) while antimicrobial susceptibility testing was performed using MicroScan Walkaway (Siemens, Frimley, Camberley, UK) and/or disc diffusion method. Minimum inhibitory concentrations breakpoints for different antimicrobials and interpretation of results were done according to CLSI guidelines.[15] The US National Healthcare Safety Network (NHSN) definition of multi-drug resistance was adopted.[10] Three- and four-class resistance was determined in Acinetobacter spp., P. aeruginosa and Klebsiella pneumoniae. Antimicrobial classes considered included penicillins, cephalosporins, carbapenems, fluoroquinolones and aminoglycosides. To be included in the analysis the isolates were required to be tested for at least 1 antimicrobial agent in 3 or more classes. Single-class resistance was defined as resistant to all antimicrobial agents tested in that class.
Data analysis
The frequency of resistant isolates was calculated as the sum of resistant isolates relative to the total number of isolates tested. The susceptibility results for any antimicrobial that were based on <30% of a given pathogen were not reported. Significant changing trends of MDR prevalence were examined using Mantel-Haenszel Chi-square for linear trend. Significant differences in the frequency of selected drug-pathogen resistance combination as well as multi-drug resistance between KAMC and the figures published by NHSN[9,10] or International Nosocomial Infection Control Consortium (INICC)[2] were evaluated using Chi-square or Fisher exact tests, as appropriate. Significant differences between patients by pathogen resistance were evaluated using Chi-square or Fisher exact tests, as appropriate, for categorical data and t-test for continuous data. All P values were two-tailed. P < 0.05 was considered to be significant. SPSS (release 20.0, SPSS Inc., Chicago, U.S.) and OpenEpi (Version 2.2, www.OpenEpi.com, Atlanta, GA, U.S.) softwares were used for all statistical analyses.
Results
A total of 297 VAP-associated bacterial pathogens were collected during the period from October 2004 to June 2009. The majority of isolates were obtained from tracheal aspirates (82.7%), followed by blood (16.8%) and BAL (0.4%). A total of 20 (6.7%) out of 297 VAP-associated pathogens identified were excluded due to small number (<5) of isolates. Another 29 (9.8%) isolates were further excluded due to lack of antimicrobial resistance data.
A total of 248 isolates were included in the current analysis; Acinetobacter spp. (n = 87, 35.1%), P. aeruginosa (n = 63, 25.4%), Staphylococcus aureus including methicillin-resistant Staphylococcus aureus (MRSA) (n = 43, 17.3%), Klebsiella spp. (n = 15, 6.0%), Enterobacter spp. (n = 10, 4.0%), Haemophilus spp. (n = 9, 3.6%), Stenotrophomonas maltophilia (n = 8, 3.2%), Coagulase-negative staphylococci (n = 7, 2.8%) and Escherichia coli (n = 6, 2.4%). The number of pathogens tested against individual antimicrobials and the percentage of tested pathogens out of all identified pathogens were shown in Table 1. At least one member of each of the five antimicrobial classes were tested in the majority (>85%) of isolates.
Table 1.
Number of pathogens (and percentage) tested for antimicrobial resistance among cultures isolated from VAP between 2004 and 2009 at adult medical-surgical ICU of KAMC, Riyadh, Saudi Arabia
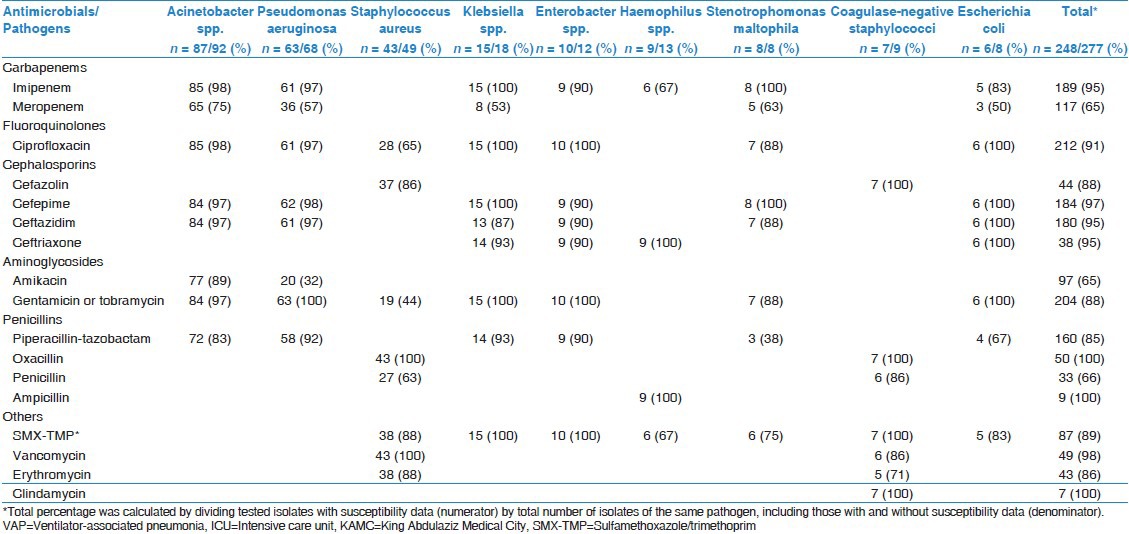
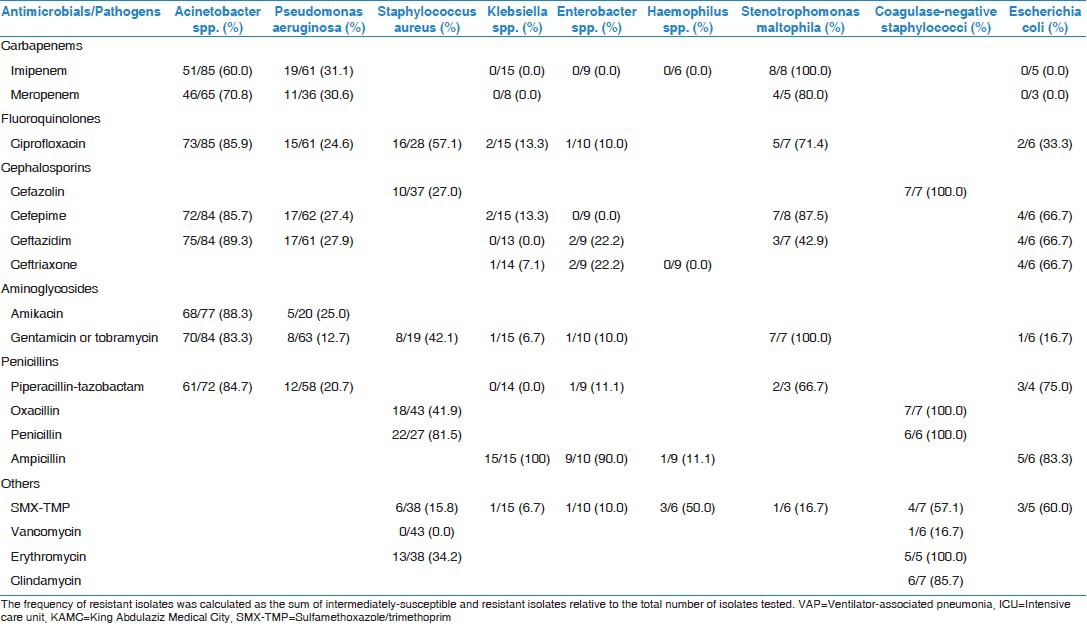
As you can be seen in Table 2 the antimicrobial resistance rates among 9 pathogens (7 Gram-negatives and 2 Gram-positives). Acinetobacter spp. had about 60-89% resistance rates to all tested antimicrobials, including carbapenems (60-71%), third-generation cephalosporins (86-89%), fluoroquinolones (86%), aminoglycosides (83-88%) and antipseudomonal penicillins (85%). P. aeruginosa had about 13-31% resistance rates to all tested antimicrobials including carbapenems (31%), third-generation cephalosporins (27-28%), fluoroquinolones (25%), aminoglycosides (13-25%) and antipseudomonal penicillins (21%). With an exception of ampicillin to which it was fully resistant, Klebsiella spp. had about 0-13% resistance rates to other tested antimicrobials. S. aureus was fully susceptible (0% resistance rate) to vancomycin with 42% resistance rate to oxacillin while Coagulase-negative staphylococci were partially susceptible (17% resistance rate) to vancomycin with full (100%) resistance to oxacillin.
Table 2.
Antimicrobial resistance percentages among pathogens associated with VAP between 2004 and 2009 at adult medical-surgical ICU of KAMC, Riyadh, Saudi Arabia
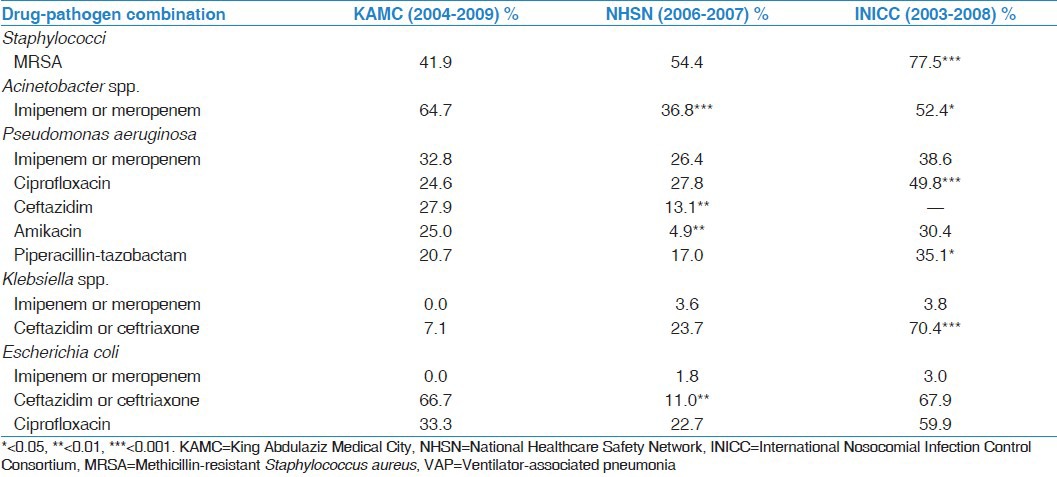
The prevalence of carbapenem-resistant Acinetobacter spp. was significantly higher in KAMC (65%) compared with the rates reported by NHSN hospitals (37%, P < 0.001) and INICC countries (52%, P = 0.028) [Table 3]. MRSA was less prevalent in KAMC (42%) compared with both NHSN (54%, P = 0.104) and INICC (78%, P < 0.001). The resistance of P. aeruginosa to ceftazidime and amikacin were significantly higher in KAMC than NHSN while the resistance to ciprofloxacin and piperacillin-tazobactam were significantly lower in KAMC than INICC. Although the resistance of both Klebsiella spp. and E. coli to carbapenems was comparable at all sites; the resistance to third-generation cephalosporins (ceftazidim or ceftriaxone) at KAMC was lower than INICC in Klebsiella spp. and was higher than NHSN in E. coli.
Table 3.
Comparisons of antimicrobial resistance rates among pathogens associated with VAP from the current study to major benchmarking reports
The prevalence of MDR Acinetobacter spp. over the years of the study [Figure 1] showed a significant increasing trend in the four-class (P for trend < 0.001) and a tendency of significant increasing trend in the three-class antimicrobial resistance (P for trend 0.064). Such significant increasing trends were not observed in P. aeruginosa. The prevalence of three- and four-class MDR Acinetobacter spp. was significantly higher in KAMC (86% and 69%, respectively) compared to NHSN hospitals [49% and 50%, respectively, Figure 2] while the prevalence of three- and four-class MDR P. aeruginosa was significantly lower in KAMC (13% and 10%, respectively) compared to NHSN hospitals (36% and 40%, respectively). Interestingly, MDR Klebsiella spp. was not detected at KAMC compared to 13-14% prevalence in NHSN.
Figure 1.
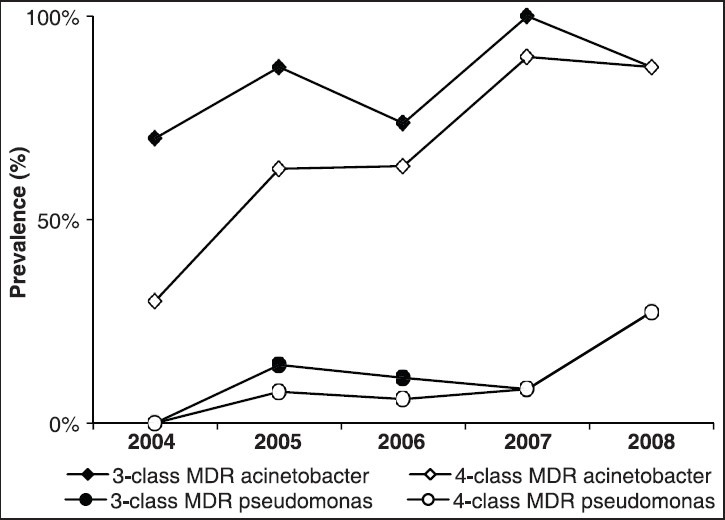
Prevalence trends of three- and four-class antimicrobial resistance among Acinetobacter spp. and Pseudomonas aeruginosa associated with ventilator-associated pneumonia between 2004 and 2008 at adult medical-surgical intensive care unit of King Abdulaziz Medical City, Riyadh, Saudi Arabia
Figure 2.
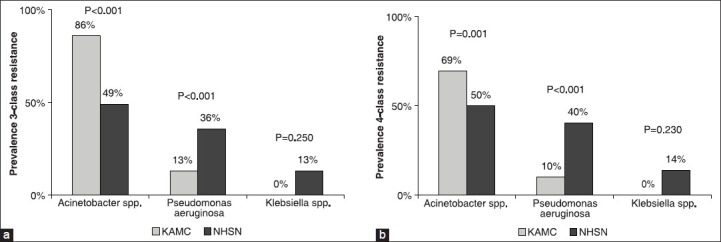
Prevalence of three-class (a) and four-class (b) antimicrobial resistance among pathogens associated with ventilator-associated pneumonia between 2004 and 2009 at adult medical-surgical intensive care unit of King Abdulaziz Medical City, Riyadh, Saudi Arabia
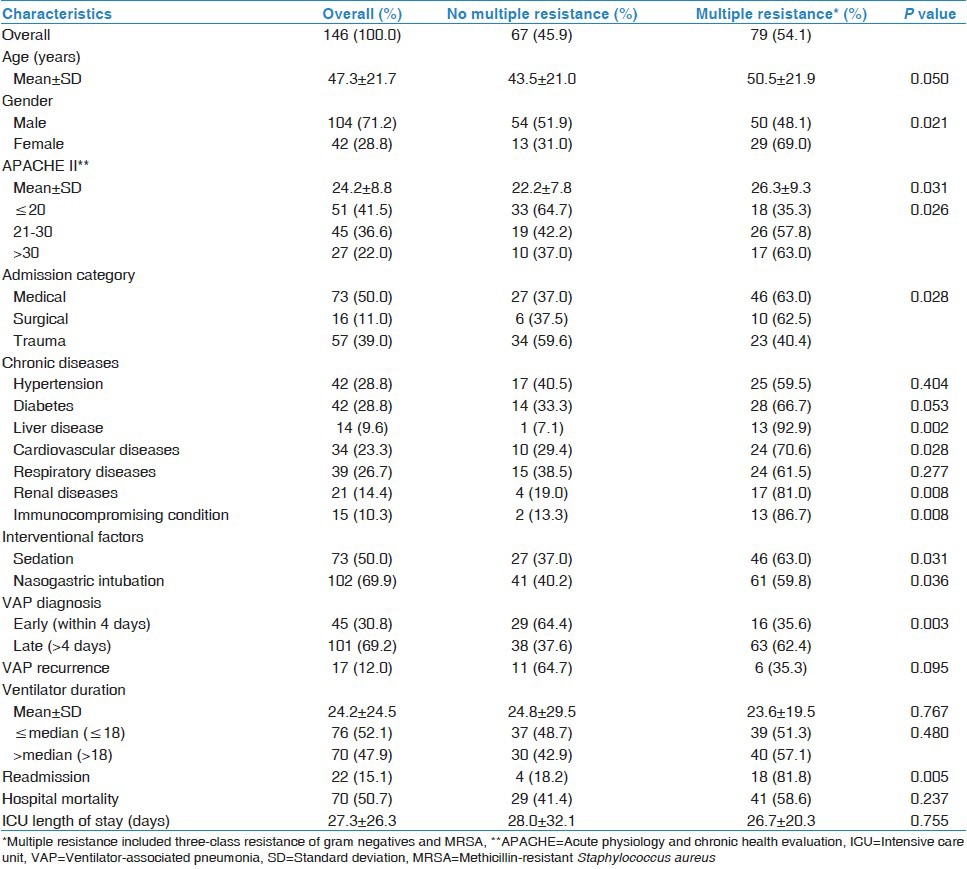
Table 4 compares demographic, clinical and outcome characteristics of the patients with primary VAP by the multiple resistance of associated pathogens. Resistant group (defined as three-class MDR Gram-negatives or MRSA) represented 54.1%. Resistance was associated with older age, female gender, higher APACHE II score, non-traumatic admission, readmission, comorbidity (liver, renal, cardiovascular and immunosuppression), sedation, nasogastric intubation and late-onset VAP. However, resistance was not associated with mortality or ICU stay.
Table 4.
Comparison of demographic, clinical and outcome characteristics of patients with primary ventilator-associated pneumonia by the pathogen resistance
Discussion
We are reporting single- and multiple-drug resistance in 9 common bacterial causes of VAP among patients of a general adult ICU over a period of 6 years. Acinetobacter spp., the most commonly VAP-associated pathogen in the current study, had very high (60-89%) resistance rates to all tested antimicrobials, including carbapenems. Despite the decline in VAP rates reported before[13] such resistance was increasing over time. In addition, the resistance level of Acinetobacter spp. was considerably higher than seen in many parts of the world.[2,9,10,16] Similarly high[8,11,12] and yet increasing[8] resistance rates in Acinetobacter spp. were reported in the local hospital isolates. Carbapenem-resistance in Acinetobacter spp. world-wide is mediated through integral membrane protein-type metalloenzymes or carbapenemases of the oxacillinase type.[17]
The current finding could have great impact on local choice of initial empiric antibiotic. Combined antibiotics regimens or more risky antibiotics as colistin or polymyxin may be the only available choice when MDR Acinetobacter spp. is suspected.[5] Acinetobacter spp. is more likely to be seen in late-onset VAP and is associated with usually affects deteriorated patients with long periods of hospitalization and ventilation.[18] All these factors promote Acinetobacter spp. colonization, growth and invasiveness.[19] The higher prevalence of MDR Acinetobacter spp. in the current study may be related to the observed high carbapenem consumption[20] and the longer ventilation duration (average 19.3 days in our patients compared to an average 14.3 days in US patients).[13,21] Acinetobacter spp. in another report from the same ICU showed was associated with prolonged ventilation.[22]
P. aeruginosa in the current study had about 13-31% resistance rates to all tested antimicrobials, including antipseudomonal penicillins. These rates were generally comparable or slightly lower than reported in local[8,11,12] and international hospital isolates.[2,16] Unlike Acinetobacter spp. MDR P. aeruginosa was significantly lower in KAMC compared to NHSN hospitals.[10] The finding is difficult to explain, however, the authors of NHSN report could not explain the discrepancy between the reported single- and multiple-drug resistances of P. aeruginosa.[10] For example, while single-drug resistance in VAP-associated P. aeruginosa ranged between 5% in amikacin to 28% in ciprofloxacin, three- and four-class resistance ranged between 36 and 40% respectively.[9,10] Moreover, we observed a very interesting trend, unlike Acinetobacter spp. that tend to be resistant to ≥ 3 drugs at the same time, P. aeruginosa tend to be resistant to fewer drugs at the same time. Even within three-class resistant isolates, pan- (five-class) resistance was 51% in Acinetobacter spp. compared to 25% in P. aeruginosa. This may explain the concordance in our isolates of single- and multiple-drug resistance rates in Acinetobacter spp. (60-89% vs. 69-86%, respectively) but not P. aeruginosa (13-31% vs. 10-13%, respectively).
Unlike NHSN hospitals, vancomycin-resistant Enterococcus was not detected in VAP isolates in this study.[9] Similar to many developing countries, this probably could be due the low use of vancomycin.[23] In addition, Gram-positive pathogens in the current study were partially to fully susceptible to vancomycin and the rate of MRSA was generally lower than reported on many parts of the world.[2,9,24]
Although was not associated with worse outcome, pathogen resistance in the current study was associated with generally worse profile of ICU patients. Literature was controversial about the impact of bacterial resistance on outcome specially mortality.[25,26] It is believed that impact on outcome, if any, is probably due associated worse patient profile.[25] Unfortunately, our data did not allow for examining the association between resistant pathogens and VAP antimicrobial treatment. Our ICU should continue actively screening for Acinetobacter and MRSA in all admitted patients[13] together with improving currently implemented antimicrobial stewardship, isolation policies and environmental cleaning. This may limit the spread of resistant pathogens and save the few effective antimicrobials.
The current study had several strengths and some limitations. The study is by far the largest susceptibility study on VAP isolates (n = 248) in ICU patients in Saudi Arabia. Moreover, it is the first study to report local contribution of MDR pathogens to VAP and their relative change over time. However, being a single-center retrospective study may limit the generalizability of the finding. Nevertheless, we believe the study is bridging local data limitation and the findings may help local treating physician to choose the right empirical antibiotic in VAP patients.
Conclusion
Acinetobacter, the most commonly VAP-associated pathogen in the current study, had very high (60-89%) resistance rates to all tested antimicrobials, including carbapenems. These resistance rates were increasing over time and were higher than seen in many parts of the world. Resistant pathogens were associated with worse profile of ICU patients but not patients’ outcomes. The current finding may have great impact on local choice of initial empiric antibiotic.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38:95–1042. doi: 10.1016/j.ajic.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 4.Amin A. Clinical and economic consequences of ventilator-associated pneumonia. Clin Infect Dis. 2009;49(Suppl 1):S36–43. doi: 10.1086/599814. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 6.Arabi Y, Al-Shirawi N, Memish Z, Anzueto A. Ventilator-associated pneumonia in adults in developing countries: A systematic review. Int J Infect Dis. 2008;12:505–12. doi: 10.1016/j.ijid.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Akova M. Emerging problem pathogens: A review of resistance patterns over time. Int J Infect Dis. 2006;10:3–8. [Google Scholar]
- 8.Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010;30:364–9. doi: 10.4103/0256-4947.67073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 10.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006-2008. Infect Control Hosp Epidemiol. 2010;31:528–31. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 11.Asghar AH, Faidah HS. Frequency and antimicrobial susceptibility of gram-negative bacteria isolated from 2 hospitals in Makkah, Saudi Arabia. Saudi Med J. 2009;30:1017–23. [PubMed] [Google Scholar]
- 12.Saeed NK, Kambal AM, El-Khizzi NA. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med J. 2010;31:1341–9. [PubMed] [Google Scholar]
- 13.Al-Dorzi HM, El-Saed A, Rishu AH, Balkhy HH, Memish ZA, Arabi YM. The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am J Infect Control. 2012;40:794–9. doi: 10.1016/j.ajic.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Atlanta, GA: US Department of Health and Human Services, CDC; 2003. CDC. NNIS Criteria for Determining Nosocomial Pneumonia. [Google Scholar]
- 15.8th ed. Approved standard M2-A8 Wayne, PA: Clinical and Laboratory Standards Institute; 2003. CLSI. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests. [Google Scholar]
- 16.Lagamayo EN. Antimicrobial resistance in major pathogens of hospital-acquired pneumonia in Asian countries. Am J Infect Control. 2008;36:S101–8. doi: 10.1016/j.ajic.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–36. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 18.Rello J, Díaz E, Rodríguez A. Etiology of ventilator-associated pneumonia. Clin Chest Med. 2005;26:87–95. doi: 10.1016/j.ccm.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005;11:868–73. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 20.Balkhy H, El-Saed A, Jabri F. Antimicrobial consumption in four different adult intensive care units in a Saudi tertiary care hospital. Int J Infect Dis Control. 2011;7:P5. [Google Scholar]
- 21.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 22.El-Saed A, Balkhy HH, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int J Infect Dis. 2013;17:e696–701. doi: 10.1016/j.ijid.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:582–91. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 24.Meyer E, Schwab F, Gastmeier P. Nosocomial methicillin resistant staphylococcus aureus pneumonia - epidemiology and trends based on data of a network of 586 German ICUs (2005-2009) Eur J Med Res. 2010;15:514–24. doi: 10.1186/2047-783X-15-12-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combes A, Luyt CE, Trouillet JL. Impact of antibiotic-resistant bacteria on the outcome of ventilator-associated pneumonia. Semin Respir Crit Care Med. 2006;27:23–8. doi: 10.1055/s-2006-933670. [DOI] [PubMed] [Google Scholar]
- 26.Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36:1433–7. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]


