Abstract
BACKGROUND:
Obstructive sleep apnea (OSA) is an important cause of morbidity in the elderly population. Limited data are available regarding the healthcare utilization and predisposing conditions related to OSA in the elderly. Our aim was to evaluate the healthcare utilization and the conditions associated with new and chronic diagnosis of OSA in a large cohort of elderly patients in the Veterans Health Administration (VHA).
MATERIALS AND METHODS:
This retrospective cohort study used inpatient and outpatient VHA data to identify the individuals diagnosed with OSA using ICD-9 codes during the fiscal years 2003-2005. Primary outcomes were emergency department (ED) visits and hospitalizations. Multivariable logistic regression analysis was performed to identify the demographic and clinical characteristics associated with new and chronic diagnosis of OSA.
RESULTS:
Of 1,867,876 elderly veterans having 2 years of care, 82,178 (4.4%) were diagnosed with OSA. Individuals with OSA were younger and more likely to have chronic diseases than those without OSA. Individuals with chronic OSA were more likely to have diagnoses of congestive heart failure (CHF), pulmonary circulation disorders, COPD, and obesity and less likely to have diagnoses of hypertension, osteoarthritis, and stroke than individuals with newly diagnosed OSA. The proportion of patients with new OSA diagnosis who required at least one ED visit was higher than the proportion of chronic OSA and no OSA patients (37%, 32%, and 15%, respectively; P-value <0.05). The proportion of new OSA patients who required at least one hospitalization was also higher than the proportion of chronic OSA and no OSA patients (24%, 17%, and 7%, respectively; P-value <0.05).
CONCLUSION:
Patients with OSA had a higher incidence of healthcare utilization compared to patients without OSA. New OSA patients had a higher rate of healthcare utilization in the year of diagnosis compared to chronic patients and patients without OSA. Early OSA recognition may reduce healthcare utilization in these patients.
Keywords: Elderly, emergency department visit, healthcare utilization, hospitalization, obstructive sleep apnea
Snoring used to be viewed as a nuisance, but with each study examining the impact of obstructive sleep apnea (OSA), it becomes increasingly clear that snoring, a symptom of OSA, is an indicator that has a profound impact on the patient. OSA affects more than 12 million Americans and is estimated to occur in up to 20% of adults.[1,2] Several studies have consistently shown that severe and untreated OSA is associated with increased healthcare utilization and cost,[3,4] reaching up to average annual cost of $2720 per case.[5] This is mainly due to the higher disease burden of OSA patients. Untreated OSA is associated with multiple comorbid conditions such as cardiovascular diseases, stroke, diabetes, depression, and cognitive impairment.[6,7,8,9]
In 2010, 39 million Americans (13% of the entire population) were 65 years of age or older (http://www.census.gov), and this number is expected to grow quickly. In the elderly (≥65 years of age), OSA prevalence found in small sample studies has been estimated to be between 20 and 60%.[10,11] As the obesity epidemic continues and the population ages, the number of elderly patients with OSA will increase in the future and will impact healthcare utilization and costs. A better understanding of the kind and frequency of healthcare utilization in the elderly population affected with OSA could help to implement preventive measures and to better distribute economic resources.
The Veterans Health Administration (VHA) is the largest integrated healthcare system in the United States. VHA cares for mostly male and elderly patients with multiple chronic conditions.[12] The availability of population-level data on a clinical sample that receives care in an integrated system suggests that VHA is an ideal setting to further understand the epidemiology of newly diagnosed and chronic OSA in an elderly population and the effect of OSA in healthcare.
The first goal of this study was to build upon the epidemiology of OSA by identifying the conditions associated with new and chronic OSA and to determine if these comorbid conditions were similar for obese and non-obese patients. The second goal was to compare acute healthcare utilization (emergency/urgent care and hospitalization) for older veterans with OSA (new and chronic) and without OSA in a large cohort of elderly VHA patients.
Materials and Methods
Study design
We performed a retrospective cohort study using national VHA inpatient and outpatient data from 1 October 2002 to 30 September 2005 [fiscal years (FY) 2003-2005]. Inpatient and outpatient data allowed identification of OSA in addition to the comorbid conditions and demographic variables that are associated with OSA. We first identified our cohort of older veterans (≥65 years) who received healthcare within the VA medical system from FY2003 to FY2005. We linked inpatient, outpatient, and pharmacy data using an encrypted identifier that is consistent in all files. The Institutional Review Board at the University of Texas Health Science Center at San Antonio approved the study.
Measures
OSA
From this cohort of older veterans, we included individuals with diagnoses indicative of OSA based on an algorithm previously validated by other cohort studies[13,14] (ICD-9-CM codes 327.23- OSA, 780.57 and 786.09- sleep apnea not otherwise specified) in the VA electronic medical record system. Individuals with two outpatient diagnoses at least 7 days apart or with one inpatient diagnosis were classified as having OSA. In order to control for time from disease onset, we further identified patients with “newly diagnosed OSA” as those with an OSA diagnosis in FY2004, but not in FY2003. Individuals with a diagnosis of OSA in FY2004 and/or FY2003 were classified as having “chronic OSA”. Individuals without a diagnosis of OSA in FY2003 and FY2004 were identified as “no OSA”. Therefore, the year of evaluation was FY2004 (±1 year) in order to differentiate pre-existing or “chronic” OSA in FY2004 versus “new” OSA in subjects with no prior diagnosis of OSA.
Demographic characteristics and comorbid conditions
Age, gender, race/ethnicity, and marital status were included in the analyses using VA data sources. Race/ethnicity was classified as White, Black, Hispanic, Other, and Missing. Because missing race was associated with lower rates of utilization and lesser comorbidities in general, we included missing race as a separate category in the analyses in order to avoid biasing toward a cohort with higher disease burden.
We used inpatient and outpatient data for FY2003 to identify the comorbid conditions thought to be associated with both OSA and acute healthcare utilization. Conditions were identified using ICD-9-CM code–based algorithms developed for use with administrative data (e.g. Charlson/Deyo and Elixhauser indices). Individuals with two outpatient diagnoses at least 7 days apart or with one inpatient diagnosis were identified as having comorbid condition.
Healthcare utilization variables and outcomes
We included two primary outcomes regarding healthcare utilization data: Emergency care visits and hospitalizations. Hospitalization was identified when individuals had received hospital care documented in VA inpatient data in FY2005. We also counted the number of visits in emergency or urgent care [emergency department (ED)] settings in FY2005, and identified individuals with one or more ED visits. All the demographic characteristics and comorbid conditions listed in Table 1 were considered as predictor variables. A subgroup analysis was performed according to the obesity status. We defined obesity as having a Body Mass Index (BMI) ≥30 Kg.m-2.
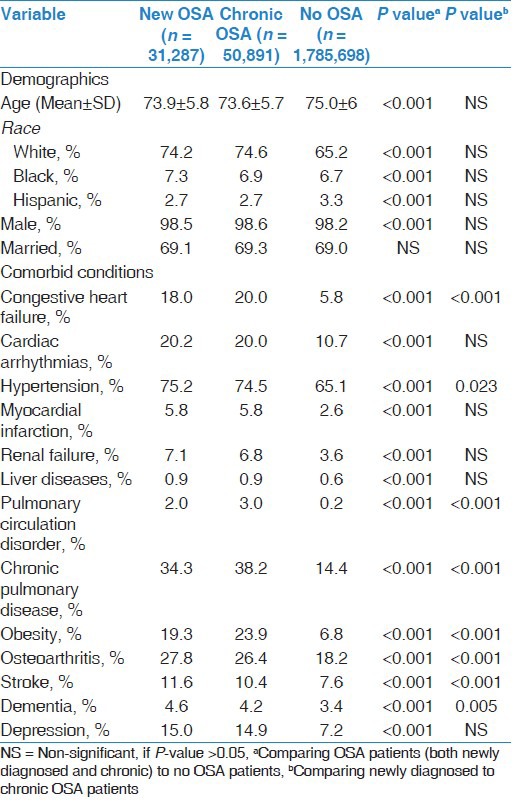
Table 1.
Descriptive statistics for patients with newly diagnosed OSA, chronic OSA, and no OSA
Statistical analysis
Data were presented as percentage of the total or as Mean ± SD. Pearson Chi-square tests were performed for categorical variables. Analysis of variance (ANOVA) and Levene's tests were used for continuous variables. P values <0.05 were considered to represent statistical significance. Multivariable logistic regression analysis [expressed in Odds Ratio (OR) and 99% Confidence Interval (CI)] was performed for predicting new diagnosis and chronic diagnosis of OSA. Demographics and comorbid conditions listed in Table 1 were considered as the dependent variables. The outcomes were new OSA diagnosis, chronic OSA diagnosis, and no OSA diagnosis. Data were analyzed using SPSS (SPSS Inc., Chicago, IL, USA).
Results
Demographics and comorbid conditions
Out of 1,867,876 patients of age 65 years or older, who received care in the VA system in FY 2003-FY2005, 82,178 (4.4%) met the diagnostic criteria for OSA. Of those with OSA, 31,287 (38%; 1.7% of the population) had newly diagnosed OSA. Patients demographics and the most frequent comorbid conditions are summarized in Table 1. OSA patients were significantly more likely to be younger males with a higher burden of comorbidities when compared to no OSA patients. Newly diagnosed OSA patients were significantly less likely to have diagnoses of obesity, diabetes, cardiovascular and respiratory diseases, but a higher rate of hypertension, osteoarthritis, cerebrovascular disease, dementia, hypothyroidism, and alcohol abuse, compared to chronic OSA patients.
Risk factors associated with new and chronic OSA diagnosis
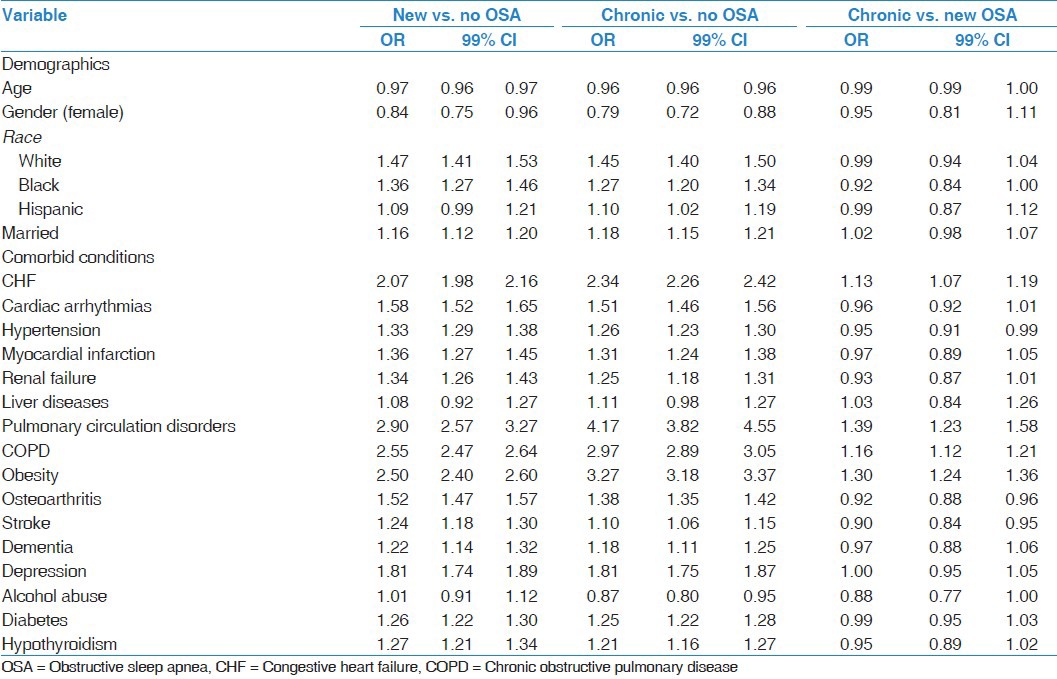
Multivariable logistic regression analyses were performed to examine the association between demographic/clinical characteristics and OSA, controlling for other variables [Table 2]. Characteristics independently associated with newly diagnosed and chronic OSA when compared to no OSA were similar, with the exception of Hispanic ethnicity and alcohol abuse, which were significantly associated with chronic OSA but not with new OSA [Table 2]. All the demographic and comorbid conditions tested were independently associated with new OSA and chronic OSA compared to no OSA, with the exception of liver disease. The model examining chronic OSA compared to newly diagnosed OSA demonstrated that individuals with chronic OSA had higher odds of having previously diagnosed congestive heart failure (CHF), pulmonary circulation disorders, chronic obstructive pulmonary disease (COPD), and obesity, and were less likely to have previously diagnosed hypertension, osteoarthritis, and stroke, when compared to newly diagnosed OSA patients.
Table 2.
Multivariable logistic regression analysis evaluating the risk factors predictive of new diagnosed OSA status versus no OSA and chronic OSA
Subgroup analysis: Obesity status
When groups were stratified by obesity status, certain patterns were different for obese and non-obese patients with OSA. Obese patients with new OSA were more likely to have depression compared to no OSA patients [Table 3], while obese chronic OSA patients were less likely to have a diagnosis of depression compared to no OSA patients [Table 4]. Obese patients with new OSA were more likely to have hypertension and less likely to have pulmonary circulation disorders and COPD compared to chronic OSA patients. Non-obese patients with new OSA were more likely to have osteoarthritis and stroke, but less likely to have CHF, pulmonary circulation disorders, and COPD, compared to chronic OSA non-obese patients [Table 5].
Table 3.
Multivariable logistic regression evaluating the risk factors predictive of new diagnosis of OSA in the non-obese and obese subgroups
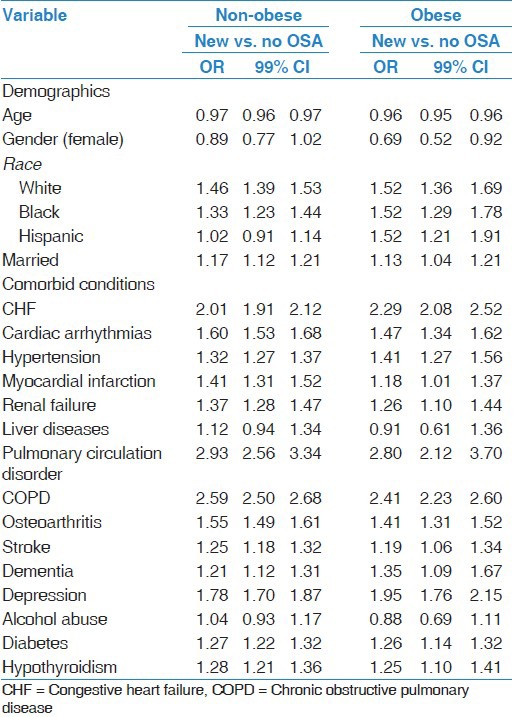
Table 4.
Multivariable logistic regression evaluating the risk factors predictive of chronic diagnosis of OSA in the non-obese and obese population
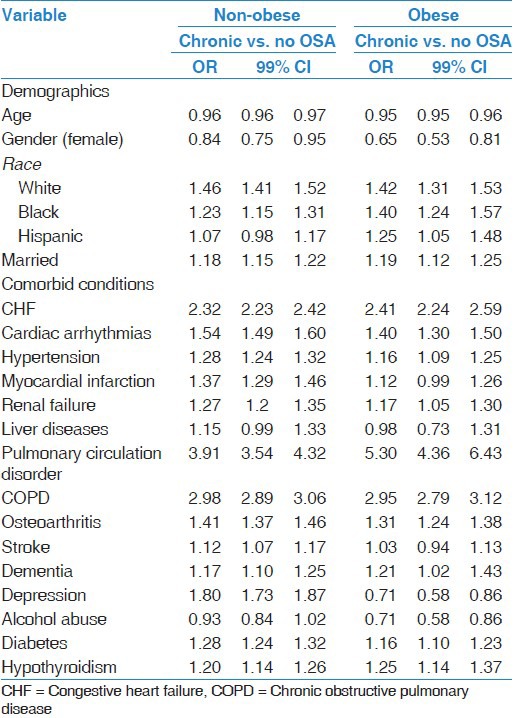
Table 5.
Multivariable logistic regression evaluating the risk factors predictive of chronic diagnosis of OSA versus new diagnosis in the non-obese and obese population

Healthcare utilization
Healthcare utilization for our cohort of newly diagnosed OSA patients was evaluated for the year of diagnosis (FY2004) and was compared to the healthcare utilization in the same time span for chronic OSA and no OSA patients [Figure 1]. Visits to the ED and hospital admissions were significantly more frequent in those with OSA (both new and chronic) compared to those without OSA. In addition, new OSA patients were significantly more likely to be hospitalized (OR 4.48, 99% CI 4.33-4.64 vs. OR 2.88, 99% CI 2.79-2.97) or receive emergency/urgent care (OR 3.27, 99% CI 3.17-3.37 vs. OR 2.56, 99% CI 2.49-2.62) when compared to chronic OSA patients.
Figure 1.

Proportion of elderly OSA patients who require at least one emergency department visit and hospitalization
Discussion
In this study, we found differences in the burden of comorbidities among OSA groups and patients without OSA diagnosis, in addition to slightly different risk patterns of comorbidity between those diagnosed with new and chronic OSA. Moreover, we found that elderly patients with OSA had a significantly increased use of acute healthcare resources including both emergency/urgent care and hospitalizations in the year of diagnosis compared to both patients with chronic OSA and without OSA.
The economic burden of sleep disorders, when extrapolated to the US population, is greater than that of other chronic pulmonary conditions such as asthma and COPD, and is similar in magnitude to that of diabetes ($132 billion in 2002).[3] Moreover, untreated or undiagnosed OSA was associated with higher rate of comorbidities, including cardiovascular and cerebrovascular events,[6,7,15] which may further increase healthcare utilization. Kapur et al. estimated that untreated OSA may cause additional medical costs of $3.4 billion in the US.[16] Given the recent global trend of increasing obesity and aging of populations, it is likely that there will be a substantial increase in the prevalence of OSA. It has been suggested that OSA prevalence is higher in older patients,[17] and that sleep-disordered breathing in the elderly is a distinct condition from that in middle-aged adults.[18] Our results showed that newly diagnosed OSA patients were less likely to be independently associated with CHF, pulmonary circulation disorders, COPD, and obesity, but were more likely to be independently associated with hypertension, osteoarthritis, and stroke, when compared to chronic OSA patients. Our results confirmed the recent interest linking OSA to hypertension, diabetes, hyperlipidemia, stroke, coronary artery disease, and increased mortality.[15] However, chronic OSA was independently associated with obesity, when compared to new OSA diagnosis. This finding suggests that new OSA patients may have some characteristics that differ from chronic OSA patients (data shown in Tables 2-5). It is unclear whether obesity style-modifying therapies have influenced these results. Furthermore, our data confirmed the association of OSA with depression.[19,20] In subgroup analysis, it was found that obese patients with new OSA were more likely to have depression compared to no OSA patients; OSA and obesity are known causes of poor sleep quality that may contribute to depression.[21] However, obese chronic OSA patients were less likely to have a diagnosis of depression compared to no OSA patients. A possible explanation for such finding is that patients who received treatment for OSA had a better quality of sleep. Alcohol abuse is an important modifiable risk factor for OSA, but our results differ from prior evidence showing that elderly OSA patients were less likely to have alcohol abuse.[22,23]
The reason why elderly patients with a new diagnosis of OSA have higher likelihood of receiving emergency care is a matter of clinical interest. It is clear from these and other data that patients with comorbid conditions carry a significant burden in healthcare utilization.[24,25] Hospital and emergency (ED) care provides a spectrum of medical care, some of which are for non-urgent conditions including chronic illnesses.[26] According to federal legislation, anyone requesting services at the ED should be properly screened and treated within the means of the hospital's ED, regardless of the ability to pay for the services.[27] Thus, EDs deal not only with individuals requiring immediate and necessary medical care, but also with preventable and non-emergent visits that may be managed by primary care providers. Not all ED preventable visits are avoidable. In some instances, even the best care cannot prevent the progression of a health condition to a stage that requires an ED visit and possibly hospitalization.[28] Therefore, any increasing trend in ED visits for chronic conditions such as OSA that may potentially be avoidable necessitates attention and careful evaluation as these visits place an undue organizational and financial burden on hospitals and healthcare systems.
OSA is a common disorder that meets the characteristics of a chronic disease, as it is a disease which is prolonged, does not resolve spontaneously, and is rarely completely cured.[29] Our data reveal that elderly patients with new and chronic OSA were more likely to require hospitalization during the year of assessment. Previous studies showed that higher risk of hospitalizations and ED visits may be seen among those patients with more severe OSA.[30] High hospitalization rates are seen in other comorbid conditions such as cardiovascular and pulmonary diseases. Hall et al.[31] showed that congestive heart failure is one of the most common reasons for hospitalization among patients 65 years and older. Similar evidence has been shown for COPD patients by Yeatts et al.[32] We suggest that healthcare providers should not only include OSA among the reasons associated with hospitalization, but also screen patients for sleep disorder breathing (e.g. OSA) during the hospital admission, in order to institute an early and appropriate management and follow-up that could prevent further ED visits and hospitalizations.
Although our study was a large database analysis, subject to the recognized limitations of such studies, we carefully assembled our cohort from ICD-9 codes to avoid ascertainment bias. Our sample was predominantly elderly men because of our use of VA administrative data, and it is possible that women with OSA and younger groups may have different healthcare resource utilization. However, due to the large sample size, we included females and adjusted the variable “gender” in the multivariable analysis. Several variables were not available in this secondary data analysis, including polysomnography, methods to assess the severity of the disease, the severity and number of OSA-related events per year of disease, and the use and compliance of OSA treatment strategies. Another important limitation was reliance on ICD-9 codes for the diagnosis of OSA and other comorbid conditions. Further studies should use different designs in order to conclusively demonstrate that this increase in healthcare utilization is due to the diagnosis of OSA.
In conclusion, patients with OSA, newly diagnosed or chronic, have a higher incidence of healthcare utilization compared to patients without OSA. New OSA patients have a higher rate of healthcare utilization in the year of diagnosis compared to chronic patients and patients without OSA in the same time span. Despite the intrinsic limitations of the observational studies using ICD-9 codes, these findings should serve as hypothesis-generating data and contribute to solve ongoing controversies. Primary care clinicians should consider early diagnosis of OSA and initiation of treatment strategies that may potentially reduce the healthcare utilization of these patients with OSA. Additional studies are needed to understand the underlying mechanisms that are responsible for these findings.
Footnotes
Source of Support: Nil
Conflict of Interest: Dr Restrepo was partly supported by grant K23HL096054 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, the Department of Veterans Affairs, or the University of Texas Health Science Center at San Antonio.
References
- 1.Ho ML, Brass SD. Obstructive sleep apnea. Neurol Int. 2011;3:e15. doi: 10.4081/ni.2011.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung. 2008;186:7–12. doi: 10.1007/s00408-007-9055-5. [DOI] [PubMed] [Google Scholar]
- 4.Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121:164–72. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 5.Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 6.Parati G, Lombardi C, Narkiewicz K. Sleep apnea: Epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671–83. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 10.Bailes S, Baltzan M, Alapin I, Fichten CS, Libman E. Diagnostic indicators of sleep apnea in older women and men: A prospective study. J Psychosom Res. 2005;59:365–73. doi: 10.1016/j.jpsychores.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14:95–103. doi: 10.1097/01.JGP.0000196627.12010.d1. [DOI] [PubMed] [Google Scholar]
- 12.Ashton CM, Petersen NJ, Wray NP, Yu HJ. The veterans affairs medical care system: Hospital and clinic utilization statistics for 1994. Med Care. 1998;36:793–803. doi: 10.1097/00005650-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Liu SS, Chisholm MF, John RS, Ngeow J, Ma Y, Memtsoudis SG. Risk of postoperative hypoxemia in ambulatory orthopedic surgery patients with diagnosis of obstructive sleep apnea: A retrospective observational study. Patient Saf Surg. 2010;4:9. doi: 10.1186/1754-9493-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam DJ, Jensen CC, Mueller BA, Starr JR, Cunningham ML, Weaver EM. Pediatric sleep apnea and craniofacial anomalies: A population-based case-control study. Laryngoscope. 2010;120:2098–105. doi: 10.1002/lary.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 17.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33:907–14. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 18.Young T. Sleep-disordered breathing in older adults: Is it a condition distinct from that in middle-aged adults? Sleep. 1996;19:529–30. doi: 10.1093/sleep/19.7.529. [DOI] [PubMed] [Google Scholar]
- 19.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 21.Gonnissen HK, Adam TC, Hursel R, Rutters F, Verhoef SP, Westerterp-Plantenga MS. Sleep duration, sleep quality and body weight: Parallel developments. Physiol Behav. 2013;121:112–6. doi: 10.1016/j.physbeh.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Larrabee TM, Liu SS, Torres-Gorena A, Soto-Rojas A, Eckert GJ, Stewart KT. The effects of varying alcohol concentrations commonly found in mouth rinses on the force decay of elastomeric chain. Angle Orthod. 2012;82:894–9. doi: 10.2319/062211-407.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bausmer U, Gouveris H, Selivanova O, Goepel B, Mann W. Correlation of the Epworth Sleepiness Scale with respiratory sleep parameters in patients with sleep-related breathing disorders and upper airway pathology. Eur Arch Otorhinolaryngol. 2010;267:1645–8. doi: 10.1007/s00405-010-1250-y. [DOI] [PubMed] [Google Scholar]
- 24.Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22:740–7. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- 25.Skaer TL, Sclar DA. Economic implications of sleep disorders. Pharmacoeconomics. 2010;28:1015–23. doi: 10.2165/11537390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Suruda A, Burns TJ, Knight S, Dean JM. Utah Office of Health Care Statistics. Primary Care Sensitive Emergency Department Visits in Utah, 2001. Utah Department of Health. April 2004. health.utah.gov/hda/Reports/Primary_Care_ERvisits_Utah2001.pdf. Health insurance, neighborhood income, and emergency department usage by Utah children 1996-1998. BMC Health Serv Res. 2005;5:29. doi: 10.1186/1472-6963-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Code Collection. [Last accessed on 2008 Dec 11]. available from: http://www4.law.cornell.edu/uscode .
- 28.Ambulatory care sensitive emergency department visits chronic disease conditions new hampshire, 2001-2005. [Last accessed on 15 April 2013]. available from: http://www.dhhs.state.nh.us/dphs/cdpc/documents/emergencydeptvisits.pdf .
- 29.Dowrick C, Dixon-Woods M, Holman H, Weinman J. What is chronic illness? Chronic Illn. 2005;1:1–6. doi: 10.1177/17423953050010010901. [DOI] [PubMed] [Google Scholar]
- 30.Santos-Silva R, Castro LS, Taddei JA, Tufik S, Bittencourt LR. Sleep disorders and demand for medical services: Evidence from a population-based longitudinal study. PLoS One. 2012;7:e30085. doi: 10.1371/journal.pone.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;108:1–8. [PubMed] [Google Scholar]
- 32.Yeatts KB, Lippmann SJ, Waller AE, Lich KH, Travers D, Weinberger M, et al. Population-based Burden of COPD-related Visits in the Emergency Department (ED): Return ED Visits, Hospital Admissions, and Comorbidity Risks. Chest. 2013;144:784–93. doi: 10.1378/chest.12-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]


