Abstract
Aim of Study:
The aim of current work was to evaluate in vitro anticataract potential of Moringa oliefera extract.
Materials and Methods:
Goat eye lenses were divided into 4 groups; Group served as control, Group II as toxic control, Group III and Group IV were incubated in extract (250 μg/ml and 500 μg/ml of extract of M. oliefera) Group II, III and IV were incubated in 55 mM glucose in artificial aqueous humor to induce lens opacification. Estimation of total, water soluble protein, catalase, glutathione and malondialdehyde along with photographic evaluation of lens was done.
Results:
Group II (toxic control) lenses showed high amount of MDA (Malondialdehyde), soluble, insoluble protein, decreased catalase and glutathione levels, while lenses treated with Moringa oliefera extract (Group III and Group IV) showed significant (* P < 0.05) reduction in MDA and increased level of catalase, glutathione, total and soluble protein.
Conclusion:
Results of present findings suggest protective effect of Moringa oliefera in prevention of in vitro glucose induced cataract.
Keywords: Glucose, in vitro anticataract, lens, Moringa oliefera
Cataract is visual impairment which arises as a result of a disturbance of lens transparency. It is one of the leading causes of blindness worldwide which is one of the major health problems and has drawn a little attention in worldwide. The loss of vision from cataract is a major cause of blindness in developing countries.[1] Cataract is accompanied by a decrease in the activities of protective antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and reduced glutathione (GSH).[3,4,5] The high level of glucose is one of the important reasons behind progression of diabetic cataract.[6,7] Glycation of sugars and binding with amino groups on proteins to produce adducts disrupts biological properties of proteins. This results in structural changes in enzymes which ultimately causes enzyme inactivation.[8]
During hyperglycemia or osmotic stress the pathway activity increases several times. Association of this condition with chronic complications in diabetes has been observed in experimental models of hyperglycemia, where significant alterations affected the opacity of lens. These changes are characterized by high sorbitol levels, alterations on the membrane permeability, lost of glutathione (GSH) and a diminution of the protein synthesis.[9] By the other side, oxidative stress is associated with cataracts formation by production of hydrogen peroxide through glucose auto-oxidation.[8] Hyperglycemia can induce oxidative stress via several mechanisms. These include glucose autoxidation, formation of advanced glycation end-products (AGE), and activation of the polyol pathway.
Natural antioxidants are famous for their capacity to protect organisms and cells from damage due to oxidative stress, the latter being considered a cause of ageing and degenerative diseases. The antioxidants present in food especially vegetables, are phenolic compounds (phenolic acids and flavonoids), carotenoids, tocopherol and ascorbic acid.[9,3]
Moringa oleifera is the most widely cultivated species of a monogeneric family, the Moringaceae, which is native to the sub-Himalayan tracts of India, Pakistan, Bangladesh and Afghanistan.[10] The plant is rich source of vitamin A, vitamin C and minerals like calcium and potassium.[11,12] Moringa preparations have been demonstrated to have potential antihypertensive, anti-inflammatory, hypolipidemic and antidiabetic properties.[13,14]
Based on previous studies, it is observed that, treatment with Moringa oleifera effectively retarded the prognosis of disease due to increment in antioxidant enzymes.[15,16,17]
Therefore present work is undertaken to evaluate that M. oleifera, which is a rich source of antioxidant is able to inhibit in vitro cataractogenesis in experimental isolated goat eye lens model.
Materials and Methods
Preparation of extract
The fresh whole plant of Moringa oleifera was purchased from local market. It was then identified and authenticated by Dr. A.B. Tiwari, Sr. Scientist, Department of Crop and Herbal Physiology, Jawaharlal Nehru Krishi Vishwa Vidyalaya, Jabalpur (M.P). The Specimen Voucher no. HD/CHPY/980 was deposited at the Jawaharlal Nehru Krishi Vishwavidyalaya (JNKVV), Jabalpur (M.P.). M. oleifera was dried in shade, coarsely powered and used for preparation of extract. The powder was extracted with ethanol: Water (1:1) by stirring for 48 h and filtered through Whatman No. 4 filter paper. The residue was then extracted with two additional 200 ml portions of ethanol: Water (1:1) as described above. The combined extracts were then evaporated (Buchi Type, York Science C., Mumbai) at 40°C to dryness, and stored at 4°C for further use. Phytochemical evaluation was done by reported method.[18]
Chemicals
Potassium chloride, sodium chloride, sodium bicarbonate, sodium phosphate, and calcium chloride were purchased from Central Drug House (CDH), India; glucose was purchased from Fischer scientific (India), trichloroacetic acid, and ethylenediaminetetraacetic acid (EDTA) were purchased from Qualigens, India; thiobarbituric acid was purchased from Sigma, US. All other chemicals used were of analytical grade. Triple distilled water was used in the experiment.
Lens culture
Fresh goat eyeballs were obtained from slaughterhouse immediately after slaughter and transported to the laboratory at 0-4°C. The lenses were removed by extra capsular extraction and incubated in artificial aqueous humor (NaCl 140 mM, KCl 5 mM, MgCl 2 2 mM, NaHCO3 0.5 mM, NaH (PO4) 2 0.5 mM, CaCl2 0.4 mM and Glucose 5.5 mM) at room temperature and pH 7.8 for 72 h. Penicillin 32mg % and streptomycin 250 mg % were added to the culture media to prevent bacterial contamination.[19,20]
Generation of cataract
Glucose in a concentration of 55 mM was used to induce cataract.[19] At high concentrations, glucose in the lens was metabolized through sorbitol pathway and accumulation of polyols (sugar alcohols), causing over hydration and oxidative stress. This led to cataractogenesis. Extract was dissolved in Dimethyl sulfoxide (DMSO) and used for the study.
Study drugs and groups design
In present study, a dose of 250 μg/ml and 500 μg/ml of extract of M. oleifera were used.
A total of 24 lenses were divided into following categories (n = 6 in each category):
Group I : Normal lens [Control (Glucose 5.5 mM)]
Group II : Glucose 55 mM
Group III : Glucose 55 mM + Extract 250 μg/ml
Group IV : Glucose 55 mM + Extract 500 μg/ml
Biochemical estimation
The protein content of the samples was determined by the method of Lowry et al., using bovine serum albumin as the standard.[20] Glutathione estimation was done as reported by Ellman with slight modifications.[22] Lens catalase activities were determined by Goth's colorimetric method.[23] The lipid peroxide formed was estimated by measuring thiobarbituric acid reacting substances (TBARS).[24]
Statistical analysis
All data were expressed as mean ± SD. The groups were compared using one-way ANOVA followed by Bonferroni's test using glucose 55 mM group as control. Where P < 0.05 was considered significant.
Results
Phytochemical screening
Phytochemical studies of M. oleifera extract revealed the presence of glycosides, alkaloids, phenolics and flavonoids.
Protein content
The lens protein level of glucose (55 μM) treated lens (Group II) showed significant decrease (P < 0.005) as compared to normal control group (Group I) M. oleifera extract at the concentration of 250 μg/ml and 500 μg/ml showed significant increase (P < 0.05) in lens protein as compared to toxic control (Group II) [Fig. 1].
Figure 1.

Effects of M.oleifera extract on total protein level on isolated goat lens; all values are mean ± SD, n = 6; Statistical comparison was performed using analysis of variance (ANOVA) followed by Bonferroniæs test (*P < 0.05)
Glutathione level
The lens glutathione level of glucose (55 μM) treated lens (Group II) showed significant decrease (P < 0.05) as compared to normal control group (Group I) M. oleifera extract at the concentration of 250μg/ml and 500 μg/ml showed significant increase (P < 0.05) in lens glutathione as compared to toxic control (Group II). Only treated group (Group IV) showed almost the same levels of glutathione as that of control [Fig. 2].
Figure 2.
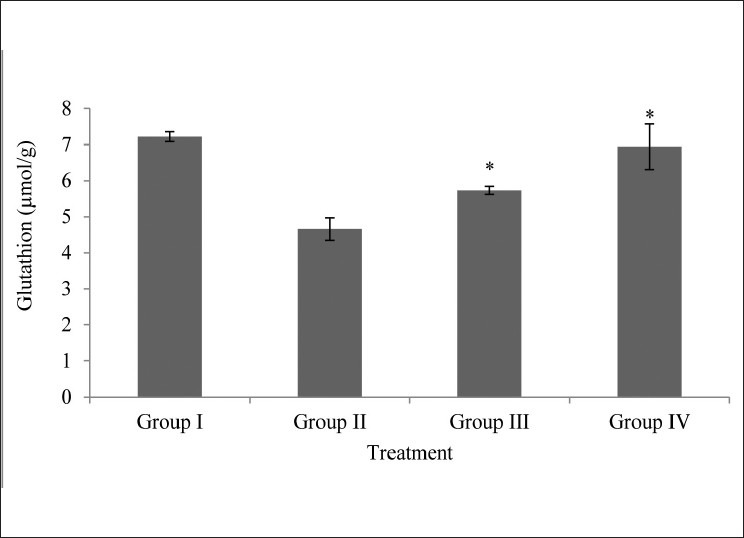
Effects of M.oleifera extract on glutathione level on isolated goat lens; all values are mean ± SD, n = 6; Statistical comparison was performed using analysis of variance (ANOVA) followed by Bonferroniæs test (*P < 0.05)
Catalase levels
In this study, incubation by sugars resulted in a time dependent inactivation of the enzymes.[23] Lens catalase activities were also significantly lower in the lens of Group II as compared to extract treated groups (Group III and IV; P < 0.05) [Fig. 3].
Figure 3.
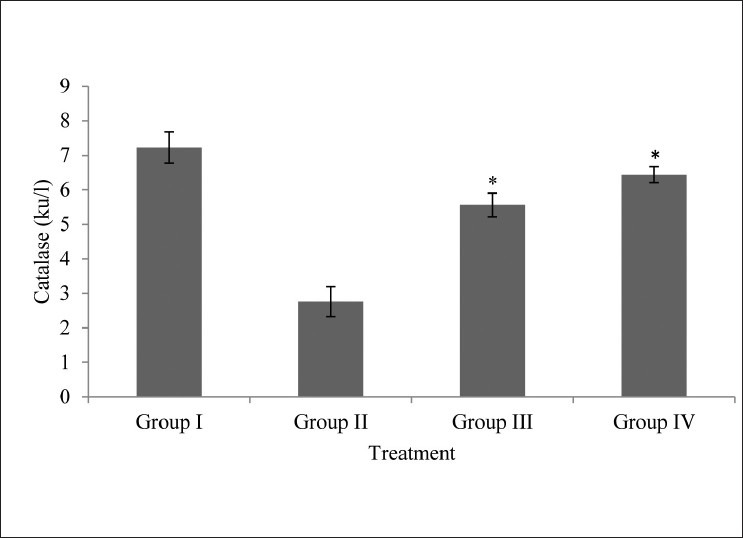
Effects of M.oleifera extract on catalase level on isolated goat lens; all values are mean ± SD, n = 6; Statistical comparison was performed using analysis of variance (ANOVA) followed by Bonferroniæs test (*P < 0.05)
MDA Levels
MDA Levels were found to be very high in glucose 55 mM treated lens compared with normal lens. Lens treated with M. oleifera extract had significantly reduced MDA content (P < 0.05) at both concentrations compared with high glucose group [Fig. 4].
Figure 4.
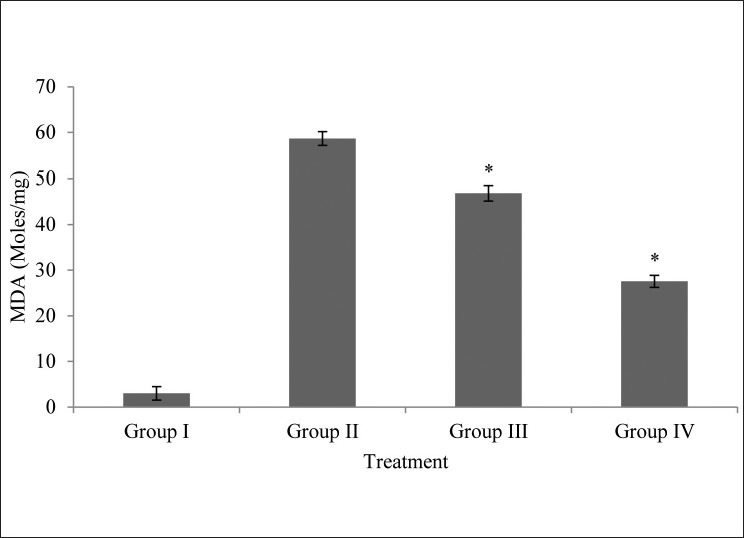
Effects of M.oleifera extract on MDA level on isolated goat lens; all values are mean ± SD, n = 6; Statistical comparison was performed using analysis of variance (ANOVA) followed by Bonferroniæs test (*P < 0.05)
Photographic evaluation
Lenses incubated in glucose 5.5 μM remained transparent, whereas, the lens incubated in 55 μM glucose developed dense opacities. The opacity increased towards centre with complete opacification at the end of 72 h. Incorporation of M. oleifera extract (250 μg/ml and 500 μg/ml) retarded the development of opacity [Fig. 5].
Figure 5.
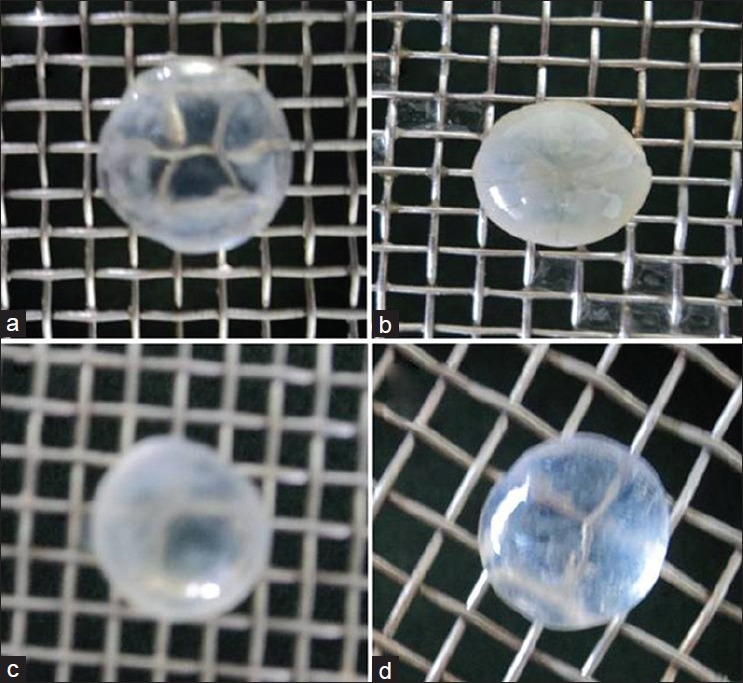
(a) Group I: Normal lens after 72 hours of incubation in glucose 5.5 mM (Transparency maintained, squares clearly visible). (b) Group II: Complete cataractogenesis after 72 hours of incubation in glucose 55 mM (Absolute loss of transparency, no squares visible through lens). (c) Group III: After 72 hours of incubation in glucose 55 mM + Extract 250 μg/ml, lens appears slightly hazy (Very less no. of squares slightly visible). (d) Group IV: After 72 hours of incubation in glucose 55 mM + Extract 500 μg/ml, lens appears slightly hazy (More no. of squares visible)
Discussion
Cataract is the most prevalent disorder leading to visual impairment, the prospect of pharmacological intervention to inhibit or to delay the onset of cataract is still at the experimental stage.[26]
CAT, SOD, and GSH are important components of the innate enzymatic defenses of the lens. CAT has been shown to be responsible for the detoxification of significant amounts of H2O2[27] SOD catalyzes the removal of superoxide radicals (O-), which would otherwise damage the membrane and biological structures.[28] The enzyme GSH, first demonstrated in the lens by Pirie,[29] has been reported to maintain the integrity of the phospholipid bilayer of membranes by inhibiting lipid peroxidation.
CAT and GSH catalyze the transformation of H2O2 within the cell to harmless by products, thereby curtailing the quantity of cellular destruction inflicted by products of lipid peroxidation. A reduction in the activities of these enzymes in tissues has been associated with the accumulation of highly reactive free radicals, leading to deleterious effects such as loss of integrity and function of cell membranes.[27,30,31]
Reduction in the levels of reduced glutathione is observed during cataract of any etiology. GSH plays a leading role in preserving lens clarity. It also acts as antioxidant and stabilize proteins in reduced form.[32,33] Phytoconstituents from herbal drugs may ultimately inhibit expenditure of GSH through oxidation leaving the–SH groups intact. On the other hand, they may directly stimulate reduced glutathione synthesis which may be due to a modulating effect on reduced glutathione related enzymes in the lens.[34] The restoration of reduced glutathione levels by M. oleifera extract also warranted its anticataract potential.
During oxidative stress, denaturation and aggregation of lens proteins is observed which is further observed by precipitation and lens opalescence. The situation is however improved along with an increase in the protein level in lens treated with M.oleifera. The preventive role of extract has also been demonstrated by the restoration of protein. In this study the levels of MDA were more in Group II when compared with Group I, III and IV which suggest preventive role against cataract.
In vitro anti-cataract effect of M. oleifera on isolated goat lenses incubated in a high glucose medium was dose-dependent. This effect was manifested as amelioration of glucose-induced lens opacity. Hence it can be conclude that oxidative stress is an important factor in the development of glucose induced cataract and the use of anti-oxidants may be advocated in patients to delay or prevent formation of cataract. In conclusion M. oleifera showed promising in vitro activity against glucose cataract in an isolated goat lens model. Result of study is encouraging, but further studies are required to access the use of M. oleifera in humans for prevention of cataract.
Footnotes
Source of Support: Authors are thankful to Rewa Shiksha Samiti for providing necessary support during studies
Conflict of Interest: None declared.
References
- 1.Kyselova Z, Stefek M, Bauer V. Pharmacological prevention of diabetic cataract. J Diabetes Complications. 2004;18:129–40. doi: 10.1016/S1056-8727(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 2.Elanchezhian R, Ramesh E, Sakthivel M, Isai M, Geraldine P, Rajamohan M, et al. Acetyl-Lcarnitine prevents selenite-induced cataractogenesis in an experimental animal model. Curr Eye Res. 2008;32:961–71. doi: 10.1080/02713680701673470. [DOI] [PubMed] [Google Scholar]
- 3.Sakthivel M, Elanchezhian R, Ramesh E, Isai M, Nelson Jesudasan C, Thomas PA, et al. Prevention of selenite induced cataractogenesis in Wistar rats by the polyphenol, ellagic acid. Exp Eye Res. 2008;86:251–9. doi: 10.1016/j.exer.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. New Engl J Med. 1988;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 5.Harding JJ. London: Chapman and Hall; 1991. Cataract: Biochemistry, Epidemiology and Pharmacology; pp. 125–47. [Google Scholar]
- 6.Kinoshita JH. Aldose reductase in the diabetic eye. Am J Ophthalmol. 1986;102:685–92. doi: 10.1016/0002-9394(86)90394-6. [DOI] [PubMed] [Google Scholar]
- 7.Ohguro N, Fukuda M, Sasabe T, Tano Y. Concentration dependent effects of hydrogen peroxide on lens epithelial cells. Br J Ophthalmol. 1999;83:1064–8. doi: 10.1136/bjo.83.9.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazzi R, Ricardy R, Aglitti T, Gatta V, Petricone P, De Salvia R. Ascorbic acid and β-carotene as modulators of oxidative damage. Carcinogenesis. 1997;18:223–8. doi: 10.1093/carcin/18.1.223. [DOI] [PubMed] [Google Scholar]
- 9.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 10.Sasikala V, Rooban BN, Priya SG, Sahasranamam V, Abraham A. Moringa oleifera prevents selenite-induced cataractogenesis in rat pups. J Ocul Pharmacol Ther. 2010;26:441–7. doi: 10.1089/jop.2010.0049. [DOI] [PubMed] [Google Scholar]
- 11.Faizi S. Bioactive Compounds from the leaves and pods of Moringa oleifera. New Trends in Natural Products Chemistry. 1998:175–83. [Google Scholar]
- 12.Palada MC. Moringa (Moringa oleifera Lam.): A versatile tree crop with horticultural potential in the subtropical United States. Hort Science. 1996;31:794–7. [Google Scholar]
- 13.Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–11. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan SG, Mali RG, Mehta AA. Effect of Moringa oleifera Lam. seed extract on toluene diisocyanate-induced immune-mediated inflammatory responses in rats. J Immunotoxicol. 2007;4:85–96. doi: 10.1080/15476910701337472. [DOI] [PubMed] [Google Scholar]
- 15.Ashok KN, Pari L. Antioxidant action of Moringa oleifera Lam. (drumstick) against antitubercular drugs induced lipid peroxidation in rats. J Med Food. 2003;6:255–9. doi: 10.1089/10966200360716670. [DOI] [PubMed] [Google Scholar]
- 16.Hamza AA. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol. 2010;48:345–55. doi: 10.1016/j.fct.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Harborne JB. New Delhi: Springer Private Limited Press; 1998. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 18.Chandorkar AG, Albal MV, Bulakh PM, Muley MP. Lens Organ Culture. Indian J Opthalmol Res. 1981;29:151–2. [PubMed] [Google Scholar]
- 19.Ganeshpurkar A, Bhadoriya SS, Pardhi P, Jain AP, Rai G. In vitro prevention of cataract by Oyster Mushroom Pleurotus florida extract on isolated goat eye lens. Indian J Pharmacol. 2011;43:667–70. doi: 10.4103/0253-7613.89823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellman GL. Tissue Sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–52. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Yan H. Glycation induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem J. 1997;328:599–605. doi: 10.1042/bj3280599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hightower KR, McCready JP, Goudsmit EM. Calcium induced opacification is dependent upon lens pH. Curr Eye Res. 1987;6:1415–20. doi: 10.3109/02713688709044505. [DOI] [PubMed] [Google Scholar]
- 25.Cheng LE, Kellogg I, Packer L. Photo inactivation of catalase. Photochem Photobiol. 1981;34:125–9. [PubMed] [Google Scholar]
- 26.Krishnakantha TP, Lokesh BR. Scavenging of superoxide anions by spice principles. Ind J Exp Biol. 1993;30:133–4. [PubMed] [Google Scholar]
- 27.Pirie A. Glutathione peroxidase in lens and a source of hydrogen peroxide in aqueous humour. Biochem J. 1965;96:244–53. doi: 10.1042/bj0960244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reedy AC, Lokesh BR. Studies on spice principle as an antioxidant in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem. 1992;111:117–24. doi: 10.1007/BF00229582. [DOI] [PubMed] [Google Scholar]
- 29.Sheela CG, Angusti K. Antiperoxide effects of S-allyl cystein sulphoxide isolated from Allium sativum Linn and gugulipid in chlosterol diet fed rats. Ind J Exp Biol. 1995;33:337–41. [PubMed] [Google Scholar]
- 30.Xie PY, Kanai A, Nakajima A, Kitahara S, Ohtsu A, Fujii K. Glutathione and glutathione related enzymes in human cataractous lenses. Ophthalmic Res. 1995;31:147–53. doi: 10.1159/000267112. [DOI] [PubMed] [Google Scholar]
- 31.Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970;47:389–94. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soderberg PG. Experimental cataract induced by ultraviolet radiation. Acta Ophthalmol Suppl. 1990;196:210–308. [PubMed] [Google Scholar]
- 33.Geraldine P, Sneha BB, Elanchezhian R, Ramesh E, Kalavathy CM, Kaliamurthy J, et al. Prevention of selenite-induced cataractogenesis by acetyl-L-carnitine: An experimental study. Exp Eye Res. 2006;83:1340–9. doi: 10.1016/j.exer.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]


