Abstract
For some pneumococci the fluoroquinolone MICs are low but the mutant prevention concentrations (MPCs) are high; this difference defines in vitro the mutant selection window (MSW). We investigated in vivo the bacterial reduction and the occurrence of resistant mutants with moxifloxacin (MFX; 400 mg once daily) or levofloxacin (LVX; 500 mg twice daily) in treatments similar to those in humans with experimental pneumonia due to pneumococci (expPP) exhibiting various MICs and MPCs. The MIC/MPC for MFX and LVX and genotypes were as follows: strain 16089, 0.125/0.125 and 0.5/0.5 (wild type); strain MS1A, 0.25/0.25 and 1/2 (efflux); strain MS2A, 0.25/4 and 1.75/28 (parC79); strain MR3B4, 0.25/4 and 2/32 (parC79); strain M16, 0.5/2 and 8/32 (parC83); strain Gyr-1207, 1.5/3 and 8/16 (gyrA); and strain MQ3A, 4/4 and 16/64 (parC and gyrA). Both drugs were efficient with wild type-expPP, but only MFX was efficient with efflux-expPP. No bacterial reduction was observed for parC-expPPs due to mutants observed in 18 to 100% of animals, depending on the strain and the drug tested. These mutants showed unbound area under the concentration-time curve and MICs of from 50 to 164 for MFX. The in vivo pharmacodynamic boundaries of the MSW were different for MFX and LVX. We conclude that, after LVX or MFX treatment, mutants occur in vivo if there is a preexisting parC mutation, since the drug concentrations fall below the MPCs of these strains. Since the MPC determination cannot be routinely determined, these phenotypes or genotypes should be detected by simple tests to guide the therapeutic options.
Pneumonia remains a leading cause of death throughout the world, and Streptococcus pneumoniae is the most common cause of significant morbidity and mortality (36, 53-55, 70). The prevalence of antibiotic-resistant S. pneumoniae has increased over the last decade (18, 25, 28, 44, 59, 72), and beta-lactam resistance could be associated with increased morbidity and mortality (20, 36, 48, 51, 57). Newer fluoroquinolones, because of their antipneumococcal activities, are also part of the therapeutic arsenal for pneumococcal pneumonia. Among these compounds, levofloxacin (LVX) and moxifloxacin (MFX) were shown to be as effective as comparators in phase III studies (10, 21, 22, 56, 61). However, because of the very low frequency of pneumonia due to strains with low-level fluoroquinolone resistance (efflux and/or mutation in the topoisomerase IV gene), these studies cannot provide measurements of the real efficacy of these fluoroquinolones versus infections due to such strains.
Recently, two major observations have emerged: first, failures of LVX treatment for pneumococcal pneumonia have been described in patients, most of whom were previously exposed to fluoroquinolones (16, 24, 37, 39, 64, 69, 71; N. Fishman, B. Suh, L. Weigel, B. Lorber, S. Gelone, A. Truant, T. Gootz, J. Christie, and P. Edelstein, Abstr. 39th, Intersci. Conf. Antimicrob. Agents Chemother., abstr. 825, p. 111, 1999) and, second, an increasing rate of low-level fluoroquinolone-resistance that occurs in some areas of the world (9, 11, 25, 28, 30, 34, 44, 60, 73).
New chemical developments on fluoroquinolones, especially C-8-OMe-fluoroquinolones, have led to a stronger intrinsic in vitro activity associated with a low frequency of mutation (1, 15, 19). However, further information concerning the in vivo efficacy of new fluoroquinolones is needed. Especially, the risk of in vivo mutations has to be investigated, taking into account the high in vitro rate of mutation of pneumococci when exposed to quinolones (1, 6, 41, 42, 45, 46, 55, 66, 68, 74) compared to beta-lactam agents.
The aim of the present study was to investigate both the efficacy of LVX and MFX and the pharmacodynamic (PK-PD) conditions associated with the eventual occurrence of resistant mutants in a simulated human-like treated experimental pneumonia induced with pneumococcal strains with various degrees of susceptibility to fluoroquinolones. For this investigation, in addition to the conventional PK-PD parameters using MIC, we also applied in vivo the novel in vitro concept of the mutant selection window (MSW), which is defined as the zone between MIC and mutant prevention concentration (MPC) (5, 41, 76, 78) and which provides a means for defining the ability of antibiotics in preventing the emergence of mutants.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and antibiotics.
The bacteria used in the experiments are listed in Table 1. These strains include strain 16089, a clinical strain of Streptococcus pneumoniae, isolated from a patient with pneumonia and kindly provided by the Centre National de Référence des Pneumocoques (Dr Geslin); five ciprofloxacin (CIP)-resistant laboratory derivatives of strain 16089 (MS1A, MS2A, MR3B4, M16, and MQ3A), selected by serial exposure of the 16089 parent to this drug (14); and a clinical strain, Gyr-1207, kindly provided by G. Doern (18). All of these strains were resistant to penicillin (MIC = 4 mg liter−1). Bacteria were grown in 5% CO2 either in brain heart infusion broth (BioMérieux, Marcy l'Etoile, France) or on sheep blood agar plates (BioMérieux). Bacterial stocks were kept at −70°C in a 15% (vol/vol) glycerol-supplemented brain heart infusion broth.
TABLE 1.
In vitro susceptibilities of S. pneumoniae strains to antibiotics and mutations identified in the QRDRs (parC, parE, gyrA, and gyrB)
| Strain | Concn (mg liter−1) |
SI |
Effluxa | QRDR genotypic mutationsb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC |
MPC |
|||||||||||
| CIP | LVX | MFX | LVX | MFX | LVX | MFX | parC | parE | gyrA | gyrB | ||
| 16089 | 0.5 | 0.5 | 0.125 | 0.5 | 0.125 | 1 | 1 | − | - | - | - | - |
| MS1A | 2 | 1 | 0.25 | 2 | 0.25 | 2 | 1 | + | - | - | - | - |
| MS2A | 8 | 1.75 | 0.25 | 28 | 4 | 16 | 16 | + | Ser-79→Phe | - | - | - |
| MR3B4 | 10 | 2 | 0.25 | 32 | 4 | 16 | 16 | + | Ser-79→Tyr | - | - | - |
| M16 | 64 | 8 | 0.5 | 32 | 2 | 4 | 4 | + | Asp-83→Asn | - | - | - |
| Gyr-1207 | 6 | 8 | 1.5 | 16 | 3 | 2 | 2 | − | - | - | Ser-81→Phe | - |
| MQ3A | >64 | 16 | 4 | 64 | 4 | 4 | 1 | + | Ser-79→Tyr | - | Ser-81→Phe | - |
Efflux reserpine-CIP.
-, No mutation.
LVX and MFX were provided by Aventis (Paris La Défense, France) and Bayer AG (Puteaux, France), respectively. The drugs were reconstituted according to the manufacturer's instructions. Stock solutions were used as fresh preparations.
In vitro susceptibility studies. (i) MIC determination.
The MICs of LVX and MFX for the various isolates were determined by the standard dilution method in agar (12).
(ii) MPC determination.
For MPC determination (5), 200 μl of a culture containing ≥10 log10 CFU/ml was applied to tryptic soy agar plates containing 5% sheep blood and drugs at various concentrations. MPCs were read after 24 and 48 h of incubation at 37°C in 5% CO2. MPCs were recorded as the lowest antibiotic concentration that prevented bacterial colony formation at 48 h. All determinations were done in triplicate, and the results were identical. The absence of visible growing bacteria at 24 h allowed us to eliminate the presence of subpopulations in the inoculum.
The selection index (SI) was calculated for each strain as the MPC/MIC ratio.
Evaluation of efflux for CIP.
MIC determination was done in parallel both with or without an efflux inhibitor (10 μg of reserpine/ml) (8, 27, 47). Efflux was considered as present when a ≥2-fold reduction of the MIC was observed.
PCR amplification of quinolone resistance-determining regions (QRDRs) and DNA sequencing.
Genomic DNA was isolated from bacterial strains and used as the template in PCR amplification of the QRDRs of parC, parE, gyrA, and gyrB genes as previously described (58). Nucleotide sequencing (Génôme Express S.A., Grenoble, France) was carried out with an automated sequencer (ABI Prism 377 DNA sequencer) with published primers (52).
Preparation of the inoculum.
Before each animal experiment, several S. pneumoniae strains from one aliquot (per strain) were inoculated into brain heart infusion broth, cultured on agar plates, and incubated for 24 h at 37°C in 5% CO2. Twenty-five to thirty colonies were taken and inoculated into 9 ml of brain heart infusion broth, incubated for 6 h at 37°C, and then cultured on agar plates for 18 h at 37°C in 5% CO2. This culture was diluted in physiologic saline in order to obtain final concentrations of 10 log10 CFU/ml. No adjuvant was used. These concentrations were first determined by using optical density measurements, in reference to a standard curve, and then confirmed by using successive dilution cultures.
Animals.
Male New Zealand White rabbits (body weight, 2.5 to 3 kg) were obtained from Elevage Scientifique des Dombes (Romans, France). Animals were not immunosuppressed and were virus antibody and specific pathogen free. They were placed in individual cages and were nourished ad libitum with drinking water and feed according to current recommendations.
Production and humanized treatment of experimental pneumococcal pneumonia in rabbits.
Production of pneumonia in immunocompetent rabbits and the installation of the central venous catheters were performed as previously described (14, 62). Briefly, 24 h after jugular catheterization, bacterial pneumonia was induced by endobronchial challenge of the animals with 0.5 ml of saline containing 10 log10 CFU/ml of either tested strain (day-to-day variability of 0.3 log10 CFU. Treatment was started 5 h after bacterial challenge and lasted for 2 days. Antibiotics were delivered through the first central venous catheter with changing infusion rates obtained by a computer-controlled electric pump and at doses that simulated antibiotic kinetics observed in human serum, as follows: (i) 500 mg of LVX given intravenously (i.v.) twice a day (Cmax = 6 mg/liter; area under the concentration-time curve from 0 to 24 h [AUC0-24] = 50 mg · h · liter−1), (ii) 400 mg of MFX given i.v. once a day (Cmax = 4.5 mg/liter; AUC0-24: 40 mg h liter−1).
Pharmacokinetic analysis.
For each animal, the concentrations of antibiotics in the serum were determined on iterative blood samples, obtained through the second central catheter. LVX and MFX concentrations were determined by a disk plate bioassay method with antibiotic medium II (Difco Laboratories) and Escherichia coli NIJJHC2 as the indicator organism. The limit of detection was 0.4 mg/liter. Standard curves were established with solutions (progression from 0.5 to 7 mg/liter) in serum. The linearity of the standard curves used for disk plate bioassays was at least 0.98 (r2). The serum samples were diluted in serum water to ensure that their concentrations would be within the range of those on the standard curve. The standard samples were assayed for each experiment, and concentrations were assayed in duplicate. The between- and within-day coefficients of variation for replicates were equal to 5 and 8%, respectively. The level of plasma protein binding was determined ex vivo for LVX and MFX by a membrane filtration method (67). Pharmacokinetics data were analyzed by using Kinetica software (Innaphase, Philadelphia, Pa.).
Evaluation of infection.
The rabbits were anesthetized and sacrificed 2 h after the end of the antibiotic infusion.
(i) Bacterial content in the lungs and spleen.
The spleen and each pulmonary lobe were weighed and homogenized in sterile serum saline. Bacteria were counted in a sample of this crude homogenate by plating 10-fold dilutions on sheep blood agar and incubating the plates for 24 h at 37°C. Bacterial concentrations in each lobe and in the spleen were determined after adjusting for weight. The threshold value was 1 log10 CFU/ml since a large volume of pulmonary homogenate was plated. For statistical comparisons of the difference between the pulmonary bacterial densities, culture-negative lobes were considered to contain 1 log10 CFU/g, whereas no carryover effect was found. For each rabbit, the mean pulmonary pneumococcal concentration was calculated according to each lobar bacterial concentration with lobar weight [e.g., mean concentration = Σ (lobar concentration × lobar weight)/Σ (lobar weights)].
(ii) In vivo mutants.
In the treated animals and for each lobe or spleen with residual surviving bacteria, emerging mutants were detected by plating 1 ml of the crude tissue homogenate and 10-fold dilutions on sheep blood agar containing two and four times the MIC of the tested strain; a 48-h incubation period was used.
PK-PD analysis.
From the individual pharmacokinetics of each treated animal, the following PK-PD parameters (unbound drug) were calculated: Cmax/MIC, Cmax/MPC, Cmax/SI, AUC/MIC, AUC/MPC, AUC/SI, T>MIC, and T>MPC. The examination of the area between the MIC and MPC on one hand and under the concentration-time curve in serum on the other hand made it possible to calculate the following PK-PD parameters of the MSW: time of concentration within the window (TMSW) and AUCMSW (76).
Statistical analysis.
The results were expressed as the mean ± the standard deviation. Quantitative variables were compared to Mann-Whitney or analysis of variance and eventually completed by a post hoc analysis by using the Bonferroni test. Percentages were compared by using the chi-square test with the Yates correction or by using the Fisher exact test. The quantitative relationships between antimicrobial efficacy and each of the PK-PD parameters were determined by using an Emax model (Hill formula) or a peak model (Gauss formula), as appropriate, by using SigmaPlot software (version 7.101). To define the PK-PD zones associated with the occurrence of mutants, a qualitative analysis (mutants versus nonmutants) was performed by using classification and regression tree (CART) methodology (7, 43). For all of the tests, a P value of <0.05 was considered significant.
RESULTS
Antimicrobial susceptibility and resistance mutations in the QRDR.
The MICs of CIP, LVX, and MFX and the MPCs of LVX and MFX for the seven strains used to induce pneumonia in animals are shown in Table 1. All of these strains, except for strain 16089, were resistant to CIP. LVX and MFX retained their lower MICs against CIP-resistant strains. As expected, the MICs of MFX were lower than those of LVX. Indeed, the first four strains (16089, MS1A, MS2A, and MR3B4) were susceptible to LVX (≤2 mg liter−1), and the last three strains (M16, Gyr-1207, and MQ3A) were resistant to LVX, whereas all strains, except for strains Gyr-1207 and MQ3A, were susceptible to MFX (≤1 mg liter−1). Although a single parC mutation (Ser-79→Phe or Asp-83→Asn) increased the MIC of LVX 2.5 to 16 times, it increased the MIC of MFX only 2 to 4 times. The presence of a gyrA mutation (Ser-81→Phe) was associated with a 16-fold increase in the MIC of LVX and a 12-fold increase in the MIC of MFX. However, a double parC (Ser-79→Tyr) and gyrA (Ser-81→Phe) mutation increased the MICs of both LVX and MFX by a factor of 32. The presence of only one CIP efflux mechanism (strain MS1A) slightly increased the MICs of both LVX and MFX, respectively, from 0.5 to 1 mg liter−1 and from 0.125 to 0.25 mg liter−1.
MPCs for MFX were lower than those for LVX. The presence of a parC mutation was associated with a greater increase in MPC values than was the presence of a gyrA mutation. Of note, the parC-83 mutation (strain M16) led to a lesser increase in the MPCs than did the parC-79 mutations (strains MS2A and MR3B4). Furthermore, the MFX MICs and MPCs were identical for the highly resistant doubly mutated strain (MQ3A); this was not the case for LVX. Finally, the SIs were similar for LVX and MFX.
LVX and MFX simulated human-like treatments.
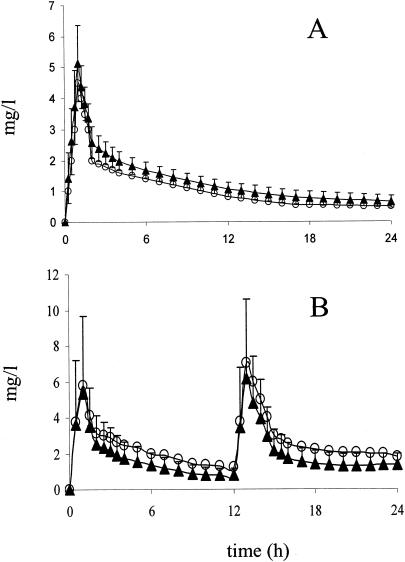
The concentration-time curves of both antibiotics observed for the sera of infected rabbits superimposed on the human pharmacokinetic data as shown in Fig. 1.
FIG. 1.
Pharmacokinetics of MFX (equivalent to the pharmacokinetics in humans at 400 mg once daily [o.d.]) (A) and LVX (equivalent to the pharmacokinetics in humans at 500 mg twice daily [b.i.d.]) (B) in a rabbit model of human therapy. Symbols: ○, desired human concentrations; ▴, obtained concentrations in rabbits.
There was no difference between rabbits groups as defined by strains (Table 2; P > 0.4 for both treatments) with regard to exposure to antibiotics measured by AUC0-24.
TABLE 2.
Pharmacokinetics of a model of human therapy with LVX (equivalent to 500 mg b.i.d. for 48 h) and with MFX (equivalent to 400 mg o.d. for 48 h) in infected rabbits
| Strain | LVX |
MFX |
||||
|---|---|---|---|---|---|---|
| n | AUC0-24 (mg/h liter−1) | Cmax (mg liter−1) | n | AUC0-24 (mg/h liter−1) | Cmax (mg liter−1) | |
| 16089 | 12 | 42.05 ± 14.28 | 5.23 ± 1.39 | 13 | 35.77 ± 2.98 | 5.53 ± 1.24 |
| MS1A | 6 | 56.68 ± 22.36 | 9.38 ± 4.08 | 14 | 38.97 ± 5.96 | 5.69 ± 0.67 |
| MS2A | 10 | 35.55 ± 15.58 | 3.79 ± 1.90 | 9 | 41.30 ± 5.51 | 6.15 ± 1.85 |
| MR3B4 | 10 | 47.39 ± 9.42 | 5.02 ± 1.44 | 11 | 40.32 ± 7.73 | 5.79 ± 0.93 |
| M16 | 8 | 53.93 ± 19.11 | 6.17 ± 2.69 | 11 | 35.82 ± 3.74 | 5.25 ± 0.48 |
| Gyr-1207 | NDa | ND | 10 | 42.50 ± 9.54 | 5.97 ± 1.05 | |
| MQ3A | 7 | 46.32 ± 25.88 | 7.60 ± 4.47 | 5 | 41.66 ± 5.06 | 6.30 ± 1.77 |
| Totalb | 51 | 46.45 ± 18.46 | 6.06 ± 3.20 | 39.03 ± 6.21 | 5.75 ± 1.14 | |
ND, not done.
No statistically significant difference.
The protein bindings (from 0.5 to 8 mg/liter) were 45 and 30% for LVX and MFX, respectively.
Antimicrobial effects of LVX and MFX in a rabbit model of human therapy on experimental pneumococcal pneumonia.
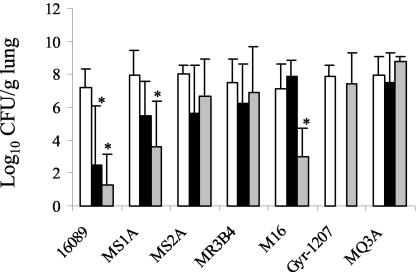
The results are shown in Fig. 2.
FIG. 2.
Pulmonary residual bacterial content (log10 CFU/g) in rabbits infected with pneumococcal strains exhibiting various levels of susceptibility to fluoroquinolones (see Table 1) treated for 48 h in a model of human therapy with LVX (▪; equivalent to 500 mg b.i.d.) or MFX (░⃞; equivalent to 400 mg o.d.) or in a control sample without treatment (□). ✽, P < 0.05 versus controls.
(i) Bacterial reduction after treatment.
As expected, both antibiotics were strongly effective on pulmonary infection due to the fully susceptible strain 16089. The efficacy of MFX seemed better than that of LVX but was not statistically significant (P = 0.21).
For the efflux derivative strain MS1A, in contrast to treatment with LVX, which had little effect, MFX treatment was associated with a significant bacterial reduction (>3 log10 CFU/g) compared to controls without treatment (P < 0.05).
As anticipated, both antibiotics were ineffective in rabbits infected with resistant or intermediate strains, i.e., strains M16 and MQ3A (Gyr-1207 strain not tested) for LVX and strains Gyr-1207 and MQ3A for MFX.
For the rabbits infected with parC mutant strains, the situation was more difficult to describe. For the LVX-resistant and MFX-susceptible strain M16, which harbored a parC83 mutation, a significant bacterial reduction was observed with MFX. For the LVX-borderline-susceptible and MFX-susceptible strains, MS2A and MR3B4, which both harbored a parC79 mutation, there was no significant bacterial reduction with either antibiotic. Similar results were obtained in the spleen (data not shown). The emergence of mutants was looked for to explain these unexpected results.
(ii) In vivo mutants.
Mutants were detected only in rabbits infected with strains already harboring parC mutations. No emerging mutants were detected in animals infected with the MQ3A strain. With both parC79 mutated strains (MS2A and MR3B4), 88 and 100% of the rabbits treated with MFX exhibited resistant mutants, and 20 and 100% of the rabbits treated with LVX exhibited resistant mutants, respectively. The mean pulmonary concentration of these mutants was approximately 5 to 6 log10 CFU/g.
As expected, LVX was completely ineffective in rabbits infected with M16 strain (parC83), since this strain was resistant, and no emerging mutant was detected. MFX treatment was associated with the emergence of mutants in 18% of rabbits infected with this latter strain, with a mean pulmonary concentration of approximately 3 log10 CFU/g. Furthermore, fewer rabbits harbored emerging resistant mutants when infected with M16 strain (parC83) than when infected by MS2A and MR3B4 strains (parC79) (18, 88, and 100%, respectively, P < 0.001).
However, there was no significant difference (P = 0.11) between LVX and MFX in terms of the percentage of rabbits harboring resistant mutants, when rabbits infected with parC79 and parC83 mutated strains were considered as a whole.
All of these mutants acquired a new gyrA mutation with a high MIC (LVX MIC = 16 mg liter−1 and MFX MIC = 4 mg liter−1). Very similar results were obtained in the spleen (data not shown).
PK-PD analysis.
For this analysis, only the free fraction of each antibiotic was considered.
(i) PK-PD with MIC (PK-PDMIC).
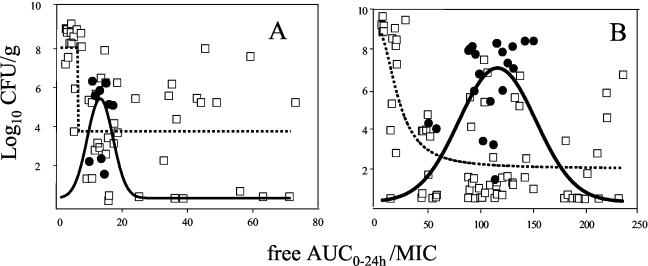
The results of AUC/MIC, Cmax/MIC, and T>MIC analyses for both antibiotics are shown in Table 3. As expected, the values of all of these parameters decreased when the MICs for both antibiotics increased and were higher for MFX than for LVX (for example, from 7.3 to 200 and from 1.7 to 46 for the AUC/MIC ratios, respectively). When these PK-PDMIC results are compared to the antimicrobial efficiency, no clear relationship can be found, either for the pulmonary bacterial reduction or for the presence or absence of emerging mutants. For a more comprehensive analysis, the bacterial contents in the lungs were split into nonmutated and mutated bacterial populations. Under these conditions, a clear relationship in the form of an Emax curve for the nonmutated population and a peak curve for the mutated population was observed for both antibiotics (Fig. 3).
TABLE 3.
Pharmacodynamic parameters with the MICa
| Strain | Mutantb | Mean value ± SD |
|||||
|---|---|---|---|---|---|---|---|
| AUC/MIC |
Cmax/MIC |
T>MIC |
|||||
| LVX | MFX | LVX | MFX | LVX | MFX | ||
| 16089 | −/− | 46 ± 15 | 200 ± 17 | 5.8 ± 1.5 | 31 ± 7 | 36 ± 15 | >100 |
| MS1A | −/− | 31 ± 13 | 117 ± 37 | 5.2 ± 2.4 | 16 ± 2.5 | 35 ± 15 | >100 |
| MS2A | +/+ | 11 ± 5 | 115 ± 15 | 1.2 ± 0.6 | 17 ± 5 | 13 ± 12 | >100 |
| MR3B4 | +/+ | 13 ± 3 | 113 ± 22 | 1.4 ± 0.4 | 16 ± 2.6 | 14 ± 7 | >100 |
| M16 | −/+ | 3.7 ± 1.3 | 50 ± 5 | 0.4 ± 0.2 | 7.6 ± 1.2 | 0 | 36 ± 5.6 |
| Gyr-1207 | −/− | ND | 19 ± 4 | ND | 2.7 ± 0.4 | ND | 44 ± 16 |
| MQ3A | −/− | 1.7 ± 1 | 7.3 ± 0.8 | 0.27 ± 0.17 | 1.1 ± 0.3 | 0.5 ± 0.7 | 4.6 ± 2.3 |
The pharmacodynamic parameters of LVX (500 mg b.i.d.)- and MXF (400 mg o.d.)-treated rabbits with pneumonia exhibiting various susceptibilities to fluoroquinolones were determined and are presented in Tables 3 to 6. In these four tables, all P values as determined by analysis of variance (vertical analysis [treatment between strains]) and the Mann-Whitney test (horizontal analysis [between treatments]) are significant (P < 0.05). †, P > 0.31. ND, not determined. The numbers of animals per strain and per treatment are indicated in Table 2. Results are expressed as the free fraction of antibiotic concentration.
The occurrence of mutant(s) in treated rabbits (LVX/MFX).
FIG. 3.
Relationships of the free 24-h AUC/MIC obtained after treatment with LVX at 500 mg b.i.d. (A) or MFX at 400 mg o.d. (B) in a rabbit model of human therapy, with the residual bacterial pulmonary concentration (log10 CFU/gram) after 48 h of therapy. Symbols: □, the nonmutated bacterial population; •, represent the mutated bacterial population. The dose effect relationship is represented as an Emax curve for the nonmutated bacterial population (dashed line, R2 = 0.45 and 0.45, P < 0.05, for LVX and MFX, respectively) and a peak curve for the mutated bacterial population (solid line, R2 = 0.72 and 0.85, P < 0.05, for LVX and MFX, respectively).
Of note, the PK-PDMIC zone for the occurrence of mutants started at around the EC 90% of the Emax curve for the nonmutated bacterial population and were 52 and 11 for AUC/MIC and 5 and 1 for Cmax/MIC for MFX and LVX, respectively. When the nonmutated population was considered, the efficacy of MFX was clearly higher than for LVX. Indeed, the Emax was only 4.4 log10 CFU/g for LVX and 6.9 log10 CFU/g for MFX.
(ii) PK-PD with MPC (PK-PDMPC) and SI (PK-PDSI).
PK-PD parameters with MPC and SI are shown in Tables 4 and 5. The values of these parameters decreased with the increase in MPCs for both antibiotics. When there was a preexisting parC mutation, the PK-PDMPC values were not proportional to PK-PDMIC due to a sharp increase in the MPCs for these strains. In contrast, for the other strains, either susceptible (16089 and MS1A) or with a gyrA mutation (Gyr-1207) or a double parC-gyrA mutation (MQ3A), the MPCs were close to the MICs and so the PK-PDMPC parameters were close to those for PK-PDMIC.
TABLE 4.
Pharmacodynamic parameters with the MPCa
| Strain | Mean value ± SD |
|||||
|---|---|---|---|---|---|---|
| AUC/MPC |
Cmax/MPC |
T>MPC (h) |
||||
| LVX | MFX | LVX | MFX | LVX | MFX | |
| 16089 | 46 ± 15 | 200 ± 17 | 5.8 ± 1.5 | 31 ± 7 | 36 ± 15 | >100 |
| MS1A | 7.7 ± 3.4 | 117 ± 37 | 1.3 ± 0.6 | 16 ± 2.5 | 5.2 ± 4 | 83 ± 34 |
| MS2A | 0.7 ± 0.3 | 7.2 ± 1 | 0.07 ± 0.03 | 1 ± 0.3 | 0 | 4 ± 1.3 |
| MR3B4 | 0.8 ± 0.16 | 7 ± 1.3 | 0.08 ± 0.02 | 1 ± 0.1 | 0 | 3.6 ± 1.2 |
| M16 | 0.9 ± 0.3 | 12.5 ± 1.3 | 0.1 ± 0.04 | 1.9 ± 0.3 | 0 | 19 ± 5 |
| Gyr-1207 | ND | 9.5 ± 2 | ND | 1.3 ± 0.2 | ND | 9 ± 6 |
| MQ3A | 0.4 ± 0.24 | 7.3 ± 0.8 | 0.06 ± 0.04 | 1.1 ± 0.3 | 0 | 4.6 ± 2.3 |
See Table 3, footnote a.
TABLE 5.
Pharmacodynamic parameters with the SIa
| Strain | Mean value ± SD |
|||
|---|---|---|---|---|
| AUC/SI |
Cmax/SI |
|||
| LEV | MFX | LEV | MFX | |
| 16089 | 23 ± 7.8 | 25 ± 2† | 2.9 ± 0.7 | 3.8 ± 0.8 |
| MS1A | 7.7 ± 3.3 | 29 ± 9 | 1.3 ± 0.6 | 4 ± 0.6 |
| MS2A | 1.2 ± 0.5 | 1.8 ± 0.2 | 0.1 ± 0.06 | 0.2 ± 0.08 |
| MR3B4 | 1.6 ± 0.3 | 1.7 ± 0.3† | 0.1 ± 0.05 | 0.2 ± 0.04 |
| M16 | 7.5 ± 2.6 | 6.2 ± 0.6† | 0.8 ± 0.3 | 0.9 ± 0.1† |
| Gyr-1207 | ND | 14 ± 3 | ND | 2 ± 0.3 |
| MQ3A | 6.8 ± 3.8 | 29 ± 3.5 | 1 ± 0.6 | 4.4 ± 1.2 |
See Table 3, footnote a.
When the PK-PD parameters were calculated by using SI (PK-PDSI), the obtained values for LVX and MFX were close to one another when the MICs and MPCs were also close, whether the strains were susceptible or resistant. When the MPCs were higher than the MICs, these PK-PDSI values decreased.
(iii) PK-PD with the MSW (PK-PDMSW).
TMSW (expressed as a percentage) is the time during which the concentration in serum is between MIC and MPC; the AUCMSW is the area between the MIC and MPC values and the concentration-time curve. These values represent the extent of drug exposure within the MSW. The results are shown in Table 6. The observed values of TMSW and AUCMSW increased as the difference between MICs and MPCs increased for both antibiotics (i.e., parC strains). Of note, the values for each drug were different. For example, the TMSW ranged from 0 to 27% for LVX and from 0 to 96% for MFX.
TABLE 6.
Pharmacodynamic parameters with the MSWa
| Strain | Mean value ± SD |
|||
|---|---|---|---|---|
| AUCMSW |
TMSW |
|||
| LVX | MFX | LVX | MFX | |
| 16089 | 0 | 0† | 0 | 0† |
| MS1A | 15 ± 8 | 3.1 ± 6 | 27 ± 14 | 17.2 ± 34 |
| MS2A | 11 ± 13 | 25 ± 2.6 | 14.8 ± 15 | 96 ± 1 |
| MR3B4 | 12 ± 7.5 | 25 ± 4.2 | 13.8 ± 7.8 | 96.3 ± 1.2 |
| M16 | 0 | 15 ± 1.3 | 0 | 77.9 ± 8 |
| Gyr-1207 | ND | 12 ± 4 | ND | 34.7 ± 10.8 |
| MQ3A | 1.2 ± 2.7 | 0† | 0.4 ± 0.8 | 0† |
See Table 3, footnote a.
Determination of the boundaries of the MSW for the LVX and MFX treatments.
In order to delineate the zone associated with the occurrence of mutants with PK-PD parameters, a qualitative approach was done by using CART analysis.
The results are shown in Table 7. As can be seen, the results for the two antibiotics, regardless of the criteria considered, were very different. For example, the MSW, expressed as the free AUC/MIC ratio, was between 7.6 and 19.4 for LVX and between 50 and 164.5 for MFX.
TABLE 7.
Determination of in vivo boundaries of MSW by using CART analysis from PK-PD results obtained in rabbits infected with pneumococci exhibiting various degrees of susceptibility to fluoroquinolones and treated with LVX (500 mg b.i.d.) or MXF (400 mg o.d.)
| Parameter | MSW |
|||||
|---|---|---|---|---|---|---|
| LVX |
MXF |
|||||
| Lower bound | Superior bound | P | Lower bound | Superior bound | P | |
| AUC/MIC | 7.6 | 19.4 | 0.13 | 50.0 | 164.5 | 0.12 |
| Cmax/MIC | 0.8 | 1.8 | 0.07 | 7.3 | 21.5 | 0.21 |
| T>MIC | 3.5 | 25.5 | 0.13 | 98 | 0.28 | |
| AUC/MPC | 0.5 | 1 | 0.17 | 5.9 | 14.5 | 0.16 |
| Cmax/MPC | 0.05 | 0.1 | 0.05 | 0.9 | 1.9 | 0.10 |
| T>MPC | 26 | 0.1 | ||||
| AUC/SI | 1.1 | 2.25 | 0.11 | 2.2 | 7.3 | 0.04 |
| Cmax/SI | 0.1 | 0.2 | 0.07 | 0.4 | 1 | 0.02 |
| AUCMSW | 0.55 | 0.21 | 14.3 | 27.6 | 0.04 | |
| TMSW | 2 | 25 | 0.10 | 72.5 | 93.5 | 0.02 |
The limits of the MSW were not significantly defined when PK-PDMIC parameters were used. However, significant results were observed for MFX when PK-PDSI, or PK-PDMSW and, to a lesser extent, PK-PDMPC parameters were used. For example, when Cmax/SI was considered, the MSW ranged from 0.1 to 0.2 for LVX (P = 0.07) and from 0.4 to 1 for MFX (p 0.02). When TMSW was used to express the MSW, the percentages ranged from 2 to 25 for LVX (P = 0.1) and from 72.5 to 93.5 for MFX (P = 0.02).
DISCUSSION
Despite the currently low but increasing frequency of pneumococci with low-level resistance to fluoroquinolones, especially parC mutant strains, investigations on the efficacy of new fluoroquinolones on these organisms and patients infected with these infections is worthy of investigation.
In the present study with a rabbit model of human therapy, we showed that the occurrence of mutants is very high in animals when there is a preexisting parC mutation and that the values of the limits of the MSW are higher than the usual values of PK-PD criteria associated with the efficacy of fluoroquinolones (2, 3, 13, 14, 23, 40, 63, 74; O. Vesga and W. Craig, 36th ICAAC). Of note, all of these new in vivo mutants harbored a mutation in the gyrase gene, a finding which is concordant with in vitro data (6, 32, 33, 35, 46, 55).
In vitro, the mutation frequency of pneumococcal strains, with or without the presence of a parC mutation, is ca. 10−7 to 10−8 when the strains are exposed to fluoroquinolones (17, 31, 55). These observations contrast with the high rate of mutant emergence in our animal model, whatever the antibiotic studied. Indeed, after MFX treatment, mutations occurred in 88 to 100% of rabbits infected with a parC79 mutant strain, whereas only 18% of rabbits infected with the parC83 mutated strain exhibited resistant mutants. The mutation rate observed in this in vivo study seems to be higher than that reported in the in vitro PK-PD models (40, 46, 75).
This high in vivo mutation rate is likely to be explained by the high pneumococcal concentration in the lungs in our model (ca. 8 log10 CFU/g). These concentrations allow detection of emerging mutants since the mutation frequency is in the range of 10−7 to 10−8. Importantly, these bacterial concentrations are of the same order of magnitude as those observed in acute exacerbation of chronic bronchitis (50) and less than those observed in pneumococcal human lobar pneumonia (26, 38). Other factors, such as the duration of antibiotic exposure (48 h), sub-MIC concentrations, antibiotic tissue fixation, and ischemic pulmonary phenomena, can interfere with the efficacy of these molecules.
Moreover, the occurrence of resistant mutants in vivo could be anticipated based on the MPC results. Indeed, the MPCs of parC mutant strains were very high. These MPCs are far above the Cmax obtained during standard LVX regimens in humans and close to the Cmax for MFX. These concentrations in humans were reproduced in our rabbit model and were higher than the MICs for the mutated strains but not for the resistant strains, and mutations occurred for those strains with high MPC values (i.e., with a preexisting parC mutation), in other words, when the MSW was wide (measured as a high value of SI, from 4 to 32, depending on the strains and the antibiotic used [see Table 1]). For strains with a low SI, no mutants were recovered in treated rabbits, either because these strains were resistant or because the MPCs were very close or equal to the MICs.
Although the MSW is less well delineated for LVX (perhaps due to the limited activity of this antibiotic), our results confirm the concept of the MSW since the PK-PDMPC, PK-PDSI, and PK-PDMSW parameters are associated with the occurrence of mutants in animals infected with parC strains, whereas the PK-PDMIC parameters are not (Table 7). Indeed, the MSW defined by using the MICs is very large and begins at AUC/MIC ratios that are much higher than the 30 to 50 values usually considered as predictive for the efficacy of fluoroquinolones against pneumococcal infections (3, 13, 22, 40, 74; D. Croisier, D., C. Lequeu, C. Neuwirth, L. Piroth, M. Froidure, H. Portier, and P. Chavanet, 42nd ICAAC, abstr. B-708, 2002; O. Vesga and W. Craig, 36th ICAAC); as suggested, the AUC/MIC ratio is not adequate to predict the emergence of resistant mutants (45). For example, in our model, resistant mutants were recovered for AUC/MICs from 50 to 164.5 with MFX. These findings are completely concordant with the in vitro theory of Zhao et al. (76, 77). Indeed, in our in vivo model, we also observed that, as the antibiotic exposure increases, pulmonary concentrations of susceptible pneumococci first decreased, and then emerging mutants were recovered (Fig. 2). In these conditions, it is not surprising that PK-PD parameters with MPCs, SI, or MSW are more adequate to delineate the MSW (Tables 4 to 6).
Although only the free fraction of antibiotics was used for calculations, it is noteworthy that values of these PK-PD parameters and the MSW bounds are very different for LVX and MFX. This observation can be related to the higher intrinsic antibacterial activity of MFX, as shown by relatively low MIC and MPC values compared to the values of LVX. Furthermore, these differences of in vitro intrinsic activities are also well translated in vivo; indeed, when the bacterial population without emerging mutants was considered, MFX was more active than LVX. Considering these results together and recognizing that only two molecules have been tested to date, we suggest that it will likely be difficult to establish a universal PK-PD parameter for MSW delineation for antibiotics with different intrinsic activities.
Recently, reports of failures to LVX treatment of pneumococcal diseases have been occurring (16, 23, 25, 37, 39, 64, 69, 71). Some of these failures occurred in patients previously exposed to fluoroquinolones and/or when the pneumococcus exhibited an decreased susceptibility to fluoroquinolones, which was sometimes related to a preexisting parC mutation. In our experimental study, we reproduced both situations: mutation and a failure to reduce the pulmonary pneumococcal concentration. It is generally considered that a bacterial reduction of 2 log10 CFU/g is associated with antibacterial efficacy. According to these criteria, MFX remained highly active for pneumonia due to wild-type strain and the strain exhibiting efflux (Fig. 2). However, when there was a preexisting parC mutation, both LVX and MFX treatments were associated with both emerging mutations and a failure to reduce the bacterial pulmonary concentrations (Fig. 1 and 2). These experimental observations are completely concordant with those from clinical situations (29). Considering the current low rate of pneumococci with low-level resistance to fluoroquinolones, a clinical trial to assess the efficacy of these drugs on pneumonia due to such strains is probably unrealistic. Consequently, we think that these experimental investigations in a rabbit model of human therapy, one used for studying difficult clinical situations (i.e., strains with low level of resistance), have to be done in order to determine the clinical situations for which the use of quinolones should or should not be supported.
From our in vivo experimental results based on a rabbit model of human therapy, we conclude that, despite the higher intrinsic efficacy of MFX on pneumococcal pneumonia compared to LVX, the rate of emerging mutation is high and the same for both drugs when a low-level fluoroquinolone resistance due to parC mutations is preexisting. These strains exhibit high MPCs and are associated with the occurrence of mutants when the drug concentrations in serum are between MICs and MPCs (that is, in the MSW), which confirms the in vitro concept. However, the MPC methodology is currently too time-consuming to be done routinely. Thus, because of the limited interest of the MIC tests for the detection of parC mutant strains (4, 49), other rapid tests are needed for the detection of these particular pneumococcal strains to confirm or complement the clinical suspicion of infections due to such strains in order to avoid pneumococcal mutations and/or clinical failures (65). Experimental investigations can bring arguments to approximate PK-PD parameters (using MPCs in addition to MICs) associated with the absence of mutation.
REFERENCES
- 1.Allen, G., G. Kaatz, and M. Rybak. 2003. Activities of mutant prevention concentration-targeted moxifloxacin and levofloxacin against Streptococcus pneumoniae in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 47:2606-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose, P., D. Grasela, T. Grasela, J. Passarell, H. Mayer, and P. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tact infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., and W. Craig. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 46:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bast, D., D. Low, C. Duncan, L. Kilburn, L. Mandell, R. Davidson, and J. de Azvedo. 2000. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contribution of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. 44:3049-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blondeau, J., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boos, M., S. Mayer, A. Fischer, K. Kohrer, S. Scheuring, P. Heisig, J. Verhoef, A. Fluit, and F. Schmitz. 2001. In vitro development of resistance to six quinolones in Streptococcus pneumoniae, Streptococcus pyogenes, and Staphylococcus aureus. Antimicrob. Agents Chemother. 45:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breiman, L., J. Friedman, R. Olshen, and C. Stone. 1984. Classification and regression trees. Wadsworth, Belmont, Calif.
- 8.Brenwald, N., M. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brueggmann, A., S. Coffman, P. Rhomberg, H. Huynh, L. Almer, A. Nilius, and R. Flamm. 2002. Fluoroquinolone resistance in Streptococcus pneumoniae in United States since 1994-1995. Antimicrob. Agents Chemother. 46:680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbon, C., H. Ariza, W. Rabie, C. Salvarezza, D. Elkharrat, M. Rangaraj, and P. Decosta. 1999. Comparative study of levofloxacin and amoxicillin/clavulanic acid in adults with mild-to-moderate community-acquired pneumonia. Clin. Microbiol. Infect. 5:724-732. [Google Scholar]
- 11.Chen, D., A. McGeer, J. de Azavedo, and D. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 12.Comité de l'Antibiogramme de la Société Française de Microbiologie. 1996. Report of the Comité de l'Antibiogramme de la Société Française de Microbiologie: technical recommendations for in vitro susceptibility testing. Clin. Microbiol. Infect. 251:11-25. [Google Scholar]
- 13.Coyle, E., G. Kaatz, and M. Rybak. 2001. Activities of newer fluoroquinolones against ciprofloxacin-resistant Streptococcus pneumoniae Antimicrob. Agents Chemother. 45:1654-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croisier, D., P. Chavanet, C. Lequeu, A. Ahanou, A. Nierlich, C. Neuwirth, L. Piroth, M. Duong, M. Buisson, and H. Portier. 2002. Efficacy and pharmacodynamics of simulated human-like treatment with levofloxacin on experimental pneumonia induced with penicillin-resistant pneumococci with varying susceptibilities to fluoroquinolones J. Antimicrob. Chemother. 50:349-360. [DOI] [PubMed] [Google Scholar]
- 15.Dalhoff, A. 2001. Comparative in vitro and in vivo activity of the C-8 methoxy quinolone moxifloxacin and the C-8 chlorine quinolone BAY y3118. Clin. Infect. Dis. 32(Suppl. 1):S16-S21. [DOI] [PubMed] [Google Scholar]
- 16.Davidson, R., R. Cavalcanti, J. Brunton, D. Bast, J. de Azvedo, P. Kibsey, C. Fleming, and D. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 17.Davies, T., G. Pankuch, B. Dewasse, M. Jacobs, and P. Appelbaum. 1999. In vitro development of resistance to five quinolones and amoxicillin-clavulanate in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:1177-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doern, G., K. Heilman, H. Huynh, P. Rhomberg, S. Coffman, and A. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rate since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong, Y., X. Zhao, J. Domagala, and K. Drlica. 1999. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feikin, D., A. Schuchat, M. Lolczak, N. Barrett, L. Harrison, L. Lefkowitz, A. McGeer, M. Farley, D. Vugia, C. Lexau, K. Stefonek, J. Patterson, and J. Jorgensen. 2000. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am. J. Public Health 90:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.File, T., J. Segreti, L. Dunbar, R. Player, R. Kohler, R. Williams, C. Kojak, and A. Rubin. 1997. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community acquired pneumonia. Antimicrob. Agents Chemother. 41:1965-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finch, R., D. Schürmann, O. Collins, R. Kubin, J. McGivern, H. Bobbaers, J. Izquierdo, P. Nikolaides, F. Ogundare, R. Raz, P. Zuck, and G. Hoeffken. 2002. Randomized controlled trial of sequential intravenous (i.v.) and oral moxifloxacin compared with sequential i.v. and oral co-amoxiclav with or without clarithromyin in patients with community-acquired pneumonia requiring initial parental treatment. Antimicrob. Agents Chemother. 46:1746-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firsov, A., I. Lubenko, S. Vostrov, O. Kononenko, S. Zinner, and Y. Portnoy. 2000. Comparative pharmacodynamics of moxifloxacin and levofloxacin in an in vitro dynamic model: prediction of the equivalent AUC/CMI breakpoints and equifficient doses J. Antimicrob. Chemother. 46:725-732. [DOI] [PubMed] [Google Scholar]
- 24.Frank, E., J. Liu, G. Kinasewitz, G. Moran, M. Oross, W. Olson, V. Reichl, S. Freitag, N. Bahal, B. Wiesinger, A. Tennenberg, and J. Kahn. 2002. A multicenter, open-label, randomized comparison of levofloxacin and azithromycin plus ceftriaxone in hospitalized adults with moderate to severe community-acquired pneumonia. Clin. Therapeut. 24:1292-1308. [DOI] [PubMed] [Google Scholar]
- 25.Fredenucci, I., M. CHomarat, R. Bercion, A. Carricajo, M. Celard, J. Croize, F. Delubac, D. Fevre, C. Fuhrmann, M. Helfre, M. Letouzey, H. Lelievre, A. Mandjee, P. Marthelet, R. Meley, J. Perrier-Gros-Claude, A. Ros, C. Roure, S. Smati, J. Thierry, and J. Tous. 2002. Comparative in vitro activity of penicillin G, levofloxacin, moxifloxacin, telithromycin, pristinamycin, quinupristin-dalfopristin and linezolid against ofloxacin-intermediate and -resistant Streptococcus pneumoniae. Clin. Microbiol. Infect. 8:680-683. [DOI] [PubMed] [Google Scholar]
- 26.Frisch, A., J. Tripp, C. Barrett, and B. Pidgeon. 1942. The specific polysaccharide content of pneumonic lungs. J. Exp. Med. 76:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill, M., N. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glatz, K., D. Szabo, G. Szabo, D. Boriszova, and F. Rozgonyi. 2001. Emergence of extremely high penicillin and cefotaxime resistance and high-level levofloxacin resistance in clinical isolates of Streptococcus pneumoniae. J. Antimicrob. Chemother. 48:731-734. [DOI] [PubMed] [Google Scholar]
- 29.Ho, P., W. Tse, K. Tsang, T. Kwok, T. Ng, V. Cheng, and R. Chan. 2001. Risk factors for acquisition of levofloxacin-resistant Streptococcus pneumoniae: a case-control study. Clin. Infect. Dis. 32:701-707. [DOI] [PubMed] [Google Scholar]
- 30.Ho, P., R. Yung, D. Tsang, T. Que, M. Ho, W. Seto, T. Ng, W. Yam, and W. Ng. 2001. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J. Antimicrob. Chemother. 48:659-665. [DOI] [PubMed] [Google Scholar]
- 31.Houssaye, S., L. Gutmann, and E. Varon. 2002. Topoisomerase mutations associated with in vitro selection of resistance to moxifloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:2712-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janoir, C., E. Varon, M. Kitzis, and L. Gutmann. 2001. New mutation in parE in a pneumococcal in vitro mutant resistant to fluoroquinolones. Antimicrob. Agents Chemother. 45:952-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janoir, C., V. Zeller, M. Kitzis, N. Moreau, and L. Gutmann. 1996. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob. Agents Chemother. 40:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, M., J. Karlowsky, R. Blosser-Middleton, I. Critchley, E. Karginova, C. Thornsberry, and D. Sahm. 2002. Longitudinal assessment of antipneumococcal susceptibility in the united states. Antimicrob. Agents Chemother. 46:2651-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorgensen, J., L. Weigel, J. Swenson, C. Whitney, M. Ferraro, and F. Tenover. 2000. Activities of clinafloxacin, gatifloxacin, gemifloxacin, and trovafloxacin against recent clinical isolates of levofloxacin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2962-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalin, M., A. Ortqvist, M. Almela, E. Aufwerber, R. Dwyer, B. Henriques, C. Jorup, I. Julander, T. Marrie, M. Mufson, R. Riquelme, A. Thalme, A. Torres, and M. Woodhead. 2000. Prospective study of prognostic factors in community-acquired bacteremic pneumococcal disease in five countries. J. Infect. Dis. 182:840-847. [DOI] [PubMed] [Google Scholar]
- 37.Kays, M., D. Smith, M. Wack, and G. Denys. 2002. Levofloxacin treatment failure in a patient with fluoroquinolone-resistant Streptococcus pneumoniae pneumonia. Pharmacotherapy 22:395-399. [DOI] [PubMed] [Google Scholar]
- 38.Knapp, B., and T. Kent. 1968. Postmortem lung cultures. Arch. Pathol. 85:200-203. [PubMed] [Google Scholar]
- 39.Kuehnert, M., F. Nolte, and C. Perlino. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. Ann. Intern. Med. 131:312-313. [DOI] [PubMed] [Google Scholar]
- 40.Lacy, M., W. Lu, X. Xu, P. Tessier, D. Nicolau, R. Quintiliani, and C. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, X., X. Zhao, and K. Drlica. 2002. Selection of Streptococcus pneumoniae mutants having reduced susceptibility to moxifloxacin and levofloxacin. Antimicrob. Agents Chemother. 46:522-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lister, P. 2002. Pharmacodynamics of gatifloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model: impact of area under the curve/MIC ratios on eradication. Antimicrob. Agents Chemother. 46:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loh, W., and Y. Shih. 1997. Split selection methods for classification trees. Statistica Sinica 7:815-840. [Google Scholar]
- 44.Low, D., K. Weiss, T. Mazzulli, M. Kuhn, D. Church, K. Forward, G. Zhanel, A. Simor, and A. Mcgeer. 2002. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in Canada during 2000. Antimicrob. Agents Chemother. 46:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacGowan, A., C. Rogers, H. Holt, and K. Bowker. 2003. Activities of moxifloxacin against, and emergence of resistance in Streptococcus pneumoniae and Pseudomonas aeruginosa in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 47:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madaras-Kelly, K., C. Daniels, M. Hegbloom, and M. Thompson. 2002. Pharmacodynamics characterization of efflux and topoisomerase IV-mediated fluoroquinolone resistance in Streptococcus pneumoniae J. Antimicrob. Chemother. 50:211-218. [DOI] [PubMed] [Google Scholar]
- 47.Markham, P. 1999. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob. Agents Chemother. 43:988-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metlay, J., J. Hofmnn, M. Cetron, M. Fine, M. Farley, C. Whitney, and R. Breiman. 2000. Impact of penicillin susceptibility on medical outcomes for adult patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 30:520-528. [DOI] [PubMed] [Google Scholar]
- 49.Millichap, J., E. Pestova, F. Siddiqui, G. Noskin, and L. Peterson. 2001. Fluoroquinolone resistance is a poor surrogate marker for type II topoisomerase mutations in clinical isolates of Streptococcus pneumoniae. J. Clin. Microbiol. 39:2719-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monso, E., J. Ruiz, A. Rosell, J. Manterola, J. Fiz, J. Morera, and V. Ausina. 1995. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 152:1316-1320. [DOI] [PubMed] [Google Scholar]
- 51.Morita, J., E. Zell, R. Danila, M. Farley, J. Hadler, L. Harrison, L. Leftkowitz, A. Reingold, B. Kupronis, A. Schuchat, and C. Whitney. 2002. Association between antimicrobial resistance among pneumococcal isolates and burden of invasive pneumococcal disease in the community. Clin. Infect. Dis. 35:420-427. [DOI] [PubMed] [Google Scholar]
- 52.Morrissey, I., and J. George. 1999. Activities of fluoroquinolones against Streptococcus pneumoniae type II topoisomerase purified as recombinant proteins. Antimicrob. Agents Chemother. 43:2579-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mufson, M., and R. Stanek. 1999. Bacteremic pneumococcal pneumonia in one American city: a 20-year longitudinal study, 1978-1997. Am. J. Med. 107(1A):34S-43S. [DOI] [PubMed] [Google Scholar]
- 54.Musher, D. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-809. [DOI] [PubMed] [Google Scholar]
- 55.Nagai, K., T. Davies, B. Dewasse, M. Jacobs, and P. Appelbaum. 2001. Single- and multistep resistance selection study of gemifloxacin compared with trovafloxacin, ciprofloxacin, gatifloxacin, and moxifloxacin in Streptococcus pneumoniae. J. Antimicrob. Chemother. 48:365-374. [DOI] [PubMed] [Google Scholar]
- 56.Norrby, S., W. Petermann, P. Willcox, N. Vetter, and E. Salewski. 1998. A comparative study of levofloxacin and ceftriaxone in the treatment of hospitalized patients with pneumonia. Scand. J. Infect. Dis. 30:397-404. [DOI] [PubMed] [Google Scholar]
- 57.Pallares, R., O. Capdevila, J. Linares, I. Grau, H. Onaga, F. Tubau, M. Schulze, P. Hohl, and F. Gudiol. 2002. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am. J. Med. 113:120-126. [DOI] [PubMed] [Google Scholar]
- 58.Pan, X., J. Ambler, S. Mehtar, and L. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez-Trallero, E., C. Fernandez-Mazarrasa, C. Garcia-Rey, E. Bouza, L. Aguilar, J. Garcia-De-Lomas, F. Baquero, A. T. Spanish, et al. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob. Agents Chemother. 45:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez-Trallero, E., C. Garcia-Rey, A. Martin-Sanchez, L. Aguilar, J. Garcia-de-Lomas, J. Ruiz, et al. 2002. Activities of six different quinolones against clinical respiratory isolates of Streptococcus pneumoniae with reduced susceptibility to ciprofloxacin in Spain. Antimicrob. Agents Chemother. 46:2665-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petitpretz, P., P. Arvis, M. Marel, J. Moita, J. Urueta, et al. 2001. Oral moxifloxacin versus high-dosage amoxicillin in the treatment of mild-to-moderate, community-acquired, suspected pneumococcal pneumonia in adults. Chest 119:185-195. [DOI] [PubMed] [Google Scholar]
- 62.Piroth, L., L. Martin, A. Coulon, C. Lequeu, M. Duong, M. Buisson, H. Portier, and P. Chavanet. 1999. Development of a new experimental model of penicillin-resistant Streptococcus pneumoniae pneumonia and amoxicillin treatment by reproducing human pharmacokinetics. Antimicrob. Agents Chemother. 43:2484-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preston, S., G. Drusano, A. Bernam, C. Fowler, A. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 64.Ross, J., M. Worthington, and S. Gorbach. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 347:65-66. [DOI] [PubMed] [Google Scholar]
- 65.Scheld, W. 2003. Maintaining fluoroquinolone class efficacy: review of influencing factors. Emerg. Infect. Dis. 9:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmitz, F., M. Boos, S. Mayer, D. Hafner, H. Jagusch, J. Verhoef, and A. C. Fluit. 2001. Propensity of fluoroquinolones with different moieties at position 8 to cause resistance development in clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2666-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stass, H., and D. Kubitza. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 43(Suppl. B):83-90. [DOI] [PubMed] [Google Scholar]
- 68.Thorburn, C., and D. Edwards. 2001. The effect of pharmacokinetics on the bactericidal activity of ciprofloxacin and sparfloxacin against Streptococcus pneumoniae and the emergence of resistance. J. Antimicrob. Chemother. 48:15-22. [DOI] [PubMed] [Google Scholar]
- 69.Urban, C., N. Rahman, X. Zhao, N. Mariano, S. Segal Maurer, K. Drlica, and J. Rahal. 2001. Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. J. Infect. Dis. 184:794-798. [DOI] [PubMed] [Google Scholar]
- 70.Watanakunakorn, C., and T. Bailey. 1997. Adult bacteremic pneumococcal pneumonia in a community teaching hospital, 1992-1996: a detailed analysis of 108 cases. Arch. Intern. Med. 157:1965-1971. [PubMed] [Google Scholar]
- 71.Weiss, K., C. Restieri, R. Gauthier, M. Laverdière, A. McGeer, R. Davidson, L. Kilburn, D. Bast, J. de Azavedo, and D. Low. 2001. A nosocomial outbreak of fluoroquinolone-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 33:517-522. [DOI] [PubMed] [Google Scholar]
- 72.Whitney, C., M. Farley, J. Hadler, L. Harrisson, C. Lexau, A. Reingold, L. Lefkowitz, P. Cieslak, M. Cetron, E. Zell, J. Jorgensen, A. Schuchat, et al. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1971-1974. [DOI] [PubMed] [Google Scholar]
- 73.Yokota, S., K. Sato, O. Kuwahara, S. Habadera, N. Tsukamoto, H. Ohuchi, H. Akizawa, T. Himi, and N. Fujii. 2002. Fluoroquinolone-resistant Streptococcus pneumoniae strains occur frequently in elderly patients in Japan. Antimicrob. Agents Chemother. 46:3311-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhanel, G., D. Roberts, A. Waltky, N. Laing, K. Nichol, H. Smith, A. Noreddin, T. Bellyou, and D. Hoban. 2002. Pharmacodynamic activity of fluoroquinolones against ciprofloxacin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 49:807-812. [DOI] [PubMed] [Google Scholar]
- 75.Zhanel, G., M. Walters, N. Laing, and D. Hoban. 2001. In vitro pharmacodynamic modeling simulating free serum concentrations of fluoroquinolones against multidrug-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 47:435-440. [DOI] [PubMed] [Google Scholar]
- 76.Zhao, X., and K. Drlica. 2002. Restricting the selection of antibiotic-resistant mutant bacteria: measurement and potential use of the mutant selection window. J. Infect. Dis. 185:561-565. [DOI] [PubMed] [Google Scholar]
- 77.Zhao, X., and K. Drlica. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl. 3):S147-S156. [DOI] [PubMed] [Google Scholar]
- 78.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]